Introduction
Atherosclerosis is characterized by the hardening
and narrowing of arterial lumen, due to fatty deposits called
plaques that form on the inner walls of arteries (1). It is responsible for most
cardiovascular diseases such as coronary artery disease, stroke and
peripheral vascular disease. However, the exact cause of
atherosclerosis remains controversial. From pathological
perspectives, atherosclerosis has been defined as a chronic
inflammatory disease involved in endothelial cell dysfunction,
lipid infiltration, macrophage recruitment and vascular smooth
muscle cells migration (2).
Vascular endothelial cells become dysfunctional under the
stimulation of several factors, such as mechanical stress and
oxidative stress (3,4). The modified lipid in blood enters the
endothelial layer and stimulates endothelial cells to release
chemokines and adhesion molecules, which recruits monocytes in
blood to migrate into the intima and transform into macrophages
(5). Macrophages phagocytose lipids
cholesterol by CD36 or scavenger receptor. Excessive lipid
accumulation transforms macrophage to foam cells (6). Necrotic foam cells aggregate to form
the lipid-rich necrotic core of plaques. In addition, activated
macrophages release inflammatory factors and chemokines, which can
promote the proliferation and migration of smooth muscle cells in
the media layer to intima (7).
Smooth muscle cells, macrophages and extracellular matrix make up
the fibrous cap. The lesions continue to develop and eventually
form plaques. Vascular endothelial dysfunction is considered as an
early marker for atherosclerosis (8). In humans, atherosclerosis often occurs
in the bifurcation or bending of artery, where turbulence is prone
to occur. This may result from continuous shear stress leading to
vascular endothelial cell dysfunction. In addition, oxidative
stress is also the main cause of endothelial dysfunction (3). In turn, endothelial cell dysfunction
can produce more reactive oxygen species (ROS) and aggravate
oxidative stress (9). Oxidative
stress can promote inflammation and increase the modified
lipoproteins (10). Oxidized low
density lipoprotein (ox LDL) has been shown to aggravate
atherosclerosis through a variety of ways, such as aggravating
vascular inflammation and form cell formation (11). In vivo, dyslipidemia,
characterized by high triglyceride and lipid cholesterol, can
aggravate inflammation and atherosclerosis (12). Activated inflammation recruits more
macrophages to the injured blood vessel. The aggregation of
macrophages intensified the process of lipid phagocytosis,
inflammatory mediators release, foam cell formation and
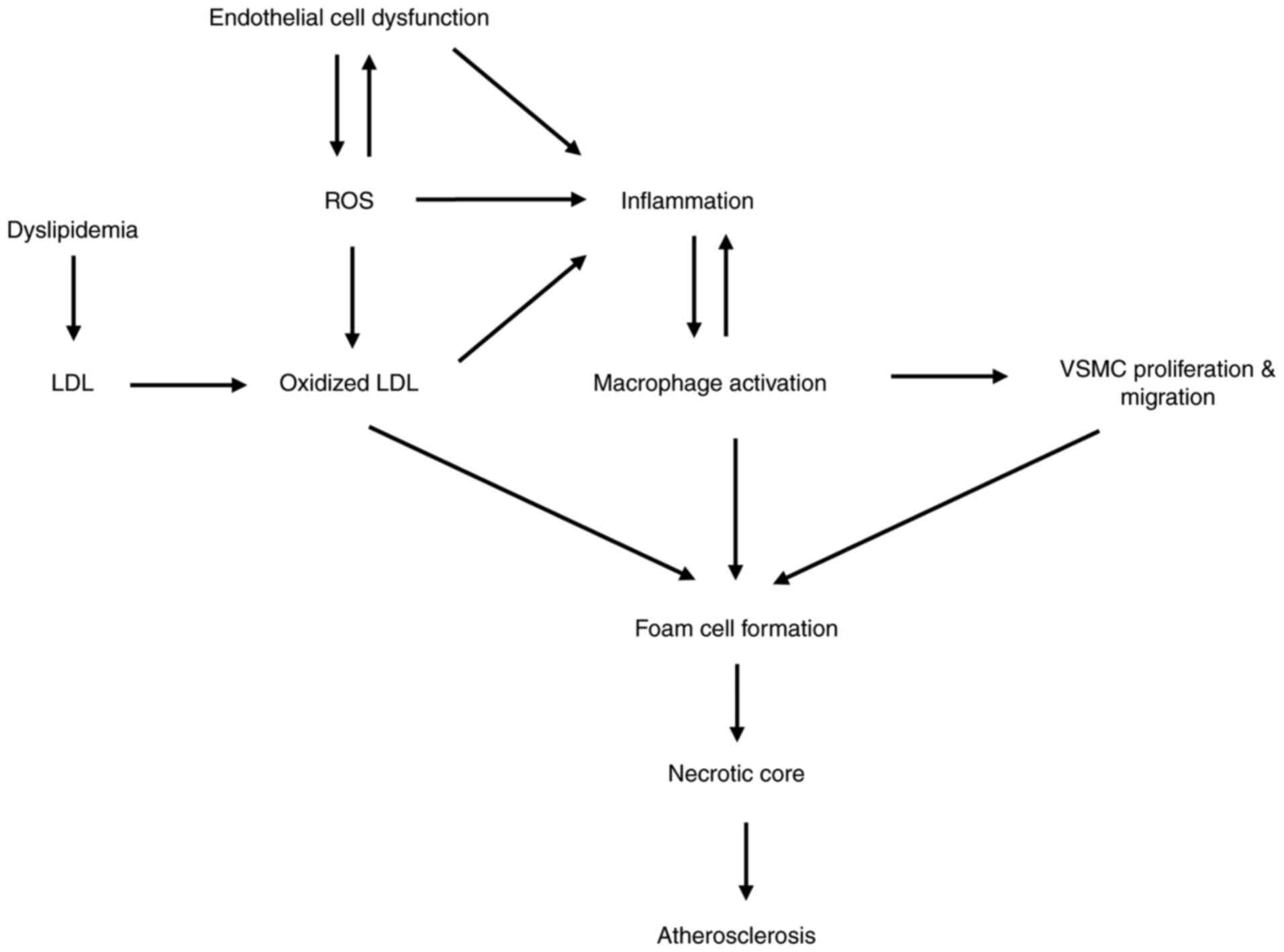
atherosclerosis exacerbation. Fig.
1 shows the association between lipid abnormality, oxidative
stress, endothelial dysfunction and inflammation in the development
of atherosclerosis. High levels of low-density lipoprotein (LDL)
cholesterol, as well as inflammation, smoking, hypertension and
diabetes, have been shown to be risk factors of atherosclerosis
(13–17). Currently, atherosclerosis is mainly
treated by altering lifestyle, taking statin medications and
undergoing surgeries.
Despite considerable advances in the treatment of
cardiovascular diseases, it remains the leading cause of mortality
and morbidity worldwide (18). The
main challenge in atherosclerosis research is that evident clinical
manifestations usually occur after decades, and the arterial wall
changes profoundly during the development and progression of the
disease. There is limited direct access to tissues from the
different evolving stages of atherosclerosis for research because
the vascular tissues obtained from individuals are under traumatic
circumstances, such as warfare or automobile accidents (19–22).
Studies have shown that atherosclerosis can occur in several
animals, and this has made it possible to obtain tissues at all
stages of atherosclerosis and cells needed for in vitro
studies. Therefore, animal models of atherosclerosis have the
potential to solve the problems of inherent restrictions in human
research.
Indeed, animal models of atherosclerosis, over the
past decades, have greatly increased the understanding on this
chronic inflammatory disease and for assessing novel
pharmacological treatments that can prevent or slow down the onset
of atherosclerosis. The first animal model to be used for
atherosclerosis research was rabbit, developed by Ignatowski, who
demonstrated lesion formation in the aortic wall of rabbits that
were fed animal protein-enriched diet (mainly meat, milk and egg
yolk) (23). Since, various animal
species, such as mice, rats, guinea pigs, hamsters, birds, dogs and
non-human primates, have been used as experimental models for
atherosclerosis (24). The
following are considered as the essential requirements for
developing animal models of atherosclerosis: i) Atherosclerotic
lesions should be easily induced; ii) lesions should mimic those in
humans; iii) lesions should have clinical sequelae; and iv) lesions
should develop spontaneously after the consumption of a diet
similar to that of humans.
The advantages and limitations of commonly used
animal models are described briefly in Table I. Among these animal models,
non-human primates closely resemble the human model of
atherosclerosis, followed by pigs or dogs, based on anatomy,
physiology, lipoprotein profile and site of lesion formation;
however, they are less widely used owing to long feeding cycles and
high costs (24). Mice are the most
commonly used species, followed by rabbits, because of the
following advantages: Ease of maintenance and breeding; genetic and
transgenic pliability; and the rapid formation of atherosclerotic
lesions. With the development in research, an increasing number of
mice and rabbit strains have been bred, and advanced methods have
been developed to provide further insights into atherosclerosis
(25,26). Moreover, appropriate lesion analysis
is necessary to decrease experimental variability and, thereby,
increase accuracy. The present review discusses the characteristics
of atherosclerosis in different mouse and rabbit models and
describes lesion analysis and cell culture methods used in
atherosclerosis-associated research.
 | Table I.Advantages and limitations of the
widely used animal species for atherosclerosis research. |
Table I.
Advantages and limitations of the
widely used animal species for atherosclerosis research.
| Species | Advantages | Limitations | Common
application |
|---|
| Mice | Explicit genomic
information; easy gene manipulation; low cost; easy breeding;
inbred strain | Lipoprotein
metabolism differs from that of humans; plaque location differs
from that of humans; limited animal samples for study; difficulty
in coronary artery research | Exploring gene
function; studies on specific cell types; signal pathway
studies |
| Rabbits | Spontaneous plaque
formation; express CETP; easy breeding; allow monitoring of lesions
by ultrasound or MRI | Inbred rabbits not
easily obtained; limited gene modification; deficient in hepatic
lipase | Pharmacological
study |
| Non-human
primates | Simulate human
pathological process maximally; non-invasive imaging is
possible | Expensive; ethical
and welfare issues; long modeling time | Pharmacological
study; social and behavioral study |
| Pigs or dogs | Lipid profile
similar to that of humans; plaque location close to that in humans;
non-invasive imaging is possible | Limited gene
modification; expensive feeding costs | Continuous
observation of lesions |
Mice
History of mice as atherosclerosis
animal models
The use of mice for atherosclerosis research started
in the late 1960s. Various research groups have attempted to
clarify the plasma lipoprotein metabolism and susceptibility to
atherosclerosis in mice. Vesselinovitch et al (27) and Vesselinovitch and Wissler
(28) used closed-colony mice,
called CF1, to induce atheroma in the aorta of the mice; however,
the results were inconsistent even when the most atherogenic diet
was used, because different animals showed different manifestations
after being fed with the diet for similar duration. As the work was
almost invariably performed on random-bred animals, it can be
assumed that a high genetic variability must exist between
individual animals in any one experiment (27,28).
