Introduction
The increasing occurrence of retinal
neovascularization (RNV) threatens the quality of vision of
individuals suffering from ocular neovascular diseases, for
instance, proliferative diabetic retinopathy (PDR), retinal vein
occlusions (RVO), retinopathy of prematurity (ROP) and wet
age-related macular degeneration (wAMD) (1). Hypoxia occurs in these types of
ocular disorders and is shown to be the key inducer in the
development of RNV (2,3). Hypoxia can stimulate diseased and
dysfunctional cells to release non-physiological dose of angiogenic
factors, thereby inducing morphological and functional alterations
in the eye and even finally causing blindness (4). Current clinical treatment
strategies, including intravitreally injection of anti-VEGF are
sight-saving, but restricted by invasiveness, late intervention and
high cost (5). Thus, it is urgent
to advance a novel therapy to simultaneously protect neuronal and
vascular functions in ischemic retinopathy diseases.
Angiogenin (ANG), a member of the pancreatic
ribonuclease superfamily, is one of the most effective angiogenic
factors in the body and has the ability to stimulate the vascular
endothelial cells to promote cell proliferation and enhance tubular
structure formation (6). ANG can
accelerate the process of angiogenesis due to its specific
capabilities as a transcription factor and a secreted protein.
After being translated to the cytoplasm, ANG is instantly
transported to the nucleus and then carries out angiogenic
functions with angiogenesis inducers (e.g., VEGF) (7). Therefore, ANG may show a crucial
role to induce angiogenesis in RNV and the molecular mechanism
requires to be explored.
Brain-derived neurotrophic factor (BDNF), a humoral
protein, can bind with tropomyosin receptor kinase B (TrkB) on
nerve cells and subsequently activates growth of neurons (8). Previous studies report that BDNF
signaling also promotes cells growth in addition to neural cells,
such as vascular endothelial cells (9,10).
BDNF has been shown to be an angiogenic inducer similar to VEGF.
For retina, a representative of neurovascular subunits, neurons and
glial cells probably interact with blood vessels to regulate
pathologic neovascularization by relieving growth factors and
guidance cues (11). Thus, the
potential role of BDNF in RNV is needed to be clearly
elucidated.
MicroRNA (miRNA) is a highly conserved endogenous,
small non-coding RNA and it can serve a role in regulating gene
expression at the transcriptional or post-transcriptional level
(12). It has been reported that
miRNAs are enrolled in various types of activities and functions of
endothelial cells, such as angiogenesis (13–15). A single miRNA can target hundreds
of mRNAs, while each mRNA sustains various miRNA-response elements
that can be targeted by different miRNAs. Therefore, different
mRNAs can be modulated by a same miRNA. These RNA transcripts serve
the part of competing endogenous RNAs (ceRNAs) (16). They can crosstalk with and
co-regulate each other by competing for binding to shared miRNAs
(17). Nevertheless, whether this
modulatory mechanism of ceRNA happens between ANG- and
BDNF-mediated angiogenesis in RNV remains to be elucidated. The
present study hypothesized that ANG and BDNF expression is
interdependently responsible for hypoxia through competition for a
common miRNA in the development of RNV.
Materials and methods
Ethics statement
All animal procedures were examined and approved by
The Animal Ethics Committee of Renji Hospital of Shanghai Jiaotong
University (approval no. SHJT-MRJ-2020-091). All surgeries were
carried out under general anesthesia by sodium pentobarbital and
best efforts were made to minimize the suffering of animals.
Oxygen-induced retinopathy (OIR) mouse
model
Pregnant female C57BL/6J mice (weight, 22–25 g; age,
10 weeks old; n=15) were purchased from the Laboratory Animal
Center of Renji Hospital. Mice were housed in standard plastic
rodent cages with free access to food and water, and maintained in
a controlled environment (24°C, 12-h light/dark cycle). The mouse
model of OIR exposed 7-day-old (P7) mouse pups to 75±2% oxygen for
5 days (until P12). The animals were returned to room air for
another 5 days until P17. The OIR model prepared by the above
method can be used to study and describe the mechanisms of initial
vessel loss (P7-P12), vascular regrowth (P12-P17) and
neovascularization (P14-P17), respectively (18). Western blotting and PCR detection
were carried out at P17. The mice were anesthetized with 2% sodium
pentobarbital in a dose of 45 mg/kg. Finally, the eyeballs were
extracted, and the cervical spine of the mice were dislocated under
anesthesia.
Cell culture and treatment
Human retinal microvascular endothelial cells
(HRECs) were supplied by Angio Proteomie and cultured with
endothelial cell medium including 5% fetal bovine serum (FBS) and
1% endothelial cell growth supplement (ScienCell Research
Laboratories, Inc.). HRECs were cultured in a humidified atmosphere
with 37°C and 5% CO2. For hypoxic stress, HRECs were
seeded into a 30-mm dish (Corning Life Sciences) and cultured in a
humidified atmosphere with 37°C and 5% CO2; the medium
was replaced when the cell confluence reached ~80%. Subsequently,
HRECs were placed in a sealed and anaerobic workstation (Ruskin
Technologies) at the atmosphere of 1% O2, 5%
CO2, 94% N2, 90% humidity and 37°C, as under
hypoxic conditions for 48 or 72 h.
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was extracted from HRECs
(~6×108 cells) exposed to hypoxia and retinal tissues by
an Eastep Super Total RNA Extraction kit, supplied by Promega
(Shanghai, China), according to the instructions of the kit. The
concentration and purity of RNA were tested by a NanoDrop 2000c
Spectrophotometer (Thermo Fisher Scientific, Inc.). For analysis of
mRNAs, 1,000 ng total RNA was reverse transcribed into cDNA by a
Perfect Real Time RT Reagent kit, obtained from Takara
Biotechnology Co., Ltd., in a 20 µl reaction volume. The reverse
transcription was performed at 37°C for 15 min, 85°C for 5 sec,
then cooled to 4°C for maintenance. To investigate the expression
of miRNA, 1,000 ng total RNA was polyadenylated and reverse
transcribed using TransScript Green miRNA RT SuperMix, which was
purchased from TransGen Biotech Co., Ltd. The reverse transcription
was performed at 37°C for 60 min, 85°C for 5 sec, then decreased to
4°C for maintenance. SYBR Green Master Mix (Roche Diagnostics GmbH)
was used for the PCR analysis of mRNAs. TransScript Green miRNA
qPCR SuperMix (TransGen Biotech Co., Ltd.) was used to explore the
expression of miRNA according to the manufacturer's instructions.
