Introduction
Cardiovascular disease (CVD) is the leading cause of
human mortality worldwide accounted for 32.9% of all deaths with a
steadily reaching of the number of CVD deaths from 12.1 million in
1990 to 18.6 million in 2019 (1),
and places a notable burden on human health (2,3).
At present, management of patients with CVD is challenging due to
the high mortality, disability rates and rising health care costs
(4). According to the World
Health Organization, three-quarters of CVD deaths could be
prevented through early detection and lifestyle interventions
(5). As a result, CVD has
attracted the attention of research communities and healthcare
professionals to ensure that measures, including developing the
care of cardiovascular disease in the ambulatory setting, improving
approaches to the prevention of cardiovascular disease, expanding
digital health interventions and universal health coverage
(6), have been taken.
Pharmaceutical developments and technological improvements are used
in the Western world to decrease CVD-associated mortality,
alongside interventions to control risk factors for CVD (7). Among risk factors for CVD,
pathological myocardial hypertrophy (MH) caused by long-term
overload is an independent risk factor for adverse cardiovascular
events (8). Therefore, reversing
MH may minimize the risk of cardiovascular events.
MH refers to an adaptive and compensatory change in
heart structure that maintains cardiac output during adverse
stimulation (9). The hypertrophic
process is categorized as physiological or pathological.
Physiological hypertrophy is key to preservation of normal heart
structure and function; it is a tightly regulated but reversible
phenomenon, such as the hypertrophic process that occurs in the
heart following continuous exercise (10). This is a compliant change (an
adaptive response to stimuli) in the heart and causes negligible
effects in the body. However, pathological hypertrophy is
associated with fibrosis, increased proinflammatory cytokine
production and cellular dysfunction (11). This type of maladaptive cardiac
hypertrophy, typically caused by chronically high, continuous
hemodynamic pressure, is irreversible and leads to heart failure in
severe cases (12).
Angiotensin-converting enzyme inhibitors (13,14), angiotensin II receptor blockers
(15,16), calcium channel antagonists,
β-receptor blockers and diuretics (17,18) are currently the primary treatments
for MH. They have all been reported to improve the clinical
symptoms of patients to a certain extent; however, the
single-target therapeutic efficacy of these agents remains
unsatisfactory as none of them significantly decrease mortality
rate (19). Therefore, MH needs
to be addressed in the CVD research. Traditional Chinese medicine
(TCM) has been reported to improve the symptoms and quality of life
of patients with heart failure (20). It has been reported that TCM
confers therapeutic benefits using natural, biologically active
ingredients that have multiple targets (21). Furthermore, agents such as
Radixastragali (Huangqi) and Ginseng (Renshen) (22,23) have gained attention due to their
stable therapeutic effects by targeting multiple targets underlying
the development of MH. Astragaloside IV(AS-IV) which is the main
component of Radixastragali, improves cardiac function and
ameliorate MH, which is mediated by attenuating inflammatory
reaction (24) and inactivating
TANK-binding kinase 1 (TBK1)/PI3K/AKT signaling pathway (25). The pharmacological properties of
ginseng are mainly attributed to ginsenosides which plays a robust
antihypertrophic role via inhibition of NHE-1-dependent calcineurin
activation (26) and the
suppression of MCT-activated calcineurin and ERK signaling pathways
(27).
Based on clinical experience and systematic theory,
advances have been achieved in the research of TCM formulas,
extracts and compounds (28). For
example, research and development of ‘compound Danshen dropping
pill’ has contributed to treatment of coronary heart disease and
angina pectoris (29). Numerous
compounds isolated from TCM products have important properties,
such as the antimalarial effects of artemisinin (30) and the anti-inflammatory properties
of licorice extract, which contains 3 triterpenes and 13 flavonoids
(31). Furthermore, the curative
effects of novel TCM compounds that exert specific pharmacological
effects may be enhanced by elucidating their mechanism individually
whilst also characterizing their target profile. Ultimately,
treatment strategies for MH may be developed using the
multi-faceted profiles of TCM compounds combined with metabolomics
and traditional pharmacological methods (32).
