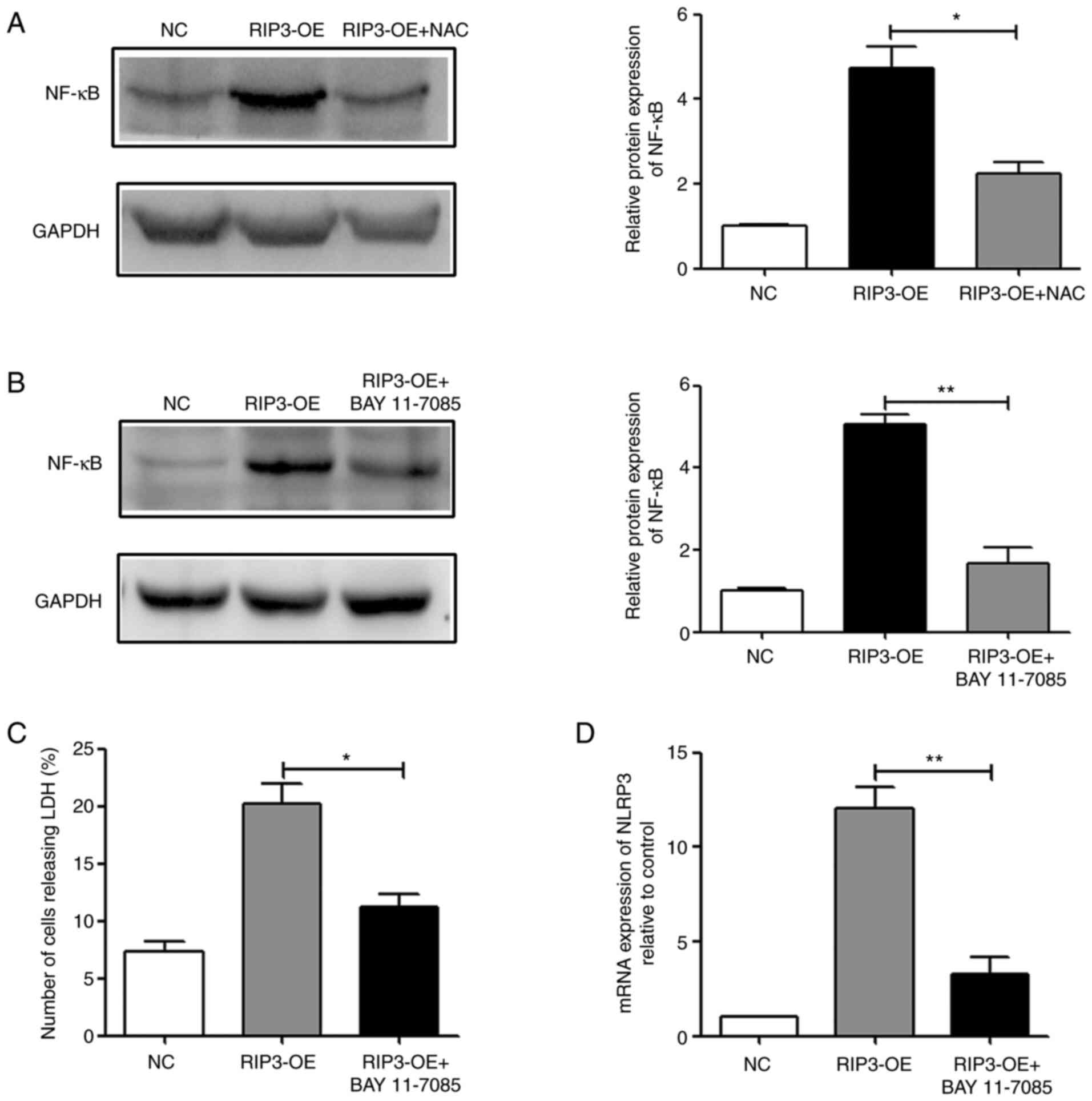
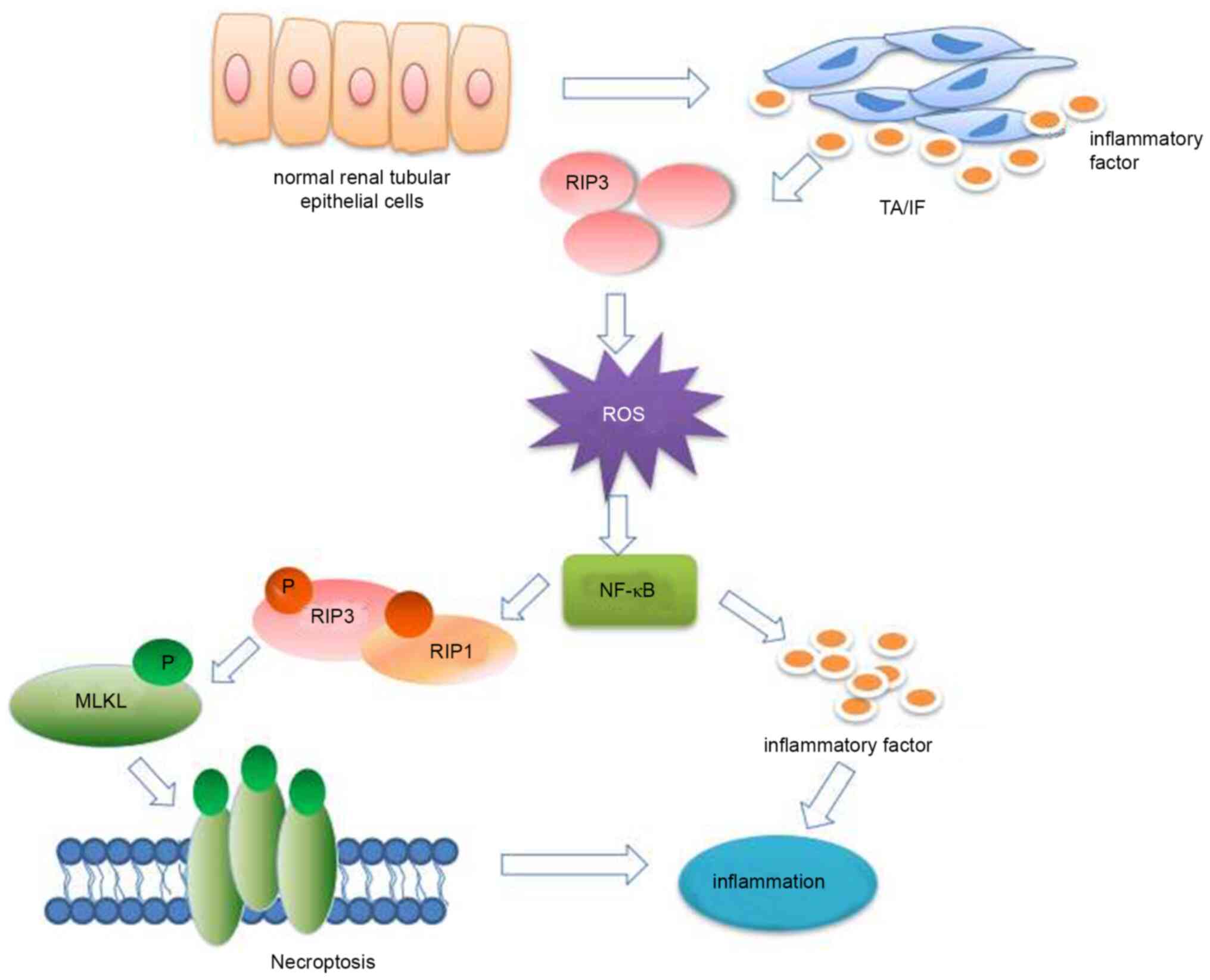
|
1
|
Colvin RB: Chronic allograft nephropathy.
