Introduction
It has been known for several centuries that
nephrolithiasis (commonly referred to as kidney stones) is a
significant health problem that may lead to loss of kidney function
(1), and that it is associated with
other morbidities such as hypertension and fractures (2,3).
Nephrolithiasis is a complex multifactorial disease that is the
result of interactions between environmental, dietary and genetic
factors. Studies have shown that the lifetime risk of kidney stones
can vary between 5–20%, and this is exhibiting an increasing trend
(4,5). Whilst men are affected twice as much
as women, in children, there is no bias towards one sex (6).
Ca2+ oxalate stones are the most
prevalent type of kidney stones, and are responsible for 70–80% of
cases of kidney stones in humans (7,8).
Ca2+ oxalate stones are caused by elevated urinary
Ca2+ and oxalate levels, and are termed hypercalciuria
and hyperoxaluria, respectively (8). Hyperoxaluria is a major risk factor of
Ca2+ oxalate stone formation, which leads to an increase
in urinary saturation of Ca2+ to form Ca2+
oxalate stones (9). However,
hyperoxaluria is primarily caused by three aspects, including
enhanced absorption of oxalate by the intestine, internal
production of oxalate by the liver and excretion of oxalate by the
kidneys (10). Additionally,
oxalate homeostasis is maintained by solute carrier family 26
member 6 (SLC26A6) in the intestinal and renal tubular epithelium,
imbalances of which result in hyperoxaluria and hyperoxalemia,
suggesting that oxalate secretion is dependent on the transcellular
mechanisms of SLC26A6c (11).
Conversely, even in the absence of hypercalciuria,
low concentrations of the Ca2+ chelator citrate in urine
can promote the formation of Ca2+ stones, as urinary
citrate can inhibit the crystallization and precipitation of
Ca2+ in the renal calculi by chelating Ca2+
ions (8). In the vast majority of
patients with Ca2+ kidney stones, they exhibit low
urinary citrate excretion, and the incidence of hypocitraturia
ranges from 19–60%. Therefore, sufficient urinary citrate
concentration is also the key to preventing stone formation.
Notably, Na+-dependent dicarboxylate-1 (NADC-1)
reabsorbs most of the citrate in the proximal tubular apical
membrane; thus, NADC-1 is one of the main determinants of renal
calculi (12,13). This is consistent with another
previous study, in which it was shown that SLC26A6 and NADC-1
transporters can function to prevent stone formation by dual method
(14).
Similarly, succinate, an intermediate of the
tricarboxylic acid cycle, is also absorbed by NADC-1 in the apical
membrane of the proximal tubule (15). Previously, succinate was only
regarded as an intermediate of the tricarboxylic acid cycle, but
more recent data has suggested that it may function as a crucial
extracellular signaling molecule, which is consistent with the
discovery of the succinate-specific G-protein-coupled receptor
succinate receptor 1 (SUCNR1), in the epithelium of several organs,
such as the kidneys and intestines (16). Hyperperfusion studies and
intravenous results suggest that succinate stimulates renin
secretion from granular cells at the juxtaglomerular apparatus
(17), confirming that an increase
in blood pressure can be induced through the SUCNR1 signaling
pathway (18,19), proving a novel direction for the
association between NADC-1 and calculus-related hypertension.
The question as to how the formation of renal
calculi and Ca2+ oxalate stones are associated with
hypertension has not been fully addressed. The emergence of
SLC26A6, and in particular, the synergistic function of SLC26A6 and
NADC-1, has shed light on the current understanding of the
mechanisms underlying the processes involved in the formation of
kidney stones, as well as the association between nephrolithiasis
and hypertension. In the present review, the family, structure and
functional expression of the two proteins are first described in
order to further understand the significance of SLC26A6 and NADC-1
in human physiology. Next, this review examined the results from
studies on oxalate and citrate transport by the kidney tubule,
highlighting areas where the transporters may be involved in the
processes of Ca2+ oxalate formation, and summarized the
reported molecular mechanisms of the synergistic action between
SLC26A6 and NADC-1 in renal tubular epithelial cells in the
literature. Additionally, a summary of the function of
SLC26A6/NADC-1 in hypertension associated with Ca2+
oxalate kidney stones is provided, indicating the possible role of
the two transporters in the formation of Ca2+ oxalate
kidney stones and their implications for hypertension.
SLC26A6 and NADC-1: Family, localization,
structure and functional expression
The phylogenetically ancient SLC26-sulfate
transporter (SulP) gene family is a part of the adenomatous
polyposis coli gene superfamily, encoding membrane proteins that
exchange electroneutral or univalent and bivalent anionic
substrates, and are of crucial importance in metabolic processes,
pH regulation and electrolyte homeostasis. Notably, the SLC26 or
SulP proteins are universally expressed in prokaryotes and
eukaryotes (20–22). Bacterial SLC26-related SulP proteins
and SLC26-related Sultr proteins are the major contributors to the
marine carbon cycle and sulfate transport by yeast, algae and
plants (20). In humans, the SLC26
family plays an important role as a multifunctional anion
transporter in various physiological activities to maintain
homeostasis in the body, including 11 proteins (SLC26A1-A11)
(Table I), of which A10 is a
pseudogene (23). Amongst these,
the protein encoded by the gene SLC26A6 exhibits the most
extensive exchange function of the SLC26 family members,
particularly with regard to oxalate, where it has a high affinity
(24).
 | Table I.SLC26 multifunctional anion
exchanger/anion channel gene family. |
Table I.
SLC26 multifunctional anion
exchanger/anion channel gene family.
