Animals, including humans, have been constantly
exposed to the danger of starvation during the course of evolution.
As a result, animals have acquired the ability to survive by
storing excess energy in the form of fat and utilizing it in the
starvation status (1,2). Ironically, however, in today's
advanced society, where excessive energy intake and lack of
exercise have become the norm, this mechanism has become the cause
of obesity, muscle weakness and insulin resistance (IR), etc.
(1,2).
Skeletal muscle is the largest organ in the human
body and plays an important role in energy metabolism and glucose
uptake. Skeletal muscle is the most energy-consuming organ in the
human body, with a heat expenditure of 30% of the total body in the
basal metabolism (3). In patients
with type 2 diabetes mellitus (DM), there is a marked decrease in
glucose uptake in the skeletal muscle (4,5).
It is well known that moderate exercise increases energy
expenditure, reduces obesity, and increases glucose metabolism,
thereby preventing and improving type 2 DM. Skeletal muscle
accounts for about 50% of the body weight, and about 15% of the
circulating blood volume is supplied to the skeletal muscle at rest
(6). Approximately 20% of the
oxygen consumed by the entire body is consumed by the skeletal
muscle. Glucose in the blood is transported to the skeletal muscle
and metabolized by oxygen. Continuous aerobic exercise increases
oxygen uptake and improves glucose metabolism in the skeletal
muscle (6). Endurance exercise
can improve aerobic metabolism and thus can reduce hyperglycemia in
patients with type 2 DM.
Aging-related changes in the body composition are
characterized by the followings: (1) a 40% decrease in lean body mass and
an increase in fat mass between the ages of 20 and 70 (7). (2)
The overall fat mass increases between the ages of 20 and 70, but
the peripheral fat tends to decrease compared to the central fat
(7). (3) After the age of 70, both lean body
mass and fat mass decrease (7).
(4) Visceral fat increases with
aging (8). (5) Fat is more likely to be deposited in
the skeletal muscle and the liver (8). (6)
Increased visceral fat is associated with impaired glucose
metabolism, and intramuscular and intrahepatic fat deposition is
associated with IR through the release of adipokines and free fatty
acids (9). (7) Decreased skeletal muscle mass results
in a decrease in the basal metabolism of 2–3% per decade after the
age of 20, and 4% per decade after the age of 50 (10,11).
The effects of insulin and insulin-like growth
factor-1 (IGF-1) can be roughly divided into metabolic and
proliferative effects, with insulin mainly exerting the former.
Insulin exerts a variety of effects, many of which are mediated by
Akt, including increasing glucose uptake, promoting glycogen
synthesis and inhibiting glycogen degradation, increasing free
fatty acid uptake, increasing protein synthesis, promoting muscle
hypertrophy, and inhibiting protein degradation (12,13). In addition, the mitogen-activated
protein kinases (MAPKs) pathway promotes cell proliferation.
Insulin, together with IGF-1, can increase skeletal muscle mass
(12,13). In addition to skeletal muscle,
liver and adipose tissue are important insulin target organs, and
insulin signaling in these organs mainly promotes protein
anabolism. Insulin signaling promotes lipid synthesis and inhibits
lipid degradation in the liver and adipose tissue, leading to the
storage of excess energy. Inhibition of glycogenesis in the liver
is also an important role of insulin (12,13). Fat, on the other hand, contains
more energy per equal weight than glucose and other substances
(protein: 4.1 kcal/g, fat: 9.3 kcal/g, and glucose: 4.1 kcal/g),
making fat the most suitable substance for storing large amounts of
energy (14,15). Glycogen is a short-term form of
energy storage, whereas fat is a long-term, high-volume form of
storage (14,15). Amino acid intake is expected to
inhibit muscle atrophy by inhibiting the degradation of skeletal
muscle protein as well as promoting the synthesis of skeletal
muscle protein (16–18).
Skeletal muscle mass progressively declines with
aging, leading to a decline in muscle strength and physical
function. In 1989, Rosenberg coined the term ‘sarcopenia’ (from the
Greek words ‘sarx’ meaning muscle, and ‘penia’ meaning loss) to
describe the loss of muscle mass associated with aging. The term
‘sarcopenia’ has been widely accepted, and sarcopenia is now
defined as a condition associated with loss of skeletal muscle mass
and muscle weakness or loss of physical function (19,20). Guidelines for sarcopenia of the
European Working Group on Sarcopenia in Older People (EWGSOP)
(21) and the Asian Working Group
for Sarcopenia (AWGS) (22) were
published in 2010 and 2014, respectively, and in 2016, the Japanese
Society of Hepatology issued sarcopenia assessment criteria
specific to liver disease (23).
In addition, a revised EWGSOP guideline was issued in 2019
(24) and a revised AWGS
guideline in 2020 (25). The
etiology of sarcopenia is thought to involve several complex
factors in addition to aging. These include changes in sex
hormones, underlying diseases (i.e., secondary sarcopenia),
myokines, IR, poor nutritional intake of proteins and other
nutrients, and disuse syndrome due to reduced motor function caused
by loss of skeletal muscle mass, etc. (23). Considerable attention has been
paid to the relationship between type 2 DM-related IR and skeletal
muscle (26). On the other hand,
frailty, as well as sarcopenia, is another condition that has
received much attention in recent years (20,27,28). Decreased organ function leads to
vulnerability to external stress. A condition, in which acute
stresses such as infections, surgeries and accidents make it easier
for physical functions to decline with decreased organ function, is
called frailty (27,28). Frailty is also associated with
outcomes such as need for care, death, falls and fractures, and
hospitalization, but it encompasses the reversibility of returning
to a healthy state with appropriate interventions (27,28). Frailty can be divided into the
three main categories: physical frailty, social frailty and
cognitive frailty, and sarcopenia is the main component of physical
frailty (29). As in the case of
sarcopenia, much attention has been paid to the relationship
between type 2 DM and frailty. This review outlines the
relationship between sarcopenia, frailty and type 2 DM.
