Mesenchymal stem cells (MSCs) originate in the
embryonic mesoderm and can be isolated from several mesenchymal
tissues in adults, including the bone marrow (1), adipose tissue (2), craniofacial sutures (3), mouse incisor cervical loop (4), periodontal ligament (5), synovial membrane (6), menstrual fluid (7), dental pulp (8) and umbilical cord blood (9). The definition of classical MSCs was
established by the International Society of Cellular Therapy based
on in vitro studies in 2006 (10). Briefly, three criteria must be
satisfied: i) Typical MSCs must adhere to the plastic plate under
standard tissue culture conditions; ii) MSCs must express specific
cell surface markers, such as cluster of differentiation (CD)73,
CD90 and CD105, and lack certain hematopoietic stem cell markers,
including the lipopolysaccharide receptor CD14, CD34 and the
leukocyte common antigen CD45; and iii) these cells must have the
capacity to be induced to differentiate into adipocytes,
osteoblasts and chondrocytes (10,11).
Recently, due to MSCs' high self-renewal ability, multi-lineage
differentiation potential and immunomodulatory capacity, studies
have been devoted to improving the clinical applications of MSCs in
tissue regeneration, with or without the aid of a bioengineering
scaffold. Several studies have reported the positive therapeutic
effects of MSCs (12,13); however, certain questions and
challenges arise during the application of MSC therapy, such as the
risk for MSC transformation, tumor formation, potential adverse
inflammatory effects and thrombosis associated with intravenous
infusion of MSCs (14). A previous
study reported that the majority of engrafted MSCs died within a
few days, making it very difficult to replace the lost tissues, but
some of the cells were incorporated into tissues following
long-term observation (15). To
date, the safety of MSC treatment has been proven, but the efficacy
and consequent interactions within the host microenvironment remain
controversial to a certain degree (16).
Recently, the majority of studies have attributed
the failure of stem cell therapy to the imbalances in the MSC niche
(12–14). Over 40 years ago, a specialized
regulatory bone-marrow (BM) microenvironmental niche was proposed,
where stem cells reside, receive appropriate support for
maintaining self-renewal and multi-lineage differentiation
capacity, and are protected from environmental stress (16). Crosstalk between various niche
signals maintains the stem cells in a dynamic balance (17–19).
The niche components, including perivascular nerve, endothelial
cells and special megakaryocytes, secrete various bioactive
proteins, such as mitochondrial inner membrane protein (also known
as Sonic hedgehog) (4), WNT, stem
cell factors (20), chemokines
(C-X-C motif) ligand (CXCL)12 (21)
and transforming growth factor-β (TGF-β) (22), to participate in MSC maintenance,
quiescence, activation and lineage commitment activity. When niche
components are ablated, stem cells fail to respond to tissue
regeneration cues (23),
underscoring the significance of the niche in dictating stem cell
behavior (22–24). The activation of signaling pathways
is usually switched on, with these pathways mediating stem cell
status. Several signaling pathways participate in stem cell
activity, including the Notch, Hedgehog (Hh) and bone morphogenetic
protein (BMP) signaling pathways. Of note, these signaling pathways
exhibit crosstalk with each other, and this determines the activity
of cells (25). The Hh signaling
pathway is associated with the risk of developing several diseases.
The biological and pathogenic importance of Hh signaling emphasizes
the need to control its action tightly, both physiologically and
therapeutically (26). Notch
signaling contains both canonical and noncanonical pathways, is
involved in the proliferation, differentiation and survival of
multiple types of tissues, and can increase the survival and
self-renewal of hematopoietic progenitors in the hematopoietic
system (27). The BMP signaling
pathway is a well studied pathway, includes the family members BMP2
and 4, and is associated with the TGF-β family. The TGF family
plays important roles in embryonic development and in the
maintenance of tissue homeostasis (28).
During homeostasis, the concerted action of local
positive and negative regulatory niche signals helps maintain adult
MSCs in dynamic balance. However, if one certain stimulus is out of
control, stem cells cannot maintain their normal function. To cite
an example, under periodontal tissue infection (periodontitis), the
regeneration of periodontium is hard to achieve (29), which indicates that severe infection
may inhibit adult stem cells. Currently, various studies have
attempted to explain the way in which the MSCs modify immune
responses to improve tissue regeneration ability, with less
research focusing on the way in which infectious niche environments
influence the activities of adult MSCs. To date, several in
vivo and in vitro preliminary studies have shown that
the downregulation of MSC bioactivity by infection is mainly
mediated by the inhibition of the WNT pathway (30). The role of WNT signaling in the
control of MSC biology has been well documented (19,30).
Transcriptomic and proteomic approaches, such as ELISA and western
blotting, have revealed the enrichment of both canonical and
noncanonical WNT pathway components in MSCs (31). The activation of the WNT pathway
plays a critical role in cell fate decisions, particularly for MSC
proliferation, self-renewal and differentiation. Furthermore, WNT
signaling modulation in MSCs has been widely investigated to fully
exploit the regenerative properties of MSCs in different fields,
such as bone, lung and heart biology (32,33).
Our previous study also demonstrated that a decreased expression
level of the WNT pathway during periodontitis and overactivation
may rescue periodontal tissue loss. In the present review, the WNT
pathway and the recent discoveries regarding its role in adult MSCs
were summarized. Additionally, the means by which inflammatory
signaling can alter MSCs by modifying WNT signaling was
explored.
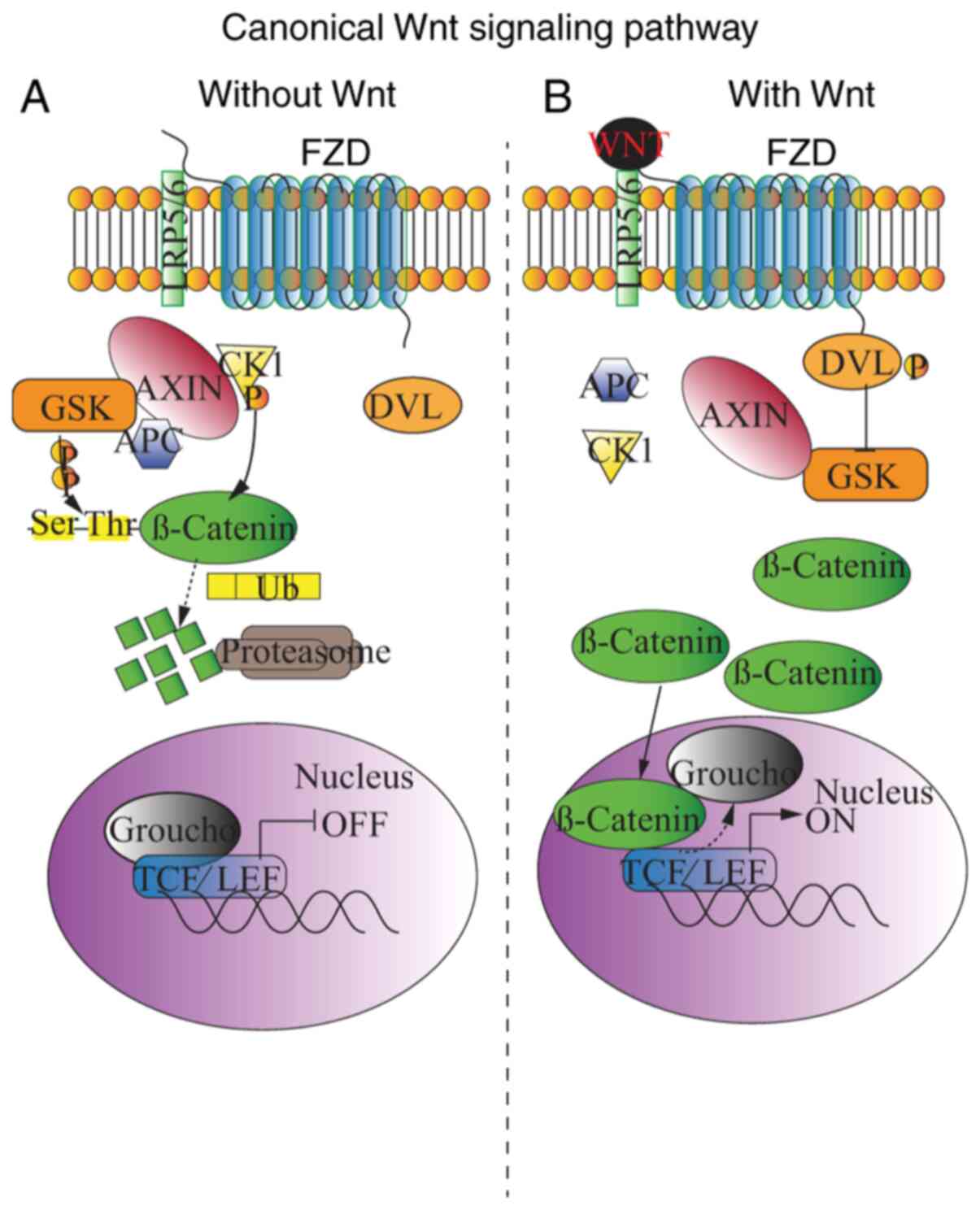
The β-catenin-dependent canonical WNT pathway
appears to be the most conserved pathway in both vertebrates and
invertebrates and plays a dual role in adherent junctions and
transcriptional regulation (37–39).
The receptors in the WNT signaling pathway include single-pass
transmembrane co-receptor LRP5/6 and seven transmembrane signaling
receptors, FZD (44). The key
element is the destruction complex consisting of the scaffold
protein Axis inhibitor (AXIN), adenomatous polyposis coli (APC) and
casein kinase 1 (CK1). Without WNT ligands, the destruction complex
in the cytoplasm is activated to phosphorylate downstream
β-catenin. APC binds β-catenin and CK1 and primes the protein for
the subsequent phosphorylation mediated by recruited glycogen
synthase kinase 3 (GSK3) at threonine and serine residues. The
phosphorylated β-catenin is then degenerated by E3 ubiquitin ligase
and transferred to proteasomes (Fig.
1A). When the WNT ligands are bonded, dishevelled (DVL) protein
is recruited by FZD, and the phosphorylated DVL then phosphorylates
and inhibits GSK3. β-catenin is thus protected from GSK3-dependent
phosphorylation. Subsequently, non-phosphorylated β-catenin is
translocated into the nucleus, where it displaces the co-repressor
groucho from the transcriptional factor groucho/T cell
factor/lymphoid-enhancing factor (TCF/LEF) complex, thereby
triggering the transcription of WNT target genes, which mediate MSC
bioactivity (Fig. 1B) (45–47).