The following thermocycling conditions were used for the qPCR:
Initial denaturation at 94°C for 3 min, followed by 40 cycles at
94°C for 20 sec, 56°C for 20 sec and 72°C for 20 sec. Melting curve
analysis was conducted after every run by heating to 95°C to
monitor the presence of unspecific products. The relative
expression level of mRNAs or miRNAs was represented via the
equation of 2−ΔΔCq (19). The primer sequences were: Human
GAPDH: sense 5′-TGCACCACCAACTGCTTAGC-3′, anti-sense
5′-GGCATGGACTGTGGTCATGAG-3′; Human ANG: sense
5′-ATGGCAACAAGCGCAGCATC-3′, anti-sense 5′-CGGACGACGGAAAATTGACTG-3′;
Human BDNF: sense 5′-GTTTGTGTGGACCCCGAGTT-3′, anti-sense
5′-GCAGCCTTCATGCAACCAAA-3′; Mouse β-actin: sense
5′-GGCTGTATTCCCCTCCATCG-3′,
anti-sense:5′-CCAGTTGGTAACAATGCCATGT-3′; Mouse ANG: sense
5′-CATCCCAACAGGAAGGAAGGA-3′, anti-sense 5′-ACCTGGAGTCATCCTGAGCC-3′;
Mouse BDNF: sense 5′-AATGTCTGACCCCAGTGCCT-3′, anti-sense
5′-ATGTTTGCGGCATCCAGGTA-3′.
Western blotting
The samples of cells or retinal tissues were lysed
on ice for 60 min using Total Protein Extraction Buffer with
protease inhibitor (TransGen Biotech Co., Ltd.) and followed by
being sonicated (20 KHz; 4°C; 5 sec; three times) referring to the
manufacturer's instructions. Protein concentrations were determined
using a BCA protein assay kit (Beyotime Institute of
Biotechnology). The protein lysates (15 µg) were obtained and
electrophoresed on 10% SDS polyacrylamide gels and then transferred
onto polyvinylidene difluoride membranes (MilliporeSigma).
Subsequently, the samples were blocked with 5% skimmed milk for 1 h
at room temperature. Primary antibodies against ANG (1:500; cat.
no. DF6449; Affinity Biosciences, Ltd.), BDNF (1:500; cat. no.
ab205067; Abcam) and β-actin (1:1,000; cat. no. AT0001; CMCTAG)
were added at 4°C overnight. Membranes were then incubated with
secondary antibodies (1:5,000; cat. nos. 32230 and 32260;
Invitrogen; Thermo Fisher Scientific, Inc.) for 40 min at room
temperature. Finally, an enhanced chemiluminescence (ECL) Plus kit
(EMD Millipore) was used for visualization according to the
manufacturer's instructions. The gray bands were analyzed by the
ImageJ software (1.50i version; National Institutes of Health). The
fold changes between different groups were demonstrated as the
ratio of experimental group vs. the control group.
miRNA mimic transfection
HRECs growing at logarithmic growth stage were
plated in a 6-well plate. On reaching ~80% confluence, HRECs were
transfected with 50 nmol miRNA mimic or scramble mimic using
Lipofectamine® RNAiMAX transfection reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. Following transfection for 5 h in a humidified
atmosphere with 37°C and 5% CO2, the cells were changed
with fresh medium and cultured under hypoxia. After transfection
for 48 h, the cell samples were collected for subsequent mRNA
detection. Following transfection for 72 h, the samples were
gathered and protein level investigated. The sequence of miR-182-5p
mimic was 5′-UUUGGCAAUGGUAGAACUCACACU-3′ and that of the scramble
was 5′-CUCUCCGAACGUGUCACGUTTUUC-3′; they were chemically
synthesized by Shanghai GenePharma Co., Ltd.
Luciferase assay
TargetScan (http://www.targetscan.org/vert_72) was used to predict
the targets of miRNA. The constructs of luciferase were constructed
by combining oligonucleotides containing the wild-type (WT) or
mutant (MUT) putative target sites of the ANG or BDNF
3′-untranslated region (3′-UTR) into the multiple cloning site of
the pmirGLO vector (Promega Corporation). 293 cells were
cotransfected with WT constructs, or MUT constructs, or vector and
miRNA mimics or scramble miRNA using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). At 48 h after
transfection, luciferase detections were carried out by the
Dual-Glo Luciferase Assay System (E2920; Promega Corporation)
according to the manufacturer's instructions. Light emission was
determined by the GloMax 96 Microplate Luminometer (Promega
Corporation). Firefly luciferase activity was represented by
normalizing to that of Renilla luciferase.
Cell viability assessment
MTS assay (Promega Corporation) was used to detect
cell proliferation activity. HRECs were cultured under normal or
hypoxic conditions with transfections as previously described and
then plated at a density of 2×103 cells/well in a
96-well plate. Prior to evaluation, the medium was replaced by 100
µl fresh medium and 20 µl MTS was added into the samples. After
culturing for 1 h in the incubator under normal oxygen, the
absorbance of each samples was detected at 490 nm.
Transwell assay
The migratory capacity of HRECs under hypoxic
conditions was evaluated by the Transwell assay according to the
manufacturer's protocols. In brief, 5×103 cells from
each sample were placed in the top chambers of the Transwell
chamber with 8.0-µm pore polycarbonate membrane inserts (3422;
Corning Life Sciences) and incubated with 200 µl medium with 5%
FBS. The bottom chambers of the Transwell were filled with 500 µl
medium containing 20% FBS. After incubating for 24 h in a
humidified atmosphere with 37°C and 5% CO2, HRECs that
remained on the top chamber were removed and migrated cells were
fixed by 4% paraformaldehyde for 20 min and then stained with 0.1%
crystal violet solution for 10 min for observation, both at room
temperature. The number of migrated cells were observed using a
light microscope and counted in five fields randomly at ×10
magnification. Quantitative analysis was conducted by Image J
software (1.50i version; National Institutes of Health).