Thompson (29) hypothesized that
the genotype of animals is important in the development of atheroma
by inducing atherosclerosis in inbred strain mice (C57BL/6) through
a high-fat/high-cholesterol (HFHC) diet and found that
atherosclerosis was observed in all mice after 25 weeks of HFHC
diet treatment. Thompson concluded that all the C57BL/6 mice showed
lesions in the aortic valve region (29). In the past 20 years from the study
by Thompson (29), mice have been
used as a favorable system for a combined genetic and biochemical
analysis of atherosclerosis and lipoprotein metabolism. In 1985,
Paigen et al (30) found
that C57BL/6 mice were the most susceptible to development of
diet-induced atherosclerosis among the ten inbred strains examined.
Based on this original finding, it has become a common practice to
perform atherosclerosis studies on C57BL/6 inbred strain mice, and
this includes studies using genetically modified (GM) mice, despite
the fact that in several instances this required founder GM mice to
be continually backcrossed with the C57BL/6 strain. In the early
1990s, mouse models exhibiting very high cholesterol levels and
relatively advanced lesions were created through genetic
engineering, including apolipoprotein E knockout (KO)
(ApoE−/−) and LDL receptor KO (LDLR−/−) mice
(31–33). Shortly afterwards, ApoE/LDLR
double-KO mice with more severe hyperlipidemia and atherosclerosis
were developed (34,35). Moreover, ApoE* 3-Leiden transgenic
mice and SRB-1−/−/ApoE−/− mice have also been
used in various studies (36–38).
Recently, the proprotein convertase subtilisin/kexin type 9
(PCSK9)-adeno-associated virus (AAV) mice have been used as a
rapid, versatile and cost-effective animal model for
atherosclerosis research (39). The
following section will focus on the characteristics of
ApoE−/−, LDLR−/−, PCSK9-AAV mice and other GM
mouse models used in atherosclerosis research.
ApoE−/− mice
The most extensively used mouse model of
atherosclerosis is ApoE−/− mice, which was developed in
1992 using gene targeting technique in mouse embryonic stem (ES)
cells to inactivate the endogenous Apoe gene (32,33).
ApoE is a glycoprotein, with a molecular size of ~34 kDa, that
serves as a ligand for cell-surface lipoprotein receptors and
clears chylomicrons and very low-density lipoprotein (VLDL)
remnants (40). Additionally, ApoE
is involved in other functions, including cholesterol homeostasis,
local redistribution of cholesterol within tissues,
immunoregulation, dietary absorption and biliary excretion of
cholesterol (41). Deletion of the
Apoe gene impaired the ability to clear plasma lipoproteins,
resulting in plasma cholesterol levels to reach 400–600 mg/dl
mostly in the VLDL and chylomicron remnant fractions upon feeding a
normal diet, whereas the plasma cholesterol levels in wild-type
mice were estimated to be 75–110 mg/dl (42,43).
Upon administration of a chow diet, the ApoE−/− mice
were first observed to develop foam cell lesions at 10 weeks,
followed by fatty streaks in the proximal aorta at 12 weeks,
intermediate lesions containing foam cells and smooth muscle cells
at 15 weeks, and fibrous plaques at 20 weeks of age (43). In highly advanced lesions,
fibro-fatty nodules are a nidus for calcification and plaques
become increasingly calcified with time (44). Moreover, a western diet (for
example, consisting of 21% fat and 0.15% cholesterol) can
accelerate the atherosclerotic process (45). Therefore, the western diet-fed
ApoE−/− mice showed total plasma cholesterol
concentration reaching >1,000 mg/dl and the formation of more
foam cell-rich atherosclerotic lesions containing cholesterol
crystals, necrotic cores and calcifications (46).
The western diet-fed ApoE−/− mice can
develop severe atherosclerosis in a short duration (12–16 weeks)
and have become a favorable animal model for atherosclerosis
research. Various studies on investigating the function of genes
involved in developing atherosclerosis have been conducted on
ApoE−/− mice (47). For
instance, western diet-fed ApoE−/− mice lacking SR-A or
CD36 demonstrated decreased lipid accumulation in peritoneal
macrophages under in vivo conditions, and this effect was
associated with increased areas of aortic sinus lesion, which is in
contrast with the results of previous studies performed on C57BL/6
mice (48).
LDLR−/− mice
LDLR−/− mice are another commonly used
animal model for atherosclerosis research. LDLR is a membrane
receptor that mediates the endocytosis of cholesterol-rich LDL and
to clear LDL in the liver (49). In
1993, similar to the method used in ApoE−/− mice
generation, LDLR−/− mice were created by homologous
recombination in ES cells (31).
Chow diet-fed LDLR−/− mice displayed modestly elevated
plasma cholesterol levels (200–300 mg/dl) and developed no or only
mild atherosclerosis, even at an advanced age (50,51).
In terms of lipoprotein particles, the levels of
intermediate-density lipoprotein and LDL-sized particles were
increased, whereas the levels of high-density lipoprotein (HDL) and
triglycerides remained unaffected (31,50).
The western diet-fed LDLR−/− mice showed accumulation of
larger VLDL remnants with elevated total plasma cholesterol
concentrations of >1,000 mg/dl and the formation of mostly foamy
lesions (52).
In 1997, researchers suggested that when the bone
marrow from wild-type mice and LDLR−/− mice were
transplanted into irradiated LDLR−/− mice, the two
groups showed similar lesions, indicating that LDLR expressed by
bone marrow cells had no effect on the lesions (53,54).
Thus, bone marrow transplantation is used as a tool to replace
endogenous bone marrow-derived cells in the artery wall with those
of the donor origin. This method is applicable for studying the
effect of genes in white blood cells, for instance, the bone marrow
transplantation from LDLR+/+ GM mice into irradiated
LDLR−/− mice provided a background of hyperlipidemia in
the study of target genes. Of note, in this method,
LDLR−/− mice are not recommended to be replaced by
ApoE−/− mice, because macrophage-derived ApoE has an
independent role in lesion development (55,56).
PCSK9-AAV mice
PCSK9-AAV mice were a new line of mouse models for
atherosclerosis research created by two groups (57,58).
One of the best advantages of these mice is that atherosclerotic
lesions can be formed by injecting AAV, without performing gene
manipulation in animals. PCSK9 is an enzyme encoded by the
PCSK9 gene in humans on chromosome 1. It binds with the LDL
receptor, which blocks the ingestion of LDL-particles from
extracellular fluid into cells. Following a single intravenous
injection of human D374Y (57) or
murine D377Y (58) gain-of-function
mutant PCSK9, mice stably expressed Pcsk9DY mRNA
in the liver. Compared with control mice, the total serum
cholesterol level in PCSK9DY-AAV transgenic mice was doubled after
30 days to 1 year of the injection (57). The western diet-fed PCSK9DY-AAV mice
showed exacerbated hyperlipidemia with total cholesterol levels up
to 1,165 mg/dl and the formation of lesions throughout the
vasculature. Aortic root lesions showed advanced plaque
development, with the presence of foam cells and smooth muscle
cells in addition to macrophage infiltration and fibrous tissue
formation (57,58). Moreover, lesions progressed to the
fibro-atheromatous stage, and vascular calcification occurred
within 15–20 weeks (59,60).
Other GM mice models
In addition to the aforementioned models, some
emerging GM mice have been used in atherosclerosis research. For
example, the SR-BI−/−/ApoER61(h/h) mice were generated
by Zhang et al in 2005 (61)
and were characterized by the development of diet-induced occlusive
coronary atherosclerosis and myocardial infarction, compensating
for the flaw that atherosclerotic plaques in mice are not prone to
rupture. ApoE* 3-Leiden GM mice carrying the ApoE3-Leiden gene
could develop severe hypercholesterolemia when fed an HFHC diet,
exhibiting a more humanized form of lipoprotein cholesterol
distribution system when crossbred with the human cholesterol ester
transfer protein (CETP)-encoding transgenic mice (62,63).
CETP expression in ApoE3-Leiden mice shifts the distribution of
cholesterol from HDL to VLDL/LDL, which resembles the cholesterol
profile of humans (64).
ApoE−/−Fbn1C1039G+/− mouse is an
ApoE−/− mouse model with a mutation (C1039G+/-) in the
fibrillin-1 (Fbn1) gene, which is characterized by the
formation of vulnerable atherosclerotic plaques that are prone to
rupture (65). Therefore,
ApoE−/−Fbn1C1039G+/− mouse can be used to
study the features of unstable human plaques (66).
As a model of atherosclerosis research, mice have
several advantages; however, they also have some limitations that
cannot be ignored. Firstly, lipid metabolism in mice is very
different from that in humans. The lipids present in the plasma of
mice are mainly HDL, whereas those in humans are mainly LDL and
VLDL. Moreover, natural CETP is absent in mice. Secondly, although
atherosclerotic lesions tend to occur in disturbed blood flow
regions in both humans and mice, the primary sites of lesion in
mice are the aorta and carotids. Thirdly, mouse models rarely show
evidence of lesion rupture, whereas in humans most of the mortality
due to atherosclerosis results from plaque rupture.
Rabbits
Rabbit is the first developed and commonly used
animal model for atherosclerosis research. The first report of
diet-induced atherosclerosis in rabbits was provided by Ignatowski
in 1908 (23). A few years later,
in 1913, Anitschkow and Chalatows fed rabbits with cholesterol
purified from egg and found that rabbits with atherosclerosis
exhibited cholesterol accumulation in their livers (67). This was the first study to propose
the role of cholesterol alone in inducing atherosclerosis.
Subsequently, increasing research suggested that rabbits are an
appropriate model for studying atherosclerosis because they easily
develop atherosclerotic lesions when fed an HFHC diet, can be
easily handled and require relatively inexpensive maintenance.
Moreover, rabbits transport considerable amounts of cholesterol via
ApoB-containing particles (VLDL and LDL) and express CETP, which is
similar to that in humans. When compared with mice, the larger size
of rabbits can provide some advantages such as noninvasive arterial
analysis, providing sufficient arterial tissues and atherosclerotic
lesions for harvest and enabling implantation of stents for
biomechanical or pharmaceutical designing and testing. Currently,
there are three types of rabbit models commonly used in
atherosclerosis research: i) Cholesterol-fed rabbits; ii) GM
rabbits; and iii) Watanabe heritable hyperlipidemic (WHHL)
rabbits.
Cholesterol-fed rabbits
The normal range of plasma cholesterol in rabbits is
30–90 mg/dl at the age of 3–16 months, but it can increase up to
1,000 mg/dl following the administration of 0.3–0.5%
cholesterol-enriched diet. Additionally, supplementing the diet
with 1–1.5% cholesterol for ~8 weeks increases the plasma
cholesterol levels to 1,500-3,000 mg/dl (68). Monocyte adhesion to intimal
endothelial cells and migration of monocytes into the subintima of
the aorta could be observed under a microscope after
high-cholesterol diet treatment. Lesion morphology is determined by
the percentage of cholesterol added to the diet and the duration of
the diet. Aortic lesions could be clearly visualized after feeding
the rabbits with cholesterol diet for ~6 weeks (69). Additionally, coronary
atherosclerosis was observed in cholesterol-fed rabbits but was
usually restricted to the left coronary arterial trunk (69). Depending on the duration of
cholesterol diet treatment, plaque calcification can occur.