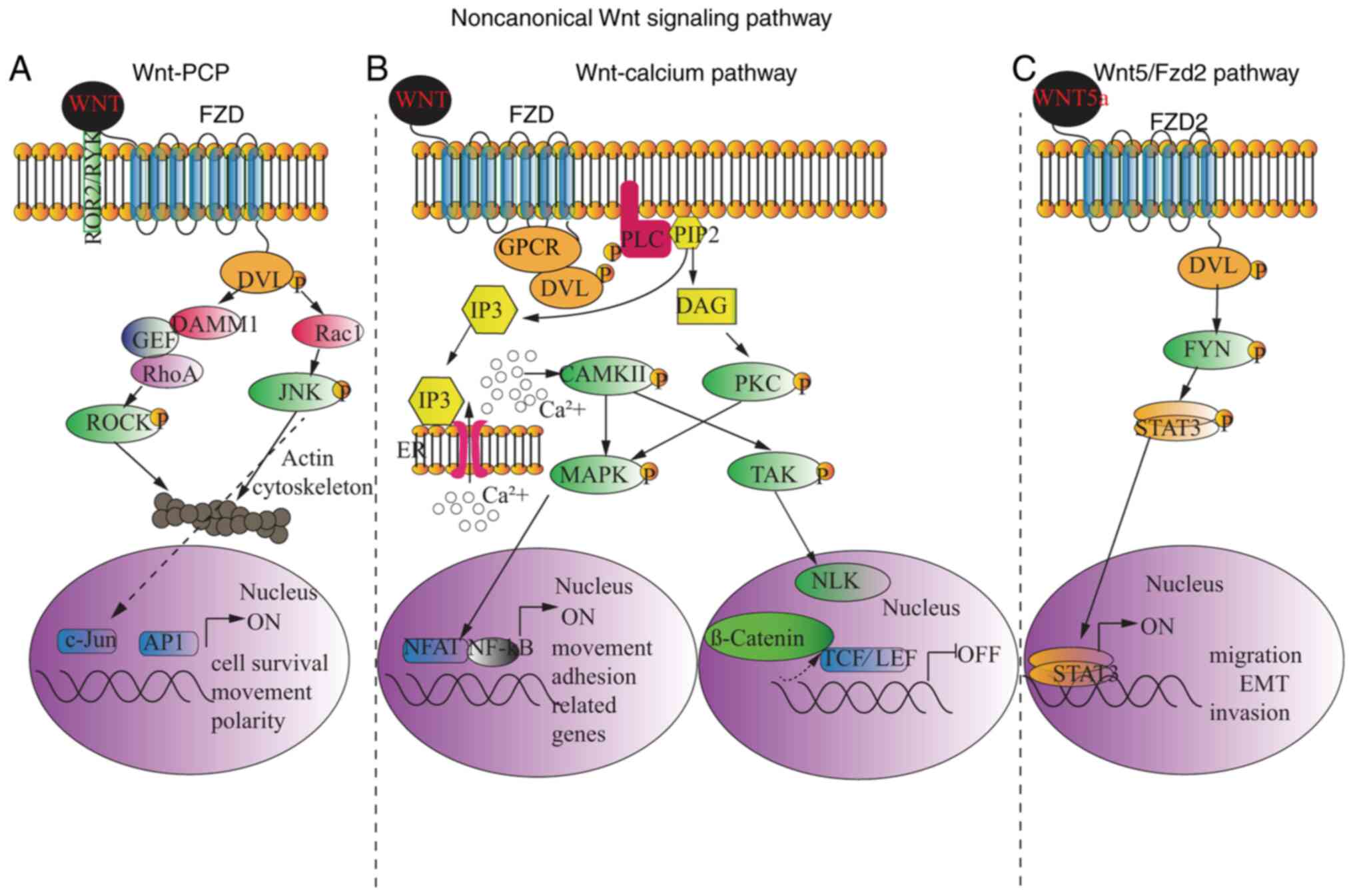
To date, two distinct noncanonical WNT signaling
pathways have been identified, with different impacts on cell
activities. Similar to canonical WNT signaling, DVL is recruited
and activated, and the subsequent cascade is meditated by the
DVL-associated activator of morphogenesis 1 or DVL-associated small
signaling G-protein Rac1. One of these pathways is the WNT-PCP
pathway, which is initiated following binding of WNT to FZD
together with a transmembrane co-receptor, followed by the
activation of the small G-protein Rho by either orphan receptor
tyrosine kinase-like receptor 2 (ROR2) or receptor tyrosine kinase
through a guanine exchange factor (GEF), followed by the activation
of Rho-associated protein kinase (48), an important regulator of the
cytoskeleton. As the alternative pathway, Rac1 activates
transcription factors through cJNK activation, which is involved in
cytoskeleton modification, as well as targets the expression of
genes regulating cell survival, movement and polarity (Fig. 2A) (49,50).
Another signaling pathway is the WNT-calcium
pathway, which reduces cell adhesion (Fig. 2B). Phosphorylated DVL, following the
binding of WNT ligands to FZD, leads to phospholipase C activation
and the subsequent formation of inositol-1,4,5-triphosphate (IP3)
and diacylglycerol (DAG) from the cell membrane component,
phosphatidylinositol-4,5-bisphosphate (PIP2). IP3 binds to its
receptor in the endoplasmic reticulum (ER) membrane, resulting in
the release of intracellular calcium (47,49,50).
Calcium movement in the cytoplasm then activates
calcium-/calmodulin-dependent protein kinase II (CAMKII), which
causes nuclear translocation of activated T cells (NFAT) family of
transcription factors through the phosphorylation of MAPKs
(51), while DAG activates PKC and
MAPKs, which all target NFAT inside the nucleus to govern cell
movement and adhesion. In addition, the noncanonical WNT-calcium
pathway has been reported to inhibit the canonical WNT/β-catenin
pathway, since CAMKII can trigger TGF-β activated kinase 1 (TAK1)
phosphorylation, which in-turn enhances the activity of Nemo-like
kinase (NLK), leading to LEF1-β-catenin/DNA dissociation (Fig. 2B) (52). Recently, another special
noncanonical pathway leading to epithelial-mesenchymal transition
in cancer cells was discovered (53). Upon WNT5a ligand binding, FZD2
exclusively recruits and phosphorylates DVL. The pathway is
achieved through the activation of STAT3 by FYN. STAT3 is
translocated to the nucleus and triggers cell migration-related
target gene expression, epithelial-mesenchymal transition and tumor
cell invasion responsible for tumor metastasis (Fig. 2C) (53).
Canonical WNT signaling is known to be a critical
stem cell niche-regulating pathway in several tissues, such as bone
marrow and craniofacial sutures (3,54). WNT
signaling has been shown to enhance the effect of osteogenic
differentiation of bone marrow BMSC through the canonical WNT
pathway. Impaired osteogenic differentiation of zinc
metallopeptidase STE24−/−BM-MSCs can be partly
attributed to decreased calcium expression, which leads to the
inhibition of the canonical WNT pathway (54). Gli1+ cells have been proven to
localize MSCs throughout the craniofacial sutures, including
sagittal, frontal-premaxilla, palatal, coronal and lambdoid sutures
(3). Another study supporting these
findings also mentioned that suture MSCs express AXIN2 and are
characteristic of long-term self-renewal and clonal expansion
during calvarial development and homeostatic maintenance (55). Recently, an exogenous WNT3a protein
delivered through liposomes was found to accelerate craniofacial
tissue healing (47,56), providing potential therapeutic
strategies to promote MSC-based tissue regeneration through the
high expression of the WNT signaling pathway (57).
Numerous studies have underscored the importance of
WNT signaling as a critical mediator of the homeostasis of MSCs,
which are powerful stem cell factors that control the stemness,
self-renewal and proliferation of multiple adult stem cell
populations (58). Furthermore, the
WNT signaling pathway has different impacts on MSCs tri-lineage
differentiation (26).
Overall, the WNT signaling pathway inhibits MSC
adipogenic differentiation. It has been demonstrated that the
expression levels of WNT antagonists sFRP4 and dickkopf WNT
signaling pathway inhibitor 1 (Dkk1) were higher in adipogenically
differentiated than in undifferentiated MSCs (59). Moreover, a short 48 h treatment of
human MSCs with secreted frizzled-related protein (sFRP)1 and sFRP4
was reported to upregulate adiponectin secretion, thereby promoting
adipogenesis (60,47). A few clinical trials explored the
association between WNT activity, and obesity and diabetes. One of
these trials concluded that sFRP4 levels were positively correlated
with impaired glucose and triglyceride metabolism (61). Certain studies have used sFRP4 as a
predictor of type II diabetes mellitus (62,63).
Conversely, a previous study showed that the GSK-3β inhibitor
6-bromo-indirubin-3α-oxime could inhibit MSC adipogenesis, as a
result of enhanced WNT signaling (64). Collectively, this evidence showed
the inhibitory regulation of WNT on the differentiation of MSCs
into adipocytes, suggesting that WNT activators can be used for the
treatment of obesity, although this remains under extensive
investigation.
There are a number of controversies regarding the
function of the WNT pathway in MSC chondrogenesis, which may be a
result of the complicated interactions within the WNT signaling
network. It was previously shown that an enhanced chondrogenic
activity on sFRP1-deficient mice highlighted the positive effect of
WNT signaling on MSC chondrogenic differentiation (65). In addition, the activation of
noncanonical WNT following WNT5a ligand binding was also found to
induce the chondrogenesis of MSCs derived from the chicken wing bud
(66). Similarly, an in
vitro study showed that WNT3a overexpression was associated
with the chondrogenic differentiation of C3H10T1/2 murine
mesenchymal cells (67). The
increase in pre-cartilage condensation in an ex vivo MSC
culture in the presence of WNT5a protein further confirmed a
promoting effect of WNT signaling on MSC chondrogenesis (66). The underlying mechanism indicates
that WNT5a activates the noncanonical WNT pathway and blocks the
canonical pathway. However, a different study reported a reduced
expression of chondrogenic-specific markers, such as collagen II,
SRY-box transcription factor (Sox)9 and aggrecan following
continuous treatment with WNT1 protein for 21 days, whereas
treatment with the WNT antagonist Dkk1 increased their expression
in human adipose-derived MSCs (68). Consistently, a large number of in
vitro studies emphasized the importance of suppressed WNT
signaling during chondrogenesis. For example, sFRP1 and Dkk1 could
accelerate the initial stages of human MSC chondrogenesis, as
indicated by the enhanced glycosaminoglycan synthesis, Sox9 and
type II collagen expression (69);
these WNT antagonists also exhibited a chondrogenesis-promoting
effect in long-term pellet cultures (70). Most of these conclusions were made
based on in vitro studies, where the concentration of the
WNT pathway regulators and the absence of other interacting
pathways could be the reason for the controversial findings.
Therefore, these findings warrant additional and more accurate
investigations.
It has been well-established that the WNT signaling
pathway is indispensable for MSC osteogenesis. The canonical WNT
signaling pathway activates the differentiation of MSCs into
osteoblasts (71). Current research
also suggests that the canonical WNT pathway is essential for the
contribution of Gli1+ MSCs to alveolar bone development and the
blocking of the canonical WNT pathway in catenin conditional
knockout mice, which caused severe bone loss (72). The disruption of WNT signaling by a
functional mutation or targeted destruction of LRP5 in mice has
been shown to promote osteoporosis and a low bone mass phenotype
(73,74), whereas its overexpression has been
shown to lead to a high bone mass syndrome (75). The WNT/β-catenin signaling functions
like a switch, favoring MSC osteogenesis at the expense of
adipogenesis by modulating the availability of cell-type-specific
transcription factors (76).