N Engl J Med. 349:2288–2290. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nankivell BJ, Borrows RJ, Fung CL,
O'Connell PJ, Allen RDM and Chapman JR: The natural history of
chronic allograft nephropathy. N Engl J Med. 349:2326–2333. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pascual M, Theruvath T, Kawai T,
Tolkoff-Rubin N and Cosimi AB: Strategies to improve long-term
outcomes after renal transplantation. N Engl J Med. 346:580–590.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gaston RS, Fieberg A, Helgeson ES,
Eversull J, Hunsicker L, Kasiske BL, Leduc R, Rush D and Matas AJ;
DeKAF Investigators*, : Late Graft loss after kidney
transplantation: Is ‘death with function’ really death with a
functioning allograft? Transplantation. 104:1483–1490. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koyama Y and Brenner DA: Liver
inflammation and fibrosis. J Clin Invest. 127:55–64. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mack M: Inflammation and fibrosis. Matrix
Biol. 68-69:106–121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mannon RB, Matas AJ, Grande J, Leduc R,
Connett J, Kasiske B, Cecka JM, Gaston RS, Cosio F, Gourishankar S,
et al: Inflammation in areas of tubular atrophy in kidney allograft
biopsies: A potent predictor of allograft failure. Am J Transplant.
10:2066–2073. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sellarés J, de Freitas DG, Mengel M, Sis
B, Hidalgo LG, Matas AJ, Kaplan B and Halloran PF: Inflammation
lesions in kidney transplant biopsies: Association with survival is
due to the underlying diseases. Am J Transplant. 11:489–499. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Naesens M, Kuypers DR, De Vusser K,
Evenepoel P, Claes K, Bammens B, Meijers B, Sprangers B, Pirenne J,
Monbaliu D, et al: The histology of kidney transplant failure: A
long-term follow-up study. Transplantation. 98:427–435. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feng S, Yang Y, Mei Y, Ma L, Zhu D, Hoti
N, Castanares M and Wu M: Cleavage of RIP3 inactivates its
caspase-independent apoptosis pathway by removal of kinase domain.
Cell Signal. 19:2056–2067. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Galluzzi L and Kroemer G: Necroptosis: A
specialized pathway of programmed necrosis. Cell. 135:1161–1163.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu XN, Yang ZH, Wang XK, Zhang Y, Wan H,
Song Y, Chen X, Shao J and Han J: Distinct roles of RIP1-RIP3
hetero- and RIP3-RIP3 homo-interaction in mediating necroptosis.
Cell Death Differ. 21:1709–1720. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang H, Sun L, Su L, Rizo J, Liu L, Wang
LF, Wang FS and Wang X: Mixed lineage kinase domain-like protein
MLKL causes necrotic membrane disruption upon phosphorylation by
RIP3. Mol Cell. 54:133–146. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen X, Li W, Ren J, Huang D, He WT, Song
Y, Yang C, Li W, Zheng X, Chen P and Han J: Translocation of mixed
lineage kinase domain-like protein to plasma membrane leads to
necrotic cell death. Cell Res. 24:105–121. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pasparakis M and Vandenabeele P:
Necroptosis and its role in inflammation. Nature. 517:311–320.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schulze-Osthoff K, Bakker AC,
Vanhaesebroeck B, Beyaert R, Jacob WA and Fiers W: Cytotoxic
activity of tumor necrosis factor is mediated by early damage of
mitochondrial functions. Evidence for the involvement of
mitochondrial radical generation. J Biol Chem. 267:5317–5323. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goossens V, Grooten J, De Vos K and Fiers
W: Direct evidence for tumor necrosis factor-induced mitochondrial
reactive oxygen intermediates and their involvement in
cytotoxicity. Proc Natl Acad Sci USA. 92:8115–8119. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fulda S: Regulation of necroptosis
signaling and cell death by reactive oxygen species. Biol Chem.
397:657–660. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Su SS, Zhao S, Yang Z, Zhong CQ,
Chen X, Cai Q, Yang ZH, Huang D, Wu R and Han J: RIP1
autophosphorylation is promoted by mitochondrial ROS and is
essential for RIP3 recruitment into necrosome. Nat Commun.