However, there is no evidence of spontaneous plaque rupture in
these rabbits.
GM rabbits
GM rabbits have been reported as a model for
studying cardiovascular diseases since 1994. To date, dozens of GM
rabbits have been developed, including ApoE−/− rabbits
and various transgenic rabbit strains (70,71).
Moreover, GM rabbit strains expressing nearly a dozen proteins
involved in atherogenesis have been established in the laboratory,
including those for human ApoAII, human ApoCIII, human CETP,
endothelial lipase, MMP9 and human UII (72–77).
Thus, these models provided insights into the molecular mechanisms
involved in lipoprotein metabolism and function in
atherosclerosis.
WHHL rabbits
The WHHL rabbits were developed by Watanabe
(78), Kobe University, and exhibit
familial hypercholesterolemia due to LDLR deficiency. The most
popular of these studies were those confirming the hypothesis of
the LDLR pathway formulated by Goldstein and Brown and elucidating
the effects of statins on lowering the blood lipid levels (79). Moreover, after selective breeding,
the coronary plaques changed to thin-cap fibroatheromas, and
myocardial infarction developed spontaneously, which was rarely
observed in other animal models (80). Interestingly, high-fructose and
high-fat diet-fed WHHL rabbits developed early insulin resistance
and glucose tolerance and showed aortic lesions with a lipid core
and calcification (81). Therefore,
this model has allowed researchers to investigate the effect of
insulin resistance on atherosclerotic lesion formation. However,
WHHL rabbits are less widely used, owing to the availability of few
suppliers and breeding difficulties.
Practical methods of using mice in
atherosclerosis research
The commonly used mice were obtained from the
Jackson Laboratory and Charles River Laboratories. However, during
research, more complex GM mouse models could provide insights into
the mechanisms of atherosclerosis. A common method is to backcross
a specific KO or transgenic mouse with ApoE−/− or
LDLR−/− mice, thereby obtaining a double-KO/transgenic
mouse model with a condition of hyperlipidemia for studying target
gene function in atherosclerosis. Moreover, PCSK9-AAV injection is
an alternative method for obtaining transgenic mouse models.
With the advancements in research, tissue-specific
GM mice are increasingly used, most of which can be obtained
through Cre-loxP recombination. Cre-loxP recombination is an
approach through which the mice carrying the LoxP-flanked
gene are crossbred with the mice carrying the Cre transgene,
which is driven by a specific promoter to obtain tissue or
cell-specific genetic manipulations. Tissue-specific gene KO mice
can be obtained and then crossed with ApoE−/− or
LDLR−/− mice for atherosclerosis research (25).
Furthermore, choosing an appropriate diet for animal
models is an important part, as it induces atherosclerosis. There
is no doubt that western diet is most commonly used in
atherosclerosis research since the 1990s. In general, a western
diet containing 21% milk fat and 0.15% or 0.2% cholesterol is
recommended (52), which
approximately mimics the daily diet in western countries. Various
studies have shown that there is a positive association between
total plasma cholesterol levels and the extent of aortic lesion
formed upon consuming different diets (32,82,83).
The cholesterol in this diet majorly consists of a proatherogenic
agent. According to a previous research, the cholesterol content in
all diet fed to mouse models of atherosclerosis was estimated to be
1.25% at most, owing to the toxicity of high doses of cholesterol
feed (52).
For general atherosclerotic models, HFHC diet is
provided for 12–16 weeks; however, a sustained HFHC diet is likely
to aggravate atherosclerotic lesion formation. The concentration of
lipids present in blood, including total cholesterol,
triglycerides, HDL-cholesterol and LDL-cholesterol, should be
measured every 2 or 4 weeks while feeding an HFHC diet (84).
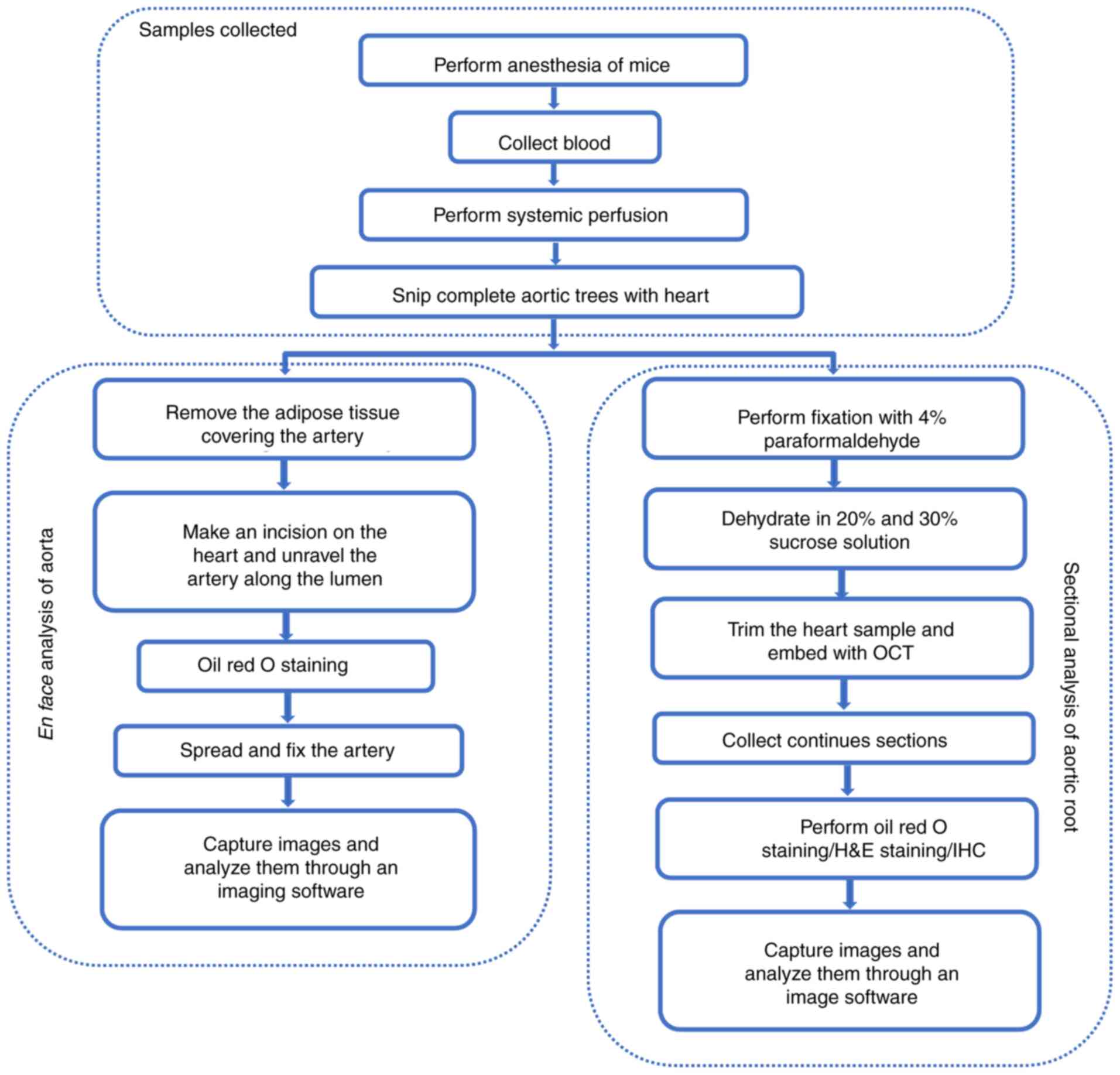
Fig. 2 elucidates
the methods used for quantitative analysis of the pathological
atherosclerotic lesions formed in mice: i) Aortic root
cross-section; and ii) en face analysis of the aorta,
‘aortic tree,’ including whole aorta, aortic arch, thoracic artery,
abdominal artery and left/right common carotid artery. Aortic roots
are the most common region for the quantification of
atherosclerotic lesions, as the lesions are stably observed in
these areas. The lesions in the aortic roots vary with the location
and size of plaques; therefore, it is necessary to cut continuous
sections throughout the aortic root. The detailed procedure has
been provided by Daugherty et al, Lin et al and Centa
et al (24,84,85).
Briefly, the procedure for obtaining frozen sections of the
embedded heart containing the aortic root and observing tissue
staining is simple. In particular, 7-µm thick sections are used,
and the complete aortic root is collected through creating 60
sections for each sample. Ten consecutive sections were
sequentially distributed in the same position on 10 numbered
slides, starting from the appearance of a complete tricuspid valve,
until each slide had six tissue sections. Slides with the same
number were used for statistical analysis of plaque area and plaque
composition. The thinner the section, the easier it is to
accurately observe components in the plaque, since the components
are spatially distributed in the plaque, but through
immunostaining, the distribution of the target component could be
observed in a plane. The sections were then stained with oil red O
stain to assess the severity of atherosclerosis. The remaining
frozen sections of aortic roots can be used to analyze the
distribution and proportion of cells, such as smooth muscle cells
and macrophages in plaques. The staining results of these sections
were reported in previous studies (84,86–88).