Recently, Src homology region 2 domain-containing phosphatase-1
(SHP1) was found to bind with GSK3 and suppress its kinase
activity. In SHP1 partial deficient mice (mev/mev), phosphorylated
GSK3 mediated increased β-catenin degradation, thus these mice
developed osteoporosis. Lineage differentiation culture of the bone
marrow MSCs extracted from mev/mev mice displayed less osteogenesis
and more adipogenesis (77). As
expected, WNT antagonism was reported to inhibit osteogenesis. This
inhibitory effect was demonstrated by an in vitro study,
where sFRP4 inhibited the periodontal MSCs committed to osteogenic
progenitor cells (78). Amongst
other WNT antagonists, sFRP1 overexpression was found to inhibit
in vivo bone formation (79)
and deteriorate osteoblast and osteocyte apoptosis (80). Comparatively, the lack of sFRP1
reduced apoptosis, accelerated MSC osteogenic differentiation
(81), enhanced trabecular bone
formation and improved fracture healing (82–84),
confirming the inhibitory effect of WNT antagonists on
osteogenesis. In addition, sFRP3 has been shown to repress the
noncanonical WNT pathway by binding to WNT5a, which in turn blocks
the inhibitory effect of WNT5a on the canonical pathway, hence
promoting osteogenesis (78).
Collectively, this evidence supported the positive regulation of
the canonical WNT pathway on MSC osteogenic differentiation. The
extensive crosstalk within the WNT signaling network during
osteogenic differentiation should be considered when devising
possible new therapeutic approaches for bone-related diseases.
MSCs' niche homeostasis is influenced by the
internal and external microenvironments, including pathogens, DNA
damage and metabolic stress. Microbiota, as an external stimulus,
plays a regulatory role in MSC health. The absence of MHC class-II
and low expression levels of class-I antigens endow MSCs with low
immunogenic potential, rendering them more suitable for MSC-based
tissue regeneration medicine (84).
However, unexpected infection is the cause of failure of tissue
repair, suggesting that implanted MSCs may be affected by the
recipient immune system (78).
During this repair process, coordinated and precise crosstalk
between endotoxins of microorganisms, inflammatory cells, cytokines
and regulatory signaling cascades with MSCs is critical for the
success of tissue regeneration (85,86).
The failure in this communication is associated with arrested MSC
activity and may further lead to wound healing defects,
inflammatory disorders, and even malignant transformation (87,88).
Particularly, the regenerative capacities of endogenous or
transplanted stem cells are significantly modified by the immune
microenvironment at the site of injury (89–92).
For example, it has been shown that recipient T cells negatively
regulate autologous MSCs mediating bone formation in the mouse, and
that NF-κB, the major transcription factor in both the innate and
adaptive immune response, represses bone formation in rats
(93). Lung tumor cell-derived
exosomes can ‘educate̛ naive MSCs into pro-inflammatory MSCs by
activating Toll-like receptor (TLR)2/IL-1/myeloid-differentiation
primary-response protein 88 (MyD88) signaling through HSP70
(94). Radiation-induced bowel
injury may damage RNA, causing crypt stem cells death via TLR3
signaling (91). Hence, an in-depth
understanding of the pattern of how the immune system governs MSC
behavior could provide insights into therapeutic avenues for
restoring damaged tissues efficiently (87,90).
In addition, previous studies have provided evidence
of the association between inflammatory signaling pathways and the
WNT pathway, which may underlie the regulation of the immune system
on MSCs. The examples include tissue repair by
macrophage-activating stem cells via WNT pathway upregulation
through the secretion of WNT7b and WNT10a, and progenitor cells
through WNT3a (95). Furthermore,
certain studies have suggested that under the attack of replicative
pathogenic microorganisms, MSCs undergo rapid aging (96–98).
The aged stem cells have been reported to exhibit downregulated WNT
pathway activity, indicating the inhibitory regulation of stem cell
infection via the WNT pathway (99).
The complex pathophysiological mechanism of tissue
injury has been delineated through the continuous efforts of
immunological studies (100,101). This complex process involves the
recognition of the molecules of typical pathogens', recruited
immune cells, secreted inflammatory cytokines and molecular pathway
interplay with local tissue cells, including MSCs. First, there are
five classes of germline-encoded host-pathogen sensors, which are
collectively termed pattern recognition receptors (PRRs) (102–104). These elements can recognize the
specific molecules on microorganisms known as pathogen-associated
molecular patterns (PAMPs). PRRs include the following: i) TLRs,
transmembrane proteins located primarily at the cell surface or in
endosomes; ii) nucleotide-binding oligomerization domain-like
receptors (NLRs), the intracellular sensors inside the cytoplasm;
iii) retinoic acid-inducible gene-I-like receptors, which are
intracellularly located and primarily involved in antiviral
responses; iv) C-type lectin receptors, carbohydrate-binding
transmembrane receptors functioning in the immune response to
pathogens and apoptosis; and v) absence in melanoma 2-like
receptors, characterized by a pyrin domain and a DNA-binding HIN
domain detecting intracellular microbial DNA. Each PRR triggers
inflammatory pathways to fight against microorganisms in various
ways, amongst which, TLRs and NLRs were key components of the
innate immune response (105).
TLRs are dominantly expressed on the membranes of
leukocytes, including dendritic cells, macrophages, natural killer
cells, and T and B lymphocytes. To date, 13 members of the TLR
family have been identified, of which TLR1, TLR2, TLR4, TLR5, TLR6
and TLR11 are expressed on the cell surface, and TLR3, TLR7, TLR8
and TLR9 are localized to the endosomal/lysosomal compartments.
TLR12 and TLR13 are not found in humans (106). Each of these TLRs binds to and
becomes activated by different ligands. For example,
lipopolysaccharides (LPSs) exclusively bind to TLR4, but TLR4 can
also be activated by multiple other pathogens. TLR3 can be
activated by Poly I:C-a mismatched double-stranded RNA in viral
infection (107). Lipoteichoic
acid (108), the immunostimulant
of Gram-positive bacteria, is recognized by TLR2. The inflammatory
pathways initiated after this receptor-ligand binding and their
downstream responsive adaptors are also different for each TLR.
However, overall, all the TLR inflammatory pathways act through two
major signaling cascades: A MyD88-dependent pathway inducing
pro-inflammatory cytokines, such as TNF-α via NF-κB and a MyD88
independent pathway that acts through type I interferons (109). The dominant MyD88-dependent
pathway originates from the cytoplasmic Toll/IL-1 receptor (TIR)
domain. Upon stimulation with extracellular PAMPs, MyD88 attaches
to TLRs through the TIR domain and recruits IL-1
receptor-associated kinase-4 (IRAK-4) to TLRs. Following
phosphorylation, the IRAK1/2/4 complex is connected to TNF
receptor-associated factor 6 (TRAF6), which then activates TAK1,
leading to the activation of the IκB kinase (IKK) complex. The
downstream IκB kinase phosphorylates the inhibitory IκBα protein
(73,110). This phosphorylation dissociates
IκBα from NF-κB, with the free NF-κB then able to translocate into
the nucleus to activate the target genes responsible for
pro-inflammatory cytokine transcription, such as pro-IL-1β and
pro-IL-18. The enhancement of the transcription of the same target
genes can also be mediated by activated mitogen-activated protein
kinase kinases to phosphorylate MAPKs including JNK, ERK and p38
(111). The activation of
MyD88-independent pathways occurs via TIR-domain-containing
adapter-inducing interferon-β and TRAF3 in case of MyD88
deficiency. It induces the recruitment of IKKε/TBK1,
phosphorylation of interferon regulatory factor 3 (IRF3) and
expression of interferon-β. Thus far, most TLR-related inflammation
is mediated by the MyD88-dependent pathway, whereas PolyI: C-TLR3
antiviral innate immunity has been found to be independent of MyD88
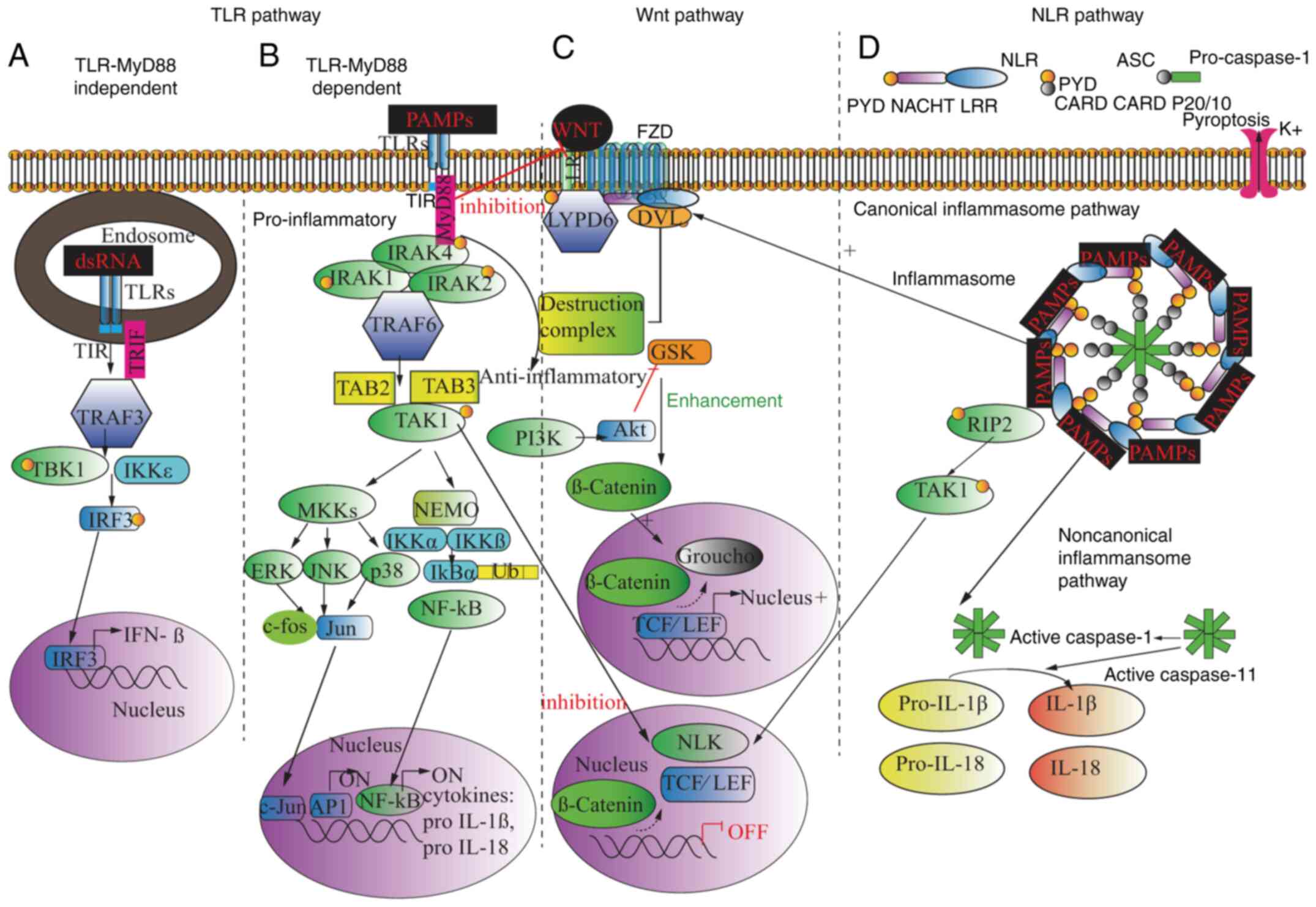
(Fig. 3A) (112).