Tube formation analysis
The ability of angiogenesis of HRECs was detected by
the tube formation assay. In brief, the 96-well plate was coated
with 50 µl of Matrigel Basement Membrane Matrix (BD Biosciences)
and polymerized for 1 h at 37°C. Subsequently, HRECs were added
gently in the Matrigel-treated plate at the density of
8×103 cells per well with 100 µl medium and cultured for
8 h at 37°C. The angiogenic network of tubes was observed and
images captured using a light microscope and counted in five fields
randomly at ×10 magnification. Quantitative analysis of tube
formation was calculated by Image J software (1.50i version;
National Institutes of Health).
Statistical analysis
All experiments in the present study were carried
out at least three times independently. The data was demonstrated
as the mean ± standard deviation. Unpaired Student's t-test or
one-way analysis of variance followed by Tukey's test were
conducted by the GraphPad Prism 8.0 software (GraphPad Software,
Inc.) to evaluate statistical differences. P<0.05 was considered
to indicate a statistically significant difference.
Results
Downregulated expression of miR-182-5p
observed with the upregulation of ANG and BDNF in OIR mouse
model
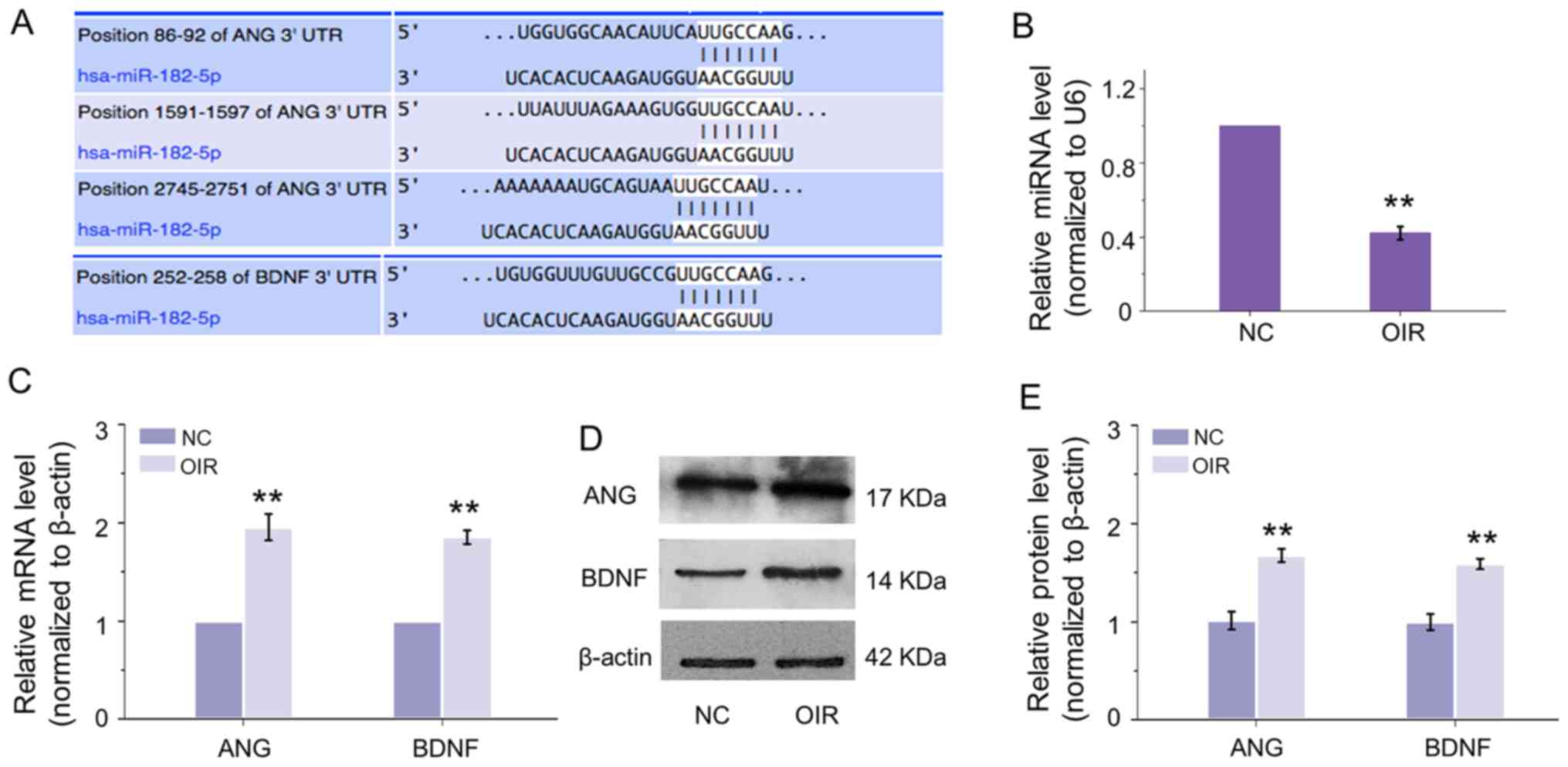
To predict the potential regulation of miRNA on ANG
and BDNF, bioinformatics analysis was conducted. From the result
shown in Fig. 1A, there are
binding sites on the 3′-UTR of ANG and BDNF of a common miRNA,
which is miR-182-5p. To explore the molecular mechanism of
miR-182-5p on the regulation of ANG and BDNF in RNV, an OIR mouse
model was firstly established. It was found that the expression
level of miR-182-5p was inhibited in retinas of OIR (Fig. 1B), while the mRNA expression of
ANG and BDNF was upregulated (Fig.
1C). Consistent with the transcriptional expression, the
protein levels of ANG and BDNF were enhanced significantly
(Fig. 1D and E). These
observations revealed that miR-182-5p may regulate ANG and BDNF
negatively and showed a critical function in the progression of
RNV.