The other commonly used method is to determine the size of the
lesions in the aortic tree. This approach was introduced after the
advent of GM animals, because the lesions in early atherosclerosis
mice model were found only in the aortic roots. Therefore, this
assessment method is relatively convenient. The aortic tree of mice
was completely isolated, and the vessel wall was unraveled along
the lumen. After fixation, oil red O staining, and imaging, the
area of atherosclerotic plaque in the entire aortic tree was
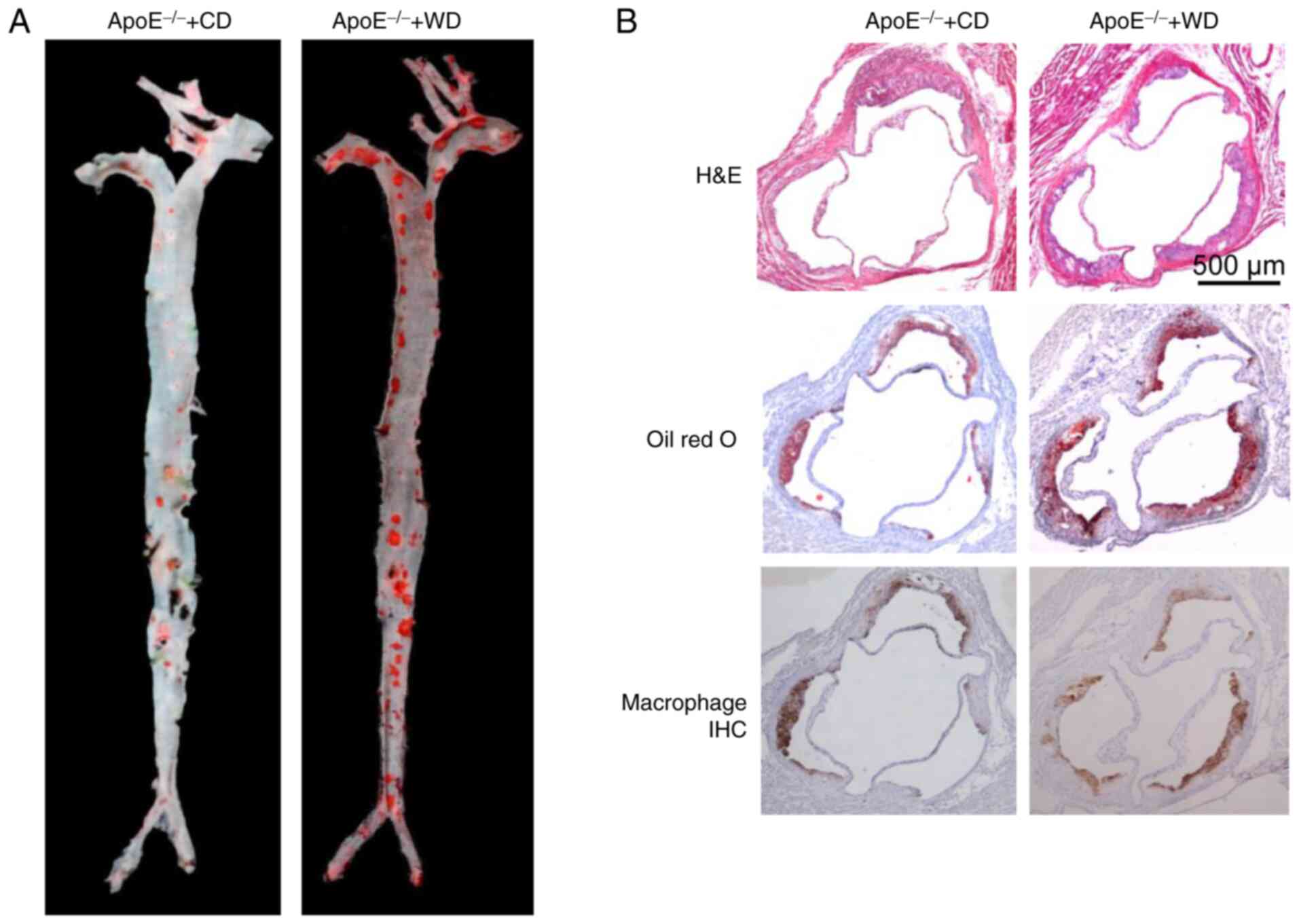
estimated using an image processing software. Representative images
of aortic tree lesions and aortic root lesions are shown in
Fig. 3A and B.
Practical methods of using rabbits in
atherosclerosis research
Most of the rabbits used in atherosclerosis research
were outbred strains. Therefore, it is necessary to screen rabbits
before the formal experiment to exclude rabbits that are
insensitive and extremely sensitive to HFHC diets. Male rabbits are
often selected for atherosclerosis research. The cholesterol
content in HFHC diet-fed mice is generally <1.0% to decrease the
liver damage caused by cholesterol. In the following section, the
method that was used to develop a rabbit model of atherosclerosis
in the laboratory will be elucidated (70,72,89,90).
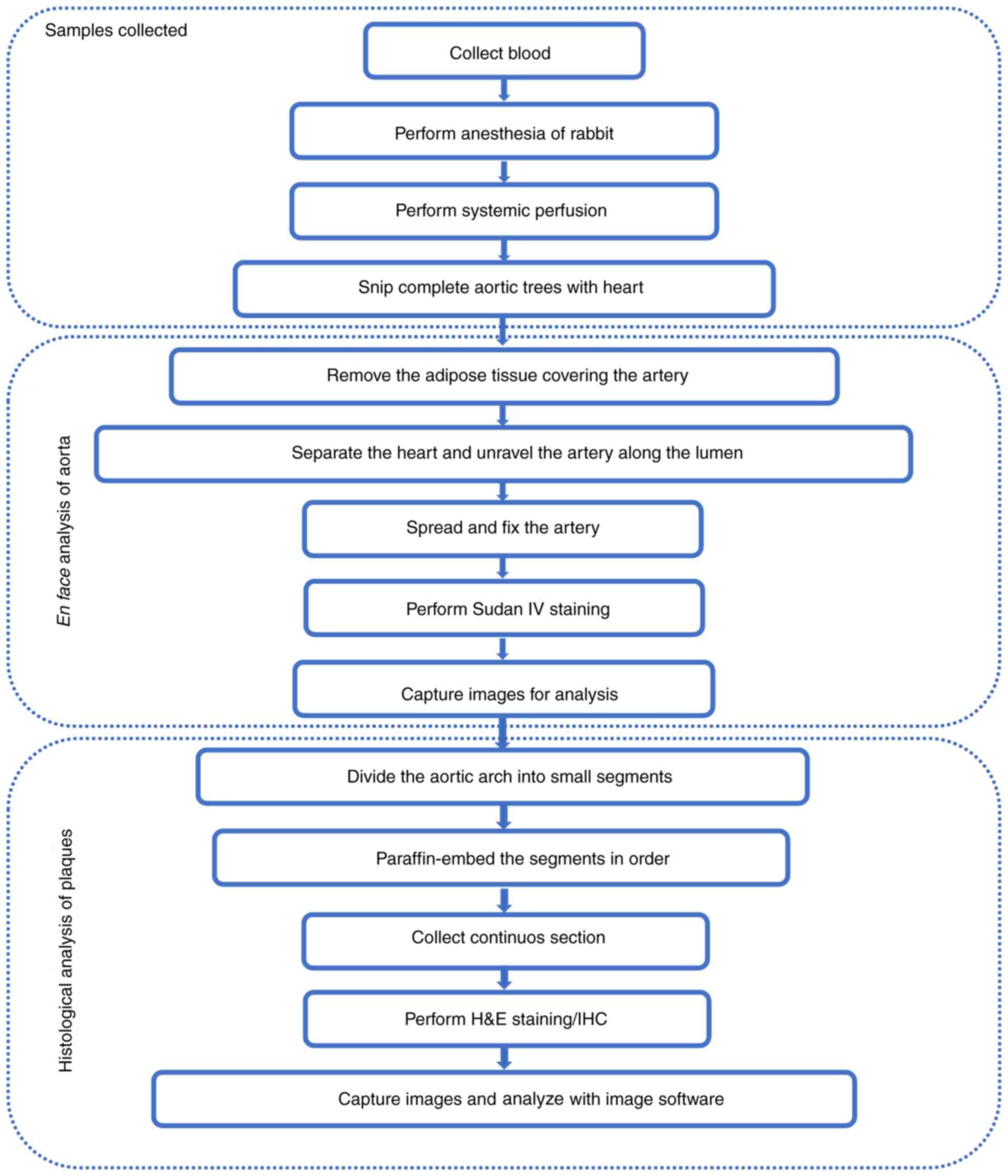
In the laboratory, 4-month-old male rabbits were fed
a diet containing 0.3% cholesterol, for 16 weeks (for aorta lesion)
or 28 weeks (for coronary lesion). During HFHC feeding, blood lipid
levels were measured every 2 weeks. Similar to that in mice, the
distribution of lesions in rabbit aorta is an important indicator
for assessing whether diets have an impact on atherosclerosis. The
practical operation process for rabbit aortic tree separation and
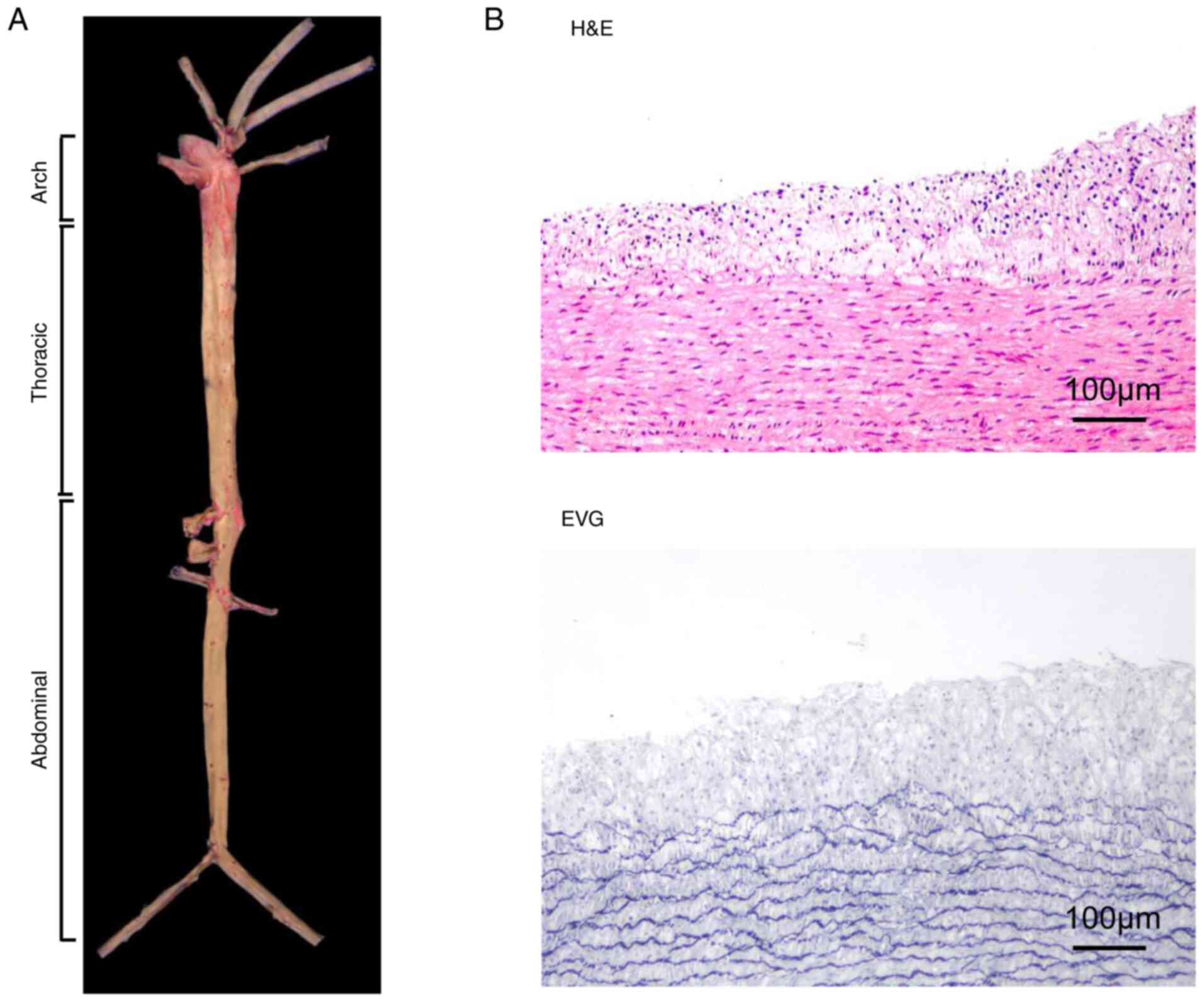
atherosclerotic lesion analysis is shown in Fig. 4. Briefly, after the rabbits were
euthanized and the organs (except the heart and kidney) were
dissected, the entire aorta was separated from the heart to the
iliac bifurcation, and the adipose tissues covering the aorta were
removed. Thereafter, the intimal surface of the artery was exposed
by making a longitudinal cut. After fixation, Sudan IV staining,
and imaging, atherosclerotic lesions were analyzed using an imaging
software. Representative images of atherosclerotic lesions in the
aorta of rabbits on a normal or HFHC diet are shown in Fig. 5A. For histological examination, the
entire aortic arch was serially sectioned at 1–2 mm intervals.