TLRs and IL-1R ligands are usually found to be
highly expressed in sites of tissue injury and wound repair
(113,114), and it has been shown that they
could influence the repair process (115,116). Thus, it is reasonable to conclude
that TLR inflammatory pathways have an impact on the behavior of
MSCs. It has been well established by several in vitro
studies that MSCs express a number of TLRs. Thus far, consistent
results have demonstrated the expression of TLR1-6 in adipose- and
bone marrow-MSCs in both humans and mice and at both the mRNA and
protein levels, whereas inconsistent results have been reported on
the expression of TLR7-10 (84,117).
TLR/Myd88/IL receptor 1 (ILR1) signaling inhibits BM-MSC colony
formation, proliferation, migration and osteoblastic
differentiation after being activated by IL-1β. It is worth
mentioning that BM-MSCs are more vulnerable to the inhibitory
effects of TLR/Myd88/ILR1 signaling than osteoblasts (30). Pevsner-Fischer et al
(118) found that TLR2 activation
reduced mouse BM-MSC differentiation into the three mesodermal
lineages. Of note, BM-MSCs collected from MyD88-deficient mice were
found to effectively differentiate into adipocytes, but not
osteocytes or chondrocytes, even without the additional stimulation
with TLR ligands. Similarly, another two studies reported that the
activation of the TLR4 or 2 pathway in the proinflammatory
environment negatively regulated human adipose MSC (hAD-MSC)
differentiation to adipocytes (119,120). Collectively, these findings
indicated that the effects of the TLR pathway on MSC lineage
commitment potentials varies depending on different culture
conditions, tissue origins and species (118). However, most of the available
evidence on the association between the infectious TLR pathway and
MSCs are based on in vitro studies. In vitro cultures
do not completely replicate in vivo MSC behavior, as it is
unable to incorporate the complicated interactions between the
numerous signaling pathways employed by the various cell types. A
good example of this difference between in vivo and in
vitro studies is the fact that in BM-MSC-based calvaria
regeneration, delivering Myd88−/−MSCs induced a
significantly higher regeneration capacity compared with wild-type
(wt) MSCs, whereas the in vitro culture of
Myd88−/− and wt MSCs resulted in equal differentiation
and proliferation ability (30).
It is well known that the oral cavity is subjected
to daily assaults from the external microbiota, which inevitably
causes periodontitis. In periodontitis, LPS can cause periodontal
tissue degeneration in a dose-time-dependent manner (121), which indicates the failure of
periodontal MSCs to support physiological turnover. The modulation
of WNT/β-catenin signaling helps attenuate periapical bone lesions
(122). An in vitro study
demonstrated that LPS activated p38-MAPK and inhibited the
canonical WNT/β-catenin signaling pathway with an increase in the
levels of p38, c-myc, cyclin D1 mRNA and phosphorylated GSK-3β
(123). During the process of
wound repair, prostaglandin E2 (PGE2) released from neighboring
apoptotic cells can activate the WNT/β-catenin pathway in MSCs via
WNT3. LPS was also reported to enhance WNT5a expression through the
TLR4/MyD88/phosphatidylinositol 3-OH kinase/Akt/NF-κB/MAPK pathway,
based on in vitro studies of human dental pulp stem
(124,125) and osteoblast (126) cells. However, there are a number
of controversies on the influence of Pg-LPS on dental MSC function
and the pathways involved. It has been shown that Pg-LPS modified
periodontal ligament stem cell lineage commitment during the
inhibition of osteogenesis and stimulation of fibrosis via ERK1/2
signaling (127). Moreover, Pg-LPS
inhibited the osteogenic differentiation of BM-MSCs (127), whereas LPS upregulated gingival
MSC proliferation without attenuating their regenerative capacity,
and this positive effect was mediated by the NF-κB but not the
WNT/β-catenin pathway (127).
The digestive system is another non-sterile
environment that is exposed to a wide array of microorganisms, with
infectious bowel diseases being relatively common as a result
(128). Under infectious
conditions, intestinal MSCs can differentiate into inflammatory
cells and secrete inflammatory cytokines, such as IL-1b, IL-8 and
TNF-α (129). When treating
intestinal MSCs with LPS, flow cytometry analysis showed that the
LPS-induced MSC cell cycle progression was arrested at the G1
phase, and that cell pyroptosis was enhanced. Studies have shown
that LPS inhibits WNT signaling through the activation of GSK3β,
causing a marked inhibition in enterocyte proliferation, both in
vitro and in vivo (130). This particular phenomenon links
the LPS/TLR4 pathway to the regulation of intestinal MSC lineage
commitment by WNT.
The above findings suggested an influence of the TLR
inflammatory pathway on the regulatory WNT pathway in MSCs, with
the molecular mechanism of this interaction partially revealed.
TLRs can modulate WNT activity at different points with various
effects. First, TLRs can modulate the activity of WNT degradation
of β-catenin by binding to the LRP5/6 FZD receptor complex, thus
promoting the function of the β-catenin destruction complex, with
subsequent blocking of the WNT target gene expression. However,
this interactive mechanism helps explain the fact that transiting
WNT3a could counteract the inhibitory effect of Pg-LPS. TAK1
phosphorylation in TLR signaling attributes to NLK activation,
which is negatively correlated with the canonical WNT pathway
downstream TCF/LEF transcription (133). By contrast, TLRs can activate AKT
through PI3K and IKK, consequently inhibiting GSK3β, enhancing
β-catenin expression and upregulating TCF/LEF transcription.
TLR4/Myd88/leucine-rich repeat (LRR) binding FLII interacting
protein 2 has also been revealed as an activator of WNT by
interacting with DVL to activate catenin/LEF/TCF-dependent
transcriptional activity (Fig. 3A and
B) (134,135).
In addition to TLRs, vertebrates have developed
several alternative strategies to sense pathogens in the cytosol
(136,137). NLRs are the intracellular
receptors playing key roles in innate immune system regulation by
sensing PAMPs that enter the cell via phagocytosis or through
pores, and cell stress-related damage-associated molecular patterns
(106). NLRs are composed of 3
major domains: The central NOD nucleotide-binding domain (NACHT)
domain is common to all NLRs; most NLRs also have similar
C-terminal LRR and variable N-terminal interaction domains
(114). Each domain has a specific
function; LRR recognizes ligands, the central NACHT domain mediates
adenosine triphosphate (ATP)-dependent self-oligomerization, and
N-terminal domain triggers homotypic protein interaction amongst
the caspase recruitment domain (CARD; from the NLRC subfamily),
pyrin domain (PYD; from the NLRP subfamily), acidic transactivating
domain (from the NLRA subfamily) or baculovirus inhibitor repeats
(BIRs; from the NLRB subfamily) (138,139).
NLRs mediate inflammasome formation and downstream
caspase-1 activation, resulting in programmed cell death under
infectious conditions, a phenomenon known as pyroptosis. The
manifestation of pyroptosis can be induced by pyknosis, chromatin
condensation, DNA breaks, plasma membrane permeabilization and
cellular swelling (140).
Following TLR-related ‘priming̛ and ‘activating̛, NLR ligand uptake
triggers inflammasome assembly, and maturation of IL-1β and IL-18.
NLRs directly bind to apoptosis-associated speck-like protein (ASC)
through PYD, and ASC binds to pro-caspase-1 through its CARD domain
(141). NLR signaling-driven
pyroptosis can be mediated by the canonical and noncanonical
inflammasome pathway. In the canonical inflammasome pathway, the
combination of NLRs/ASC activates pro inflammasome 1 and organizes
the functional caspase-1 into a multiprotein oligomer complex. The
activated caspase-1 induces IL-1β and IL-18 secretion from
pro-IL-1β and pro-IL-18. As a consequence, inflammatory immune
cells are attracted, and pro-inflammatory cytokines (such as TNF-α
and IL-6), chemokines (such as IL-8, CXCL8 and monocyte
chemoattractant protein 1) and adhesion molecules are released. In
addition, the K+ selective hemichannel on the
inflammatory cell surface is opened upon ATP binding, with the
subsequent K+ efflux inducing pore formation on the cell
membrane via pannexin-1, and extracellular pathogen influx further
deteriorating pyroptosis. The noncanonical pathway works in synergy
with the canonical pathway. In the non-canonical pathway,
caspase-11 activation directly enhances IL-1β and IL-18 release and
promotes the canonical caspase-1 pathway (142). Subsequently, the release of IL-1β
and IL-18, and proteolytic cleavage of Gasdermin-D into Gasdermin-N
domain by caspase-4, 5 and 11 either directly activate caspase-1 or
indirectly activate the canonical inflammasome/NLR pathway to
induce pyroptosis (142,143).
The activation of TLR and NLR pathways may trigger
the production of a common by-product, antimicrobial ROS (160). ROS are short-lived
oxygen-containing molecules existing in the form of free redials,
including superoxide anion (O2−), hydroxyl
radical (OH), hydroxyl ion (OH−) and nitric oxide (NO),
as well as non-radical ROS, such as hydrogen peroxide
(H2O2) (161). ROS production occurs under
different physiological and pathological circumstances aided by
active enzymes (162). ROS are
ubiquitously found in the extracellular space (163), plasma membrane (164) and intracellular compartments
[including mitochondria, where nicotinamide adenine dinucleotide
phosphate (NADPH) oxidases are concentrated] (165), peroxisomes, ER (166) and cytosol (NO synthases and
lipoxygenases) (167). However,
amongst these, mitochondrial complexes I and III, and the
corresponding NADPH oxidase isoform NADPH oxidase 4, are the major
sources of ROS production that play pivotal roles in MSC regulatory
niches (168). Several studies
have concluded that local hypoxia is the culprit for the failure of
MSC-based tissue regeneration and MSC ageing (169,170).
ROS influence MSC survival in a
concentration-dependent manner. An appropriate level of ROS is
necessary and advantageous to maintain cellular proliferation and
survival; however, excessive ROS may cause cellular damage and
dysfunction after initiating chemical reactions involving RNA, DNA,
proteins and lipids (161,171,172). Eto et al (173) demonstrated that AD-MSCs were
extremely sensitive to oxygen concentration, with only cells
implanted <300 mm from an oxygen source surviving and the rest
undergoing apoptosis. However, excess ROS has been confirmed to
activate MAPK pathways and apoptotic proteins, and suppress
antiapoptotic pathways (173,174). Consistently, antioxidants
stimulate MSC proliferation (175).
ROS involvement in the regulation of MSC
differentiation may stem from the difference in ROS levels within
the differentiated and undifferentiated MSCs. During homeostasis,
undifferentiated MSCs are predominantly supported by glycolytic
energy with higher levels of glycolytic enzymes and increased
lactate production. By contrast, MSC-differentiated osteoblasts
rely more on oxidative mitochondrial metabolism (176). Therefore, MSCs exhibit relatively
low levels of ROS, but low antioxidant activity may suggest that
they are more sensitive to oxidative stress (177–179). In fact, the impact of ROS on MSC
lineage differentiation significantly varies depending on the
dosage. The in vivo and in vitro studies collectively
suggested that excessive ROS could suppress osteogenesis but induce
adipogenic differentiation (180).
