Introduction
Aplastic anemia (AA) is a bone marrow failure
disorder characterized by peripheral blood pancytopenia and bone
marrow (BM) aplasia (1). As the
disease progresses, the marrow is severely destroyed, exhibiting
enhanced hematopoietic stem/progenitor cell apoptosis, decreased
capillaries, excessive adipocytes and reduced BM function (2–4).
BM-mesenchymal stem cells (MSCs) serve as multipotent cells with
the potential to differentiate into several cells, such as
osteoblasts and adipocytes, and constitute the principal cellular
components of the BM microenvironment (5). BM-MSCs from patients with AA display
fundamental deficiencies that lead to susceptibility to progressive
BM failure (6,7). Increasing evidence has revealed that
osteogenic-adipose balance dysregulation is associated with the
pathophysiological processes of AA (8–10).
Therefore, understanding the modulation mechanism of AA BM-MSC
adipogenic differentiation is of importance. However, to the best
of our knowledge, advancements in this field are still limited.
MicroRNAs (miRNAs/miRs) are small non-coding RNAs
with a length of 20–25 nucleotides that influence several
biological processes (11).
miRNAs can control gene expression at the post-transcriptional
level by pairing with target mRNAs at the 3′-untranslated region
(3′-UTR) (12). miRNAs also
modulate numerous targets that have essential functions in a broad
spectrum of biological processes, including cell proliferation,
apoptosis, differentiation, invasion, metastasis and tumorigenesis
(13). Previous studies have
revealed that miRNAs are involved in the progression of AA
(14,15). miR-1, miR-146b-5p and miR-150-5p
serve as biomarkers for the diagnosis of AA (16). miR-144-3p represses BM-MSC
osteogenic differentiation in patients with AA by suppressing Tet
methylcytosine dioxygenase 2 (17). miR-204 controls AA BM-MSC
osteogenic and adipogenic differentiation (18). Furthermore, miR-30a-5p is a
well-studied miRNA, and has been shown to serve a role in the
development of cancer (19–21). A previous study demonstrated that
miR-30a-5p promotes adipocyte differentiation and adipogenesis
(22). However, the effect of
miR-30a-5p on the adipogenic differentiation of AA BM-MSCs remains
unclear.
Family with sequence similarity 13, member A
(FAM13A) is highly expressed in thyroid, placenta, duodenum and
adipose tissue, and serves as a crucial factor in multiple cellular
processes, such as cell proliferation, invasion and differentiation
(23–25). Two splicing variants of FAM13A
have been identified in humans, namely isoform 1 (v1) and isoform 2
(v2) (26). FAM13A v1 includes a
Ras homologous GTPase-activating protein (RhoGAP) domain that is
essential for the control of cell survival and proliferation,
whereas FAM13A v2 does not carry the RhoGAP domain, suggesting a
different function in the control of cellular signaling (27,28). It has been reported that miR-328
in exosomes of M2 macrophages stimulates the development of
pulmonary fibrosis by FAM13A (29). FAM13A influences the distribution
of body fat and the function of adipocytes, and FAM13A depletion
promotes adipocyte differentiation (30). However, the role of FAM13A in the
adipogenic differentiation of AA BM-MSCs, and the association
between FAM13A and miR-30a-5p remains elusive.
It is well recognized that the Wnt/β-catenin
signaling pathway is a crucial regulator of MSC osteogenesis and
adipogenesis (31). miR-210-3p
increases adipogenic differentiation by modulating Wnt signaling in
estrogen receptor α-deficient MSCs (32). Histone demethylase 7A mediates
osteogenic and adipogenic differentiation by regulating Wnt
signaling (33). Cysteine-rich
protein 61 modulates the adipogenic differentiation of MSCs by
mediating canonical Wnt signaling and rapamycin complex 1 (34). Furthermore, it has been determined
that FAM13A can activate the Wnt/β-catenin signaling pathway
(35). miR-30a-5p-targeted PR
domain zinc finger protein 1 (PRDM1) regulates Wnt/β-catenin
signaling in a Dickkopf-1 (DKK1)-dependent manner in glioma
development (36). However, the
association between Wnt/β-catenin signaling, FAM13A and miR-30a-5p
in the regulation of BM-MSC adipogenic differentiation remains
unclear.
The present study aimed to explore the role and
underlying mechanism of miR-30a-5p in the modulation of AA BM-MSC
adipogenic differentiation. The present results revealed a novel
function of miR-30a-5p in promoting adipogenic differentiation of
AA BM-MSCs by targeting the FAM13A/Wnt/β-catenin signaling
pathway.
Materials and methods
AA clinical samples and AA BM-MSC
culture
Human clinical samples of BM, including 11 patients
with AA (7 men and 4 women; age, 22–48 years) and 14 patients with
iron deficiency anemia (7 men and 7 women; age, 20–42 years), were
obtained from The First People's Hospital of Lianyungang
(Lianyungang, China) between January and December 2020 (37). There are several ethical concerns
with BM aspiration from healthy donors for MSCs isolation and
expansion. Thus, the patients with iron deficiency anemia served as
control subjects. All patients provided informed consent for the
use of these samples in the present study. The present study
conformed to the experimental guidelines of the World Medical
Association, and was approved by the Ethics Committee of The First
People's Hospital of Lianyungang (approval no. LYGDYYY-2020-017).
BM-MSCs were obtained from the BM of patients with AA and control
subjects as previously described (38,39). Briefly, BM samples were mixed with
DMEM (MilliporeSigma) supplemented with 10% fetal bovine serum
(FBS; MilliporeSigma) at 20°C for 30 min. Following centrifugation
at 110 × g at 20°C for 5 min, the cells were resuspended and
maintained in DMEM supplemented with 10% FBS, 0.1 mg/ml
streptomycin (MilliporeSigma) and 100 U/ml penicillin
(MilliporeSigma) at 37°C with 5% CO2. The medium was
refreshed every 2–3 days until the cells reached 80% confluence.
BM-MSCs of passage 3 were used in subsequent experiments.
Cell transfection and treatment
Mimic control, miR-30a-5p mimic, inhibitor control,
miR-30a-5p inhibitor, lentiviral plasmids containing short hairpin
RNA (shRNA/sh) FAM13A or the corresponding scrambled shRNA [sh
negative control (NC)] and the vector overexpressing (OE) the
FAM13A coding sequence (OE-FAM13A) or the corresponding NC (OENC)
were all synthesized by GenScript. The following sequences were
used: mimic control, 5′-UUCUCCGAACGUGUCACGUTT −3′; inhibitor
control, 5′-CAGUACUUUUGUGUAGUACAA −3′; miR-30a-5p mimic,
5′-UGUAAACAUCCUCGACUGGAAG-3′; miR-30a-5p inhibitor,
5′-CUUCCAGUCGAGGAUGUUUACA-3′; shNC,
5′-TGAAGCCCTGTCTCATCTTCAATAT-3′; shFAM13A,
5′-GGAGAACTCTTAGAAAGAA-3′. In brief, BM-MSCs (2×106
cells/ml) were seeded into 6-well plates and maintained in DMEM at
37°C for 24 h. Mimic control (50 pmol), miR-30a-5p mimic (50 pmol),
inhibitor control (50 pmol), miR-30a-5p inhibitor (50 pmol),
shFAM13A (5 nM), shNC (5 nM), OENC (5 nM) and OE-FAM13A (5 nM) were
transfected into BM-MSCs at 37°C for 6 h using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Following transfection for 48 h, reverse transcription-quantitative
PCR (RT-qPCR) was used to determine the transfection efficiency.
The Wnt/β-catenin signaling inhibitor, DKK1 (200 ng/ml; PeproTech,
Inc.) was added to the medium at 37°C for 24 h when required
(40).
Adipogenic differentiation assays
The induction of adipogenic differentiation was
initiated as previously described (41–43). Briefly, BM-MSCs (3×105
cells) were plated in 6-well plates and cultured in an adipogenic
medium comprising DMEM supplemented with 0.5 mM
3-isobutyl-1-methyl-xanthine (Sigma-Aldrich; Merck KGaA), 10% FBS,
100 mg/ml indomethacin (MilliporeSigma), 0.01 mg/ml insulin
(MilliporeSigma) and 1 mM dexamethasone (MilliporeSigma) at 37°C
for 12 days. Adipogenic differentiation was analyzed via the
detection of fatty acid-binding protein 4 (FABP4), lipoprotein
lipase (LPL), perilipin-1 (PLIN1), peroxisome
proliferator-activated receptor γ (PPARγ) and CCAAT/enhancer
binding protein α (C/EBPα) mRNA expression levels using
RT-qPCR.
The accumulation of lipid droplets was measured
using Oil Red O staining. Briefly, BM-MSCs were washed twice with
PBS at the end of the adipogenic differentiation. Subsequently,
cells were fixed with 4% paraformaldehyde for 30 min at room
temperature and stained with Oil Red O staining solution (Beijing
Solarbio Science & Technology Co., Ltd.) for 15 min at room
temperature. Images were captured using a light microscope.