Skeletal muscle takes up more than 80% of the
glucose in the peripheral tissues by insulin and uses it for energy
or stores the excess energy as glycogen (30). In diabetic patients, the total
utilization of insulin in the whole body is about half of that in
healthy individuals, which is due to the reduced utilization in the
skeletal muscle (30). In other
words, skeletal muscle is the main target of insulin and the
largest organ that utilizes blood glucose. Therefore, the loss of
skeletal muscle mass due to sarcopenia leads to decreased glucose
utilization by insulin (i.e., IR). Decreased physical activity due
to decreased skeletal muscle mass is also responsible for decreased
glucose and lipid metabolism in the skeletal muscle (29). In addition, it is reported that
IL-6 secreted by skeletal muscle stimulates the secretion of
glucagon like peptide-1 (GLP-1) during exercise, and exercise is
also associated with changes in insulin and glucagon secretion
(31,32). Liver cirrhosis (LC), which is
considered to be a typical form of secondary sarcopenia because of
its tendency to cause protein-energy malnutrition, is also
associated with a high rate of IR (20,23,33–35). LC patients are characterized by
postprandial hyperglycemia (33).
The organ network between liver and skeletal muscle has recently
received much attention (23).
The glucose transporter 4 (GLUT4) is important for
the uptake of blood glucose into skeletal muscle cells (36). GLUT4 is normally intracellular,
but upon stimulation of glucose uptake, it moves to the cell
membrane and acts as a transport pathway for glucose uptake into
the cell. When the stimulus subsides, GLUT4 returns to the cell and
waits for another opportunity to mobilize. When insulin binds to
insulin receptors on the surface of the cell membrane of skeletal
muscle, intracellular signaling molecules such as IRS, PI3 kinase,
and Akt are activated in turn (36). This information is ultimately
transmitted to GLUT4 stores in the cell, resulting in glucose
uptake. IR in patients with type 2 DM is thought to be due to
abnormalities in this signaling pathway (18,36). In patients with type 2 DM, glucose
uptake in peripheral tissues by insulin is reduced. As mentioned
earlier, decreased glucose uptake has been shown to originate
primarily in the skeletal muscle among the organs of the body, and
exercise is a powerful way to increase glucose uptake and improve
IR (3). The effects of prolonged
exercise, such as marathon running, include the conversion of
skeletal muscle to red muscle, an increase in the number of
mitochondria, and an increase in the amount of GLUT4 (36,37). Moderate exercise improves
mitochondrial function and also increases the ability of blood
glucose uptake by increasing GLUT4 levels (36,37). Activation of mitochondrial
function increases the beta-oxidation of fatty acids, facilitating
the processing of free fatty acids being released from adipose
tissue and reducing the accumulation of triglycerides in the liver
(38,39). Thus, moderate exercise can
efficiently improve dysfunction in the skeletal muscle.
The protein peroxisome proliferator-activated
receptor-γ coactivator-1α (PGC-1α) plays an important role in
increasing mitochondrial mass and is thought to be the key to
improving the condition of lifestyle-related diseases by exercise
(40). In other words, increased
expression of PGC-1α causes increased mitochondria, change to red
muscle, increased energy expenditure, and weight loss as seen in
exercise (40). PGC-1α is highly
expressed in organs with active metabolism, such as brown
adipocytes, skeletal muscle, and liver, and is especially highly
expressed in soleus muscle, which has many type I myofibers
(described later) among skeletal muscles (41). It is known that PGC-1α expression
is increased by sustained exercise (42,43). Mice overexpressing PGC-1α in the
skeletal muscle (PGC-1α transgenic mice) showed increased
slow-twitch troponin I, myoglobin, and mitochondrial mass (44), characteristics of type I and IIA
myofibers (described later), as well as enhanced expression of
branched-chain amino acid (BCAA) metabolizing enzymes (45), resulting in increased endurance
exercise capacity. It has been reported that expression of PGC-1α
improves insulin sensitivity in diabetic mouse models (46).
Skeletal muscle can be roughly classified into the
type I myofibers, which have a slow contraction rate, and the type
II myofibers, which have a fast contraction rate and a strong
contractile force (47). Type II
myofibers can be further divided into the type IIA myofibers with
high aerobic glycolytic capacity and the type IIB myofibers with
low aerobic glycolytic capacity (47). Type I and IIA myofibers with high
aerobic glycolytic capacity have higher mitochondrial density and
activity, actively metabolize glucose and lipids, and are less
fatigued during endurance exercise (48). Type IIB myofibers have low
mitochondrial density and activity, and are mainly involved in
anoxic glycolysis in the cytoplasm, making them suitable for
exercise that requires instantaneous contraction such as
short-distance running (48). The
distribution of these myofibers varies from region to region in the
body. In the deep layers close to the bone, the proportion of type
I and IIA myofibers is higher, and in the superficial layers close
to the skin, the proportion of type IIB myofibers is higher
(48). The composition of
myofiber types changes with various stimuli. For example, the
proportion of type I and IIA fibers increases with continuous
aerobic exercise (49). On the
other hand, it is known that the percentage of type IIB myofibers
is higher and the percentage of type I and IIA myofibers is lower
in patients with type 2 DM (49).
Therefore, increasing the proportion of type I and IIA myofibers
may be an effective means of preventing type 2 DM by increasing the
number of mitochondria and energy metabolism. The skeletal muscle
in patients with type 2 DM has fewer capillaries, regardless of the
type of myofiber (50).
According to the data released by the Japan Ministry
of Health, Labour and Welfare in 2017, there are 3.289 million
people with type 2 DM in Japan, and one in five adults has impaired
glucose tolerance. The global diabetic population in 2019 is about
463 million. The International Diabetes Federation predicts that
the number of people with type 2 DM will approach 600 million by
2035. Previous reports (Asian subjects) using the AWGS criteria for
the diagnosis of sarcopenia reported the frequency of sarcopenia in
patients with type 2 DM to be 7–28.8% (51–67). Among these reports, the average
HbA1c of the target patients was the lowest in the report by
Fukuoka et al (average HbA1c=7.0) (66) and was the highest in the report by
Murai et al (average HbA1c=9.5) (63).
A 6-year follow-up study of limb skeletal muscle
mass using dual-energy X-ray absorption (DXA) in 2,675 elderly
people aged 70–79 years reported that the amount of limb muscle
mass loss in elderly people with type 2 DM was significantly
greater than that in elderly people without type 2 DM (68). In a cross-sectional study in
Indians, the prevalence of sarcopenia in patients with type 2 DM
was higher than in controls with an odds ratio (OR) of 3.48
(69). A report from Netherlands
found significant decrease in skeletal muscle mass and muscle
strength in patients with type 2 DM, even after adjusting for age,
body mass index (BMI), fasting glucose, HDL cholesterol, BCAAs, and
protein intake (70). An analysis
of 14,528 individuals in the National Health and Nutrition
Examination Surveys III in the United States indicated that
sarcopenia is involved in glucose metabolism independently of
obesity, that this tendency is stronger in individuals younger than
60 years, and that loss of skeletal muscle mass is a predictor of
the type 2 DM incidence (71).