Highly well-defined levels of ROS may activate MSC differentiation
into chondrocytes (144),
adipocytes (181), osteocytes
(46) and neurons (182) through the enhanced activity of
regulatory signaling pathways, including the WNT pathway. A
previous in vitro study verified that the exogenous addition
of H2O2 inhibits TCF-mediated transcription
and that β-catenin overexpression and use of antioxidants could
rescue that inhibition. In addition, ROS concomitantly increases
with ageing, with the aged MSCs reported to exhibit a reduced
expression of WNT target genes, such as AXIN2 and TNF receptor
superfamily member 11b, in 31-month-old mice compared with
4-month-old mice, thus diminishing osteogenesis (183). An in vivo study revealed
that irradiation injury could cause intestinal tissue regeneration
via the activation of WNT signaling, which was achieved through the
ROS/hypoxia-inducible factor/WNT2b signaling axis (132). One explanation of this mechanism
could be that superoxide-generating NADPH oxidase (Nox) induces ROS
production, leading to the inactivation of nucleoredoxin (NRX) in
the β-catenin destruction complex to activate the WNT/β-catenin
pathway (184). Conversely, in the
MSC niche, the binding of WNT ligand to its receptor complexes may
trigger the sequential activation of Src kinase, Rac1-GEF-vav
guanine nucleotide exchange factor 2 through Src-dependent tyrosine
phosphorylation and Rac1. Activated Rac1 may in turn induce
Nox1-derived ROS, which may result in the oxidation of NRX, with
the oxidized NRX then detaching from DVL. Subsequently, liberated
DVL suppresses the β-catenin destruction complex, resulting in the
stabilization of β-catenin and the activation of WNT signaling
(184,185).
MSCs are well known for their multipotency,
regenerative ability and immunomodulatory properties. The stem cell
niches may play a crucial role in regulating their self-renewal,
differentiation and cell fate. Although MSC-based tissue
regeneration has led to notable developments over the past decade,
no associated clinical standard therapy has been developed, due to
the low effectiveness of MSCs. Insights into the crosstalk between
regulatory factors within MSC niches and MSCs are important for
both normal tissue homeostasis and disease conditions, such as
tissue infection. WNT signaling is broadly involved in the
regulation of adult MSC fate in various tissues. The canonical
WNT/β catenin pathway may help with the maintenance of MSC stemness
and proliferation ability. However, WNT signaling has been shown
several times to inhibit adipogenic activity and enhance osteogenic
differentiation. Inflammation is the protective response of the
host body against various harmful stimuli. However, the host
defense may not be able to deal with the constant attack by
pathogens, which could lead to harmful infection, and this may
break down or upset the balance of MSC niches. MSCs are regulated
by inflammatory factors. Currently, most studies are focusing on
the investigation of MSC modulatory effects on the immune system
and their application in the treatment of a variety of
immunoproliferative diseases and improvement of tissue injury
repair efficiency. Less attention has been paid to establishing how
the immune response to infection affects the functional restorative
capacity of MSCs. In the present review, the current knowledge on
the behavioral changes of MSCs under the influence of various
immune response-mediated pathways and immune regulatory reactions,
such as the TLR pathway, NLR pathway, pro-inflammatory cytokines,
ROS by-products and the modifications mediating WNT signaling, were
summarized.
Following the recognition of PAMPs and PRRs, the
active TLR pathway inhibits MSC proliferation and tissue-specific
differentiation by interrupting WNT signaling at different points,
including the LRP5/6 FZD receptor and GSK3β-destruction complexes
or by directly terminating TCF/LEF transcription. NLR pathways
mediate inflammasome formation and the resulting pyroptosis. NLRs
modify MSC lineage distribution profiles that vary greatly amongst
different tissue origins. Numerous proinflammatory cytokines have
been found to inhibit the normal functions of MSCs and exert
undesirable tissue regenerative effects. ROS, as one of the common
by-products of immune infection, may function as a positive
regulator of the WNT pathway and MSC activity at an appropriate
level; however, extremely high levels of ROS may deplete MSC
survival and damage TCF transcription, which are crucial for MSC
differentiation. Future prospective research is required to unravel
the regulation of MSCs in relation to the pathophysiology of immune
defense response to infections and their terminal fate, as
MSC-based tissue regeneration may have a therapeutic effect.
Not applicable.
No funding was received.
The datasets used and/or analyzed during the study
are available from the corresponding author on reasonable
request.
WL, QZ and HY designed and supervised the study.
JY, QZ, QC and HD analyzed and interpreted the data. QZ and JY
wrote the manuscript. All authors read and approved the final
manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Bianco P, Riminucci M, Gronthos S and
Robey PG: Bone marrow stromal stem cells: Nature, biology, and
potential applications. Stem Cells. 19:180–192. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell
JW, Katz AJ, Benhaim P, Lorenz HP and Hedrick MH: Multilineage
cells from human adipose tissue: Implications for cell-based
therapies. Tissue Eng. 7:211–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao H, Feng J, Ho TV, Grimes W, Urata M
and Chai Y: The suture provides a niche for mesenchymal stem cells
of craniofacial bones. Nat Cell Biol. 17:386–396. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao H, Feng J, Seidel K, Shi S, Klein O,
Sharpe P and Chai Y: Secretion of shh by a neurovascular bundle
niche supports mesenchymal stem cell homeostasis in the adult mouse
incisor. Cell Stem Cell. 14:160–173. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seo BM, Miura M, Gronthos S, Bartold PM,
Batouli S, Brahim J, Young M, Robey PG, Wang CY and Shi S:
Investigation of multipotent postnatal stem cells from human
periodontal ligament. Lancet. 364:149–155. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Harvanová D, Tóthová T, Sarišský M,
Amrichová J and Rosocha J: Isolation and characterization of
synovial mesenchymal stem cells. Folia Biol (Praha). 57:119–124.
2011.
|
|
7
|
Patel AN, Park E, Kuzman M, Benetti F,
Silva FJ and Allickson JG: Multipotent menstrual blood stromal stem
cells: Isolation, characterization, and differentiation. Cell
Transplant. 17:303–311. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Agha-Hosseini F, Jahani MA, Jahani M,
Mirzaii-Dizgah I and Ali-Moghaddam K: In vitro isolation of stem
cells derived from human dental pulp. Clin Transplant. 24:E23–E28.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weiss ML, Medicetty S, Bledsoe AR,
Rachakatla RS, Choi M, Merchav S, Luo Y, Rao MS, Velagaleti G and
Troyer D: Human umbilical cord matrix stem cells: Preliminary
characterization and effect of transplantation in a rodent model of
Parkinson's disease. Stem Cells. 24:781–792. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Divya MS, Roshin GE, Divya TS, Rasheed VA,
Santhoshkumar TR, Elizabeth KE, James J and Pillai RM: Umbilical
cord blood-derived mesenchymal stem cells consist of a unique
population of progenitors co-expressing mesenchymal stem cell and
neuronal markers capable of instantaneous neuronal differentiation.
Stem Cell Res Ther. 3:572012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tavakoli S, Ghaderi Jafarbeigloo HR,
Shariati A, Jahangiryan A, Jadidi F, Jadidi Kouhbanani MA,
Hassanzadeh A, Zamani M, Javidi K and Naimi A: Mesenchymal stromal
cells; a new horizon in regenerative medicine. J Cell Physiol.
235:9185–9210. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hoogduijn MJ and Lombardo E: Mesenchymal
stromal cells anno 2019: dawn of the therapeutic Era? concise
review. Stem Cells Transl Med. 8:1126–1134. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Najar M, Bouhtit F, Melki R, Afif H, Hamal
A, Fahmi H, Merimi M and Lagneaux L: Mesenchymal stromal cell-based
therapy: New perspectives and challenges. J Clin Med. 8:6262019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen C and Hou J: Mesenchymal stem
cell-based therapy in kidney transplantation. Stem Cell Res Ther.
7:162016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schofield R: The relationship between the
spleen colony-forming cell and the haemopoietic stem cell. Blood
Cells. 4:7–25. 1978.PubMed/NCBI
|
|
17
|
Martino MM, Briquez PS, Güç E, Tortelli F,
Kilarski WW, Metzger S, Rice JJ, Kuhn GA, Müller R, Swartz MA and
Hubbell JA: Growth factors engineered for super-affinity to the
extracellular matrix enhance tissue healing. Science. 343:885–888.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Adam RC and Fuchs E: The yin and yang of
chromatin dynamics in stem cell fate selection. Trends Genet.
32:89–100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim JH, Liu X, Wang J, Chen X, Zhang H,
Kim SH, Cui J, Li R, Zhang W, Kong Y, et al: Wnt signaling in bone
formation and its therapeutic potential for bone diseases. Ther Adv
Musculoskelet Dis. 5:13–31. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ding L and Morrison SJ: Haematopoietic
stem cells and early lymphoid progenitors occupy distinct bone
marrow niches. Nature. 495:231–235. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bruns I, Lucas D, Pinho S, Ahmed J,
Lambert MP, Kunisaki Y, Scheiermann C, Schiff L, Poncz M, Bergman A
and Frenette PS: Megakaryocytes regulate hematopoietic stem cell
quiescence through CXCL4 secretion. Nat Med. 20:1315–1320. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao M, Perry JM, Marshall H, Venkatraman
A, Qian P, He XC, Ahamed J and Li L: Megakaryocytes maintain
homeostatic quiescence and promote post-injury regeneration of
hematopoietic stem cells. Nat Med. 20:1321–1326. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rompolas P, Deschene ER, Zito G, Gonzalez
DG, Saotome I, Haberman AM and Greco V: Live imaging of stem cell
and progeny behaviour in physiological hair-follicle regeneration.
Nature. 487:496–499. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Spradling A, Drummond-Barbosa D and Kai T:
Stem cells find their niche. Nature. 414:98–104. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bitgood MJ and McMahon AP: Hedgehog and
Bmp genes are coexpressed at many diverse sites of cell-cell
interaction in the mouse embryo. Dev Biol. 172:126–138. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Petrova R and Joyner AL: Roles for
Hedgehog signaling in adult organ homeostasis and repair.
Development. 141:3445–3457. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ohishi K, Varnum-Finney B, Flowers D,
Anasetti C, Myerson D and Bernstein ID: Monocytes express high
amounts of Notch and undergo cytokine specific apoptosis following
interaction with the Notch ligand, Delta-1. Blood. 95:2847–2854.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Verrecchia F and Mauviel A: Transforming
growth factor-β and fibrosis. World J Gastroenterol. 13:3056–3062.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Han J, Menicanin D, Gronthos S and Bartold
PM: Stem cells, tissue engineering and periodontal regeneration.
Aust Dent J. 59 (Suppl 1):S117–S130. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Martino MM, Maruyama K, Kuhn GA, Satoh T,
Takeuchi O, Müller R and Akira S: Inhibition of IL-1R1/MyD88
signalling promotes mesenchymal stem cell-driven tissue
regeneration. Nat Commun. 7:110512016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kuljanin M, Bell GI, Sherman SE, Lajoie GA
and Hess DA: Proteomic characterisation reveals active
Wnt-signalling by human multipotent stromal cells as a key
regulator of beta cell survival and proliferation. Diabetologia.