Hypoxia induces downregulation of
miR-182-5p and overexpression of ANG and BDNF in HRECs in
vitro
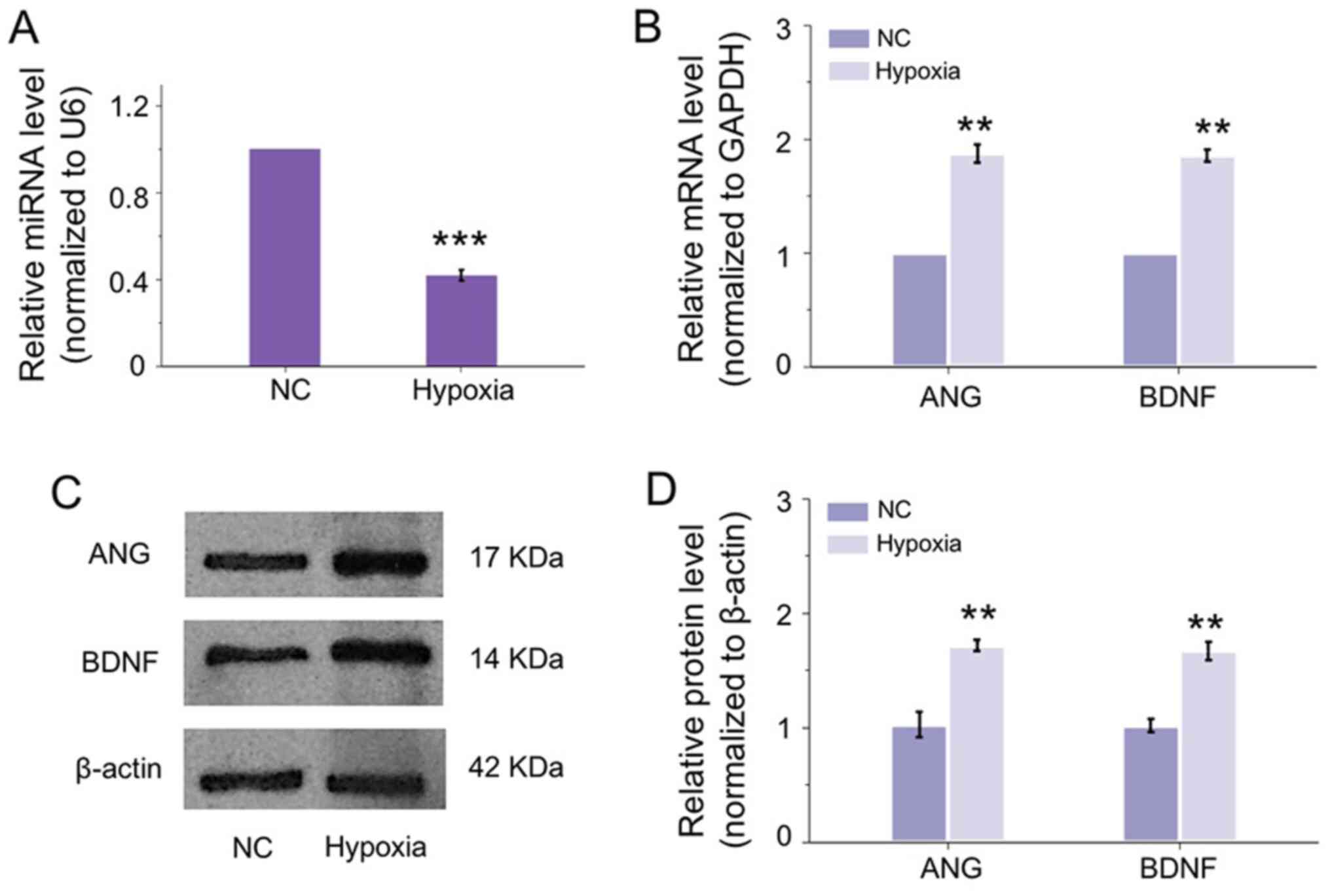
To verify whether the observation in the OIR mouse
model in vivo existed in vitro, HRECs were applied
and exposed to hypoxia to mimic the circumstance of cells under RNV
condition. Unsurprisingly, miR-182-5p was inhibited significantly
by hypoxia in HRECs (Fig. 2A). In
accordance with the changes in OIR retina, the mRNA expressions of
ANG and BDNF were upregulated induced by hypoxia (Fig. 2B). In addition, the translational
expression of ANG and BDNF in HRECs was correspondingly increased
under hypoxia (Fig. 2C and D).
These results indicated the miR-182-5p may regulate the expression
of ANG and BDNF in the development of RNV in vivo and in
vitro. The molecular mechanism requires further
exploration.
miR-182-5p targets ANG and BDNF
directly
As the downregulation of miR-182-5p may be relevant
to the upregulation of ANG and BDNF in HRECs and in OIR retina, the
transfection interference experiment was performed to detect the
regulatory effect of miR-182-5p on ANG and BDNF. With the
transfection of miR-182-5p mimic, its expression level was
increased >20-fold compared with either hypoxic or hypoxic with
scramble transfection group. While scramble transfection did not
result in any prominent change in miR-182-5p expression of HRECs
under hypoxia compared with hypoxic group the two groups were
inhibited compared with normal group (Fig. 3A). In addition, the
hypoxia-induced increased mRNA expression and protein levels of ANG
and BDNF in HRECs were clearly reduced with the upregulation of
miR-182-5p (Fig. 3B-D). These
revealed miR-182-5p could regulate the expression of ANG and BDNF
in HRECs exposed to hypoxic condition. Subsequently, luciferase
assay was performed to explore if miR-182-5p can target ANG and
BDNF directly. By constructing the WT or MUT version of the
predicted binding region in the ANG or BDNF 3′-UTR, the luciferase
activity results demonstrated that co-transfection of 293 cells
with 3′-UTR WT plasmid of ANG or BDNF and miR-182-5p mimic caused a
significant downregulation in luciferase activity compared with the
scramble mimic. By contrast, miR-182-5p mimic indicated no
inhibition on the luciferase activity of the MUT plasmid or vector
(Fig. 3E and F). Therefore, ANG
and BDNF could be suppressed by miR-182-5p directly in HRECs under
hypoxic condition.