When 932 subjects aged 40 years or older were classified into
sarcopenia and normal groups using DXA, HbA1c level in the male
sarcopenia group were significantly higher than that in the normal
group (72). Sugimoto et
al (59) examined the
relationship between HbA1c levels and the prevalence of sarcopenia
in 2,813 subjects aged 40 years and older, and the summary of their
results were: i) the prevalence of sarcopenia increased with
increasing HbA1c levels, and these linear relationships were
particularly marked in non-obese type 2 DM patients with a BMI
<22.3 kg/m2 (the prevalence of sarcopenia was: 7.0%
in the HbA1c <6.5% group; 18.5% in the HbA1c 6.5–7.0% group;
20.3% in the HbA1c 7.0–8.0% group; and 26.7% in the HbA1c 8.0% or
higher group), ii) higher HbA1c levels were more strongly
associated with lower skeletal muscle index (OR=5.42) than lower
grip strength (OR=1.89) or walking speed (OR=1.13), and iii) no
association was found between blood glucose levels and the
prevalence of sarcopenia in the elderly people with normal blood
glucose levels (59). Lee et
al (73) divided 3,132
elderly non-diabetic men aged 65 years or older into quartiles
according to homeostasis model assessment of insulin resistance
(HOMA-IR) levels, followed them for 5 years using DXA, and they
found that elderly men with higher HOMA-IR had greater loss of limb
skeletal muscle mass (73). These
results suggest that aging-related muscle mass loss may affect
HbA1c levels through reduced glucose utilization, or that increased
IR may further contribute to sarcopenia by decreasing skeletal
muscle protein synthesis and accelerating protein degradation.
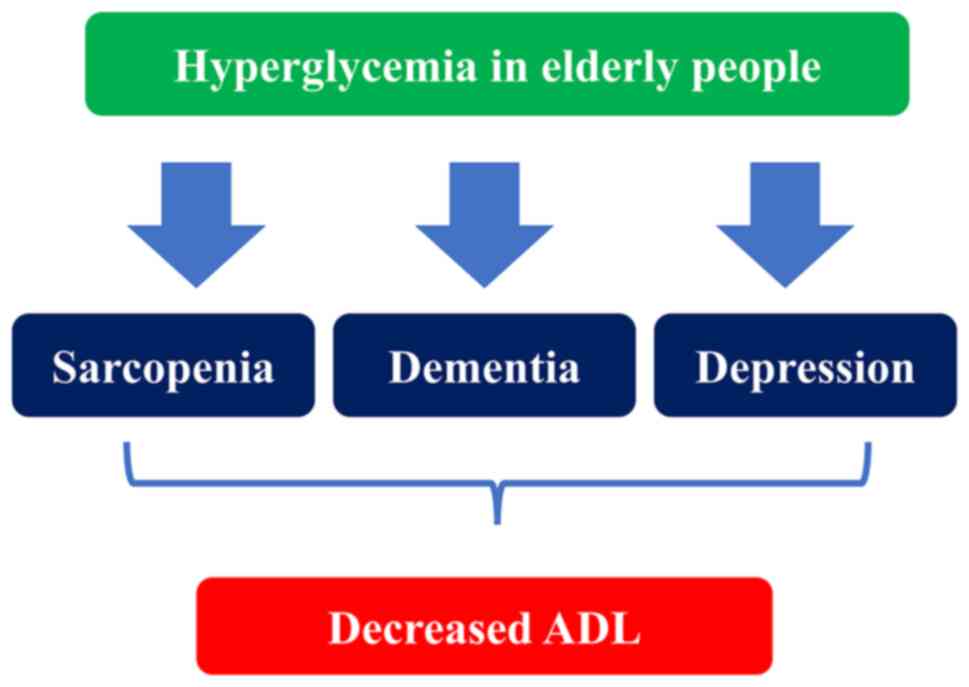
Hyperglycemia in the elderly is an aggravating factor for
sarcopenia, dementia, and depression, and leads to ADL decline
(74) (Fig. 1).
Sarcopenia is a risk factor for developing type 2
DM, and type 2 DM is a risk factor for developing sarcopenia
(71,75). Regarding the mechanism, Ogawa's
research group revealed for the first time that elevated blood
glucose levels cause muscle mass loss through the action of two
proteins, KLF15 and WWP1 (76).
IR prevents the proliferation and growth of skeletal muscle cells,
leading to a decrease in the skeletal muscle mass. They reported
that in mice with type 2 DM, the amount of a transcription factor
called KLF15 protein increases in the skeletal muscle as skeletal
muscle mass decreases (76).
KLF15 promotes skeletal muscle loss by increasing the expression of
genes that cause skeletal muscle degradation and muscle atrophy.
Their research confirmed that mice without KLF15 did not lose
skeletal muscle mass in diabetes. They also found that (1) the degradation of KLF15 is inhibited
by elevated blood glucose levels, (2) it is accumulated in the skeletal
muscle, and (3) a protein called
WWP1 plays an important role in regulating its degradation. WWP1 is
one of the proteins called ‘ubiquitin ligase’, which binds
ubiquitin to another protein. Proteins with large amounts of
ubiquitin are degraded faster (77). Their study showed that WWP1
specifically binds ubiquitin to KLF15. When blood glucose levels
increase, the amount of WWP1 is reduced, resulting in less
ubiquitin binding to KLF15, which inhibits KLF15 degradation
(76). The characteristics of the
skeletal muscle in patients with type 2 DM are listed in Table I.