60:1987–1998. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Volleman TNE, Schol J, Morita K, Sakai D
and Watanabe M: Wnt3a and wnt5a as potential chondrogenic
stimulators for nucleus pulposus cell induction: A comprehensive
review. Neurospine. 17:19–35. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sato A, Yamamoto H, Sakane H, Koyama H and
Kikuchi A: Wnt5a regulates distinct signalling pathways by binding
to Frizzled2. EMBO J. 29:41–54. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sharma RP and Chopra VL: Effect of the
Wingless (wg1) mutation on wing and haltere development in
Drosophila melanogaster. Dev Biol. 48:461–465. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cadigan KM and Nusse R: WNT signaling: A
common theme in animal development. Genes Dev. 11:3286–3305. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wodarz A and Nusse R: Mechanisms of WNT
signaling in development. Annu Rev Cell Dev Biol. 14:59–88. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rao TP and Kühl M: An updated overview on
Wnt signaling pathways: A prelude for more. Circ Res.
106:1798–1806. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Heethoff M, Helfen L and Norton RA:
Description of Neoliodes dominicus n.sp. (Acari, Oribatida) from
Dominican amber, aided by synchrotron X-ray microtomography. J
Paleontol. 83:153–159. 2009. View Article : Google Scholar
|
|
39
|
Nelson WJ and Nusse R: Convergence of WNT,
beta-catenin, and cadherin pathways. Science. 303:1483–1487. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Komiya Y and Habas R: Wnt signal
transduction pathways. Organogenesis. 4:68–75. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
He X, Semenov M, Tamai K and Zeng X: LDL
receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling:
Arrows point the way. Development. 131:1663–1677. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Matsui T, Raya A, Kawakami Y,
Callol-Massot C, Capdevila J, Rodríguez-Esteban C and Izpisúa
Belmonte JC: Noncanonical Wnt signaling regulates midline
convergence of organ primordia during zebrafish development. Genes
Dev. 19:164–175. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kaur P, Jin HJ, Lusk JB and Tolwinski NS:
Modeling the role of wnt signaling in human and drosophila stem
cells. Genes (Basel). 9:1012018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nusse R: Wnt signaling in disease and in
development. Cell Res. 15:28–32. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Baron R and Kneissel M: WNT signaling in
bone homeostasis and disease: From human mutations to treatments.
Nat Med. 19:179–192. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Takada I, Kouzmenko AP and Kato S: WNT and
PPARgamma signaling in osteoblastogenesis and adipogenesis. Nat Rev
Rheumatol. 5:442–447. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Visweswaran M, Pohl S, Arfuso F, Newsholme
P, Dilley R, Pervaiz S and Dharmarajan A: Multi-lineage
differentiation of mesenchymal stem cells-To WNT, or not WNT. Int J
Biochem Cell Biol. 68:139–147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ko JH, Lee HJ, Jeong HJ, Kim MK, Wee WR,
Yoon SO, Choi H, Prockop DJ and Oh JY: Mesenchymal stem/stromal
cells precondition lung monocytes/macrophages to produce tolerance
against allo- and autoimmunity in the eye. Proc Natl Acad Sci USA.
113:158–163. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Huelsken J and Behrens J: The WNT
signalling pathway. J Cell Sci. 115:3977–3978. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Komiya Y and Habas R: WNT signal
transduction pathways. Organogenesis. 4:68–75. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Murphy LL and Hughes CC: Endothelial cells
stimulate T cell NFAT nuclear translocation in the presence of
cyclosporin A: Involvement of the WNT/glycogen synthase kinase-3
beta pathway. J Immunol. 169:3717–3725. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Veltri A, Lang C and Lien WH: Concise
review: WNT signaling pathways in skin development and epidermal
stem cells. Stem Cells. 36:22–35. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kim KA, Kakitani M, Zhao J, Oshima T, Tang
T, Binnerts M, Liu Y, Boyle B, Park E, Emtage P, et al: Mitogenic
influence of human R-spondin1 on the intestinal epithelium.
Science. 309:1256–1259. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Fei D, Zhang Y, Wu J, Zhang H, Liu A, He
X, Wang J, Li B, Wang Q and Jin Y: Cav 1.2 regulates osteogenesis
of bone marrow-derived mesenchymal stem cells via canonical Wnt
pathway in age-related osteoporosis. Aging Cell. 18:e129672019.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Maruyama T, Jeong J, Sheu TJ and Hsu W:
Stem cells of the suture mesenchyme in craniofacial bone
development, repair and regeneration. Nat Commun. 7:105262016.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Jing H, Liao L, An Y, Su X, Liu S, Shuai
Y, Zhang X and Jin Y: Suppression of EZH2 Prevents the Shift of
Osteoporotic MSC Fate to adipocyte and enhances bone formation
during osteoporosis. Mol Ther. 24:217–229. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Niehrs C and Acebron SP: Mitotic and
mitogenic WNT signalling. EMBO J. 31:2705–2713. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Li L and Clevers H: Coexistence of
quiescent and active adult stem cells in mammals. Science.
327:542–545. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Park JR, Jung JW, Lee YS and Kang KS: The
roles of WNT antagonists Dkk1 and sFRP4 during adipogenesis of
human adipose tissue-derived mesenchymal stem cells. Cell Prolif.
41:859–874. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ehrlund A, Mejhert N, Lorente-Cebrián S,
Aström G, Dahlman I, Laurencikiene J and Rydén M: Characterization
of the WNT inhibitors secreted frizzled-related proteins (SFRPs) in
human adipose tissue. J Clin Endocrinol Metab. 98:E503–E508. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hoffmann MM, Werner C, Böhm M, Laufs U and
Winkler K: Association of secreted frizzled-related protein 4
(SFRP4) with type 2 diabetes in patients with stable coronary
artery disease. Cardiovasc Diabetol. 13:1552014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Mahdi T, Hänzelmann S, Salehi A, Muhammed
SJ, Reinbothe TM, Tang Y, Axelsson AS, Zhou Y, Jing X, Almgren P,
et al: Secreted frizzled-related protein 4 reduces insulin
secretion and is overexpressed in type 2 diabetes. Cell Metab.
16:625–633. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Eizirik DL and Cnop M: Mining genes in
type 2 diabetic islets and finding gold. Cell Metab. 16:555–557.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zaragosi LE, Wdziekonski B, Fontaine C,
Villageois P, Peraldi P and Dani C: Effects of GSK3 inhibitors on
in vitro expansion and differentiation of human adipose-derived
stem cells into adipocytes. BMC Cell Biol. 9:112008. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Gaur T, Rich L, Lengner CJ, Hussain S,
Trevant B, Ayers D, Stein JL, Bodine PV, Komm BS, Stein GS and Lian
JB: Secreted frizzled related protein 1 regulates WNT signaling for
BMP2 induced chondrocyte differentiation. J Cell Physiol.
208:87–96. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Jin EJ, Park JH, Lee SY, Chun JS, Bang OS
and Kang SS: WNT-5a is involved in TGF-beta3-stimulated
chondrogenic differentiation of chick wing bud mesenchymal cells.
Int J Biochem Cell Biol. 38:183–195. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Fischer L, Boland G and Tuan RS: WNT
signaling during BMP-2 stimulation of mesenchymal chondrogenesis. J
Cell Biochem. 84:816–831. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Luo S, Shi Q, Zha Z, Yao P, Lin H, Liu N,
Wu H and Sun S: Inactivation of WNT/β-catenin signaling in human
adipose-derived stem cells is necessary for chondrogenic
differentiation and maintenance. Biomed Pharmacother. 67:819–824.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Im GI and Quan Z: The effects of WNT
inhibitors on the chondrogenesis of human mesenchymal stem cells.
Tissue Eng Part A. 16:2405–2413. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Im GI, Lee JM and Kim HJ: WNT inhibitors
enhance chondrogenesis of human mesenchymal stem cells in a
long-term pellet culture. Biotechnol Lett. 33:1061–1068. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Liu G, Vijayakumar S, Grumolato L,
Arroyave R, Qiao H, Akiri G and Aaronson SA: Canonical WNTs
function as potent regulators of osteogenesis by human mesenchymal
stem cells. J Cell Biol. 185:67–75. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Men Y, Wang Y, Yi Y, Jing D, Luo W, Shen
B, Stenberg W, Chai Y, Ge WP, Feng JQ and Zhao H: Gli1+
periodontium stem cells are regulated by osteocytes and occlusal
force. Dev Cell. 54:639–654.e6. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Gong Y, Slee RB, Fukai N, Rawadi G,
Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, et
al: LDL receptor-related protein 5 (LRP5) affects bone accrual and
eye development. Cell. 107:513–523. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kato M, Patel MS, Levasseur R, Lobov I,
Chang BH, Glass DA II, Hartmann C, Li L, Hwang TH, Brayton CF, et
al: Cbfa1-independent decrease in osteoblast proliferation,
osteopenia, and persistent embryonic eye vascularization in mice
deficient in Lrp5, a WNT coreceptor. J Cell Biol. 157:303–314.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Boyden LM, Mao J, Belsky J, Mitzner L,
Farhi A, Mitnick MA, Wu D, Insogna K and Lifton RP: High bone
density due to a mutation in LDL-receptor-related protein 5. N Engl
J Med. 346:1513–1521. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Bennett CN, Longo KA, Wright WS, Suva LJ,
Lane TF, Hankenson KD and MacDougald OA: Regulation of
osteoblastogenesis and bone mass by WNT10b. Proc Natl Acad Sci USA.
102:3324–3329. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Jiang M, Zheng C, Shou P, Li N, Cao G,
Chen Q, Xu C, Du L, Yang Q, Cao J, et al: SHP1 regulates bone mass
by directing mesenchymal stem cell differentiation. Cell Rep.
16:769–780. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Yamada A, Iwata T, Yamato M, Okano T and
Izumi Y: Diverse functions of secreted frizzled-related proteins in
the osteoblastogenesis of human multipotent mesenchymal stromal
cells. Biomaterials. 34:3270–3278. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Yao W, Cheng Z, Shahnazari M, Dai W,
Johnson ML and Lane NE: Overexpression of secreted frizzled-related
protein 1 inhibits bone formation and attenuates parathyroid
hormone bone anabolic effects. J Bone Miner Res. 25:190–199. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Nakanishi R, Akiyama H, Kimura H, Otsuki
B, Shimizu M, Tsuboyama T and Nakamura T: Osteoblast-targeted
expression of Sfrp4 in mice results in low bone mass. J Bone Miner
Res. 23:271–277. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Bodine PV, Billiard J, Moran RA,
Ponce-de-Leon H, McLarney S, Mangine A, Scrimo MJ, Bhat RA,
Stauffer B, Green J, et al: The WNT antagonist secreted
frizzled-related protein-1 controls osteoblast and osteocyte
apoptosis. J Cell Biochem. 96:1212–1230. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Gaur T, Wixted JJ, Hussain S, O'Connell
SL, Morgan EF, Ayers DC, Komm BS, Bodine PV, Stein GS and Lian JB:
Secreted frizzled related protein 1 is a target to improve fracture
healing. J Cell Physiol. 220:174–181. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Trevant B, Gaur T, Hussain S, Symons J,
Komm BS, Bodine PV, Stein GS and Lian JB: Expression of secreted
frizzled related protein 1, a WNT antagonist, in brain, kidney, and
skeleton is dispensable for normal embryonic development. J Cell
Physiol. 217:113–126. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Rashedi I, Gómez-Aristizábal A, Wang XH,
Viswanathan S and Keating A: TLR3 or TLR4 activation enhances
mesenchymal stromal cell-mediated treg induction via notch
signaling. Stem Cells. 35:265–275. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Di Meglio P, Perera GK and Nestle FO: The
multitasking organ: Recent insights into skin immune function.