Puerarin (PU) is an isoflavone chemical component
(33) found within the
Pueraria genera of plants (34). PU has been reported to exert
numerous therapeutic effects, including anti-cancer, vasodilatory
and heart protective effects (35). Furthermore, it has been previously
reported that PU ameliorates pressure overload-induced MH in
ovariectomized rats via activation of the peroxisome
proliferator-activated receptor-α/peroxisome proliferator-activated
receptor γ-1 signaling pathway and regulation of energy metabolism
remodeling (36). However, the
multi-target characteristics of TCM monomers suggest that PU has
multiple therapeutic targets (37). Numerous signaling pathways are
associated with the pathophysiology of MH, including the
5′AMP-activated protein kinase/mTOR (38), PI3K/AKT/mTOR (39) and insulin-like growth factor
1/PI3K/AKT (40) signaling
pathways. Therefore, the exploration of the mechanism of PU on MH
to achieve the effect of precise treatment is urgent. Numerous
studies have reported that the Wnt signaling pathway, which is
activated in early embryonic development to promote cardiac
development and silenced in adulthood to maintain normal cardiac
function, serves an important role in CVD (41,42). As experimental data on the effects
of PU on the Wnt signaling pathway in the context of MH remain
incomplete, the present study was preformed to address this.
Materials and methods
Chemicals and reagents
PU (purity, 99.00%) was purchased from TargetMol
Chemicals, Inc. Isoproterenol hydrochloride (ISO), Dickkopf-1
(DKK1) and IM-12 were purchased from MedChemExpress. Cell Counting
Kit-8 (CCK-8) assay was purchased from Dojindo Molecular
Technologies, Inc. PVDF membranes, RIPA buffer, Actin-Tracker Green
(microfilament green fluorescent probe), DAPI Staining Solution and
BCA Protein Assay kit were purchased from Beyotime Institute of
Biotechnology. TransScript® Green Two-Step qRT-PCR
SuperMix kit was purchased from TransGen Biotech Co., Ltd.
Antibodies against low-density lipoprotein receptor-related protein
6 (LRP6; cat. no. 3395), phosphorylated (p-)p65 (cat. no. 3033),
NF-κB p65 (cat. no. C22B4), Tubulin (cat. no. 2128S), β-catenin
(cat. no. 8480), Wnt5a/b (cat. no. 2530), c-Myc (cat. no. 5605),
glycogen synthase kinase-3β (GSK3β; cat. no. 12456) and p-GSK3β
(cat. no. 9322) were purchased from Cell Signaling Technology, Inc.
Atrial natriuretic peptide (ANP; cat. no. ab189921) and brain
natriuretic peptide (BNP, cat. no. ab236101) were purchased from
Abcam. GAPDH (cat. no. BM1623) and the secondary antibodies
including HRP Conjugated AffiniPure Goat Anti-Rabbit IgG (cat. No.
BA1054) and HRP Conjugated AffiniPure Mouse Anti-Human IgG (cat.
No. BM2002) was from Boster.
Cells
The human cardiomyocyte AC16 cell line (MingzhouBio
Inc., cat. no. MZ-4038) was used. Cells were cultured in culture
flasks with DMEM (BasalMedia Inc.) at 37°C under 5% CO2
in a humidified atmosphere. When cells reached the logarithmic
growth phase, they were evenly distributed into six-well plates.
Experimental treatments were performed after 24 h.
In vitro cardiac hypertrophy model and
drug treatment
Cells were randomly divided as follows: i) Control,
cells treated with DMEM; ii) ISO, cells treated with 10 µM ISO for
24 h; iii) PU, cells treated with 40 µM PU for 6 h followed by 10
µM ISO for 24 h; iv) DKK1, cells treated with 780 nM DKK1 for 6 h
followed by 10 µM ISO for 24 h; v) IM12, cells treated with 30 nM
IM-12 for 24 h; vi) PU + IM12, cells treated with 40 µM PU for 6 h
followed by 30 nM IM-12 for 24 h and vi) PU + ISO + IM12, cells
treated with 40 µM PU for 6 h followed by 10 µM ISO for 24 h and 30
nM IM-12 for 6 h. A total of 1.5×105 cells were plated
in culture flasks with DMEM at 37°C under 5% CO2 in a
humidified atmosphere. The cells were washed three times with PBS
before addition of new drugs to avoid mixing of different drug
components.
CCK-8 assay
PU was dissolved in DMSO and DMEM to produce a range
of concentrations (5, 10, 20, 40 and 80 µM) for subsequent use. The
final DMSO content was 0.2%. Cells were evenly dispersed into six
groups containing different concentrations of PU (0, 5, 10, 20, 40
and 80 µM) and inoculated into a 96-well plate (~5,000 cells/well).