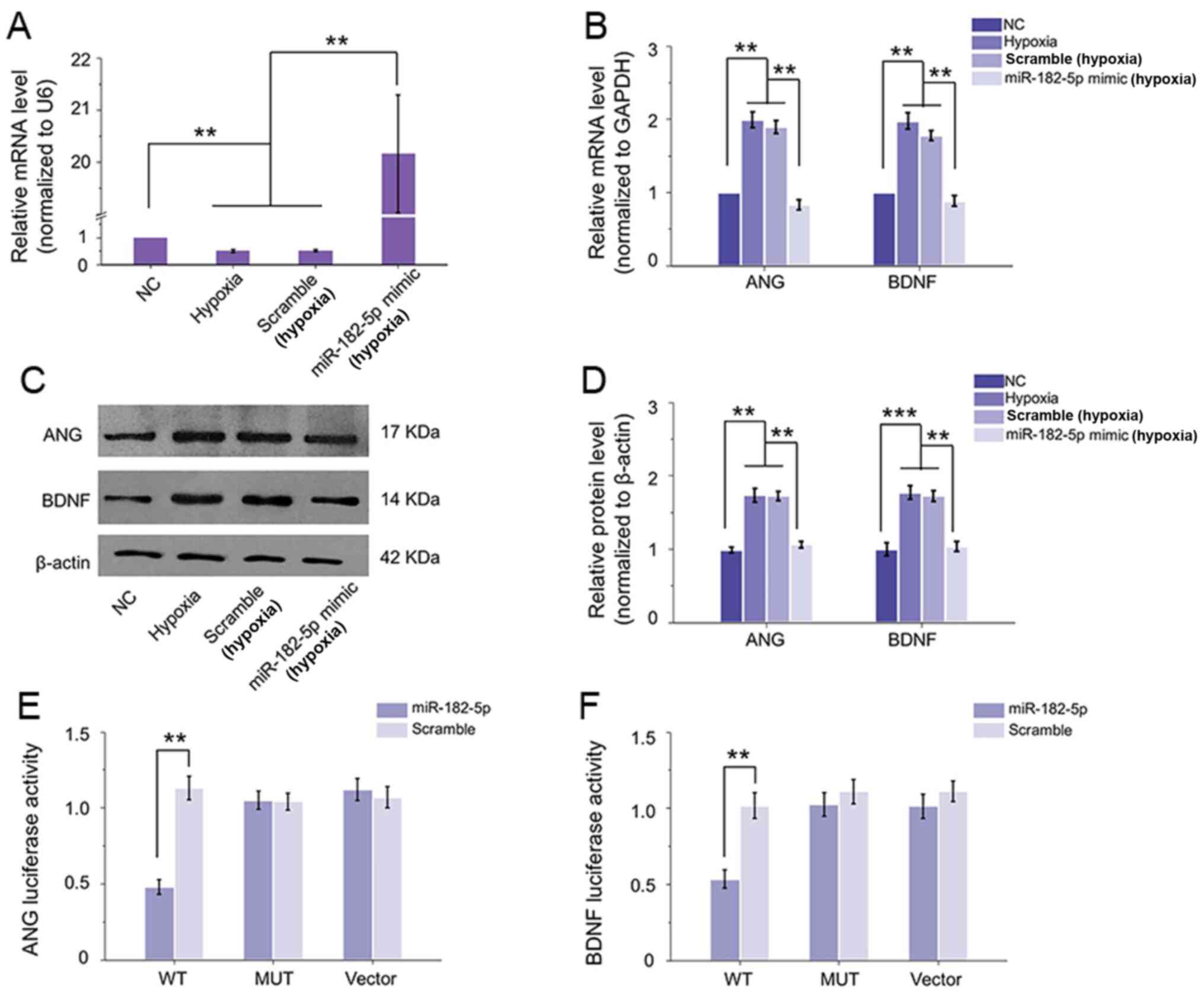 | Figure 3.miR-182-5p can target ANG and BDNF
directly. (A) Following transfection with miR-182-5p mimic, its
expression was significantly elevated compared with hypoxic
condition, while scramble did not induce any change. (B)
Overexpression of miR-182-5p inhibited hypoxia-caused high mRNA
levels of ANG and BDNF in HRECs, while scramble could not affect
their expression. (C and D) Western blotting revealed the
inhibitory effect of miR-182-5p on ANG and BDNF protein expression.
(E and F) Luciferase activity with various reporters was detected
in the presence or absence of miR-182-5p mimic in 293 cells.
miR-182-5p mimic could effectively inhibit the luciferase activity
of WT vectors of both ANG and BDNF compared with scramble
transfection, neither MUT vector or vector showed a change with
miR-182-5p mimic or scramble. **P<0.01; ***P<0.001 vs.
relative control group (n=6). miR, microRNA; ANG, angiogenin; BDNF,
brain-derived neurotrophic factor; HRECs, human retinal
microvascular endothelial cells; WT, wild-type; MUT, mutant; NC,
negative control. |
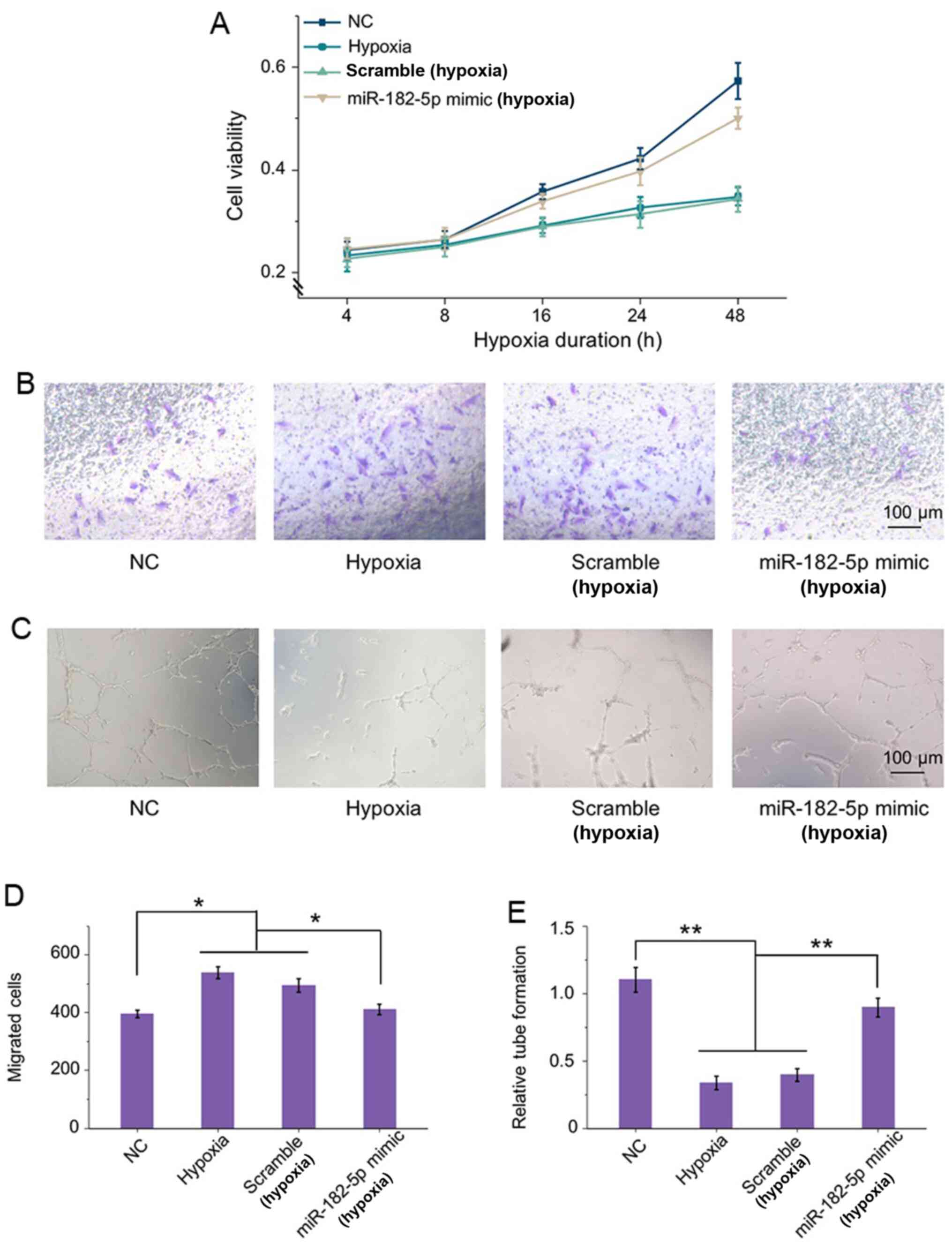
Inhibition of ANG and BDNF by
miR-182-5p improves cell functions in HRECs exposed to hypoxia
The effects of miR-182-5p inhibiting ANG and BDNF on
HRECs under RNV condition were then evaluated. Compared with
normoxic condition, there was a significant effect on cell
viability of HRECs under hypoxia. Knockdown of ANG and BDNF by
miR-182-5p mimic transfection elevated the viability for >48 h.
While the scramble transfection did not induce any significant
change in cell viability compared with the hypoxic cells (Fig. 4A).
Transwell assays demonstrated that hypoxia can
significantly enhance cell migration compared with that in normal
groups (Fig. 4B). However, there
was no significant difference in the results of migration in
untransfected cells and scramble cells under hypoxia. In addition,
under hypoxic condition, miR-182-5p overexpression protected
against hypoxia-stimulated HRECs migration. The quantification also
confirmed that the migrated cells were stimulated by hypoxia and
could be significantly ameliorated by miR-182-5p transfection
(Fig. 4D). Thus, inhibition of
ANG and BDNF by miR-182-5p targeting under hypoxia reduced cell
motility effectively.
In addition, Matrigel tube formation was evaluated
in HRECs incubated under the same circumstance as previously
described. As shown in Fig. 4C,
hypoxic conditions could lead to a morphological change in HRECs
and cause damage on the tube network formation compared with the
normal group. In addition, ANG and BDNF intervention by miR-182-5p
clearly enhanced the angiogenic ability of HRECs by inducing tube
formation to 2-fold relative to that of the negative transfection
cells in hypoxic conditions (Fig.
4E). These findings indicated that knockdown of ANG and BDNF by
miR-182-5p showed a protective function in HREC viability,