Immunity. 35:857–869. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Gurtner GC, Werner S, Barrandon Y and
Longaker MT: Wound repair and regeneration. Nature. 453:314–321.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Aurora AB and Olson EN: Immune modulation
of stem cells and regeneration. Cell Stem Cell. 15:14–25. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Forbes SJ and Rosenthal N: Preparing the
ground for tissue regeneration: From mechanism to therapy. Nat Med.
20:857–869. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Takemura N, Kawasaki T, Kunisawa J, Sato
S, Lamichhane A, Kobiyama K, Aoshi T, Ito J, Mizuguchi K,
Karuppuchamy T, et al: Blockade of TLR3 protects mice from lethal
radiation-induced gastrointestinal syndrome. Nat Commun.
5:34922014. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Burzyn D, Kuswanto W, Kolodin D, Shadrach
JL, Cerletti M, Jang Y, Sefik E, Tan TG, Wagers AJ and Mathis D: A
special population of regulatory T cells potentiates muscle repair.
Cell. 155:1282–1295. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Liu Y, Wang L, Kikuiri T, Akiyama K, Chen
C, Xu X, Yang R, Chen W, Wang S and Shi S: Mesenchymal stem
cell-based tissue regeneration is governed by recipient T
lymphocytes via IFN-γ and TNF-α. Nat Med. 17:1594–1601. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Li X, Wang S, Zhu R, Li H, Han Q and Zhao
RC: Lung tumor exosomes induce a pro-inflammatory phenotype in
mesenchymal stem cells via NFκB-TLR signaling pathway. J Hematol
Oncol. 9:422016. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Wynn TA and Vannella KM: Macrophages in
tissue repair, regeneration, and fibrosis. Immunity. 44:450–462.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Mejia-Ramirez E and Florian MC:
Understanding intrinsic hematopoietic stem cell aging.
Haematologica. 105:22–37. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Oh J, Lee YD and Wagers AJ: Stem cell
aging: Mechanisms, regulators and therapeutic opportunities. Nat
Med. 20:870–880. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Liu L and Rando TA: Manifestations and
mechanisms of stem cell aging. J Cell Biol. 193:257–266. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Keyes BE and Fuchs E: Stem cells: Aging
and transcriptional fingerprints. J Cell Biol. 217:79–92. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Janeway CA Jr: Approaching the asymptote?
Evolution and revolution in immunology. Cold Spring Harb Symp Quant
Biol. 54:1–13. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Kubelkova K and Macela A: Innate immune
recognition: An issue more complex than expected. Front Cell Infect
Microbiol. 9:2412019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Unterholzner L, Keating SE, Baran M, Horan
KA, Jensen SB, Sharma S, Sirois CM, Jin T, Latz E, Xiao TS, et al:
IFI16 is an innate immune sensor for intracellular DNA. Nat
Immunol. 11:997–1004. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Schroder K and Tschopp J: The
inflammasomes. Cell. 140:821–832. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Martinon F, Mayor A and Tschopp J: The
inflammasomes: Guardians of the body. Annu Rev Immunol. 27:229–265.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Davis BK, Wen H and Ting JP: The
inflammasome NLRs in immunity, inflammation, and associated
diseases. Annu Rev Immunol. 29:707–735. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Mahla RS, Reddy MC, Prasad DV and Kumar H:
Sweeten PAMPs: Role of sugar complexed PAMPs in innate immunity and
vaccine biology. Front Immunol. 4:2482013. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Fortier ME, Kent S, Ashdown H, Poole S,
Boksa P and Luheshi GN: The viral mimic, polyinosinic:polycytidylic
acid, induces fever in rats via an interleukin-1-dependent
mechanism. Am J Physiol Regul Integr Comp Physiol. 287:R759–R766.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Sultan M, Coyle KM, Vidovic D, Thomas ML,
Gujar S and Marcato P: Hide-and-seek: The interplay between cancer
stem cells and the immune system. Carcinogenesis. 38:107–118. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Feng G, Zheng K, Cao T, Zhang J, Lian M,
Huang D, Wei C, Gu Z and Feng X: Repeated stimulation by LPS
promotes the senescence of DPSCs via TLR4/MyD88-NF-κB-p53/p21
signaling. Cytotechnology. 70:1023–1035. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Kain M: How NF-kappaB is activated: The
role of the IkappaB kinase (IKK) complex. Oncogene. 18:6867–6874.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Blasius AL and Beutler B: Intracellular
toll-like receptors. Immunity. 32:305–315. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Lester SN and Li K: Toll-like receptors in
antiviral innate immunity. J Mol Biol. 426:1246–1264. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Piccinini AM and Midwood KS: DAMPening
inflammation by modulating TLR signalling. Mediators Inflamm.
2010:6723952010. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Chen GY and Nuñez G: Sterile inflammation:
Sensing and reacting to damage. Nat Rev Immunol. 10:826–837. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Arslan F, Smeets MB, Riem Vis PW, Karper
JC, Quax PH, Bongartz LG, Peters JH, Hoefer IE, Doevendans PA,
Pasterkamp G and de Kleijn DP: Lack of fibronectin-EDA promotes
survival and prevents adverse remodeling and heart function
deterioration after myocardial infarction. Circ Res. 108:582–592.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Oyama J, Blais C Jr, Liu X, Pu M, Kobzik
L, Kelly RA and Bourcier T: Reduced myocardial ischemia-reperfusion
injury in toll-like receptor 4-deficient mice. Circulation.
109:784–789. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
DelaRosa O and Lombardo E: Modulation of
adult mesenchymal stem cells activity by toll-like receptors:
Implications on therapeutic potential. Mediators Inflamm.
2010:8656012010. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Pevsner-Fischer M, Morad V, Cohen-Sfady M,
Rousso-Noori L, Zanin-Zhorov A, Cohen S, Cohen IR and Zipori D:
Toll-like receptors and their ligands control mesenchymal stem cell
functions. Blood. 109:1422–1432. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Poulain-Godefroy O, Le Bacquer O, Plancq
P, Lecoeur C, Pattou F, Frühbeck G and Froguel P: Inflammatory role
of Toll-like receptors in human and murine adipose tissue.
Mediators Inflamm. 2010:8234862010. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
S Purohit J, Hu P, Burke SJ, Collier JJ,
Chen J and Zhao L: The effects of NOD activation on adipocyte
differentiation. Obesity (Silver Spring). 21:737–747. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Chang LY, Lai YL, Yu TH, Chen YT and Hung
SL: Effects of areca nut extract on lipopolysaccharides-enhanced
adhesion and migration of human mononuclear leukocytes. J
Periodontol. 85:859–867. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Tang Y, Zhou X, Gao B, Xu X, Sun J, Cheng
L, Zhou X and Zheng L: Modulation of WNT/β-catenin signaling
attenuates periapical bone lesions. J Dent Res. 93:175–182. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Wang J, Dai J, Liu B, Gu S, Cheng L and
Liang J: Porphyromonas gingivalis lipopolysaccharide activates
canonical WNT/β-catenin and p38 MAPK signalling in stem cells from
the apical papilla. Inflammation. 36:1393–1402. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Li D, Fu L, Zhang Y, Yu Q, Ma F, Wang Z,
Luo Z, Zhou Z, Cooper PR and He W: The effects of LPS on adhesion
and migration of human dental pulp stem cells in vitro. J Dent.
42:1327–1334. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
He W, Wang Z, Zhou Z, Zhang Y, Zhu Q, Wei
K, Lin Y, Cooper PR, Smith AJ and Yu Q: Lipopolysaccharide enhances
WNT5a expression through toll-like receptor 4, myeloid
differentiating factor 88, phosphatidylinositol 3-OH kinase/Akt and
nuclear factor kappa B pathways in human dental pulp stem cells. J
Endod. 40:69–75. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
He W, Qu T, Yu Q, Wang Z, Wang H, Zhang J
and Smith AJ: Lipopolysaccharide enhances decorin expression
through the Toll-like receptor 4, myeloid differentiating factor
88, nuclear factor-kappa B, and mitogen-activated protein kinase
pathways in odontoblast cells. J Endod. 38:464–469. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Xing Q, Ye Q, Fan M, Zhou Y, Xu Q and
Sandham A: Porphyromonas gingivalis lipopolysaccharide inhibits the
osteoblastic differentiation of preosteoblasts by activating Notch1
signaling. J Cell Physiol. 225:106–114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Wlodarska M, Kostic AD and Xavier RJ: An
integrative view of microbiome-host interactions in inflammatory
bowel diseases. Cell Host Microbe. 17:577–591. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Kedia S, Rampal R, Paul J and Ahuja V: Gut
microbiome diversity in acute infective and chronic inflammatory
gastrointestinal diseases in North India. J Gastroenterol.
51:660–671. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Sodhi CP, Shi XH, Richardson WM, Grant ZS,
Shapiro RA, Prindle T Jr, Branca M, Russo A, Gribar SC, Ma C and
Hackam DJ: Toll-like receptor-4 inhibits enterocyte proliferation
via impaired beta-catenin signaling in necrotizing enterocolitis.
Gastroenterology. 138:185–196. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Liu X, Lu R, Xia Y, Wu S and Sun J:
Eukaryotic signaling pathways targeted by Salmonella effector
protein AvrA in intestinal infection in vivo. BMC Microbiol.
10:3262010. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Suh HN, Kim MJ, Jung YS, Lien EM, Jun S
and Park JI: Quiescence Exit of Tert+ stem cells by WNT/β-catenin
is indispensable for intestinal regeneration. Cell Rep.
21:2571–2584. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Marchetti B and Pluchino S: WNT your brain
be inflamed? Yes, it WNT! Trends Mol Med. 19:144–156. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Jin J, Yu Q, Han C, Hu X, Xu S, Wang Q,
Wang J, Li N and Cao X: LRRFIP2 negatively regulates NLRP3
inflammasome activation in macrophages by promoting
Flightless-I-mediated caspase-1 inhibition. Nat Commun. 4:20752013.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Zolezzi JM and Inestrosa NC: WNT/TLR
dialog in neuroinflammation, relevance in Alzheimer's disease.
Front Immunol. 8:1872017. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Kanneganti TD, Lamkanfi M and Núñez G:
Intracellular NOD-like receptors in host defense and disease.