Representative samples from these sections were processed routinely
and embedded longitudinally in paraffin. The sections (4 µm) were
then stained with hematoxylin-eosin (H&E) and elastic van
Gieson (Fig. 5B) or
immunohistochemistry.
Noninvasive imaging of animal model in
atherosclerosis
More recently, researchers have paid more attention
to the study of plaque composition and vulnerability, rather than
the severity of stenosis of atherosclerotic plaque (91). Benefit from the development of
imaging technology, multiple non-invasive systems have been
developed to detect morphology and component of atherosclerosis
lesions, including ultrasound, computed tomography (CT), magnetic
resonance imaging (MRI), positron emission computed tomography
(PET). Ultrasound is a relatively inexpensive, radiation-free test
that can be used to determine the location of lesions and the
thickness of blood vessel walls. Ultrasound has been reported to
detect atherosclerosis in mice and rabbits (92–94).
CT can provide quantitative assessment of the extent of vascular
calcification (95). MRI and PET
were the modalities used to perform molecular imaging. MRI can
provide high-resolution vascular morphology images that can
distinguish the lipid-rich necrotic core, fibrous cap,
calcification and intraplaque hemorrhage (96,97).
Targeted specific MRI contrast agents can be used to detect
components in plaques, but they are insensitive. PET is a
quantitative nuclear imaging technique that allows the
visualization of radioisotopes and is mostly applied to investigate
tissue metabolic and physiological state at the molecular level
with high sensitivity, but PET cannot be used to detect plaque
morphology (98). Imaging of PET
must be combined with CT imaging (PET/CT) or magnetic resonance
imaging (PET/MRI) to localize the pathophysiological processes to
an anatomical location. 18F-fluorodeoxyglucose (FDG) is the most
common radioligand used in imaging studies of atherosclerosis
(98). Hybrid PET/CT or PET/MRI has
been reported to use in mice and rabbit atherosclerosis models to
identify macrophages or chemokines in plaques (99–102).
Non-invasive imaging has a great application prospect in
atherosclerosis. Previous research revealed that vulnerable plaques
were characterized by increased content of macrophages and
angiogenesis. In the future, molecular imaging techniques have a
high potential to shed light on specific molecular/cellular
processes and biomarkers of vulnerable plaques. Preclinical studies
of medication and contrast agent need validation in animal models,
thus, it is very valuable to develop applicable non-invasive
imaging systems for small animals.
In vitro study of atherosclerosis
Cell cultures are essential for studying the
specific molecular mechanisms of diseases. The cell types that are
usually employed in atherosclerosis research include endothelial
cells, macrophages and smooth muscle cells. Atherosclerosis begins
with endothelial dysfunction, which causes endothelial cells to
release adhesion factors and chemokines (2) that recruit macrophages to accumulate
in the intima. Macrophages induce the release of growth factors and
chemokines to promote smooth muscle cell proliferation and
migration (5,7). Simultaneously, macrophages swallowing
lipids lead to foam formation (103).
In the following section, the methods of primary
cell culture and some applications are mentioned.
Endothelial cell
The endothelium acts as the first barrier to
vascular protection and has multiple important physiological
functions (104). Endothelial
injury and dysfunction are considered initial events in the
development of atherosclerotic lesions (2).
Human umbilical vein endothelial cells (HUVECs) are
the most widely used model in endothelial cell-based studies. This
wide application is due to the easy access to fetal umbilical cord,
convenient cell extraction operation, as the acquisition of
abundant endothelial cells is the guarantee for the establishment
of cell models in vitro. In addition, HUVECs have
representative physiological and pathological characteristics of
adult endothelial cells. This model has been employed in the study
of endothelial cell function and in elucidating the role of
endothelium in the blood vessel wall response towards stretching,
shear forces and reactive oxygen species generation (105–107). Since HUVECs are derived from the
fetus, differences with adult endothelial cells should be
considered. It was reported that HUVECs were unable to express ABO
blood group antigens, which may significantly affect their surface
function (108). It has also been
reported that the sex of the fetus may affect the physiological
function of endothelial cells (105,109).
Several protocols for HUVEC isolation have been
established (107,110–112). In short, the first step involved
rinsing the umbilical cord (~10–30 cm) with phosphate-buffered
saline (PBS) in a sterile environment. Thereafter, one end of the
umbilical cord was closed with hemostatic forceps, and 0.1 or 0.2%
collagenase solution was poured from the other end. After the
completion of perfusion, both ends of the umbilical cord were
closed and incubated at 37°C for 10–20 min. After the completion of
digestion, culture medium containing fetal bovine serum (FBS) was
injected into the umbilical cord to terminate the digestion
process. The endothelium was further eluted with 30 ml PBS, and the
collected cells were centrifuged and counted. Finally, the cells
were cultured in M199 complete medium supplemented with FBS and
penicillin-streptomycin and incubated at 37°C in a 5%
CO2 atmosphere. The most commonly used method for HUVEC
identification is the immunofluorescence staining of von Willebrand
factor, VIII factor, and CD31 (113).
Macrophage
Macrophages play crucial roles at all stages of
atherosclerosis, from initiation of lesion formation and expansion
to necrosis leading to rupture, the clinical manifestations of
atherosclerosis, and resolution and regression of atherosclerotic
lesions (114). Macrophages that
phagocytose oxidized LDL are the main source of foam cells, which
are the main components of atherosclerotic plaques (115). During the activation of
macrophages, more growth factors and chemokines are released,
leading to platelet aggregation at the site of injury, while
promoting smooth muscle cell proliferation and migration (116).
Mouse peritoneal macrophages, bone marrow-derived
macrophages, mouse mononuclear macrophage cell lines (RAW264.7),
and human monocyte cell lines (THP-1) are commonly used in
macrophage-based studies.
The procedure for isolating murine macrophages has
been reported (86,117). For PM isolation, mice were
injected with 1.0 ml 3% sodium thioglycolate for 3 days before
isolating macrophages. Peritoneal lavage was performed with 5–8 ml
sterile PBS or RPMI-1640 medium. After centrifugation and washing
with PBS, the cells were resuspended in culture medium. Finally,
the cells were added to a culture plate in order to allow the
macrophages to adhere to the wall, and the medium was changed after
2 h to obtain macrophages with high purity. For BMDM isolation from
mice, bone marrow cells were harvested and cultured in medium
supplemented with macrophage colony-stimulating factor. After 7
days of culturing, contaminating non-adherent cells were eliminated
and adherent cells were harvested for further assays. Macrophages
were then identified via immunostaining using F4/80 antibodies
(118).
Smooth muscle cell
Smooth muscle cells constitute the media layer of
the arteries. In atherosclerotic lesions, smooth muscle cells
proliferate and migrate to the intima upon inflammation, and
collagen fibers are secreted to form plaque fibrous caps (7). Therefore, the number and function of
smooth muscle cells often affect the stability of atherosclerotic
plaques. In an atherogenic environment, smooth muscle cells present
in the lesion phagocytose the modified lipoprotein to form smooth
muscle-derived foam cells, which then secrete inflammatory factors,
thereby aggravating the inflammation of the lesion (119).
Rat and mouse primary smooth muscle cells are
commonly used in atherosclerosis research, including cell
proliferation, migration, calcification and phenotypic
transformation studies.
The commonly used method for the isolation of rat
smooth muscle cells is tissue transplantation (120). The procedure of tissue
transplantation included the following steps: Isolating the aortic
artery; separating the media from it; and cutting the aortic artery
into small pieces, followed by incubation in complete medium until
the cells reach confluence. The rats were euthanized by anesthesia,
and their thoracic aorta was isolated. The aorta was cut in
Dulbecco's modified Eagle's medium (high glucose) supplemented with
20% FBS, and the inner wall of the blood vessel was slightly
scraped to destroy the endothelium. After carefully removing the
outer membrane of the blood vessel, the medium layer was cut into
small pieces of 2–4 mm2 and spread evenly on the bottom
of a cell culture flask. After 4 h, the tissue block was gently
attached, and the flask was slowly inverted to cover the tissue.
Thereafter, the culture medium was changed every 3 days, and after
~1 week, the culture flask was examined under a microscope. The
smooth muscle cells were observed to grow out of the tissue block
and cover the surrounding bottle wall. The cells were passaged once
they reached a confluence of ~70–80%. Smooth muscle cells were then
identified via immunostaining with an anti-α-actin antibody.
The primary cells, cell lines, and the corresponding
experiments commonly used for atherosclerosis research are shown in
Table II.
 | Table II.Cell types commonly used in
atherosclerosis research. |
Table II.
Cell types commonly used in
atherosclerosis research.
| Cell type | Commonly used
primary cells | Commonly used cell
lines | Application |
|---|
| Endothelial
cell | Human umbilical
vein endothelial cells; human microvascular endothelial cells |
| Endothelial cell
dysfunction; inflammatory response |
| Macrophage | Peritoneal
macrophages Bone marrow-derived macrophages | THP1, J774a.1 and
U937 | Phagocytosis;
proliferation; migration; adhesion; polarization |
| Smooth muscle
cell | Vascular smooth
muscle cells from rat; vascular smooth muscle cells from mice | A7r5 and
MOVAS-1 | Calcification;
phenotype transformation; proliferation; migration |
Conclusion
The generation of ApoE−/− and
LDLR−/− mice was a milestone in atherosclerosis
research, because of their ease of gene manipulation, which
expanded the scope of research into atherosclerosis and provided
elaborate insights into molecular mechanisms, especially in lipid
metabolism and inflammatory pathways. HFHC diet-induced rabbit
models are widely used, and WHHL rabbits showing symptoms of plaque
rupture and myocardial infarction are excellent animal models for
mimicking human atherosclerosis. Other animals used for
atherosclerosis research include pigs, non-human primates, rats,
dogs, and quails; however, they are not widely used owing to high
costs, ethical issues, slow modeling, and genetic background. As
different animal models have their own advantages and limitations,
suitable animals need to be chosen according to the purpose of the
study. Mice are most commonly used to elucidate molecular
mechanisms, because they have a clear genetic background and are
easy to genetically modify. For drug development studies, mice and
rabbits are chosen because of their small size and requirement of
decreasing drug dosage. In general, dogs are commonly used to
simulate clinical surgeries because of the ease to operate given
their large size. In brief, animal models of each species can only
mimic some characteristics of human atherosclerosis. Therefore, a
scientific problem can be verified through performing research at
different levels (in vivo vs. in vitro) and in animal
models of different species. With the development of gene editing
technology, especially with the emergence of the clustered
regularly interspaced short palindromic repeats (CRISPR)-associated
protein 9 system (CRISPR/Cas9) and somatic cell nuclear transfer,
more laboratory animal strains may be used as study models of
atherosclerosis in the future. For instance, ApoE−/−
pigs and ApoE−/− dogs were produced using the
CRISPR/Cas9 system in 2018 (121,122). Further research on atherosclerosis
still relies on animal models; however, bridging the gap between
basic research and clinical applications is an important issue for
future researchers to consider, which can be achieved by developing
a suitable animal model.