RT-qPCR
Total RNA was extracted from BM-MSCs cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Total RNA was
reverse transcribed into cDNA using Maxima First Strand cDNA
Synthesis kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. qPCR was subsequently performed using the
SYBR Real-Time PCR I Kit (Takara Bio, Inc.) according to the
manufacturer's protocol. The reaction conditions were as follows:
Initial denaturation at 95°C for 10 min, followed by 35 cycles of
94°C for 30 sec, 60°C for 50 sec and 72°C for 30 sec, and final
extension at 72°C for 10 min. The primer sequences used were as
follows: miR-30a-5p forward (F), 5′-ACACTCCAGCTGGGTGTAAACATCCTCGAC
−3′ and miR-30a-5p reverse (R), 5′-CAGTGCGTGTCGTGGAGT-3′; FAM13A F,
5′-ACCCTGTTTGAAGTAGAGTATACAG-3′ and FAM13A R,
5′-AGACCTCTTTTACTATGATAAGCCT-3′; C/EBPα F,
5′-TAGGATAACCTTGTGCCTTGGAAAT-3′ and C/EBPα R,
5′-GTCTGCTGTAGCCTCGGGAA-3′; PPARγ F, 5′-GCTGACCAAAGCAAAGGCG-3′ and
PPARγ R, 5′-GCCCTGAAAGATGCGGATG-3′; LPL F,
5′-TCATTCCCGGAGTAGCAGAGT-3′ and LPL R, 5′-GGCCACAAGTTTTGGCACC-3′;
PLIN1 F, 5′-GCGAGGATGGCAGTCAACAAA −3′ and PLIN1 R,
5′-GCACGCCCTTCTCATAGGCAT −3′; FABP4 F, 5′-ACTGGGCCAGGAATTTGACG-3′
and FABP4 R, 5′-CTCGTGGAAGTGACGCCTT-3′; GAPDH F,
5′-AAGAAGGTGGTGAAGCAGGC-3′ and GAPDH R, 5′-TCCACCACCCAGTTGCTGTA-3′;
and U6 F, 5′-GCTTCGGCAGCACATATACTAA-3′ and U6 R,
5′-AACGCTTCACGAATTTGCGT-3′. The expression levels were normalized
to the internal reference genes for miRNA and mRNA, namely U6 and
GAPDH, respectively. The 2−ΔΔCq method was used to
quantify the relative expression levels (44).
Dual-luciferase reporter assay
A dual-luciferase reporter assay was used to
determine the relationship between miR-30a-5p and FAM13A. The
miR-30a-5p-targeted site in the FAM13A 3′-UTR was identified using
TargetScan (http://www.targetscan.org/vert_72/). The pmirGLO
vectors carrying the wild-type (WT) 3′-UTR of FAM13A and the FAM13A
3′-UTR with the mutant (MUT) miR-30a-5p-binding site were
synthesized by Guangzhou RiboBio Co., Ltd. Briefly, the 293T cells
were co-transfected with either miR-30a-5p mimic or mimic control,
and vectors containing FAM13A-WT or FAM13A-MUT fragments at 37°C
for 6 h using Lipofectamine® 3000. After
co-transfection, the cells were maintained at 37°C for 48 h.
Luciferase activity was detected using Dual-Luciferase Assay Kit
System (Promega Corporation) and normalized to Renilla
luciferase activity.
Western blotting
Total protein was extracted from BM-MSCs using RIPA
buffer (Cell Signaling Technology, Inc.). Nuclear and cytoplastic
proteins were extracted using the Nuclear and Cytoplastic Protein
Extraction Kit (Abbkine Scientific Co., Ltd.). Protein
concentrations were measured using a BCA Protein Quantification Kit
(Abbkine Scientific Co. Ltd.). Proteins (30 µg) were separated by
12% SDS-PAGE and subsequently transferred onto polyvinylidene
difluoride membranes (MilliporeSigma). Membranes were blocked with
5% skimmed milk for 1 h at room temperature and incubated overnight
at 4°C with primary antibodies against FAM13A (1:1,000, cat. no.
55401-1-AP; Wuhan Sanying Biotechnology), total β-catenin (1:1,000,
cat. no. 37447; Cell Signaling Technology, Inc.), non-phospho
(active) β-catenin (1:1,000, cat. no. 19807; Cell Signaling
Technology, Inc.), low density lipoprotein receptor-related protein
6 (LRP6; 1:1,000, cat. no. 3395; Cell Signaling technology, Inc.),
disheveled segment polarity protein 3 (DVL3; 1:1,000, cat. no.
3218; Cell Signaling Technology, Inc.), histone H3 (1:1,000, cat.
no. 17168-1-AP; Wuhan Sanying Biotechnology) and GAPDH (1:5,000,
cat. no. 10494-1-AP; Wuhan Sanying Biotechnology), wherein GAPDH
and histone H3 served as loading controls. Following the primary
incubation, membranes were washed with 0.1% TBS-Tween-20
(Sigma-Aldrich; Merck KGaA), and then incubated with the
corresponding HRP-conjugated goat anti-rabbit IgG (1:2,000, cat.
no. SA00001-2; Wuhan Sanying Biotechnology) and HRP-conjugated goat
anti-mouse IgG (1:2,000, cat. no. SA00001-1, Wuhan Sanying
Biotechnology) secondary antibodies for 1 h at room temperature.
Protein bands were visualized using an Odyssey CLx Infrared Imaging
System (LI-COR Biosciences), and protein expression was
semi-quantified using ImageJ software (V1.52; National Institutes
of Health).
Statistical analysis
Data are presented as the mean ± SD, and statistical
analysis was conducted using GraphPad Prism 7 (GraphPad Software,
Inc.). In total, three independent experimental repeats were
performed for all experiments. Unpaired Student's t-tests were
performed for statistical comparisons between two groups. One-way
analysis of variance followed by Tukey's post hoc test was employed
for statistical comparisons between more than two groups. The
correlation between miR-30a-5p and FAM13A expression levels in AA
was analyzed using Pearson's correlation coefficient analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
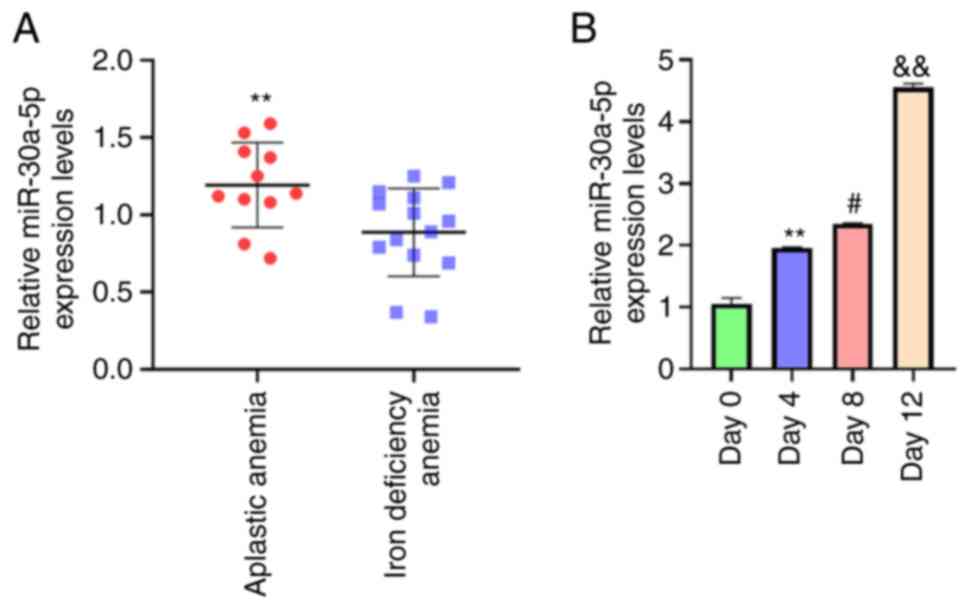
miR-30a-5p is upregulated during the
adipogenic differentiation of AA BM-MSCs
To assess the potential association of miR-30a-5p
with BM-MSC adipose differentiation, the miR-30a-5p expression
levels in patients with AA were analyzed. miR-30a-5p expression
levels were significantly elevated in BM-MSCs from patients with AA
(n=11) compared with those of control subjects with iron deficiency
anemia (n=14; P<0.01; Fig.
1A). These results implied a possible association between
miR-30a-5p and AA BM-MSC adipose differentiation. Furthermore,
miR-30a-5p expression levels in day 4 were higher than day 0
(P<0.01; Fig. 1B); miR-30a-5p
expression levels in day 8 were higher than day 4 (P<0.05;
Fig. 1B); miR-30a-5p expression
levels in day 12 were higher than day 8 (P<0.01; Fig. 1B). These data showed that
miR-30a-5p expression levels significantly increased in a
time-dependent manner in adipose-induced BM-MSCs. Taken together,
these findings indicated that miR-30a-5p may serve as a potential
modulator in AA BM-MSCs.
miR-30a-5p contributes to the
adipogenic differentiation of AA BM-MSCs
To further evaluate the effect of miR-30a-5p on the
adipogenic differentiation of AA BM-MSCs, AA BM-MSCs were
transfected with mimic control or miR-30a-5p mimic, followed by
adipose induction for 12 days. As shown in Fig. 2A, the miR-30a-5p mimic increased
miR-30a-5p expression compared with mimic control group
(P<0.01), which validated the transfection was successful. The
adipogenesis-associated factors FABP4, LPL, PLIN1, PPARγ and C/EBPα
are known markers of adipogenic differentiation (31). The mRNA expression levels of these
proteins were significantly enhanced by miR-30a-5p mimic
transfection in BM-MSCs compared with those in the mimic control
group (P<0.01; Fig. 2B).