| Gene | Protein name | Human gene
locus | Transportions | Tissue
distribution/subcellular expression | Link to
disease | (Refs.) |
|---|
| Slc26a1 | SLC26A1 | 4p16.3 |
SO42−,
OXa2− | Hepatocytes,
basolateral renal proximal tubule, intestine | Oxalate
urolithiasis, urinary sulfate wasting, hepatotoxicitya | (76,77) |
| Slc26a2 | SLC26A2 | 5q32 |
SO42−,
OXa2−, Cl− | Chondrocytes, renal
proximal tubule, intestine, pancreatic duct (apical) | Diastrophic
dysplasia, chondrodysplasia, De la Chapelle dysplasia | (78–80) |
| Slc26a3 | SLC26A3 | 7q31 | OXa2−,
Cl−, HCO3− | Enterocytes, sperm
epididymis (apical) | Congenital
chloride, diarrhea | (81,82) |
| Slc26a4 | SLC26A4 | 7q31 | I−,
Cl−, HCO3− | Cochlear,
vestibular epithelial cells, thyrocytes type B intercalated cell,
airway epithelial cell (apical) | Pendred syndrome,
deafness (DFNB4)a,
enlargement of the vestibular aqueduct | (83,84) |
| Slc26a5 | SLC26A5 | 7q22 | Cl−,
SO42−, OXa2−, For− | Cochlear hair
cells |
Deafnessa | (85) |
| Slc26a6 | SLC26A6 | 3p21.3 | Cl−,
HCO3−, oxalate, OH−, formate | Enterocytes,
Pancreatic duct, Renal proximal tubule, Cardiac myocytes,
Sperm |
Nephrolithiasisa | (42,86) |
| Slc26a7 | SLC26A7 | 8q23 | Cl−,
HCO3−, OH−,
SO42− | Gastric parietal
cells, Type A intercalated cells, Endothelial cells, apical and
lysosomal | Gastric
hypochlorhydriaa,
distal renal tubular acidosisa | (87,88) |
| Slc26a8 | SLC26A8 | 6p21 | Cl−,
HCO3−, OH− | Male germ cells,
Sperm | Male
infertilitya | (89) |
| Slc26a9 | SLC26A9 | 1q321.1 | Cl−,
HCO3− | Airway epithelial
cells, Gastric parietal cells, Kidney, unknown cell type | Gastric
hypochlorhydriaa,
cystic fibrosis-associated meconium ileus, diabetes | (90,91) |
| Slc26a10 | SLC26A10 | 12q13 |
| Unknown transcribed
pseudogene | Not reported | (86,92) |
| Slc26a11 | SLC26A11 | 17q25.3 | Cl−,
HCO3−, SO42−,
OXa2− | Renal intercalated
cells, apical Pancreatic duct, Endothelial cells, Brain,
widespread | Not reported | (93,94) |
The SLC26A6 gene was cloned on the basis of
homology to the other two members of the SLC26 family,
SLC26A3 and SLC26A4 (5). The SLC26A6 gene maps to
chromosome 3p21.3-4, which consists of 21 relatively short exons
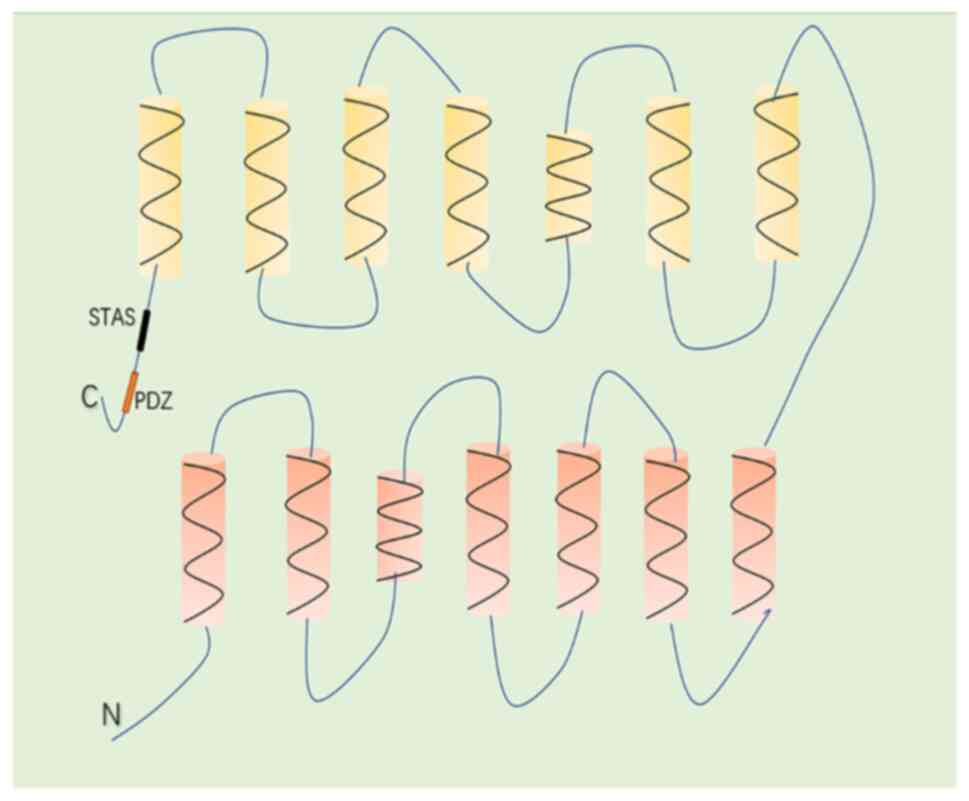
interrupted by 20 intronic sequences (25). The SLC26A6 protein has a molecular
mass of 82 kDa, functions as a secondary cytomembrane transporter
and consists of 759 amino acids with a predicted topological
structure of 14 transmembrane α-helices (the 3rd and 10th helices
do not completely span the entire cytomembrane) and an
intracellular -NH2 and -COOH terminal (26) (Fig.
1). The -COOH terminal of SLC26A6 possesses a conserved domain,
namely sulfate transporter and anti-sigma factor antagonist (STAS),
which plays a vital role in regulating protein function and
expression (21,27). Furthermore, the -COOH terminal of
SLC26A6 contains a consensus PDZ interaction motif identical to
that found in the cystic fibrosis transmembrane conductance
regulator, which provides interaction sites for other interacting
proteins and ultimately participates in the regulation of membrane
protein function (27). There are
also three alternative splicing variants of the SLC26A6
gene, termed SLC26A6A, SLC26A6C and SLC26A6D, which
consist of 12, 8 and 12 transmembrane domains, respectively.
SLC26A6A is primarily a spicing variant expressed in the
small intestine and colon (28).
SLC26A6D is primarily expressed in the kidney and pancreas,
whereas SLC26A6C is faintly expressed in the human kidney
(29), suggesting that various
slc26a6 variants are tissue specific. Similarly, the SLC26A6
transporter is widely expressed in various organs, such as the
salivary glands (30), heart
(31), intestine (32,33),
pancreas (34), kidney (35) and uterus (36), with the highest expression observed
in the apical membrane of the kidney proximal tubule and small
intestinal villi (25).
Heterologous expression studies have demonstrated that mouse
Slc26A6 and human Slc26A6 can function in multiple
transport modes, including acting as a coupled ion channel to
mediate the exchange of a cluster of anions, including
HCO3−, Cl− and OXa2− in
epithelial cells, and can also act as an uncoupled ion channel to
transport SNC−, NO3− and
Cl−, amongst others (20,31,37–42).
In the present review, a focus is placed on the function of SLC26A6
as an
Cl−(in)/OXa2−(out)
exchanger in maintaining the dynamic balance of oxalate
equilibrium, as the deletion of the SLC26A6 gene can lead to
a decrease of intestinal secretion, which will lead to
hyperoxalemia and hyperoxaluria (43). Notably, SLC26A6 is intricately
associated with renal Ca2+-oxalate stones (6).
The SLC13 gene family consists of five
sequence-related members that have been identified in several
animals, plants, yeast and bacteria. The proteins encoded by these
genes are divided into two distinct groups: The
Na+-sulphate co-transporters and the
Na+-carboxylate co-transporters. Members of the SLC13
family include renal Na+-dependent inorganic sulphate
transporter-1 (SLC13A1), Na+-dependent dicarboxylate
transporters NADC-1/SDCT1 (SLC13A2), NADC-3/SDCT2 (SLC13A3),
sulphate transporter-1 (SLC13A4) and Na+-coupled citrate
transporter (SLC13A5) (Table II)
(44).
 | Table II.SLC13 sodium sulphate/carboxylate
cotransporter gene family. |
Table II.