motility, as well as tube integrity under RNV conditions.
Correspondingly, inhibition of ANG and BDNF by miRNA regulation
improved HREC morphology in RNV and might be a potential strategy
for clinical treatment.
Discussion
Ocular diseases with RNV, including PDR, ROP and
RVO, are commonly characterized by the pathological angiogenesis in
the retina, finally leading to vision loss (20). Hypoxia is a crucial pathologic
circumstance and stimulates the stimulation of proangiogenic
factors supporting the formation of neovascularization in the
progress of retinal neovascular diseases (21). Retinal angiogenesis is formed by
the coordinate induction of a group of growth factor genes,
especially VEGF. Therefore, the majority studies focus on finding
alternative strategies for the development of anti-angiogenic VEGF
inhibitors for curing retinal neovascularization (22,23). However, a large number of
preclinical investigations and clinical researches about retinal
disorders indicate that limitations to anti-VEGF strategies may
exist. Some patients do not respond to anti-VEGF treatment and some
suffered from recurrences of neovascularization and bleeding. In
addition, the secretion of growth factors besides VEGF may
influence the response to anti-VEGF strategies in RNV diseases
(24–26). Thus, it remains crucial for
investigating the production of other proangiogenic factors and the
breach of blood-retina barrier.
ANG is one of the most potential factors related to
angiogenesis and it stimulates vessel formation by inducing the
vessel endothelial and smooth muscle cells and activating a series
of biological stages, including cell proliferation, migration and
invasion, as well as tubular structures formation (27,28). ANG also accelerates the
degradation of basement membrane and extracellular matrix and
promotes the migration capacity of the cells (29). Thus, the effects of ANG indicate
its potential role in retinal microvascular endothelial cells in
RNV. Angiogenesis is modulated by various types of factors,
including growth factors and neurotrophins. BDNF is a type of
neurotrophin and is recognized to regulate the nervous system in
development, maintenance and plasticity (30). In addition, there are several
types of cancer, such as multiple myeloma, breast cancer and
thyroid cancer, that overexpress neurotrophins, including BDNF,
which can be conducive to angiogenesis and tumor progression
(31). Additionally, it is
reported that BDNF displayed basic functions in non-neuronal
tissues (32). It can be
hypothesized that BDNF has a critical function in the progression
of angiogenesis in RNV. Therefore, the interdependent crosstalk
between ANG and BDNF in retinal angiogenesis deserves further
exploration. The present study revealed a significant elevation of
ANG and BDNF in vivo in the OIR model and in vitro in
HRECs under hypoxic condition. To detect the modulatory mechanism
on ANG and BDNF in RNV, non-coding RNA regulation stimulated was
investigated.