Furthermore, Oil Red O staining demonstrated that the miR-30a-5p
mimic markedly increased the accumulation of lipid droplets in
BM-MSCs (P<0.01; Fig. 2C).
Together, these findings suggested that miR-30a-5p promoted BM-MSC
adipose differentiation.
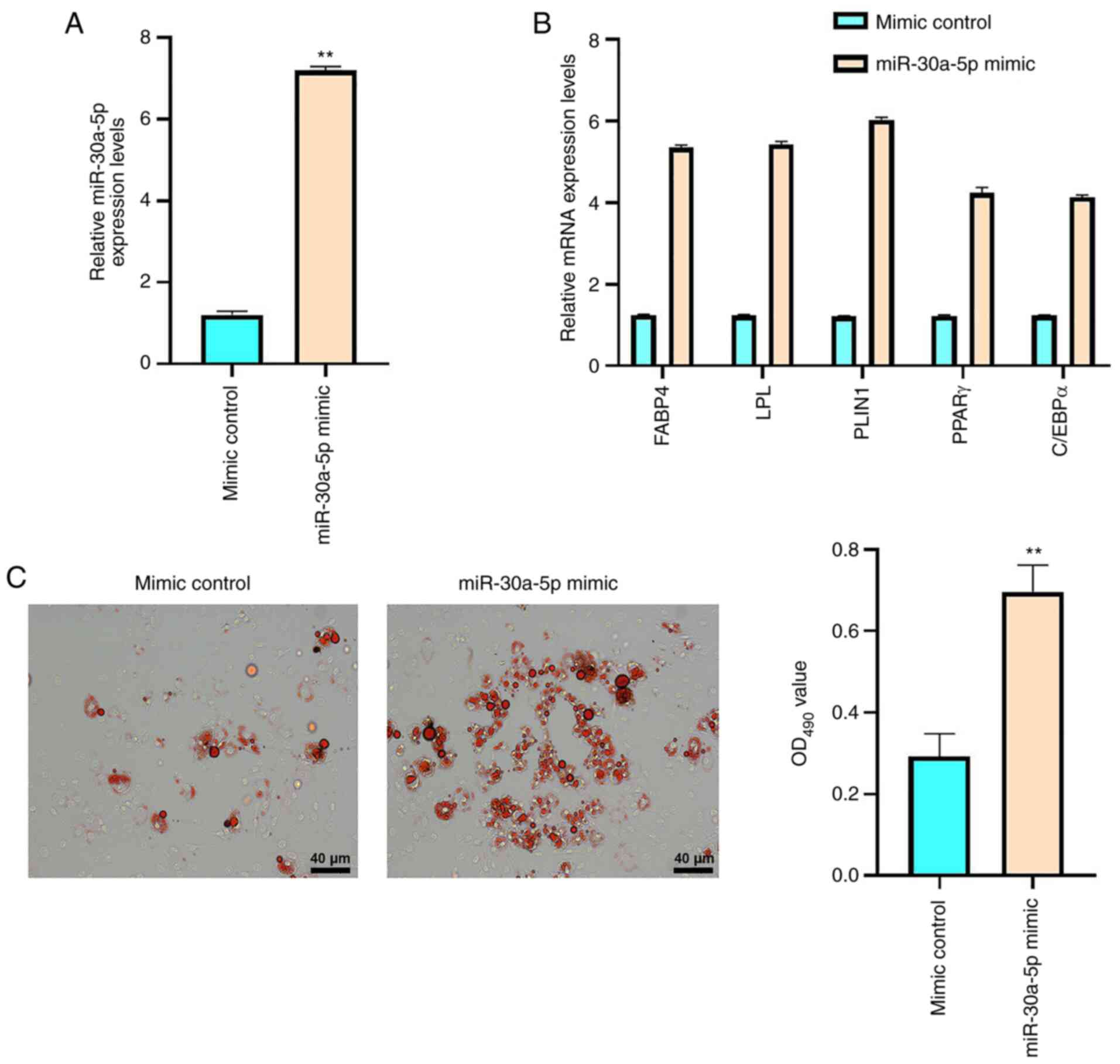 | Figure 2.miR-30a-5p contributes to the
adipogenic differentiation of AA BM-MSCs. AA BM-MSCs were treated
with mimic control or miR-30a-5p mimic. (A) BM-MSC miR-30a-5p
expression levels were analyzed using RT-qPCR. (B) BM-MSC FABP4,
LPL, PLIN1, PPARγ and C/EBPα mRNA expression levels were analyzed
using RT-qPCR. (C) Lipid droplet accumulation was evaluated using
Oil Red O staining in BM-MSCs. Data are presented as the mean ± SD.
**P<0.01 vs. mimic control. miR, microRNA; AA, aplastic anemia;
BM-MSCs, bone marrow-mesenchymal stem cells; RT-qPCR, reverse
transcription-quantitative PCR; FABP4, fatty acid-binding protein
4; LPL, lipoprotein lipase; PLIN1, perilipin-1; PPARγ, peroxisome
proliferator-activated receptor γ; C/EBPα, CCAAT/enhancer binding
protein α. |
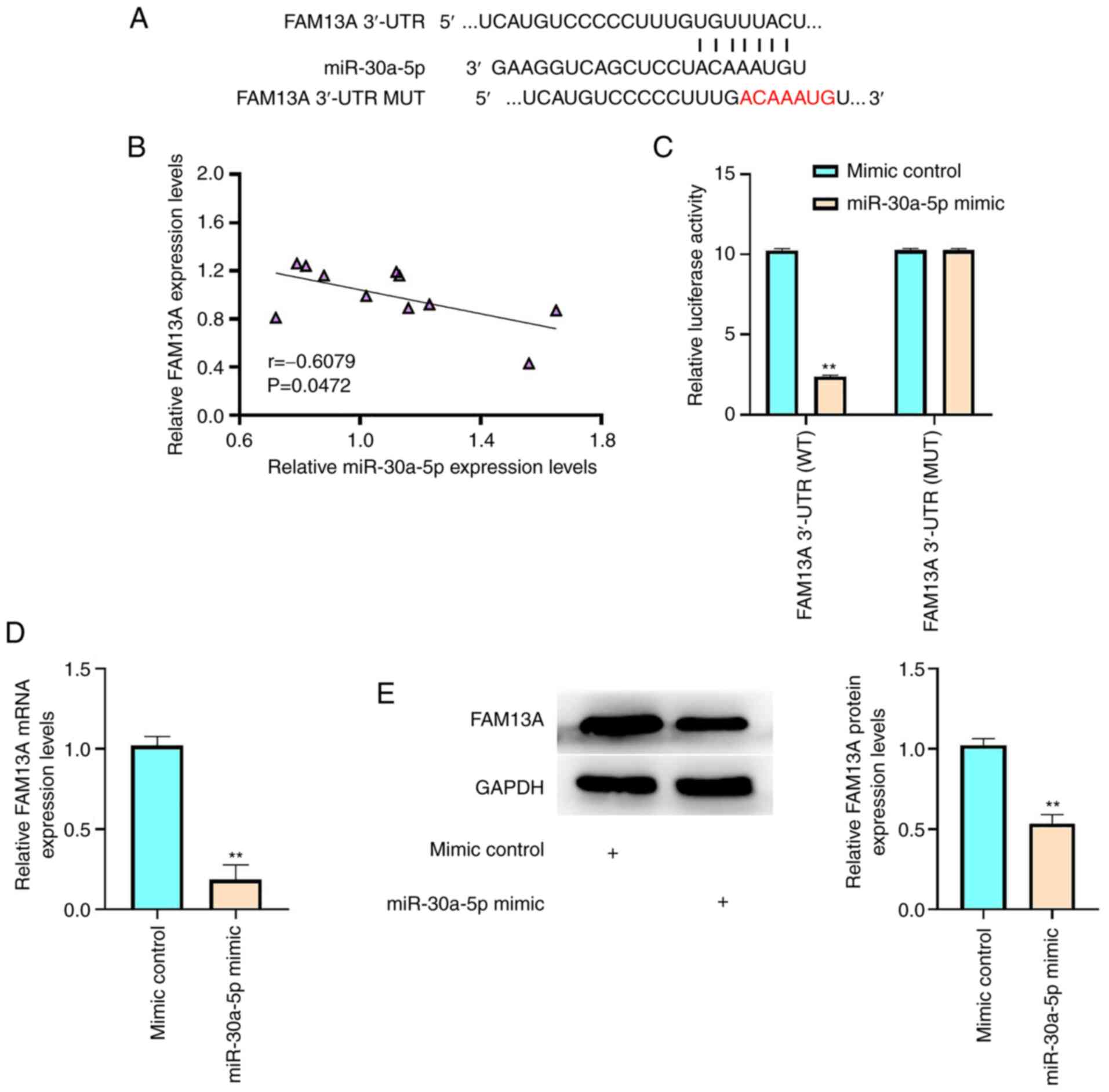
miR-30a-5p targets FAM13A in AA
BM-MSCs
The underlying mechanism of miR-30a-5p-regulated
adipogenic differentiation of AA BM-MSCs was further investigated.