SLC13 sodium sulphate/carboxylate
cotransporter gene family.
| Gene | Protein names | Human gene
locus | Transportions | Tissue
distribution/subcellular expression |
|---|
| SLC13A1 | NaSi-1,
Na-sulphate | 7q31-q32 | Sulphate, selenate,
thiosulphate | Kidney, proximal
tubular cells, brush border membrane |
| SLC13A2 | NADC-1, SDCT1,
NADC-2 | 17p11.1-q11.1 | Succinate, citrate,
α-ketoglutarate | Kidney, intestine,
brush border membrane |
| SLC13A3 | NADC-3, SDCT2 | 20q12-q13.1 | Succinate, citrate,
α-ketoglutarate | Kidney proximal
tubule basolateral membrane, liver, pancreas, brain, placenta |
| SLC13A4 | SUT-1 | 7q33 | Sulphate | Placenta, tonsillar
high endothelial venules, testis, heart |
| SLC13A5 | NaCT | 12p12-13 | Citrate | Liver, brain,
testis |
Initially, the original SLC13 family members were
isolated from Xenopus oocytes. The first member was Slc13a1,
encoding a rat Na+-sulphate cotransporter (45), followed by Slc13a2, encoding a
rabbit Na+-dicarboxylate cotransporter (13), the Xenopus Slc13a2 (46) and the winter flounder Slc13a3
(47). SLC13A2 has been isolated
from five vertebrates: Humans (48), rabbits (13), mice (49), rats (50–52)
and Xenopus (46). The human
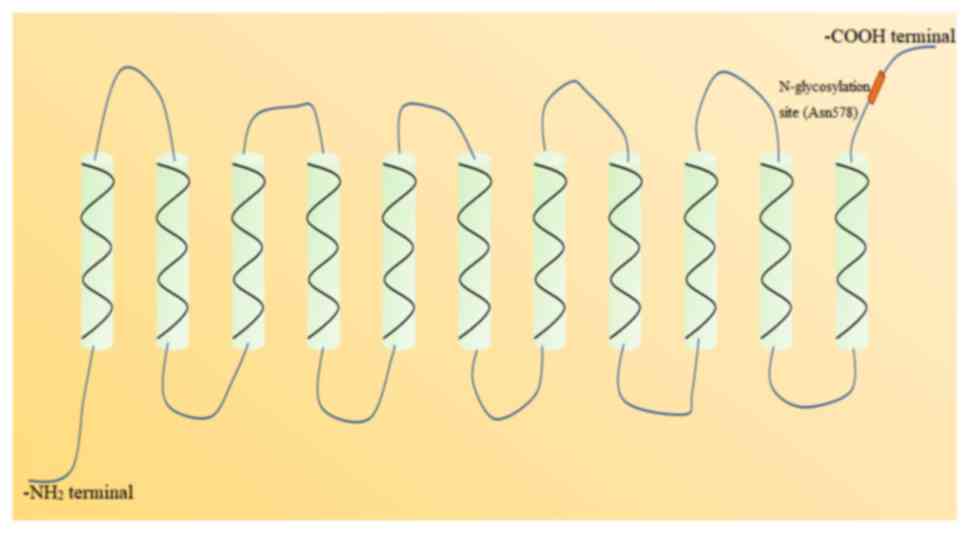
NADC-1 gene contains 12 exons consisting of 1953 base pairs,
encoding 593 amino acids (48), and
the gene is found on chromosome 17p11.1-q11.1 (53). NADC-1 possesses an 11-transmembrane
α-helices topological structure, with an intracellular
-NH2 terminal and an extracellular -COOH terminal
(Fig. 2). There is a conserved
N-glycosylation site (Asn578) in the extracellular -COOH terminal,
which is an important structure to control the function and
expression of NADC-1 (54). In
addition, there are two N-glycosylation sites in the -COOH terminal
of mouse NADC-1, namely Asn584 and Asn580 (49). NADC-1 is widely expressed in various
tissues, particularly in kidney and gastrointestinal epithelium.
Western blotting showed that human NADC-1 was present in the
kidneys and intestines (48), and
rabbit NADC-1 was strongly expressed in the kidneys and jejunum,
with weaker expression detected in the liver (13). Similarly, mouse and rat NADC-1 were
also detected in the kidneys and intestines (49). In immunocytochemical experiments and
in situ hybridization studies, rat NADC-1 protein was
confirmed to be present in the outer stripe of the outer medulla
and in the luminal membranes of the renal superficial cortex
(51). As a Na+-coupled
symporter, NADC-1 transporter exhibits strong cation selectivity
for Na+, the coupling ratio of Na+ to anions
is 3:1, and it has a preference for divalent anions, including
tricarboxylic or Krebs cycle intermediates, such as succinate and
citrate, with a high affinity for succinate and a lower affinity
for citrate (44,54,55).
It is notable that >65% of the intermediate products of the
Krebs cycle excreted in the kidney are reabsorbed by NADC-1 in the
proximal tubules for intracellular metabolism or exchange with
organic anions in the process of organic anion secretion (55). In particular, NADC-1 can affect
Ca2+ citrate chelates by regulating the concentration of
citrate to prevent the formation of kidney stones, as citrate
competes for oxalate to bind with ions with higher affinity, such
that supersaturation of stones will not be achieved at high
concentrations of citrate. Furthermore, it has been shown that ~50%
of patients with nephrolithiasis exhibit hypocitraturia, consistent
with the role of NADC-1 as a Ca2+ inhibitor (56).
Essential roles of the SLC26A6 and NADC-1
transporters in the kidney
As aforementioned, Ca2+ oxalate stones
are the most prevalent type of renal stones, and are predominately
determined by the high levels of urinary oxalate and urinary
Ca2+, or the decrease in urinary citrate concentration
(the major Ca2+ inhibitor) (8). There are two sources of oxalate in the
human body, absorption through the intestinal exogenous
paracellular pathway and endogenous liver production (57). Oxalate is primarily excreted by the
intestines and kidneys, and >90% of oxalate is excreted via
urine. Thus, the secretion of oxalate in the kidney plays a crucial
role in the development of nephrolithiasis. Jiang et al
(43) showed that the exchange of
Cl−(in)/OXa2−(out) at
the apical membrane of the proximal tubule is entirely mediated by
SLC26A6, consistent with its expression on the brush border
membrane of the renal proximal tubule cells (24). Similarly, in humans, previous
studies suggested that >65% of citrate is reabsorbed in the
renal tubule after glomerular filtration (56), whereas in vitro perfusion
studies using rabbit nephrons showed that citrate is taken up
exclusively in the proximal tubule (58). In the proximal tubule, the
reabsorption of citrate and succinate in the apical membrane is
predominantly mediated by NADC-1, via Na+ coupled
electrogenic exchange (48,49,55).
There is an increasing body of studies that have
suggested that even in the absence of hypercalciuria, the
simultaneous occurrence of hyperoxaluria and hypocitraturia can
trigger the formation of Ca2+-oxalate stones, which has
increased widespread concern amongst researchers. Ohana et
al (14) studied the molecular
mechanisms involving the oxalate transporter SLC26A6 and citrate
transporter NADC-1 in controlling the dynamic balance of urinary
citrate and oxalate. In the study, NADC-1 and SLC26A6 were
co-expressed in Xenopus laevis oocytes and the activity of
the two exchangers, the Na+-dicarboxylate transporter
and oxalate transporter, were monitored. The results showed that
NADC-1 increased SLC26A6 activity, in turn increasing
Cl−-oxalate exchange by 30% and similarly increasing
1Cl−−2HCO3− exchange, and that
there were no changes in the stoichiometry of exchange (14). Conversely, the study indicated that
SLC26A6 restricted the activity of NADC-1 and that the effect of
SLC26A6 in the active state was more significant than that in the
inactive state. Notably, other members of the SLC26 family also
exhibit an inhibitory effect on NADC-1, such as SLC26A3 (59) (Fig.