miRNAs are small, non-coding RNAs that can bind to
the 3′-UTR of target mRNAs to lead mRNA degradation or prohibit
protein translation (14). More
and more miRNAs have been identified in abnormal expression,
indicating a crucial role in retinal neovascular diseases such as
DR (15,33,34). A previous study established the
biogenesis, roles and functions of various miRNAs in the modulation
of pathological ocular NV, revealing miRNAs as both biomarkers and
therapeutic targets in vascular eye diseases (35). To compete for a common miRNA,
protein-coding mRNAs may crosstalk with others without direct
binding. By using TargetScan prediction, miR-182-5p may have a
regulatory effect on ANG and BDNF. It was found that the expression
of miR-182-5p was inhibited in vivo and in vitro and
that overexpression of miR-182-5p could significantly restrain the
expression levels of mRNA and protein of ANG and BDNF in
vitro. In addition, the inhibitory effect of miR-182-5p on ANG
and BDNF was confirmed by luciferase assay. These results
demonstrated that miR-182-5p could target ANG and BDNF directly.
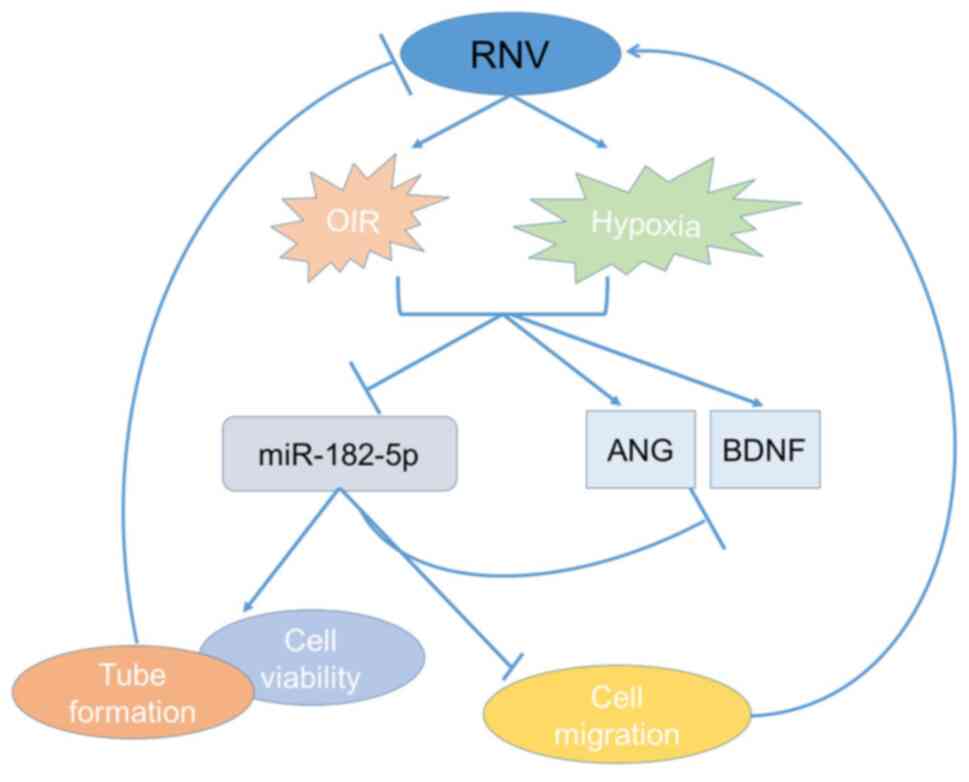
Knockdown of ANG and BDNF by miR-182-5p upregulation protected
HRECs against hypoxia-induced impairment, including enhancing cell
viability, reducing cell migration and sustaining vascular tube
network (Fig. 5). In addition, in
a previous study, miR-182-5p also serves roles in angiogenesis in
other vascular diseases (36). In
colon cancer, miR-182-5p regulates tumorigenesis partially by
regulating angiogenesis and lymphangiogenesis by targeting VEGF-C
and therefore retarding ERK and AKT signaling pathways (36). This implied that miR-182-5p could
regulate angiogenesis through different pathways. Thereby,
miR-182-5p can be a potential therapeutic target to treat RNV.
The present study suggested that there was a
cross-talk between ANG and BDNF mediated by the competition for
miR-182-5p binding. Increased miR-182-5p expression resulted in a
significant downregulation of ANG and BDNF and this regulation had
a crucial function in the development of RNV including DR, RVO, ROP
and other retinal diseases (e.g., age-related macular
degeneration). miR-182-5p-based intervention not only affects the
expression of growth factor-ANG, but also alters the level of
neurotrophins-BDNF. Thus, miR-182-5p/ANG/BDNF cross-talk can have a
clinical significance for the treatment of retinal neovascular
disease.
Acknowledgements
Not applicable.
Funding
This study was funded by Changhai Hospital Teaching Research and
Reform Project Fund in 2019 (grant no. CHJG2019012).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CL, HL and WS conceived and designed the
experiments. CL and HL contributed to the acquisition of data. CL
and HL analyzed and interpreted the data. CL, HL and WS contributed
to drafting the article. All authors have revised the manuscript
critically for important intellectual content. All authors read and
approved the final manuscript. CL, HL and WS confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by The Animal Ethics
Committee of Renji Hospital of Shanghai Jiaotong University
(approval no. SHJT-MRJ-2020-091). All surgeries were carried out
under general anesthesia by sodium pentobarbital and best efforts
were made to minimize the suffering of animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rajappa M, Saxena P and Kaur J: Ocular
angiogenesis: Mechanisms and recent advances in therapy. Adv Clin
Chem. 50:103–121. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Usui-Ouchi A, Aguilar E, Murinello S,
Prins M, Gantner ML, Wright PE, Berlow RB and Friedlander M: An
allosteric peptide inhibitor of HIF-1α regulates hypoxia-induced
retinal neovascularization. Proc Natl Acad Sci (USA).
117:28297–28306. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Berkowitz BA: Hypoxia and retinal
neovascularization. Retinal and Choroidal Angiogenesis Scientific
Symposium; Nashville, TN: pp. 151–168. 2005
|
|
4
|
Siemerink MJ, Augustin AJ and Schlingemann
RO: Mechanisms of ocular angiogenesis and its molecular mediators.