The miR-30a-5p-targeted site in the FAM13A 3′-UTR was identified
using TargetScan (Fig. 3A).
miR-30a-5p expression levels were significantly negatively
correlated with those of FAM13A in AA BM-MSC samples (P=0.0472;
Fig. 3B). These results indicated
a potential interaction between miR-30a-5p and FAM13A in the
regulation of AA BM-MSCs. Furthermore, miR-30a-5p mimic inhibited
the luciferase activity of FAM13A-WT compared with that of the
mimic control, whereas no change in luciferase activity was
observed upon transfection with FAM13A-MUT (P<0.01; Fig. 3C). In addition, the FAM13A mRNA
and protein expression levels were significantly reduced by
miR-30a-5p mimic transfection in BM-MSCs (P<0.01; Fig. 3D and E). These results therefore
suggested that miR-30a-5p may target FAM13A in AA BM-MSCs.
FAM13A inhibits the adipose
differentiation of AA BM-MSCs
To analyze the association of FAM13A with AA BM-MSC
adipose differentiation, the FAM13A expression levels in patients
with AA were determined. The results demonstrated that FAM13A mRNA
expression levels were significantly reduced in BM-MSCs from
patients with AA (n=11) compared with those of control subjects
with iron deficiency anemia (n=14; P<0.05; Fig. 4A). This implied an inverse
association between FAM13A and AA BM-MSC adipogenic
differentiation. AA BM-MSCs were then transfected with lentiviral
plasmids carrying shFAM13A or shNC to investigate the function of
FAM13A in the adipogenic differentiation of AA BM-MSCs. As shown in
Fig. 4B, shFAM13A transfection
reduced FAM13A protein expression compared with shNC group
(P<0.01), which verified the transfection was successful. The
depletion of FAM13A significantly upregulated the mRNA expression
levels of FABP4, LPL, PLIN1, PPARγ and C/EBPα (P<0.01; Fig. 4C) compared with those of the shNC
group. Furthermore, Oil Red O staining revealed that the
accumulation of lipid droplets was markedly enhanced by FAM13A
knockdown in BM-MSCs compared with that of the shNC group (Fig. 4D). These results therefore
indicated that FAM13A may attenuate AA BM-MSC adipose
differentiation.
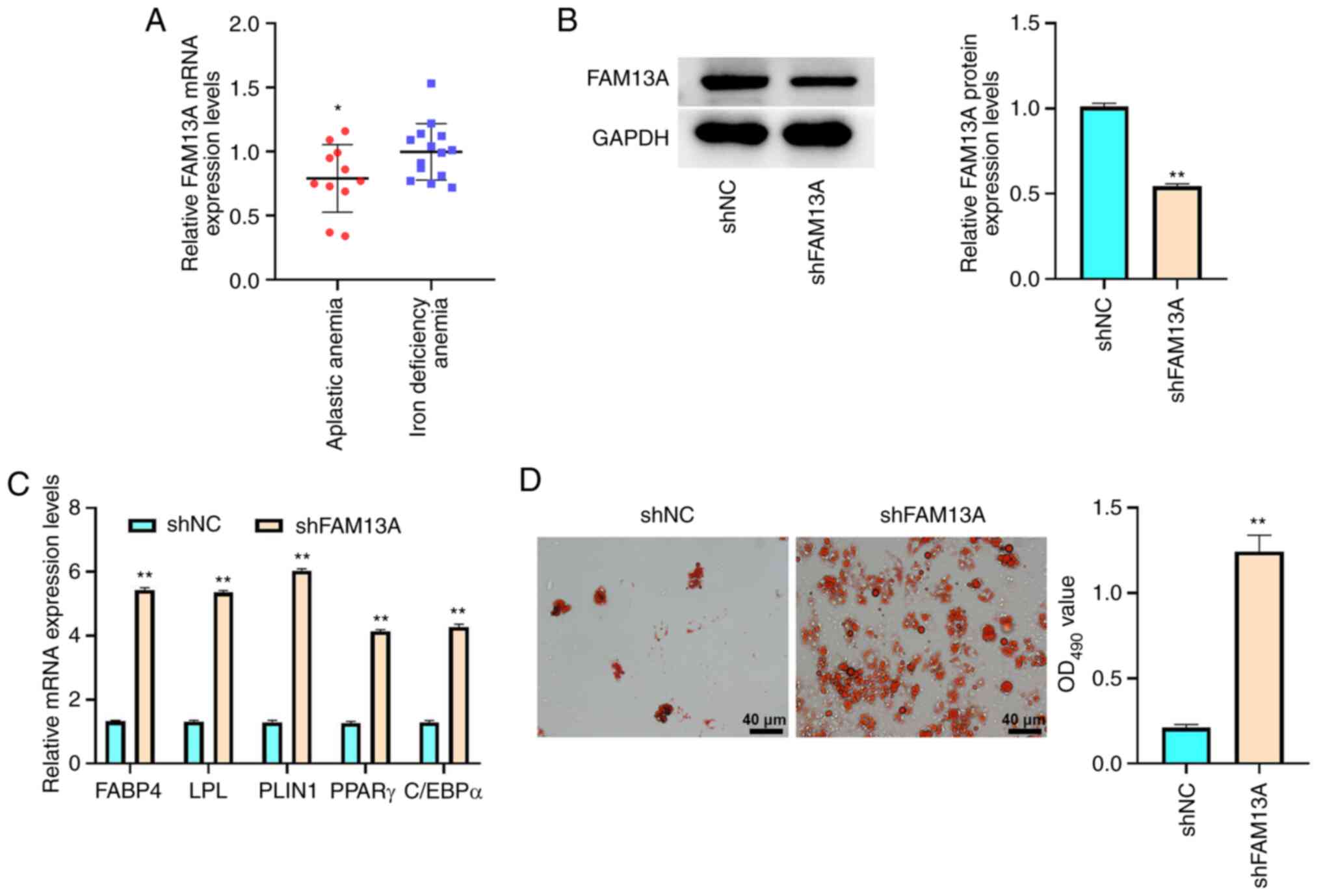 | Figure 4.FAM13A inhibits the adipogenic
differentiation of AA BM-MSCs. (A) FAM13A mRNA expression levels
were analyzed using RT-qPCR in BM-MSCs from patients with AA (n=11)
and patients with iron deficiency anemia (n=14). (B-D) BM-MSCs were
treated with lentiviral plasmids carrying shFAM13A or shNC. (B) The
protein expression levels of FAM13A were examined by western
blotting. (C) FABP4, LPL, PLIN1, PPARγ and C/EBPα mRNA expression
levels were analyzed using RT-qPCR. (D) Lipid droplet accumulation
was evaluated by Oil Red O staining. Data are presented as the mean
± SD. *P<0.05 vs. iron deficiency anemia; **P<0.01 vs. shNC.
FAM13A, family with sequence similarity 13, member A; AA, aplastic
anemia; BM-MSCs, bone marrow-mesenchymal stem cells; RT-qPCR,
reverse transcription-quantitative PCR; sh, short hairpin; NC,
negative control; FABP4, fatty acid-binding protein 4; LPL,
lipoprotein lipase; PLIN1, perilipin-1; PPARγ, peroxisome
proliferator-activated receptor γ; C/EBPα, CCAAT/enhancer binding
protein α. |
FAM13A reduces the adipogenic
differentiation of BM-MSCs by activating the Wnt/β-catenin
signaling pathway
The mechanism of FAM13A-mediated AA BM-MSC adipose
differentiation was further investigated. It was hypothesized that
FAM13A modulated the adipogenic differentiation of AA BM-MSCs via
the Wnt/β-catenin signaling pathway. FAM13A was overexpressed in AA
BM-MSCs. As shown in Fig. 5A,
FAM13A protein expression in the OE-FAM13A group was higher than
that in the OENC group (P<0.01), which validated the
transfection was successful. The protein expression levels of
non-phospho β-catenin, which is the active state of β-catenin
(45), were significantly
enhanced by OE-FAM13A compared with those in the OENC group
(P<0.01; Fig. 5B). However,
they were significantly reduced by FAM13A knockdown in AA BM-MSCs
compared with those in the shNC group (P<0.05), while total
β-catenin was unchanged (Fig.
5B). Moreover, FAM13A overexpression significantly promoted the
nuclear localization of non-phospho β-catenin and inhibited the
cytoplasmic localization of non-phospho β-catenin in the cells
compared with the findings in the OENC group (P<0.01; GAPDH
served as loading controls for the cytoplasmic protein and histone
H3 served as loading controls for the nuclear protein; Fig. 5C). Downstream proteins, including
LRP6 and DVL3, were also significantly upregulated by FAM13A
overexpression compared with the effects of OENC, and were
significantly downregulated by FAM13A knockdown (P<0.01;
Fig. 5D). These results indicated
that FAM13A activated the Wnt/β-catenin signaling pathway in AA
BM-MSCs.