Acknowledgements
Not applicable.
Funding
This work was supported by the Science Plan Project
of Shaanxi Province (grant nos. 2020PT-001 and 2019JQ-599).
Availability of data and materials
Not applicable.
Authors' contributions
YZ designed the review, prepared the tables and
figures, and wrote the manuscript. MF and SH searched the
literature and wrote the manuscript. LB and SZ provided helpful
comments and acquired data. EL conceived this review and revised
the manuscript. All authors read and approved the final manuscript.
Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
LDL
|
low-density lipoprotein
|
|
HFHC
|
high-fat/high-cholesterol
|
|
GM
|
genetically modified
|
|
KO
|
knockout
|
|
ApoE
|
apolipoprotein E
|
|
LDLR
|
low-density lipoprotein receptor
|
|
PCSK9
|
proprotein convertase
subtilisin/kexin type 9
|
|
AAV
|
adeno-associated virus
|
|
ES
|
embryonic stem
|
|
VLDL
|
very low-density lipoprotein
|
|
HDL
|
high-density lipoprotein
|
|
CETP
|
cholesterol ester transfer
protein
|
|
Fbn1
|
fibrillin-1
|
|
WHHL
|
Watanabe heritable hyperlipidemic
|
|
H&E
|
hematoxylin-eosin
|
|
CT
|
computed tomography
|
|
MRI
|
magnetic resonance imaging
|
|
PET
|
positron emission computed
tomography
|
|
HUVECs
|
Human umbilical vein endothelial
cells
|
|
CRISPR/Cas9
|
clustered regularly interspaced short
palindromic repeats-associated protein 9
|
References
|
1
|
Libby P, Ridker PM and Hansson GK:
Progress and challenges in translating the biology of
atherosclerosis. Nature. 473:317–325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gimbrone MA Jr and Garcia-Cardena G:
Endothelial cell dysfunction and the pathobiology of
atherosclerosis. Circ Res. 118:620–636. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sena CM, Leandro A, Azul L, Seica R and
Perry G: Vascular oxidative stress: Impact and therapeutic
approaches. Front Physiol. 9:16682018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chistiakov DA, Orekhov AN and Bobryshev
YV: Effects of shear stress on endothelial cells: Go with the flow.
Acta Physiol (Oxf). 219:382–408. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moore KJ, Sheedy FJ and Fisher EA:
Macrophages in atherosclerosis: A dynamic balance. Nat Rev Immunol.
13:709–721. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chistiakov DA, Melnichenko AA, Myasoedova
VA, Grechko AV and Orekhov AN: Mechanisms of foam cell formation in
atherosclerosis. J Mol Med (Berl). 95:1153–1165. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bennett MR, Sinha S and Owens GK: Vascular
smooth muscle cells in atherosclerosis. Circ Res. 118:692–702.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Davignon J and Ganz P: Role of endothelial
dysfunction in atherosclerosis. Circulation. 109 (23 Suppl
1):III27–III32. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Incalza MA, D'Oria R, Natalicchio A,
Perrini S, Laviola L and Giorgino F: Oxidative stress and reactive
oxygen species in endothelial dysfunction associated with
cardiovascular and metabolic diseases. Vascul Pharmacol. 100:1–19.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hajjar DP and Gotto AM Jr: Biological
relevance of inflammation and oxidative stress in the pathogenesis
of arterial diseases. Am J Pathol. 182:1474–1481. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rhoads JP and Major AS: How oxidized
low-density lipoprotein activates inflammatory responses. Crit Rev
Immunol. 38:333–342. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang T, Chen J, Tang X, Luo Q, Xu D and
Yu B: Interaction between adipocytes and high-density lipoprotein:
New insights into the mechanism of obesity-induced dyslipidemia and
atherosclerosis. Lipids Health Dis. 18:2232019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ference BA, Ginsberg HN, Graham I, Ray KK,
Packard CJ, Bruckert E, Hegele RA, Krauss RM, Raal FJ, Schunkert H,
et al: Low-density lipoproteins cause atherosclerotic
cardiovascular disease. 1. Evidence from genetic, epidemiologic,
and clinical studies. A consensus statement from the European
atherosclerosis society consensus panel. Eur Heart J. 38:2459–2472.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Messner B and Bernhard D: Smoking and
cardiovascular disease: Mechanisms of endothelial dysfunction and
early atherogenesis. Arterioscler Thromb Vasc Biol. 34:509–515.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Altman R: Risk factors in coronary
atherosclerosis athero-inflammation: The meeting point. Thromb J.
1:42003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hollander W: Role of hypertension in
atherosclerosis and cardiovascular disease. Am J Cardiol.
38:786–800. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Katakami N: Mechanism of development of
atherosclerosis and cardiovascular disease in diabetes mellitus. J
Atheroscler Thromb. 25:27–39. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Virani SS, Alonso A, Benjamin EJ,
Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR,
Cheng S, Delling FN, et al: Heart disease and stroke
statistics-2020 update: A report from the american heart
association. Circulation. 141:e139–e596. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lenfant C and Savage PJ: The early natural
history of atherosclerosis and hypertension in the young: National
institutes of health perspectives. Am J Med Sci. 310 (Suppl
1):S3–S7. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McNamara JJ, Molot MA, Stremple JF and
Cutting RT: Coronary artery disease in combat casualties in
Vietnam. JAMA. 216:1185–1187. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Strong JP, Mcgill HC Jr, Tejada C and
Holman RL: The natural history of atherosclerosis; comparison of
the early aortic lesions in New Orleans, Guatemala, and Costa Rica.
Am J Pathol. 34:731–744. 1958.PubMed/NCBI
|
|
22
|
Enos WF, Holmes RH and Beyer J: Coronary
disease among United States soldiers killed in action in Korea;
preliminary report. J Am Med Assoc. 152:1090–1093. 1953. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Konstantinov IE and Jankovic GM: Alexander
I. Ignatowski: A pioneer in the study of atherosclerosis. Tex Heart
Inst J. 40:246–249. 2013.PubMed/NCBI
|
|
24
|
Daugherty A, Tall AR, Daemen M, Falk E,
Fisher EA, García-Cardeña G, Lusis AJ, Owens AP III, Rosenfeld ME,
Virmani R, et al: Recommendation on design, execution, and
reporting of animal atherosclerosis studies: A scientific statement
from the american heart association. Arterioscler Thromb Vasc Biol.
37:e131–e157. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu C, Daugherty A and Lu HS: Updates on
approaches for studying atherosclerosis. Arterioscler Thromb Vasc
Biol. 39:e108–e117. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fan J, Chen Y, Yan H, Niimi M, Wang Y and
Liang J: Principles and applications of rabbit models for
atherosclerosis research. J Atheroscler Thromb. 25:213–220. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vesselinovitch D, Wissler RW and Doull J:
Experimental production of atherosclerosis in mice. 1. Effect of
various synthetic diets and radiation on survival time, food
consumption and body weight in mice. J Atheroscler Res. 8:483–495.
1968. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vesselinovitch D and Wissler RW:
Experimental production of atherosclerosis in mice. 2. Effects of
atherogenic and high-fat diets on vascular changes in chronically
and acutely irradiated mice. J Atheroscler Res. 8:497–523. 1968.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Thompson JS: Atheromata in an inbred
strain of mice. J Atheroscler Res. 10:113–122. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Paigen B, Morrow A, Brandon C, Mitchell D
and Holmes P: Variation in susceptibility to atherosclerosis among
inbred strains of mice. Atherosclerosis. 57:65–73. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ishibashi S, Brown MS, Goldstein JL,
Gerard RD, Hammer RE and Herz J: Hypercholesterolemia in low
density lipoprotein receptor knockout mice and its reversal by
adenovirus-mediated gene delivery. J Clin Invest. 92:883–893. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Plump AS, Smith JD, Hayek T, Aalto-Setälä
K, Walsh A, Verstuyft JG, Rubin EM and Breslow JL: Severe
hypercholesterolemia and atherosclerosis in apolipoprotein
E-deficient mice created by homologous recombination in ES cells.
Cell. 71:343–353. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Piedrahita JA, Zhang SH, Hagaman JR,
Oliver PM and Maeda N: Generation of mice carrying a mutant
apolipoprotein E gene inactivated by gene targeting in embryonic
stem cells. Proc Natl Acad Sci USA. 89:4471–4475. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Olszanecki R and Korbut R: The effect of
montelukast on atherogenesis in apoE/LDLR-double knockout mice. J
Physiol Pharmacol. 59:633–639. 2008.PubMed/NCBI
|
|
35
|
Olszanecki R, Jawien J, Gajda M, Mateuszuk
L, Gebska A, Korabiowska M, Chłopicki S and Korbut R: Effect of
curcumin on atherosclerosis in apoE-LDLR-double knockout mice. J
Physiol Pharmacol. 4:627–635. 2005.PubMed/NCBI
|
|
36
|
Schilperoort M, van den Berg R, Bosmans
LA, van Os BW, Dollé ME, Smits NA, Guichelaar T, van Baarle D,
Koemans L, Berbée JF, et al: Disruption of circadian rhythm by
alternating light-dark cycles aggravates atherosclerosis
development in APOE* 3-leiden. CETP mice. J Pineal Res.
68:e126142020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Berbée JF, Wong MC, Wang Y, van der Hoorn
JW, Khedoe PP, van Klinken JB, Mol IM, Hiemstra PS, Tsikas D,
Romijn JA, et al: Resveratrol protects against atherosclerosis, but
does not add to the antiatherogenic effect of atorvastatin, in
APOE* 3-leiden. CETP mice. J Nutr Biochem. 24:1423–1430. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
de Haan W, van der Hoogt CC, Westerterp M,
Hoekstra M, Dallinga-Thie GM, Princen HM, Romijn JA, Jukema JW,
Havekes LM and Rensen PC: Atorvastatin increases HDL cholesterol by
reducing CETP expression in cholesterol-fed APOE* 3-leiden. CETP
mice. Atherosclerosis. 197:57–63. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Stein EA and Raal F: Reduction of
low-density lipoprotein cholesterol by monoclonal antibody
inhibition of PCSK9. Annu Rev Med. 65:417–431. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Getz GS and Reardon CA: Apoprotein E as a
lipid transport and signaling protein in the blood, liver, and
artery wall. J Lipid Res (50 Suppl). S156–S161. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sehayek E, Shefer S, Nguyen LB, Ono JG,
Merkel M and Breslow JL: Apolipoprotein E regulates dietary
cholesterol absorption and biliary cholesterol excretion: studies
in C57BL/6 apolipoprotein E knockout mice. Proc Natl Acad Sci USA.