Dev Ophthalmol. 46:4–20. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Antonetti DA, Klein R and Gardner TW:
Diabetic retinopathy. N Engl J Med. 366:1227–1239. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pavlov N and Badet J: Angiogenin:
Involvement in angiogenesis and tumour growth. Bull Cancer.
88:725–732. 2001.(In French). PubMed/NCBI
|
|
7
|
Kishimoto K, Liu S, Tsuji T, Olson KA and
Hu GF: Endogenous angiogenin in endothelial cells is a general
requirement for cell proliferation and angiogenesis. Oncogene.
24:445–456. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Allen SJ and Dawbarn D: Clinical relevance
of the neurotrophins and their receptors. Clin sci (Lon).
110:175–191. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kermani P, Rafii D, Jin DK, Whitlock P,
Schaffer W, Chiang A, Vincent L, Friedrich M, Shido K, Hackett NR,
et al: Neurotrophins promote revascularization by local recruitment
of TrkB+ endothelial cells and systemic mobilization of
hematopoietic progenitors. J Clin Invest. 115:653–663. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matsuda S, Fujita T, Kajiya M, Takeda K,
Shiba H, Kawaguchi H and Kurihara H: Brain-derived neurotrophic
factor induces migration of endothelial cells through a
TrkB-ERK-integrin αVβ3-FAK cascade. J Cell Physiol. 227:2123–2129.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Salis MB, Graiani G, Desortes E, Caldwell
RB, Madeddu P and Emanueli C: Nerve growth factor supplementation
reverses the impairment, induced by Type 1 diabetes, of hindlimb
post-ischaemic recovery in mice. Diabetologia. 47:1055–1063. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leung A and Natarajan R: Noncoding RNAs in
vascular disease. Curr Opin Cardiol. 29:199–206. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gong Q and Su G: Roles of miRNAs and long
noncoding RNAs in the progression of diabetic retinopathy. Biosci
rep. 37:BSR201711572017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gong Q, Xie J, Liu Y, Li Y and Su G:
Differentially Expressed MicroRNAs in the Development of Early
Diabetic Retinopathy. J Diabetes Res. 2017:p1–10. 2017. View Article : Google Scholar
|
|
16
|
Giza DE, Vasilescu C and Calin GA:
MicroRNAs and ceRNAs: Therapeutic implications of RNA networks.
Expert Opin Biol Ther. 14:1285–1293. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nitzan M, Steiman-Shimony A, Altuvia Y,
Biham O and Margalit H: Interactions between distant ceRNAs in
regulatory networks. Biophys J. 106:2254–2266. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim CB, D'Amore PA and Connor KM:
Revisiting the mouse model of oxygen-induced retinopathy. Eye
Brain. 8:67–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(T (−Delta Delta C)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Selvam S, Kumar T and Fruttiger M: Retinal
vasculature development in health and disease. Prog Retin Eye Res.
63:1–19. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Campochiaro PA: Molecular pathogenesis of
retinal and choroidal vascular diseases. Prog Retin Eye Res.
49:67–81. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lutty GA and McLeod DS: Development of the
hyaloid, choroidal and retinal vasculatures in the fetal human eye.
Prog Retin Eye Res. 62:58–76. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rattner A, Williams J and Nathans J: Roles
of HIFs and VEGF in angiogenesis in the retina and brain. J Clin
Invest. 129:3807–3820. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wallsh JO and Gallemore RP:
Anti-VEGF-Resistant Retinal Diseases: A Review of the Latest
Treatment Options. Cells. 10:10492021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Seah I, Zhao X, Lin Q, Liu Z, Su SZZ, Yuen
YS, Hunziker W, Lingam G, Loh XJ and Su X: Use of biomaterials for
sustained delivery of anti-VEGF to treat retinal diseases. Eye
(Lond). 34:1341–1356. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Michl M, Fabianska M, Seeböck P,
Sadeghipour A, Haj Najeeb B, Bogunovic H, Schmidt-Erfurth UM and
Gerendas BS: Automated quantification of macular fluid in retinal
diseases and their response to anti-VEGF therapy. Br J Ophthalmol.
0:1–8. 2020.
|
|
27
|
Viallard C and Larrivée B: Tumor
angiogenesis and vascular normalization: Alternative therapeutic
targets. Angiogenesis. 20:409–426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gao X and Xu Z: Mechanisms of action of
angiogenin. Acta Biochim Biophys Sin (Shanghai). 40:619–624. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yao X, Li D, Xiong DM, Li L, Jiang R and
Chen JX: A novel role of ribonuclease inhibitor in regulation of
epithelial-to-mesenchymal transition and ILK signaling pathway in
bladder cancer cells. Cell Tissue Res. 353:409–423. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Numakawa T, Richards M, Adachi N, Kishi S,
Kunugi H and Hashido K: MicroRNA function and neurotrophin BDNF.
Neurochem Int. 59:551–558. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pearse RN, Swendeman SL, Li Y, Rafii D and
Hempstead BL: A neurotrophin axis in myeloma: TrkB and BDNF promote
tumor-cell survival. Blood. 105:4429–4436. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Garrido MP, Torres I, Vega M and Romero C:
Angiogenesis in Gynecological Cancers: Role of Neurotrophins. Front
Oncol. 9:9132019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gong Q, Li F, Xie J and Su G: Upregulated
VEGF and Robo4 correlate with the reduction of miR-15a in the
development of diabetic retinopathy. Endocrine. 65:35–45. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gong Q, Xie J, Li Y, Liu Y and Su G:
Enhanced ROBO4 is mediated by up-regulation of HIF-1α/SP1 or
reduction in miR-125b-5p/miR-146a-5p in diabetic retinopathy. J
Cell Mol Med. 23:4723–4737. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu CH, Huang S, Britton WR and Chen J:
MicroRNAs in Vascular Eye Diseases. Int J of Mol Sci. 21:649
View Article : Google Scholar
|
|
36
|
Yan S, Wang H, Chen X, Liang C, Shang W,
Wang L, Li J and Xu D: MiR-182-5p inhibits colon cancer
tumorigenesis, angiogenesis, and lymphangiogenesis by directly
downregulating VEGF-C. Cancer Lett. 488:18–26. 2020. View Article : Google Scholar : PubMed/NCBI
|