Sodium glucose co-transporter 2 (SGLT2) inhibitors
are drugs that tilt the body toward a state of protein catabolism
by increasing the amount of urinary glucose, which also reduces
body fat, but at the same time reduces muscle mass because of the
tilt toward protein catabolism (78,79). Metformin has been shown to inhibit
sarcopenia (80–82). During acute exercise, enzyme
activity increases to burn fat and carbohydrates to compensate for
the large amount of energy consumed by the skeletal muscle. In this
process, AMP-activated protein kinase (AMPK), which senses the
energy status in the skeletal muscle, is activated (83,84). AMPK regulates energy metabolism in
cells throughout the body. When the intracellular energy state
becomes low energy (i.e., high AMP/ATP ratio) after exercise, AMPK
is activated and sends GLUT4 out onto the cell membrane to take up
glucose and break down fatty acids to obtain energy (83,84). Metformin activates AMPK (85). In other words, metformin takes
glucose into the muscle cells by the same mechanism as the effect
of exercise. Bouch et al (53) reported that in a comparison of
sarcopenic and non-sarcopenic groups in patients with type 2 DM,
the non-sarcopenic group had a significantly higher rate of
metformin use (19% vs. 54%) (53). On the other hand, in a cohort
study that followed changes in limb skeletal muscle mass in men
aged 65 years and older for 3.5 years, limb skeletal muscle mass
decreased by 4.4% in the group of diabetic patients who used drugs
other than insulin sensitizers, while in the group that used
insulin sensitizers, the decrease rate of limb skeletal muscle mass
was 1.8% (86). Out of the
previous reports using the AWGS criteria for the diagnosis of
sarcopenia in Asians (mentioned earlier) (51–67), Sugimoto et al (59) reported the lowest frequency of
sarcopenia (7%), and insulin preparations were used in about 70% of
the non-sarcopenic patients in their study (59). On the other hand, Cui et al
(58) reported the highest
frequency of sarcopenia (28.8%), and insulin preparations were used
in about 70% of sarcopenic patients. Ida et al (62) reported that the use of insulin
preparations was significantly higher in the sarcopenia group
compared to the non-sarcopenia group (79.5% vs. 62.1%). Thus,
further investigations of the effect of insulin preparations on
sarcopenia are needed.
The concept of frailty indicates ‘a vulnerable state
with reduced recovery to stress’ and ‘a condition that increases
the risk of health problems, including falls, disability, and
death’. In the elderly people, there is a risk that minor stresses
may lead to changes in health status (e.g., from independent to
needing care, from mobile to immobile, easily falling, from clear
consciousness to delirium, etc.) that are disproportionate to the
cause. It is important to take this into consideration during
medical interventions (35,87).
Type 2 DM is thought to progress physical frailty
and increase the risk of needing care and death through IR and
inflammation (88), and clinical
data supporting this relationship have been accumulated these days.
Several prospective cohort studies and cross-sectional studies have
reported the relationship between type 2 DM and frailty. In a
prospective cohort study of non-institutionalized individuals aged
60 years or older (observation period, 3.5 years), type 2 DM
increased the incidence of new cases of frailty (OR=2.18) (89). In a prospective study of subjects
aged 65 years and older, type 2 DM was a significant risk factor
for the progression from pre-frail to frailty, especially in
patients with macrovascular diseases. Inappropriate lifestyle,
abdominal obesity, and poor glycemic and lipid control were
associated with the frailty progression, and dietary therapy
reduced the risk (90). There are
also reports that HOMA-IR is associated with the onset of frailty
(91,92), and that frailty is associated with
2-hour blood glucose levels of 75 g oral glucose tolerance test
(OGTT) (93,94). On the other hand≠, some
longitudinal studies have shown that frailty is a risk factor for
the development of type 2 DM, and type 2 DM and frailty can
interact and form a vicious circle (95). In patients with type 2 DM, frailty
can be a risk factor for pathological fracture (96). The presence of osteoporosis in
female patients with type 2 DM predisposes to the development of
frailty (97). Not only frailty
but also pre-frail can be a poor prognostic factor in patients with
type 2 DM (98).
Regarding the relationship between glycemic control
and frailty, in a cross-sectional study of the Women's Health and
Aging Study, the frequency of frailty was significantly higher in
the group with HbA1c 6.5% or higher than in the group with HbA1c
less than 6.0% (99), and in a
longitudinal study, the frequency of frailty was 3.3 times higher
in the group with HbA1c >8% than in the group with HbA1c
<5.5% at baseline (100). In
a community-based cohort study, higher 5-year mean blood glucose
levels were associated with more frailty in non-diabetic patients,
whereas a U-shaped association was found in diabetic patients.
Baseline fasting blood glucose (FBS) <150 mg/dl (HbA1c=6.9%) had
an OR of 1.41, and FBS >190 mg/dl (HbA1c=8.2%) had an OR of
1.30, indicating a higher incidence of frailty, however, the
incidence of frailty was the lowest at FBS 170 mg/dl (HbA1c=7.6%)
(101). A recent cross-sectional
study of elderly diabetic patients in Japan showed that the lower
the HbA1c level, the greater the risk of developing frailty
(102). Therefore, low HbA1c may
be a risk factor for developing frailty in patients with type 2 DM.
The reason for this may be that strict glycemic control may lead to
frailty via hypoglycemia. In the Japanese guidelines for the
treatment of type 2 DM in the elderly, it states that there are no
reports that show that good glycemic control inhibits or improves
frailty. On the other hand, there are several reports on management
goals for type 2 DM patients with frailty. In type 2 DM patients
with frailty, there is a report that the group with HbA1c 8–8.9%
had preserved ADL and lower risk of death compared to the group
with HbA1c 7–7.9%, and caution against over-lowering of blood
glucose level is needed (103).
As elderly patients may not have hypoglycemic symptoms such as
sweating or palpitations despite low blood glucose levels,
hypoglycemia may be overlooked in the routine medical care, which
is associated with the risk of frailty progression (74). As lifestyle-related diseases such
as type 2 DM can be risk factors for frailty, it is important to
strictly manage them at least in the middle age. However, it is
important to note that in the old age, especially over the age of
75 with reduced physiological reserve, the adverse events
associated with strict glycemic control can lead to frailty and
even the need for nursing care. In the elderly, hypoglycemia,
depression, dementia, and frailty form a vicious cycle through
reduced physical activity and reduced dietary intake (74) (Fig.
2)
The relationship between sarcopenia, frailty and
type 2 DM was reviewed from the molecular biological and clinical
perspectives. The frequency of type 2 DM increases with aging, and
in Japan, about 20% of the population over the age of 70 suffer
from type 2 DM. In an aging society, the association between
sarcopenia or frailty and type 2 DM is an important issue. Skeletal
muscles of diabetic patients have a different distribution of
myofibers compared to healthy individuals. Hyperglycemia leads to
the degradation of muscle proteins. There is a close relationship
between the severity of type 2 DM and the frequency of sarcopenia.
Hypoglycemia in diabetic patients with frailty should be carefully
monitored. It is important to note that appropriate interventions
in patients with sarcopenia or frailty can prevent the development
of type 2 DM, and appropriate interventions in type 2 DM patients
can prevent the development of sarcopenia or frailty, which may
lead to improved healthy life expectancy.
Not applicable.
No funding was received.