97:3433–3437. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Plump AS and Breslow JL: Apolipoprotein E
and the apolipoprotein E-deficient mouse. Annu Rev Nutr.
15:495–518. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nakashima Y, Plump AS, Raines EW, Breslow
JL and Ross R: ApoE-deficient mice develop lesions of all phases of
atherosclerosis throughout the arterial tree. Arterioscler Thromb.
14:133–140. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rattazzi M, Bennett BJ, Bea F, Kirk EA,
Ricks JL, Speer M, Schwartz SM, Giachelli CM and Rosenfeld ME:
Calcification of advanced atherosclerotic lesions in the innominate
arteries of ApoE-deficient mice: Potential role of chondrocyte-like
cells. Arterioscler Thromb Vasc Biol. 25:1420–1425. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Meir KS and Leitersdorf E: Atherosclerosis
in the apolipoprotein-E-deficient mouse: a decade of progress.
Arterioscler Thromb Vasc Biol. 24:1006–1014. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Oppi S, Luscher TF and Stein S: Mouse
models for atherosclerosis research-which is my line? Front
Cardiovasc Med. 6:462019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
von Scheidt M, Zhao Y, Kurt Z, Pan C, Zeng
L, Yang X, Schunkert H and Lusis AJ: Applications and limitations
of mouse models for understanding human atherosclerosis. Cell
Metab. 25:248–261. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Moore KJ, Kunjathoor VV, Koehn SL, Manning
JJ, Tseng AA, Silver JM, McKee M and Freeman MW: Loss of
receptor-mediated lipid uptake via scavenger receptor A or CD36
pathways does not ameliorate atherosclerosis in hyperlipidemic
mice. J Clin Invest. 115:2192–2201. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Go GW and Mani A: Low-density lipoprotein
receptor (LDLR) family orchestrates cholesterol homeostasis. Yale J
Biol Med. 85:19–28. 2012.PubMed/NCBI
|
|
50
|
Ishibashi S, Goldstein JL, Brown MS, Herz
J and Burns DK: Massive xanthomatosis and atherosclerosis in
cholesterol-fed low density lipoprotein receptor-negative mice. J
Clin Invest. 93:1885–1893. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Moore RE, Kawashiri MA, Kitajima K,
Secreto A, Millar JS, Pratico D and Rader DJ: Apolipoprotein A-I
deficiency results in markedly increased atherosclerosis in mice
lacking the LDL receptor. Arterioscler Thromb Vasc Biol.
23:1914–1920. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Getz GS and Reardon CA: Diet and murine
atherosclerosis. Arterioscler Thromb Vasc Biol. 26:242–249. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Boisvert WA, Spangenberg J and Curtiss LK:
Role of leukocyte-specific LDL receptors on plasma lipoprotein
cholesterol and atherosclerosis in mice. Arterioscler Thromb Vasc
Biol. 17:340–347. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Herijgers N, Van Eck M, Groot PH,
Hoogerbrugge PM and Van Berkel TJ: Effect of bone marrow
transplantation on lipoprotein metabolism and atherosclerosis in
LDL receptor-knockout mice. Arterioscler Thromb Vasc Biol.
17:1995–2003. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Linton MF, Atkinson JB and Fazio S:
Prevention of atherosclerosis in apolipoprotein E-deficient mice by
bone marrow transplantation. Science. 267:1034–1037. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Boisvert WA, Spangenberg J and Curtiss LK:
Treatment of severe hypercholesterolemia in apolipoprotein
E-deficient mice by bone marrow transplantation. J Clin Invest.
96:1118–1124. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Roche-Molina M, Sanz-Rosa D, Cruz FM,
García-Prieto J, López S, Abia R, Muriana FJ, Fuster V, Ibáñez B
and Bernal JA: Induction of sustained hypercholesterolemia by
single adeno-associated virus-mediated gene transfer of mutant
hPCSK9. Arterioscler Thromb Vasc Biol. 35:50–59. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bjorklund MM, Hollensen AK, Hagensen MK,
Dagnaes-Hansen F, Christoffersen C, Mikkelsen JG and Bentzon JF:
Induction of atherosclerosis in mice and hamsters without germline
genetic engineering. Circ Res. 114:1684–1689. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Goettsch C, Hutcheson JD, Hagita S, Rogers
MA, Creager MD, Pham T, Choi J, Mlynarchik AK, Pieper B, Kjolby M,
et al: A single injection of gain-of-function mutant PCSK9
adeno-associated virus vector induces cardiovascular calcification
in mice with no genetic modification. Atherosclerosis. 251:109–118.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Veseli BE, Perrotta P, De Meyer GRA, Roth
L, der Donckt CV, Martinet W and De Meyer GR: Animal models of
atherosclerosis. Eur J Pharmacol. 816:3–13. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhang S, Picard MH, Vasile E, Zhu Y,
Raffai RL, Weisgraber KH and Krieger M: Diet-induced occlusive
coronary atherosclerosis, myocardial infarction, cardiac
dysfunction, and premature death in scavenger receptor class B type
I-deficient, hypomorphic apolipoprotein ER61 mice. Circulation.
111:3457–3464. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Westerterp M, van der Hoogt CC, de Haan W,
Offerman EH, Dallinga-Thie GM, Jukema JW, Havekes LM and Rensen PC:
Cholesteryl ester transfer protein decreases high-density
lipoprotein and severely aggravates atherosclerosis in
APOE*3-leiden mice. Arterioscler Thromb Vasc Biol. 26:2552–2559.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
van den Maagdenberg AM, Hofker MH,
Krimpenfort PJ, de Bruijn I, van Vlijmen B, van der Boom H, Havekes
LM and Frants RR: Transgenic mice carrying the apolipoprotein
E3-Leiden gene exhibit hyperlipoproteinemia. J Biol Chem.
268:10540–10545. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Berbee JF, Boon MR, Khedoe PP, Bartelt A,
Schlein C, Worthmann A, Kooijman S, Hoeke G, Mol IM, John C, et al:
Brown fat activation reduces hypercholesterolaemia and protects
from atherosclerosis development. Nat Commun. 6:63562015.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Van der Donckt C, Van Herck JL, Schrijvers
DM, Vanhoutte G, Verhoye M, Blockx I, Van Der Linden A, Bauters D,
Lijnen HR, Sluimer JC, et al: Elastin fragmentation in
atherosclerotic mice leads to intraplaque neovascularization,
plaque rupture, myocardial infarction, stroke, and sudden death.
Eur Heart J. 36:1049–1058. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Roth L, Rombouts M, Schrijvers DM, Lemmens
K, De Keulenaer GW, Martinet W and De Meyer GR: Chronic
intermittent mental stress promotes atherosclerotic plaque
vulnerability, myocardial infarction and sudden death in mice.
Atherosclerosis. 242:288–294. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Steinberg D: In celebration of the 100th
anniversary of the lipid hypothesis of atherosclerosis. J Lipid
Res. 54:2946–2949. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Fan J and Watanabe T: Cholesterol-fed and
transgenic rabbit models for the study of atherosclerosis. J
Atheroscler Thromb. 7:26–32. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Fan J, Kitajima S, Watanabe T, Xu J, Zhang
J, Liu E and Chen YE: Rabbit models for the study of human
atherosclerosis: From pathophysiological mechanisms to
translational medicine. Pharmacol Ther. 146:104–119. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Niimi M, Yang D, Kitajima S, Ning B, Wang
C, Li S, Liu E, Zhang J, Chen YE and Fan J: ApoE knockout rabbits:
A novel model for the study of human hyperlipidemia.
Atherosclerosis. 245:187–193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Matsuhisa F, Kitajima S, Nishijima K,
Akiyoshi T, Morimoto M and Fan J: Transgenic rabbit models: Now and
the future. Applied Sciences. 10:74162020. View Article : Google Scholar
|
|
72
|
Yu QQ, Cheng DX, Xu LR, Li YK, Zheng XY,
Liu Y, Li YF, Liu HL, Bai L, Wang R, et al: Urotensin II and
urantide exert opposite effects on the cellular components of
atherosclerotic plaque in hypercholesterolemic rabbits. Acta
Pharmacol Sin. 41:546–553. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chen Y, Waqar AB, Nishijima K, Ning B,
Kitajima S, Matsuhisa F, Chen L, Liu E, Koike T, Yu Y, et al:
Macrophage-derived MMP-9 enhances the progression of
atherosclerotic lesions and vascular calcification in transgenic
rabbits. J Cell Mol Med. 24:4261–4274. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Gao S, Wang X, Cheng D, Li J, Li L, Ran L,
Zhao S, Fan J and Liu E: Overexpression of cholesteryl ester
transfer protein increases macrophage-derived foam cell
accumulation in atherosclerotic lesions of transgenic rabbits.
Mediators Inflamm. 2017:38242762017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ding Y, Wang Y, Zhu H, Fan J, Yu L, Liu G
and Liu E: Hypertriglyceridemia and delayed clearance of fat load
in transgenic rabbits expressing human apolipoprotein CIII.
Transgenic Res. 20:867–875. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Koike T, Kitajima S, Yu Y, Li Y, Nishijima
K, Liu E, Sun H, Waqar AB, Shibata N, Inoue T, et al: Expression of
human apoAII in transgenic rabbits leads to dyslipidemia: A new
model for combined hyperlipidemia. Arterioscler Thromb Vasc Biol.
29:2047–2053. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Wang C, Nishijima K, Kitajima S, Niimi M,
Yan H, Chen Y, Ning B, Matsuhisa F, Liu E, Zhang J, et al:
Increased hepatic expression of endothelial lipase inhibits
cholesterol diet-induced hypercholesterolemia and atherosclerosis
in transgenic rabbits. Arterioscler Thromb Vasc Biol. 37:1282–1289.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Watanabe Y: Serial inbreeding of rabbits
with hereditary hyperlipidemia (WHHL-rabbit). Atherosclerosis.
36:261–268. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Shiomi M and Ito T: The Watanabe heritable
hyperlipidemic (WHHL) rabbit, its characteristics and history of
development: A tribute to the late Dr. Yoshio Watanabe.
Atherosclerosis. 207:1–7. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Masashi S and Takashi I: The Watanabe
heritable hyperlipidemic (WHHL) rabbit, its characteristics and
history of development: A tribute to the late Dr. Yoshio Watanabe.