Immunity. 27:549–559. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Inohara C, Chamaillard, McDonald C and
Nuñez G: NOD-LRR proteins: Role in host-microbial interactions and
inflammatory disease. Annu Rev Biochem. 74:355–383. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Franchi L, Warner N, Viani K and Nuñez G:
Function of Nod-like receptors in microbial recognition and host
defense. Immunol Rev. 227:106–128. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Shaw MH, Reimer T, Kim YG and Nuñez G:
NOD-like receptors (NLRs): Bona fide intracellular microbial
sensors. Curr Opin Immunol. 20:377–382. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Jorgensen I and Miao EA: Pyroptotic cell
death defends against intracellular pathogens. Immunol Rev.
265:130–142. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Franchi L, Muñoz-Planillo R and Núñez G:
Sensing and reacting to microbes through the inflammasomes. Nat
Immunol. 13:325–332. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Galluzzi L, Vitale I, Aaronson SA, Abrams
JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews
DW, et al: Molecular mechanisms of cell death: Recommendations of
the nomenclature committee on cell death 2018. Cell Death Differ.
25:486–541. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Naji A, Muzembo BA, Yagyu K, Baba N,
Deschaseaux F, Sensebé L and Suganuma N: Endocytosis of
indium-tin-oxide nanoparticles by macrophages provokes pyroptosis
requiring NLRP3-ASC-Caspase1 axis that can be prevented by
mesenchymal stem cells. Sci Rep. 6:261622016. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Kim HS, Shin TH, Yang SR, Seo MS, Kim DJ,
Kang SK, Park JH and Kang KS: Implication of NOD1 and NOD2 for the
differentiation of multipotent mesenchymal stem cells derived from
human umbilical cord blood. PLoS One. 5:e153692010. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Wang L, Chen K, Wan X, Wang F, Guo Z and
Mo Z: NLRP3 inflammasome activation in mesenchymal stem cells
inhibits osteogenic differentiation and enhances adipogenic
differentiation. Biochem Biophys Res Commun. 484:871–877. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Wu XM, Chen WQ, Hu YW, Cao L, Nie P and
Chang MX: RIP2 Is a critical regulator for NLRs signaling and MHC
antigen presentation but not for MAPK and PI3K/Akt pathways. Front
Immunol. 9:7262018. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Bist P, Dikshit N, Koh TH, Mortellaro A,
Tan TT and Sukumaran B: The Nod1, Nod2, and Rip2 axis contributes
to host immune defense against intracellular Acinetobacter
baumannii infection. Infect Immun. 82:1112–1122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Souza JA, Medeiros MC, Rocha FR, de Aquino
SG, Ávila-Campos MJ, Spolidorio LC, Zamboni DS, Graves DT and Rossa
C: Role of NOD2 and RIP2 in host-microbe interactions with
Gram-negative bacteria: Insights from the periodontal disease
model. Innate Immun. 22:598–611. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Singh V, Holla S, Ramachandra SG and
Balaji KN: WNT-inflammasome signaling mediates NOD2-induced
development of acute arthritis in mice. J Immunol. 194:3351–3360.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Prakhar P, Holla S, Ghorpade DS, Gilleron
M, Puzo G, Udupa V and Balaji KN: Ac2PIM-responsive miR-150 and
miR-143 target receptor-interacting protein kinase 2 and
transforming growth factor beta-activated kinase 1 to suppress
NOD2-induced immunomodulators. J Biol Chem. 290:26576–26586. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
McGonagle D, Savic S and McDermott MF: The
NLR network and the immunological disease continuum of adaptive and
innate immune-mediated inflammation against self. Semin
Immunopathol. 29:303–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Zhou L, Dörfer CE, Chen L and Fawzy
El-Sayed KM: Porphyromonas gingivalis lipopolysaccharides affect
gingival stem/progenitor cells attributes through NF-κB, but not
WNT/β-catenin, pathway. J Clin Periodontol. 44:1112–1122. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Chen S, Shen D, Popp NA, Ogilvy AJ, Tuo J,
Abu-Asab M, Xie T and Chan CC: Responses of multipotent retinal
stem cells to IL-1β, IL-18, or IL-17. J Ophthalmol.
2015:3693122015. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Huang J and Chen L: IL-1β inhibits
osteogenesis of human bone marrow-derived mesenchymal stem cells by
activating FoxD3/microRNA-496 to repress WNT signaling. Genesis.
552017.doi: 10.1002/dvg.23040.
|
|
155
|
Gao Y, Grassi F, Ryan MR, Terauchi M, Page
K, Yang X, Weitzmann MN and Pacifici R: IFN-gamma stimulates
osteoclast formation and bone loss in vivo via antigen-driven T
cell activation. J Clin Invest. 117:122–132. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Suzawa M, Takada I, Yanagisawa J, Ohtake
F, Ogawa S, Yamauchi T, Kadowaki T, Takeuchi Y, Shibuya H, Gotoh Y,
et al: Cytokines suppress adipogenesis and PPAR-gamma function
through the TAK1/TAB1/NIK cascade. Nat Cell Biol. 5:224–230. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Gaspersic R, Stiblar-Martincic D, Osredkar
J and Skaleric U: In vivo administration of recombinant TNF-alpha
promotes bone resorption in mice. J Periodontal Res. 38:446–448.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Wang L, Zhao Y and Shi S: Interplay
between mesenchymal stem cells and lymphocytes: Implications for
immunotherapy and tissue regeneration. J Dent Res. 91:1003–1010.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Yang SR, Park JR and Kang KS: Reactive
oxygen species in mesenchymal stem cell aging: Implication to lung
diseases. Oxid Med Cell Longev. 2015:4862632015. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Kawai T and Akira S: Toll-like receptors
and their crosstalk with other innate receptors in infection and
immunity. Immunity. 34:637–650. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Atashi F, Modarressi A and Pepper MS: The
role of reactive oxygen species in mesenchymal stem cell adipogenic
and osteogenic differentiation: A review. Stem Cells Dev.
24:1150–1163. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Brown GC and Borutaite V: There is no
evidence that mitochondria are the main source of reactive oxygen
species in mammalian cells. Mitochondrion. 12:1–4. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
McNally JS, Davis ME, Giddens DP, Saha A,
Hwang J, Dikalov S, Jo H and Harrison DG: Role of xanthine
oxidoreductase and NAD (P)H oxidase in endothelial superoxide
production in response to oscillatory shear stress. Am J Physiol
Heart Circ Physiol. 285:H2290–H2297. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
O'Donnell VB and Azzi A: High rates of
extracellular superoxide generation by cultured human fibroblasts:
Involvement of a lipid-metabolizing enzyme. Biochem J. 318:805–812.
1996. View Article : Google Scholar
|
|
165
|
Starkov AA: The role of mitochondria in
reactive oxygen species metabolism and signaling. Ann N Y Acad Sci.
1147:37–52. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Gross E, Sevier CS, Heldman N, Vitu E,
Bentzur M, Kaiser CA, Thorpe C and Fass D: Generating disulfides
enzymatically: Reaction products and electron acceptors of the
endoplasmic reticulum thiol oxidase Ero1p. Proc Natl Acad Sci USA.
103:299–304. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Roy P, Roy SK, Mitra A and Kulkarni AP:
Superoxide generation by lipoxygenase in the presence of NADH and
NADPH. Biochim Biophys Acta. 1214:171–179. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Schröder K, Wandzioch K, Helmcke I and
Brandes RP: Nox4 acts as a switch between differentiation and
proliferation in preadipocytes. Arterioscler Thromb Vasc Biol.
29:239–245. 2009. View Article : Google Scholar
|
|
169
|
Pittenger MF and Martin BJ: Mesenchymal
stem cells and their potential as cardiac therapeutics. Circ Res.
95:9–20. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Liu SP, Ding DC, Wang HJ, Su CY, Lin SZ,
Li H and Shyu WC: Nonsenescent Hsp27-upregulated MSCs implantation
promotes neuroplasticity in stroke model. Cell Transplant.
19:1261–1279. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Kobayashi CI and Suda T: Regulation of
reactive oxygen species in stem cells and cancer stem cells. J Cell
Physiol. 227:421–430. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
D'Autréaux B and Toledano MB: ROS as
signalling molecules: Mechanisms that generate specificity in ROS
homeostasis. Nat Rev Mol Cell Biol. 8:813–824. 2007. View Article : Google Scholar
|
|
173
|
Eto H, Kato H, Suga H, Aoi N, Doi K, Kuno
S and Yoshimura K: The fate of adipocytes after nonvascularized fat
grafting: Evidence of early death and replacement of adipocytes.
Plast Reconstr Surg. 129:1081–1092. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Rodrigues M, Turner O, Stolz D, Griffith
LG and Wells A: Production of reactive oxygen species by
multipotent stromal cells/mesenchymal stem cells upon exposure to
fas ligand. Cell Transplant. 21:2171–2187. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Zou X, Li H, Chen L, Baatrup A, Bünger C
and Lind M: Stimulation of porcine bone marrow stromal cells by
hyaluronan, dexamethasone and rhBMP-2. Biomaterials. 25:5375–5385.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Denu RA and Hematti P: Effects of
oxidative stress on mesenchymal stem cell biology. Oxid Med Cell
Longev. 2016:29890762016. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Valle-Prieto A and Conget PA: Human
mesenchymal stem cells efficiently manage oxidative stress. Stem
Cells Dev. 19:1885–1893. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Orciani M, Gorbi S, Benedetti M, Di
Benedetto G, Mattioli-Belmonte M, Regoli F and Di Primio R:
Oxidative stress defense in human-skin-derived mesenchymal stem
cells versus human keratinocytes: Different mechanisms of
protection and cell selection. Free Radic Biol Med. 49:830–838.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Ko E, Lee KY and Hwang DS: Human umbilical
cord blood-derived mesenchymal stem cells undergo cellular
senescence in response to oxidative stress. Stem Cells Dev.
21:1877–1886. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Hou J, Han ZP, Jing YY, Yang X, Zhang SS,
Sun K, Hao C, Meng Y, Yu FH, Liu XQ, et al: Autophagy prevents
irradiation injury and maintains stemness through decreasing ROS
generation in mesenchymal stem cells. Cell Death Dis. 4:e8442013.
View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Kanda Y, Hinata T, Kang SW and Watanabe Y:
Reactive oxygen species mediate adipocyte differentiation in
mesenchymal stem cells. Life Sci. 89:250–258. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Zhang Y, Marsboom G, Toth PT and Rehman J:
Mitochondrial respiration regulates adipogenic differentiation of
human mesenchymal stem cells. PLoS One. 8:e770772013. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Ren J, Stroncek DF, Zhao Y, Jin P,
Castiello L, Civini S, Wang H, Feng J, Tran K, Kuznetsov SA, et al:
Intra-subject variability in human bone marrow stromal cell (BMSC)
replicative senescence: Molecular changes associated with BMSC
senescence. Stem Cell Res (Amst). 11:1060–1073. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Sallman DA, Cluzeau T, Basiorka AA and
List A: Unraveling the pathogenesis of MDS: The NLRP3 inflammasome
and pyroptosis drive the mds phenotype. Front Oncol. 6:1512016.
View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Kajla S, Mondol AS, Nagasawa A, Zhang Y,
Kato M, Matsuno K, Yabe-Nishimura C and Kamata T: A crucial role
for Nox 1 in redox-dependent regulation of WNT-β-catenin signaling.
FASEB J. 26:2049–2059. 2012. View Article : Google Scholar : PubMed/NCBI
|