Not applicable.
HN drafted the initial manuscript. SF, AA, KY, HO,
SN and KH edited and reviewed the manuscript. Data authentication
is not applicable. All authors have read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Gibbons A: HUMAN EVOLUTION. Why humans are
the high-energy apes. Science. 352:6392016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roth J, Szulc AL and Danoff A: Energy,
evolution, and human diseases: An overview. Am J Clin Nutr.
93:875S–883S. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Evans PL, McMillin SL, Weyrauch LA and
Witczak CA: Regulation of skeletal muscle glucose transport and
glucose metabolism by exercise training. Nutrients. 11:24322019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Seo JA, Kang MC, Yang WM, Hwang WM, Kim
SS, Hong SH, Heo JI, Vijyakumar A, Pereira de ML, Uner A, et al:
Apolipoprotein J is a hepatokine regulating muscle glucose
metabolism and insulin sensitivity. Nat Commun. 11:20242020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Severinsen MCK and Pedersen BK:
Muscle-organ crosstalk: The emerging roles of myokines. Endocr Rev.
41:594–609. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mukund K and Subramaniam S: Skeletal
muscle: A review of molecular structure and function, in health and
disease. Wiley Interdiscip Rev Syst Biol Med. 12:e14622020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jackson AS, Janssen I, Sui X, Church TS
and Blair SN: Longitudinal changes in body composition associated
with healthy ageing: Men, aged 20–96 years. Br J Nutr.
107:1085–1091. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schosserer M, Grillari J, Wolfrum C and
Scheideler M: Age-induced changes in white, brite, and brown
adipose depots: A mini-review. Gerontology. 64:229–236. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu H and Ballantyne CM: Skeletal muscle
inflammation and insulin resistance in obesity. J Clin Invest.
127:43–54. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kawada T: Basal metabolic rate parameters,
sarcopenia, and frailty in older males. J Am Med Dir Assoc.
20:9192019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zampino M, Semba RD, Adelnia F, Spencer
RG, Fishbein KW, Schrack JA, Simonsick EM and Ferrucci L: Greater
skeletal muscle oxidative capacity is associated with higher
resting metabolic rate: Results from the baltimore longitudinal
study of aging. J Gerontol A Biol Sci Med Sci. 75:2262–2268. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saltiel AR: Insulin signaling in health
and disease. J Clin Invest. 131:e1422412021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kadowaki T, Ueki K, Yamauchi T and Kubota
N: SnapShot: Insulin signaling pathways. Cell. 148:624–624.e1.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Burke LM, Kiens B and Ivy JL:
Carbohydrates and fat for training and recovery. J Sports Sci.
22:15–30. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Volek JS, Noakes T and Phinney SD:
Rethinking fat as a fuel for endurance exercise. Eur J Sport Sci.
15:13–20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sato T, Ito Y and Nagasawa T: Regulation
of skeletal muscle protein degradation and synthesis by oral
administration of lysine in rats. J Nutr Sci Vitaminol (Tokyo).
59:412–419. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sato T, Ito Y and Nagasawa T: L-Lysine
suppresses myofibrillar protein degradation and autophagy in
skeletal muscles of senescence-accelerated mouse prone 8.
Biogerontology. 18:85–95. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kamei Y, Hatazawa Y, Uchitomi R, Yoshimura
R and Miura S: Regulation of skeletal muscle function by amino
acids. Nutrients. 12:2612020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kitamura A, Seino S, Abe T, Nofuji Y,
Yokoyama Y, Amano H, Nishi M, Taniguchi Y, Narita M, Fujiwara Y and
Shinkai S: Sarcopenia: prevalence, associated factors, and the risk
of mortality and disability in Japanese older adults. J Cachexia
Sarcopenia Muscle. 12:30–38. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nishikawa H, Fukunishi S, Asai A,
Nishiguchi S and Higuchi K: Sarcopenia and frailty in liver
Cirrhosis. Life (Basel). 11:3992021.PubMed/NCBI
|
|
21
|
Cruz-Jentoft AJ, Baeyens JP, Bauer JM,
Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y,
Schneider SM, et al: Sarcopenia: European consensus on definition
and diagnosis: Report of the European working group on sarcopenia
in older people. Age Ageing. 39:412–423. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen LK, Liu LK, Woo J, Assantachai P,
Auyeung TW, Bahyah KS, Chou MY, Chen LY, Hsu PS, Krairit O, et al:
Sarcopenia in Asia: Consensus report of the Asian working group for
sarcopenia. J Am Med Dir Assoc. 15:95–101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nishikawa H, Shiraki M, Hiramatsu A,
Moriya K, Hino K and Nishiguchi S: Japan society of hepatology
guidelines for sarcopenia in liver disease (1st edition):
Recommendation from the working group for creation of sarcopenia
assessment criteria. Hepatol Res. 46:951–963. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie
Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA,
et al: Sarcopenia: Revised European consensus on definition and
diagnosis. Age Ageing. 48:16–31. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen LK, Woo J, Assantachai P, Auyeung TW,
Chou MY, Iijima K, Jang HC, Kang L, Kim M, Kim S, et al: Asian
working group for sarcopenia: 2019 consensus update on sarcopenia
diagnosis and treatment. J Am Med Dir Assoc. 21:300–307.e2. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sampaio RAC, Sewo Sampaio PY, Uchida MC
and Arai H: Management of dynapenia, sarcopenia, and frailty: The
role of physical exercise. J Aging Res. 2020:81867692020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Satake S and Arai H: Implications of
frailty screening in clinical practice. Curr Opin Clin Nutr Metab
Care. 20:4–10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sugimoto K, Rakugi H, Kojima T, Ishii S,
Akishita M, Tamura Y, Araki A, Kozaki K, Senda K, Fukuoka H, et al:
Chapter 4 Frailty and specific diseases. Geriatr Gerontol Int. 20
(Suppl 1):S25–S37. 2020. View Article : Google Scholar
|
|
29
|
Mesinovic J, Zengin A, De Courten B,
Ebeling PR and Scott D: Sarcopenia and type 2 diabetes mellitus: A
bidirectional relationship. Diabetes Metab Syndr Obes.
12:1057–1072. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
DeFronzo RA: Lilly lecture 1987. The
triumvirate: Beta-cell, muscle, liver. A collusion responsible for
NIDDM. Diabetes. 37:667–687. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bu L, Cao X, Zhang Z, Wu H, Guo R and Ma
M: Decreased secretion of tumor necrosis factor-α attenuates
macrophages-induced insulin resistance in skeletal muscle. Life
Sci. 244:1173042020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Love KM, Liu J, Regensteiner JG, Reusch
JEB and Liu Z: GLP-1 and insulin regulation of skeletal and cardiac
muscle microvascular perfusion in type 2 diabetes. J Diabetes.