Atherosclerosis. 207:1–7. 2009. View Article : Google Scholar
|
|
81
|
Ning B, Wang X, Yu Y, Waqar AB, Yu Q,
Koike T, Shiomi M, Liu E, Wang Y and Fan J: High-fructose and
high-fat diet-induced insulin resistance enhances atherosclerosis
in Watanabe heritable hyperlipidemic rabbits. Nutr Metab (Lond).
12:302015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Lichtman AH, Clinton SK, Iiyama K,
Connelly PW, Libby P and Cybulsky MI: Hyperlipidemia and
atherosclerotic lesion development in LDL receptor-deficient mice
fed defined semipurified diets with and without cholate.
Arterioscler Thromb Vasc Biol. 19:1938–1944. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Reardon CA, Blachowicz L, Lukens J,
Nissenbaum M and Getz GS: Genetic background selectively influences
innominate artery atherosclerosis: Immune system deficiency as a
probe. Arterioscler Thromb Vasc Biol. 23:1449–1454. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Lin Y, Bai L, Chen Y, Zhu N, Bai Y, Li Q,
Zhao S, Fan J and Liu E: Practical assessment of the quantification
of atherosclerotic lesions in apoE(−)/(−) mice. Mol Med Rep.
12:5298–5306. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Centa M, Ketelhuth DFJ, Malin S and
Gisterå A: Quantification of atherosclerosis in mice. J Vis Exp.
12:doi: 10.3791/59828. 2019.PubMed/NCBI
|
|
86
|
Bai L, Li Z, Li Q, Guan H, Zhao S, Liu R,
Wang R, Zhang J, Jia Y, Fan J, et al: Mediator 1 is atherosclerosis
protective by regulating macrophage polarization. Arterioscler
Thromb Vasc Biol. 37:1470–1481. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wang R, Zhang Y, Xu L, Lin Y, Yang X, Bai
L, Chen Y, Zhao S, Fan J, Cheng X and Liu E: Protein inhibitor of
activated STAT3 suppresses oxidized LDL-induced cell responses
during atherosclerosis in apolipoprotein E-deficient mice. Sci Rep.
6:367902016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Guan H, Lin Y, Bai L, An Y, Shang J, Wang
Z, Zhao S, Fan J and Liu E: Dietary cocoa powder improves
hyperlipidemia and reduces atherosclerosis in apoE deficient mice
through the inhibition of hepatic endoplasmic reticulum stress.
Mediators Inflamm. 2016:19375722016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Li S, Wang YN, Niimi M, Ning B, Chen Y,
Kang D, Wang Z, Yu Q, Waqar AB, Liu E, et al: Angiotensin II
destabilizes coronary plaques in watanabe heritable hyperlipidemic
rabbits. Arterioscler Thromb Vasc Biol. 36:810–816. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Yan H, Niimi M, Matsuhisa F, Zhou H,
Kitajima S, Chen Y, Wang C, Yang X, Yao J, Yang D, et al:
Apolipoprotein CIII deficiency protects against atherosclerosis in
knockout rabbits. Arterioscler Thromb Vasc Biol. 40:2095–2107.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Dweck MR, Aikawa E, Newby DE, Tarkin JM,
Rudd JH, Narula J and Fayad ZA: Noninvasive molecular imaging of
disease activity in atherosclerosis. Circ Res. 119:330–340. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Chen Q, Yu J, Lukashova L, Latoche JD, Zhu
J, Lavery L, Verdelis K, Anderson CJ and Kim K: validation of
ultrasound super-resolution imaging of vasa vasorum in rabbit
atherosclerotic plaques. IEEE Trans Ultrason Ferroelectr Freq
Control. 67:1725–1729. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zhang X, Ha S, Wei W, Duan S, Shi Y and
Yang Y: Noninvasive imaging of aortic atherosclerosis by ultrasound
biomicroscopy in a mouse model. J Ultrasound Med. 34:111–116. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Punjabi M, Xu L, Ochoa-Espinosa A,
Kosareva A, Wolff T, Murtaja A, Broisat A, Devoogdt N and Kaufmann
BA: Ultrasound molecular imaging of atherosclerosis with
nanobodies: Translatable microbubble targeting murine and human
VCAM (Vascular Cell Adhesion Molecule) 1. Arterioscler Thromb Vasc
Biol. 39:2520–2530. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Borland SJ, Behnsen J, Ashton N, Francis
SE, Brennan K, Sherratt MJ, Withers PJ and Canfield AE: X-ray
micro-computed tomography: An emerging technology to analyze
vascular calcification in animal models. Int J Mol Sci.
21:45382020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Magnoni M, Ammirati E and Camici PG:
Non-invasive molecular imaging of vulnerable atherosclerotic
plaques. J Cardiol. 65:261–269. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Choudhury RP, Fuster V, Badimon JJ, Fisher
EA and Fayad ZA: MRI and characterization of atherosclerotic
plaque: Emerging applications and molecular imaging. Arterioscler
Thromb Vasc Biol. 22:1065–1074. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Evans NR, Tarkin JM, Chowdhury MM,
Warburton EA and Rudd JH: PET imaging of atherosclerotic disease:
Advancing plaque assessment from anatomy to pathophysiology. Curr
Atheroscler Rep. 18:302016. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Calcagno C, Lairez O, Hawkins J, Kerr SW,
Dugas MS, Simpson T, Epskamp J, Robson PM, Eldib M, Bander I, et
al: Combined PET/DCE-MRI in a rabbit model of atherosclerosis:
Integrated quantification of plaque inflammation, permeability, and
burden during treatment with a leukotriene A4 hydrolase inhibitor.
JACC Cardiovasc Imaging. 11:291–301. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Calcagno C, Perez-Medina C, Mulder WJM and
Fayad ZA: Whole-Body atherosclerosis imaging by positron emission
tomography/magnetic resonance imaging: From mice to nonhuman
primates. Arterioscler Thromb Vasc Biol. 40:1123–1134. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Luehmann HP, Detering L, Fors BP, Pressly
ED, Woodard PK, Randolph GJ, Gropler RJ, Hawker CJ and Liu Y:
PET/CT imaging of chemokine receptors in inflammatory
atherosclerosis using targeted nanoparticles. J Nucl Med.
57:1124–1129. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Cheng D, Li X, Zhang C, Tan H, Wang C,
Pang L and Shi H: Detection of vulnerable atherosclerosis plaques
with a dual-modal single-photon-emission computed
tomography/magnetic resonance imaging probe targeting apoptotic
macrophages. ACS Appl Mater Interfaces. 7:2847–2855. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Rahaman SO, Lennon DJ, Febbraio M, Podrez
EA, Hazen SL and Silverstein RL: A CD36-dependent signaling cascade
is necessary for macrophage foam cell formation. Cell Metab.
4:211–221. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Versari D, Daghini E, Virdis A, Ghiadoni L
and Taddei S: Endothelial dysfunction as a target for prevention of
cardiovascular disease. Diabetes Care. 32 (Suppl 2):S314–S321.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Medina-Leyte DJ, Domínguez-Pérez M,
Mercado I, Villarreal-Molina MT and Jacobo-Albavera L: Use of human
umbilical vein endothelial cells (HUVEC) as a model to study
cardiovascular disease: A review. Applied Sciences. 10:9382020.
View Article : Google Scholar
|
|
106
|
Yang Q, Xu J, Ma Q, Liu Z, Sudhahar V, Cao
Y, Wang L, Zeng X, Zhou Y, Zhang M, et al: PRKAA1/AMPKα1-driven
glycolysis in endothelial cells exposed to disturbed flow protects
against atherosclerosis. Nat Commun. 9:46672018. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Chen Y, Liu R, Zhang G, Yu Q, Jia M, Zheng
C, Wang Y, Xu C, Zhang Y and Liu E: Hypercysteinemia promotes
atherosclerosis by reducing protein S-nitrosylation. Biomed
Pharmacother. 70:253–259. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
O'Donnell J, Mille-Baker B and Laffan M:
Human umbilical vein endothelial cells differ from other
endothelial cells in failing to express ABO blood group antigens. J
Vasc Res. 37:540–547. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Addis R, Campesi I, Fois M, Capobianco G,
Dessole S, Fenu G, Montella A, Cattaneo MG, Vicentini LM and
Franconi F: Human umbilical endothelial cells (HUVECs) have a sex:
Characterisation of the phenotype of male and female cells. Biol
Sex Differ. 5:182014. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Crampton SP, Davis J and Hughes CC:
Isolation of human umbilical vein endothelial cells (HUVEC). J Vis
Exp. 183:doi: 10.3791/183. 2007.PubMed/NCBI
|
|
111
|
Jaffe EA, Nachman RL, Becker CG and Minick
CR: Culture of human endothelial cells derived from umbilical
veins. Identification by morphologic and immunologic criteria. J
Clin Invest. 52:2745–2756. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Baudin B, Bruneel A, Bosselut N and
Vaubourdolle M: A protocol for isolation and culture of human
umbilical vein endothelial cells. Nat Protoc. 2:481–485. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Liu L and Shi GP: CD31: Beyond a marker
for endothelial cells. Cardiovasc Res. 94:3–5. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Tabas I and Bornfeldt KE: Macrophage
phenotype and function in different stages of atherosclerosis. Circ
Res. 118:653–667. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Li AC and Glass CK: The macrophage foam
cell as a target for therapeutic intervention. Nat Med.
8:1235–1242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zimmerman MA, Selzman CH, Reznikov LL,
Miller SA, Raeburn CD, Emmick J, Meng X and Harken AH: Lack of
TNF-alpha attenuates intimal hyperplasia after mouse carotid artery
injury. Am J Physiol Regul Integr Comp Physiol. 283:R505–R512.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Zhang X, Goncalves R and Mosser DM: The
isolation and characterization of murine macrophages. Curr Protoc
Immunol Chapter. 14:Unit 14 11. 2008.PubMed/NCBI
|
|
118
|
Austyn JM and Gordon S: F4/80, a
monoclonal antibody directed specifically against the mouse
macrophage. Eur J Immunol. 11:805–815. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Bennett MR and Boyle JJ: Apoptosis of
vascular smooth muscle cells in atherosclerosis. Atherosclerosis.
138:3–9. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Gao S, Xu L, Zhang Y, Yu Q, Li J, Guan H,
Wang X, Cheng D, Liu Y, Bai L, et al: Salusin-alpha inhibits
proliferation and migration of vascular smooth muscle cell via
Akt/mTOR signaling. Cell Physiol Biochem. 50:1740–1753. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Feng C, Wang X, Shi H, Yan Q, Zheng M, Li
J, Zhang Q, Qin Y, Zhong Y, Mi J and Lai L: Generation of ApoE
deficient dogs via combination of embryo injection of CRISPR/Cas9
with somatic cell nuclear transfer. J Genet Genomics. 45:47–50.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Fang B, Ren X, Wang Y, Li Z, Zhao L, Zhang
M, Li C, Zhang Z, Chen L, Li X, et al: Apolipoprotein E deficiency
accelerates atherosclerosis development in miniature pigs. Dis
Model Mech. 11:dmm0366322018. View Article : Google Scholar : PubMed/NCBI
|