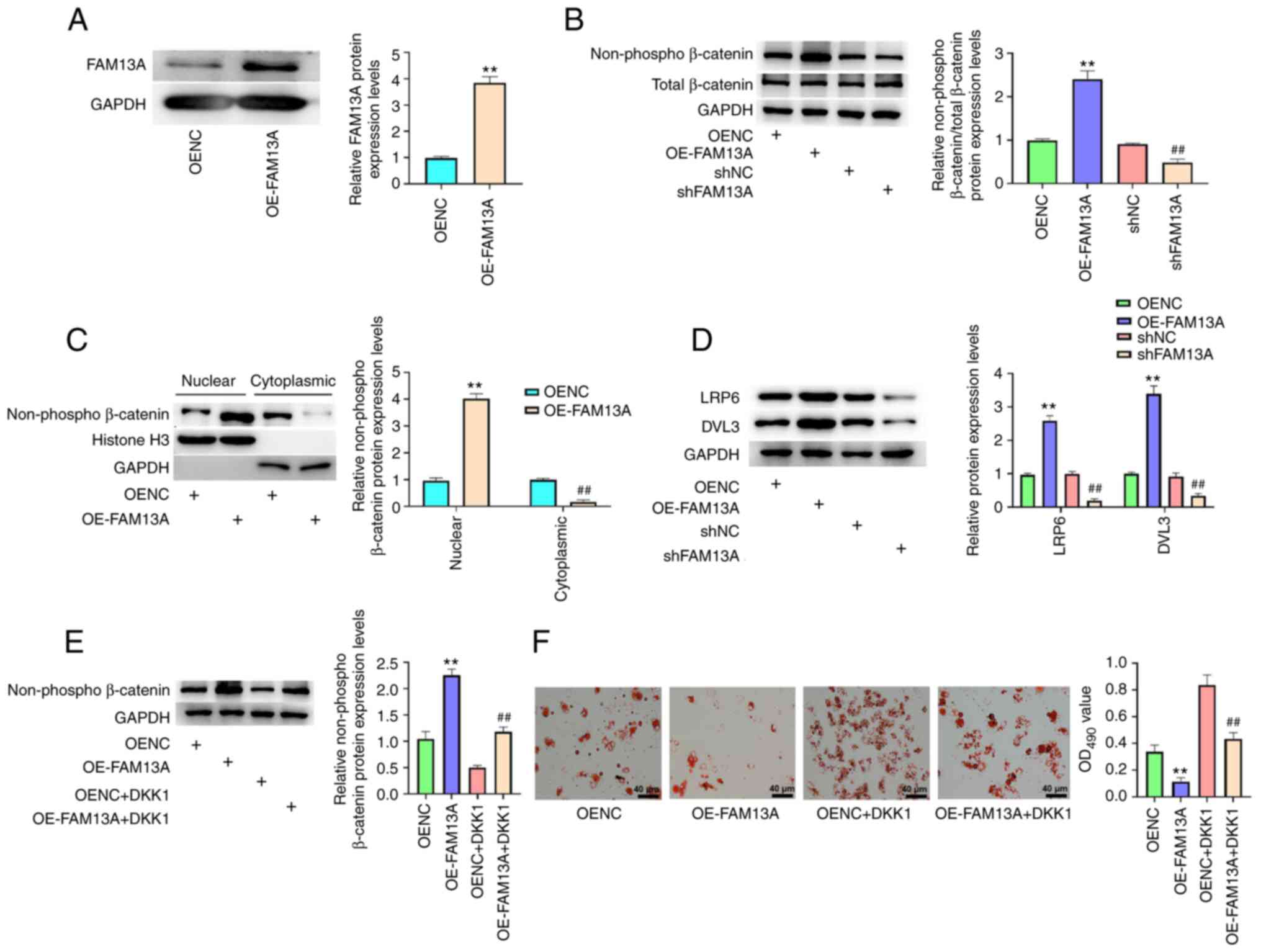 | Figure 5.FAM13A reduces the adipogenic
differentiation of BM-MSCs by activating the Wnt/β-catenin
signaling pathway. (A) AA BM-MSCs were transfected with OENC or
OE-FAM13A. FAM13A protein expression levels were analyzed via
western blotting. (B) Total β-catenin and non-phospho β-catenin
protein expression levels were analyzed via western blotting. (C)
The nuclear and cytoplasmic protein expression levels of
non-phospho β-catenin were analyzed via western blotting. (D) The
protein expression levels of LRP6 and DVL3 were analyzed via
western blotting analysis. (E and F) AA BM-MSCs were transfected
with OENC or OE-FAM13A with or without DKK1 treatment. (E)
Non-phospho β-catenin protein expression levels were determined via
western blotting. (F) Lipid droplet accumulation was analyzed by
Oil Red O staining. Data are presented as mean ± SD. **P<0.01
vs. OENC; ##P<0.01 vs. shNC or OE-FAM13A. FAM13A,
family with sequence similarity 13, member A; AA, aplastic anemia;
BM-MSCs, bone marrow-mesenchymal stem cells; sh, short hairpin;
LRP6, low density lipoprotein receptor-related protein 6; DVL3,
disheveled segment polarity protein 3; OE, overexpression; NC,
negative control; DKK1, Dickkopf-related protein 1. |
DKK1 is an inhibitor of the Wnt/β-catenin signaling
pathway (46,47). Treatment with DKK1 significantly
inhibited the expression of non-phospho β-catenin enhanced by
FAM13A overexpression in AA BM-MSCs compared with OE-FAM13A group
(P<0.01; Fig. 5E).
Furthermore, Oil Red O staining demonstrated that DKK1 markedly
increased the levels of lipid droplets and attenuated FAM13A
overexpression-reduced accumulation of lipid droplets in the cells
(Fig. 5F). These results
therefore suggested that FAM13A reduces the adipose differentiation
of BM-MSCs by activating Wnt/β-catenin signaling.
miR-30a-5p promotes the adipose
differentiation of AA BM-MSCs via FAM13A/Wnt/β-catenin
signaling
The role of the FAM13A/Wnt/β-catenin axis in
miR-30a-5p-mediated AA BM-MSC adipose differentiation was
determined. For this purpose, BM-MSCs were transfected with
miR-30a-5p mimic, OE-FAM13A or miR-30a-5p mimic + OE-FAM13A.
‘Control’ refers to untreated BM-MSCs. The RT-qPCR results
demonstrated that miR-30a-5p mimic significantly reduced FAM13A
mRNA expression levels compared with those of the control group,
while the FAM13A mRNA expression levels were significantly enhanced
by the overexpression of FAM13A in the cells compared with the
findings in the miR-30a-5p mimic group (P<0.01; Fig. 6A). miR-30a-5p mimic also
significantly enhanced the mRNA expression levels of FABP4, LPL,
PLIN1, PPARγ and C/EBPα compared with those of the control
(P<0.01), and the mRNA expression levels of FABP4, LPL, PLIN1,
PPARγ and C/EBPα were significantly attenuated by FAM13A
overexpression in the cells compared with those of the miR-30a-5p
mimic group (P<0.01; Fig.
6B).
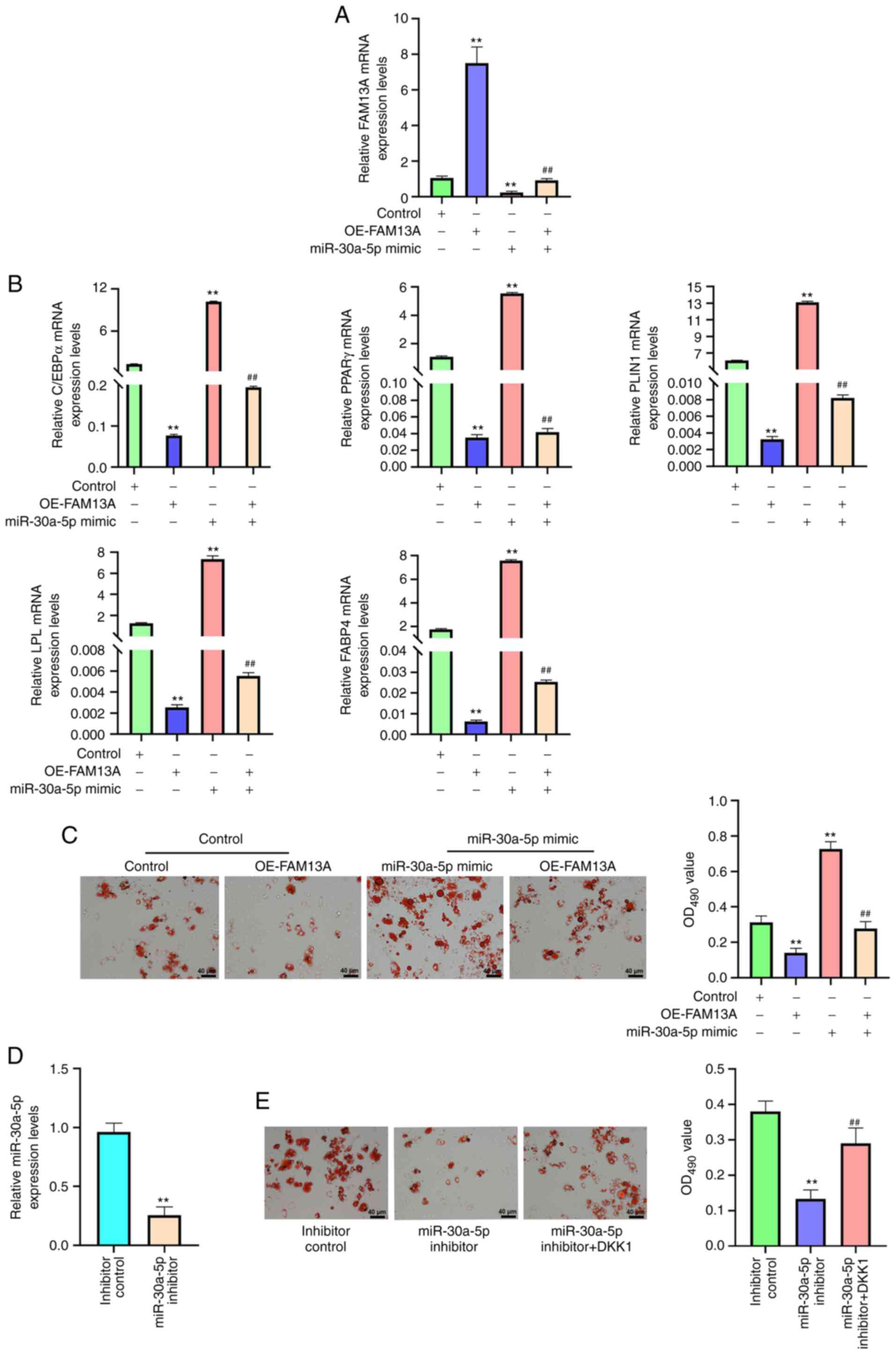 | Figure 6.miR-30a-5p promotes the adipogenic
differentiation of AA BM-MSCs via the FAM13A/Wnt/β-catenin
signaling pathway. (A-C) AA BM-MSCs were treated with OE-FAM13A,
miR-30a-5p mimic or miR-30a-5p mimic + OE-FAM13A; in the control
group, AA BM-MSCs were not treated. (A) FAM13A mRNA expression
levels were analyzed using RT-qPCR. (B) mRNA expression levels of
C/EBPα, PPARγ, PLIN1, LPL and FABP4 were analyzed using RT-qPCR.