12:488–498. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nishikawa H and Osaki Y: Clinical
significance of therapy using branched-chain amino acid granules in
patients with liver cirrhosis and hepatocellular carcinoma. Hepatol
Res. 44:149–158. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nishikawa H, Enomoto H, Ishii A, Iwata Y,
Miyamoto Y, Ishii N, Yuri Y, Takata R, Hasegawa K, Nakano C, et al:
Development of a simple predictive model for decreased skeletal
muscle mass in patients with compensated chronic liver disease.
Hepatol Res. 47:1223–1234. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nishikawa H, Yoh K, Enomoto H, Iwata Y,
Sakai Y, Kishino K, Shimono Y, Ikeda N, Takashima T, Aizawa N, et
al: Sarcopenia and frailty in chronic liver damage: Common and
different points. In Vivo. 34:2549–2559. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Richter EA and Hargreaves M: Exercise,
GLUT4, and skeletal muscle glucose uptake. Physiol Rev.
93:993–1017. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Flores-Opazo M, McGee SL and Hargreaves M:
Exercise and GLUT4. Exerc Sport Sci Rev. 48:110–118. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hood DA, Memme JM, Oliveira AN and Triolo
M: Maintenance of skeletal muscle mitochondria in health, exercise,
and aging. Annu Rev Physiol. 81:19–41. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Memme JM, Erlich AT, Phukan G and Hood DA:
Exercise and mitochondrial health. J Physiol. 599:803–817. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hatazawa Y, Tadaishi M, Nagaike Y, Morita
A, Ogawa Y, Ezaki O, Takai-Igarashi T, Kitaura Y, Shimomura Y,
Kamei Y and Miura S: PGC-1α-mediated branched-chain amino acid
metabolism in the skeletal muscle. PLoS One. 9:e910062014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schnyder S and Handschin C: Skeletal
muscle as an endocrine organ: PGC-1α, myokines and exercise. Bone.
80:115–125. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Norheim F, Langleite TM, Hjorth M, Holen
T, Kielland A, Stadheim HK, Gulseth HL, Birkeland KI, Jensen J and
Drevon CA: The effects of acute and chronic exercise on PGC-1α,
irisin and browning of subcutaneous adipose tissue in humans. FEBS
J. 281:739–749. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Booth FW, Ruegsegger GN, Toedebusch RG and
Yan Z: Endurance exercise and the regulation of skeletal muscle
metabolism. Prog Mol Biol Transl Sci. 135:129–151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Islam H, Hood DA and Gurd BJ: Looking
beyond PGC-1α: Emerging regulators of exercise-induced skeletal
muscle mitochondrial biogenesis and their activation by dietary
compounds. Appl Physiol Nutr Metab. 45:11–23. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jang C, Oh SF, Wada S, Rowe GC, Liu L,
Chan MC, Rhee J, Hoshino A, Kim B, Ibrahim A, et al: A
branched-chain amino acid metabolite drives vascular fatty acid
transport and causes insulin resistance. Nat Med. 22:421–426. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Arany Z, He H, Lin J, Hoyer K, Handschin
C, Toka O, Ahmad F, Matsui T, Chin S, Wu PH, et al: Transcriptional
coactivator PGC-1 alpha controls the energy state and contractile
function of cardiac muscle. Cell Metab. 1:259–271. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cretoiu D, Pavelescu L, Duica F, Radu M,
Suciu N and Cretoiu SM: Myofibers. Adv Exp Med Biol. 1088:23–46.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Drake JC and Yan Z: Precision remodeling:
How exercise improves mitochondrial quality in myofibers. Curr Opin
Physiol. 10:96–101. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hickey MS, Carey JO, Azevedo JL, Houmard
JA, Pories WJ, Israel RG and Dohm GL: Skeletal muscle fiber
composition is related to adiposity and in vitro glucose transport
rate in humans. Am J Physiol. 268((3 Pt 1)): E453–E457.
1995.PubMed/NCBI
|
|
50
|
Märin P, Krotkiewski M, Andersson B and
Björntorp P: Muscle fiber composition and capillary density in
women and men with NIDDM. Diabetes Care. 17:382–386. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kaji A, Hashimoto Y, Kobayashi Y, Sakai R,
Okamura T, Miki A, Hamaguchi M, Kuwahata M, Yamazaki M and Fukui M:
Sarcopenia is associated with tongue pressure in older patients
with type 2 diabetes: A cross-sectional study of the KAMOGAWA-DM
cohort study. Geriatr Gerontol Int. 19:153–158. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sazlina SG, Lee PY, Chan YM, A Hamid MS
and Tan NC: The prevalence and factors associated with sarcopenia
among community living elderly with type 2 diabetes mellitus in
primary care clinics in Malaysia. PLoS One. 15:e02332992020.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bouchi R, Fukuda T, Takeuchi T, Minami I,
Yoshimoto T and Ogawa Y: Sarcopenia is associated with incident
albuminuria in patients with type 2 diabetes: A retrospective
observational study. J Diabetes Investig. 8:783–787. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hashimoto Y, Kaji A, Sakai R, Hamaguchi M,
Okada H, Ushigome E, Asano M, Yamazaki M and Fukui M: Sarcopenia is
associated with blood pressure variability in older patients with
type 2 diabetes: A cross-sectional study of the KAMOGAWA-DM cohort
study. Geriatr Gerontol Int. 18:1345–1349. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Okamura T, Mik A, Hashimoto Y, Kaji A,
Sakai R, Osaka T, Hamaguchi M, Yamazaki M and Fukui M: Deficiency
of energy intake rather than protein is associated with sarcopenia
in early patients with type 2 diabetes: A cross-sectional study of
the KAMOGAWA-DM cohort. J Diabetes. 11:477–483. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Okamura T, Hashimoto Y, Miki A, Kaji A,
Sakai R, Iwai K, Osaka T, Kitagawa N, Ushigome E, Hamaguchi M, et
al: High brain natriuretic peptide is associated with sarcopenia in
patients with type 2 diabetes: A cross-sectional study of