8:143292017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen T: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li N, He Y, Wang L, Mo C, Zhang J, Zhang
W, Li J, Liao Z, Tang X and Xiao H: D-galactose induces necroptotic
cell death in neuroblastoma cell lines. J Cell Biochem.
112:3834–3844. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Degterev A, Hitomi J, Germscheid M, Ch'en
IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, et al:
Identification of RIP1 kinase as a specific cellular target of
necrostatins. Nat Chem Biol. 4:313–321. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hitomi J, Christofferson DE, Ng A, Yao J,
Degterev A, Xavier RJ and Yuan J: Identification of a molecular
signaling network that regulates a cellular necrotic cell death
pathway. Cell. 135:1311–1323. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He Y, Hara H and Núñez G: Mechanism and
regulation of NLRP3 inflammasome activation. Trends Biochem Sci.
41:1012–1021. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vanden Berghe T, Linkermann A,
Jouan-Lanhouet S, Walczak H and Vandenabeele P: Regulated necrosis:
The expanding network of non-apoptotic cell death pathways. Nat Rev
Mol Cell Biol. 15:135–147. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Perrea DN, Moulakakis KG, Poulakou MV,
Vlachos IS, Papachristodoulou A and Kostakis AI: Correlation
between oxidative stress and immunosuppressive therapy in renal
transplant recipients with an uneventful postoperative course and
stable renal function. Int Urol Nephrol. 38:343–348. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thoma A and Lightfoot AP: NF-κB and
inflammatory cytokine signalling: Role in skeletal muscle atrophy.
Adv Exp Med Biol. 1088:267–279. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Farris AB and Colvin RB: Renal
interstitial fibrosis: Mechanisms and evaluation. Curr Opin Nephrol
Hypertens. 21:289–300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Weinlich R, Oberst A, Beere HM and Green
DR: Necroptosis in development, inflammation and disease. Nat Rev
Mol Cell Biol. 18:127–136. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zorov DB, Juhaszova M and Sollott SJ:
Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS
release. Physiol Rev. 94:909–950. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Su LJ, Zhang JH, Gomez H, Murugan R, Hong
X, Xu D, Jiang F and Peng ZY: Reactive oxygen species-induced lipid
peroxidation in apoptosis, autophagy, and ferroptosis. Oxid Med
Cell Longev. 2019:50808432019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang T, Zhang X and Li JJ: The role of
NF-kappaB in the regulation of cell stress responses. nt
Immunopharmacol. 2:1509–1520. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moriwaki K and Chan FK: The inflammatory
signal adaptor RIPK3: Functions beyond necroptosis. Int Rev Cell
Mol Biol. 328:253–275. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li N and Karin M: Is NF-kappaB the sensor
of oxidative stress? FASEB J. 13:1137–1143. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li JX, Feng JM, Wang Y, Li XH, Chen XX, Su
Y, Shen YY, Chen Y, Xiong B, Yang CH, et al: The B-Raf(V600E)
inhibitor dabrafenib selectively inhibits RIP3 and alleviates
acetaminophen-induced liver injury. Cell Death Dis. 5:e12782014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Martens S, Jeong M, Tonnus W, Feldmann F,
Hofmans S, Goossens V, Takahashi N, Bräsen JH, Lee EW, Van der
Veken P, et al: Sorafenib tosylate inhibits directly necrosome
complex formation and protects in mouse models of inflammation and
tissue injury. Cell Death Dis. 8:e29042017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fauster A, Rebsamen M, Huber KVM,
Bigenzahn JW, Stukalov A, Lardeau CH, Scorzoni S, Bruckner M,
Gridling M, Parapatics K, et al: A cellular screen identifies
ponatinib and pazopanib as inhibitors of necroptosis. Cell Death
Dis. 6:e17672015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Morioka S, Broglie P, Omori E, Ikeda Y,
Takaesu G, Matsumoto K and Ninomiya-Tsuji J: TAK1 kinase switches
cell fate from apoptosis to necrosis following TNF stimulation. J
Cell Biol. 204:607–623. 2014. View Article : Google Scholar : PubMed/NCBI
|