(C) Lipid droplet accumulation was determined using Oil Red O
staining. (D) miR-30a-5p expression levels were analyzed using
RT-qPCR in BM-MSCs transfected with control inhibitor or miR-30a-5p
inhibitor. (E) Lipid droplet accumulation was determined using Oil
Red O staining in AA BM-MSCs treated with inhibitor control,
miR-30a-5p inhibitor, or co-treated with miR-30a-5p inhibitor and
DKK1. Data are presented as the mean ± SD. **P<0.01 vs. control
or inhibitor control; ##P<0.01 vs. miR-30a-5p mimic
or miR-30a-5p inhibitor. miR, microRNA; AA, aplastic anemia;
BM-MSCs, bone marrow-mesenchymal stem cells; FAM13A, family with
sequence similarity 13, member A; OE, overexpression; RT-qPCR,
reverse transcription-quantitative PCR; FABP4, fatty acid-binding
protein 4; LPL, lipoprotein lipase; PLIN1, perilipin-1; PPARγ,
peroxisome proliferator-activated receptor γ; C/EBPα,
CCAAT/enhancer binding protein α. |
Furthermore, the miR-30a-5p mimic markedly increased
the accumulation of lipid droplets, and this effect could be
blocked by FAM13A overexpression compared with that of the
miR-30a-5p mimic group (Fig. 6C).
AA BM-MSCs were also transfected with inhibitor control or
miR-30a-5p inhibitor. As shown in Fig. 6D, the miR-30a-5p inhibitor reduced
miR-30a-5p expression compared with inhibitor control group, which
validated the successful transfection (P<0.01). In addition,
miR-30a-5p inhibitor markedly reduced the levels of lipid droplets
in AA BM-MSCs, while DKK1 treatment rescued this phenotype
(Fig. 6E). Together, these
results suggested that miR-30a-5p contributes to the adipose
differentiation of AA BM-MSCs by regulating the
FAM13A/Wnt/β-catenin axis.
Discussion
AA is a severe hypocellularity of the BM
characterized by pancytopenia of the peripheral blood (48). MSCs serve as essential precursor
cells of the BM microenvironment, and differentiate into numerous
types of stromal cells, including osteoclasts, fibroblasts,
endothelial cells and adipocytes, thus providing a suitable
framework and a complex system of extracellular matrix proteins,
adhesion molecules and cytokines (49–51). Fatty BM and aberrant hematopoiesis
are the pathological hallmarks of AA, and the mechanisms of AA
adipose-osteogenic modulation are complex (52,53). As main members of the family of
non-coding RNAs and a important regulators of physiological and
pathological processes, miRNAs are involved in the modulation of AA
progression. For example, PPARγ-regulated miR-199a-5p contributes
to the adiposity of BM in AA (38). Maternally expressed 3 regulates
the expression of T cell immunoreceptor with Ig and ITIM domains
and the activation of CD4+ T cells by sponging miR-23a
(54). miR-126-5p may serve as a
diagnostic biomarker for AA (55), while miR-1202 is significantly
upregulated in patients with AA, and can potentially target
endo-α-1,2-mannosidase and Rap guanine nucleotide exchange factor 5
(15). IL-11 increases the
efficacy of hematopoietic stem cell transplant treatment in an AA
mouse model by modulating the NF-κB/miR-204/thrombopoietin
signaling pathway (56). miR-204
is involved in modulating the adipogenic differentiation of AA MSCs
(18). Moreover, it has been
reported that miR-30a-5p enhances adipocyte differentiation and
adipogenesis (22). The present
results demonstrated that miR-30a-5p was significantly elevated in
the BM-MSCs of patients with AA and in adipose-induced BM-MSCs.
Moreover, overexpression of miR-30a-5p promoted the expression of
adipogenesis-associated factors (FABP4, LPL, PLIN1, PPARγ and
C/EBPα) and increased the number of lipid droplets in BM-MSCs.
These data presented a novel function of miR-30a-5p in the
adipogenic differentiation of BM-MSCs, providing valuable evidence
for the fundamental role of miRNAs in the progression of AA.
FAM13A, an important factor in the modulation of
metabolic homeostasis, modulates insulin sensitivity and maintains
metabolism homeostasis in adipocytes (57,58). FAM13A also regulates lipid
metabolism and hepatic glucose by suppressing AMP-activated protein
kinase activity (59).
Obesity-related FAM13A is essential for insulin sensitivity and
adipose development (60). FAM13A
influences fat administration and adipocyte capacity, while FAM13A
knockdown stimulates adipogenic differentiation (30). The present study demonstrated that
FAM13A was downregulated in the BM-MSCs of patients with AA, and
FAM13A was targeted by miR-30a-5p in AA BM-MSCs. In addition,
knockdown of FAM13A promoted the expression of
adipogenesis-associated factors (FABP4, LPL, PLIN1, PPARγ and
C/EBPα) and increased the number of lipid droplets in BM-MSCs, and
overexpression of FAM13A reversed the promotive effects of
miR-30a-5p mimic on the expression of adipogenesis-associated
factors and the number of lipid droplets. These data suggested a
novel role of FAM13A in the modulation of AA BM-MSCs.
As a fundamental cellular pathway, the role of
Wnt/β-catenin signaling in adipogenic differentiation has been well
characterized (61). It has been
previously reported that the activation of Wnt/β-catenin signaling
inhibits the adipogenic differentiation of AA BM-MSCs (62). Taurine transporters control the
adipogenic differentiation of adipose stem cells by modifying
Wnt/β-catenin signaling (63).
Lysine demethylase 4A modulates osteogenic and adipogenic
differentiation via epigenetic modification of Wnt signaling
(64). miR-196a represses the
adipogenic differentiation of adipose stem cells by controlling
Wnt/β-catenin signaling (65).
Furthermore, polydatin increases MSC osteogenic differentiation by
stimulating the bone morphogenetic protein 2-Wnt/β-catenin pathway
(66). miR-199a-3p controls
BM-MSC adipose differentiation by regulating the Wnt/β-catenin
signaling pathway (67). The
present study demonstrated that FAM13A activated the Wnt/β-catenin
signaling pathway in AA BM-MSCs. Moreover, DKK1 (an inhibitor of
the Wnt/β-catenin signaling pathway) reversed the inhibitive role
of FAM13A overexpression or miR-30a-5p knockdown on the number of
lipid droplets. Taken together, miR-30a-5p contributed to AA BM-MSC
adipose differentiation by modulating the FAM13A/Wnt/β-catenin
signaling pathway. The current findings provided novel evidence
that Wnt/β-catenin signaling serves an important role in the
regulation of adipogenic differentiation in AA BM-MSCs.
However, there were some shortcomings in the present
study. Firstly, the size of BM samples was small, and future
studies should expand the sample size and include samples of
healthy donors. Secondly, the effects of DKK1 alone on BM-MSCs have
not been clearly investigated, which therefore warrants intensive
study in the future.