KAMOGAWA-DM cohort study. Endocr J. 66:369–377. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Bouchi R, Fukuda T, Takeuchi T, Nakano Y,
Murakami M, Minami I, Izumiyama H, Hashimoto K, Yoshimoto T and
Ogawa Y: Insulin treatment attenuates decline of muscle mass in
Japanese patients with type 2 diabetes. Calcif Tissue Int. 101:1–8.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cui M, Gang X and Wang G, Xiao X, Li Z,
Jiang Z and Wang G: A cross-sectional study: Associations between
sarcopenia and clinical characteristics of patients with type 2
diabetes. Medicine (Baltimore). 99:e187082020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sugimoto K, Tabara Y, Ikegami H, Takata Y,
Kamide K, Ikezoe T, Kiyoshige E, Makutani Y, Onuma H, Gondo Y, et
al: Hyperglycemia in non-obese patients with type 2 diabetes is
associated with low muscle mass: The multicenter study for
clarifying evidence for sarcopenia in patients with diabetes
mellitus. J Diabetes Investig. 10:1471–1479. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Fung FY, Koh YLE, Malhotra R, Ostbye T,
Lee PY, Shariff Ghazali S and Tan NC: Prevalence of and factors
associated with sarcopenia among multi-ethnic ambulatory older
Asians with type 2 diabetes mellitus in a primary care setting. BMC
Geriatr. 19:1222019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Mori H, Kuroda A, Ishizu M, Ohishi M,
Takashi Y, Otsuka Y, Taniguchi S, Tamaki M, Kurahashi K, Yoshida S,
et al: Association of accumulated advanced glycation end-products
with a high prevalence of sarcopenia and dynapenia in patients with
type 2 diabetes. J Diabetes Investig. 10:1332–1340. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ida S, Murata K, Nakadachi D, Ishihara Y,
Imataka K, Uchida A, Monguchi K, Kaneko R, Fujiwara R and Takahashi
H: Association between dynapenia and decline in higher-level
functional capacity in older men with diabetes. Geriatr Gerontol
Int. 18:1393–1397. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Murai J, Nishizawa H, Otsuka A, Fukuda S,
Tanaka Y, Nagao H, Sakai Y, Suzuki M, Yokota S, Tada H, et al: Low
muscle quality in Japanese type 2 diabetic patients with visceral
fat accumulation. Cardiovasc Diabetol. 17:1122018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Osaka T, Hamaguchi M, Hashimoto Y,
Ushigome E, Tanaka M, Yamazaki M and Fukui M: Decreased the
creatinine to cystatin C ratio is a surrogate marker of sarcopenia
in patients with type 2 diabetes. Diabetes Res Clin Pract.
139:52–58. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Fukuda T, Bouchi R, Takeuchi T, Tsujimoto
K, Minami I, Yoshimoto T and Ogawa Y: Sarcopenic obesity assessed
using dual energy X-ray absorptiometry (DXA) can predict
cardiovascular disease in patients with type 2 diabetes: A
retrospective observational study. Cardiovasc Diabetol. 17:552018.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Fukuoka Y, Narita T, Fujita H, Morii T,
Sato T, Sassa MH and Yamada Y: Importance of physical evaluation
using skeletal muscle mass index and body fat percentage to prevent
sarcopenia in elderly Japanese diabetes patients. J Diabetes
Investig. 10:322–330. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Murata Y, Kadoya Y, Yamada S and Sank T:
Sarcopenia in elderly patients with type 2 diabetes mellitus:
Prevalence and related clinical factors. Diabetol Int. 9:136–142.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Park SW, Goodpaster BH, Lee JS, Kuller LH,
Boudreau R, de Rekeneire N, Harris TB, Kritchevsky S, Tylavsky FA,
Nevitt M, et al: Excessive loss of skeletal muscle mass in older
adults with type 2 diabetes. Diabetes Care. 32:1993–1997. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Anbalagan VP, Venkataraman V, Pradeepa R,
Deepa M, Anjana RM and Mohan V: The prevalence of presarcopenia in
Asian Indian individuals with and without type 2 diabetes. Diabetes
Technol Ther. 15:768–775. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Leenders M, Verdijk LB, van der Hoeven L,
Adam JJ, van Kranenbur J, Nilwik R and van Loon LJ: Patients with
type 2 diabetes show a greater decline in muscle mass, muscle
strength, and functional capacity with aging. J Am Med Dir Assoc.
14:585–592. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Srikanthan P, Hevener AL and Karlamangla
AS: Sarcopenia exacerbates obesity-associated insulin resistance
and dysglycemia: Findings from the National health and nutrition
examination survey III. PLoS One. 5:e108052010. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Sanada K, Miyachi M, Tanimoto M, Yamamoto
K, Murakami H, Okumura S, Gando Y, Suzuki K, Tabata I and Higuchi
M: A cross-sectional study of sarcopenia in Japanese men and women:
Reference values and association with cardiovascular risk factors.
Eur J Appl Physiol. 110:57–65. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Lee CG, Boyko EJ, Strotmeyer ES, Lewis CE,
Cawthon PM, Hoffman AR, Everson-Rose SA, Barrett-Connor E and
Orwoll ES; Osteoporotic Fractures in Men Study Research Group, :
Association between insulin resistance and lean mass loss and fat
mass gain in older men without diabetes mellitus. J Am Geriatr Soc.
59:1217–1224. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Araki A and Ito H: Diabetes mellitus and
geriatric syndromes. Geriatr Gerontol Int. 9:105–114. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kim TN, Park MS, Yang SJ, Yoo HJ, Kang HJ,
Song W, Seo JA, Kim SG, Kim NH, Baik SH, et al: Prevalence and
determinant factors of sarcopenia in patients with type 2 diabetes:
The Korean sarcopenic obesity study (KSOS). Diabetes Care.
33:1497–1499. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Hirata Y, Nomura K, Senga Y, Okada Y,
Kobayashi K, Okamoto S, Minokoshi Y, Imamura M, Takeda S, Hosooka T
and Ogawa W: Hyperglycemia induces skeletal muscle atrophy via a
WWP1/KLF15 axis. JCI Insight. 4:e1249522019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Varshavsky A: The ubiquitin system,
autophagy, and regulated protein degradation. Annu Rev Biochem.