3).
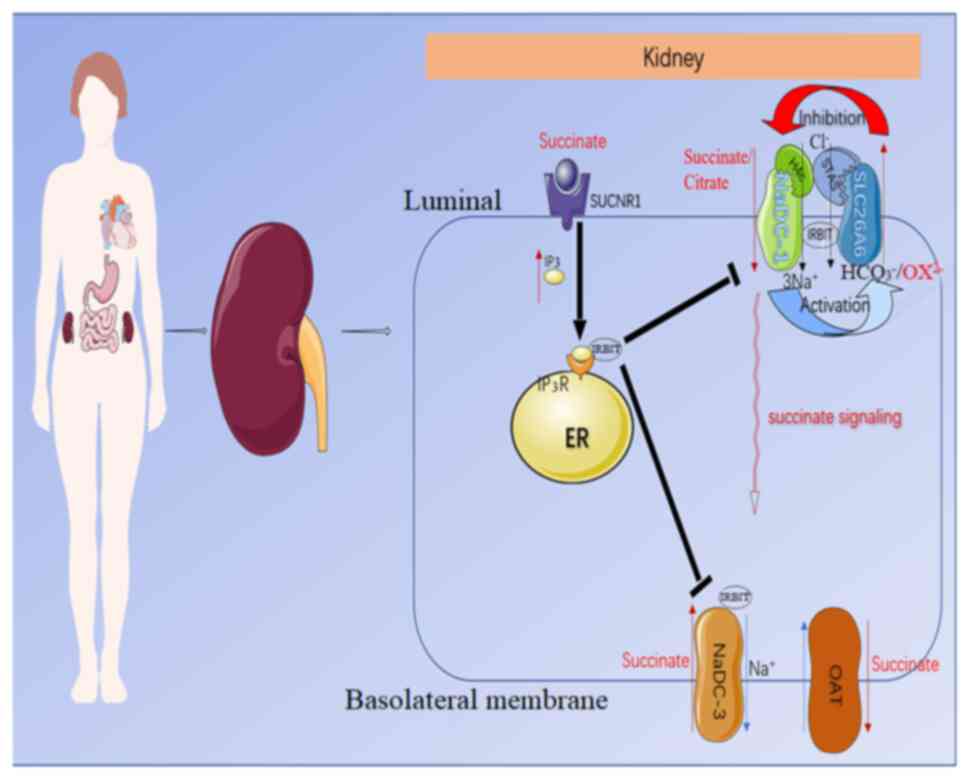 | Figure 3.Predicted molecular mechanism by
which NADC-1 and SLC26A6 interact to modulate succinate/citrate and
oxalate transport in epithelial cells. NADC-1 and SLC26A6 regulate
each other through the H4c and STAS domains, in which NADC-1
activates SLC26A6, and SLC26A6 inhibits NADC-1. Apical
succinate/citrate uptake is mediated by an NADC-1-SLC26A6 succinate
transport complex. Meanwhile, luminal succinate stimulates the
succinate receptor SUCNR1, which induces the release of IRBIT by
activating the intracellular IP3 receptor. IRBIT then
translocates to the membrane and binds to succinate transporters on
the apical and basal lateral membranes to coordinate and modulate
the absorption of succinate across the epithelium. SLC26A6, solute
carrier family 26 member 6; STAS, sulfate transporter and
anti-sigma factor antagonist; NADC-1, Na+-dependent
dicarboxylate-1; SUCNR1, succinate-specific G-protein-coupled
receptor succinate receptor 1; IP3, inositol
triphosphate; IRBIT, IP3 receptor-binding protein; ER,
endoplasmic reticulum; OAT, organic anion transporter. |
In addition, Khamaysi et al (60) recently showed that the
SLC26A6/NADC-1 complex participates in hypertension by regulating
local succinate levels (Fig. 3).
The study additionally demonstrated the synergistic structural
domain of the complex. It was concluded that the SLC26A6 and
inositol triphosphate (IP3) receptor-binding protein
(IRBIT) inhibited NADC-1-mediated succinate transport by ~50%, with
a superimposed effect that made the inhibition more potent. In
turn, NADC-1 elevated SLC26A6 transporter activity and increased
IRBIT release by transporting succinate to enrich the concentration
of IP3. In addition, the interaction between
NADC-1/SLC26A6 is largely mediated through the amino acid K107 in
the vcINDY H4c-like region of NADC-1 (61) and E613 in the SLC26A6-STAS domain,
and the STAS domain of SLC26A6 has previously been shown to be the
transport determining functional domain (60). Succinate transport by NADC-1 can
activate phospholipase C β to increase Ca2+ and IP3
levels by stimulating SUCNR1 (62,63),
whereas IRBIT competes with IP3 for binding to the
IP3 receptor protein. When IP3 levels
increase, it triggers an increase in IRBIT release (64), and IRBIT can act on various
transporters, such as activating the anion transporter SLC26A6
(65) and inhibiting the succinate
transporter NADC-1 on the apical membrane of the lumen. In
addition, IRBIT inhibits the NADC-3 transporter on the basolateral
membrane of the proximal tubule, which mediates citrate/succinate
influx from the interstitium into the epithelial cells (24), orchestrating the succinate inflow to
control succinate absorption and metabolism. The organic anion
transporters 1–3 that extrude succinate from the proximal tubule
basement membrane are also significantly inhibited by IRBIT
(66). If the regulation of the
SLC26A6 and NADC-1 transporters becomes imbalanced, it can readily
lead to an increase in serum succinate and calculus-related
salt-independent hypertension. Although several hypotheses have
been suggested to describe the association between kidney stones
and hypertension, such as tubulointerstitial damage and altered
renal handling of Ca2+, amongst others (2), succinate stimulates renin secretion
and increases the risk of developing hypertension, making the
mechanism suggested by Khamaysi et al (60), wherein NADC-1/SLC26A6 mediation of
citrate and succinate contribute to the association between renal
calculi and hypertension, more convincing.
Another association between NADC-1 and SLC26A6 is
the acid-base balance. Immunohistochemistry has shown that patients
with low pH in urine are more likely to exhibit higher NADC-1
expression (67), which is
consistent with chronic acid intake-induced renal stone formation
and upregulation of NADC-1 mRNA expression in a rat model. The
reason behind this may be that citrate, rather than the succinate,
only present in the form of tricarboxylic acid under alkaline
conditions, is not reabsorbed in the proximal tubule. That is,
citrate can only be reabsorbed by the NADC-1 transporter in the
proximal renal tubule in its divalent form (51). Notably, in vitro
microperfusion studies of proximal tubule segments in mice have
shown that SLC26A6 also acts as a major
HCO3−/Cl− exchanger (35), leading to the hypothesis that
SLC26A6 can inhibit NADC-1 by increasing the pH of the urine,
although this hypothesis remains to be confirmed.