In conclusion, miR-30a-5p was upregulated in the
BM-MSCs of patients with AA and adipose-induced BM-MSCs. Moreover,
miR-30a-5p promoted the adipogenic differentiation of AA BM-MSCs,
which may be involved in the FAM13A/Wnt/β-catenin signaling. These
results provide a theoretical basis for the mechanism by which
miR-30a-5p and FAM13A modulate AA BM-MSC adipose differentiation
and provide promising therapeutic targets for AA treatment.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
EW and XL designed the study; YZ and RD performed
the research; XW and SZ analyzed the data; and XL wrote the
manuscript. All authors read and approved the final manuscript. YZ
and RD confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The First People's Hospital of Lianyungang (approval
no. LYGDYYY-2020-017). All patients signed the written informed
consent form.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AA
|
aplastic anemia
|
|
BM
|
bone marrow
|
|
miRNA/miR
|
microRNA
|
|
MSCs
|
mesenchymal stem cells
|
|
FAM13A
|
family with sequence similarity 13,
member A
|
|
3′-UTR
|
3′-untranslated region
|
References
|
1
|
Bacigalupo A: How I treat acquired
aplastic anemia. Blood. 129:1428–1436. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Savage SA, Viard M, O'hUigin C, Zhou W,
Yeager M, Li SA, Wang T, Ramsuran V, Vince N, Vogt A, et al:
Genome-wide association study identifies HLA-DPB1 as a significant
risk factor for severe aplastic anemia. Am J Hum Genet.
106:264–271. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang L and Liu H: Pathogenesis of aplastic
anemia. Hematology. 24:559–566. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shallis RM, Ahmad R and Zeidan AM:
Aplastic anemia: Etiology, molecular pathogenesis, and emerging
concepts. Eur J Haematol. 101:711–720. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nombela-Arrieta C, Ritz J and Silberstein
LE: The elusive nature and function of mesenchymal stem cells. Nat
Rev Mol Cell Biol. 12:126–131. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li J, Lu S, Yang S, Xing W, Feng J, Li W,
Zhao Q, Wu H, Ge M, Ma F, et al: Impaired immunomodulatory ability
of bone marrow mesenchymal stem cells on CD4(+) T cells in aplastic
anemia. Results Immunol. 2:142–147. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li J, Yang S, Lu S, Zhao H, Feng J, Li W,
Ma F, Ren Q, Liu B, Zhang L, et al: Differential gene expression
profile associated with the abnormality of bone marrow mesenchymal
stem cells in aplastic anemia. PLoS One. 7:e477642012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng HC, Liu SW, Li W, Zhao XF, Zhao X,
Cheng M, Qiu L and Ma J: Arsenic trioxide regulates adipogenic and
osteogenic differentiation in bone marrow MSCs of aplastic anemia
patients through BMP4 gene. Acta Biochim Biophys Sin (Shanghai).
47:673–679. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tripathy NK, Singh SP and Nityanand S:
Enhanced adipogenicity of bone marrow mesenchymal stem cells in
aplastic anemia. Stem Cells Int. 2014:2768622014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Deng S, Zeng Y, Wu L, Hu Z, Shen J, Shen
Y, Shen Y, Zhou Y, Chen J and Lin S: The regulatory roles of
VEGF-Notch signaling pathway on aplastic anemia with kidney
deficiency and blood stasis. J Cell Biochem. Sep 19–2018.(Epub
ahead of print).
|
|
11
|
Lu TX and Rothenberg ME: MicroRNA. J
Allergy Clin Immunol. 141:1202–1207. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou Q, Huang SX, Zhang F, Li SJ, Liu C,
Xi YY, Wang L, Wang X, He QQ, Sun CC and Li DJ: MicroRNAs: A novel
potential biomarker for diagnosis and therapy in patients with
non-small cell lung cancer. Cell Prolif. 50:e123942017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y, Niu ZY, Guo YJ, Wang LH, Lin FR
and Zhang JY: IL-11 promotes the treatment efficacy of
hematopoietic stem cell transplant therapy in aplastic anemia model
mice through a NF-κB/microRNA-204/thrombopoietin regulatory axis.
Exp Mol Med. 49:e4102017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Adhikari S and Mandal P: Integrated
analysis of global gene and microRNA expression profiling
associated with aplastic anaemia. Life Sci. 228:47–52. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hosokawa K, Kajigaya S, Feng X, Desierto
MJ, Fernandez Ibanez MD, Rios O, Weinstein B, Scheinberg P,
Townsley DM and Young NS: A plasma microRNA signature as a
biomarker for acquired aplastic anemia. Haematologica. 102:69–78.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li N, Liu L, Liu Y, Luo S, Song Y and Fang
B: miR-144-3p suppresses osteogenic differentiation of BMSCs from
patients with aplastic anemia through repression of TET2. Mol Ther
Nucleic Acids. 19:619–626. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao J, Wang C, Song Y and Fang B: Arsenic
trioxide and microRNA-204 display contrary effects on regulating
adipogenic and osteogenic differentiation of mesenchymal stem cells
in aplastic anemia. Acta Biochim Biophys Sin (Shanghai).
46:885–893. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu J, Zeng Y, Li W, Qin H, Lei Z, Shen D,
Gu D, Huang JA and Liu Z: CD73/NT5E is a target of miR-30a-5p and
plays an important role in the pathogenesis of non-small cell lung
cancer. Mol Cancer. 16:342017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li L, Kang L, Zhao W, Feng Y, Liu W, Wang
T, Mai H, Huang J, Chen S, Liang Y, et al: miR-30a-5p suppresses
breast tumor growth and metastasis through inhibition of
LDHA-mediated Warburg effect. Cancer Lett. 400:89–98. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Murinello S, Usui Y, Sakimoto S, Kitano M,
Aguilar E, Friedlander HM, Schricker A, Wittgrove C, Wakabayashi Y,
Dorrell MI, et al: miR-30a-5p inhibition promotes interaction of
Fas+ endothelial cells and FasL+ microglia to decrease pathological
neovascularization and promote physiological angiogenesis. Glia.
67:332–344. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cui S, Soni CB, Xie J, Li Y, Zhu H, Wu F
and Zhi X: MiR-30a-5p accelerates adipogenesis by negatively
regulating Sirtuin 1. Int J Clin Exp Pathol. 11:5203–5212.
2018.PubMed/NCBI
|
|
23
|
Corvol H, Hodges CA, Drumm ML and Guillot
L: Moving beyond genetics: Is FAM13A a major biological contributor
in lung physiology and chronic lung diseases? J Med Genet.
51:646–649. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liang C, Li A, Raza SHA, Khan R, Wang X,
Wang S, Wang G, Zhang Y and Zan L: The Molecular characteristics of
the FAM13A gene and the role of transcription factors ACSL1 and
ASCL2 in its core promoter region. Genes (Basel). 10:9812019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin X, Li Y, Gong L, Yun JH, Xu S,
Tesfaigzi Y, Qiao D and Zhou X: Tempo-spatial regulation of the Wnt
pathway by FAM13A modulates the stemness of alveolar epithelial
progenitors. EBioMedicine. 69:1034632021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eisenhut F, Heim L, Trump S, Mittler S,
Sopel N, Andreev K, Ferrazzi F, Ekici AB, Rieker R, Springel R, et
al: FAM13A is associated with non-small cell lung cancer (NSCLC)
progression and controls tumor cell proliferation and survival.
Oncoimmunology. 6:e12565262017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Y, Wang S, Wang C, Xiao J, Zhang S
and Zhou H: High expression of FAM13A was associated with
increasing the liver cirrhosis risk. Mol Genet Genomic Med.
7:e5432019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Corvol H, Rousselet N, Thompson KE, Berdah
L, Cottin G, Foussigniere T, Longchampt E, Fiette L, Sage E,
Prunier C, et al: FAM13A is a modifier gene of cystic fibrosis lung
phenotype regulating rhoa activity, actin cytoskeleton dynamics and
epithelial-mesenchymal transition. J Cyst Fibros. 17:190–203. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yao MY, Zhang WH, Ma WT, Liu QH, Xing LH
and Zhao GF: microRNA-328 in exosomes derived from M2 macrophages
exerts a promotive effect on the progression of pulmonary fibrosis
via FAM13A in a rat model. Exp Mol Med. 51:1–16. 2019. View Article : Google Scholar
|
|
30
|
Fathzadeh M, Li J, Rao A, Cook N,
Chennamsetty I, Seldin M, Zhou X, Sangwung P, Gloudemans MJ, Keller
M, et al: FAM13A affects body fat distribution and adipocyte
function. Nat Commun. 11:14652020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Park E, Kim J, Yeo S, Kim G, Ko EH, Lee
SW, Li WY, Choi CW and Jeong SY: Antiadipogenic effects of loganic
Acid in 3T3-L1 preadipocytes and ovariectomized mice. Molecules.
23:16632018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li X, Peng B, Zhu X, Wang P, Sun K, Lei X,
He H, Tian Y, Mo S, Zhang R and Yang L: MiR-210-3p inhibits
osteogenic differentiation and promotes adipogenic differentiation
correlated with Wnt signaling in ERα-deficient rBMSCs. J Cell
Physiol. 234:23475–23484. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang X, Wang G, Wang Y, Zhou J, Yuan H, Li
X, Liu Y and Wang B: Histone demethylase KDM7A reciprocally
regulates adipogenic and osteogenic differentiation via regulation
of C/EBPα and canonical Wnt signalling. J Cell Mol Med.