86:123–128. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Sasaki T: Sarcopenia, frailty circle and
treatment with sodium-glucose cotransporter 2 inhibitors. J
Diabetes Investig. 10:193–195. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Sasaki T, Sugawara M and Fukuda M:
Sodium-glucose cotransporter 2 inhibitor-induced changes in body
composition and simultaneous changes in metabolic profile: 52-week
prospective LIGHT (Luseogliflozin: The components of weight loss in
Japanese patients with type 2 diabetes mellitus) study. J Diabetes
Investig. 10:108–117. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Chen F, Xu S, Wang Y, Chen F, Cao L, Liu
T, Huang T, Wei Q, Ma G, Zhao Y and Wang D: Risk factors for
sarcopenia in the elderly with type 2 diabetes mellitus and the
effect of metformin. J Diabetes Res. 2020:39504042020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Laksmi PW, Setiati S, Tamin TZ, Soewondo
P, Rochmah W, Nafrialdi N and Prihartono J: Effect of metformin on
handgrip strength, gait speed, myostatin serum level, and
health-related quality of life: A double blind randomized
controlled trial among non-diabetic pre-frail elderly patients.
Acta Med Indones. 49:118–127. 2017.PubMed/NCBI
|
|
82
|
Yang Y, Liao Z and Xiao Q: Metformin
ameliorates skeletal muscle atrophy in Grx1 KO mice by regulating
intramuscular lipid accumulation and glucose utilization. Biochem
Biophys Res Commun. 533:1226–1232. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Pirkmajer S, Petrič M and Chibalin AV: The
role of AMPK in regulation of Na+, K+-ATPase
in skeletal muscle: Does the gauge always plug the sink? J Muscle
Res Cell Motil. 42:77–97. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
McGee SL and Hargreaves M: Exercise
adaptations: Molecular mechanisms and potential targets for
therapeutic benefit. Nat Rev Endocrinol. 16:495–505. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
LaMoia TE and Shulman GI: Cellular and
molecular mechanisms of metformin action. Endocr Rev. 42:77–96.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Lee CG, Boyko EJ, Barrett-Connor E,
Miljkovic I, Hoffman AR, Everson-Rose SA, Lewis CE, Cawthon PM,
Strotmeyer ES and Orwoll ES; Osteoporotic Fractures in Men (MrOS)
Study Research Group, : Insulin sensitizers may attenuate lean mass
loss in older men with diabetes. Diabetes Care. 34:2381–2386. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Clegg A, Young J, Iliffe S, Rikkert MO and
Rockwood K: Frailty in elderly people. Lancet. 381:752–762. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Morley JE, Malmstrom TK, Rodriguez-Mañas L
and Sinclair AJ: Frailty, Sarcopenia and diabetes. J Am Med Dir
Assoc. 15:853–859. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
García-Esquinas E, Graciani A,
Guallar-Castillón P, López-García E, Rodríguez-Mañas L and
Rodríguez-Artalejo F: Diabetes and risk of frailty and its
potential mechanisms: A prospective cohort study of older adults. J
Am Med Dir Assoc. 16:748–754. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Espinoza SE, Jung I and Hazuda H: Frailty
transitions in the San Antonio longitudinal study of aging. J Am
Geriatr Soc. 60:652–660. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Barzilay JI, Blaum C, Moore T, Xue QL,
Hirsch CH, Walston JD and Fried LP: Insulin resistance and
inflammation as precursors of frailty: The cardiovascular health
study. Arch Intern Med. 167:635–641. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Pérez-Tasigchana RF, León-Muñoz LM,
Lopez-Garcia E, Gutierrez-Fisac JL, Laclaustra M,
Rodríguez-Artalejo F and Guallar-Castillón P: Metabolic syndrome
and insulin resistance are associated with frailty in older adults:
A prospective cohort study. Age Ageing. 46:807–812. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Walston J, McBurnie MA, Newman A, Tracy
RP, Kop WJ, Hirsch CH, Gottdiener J and Fried LP; Cardiovascular
Health Study, : Frailty and activation of the inflammation and
coagulation systems with and without clinical comorbidities:
Results from the cardiovascular health study. Arch Intern Med.
162:2333–2341. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Kalyani RR, Varadhan R, Weiss CO, Fried LP
and Cappola AR: Frailty status and altered glucose-insulin
dynamics. J Gerontol A Biol Sci Med Sci. 67:1300–1306. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Veronese N, Stubbs B, Fontana L, Trevisan
C, Bolzetta F, De Rui M, Sartori L, Musacchio E, Zambon S, Maggi S,
et al: Frailty is associated with an increased risk of incident
type 2 diabetes in the elderly. J Am Med Dir Assoc. 17:902–907.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Li G, Prior JC, Leslie WD, Thabane L,
Papaioannou A, Josse RG, Kaiser SM, Kovacs CS, Anastassiades T,
Towheed T, et al: Frailty and risk of fractures in patients with
Type 2 Diabetes. Diabetes Care. 42:507–513. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Tamura H, Miyamoto T, Tamaki A, Nawa G and
Konya H: Osteoporosis complication is a risk factor for frailty in
females with type 2 diabetes mellitus. J Phys Ther Sci. 31:621–624.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Chao CT, Wang J and Chien KL; COhort of
GEriatric Nephrology in NTUH (COGENT) study group, : Both
pre-frailty and frailty increase healthcare utilization and adverse
health outcomes in patients with type 2 diabetes mellitus.
Cardiovasc Diabetol. 17:1302018. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Blaum CS, Xue QL, Tian J, Semba RD, Fried
LP and Walston J: Is hyperglycemia associated with frailty status
in older women? J Am Geriatr Soc. 57:840–847. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Kalyani RR, Tian J, Xue QL, Walston J,
Cappola AR, Fried LP, Brancati FL and Blaum CS: Hyperglycemia and
incidence of frailty and lower extremity mobility limitations in
older women. J Am Geriatr Soc. 60:1701–1707. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zaslavsky O, Walker RL, Crane PK, Gray SL
and Larson EB: glucose levels and risk of frailty. J Gerontol A
Biol Sci Med Sci. 71:1223–1229. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Yanagita I, Fujihara Y, Eda T, Tajima M,
Yonemura K, Kawajiri T, Yamaguchi N, Asakawa H, Nei Y, Kayashima Y,
et al: Low glycated hemoglobin level is associated with severity of
frailty in Japanese elderly diabetes patients. J Diabetes Investig.
9:419–425. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Yau CK, Eng C, Cenzer IS, Boscardin WJ,
Rice-Trumble K and Lee SJ: Glycosylated hemoglobin and functional
decline in community-dwelling nursing home-eligible elderly adults
with diabetes mellitus. J Am Geriatr Soc. 60:1215–1221. 2012.
View Article : Google Scholar : PubMed/NCBI
|