Involvement of SLC26A6 and NaDC-1
transporters in the pathophysiology states of the kidney
The co-expression of NADC-1/SLC26A6 in Xenopus
laevis oocytes and extensive in vitro experiments has
further deepened the current understanding of the synergistic
molecular mechanisms involved in the formation of Ca2+
oxalate stones and the associated hypertension, whereas the
understanding of the molecular mechanism of Ca2+ oxalate
stone formation by the secretion of oxalate from SLC26A6 has been
vastly improved by numerous studies in mouse models (11,68,69).
In order to improve the current understanding of the transporter
function relevant to nephrolithiasis, a micro-perfusion study found
that the renal function of Slc26a6-null mice did not change
significantly, but the Cl−/oxalate exchange mediated by
the SLC26A6 transporter was abolished completely (35), meaning that the
Cl−/oxalate exchange in the apical membrane of the renal
proximal tubule is entirety mediated by SLC26A6. Similarly, Jiang
et al (43) and Freel et
al (33) also established
SLC26A6 null mice that demonstrated a 4-fold increase in urine
oxalate excretion. A large amount of oxalate in urine can increase
the protein expression of NADPH oxidase in the renal epithelial
cells, which leads to oxidative stress in cells to promote the
formation of renal stones (70).
This is consistent with the high expression of A6 found by Jiang
et al (71) in NRE-52 cells,
which increased damage to the cells and resulted in increased
crystal adhesion to the cells. Moreover, oxalate is also the most
common type of kidney stone, specifically Ca2+ oxalate
kidney stones (7), thus a large
amount of oxalate in urine is a high risk factor for
nephrolithiasis.
Several SLC26A6 variants were also found during the
literature review, such as the SLC26A6 (V206M) and SLC26A6 (G539R)
polymorphisms, which can generate the phenotypes of hyperoxaluria
and hyperoxalemia to promote the formation of kidney stones
(68,72,73).
Conversely, research on NADC-1 is relatively limited, and only one
related mutant has been identified. The variant I550V in the NADC-1
transporter is reported to decrease urinary citrate excretion,
although it has a mild effect on the transporter function,
resulting in a 20% decrease in transporter activity (74). Unexpectedly, the two variants were
located in the region encoding the STAS domain, as found in SLC26A6
by Shimshilashvili et al (11), further demonstrating the crucial
role of the SLC26A6/NADC-1 complex in maintaining the dynamic
balance of citrate/succinate and oxalate to prevent kidney stones
from forming. The two STAS domain polymorphisms SLC26A6 (R621G) and
SLC26A6 (D673N or D674N) both decreased SLC26A6 expression,
transport activity and mutual mediation with transporter NADC-1.
Notably, the former variant resulted in a significantly lower
concentration of urinary citrate and normal concentrations of
urinary oxalate were sufficient to induce kidney stones. However,
the latter variant had a high urinary oxalate concentration and a
50% higher citrate concentration than the former variant, but this
did not successfully induce kidney stone formation. This
demonstrates the importance of SLC26A6 in mediating urinary citrate
concentration, that is, it emphasizes the role of SLC26A6 and
NADC-1 in preventing the formation of kidney stones. Furthermore,
partner proteins that form complexes in the membrane, as
demonstrated for the cystic fibrosis transmembrane conductance
regulator (CFTR) (75), can
compensate for the weakening of the SLC26A6 (D674N) polymorphism
transport function, which makes the SLC26A6-STAS domain a potential
target for the treatment of diseases caused by transporter
dysfunction.
Discussion
In the present review, the association between the
NADC-1/SLC26A6 transporter and nephrolithiasis and calculus-related
hypertension was discussed. The roles of oxalate transporter
SLC26A6 and citrate transporter NADC-1 in nephrolithiasis and
calculus-related hypertension remain elusive, and the synergistic
molecular mechanisms between these transporters require further
investigation. Nevertheless, SLC26A6 and NADC-1 transporters may
serve as a future direction in the study of kidney stones and
calculus-related hypertension.
Various variants of SLC26A6 and NADC-1 have been
shown to be involved in the formation of kidney stones. However, to
the best of our knowledge, there are no studies on the synergistic
region of the two transporters in nephrolithiasis and
calculus-related hypertension. That is to say, the crucial role of
SLC26A6/NADC-1 in kidney stones and calculus-related hypertension
requires further study, perhaps with a particular focus on SLC26A6
and NADC-1 in the intestinal villus epithelium. Conversely, further
verification is needed with regard to whether the SLC26A6
transporter can function as an
HCO3−/Cl− exchanger to mediate the
activity of NADC-1 transporter by adjusting the pH of urine. In the
present review, discussion around the use of soluble polypeptides
for management of transport disorders caused by the functional
structural variations in the SLC26A6 transporter were discussed,
highlighting a novel treatment direction in the management of
kidney stones and calculus-related hypertension.
In conclusion, SLC26A6/NADC-1 is a promising target
and potential marker for nephrolithiasis and calculus-related
hypertension disease treatment in future. However, drugs targeting
SLC26A6/NADC-1 need to be examined further in animal experiments
and clinical studies.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 82073087 and
81960507), and the Zunyi Medical University 2017 New Academic
Cultivation and Innovation Exploration Special Project [grant no.
Qian-Ke-He-Ping-Tai-Ren-Cai (2017)5733-040].
Availability of data and materials
Not applicable.
Authors' contributions
XY and SY made substantial contributions to the
conception and design of the manuscript. JA, HJ, HW and BT were
involved in revising the manuscript critically for important
intellectual content. All authors have read and approved the final
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Evan AP, Lingeman JE, Worcester EM,
Bledsoe SB, Sommer AJ, Williams JC Jr, Krambeck AE, Philips CL and
Coe FL: Renal histopathology and crystal deposits in patients with
small bowel resection and calcium oxalate stone disease. Kidney
Int. 78:310–317. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Obligado SH and Goldfarb DS: The
association of nephrolithiasis with hypertension and obesity: A
review. Am J Hypertens. 21:257–264. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Borghi L, Meschi T, Guerra A, Briganti A,
Schianchi T, Allegri F and Novarini A: Essential arterial
hypertension and stone disease. Kidney Int. 55:2397–2406. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pak CY: Kidney stones. Lancet.
351:1797–1801. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lohi H, Kujala M, Kerkelä E,
Saarialho-Kere U, Kestilä M and Kere J: Mapping of five new
putative anion transporter genes in human and characterization of
SLC26A6, a candidate gene for pancreatic anion exchanger. Genomics.
70:102–112. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kleta R: A key stone cop regulates oxalate
homeostasis. Nat Genet. 38:403–404. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Evan AP, Lingeman JE, Coe FL, Parks JH,
Bledsoe SB, Shao Y, Sommer AJ, Paterson RF, Kuo RL and Grynpas M:
Randall's plaque of patients with nephrolithiasis begins in
basement membranes of thin loops of Henle. J Clin Invest.
111:607–616. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moe OW and Preisig PA: Dual role of
citrate in mammalian urine. Curr Opin Nephrol Hypertens.