23:2149–2162. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang Y, Qi Q, Wang Y, Shi Y, Yang W, Cen
Y, Zhu E, Li X, Chen D and Wang B: Cysteine-rich protein 61
regulates adipocyte differentiation from mesenchymal stem cells
through mammalian target of rapamycin complex 1 and canonical Wnt
signaling. FASEB J. 32:3096–3107. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jin Z, Chung JW, Mei W, Strack S, He C,
Lau GW and Yang J: Regulation of nuclear-cytoplasmic shuttling and
function of Family with sequence similarity 13, member A (Fam13a),
by B56-containing PP2As and Akt. Mol Biol Cell. 26:1160–1173. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang X, Wang K, Han L, Zhang A, Shi Z,
Zhang K, Zhang H, Yang S, Pu P, Shen C, et al: PRDM1 is directly
targeted by miR-30a-5p and modulates the Wnt/β-catenin pathway in a
Dkk1-dependent manner during glioma growth. Cancer Lett.
331:211–219. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Killick SB, Bown N, Cavenagh J, Dokal I,
Foukaneli T, Hill A, Hillmen P, Ireland R, Kulasekararaj A, Mufti
G, et al: Guidelines for the diagnosis and management of adult
aplastic anaemia. Br J Haematol. 172:187–207. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang X, Liu L, Dou C, Cheng P, Liu L, Liu
H, Ren S, Wang C, Jia S, Chen L, et al: PPAR Gamma-regulated
MicroRNA 199a-5p underlies bone marrow adiposity in aplastic
anemia. Mol Ther Nucleic Acids. 17:678–687. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nandy SB, Mohanty S, Singh M, Behari M and
Airan B: Fibroblast Growth Factor-2 alone as an efficient inducer
for differentiation of human bone marrow mesenchymal stem cells
into dopaminergic neurons. J Biomed Sci. 21:832014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang D, Wang Y, Xu S, Wang F, Wang B, Han
K, Sun D and Li L: Epigallocatechin-3-gallate protects against
hydrogen peroxide-induced inhibition of osteogenic differentiation
of human bone marrow-derived mesenchymal stem cells. Stem Cells
Int. 2016:75327982016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang H, Zhang B, Tao Y, Cheng M, Hu J, Xu
M and Chen H: Isolation and characterization of mesenchymal stem
cells from whole human umbilical cord applying a single enzyme
approach. Cell Biochem Funct. 30:643–649. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Andrews FV, Kim SM, Edwards L and
Schlezinger JJ: Identifying adipogenic chemicals: Disparate effects
in 3T3-L1, OP9 and primary mesenchymal multipotent cell models.
Toxicol In Vitro. 67:1049042020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang S, Zhao C, Liu S, Wang Y, Zhao Y,
Guan W and Zhu Z: Characteristics and multi-lineage differentiation
of bone marrow mesenchymal stem cells derived from the Tibetan
mastiff. Mol Med Rep. 18:2097–2109. 2018.PubMed/NCBI
|
|
44
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sakai T, Nishida Y, Hamada S, Koike H,
Ikuta K, Ota T and Ishiguro N: Immunohistochemical staining with
non-phospho β-catenin as a diagnostic and prognostic tool of COX-2
inhibitor therapy for patients with extra-peritoneal desmoid-type
fibromatosis. Diagn Pathol. 12:662017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yoshida Y, Yamasaki S, Oi K, Kuranobu T,
Nojima T, Miyaki S, Ida H and Sugiyama E: IL-1β enhances wnt signal
by inhibiting DKK1. Inflammation. 41:1945–1954. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jumpertz S, Hennes T, Asare Y, Schutz AK
and Bernhagen J: CSN5/JAB1 suppresses the WNT inhibitor DKK1 in
colorectal cancer cells. Cell Signal. 34:38–46. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
El-Mahgoub ER, Ahmed E, Afifi RA, Kamal MA
and Mousa SM: Mesenchymal stem cells from pediatric patients with
aplastic anemia: Isolation, characterization, adipogenic, and
osteogenic differentiation. Fetal Pediatr Pathol. 33:9–15. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Medinger M, Drexler B, Lengerke C and
Passweg J: Pathogenesis of acquired aplastic anemia and the role of
the bone marrow microenvironment. Front Oncol. 8:5872018.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gonzaga VF, Wenceslau CV, Lisboa GS, Frare
EO and Kerkis I: Mesenchymal stem cell benefits observed in bone
marrow failure and acquired aplastic anemia. Stem Cells Int.
2017:80765292017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li Y, Wang F, Guo R, Zhang Y, Chen D, Li
X, Tian W, Xie X and Jiang Z: Exosomal sphingosine 1-phosphate
secreted by mesenchymal stem cells regulated Treg/Th17 balance in
aplastic anemia. IUBMB Life. 71:1284–1292. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sieff CA: Introduction to acquired and
inherited bone marrow failure. Hematol Oncol Clin North Am.
32:569–580. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Luzzatto L and Risitano AM: Advances in
understanding the pathogenesis of acquired aplastic anaemia. Br J
Haematol. 182:758–776. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang J, Liu X, Hao C, Lu Y, Duan X, Liang
R, Gao G and Zhang T: MEG3 modulates TIGIT expression and CD4 + T
cell activation through absorbing miR-23a. Mol Cell Biochem.
454:67–76. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Giudice V, Banaszak LG,
Gutierrez-Rodrigues F, Kajigaya S, Panjwani R, Ibanez MDPF, Rios O,
Bleck CK, Stempinski ES, Raffo DQ, et al: Circulating exosomal
microRNAs in acquired aplastic anemia and myelodysplastic
syndromes. Haematologica. 103:1150–1159. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang Y, Niu ZY, Guo YJ, Wang LH, Lin FR
and Zhang JY: IL-11 promotes the treatment efficacy of
hematopoietic stem cell transplant therapy in aplastic anemia model
mice through a NF-κB/microRNA-204/thrombopoietin regulatory axis.
Exp Mol Med. 49:e4102017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lundback V, Kulyte A, Strawbridge RJ,
Ryden M, Arner P, Marcus C and Dahlman I: FAM13A and POM121C are
candidate genes for fasting insulin: Functional follow-up analysis
of a genome-wide association study. Diabetologia. 61:1112–1123.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wardhana DA, Ikeda K, Barinda AJ, Nugroho
DB, Qurania KR, Yagi K, Miyata K, Oike Y, Hirata KI and Emoto N:
Family with sequence similarity 13, member A modulates adipocyte
insulin signaling and preserves systemic metabolic homeostasis.
Proc Natl Acad Sci USA. 115:1529–1534. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lin X, Liou YH, Li Y, Gong L, Li Y, Hao Y,
Pham B, Xu S, Jiang Z, Li L, et al: FAM13A represses AMPK activity
and regulates hepatic glucose and lipid metabolism. iScience.
23:1009282020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tang J, Zhou H, Sahay K, Xu W, Yang J,
Zhang W and Chen W: Obesity-associated family with sequence
similarity 13, member A (FAM13A) is dispensable for adipose
development and insulin sensitivity. Int J Obes (Lond).
43:1269–1280. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Xu C, Wang J, Zhu T, Shen Y, Tang X, Fang
L and Xu Y: Cross-talking between PPAR and WNT signaling and its
regulation in mesenchymal stem cell differentiation. Curr Stem Cell
Res Ther. 11:247–254. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yuan Z, Li Q, Luo S, Liu Z, Luo D, Zhang
B, Zhang D, Rao P and Xiao J: PPARγ and Wnt signaling in adipogenic
and osteogenic differentiation of mesenchymal stem cells. Curr Stem
Cell Res Ther. 11:216–225. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Hou X, Wang Z, Ding F, He Y, Wang P, Liu
X, Xu F, Wang J and Yang Y: Taurine transporter regulates
adipogenic differentiation of human adipose-derived stem cells
through affecting Wnt/β-catenin signaling pathway. Int J Biol Sci.
15:1104–1112. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Qi Q, Wang Y, Wang X, Yang J, Xie Y, Zhou
J, Li X and Wang B: Histone demethylase KDM4A regulates adipogenic
and osteogenic differentiation via epigenetic regulation of C/EBPα
and canonical Wnt signaling. Cell Mol Life Sci. 77:2407–2421. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ai G, Meng M, Wang L, Shao X, Li Y, Cheng
J, Tong X and Cheng Z: microRNA-196a promotes osteogenic
differentiation and inhibit adipogenic differentiation of adipose
stem cells via regulating β-catenin pathway. Am J Transl Res.
11:3081–3091. 2019.PubMed/NCBI
|
|
66
|
Chen XJ, Shen YS, He MC, Yang F, Yang P,
Pang FX, He W, Cao YM and Wei QS: Polydatin promotes the osteogenic
differentiation of human bone mesenchymal stem cells by activating
the BMP2-Wnt/beta-catenin signaling pathway. Biomed Pharmacother.
112:1087462019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Shuai Y, Yang R, Mu R, Yu Y, Rong L and
Jin L: MiR-199a-3p mediates the adipogenic differentiation of bone
marrow-derived mesenchymal stem cells by regulating KDM6A/WNT
signaling. Life Sci. 220:84–91. 2019. View Article : Google Scholar : PubMed/NCBI
|