15:419–424. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Noori N, Honarkar E, Goldfarb DS,
Kalantar-Zadeh K, Taheri M, Shakhssalim N, Parvin M and Basiri A:
Urinary lithogenic risk profile in recurrent stone formers with
hyperoxaluria: A randomized controlled trial comparing DASH
(Dietary Approaches to Stop Hypertension)-style and low-oxalate
diets. Am J Kidney Dis. 63:456–463. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Khan A: Prevalence, pathophysiological
mechanisms and factors affecting urolithiasis. Int Urol Nephrol.
50:799–806. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shimshilashvili L, Aharon S, Moe OW and
Ohana E: Novel human polymorphisms define a key role for the
SLC26A6-STAS domain in protection from ca2+-oxalate
lithogenesis. Front Pharmacol. 11:4052020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hamm LL: Renal handling of citrate. Kidney
Int. 38:728–735. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pajor AM: Sequence and functional
characterization of a renal sodium/dicarboxylate cotransporter. J
Biol Chem. 270:5779–5785. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ohana E, Shcheynikov N, Moe OW and Muallem
S: SLC26A6 and NaDC-1 transporters interact to regulate oxalate and
citrate homeostasis. J Am Soc Nephrol. 24:1617–1626. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Prakash S, Cooper G, Singhi S and Saier MH
Jr: The ion transporter superfamily. Biochim Biophys Acta.
1618:79–92. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aguiar CJ, Andrade VL, Gomes ER, Alves MN,
Ladeira MS, Pinheiro AC, Gomes DA, Almeida AP, Goes AM, Resende RR,
et al: Succinate modulates Ca(2+) transient and cardiomyocyte
viability through PKA-dependent pathway. Cell Calcium. 47:37–46.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vargas SL, Toma I, Kang JJ, Meer EJ and
Peti-Peterdi J: Activation of the succinate receptor GPR91 in
macula densa cells causes renin release. J Am Soc Nephrol.
20:1002–1011. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He W, Miao FJ, Lin DC, Schwandner RT, Wang
Z, Gao J, Chen JL, Tian H and Ling L: Citric acid cycle
intermediates as ligands for orphan G-protein-coupled receptors.
Nature. 429:188–193. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baumbach L, Leyssac PP and Skinner SL:
Studies on renin release from isolated superfused glomeruli:
Effects of temperature, urea, ouabain and ethacrynic acid. J
Physiol. 258:243–256. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alper SL and Sharma AK: The SLC26 gene
family of anion transporters and channels. Mol Aspects Med.
34:494–515. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dorwart MR, Shcheynikov N, Yang D and
Muallem S: The solute carrier 26 family of proteins in epithelial
ion transport. Physiology (Bethesda). 23:104–114. 2008.PubMed/NCBI
|
|
22
|
Price GD and Howitt SM: The cyanobacterial
bicarbonate transporter BicA: Its physiological role and the
implications of structural similarities with human SLC26
transporters. Biochem Cell Biol. 89:178–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang J, Wang W, Wang H and Tuo B:
Physiological and pathological functions of SLC26A6. Front Med
(Lausanne). 7:6182562021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bai X, Chen X, Feng Z, Hou K, Zhang P, Fu
B and Shi S: Identification of basolateral membrane targeting
signal of human sodium-dependent dicarboxylate transporter 3. J
Cell Physiol. 206:821–830. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Waldegger S, Moschen I, Ramirez A, Smith
RJ, Ayadi H, Lang F and Kubisch C: Cloning and characterization of
SLC26A6, a novel member of the solute carrier 26 gene family.
Genomics. 72:43–50. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Geertsma ER, Chang YN, Shaik FR, Neldner
Y, Pardon E, Steyaert J and Dutzler R: Structure of a prokaryotic
fumarate transporter reveals the architecture of the SLC26 family.
Nat Struct Mol Biol. 22:803–808. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ko SB, Zeng W, Dorwart MR, Luo X, Kim KH,
Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ and Muallem S:
Gating of CFTR by the STAS domain of SLC26 transporters. Nat Cell
Biol. 6:343–350. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Malakooti J, Saksena S, Gill RK and Dudeja
PK: Transcriptional regulation of the intestinal luminal
Na+ and Cl− transporters. Biochem J.
435:313–325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lohi H, Lamprecht G, Markovich D, Heil A,
Kujala M, Seidler U and Kere J: Isoforms of SLC26A6 mediate anion
transport and have functional PDZ interaction domains. Am J Physiol
Cell Physiol. 284:C769–C779. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Poole DF and Tyler JE: Oxalic
acid-produced surface phenomena on human enamel examined by
scanning electron microscopy. Arch Oral Biol. 15:1157–1162. 1970.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sirish P, Ledford HA, Timofeyev V, Thai
PN, Ren L, Kim HJ, Park S, Lee JH, Dai G, Moshref M, et al: Action
potential shortening and impairment of cardiac function by ablation
of Slc26a6. Circ Arrhythm Electrophysiol. 10:e0052672017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Z, Petrovic S, Mann E and Soleimani
M: Identification of an apical Cl(−)/HCO3(−) exchanger in the small
intestine. Am J Physiol Gastrointest Liver Physiol. 282:G573–G579.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Freel RW, Hatch M, Green M and Soleimani
M: Ileal oxalate absorption and urinary oxalate excretion are
enhanced in Slc26a6 null mice. Am J Physiol Gastrointest Liver
Physiol. 290:G719–G728. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ishiguro H, Yamamoto A, Nakakuki M, Yi L,
Ishiguro M, Yamaguchi M, Kondo S and Mochimaru Y: Physiology and
pathophysiology of bicarbonate secretion by pancreatic duct
epithelium. Nagoya J Med Sci. 74:1–18. 2012.PubMed/NCBI
|
|
35
|
Wang Z, Wang T, Petrovic S, Tuo B,
Riederer B, Barone S, Lorenz JN, Seidler U, Aronson PS and
Soleimani M: Renal and intestinal transport defects in Slc26a6-null
mice. Am J Physiol Cell Physiol. 288:C957–C965. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gholami K, Muniandy S and Salleh N:
In-vivo functional study on the involvement of CFTR, SLC26A6, NHE-1
and CA isoenzymes II and XII in uterine fluid pH, volume and
electrolyte regulation in rats under different sex-steroid
influence. Int J Med Sci. 10:1121–1134. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Knauf F, Yang CL, Thomson RB, Mentone SA,
Giebisch G and Aronson PS: Identification of a chloride-formate
exchanger expressed on the brush border membrane of renal proximal
tubule cells. Proc Natl Acad Sci USA. 98:9425–9430. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chernova MN, Jiang L, Friedman DJ, Darman
RB, Lohi H, Kere J, Vandorpe DH and Alper SL: Functional comparison
of mouse slc26a6 anion exchanger with human SLC26A6 polypeptide
variants: Differences in anion selectivity, regulation, and
electrogenicity. J Biol Chem. 280:8564–8580. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Clark JS, Vandorpe DH, Chernova MN,
Heneghan JF, Stewart AK and Alper SL: Species differences in
Cl− affinity and in electrogenicity of SLC26A6-mediated
oxalate/Cl− exchange correlate with the distinct human
and mouse susceptibilities to nephrolithiasis. J Physiol.
586:1291–1306. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jiang Z, Grichtchenko II, Boron WF and
Aronson PS: Specificity of anion exchange mediated by mouse
Slc26a6. J Biol Chem. 277:33963–33967. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xie Q, Welch R, Mercado A, Romero MF and
Mount DB: Molecular characterization of the murine Slc26a6 anion
exchanger: Functional comparison with Slc26a1. Am J Physiol Renal
Physiol. 283:F826–F838. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Aronson PS: Ion exchangers mediating Na+,
HCO3− and Cl− transport in the
renal proximal tubule. J Nephrol. 19 (Suppl 9):S3–S10.
2006.PubMed/NCBI
|
|
43
|
Jiang Z, Asplin JR, Evan AP, Rajendran VM,
Velazquez H, Nottoli TP, Binder HJ and Aronson PS: Calcium oxalate
urolithiasis in mice lacking anion transporter Slc26a6. Nat Genet.
38:474–478. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
Markovich D and Murer H: The SLC13 gene
family of sodium sulphate/carboxylate cotransporters. Pflugers
Arch. 447:594–602. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Markovich D, Forgo J, Stange G, Biber J
and Murer H: Expression cloning of rat renal Na+/SO4(2-)
cotransport. Proc Natl Acad Sci USA. 90:8073–8077. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bai L and Pajor AM: Expression cloning of
NaDC-2, an intestinal Na(+)- or Li(+)-dependent dicarboxylate
transporter. Am J Physiol. 273((2 Pt 1)): G267–G274.
1997.PubMed/NCBI
|
|
47
|
Steffgen J, Burckhardt BC, Langenberg C,
Kühne L, Müller GA, Burckhardt G and Wolff NA: Expression cloning
and characterization of a novel sodium-dicarboxylate cotransporter
from winter flounder kidney. J Biol Chem. 274:20191–20196. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pajor AM: Molecular cloning and functional
expression of a sodium-dicarboxylate cotransporter from human
kidney. Am J Physiol. 270((4 Pt 2)): F642–F648. 1996.PubMed/NCBI
|
|
49
|
Pajor AM and Sun NN: Molecular cloning,
chromosomal organization, and functional characterization of a
sodium-dicarboxylate cotransporter from mouse kidney. Am J Physiol
Renal Physiol. 279:F482–F490. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Khatri IA, Kovacs SV and Forstner JF:
Cloning of the cDNA for a rat intestinal Na+/dicarboxylate
cotransporter reveals partial sequence homology with a rat
intestinal mucin. Biochim Biophys Acta. 1309:58–62. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sekine T, Cha SH, Hosoyamada M, Kanai Y,
Watanabe N, Furuta Y, Fukuda K, Igarashi T and Endou H: Cloning,
functional characterization, and localization of a rat renal
Na+-dicarboxylate transporter. Am J Physiol. 275:F298–F305.
1998.PubMed/NCBI
|
|
52
|
Chen XZ, Shayakul C, Berger UV, Tian W and
Hediger MA: Characterization of a rat Na+-dicarboxylate
cotransporter. J Biol Chem. 273:20972–20981. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mann SS, Hart T, Pettenati MJ, von
Kap-herr C and Holmes RP: Assignment of the sodium-dependent
dicarboxylate transporter gene (SLC13A2 alias NaDC-1) to human
chromosome region 17p11.1->q11.1 by radiation hybrid mapping and
fluorescence in situ hybridization. Cytogenet Cell Genet. 84:89–90.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Pajor AM: Molecular properties of
sodium/dicarboxylate cotransporters. J Membr Biol. 175:1–8. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Pajor AM: Sodium-coupled transporters for
Krebs cycle intermediates. Annu Rev Physiol. 61:663–682. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hamm LL: Renal handling of citrate. Kidney
Int. 38:728–735. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Aronson PS: Essential roles of
CFEX-mediated Cl(−)-oxalate exchange in proximal tubule NaCl
transport and prevention of urolithiasis. Kidney Int. 70:1207–1213.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Brennan TS, Klahr S and Hamm LL: Citrate
transport in rabbit nephron. Am J Physiol. 251((4 Pt 2)):
F683–F689. 1986.PubMed/NCBI
|
|
59
|
Shcheynikov N, Wang Y, Park M, Ko SB,
Dorwart M, Naruse S, Thomas PJ and Muallem S: Coupling modes and
stoichiometry of Cl-/HCO3− exchange by
slc26a3 and slc26a6. J Gen Physiol. 127:511–524. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Khamaysi A, Anbtawee-Jomaa S, Fremder M,
Eini-Rider H, Shimshilashvili L, Aharon S, Aizenshtein E, Shlomi T,
Noguchi A, Springer D, et al: Systemic succinate homeostasis and
local succinate signaling affect blood pressure and modify risks
for calcium oxalate lithogenesis. J Am Soc Nephrol. 30:381–392.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Mancusso R, Gregorio GG, Liu Q and Wang
DN: Structure and mechanism of a bacterial sodium-dependent
dicarboxylate transporter. Nature. 491:622–626. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Robben JH, Fenton RA, Vargas SL, Schweer
H, Peti-Peterdi J, Deen PM and Milligan G: Localization of the
succinate receptor in the distal nephron and its signaling in
polarized MDCK cells. Kidney Int. 76:1258–1267. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sundstrom L, Greasley PJ, Engberg S,
Wallander M and Ryberg E: Succinate receptor GPR91, a Gaα(i)
coupled receptor that increases intracellular calcium
concentrations through PLCβ. FEBS Lett. 587:2399–2404. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ando H, Mizutani A, Matsu-ura T and
Mikoshiba K: IRBIT, a novel inositol 1,4,5-trisphosphate (IP3)
receptor-binding protein, is released from the IP3 receptor upon
IP3 binding to the receptor. J Biol Chem. 278:10602–10612. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Park S, Shcheynikov N, Hong JH, Zheng C,
Suh SH, Kawaai K, Ando H, Mizutani A, Abe T, Kiyonari H, et al:
Irbit mediates synergy between ca(2+) and cAMP signaling pathways
during epithelial transport in mice. Gastroenterology. 145:232–241.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lungkaphin A, Lewchalermwongse B and
Chatsudthipong V: Relative contribution of OAT1 and OAT3 transport
activities in isolated perfused rabbit renal proximal tubules.
Biochim Biophys Acta. 1758:789–795. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Okamoto N, Aruga S, Tomita K, Takeuchi T
and Kitamura T: Chronic acid ingestion promotes renal stone
formation in rats treated with vitamin D3. Int J Urol. 14:60–66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Monico CG, Weinstein A, Jiang Z, Jiang Z,
Rohlinger AL, Cogal AG, Bjornson BB, Olson JB, Bergstralh EJ,
Milliner DS and Aronson PS: Phenotypic and functional analysis of
human SLC26A6 variants in patients with familial hyperoxaluria and
calcium oxalate nephrolithiasis. Am J Kidney Dis. 52:1096–1103.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Jiang H, Pokhrel G, Chen Y, Wang T, Yin C,
Liu J, Wang S and Liu Z: High expression of SLC26A6 in the kidney
may contribute to renal calcification via an SLC26A6-dependent
mechanism. PeerJ. 6:e51922018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Khan SR, Khan A and Byer KJ: Temporal
changes in the expression of mRNA of NADPH oxidase subunits in
renal epithelial cells exposed to oxalate or calcium oxalate
crystals. Nephrol Dial Transplant. 26:1778–1785. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Jiang H, Gao X, Gong J, Yang Q, Lan R,
Wang T, Liu J, Yin C, Wang S and Liu Z: Downregulated expression of
solute carrier family 26 member 6 in NRK-52E cells attenuates
oxalate-induced intracellular oxidative stress. Oxid Med Cell
Longev. 2018:17246482018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lu X, Sun D, Xu B, Pan J, Wei Y, Mao X, Yu
D, Liu H and Gao B: In silico screening and molecular dynamic study
of nonsynonymous single nucleotide polymorphisms associated with
kidney stones in the SLC26A6 gene. J Urol. 196:118–123. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Corbetta S, Eller-Vainicher C, Frigerio M,
Valaperta R, Costa E, Vicentini L, Baccarelli A, Beck-Peccoz P and
Spada A: Analysis of the 206M polymorphic variant of the SLC26A6
gene encoding a Cl− oxalate transporter in patients with
primary hyperparathyroidism. Eur J Endocrinol. 160:283–288. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Udomsilp P, Saepoo S, Ittiwut R,
Shotelersuk V, Dissayabutra T, Boonla C and Tosukhowong P:
rs11567842 SNP in SLC13A2 gene associates with hypocitraturia in
Thai patients with nephrolithiasis. Genes Genomics. 40:965–972.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Bosch B and De Boeck K: Searching for a
cure for cystic fibrosis. A 25-year quest in a nutshell. Eur J
Pediatr. 175:1–8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Bissig M, Hagenbuch B, Stieger B, Koller T
and Meier PJ: Functional expression cloning of the canalicular
sulfate transport system of rat hepatocytes. J Biol Chem.
269:3017–21. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Regeer RR and Markovich D: A dileucine
motif targets the sulfate anion transporter sat-1 to the
basolateral membrane in renal cell lines. Am. J. Physiol. 287((2)):
C365–C372. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Hästbacka J, de la Chapelle A, Mahtani MM,
Clines G, Reeve-Daly MP, Daly M, Hamilton BA, Kusumi K, Trivedi B,
et al: The diastrophic dysplasia gene encodes a novel sulfate
transporter: positional cloning by fine-structure linkage
disequilibrium mapping. Cell. 78((6)): 1073–1087. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Heneghan JF, Akhavein A, Salas MJ,
Shmukler BE, Karniski LP, Vandorpe DH and Alper SL: Regulated
transport of sulfate and oxalate by SLC26A2/DTDST. Am J Physiol
Cell Physiol. 298((6)): C1363-75. doi: 10.1152/ajpcell.00004.2010.
Epub 2010 Mar 10. Erratum in: Am J Physiol Cell Physiol. 2011 Feb;
300(2): C383. PMID: 20219950; PMCID: PMC2889644. PubMed/NCBI
|
|
80
|
Haila S, Hästbacka J, Böhling T,
Karjalainen-Lindsberg ML, Kere J and Saarialho-Kere U: SLC26A2
(diastrophic dysplasia sulfate transporter) is expressed in
developing and mature cartilage but also in other tissues and cell
types. J Histochem. Cytochem. 49((8)): 973–982. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Hoglund P, Haila S, Socha J, Tomaszewski
L, Saarialho-Kere U, Karjalainen-Lindsberg ML, Airola K, Holmberg
C, de la Chapelle A and Kere J: Mutations of the Down-regulated in
adenoma (DRA) gene cause congenital chloride diarrhoea. Nat Genet.
14:316–319. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Chernova MN, Jiang L, Shmukler BE,
Schweinfest CW, Blanco P, Freedman SD, Stewart AK and Alper SL:
Acute regulation of the SLC26A3 congenital chloride diarrhoea anion
exchanger (DRA) expressed in Xenopus oocytes. J Physiol. 549((Pt
1)): 3–19. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Sheffield VC, Kraiem Z, Beck JC, Nishimura
D, Stone EM, Salameh M, Sadeh O and Glaser B: Pendred syndrome maps
to chromosome 7q21-34 and is caused by an intrinsic defect in
thyroid iodine organification. Nat Genet. 12:424–426. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Shcheynikov N, Yang D, Wang Y, Zeng W,
Karniski LP, So I, Wall SM and Muallem S: The Slc26a4 transporter
functions as an electroneutral Cl-/I-/HCO3−
exchanger: Role of Slc26a4 and Slc26a6 in I- and
HCO3− secretion and in regulation of CFTR in
the parotid duct. J Physiol. 586:3813–3824. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Liu XZ, Ouyang XM, Xia XJ, Zheng J, Pandya
A, Li F, Du LL, Welch KO, Petit C, Smith RJ, et al: Prestin, a
cochlear motor protein, is defective in non-syndromic hearing loss.
Hum Mol Genet. 12:1155–1162. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Alvarez BV, Kieller DM, Quon AL, Markovich
D and Casey JR: Slc26a6: A cardiac chloride-hydroxyl exchanger and
predominant chloride-bicarbonate exchanger of the mouse heart. J
Physiol. 561((Pt 3)): 721–734. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Petrovic S, Amlal H, Sun X, Karet F,
Barone S and Soleimani M: Vasopressin induces expression of the
Cl-/HCO3− exchanger SLC26A7 in kidney
medullary collecting ducts of Brattleboro rats. Am J Physiol Renal
Physiol. 290:F1194–F1201. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Dudas PL, Mentone S, Greineder CF,
Biemesderfer D and Aronson PS: Immunolocalization of anion
transporter Slc26a7 in mouse kidney. Am J Physiol Renal Physiol.
290:F937–F945. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Toure A, Morin L, Pineau C, Becq F,
Dorseuil O and Gacon G: Tat1, a novel sulfate transporter
specifically expressed in human male germ cells and potentially
linked to rhogtpase signaling. J Biol Chem. 276:20309–20315. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Lohi H, Kujala M, Makela S, Lehtonen E,
Kestila M, Saarialho-Kere U, Markovich D and Kere J: Functional
characterization of three novel tissue-specific anion exchangers
SLC26A7, -A8, and -A9. J Biol Chem. 277:14246–14254. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Loriol C, Dulong S, Avella M, Gabillat N,
Boulukos K, Borgese F and Ehrenfeld J: Characterization of SLC26A9,
facilitation of Cl (−) transport by bicarbonate. Cell Physiol
Biochem. 22:15–30. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Wang J, Chen X, Liu B and Zhu Z:
Suppression of PTP1B in gastric cancer cells in vitro induces a
change in the genome-wide expression profile and inhibits gastric
cancer cell growth. Cell Biol Int. 34:747–753. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Stewart AK, Shmukler BE, Vandorpe DH,
Reimold F, Heneghan JF, Nakakuki M, Akhavein A, Ko S, Ishiguro H
and Alper SL: SLC26 anion exchangers of guinea pig pancreatic duct:
Molecular cloning and functional characterization. Am J Physiol
Cell Physiol. 301:C289–C303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ouesleti S, Brunel V, Ben Turkia H,
Dranguet H, Miled A, Miladi N, Ben Dridi MF, Lavoinne A,
Saugier-Veber P and Bekri S: Molecular characterization of MPS
IIIA, MPS IIIB and MPS IIIC in Tunisian patients. Clin Chim Acta.
412:2326–2331. 2011. View Article : Google Scholar : PubMed/NCBI
|