PU incubation was performed for 6 h at 37°C under 5% CO2
before 100 µl CCK-8 reagent was added, followed by incubation for 2
h at 37°C under 5% CO2 in a humidified atmosphere. The
96-well plate was assessed using a PT-3502PC microplate reader
(Beijing Potenov Technology Co. Ltd.) to quantify the absorption
value of each well at 450 nm. A line chart was constructed plotting
optical density (OD) values against drug concentrations to assess
the effect of PU on cell viability.
Cytoskeletal staining
The surface area of AC16 cardiomyocytes in the
different treatment groups was assessed using cytoskeletal
staining. Control, ISO and PU), cells were washed with PBS twice
and fixed using 3.7% formaldehyde solution at room temperature for
10 min in the dark according to the manufacturer's protocol for the
Actin-Tracker Green. PBS containing 0.1% Triton X-100 was added to
permeabilize the fixed cells for 10 min at room temperature. The
cells were stained with Actin-Tracker Green working solution
diluted with PBS containing 1–5% BSA and 0.1% Triton X-100 at a
ratio of 1:50 for 30 min at room temperature. Following rinsing
with PBS, 5 mg/ml DAPI (sufficient to cover each section) was added
to each well for 3–5 min at room temperature before imaging using a
fluorescence microscope (Olympus Corporation). The magnification
was 400×. A total of five non-overlapping fields located at the
upper and lower left and right and center of each well was
selected. The cells in each field were numbered and sampled by a
random number generator. A total of four cells was randomly
extracted from each field. Finally, the size of cells was
quantified using ImageJ2 (version 1.53; National Institutes of
Health). The mean area of the selected cells was determined for
each well and normalized to the control.
Reverse transcription-quantitative
(RT-q)PCR
RNA was extracted from AC16 cells (Control, ISO and
PU) using TRIzol® (Thermo Fisher Scientific, Inc.). The
concentration of RNA was detected using a NanoDrop®
spectrophotometer (Thermo Fisher Scientific, Inc.). Complementary
DNA synthesis, using incubation at 42°C for 15 min and heating at
85°C for 5 sec, was performed according to the manufacturer's
protocols of the TransScript® Green Two-Step qRT-PCR
SuperMix kit. The primer sequences were as follows: ANP forward
(F), 5′-CAACGCAGACCTGATGGATTT-3′ and reverse (R),
5′-AGCCCCCGCTTCTTCATTC-3′; BNP F, 5′-TGGAAACGTCCGGGTTACAG-3′ and R,
5′-CTGATCCGGTCCATCTTCCT-3′; β-myosin heavy chain (β-MHC) F,
5′-TCACCAACAACCCCTACGATT-3′ and R, 5′-CTCCTCAGCGTCATCAATGGA-3′ and
GAPDH F, 5′-CTGGGCTACACTGAGCACC-3′ and R,
5′-AAGTGGTCGTTGAGGGCAATG-3′. The reaction systems of each primer
sequence were added into a 96-well fluorescent qPCR plate and CFX
Connect Real-Time PCR Detection System (Bio-Rad Laboratories,
Inc.). The thermocycling conditions were as follows: 94°C initial
denaturation for 30 sec, followed by 40 cycles of 5 sec at 94°C and
30 sec at 60°C. The 2−ΔΔCq method (43) was used to assess mRNA expression
levels.
Western blotting
The cells in each group (Control, ISO, PU, DKK1,
IM12, PU + IM12, PU + ISO + IM12) were digested with 0.25% trypsin
for 25 sec at 37°C under 5% CO2 in a humidified
atmosphere, washed with PBS three times and lysed with RIPA lysis
buffer containing protease and phosphate inhibitors at 4°C for 30
min. The supernatant was collected following centrifugation (12,000
× g at 4°C for 10 min), before protein concentration was quantified
via BCA assay. An equal amount of protein (15 µg protein/lane) was
separated by 10% SDS-PAGE and transferred onto PVDF membranes. PVDF
membranes were blocked with 5% skimmed milk powder solution at room
temperature for 1 h before being incubated at 4°C overnight with
specific primary antibodies. The antibodies were as follows: LRP6
(1:1,000), p-p65 (1:1,000), p65 (1:1,000), Tubulin (1:10,000),
β-catenin (1:1,000), Wnt5a/b (1:1,000), GAPDH (1:5,000), c-Myc
(1:1,000), GSK3β (1:1,000), p-GSK3β (1:1,000), ANP (1:1,000) and
BNP (1:1,000). After washing with TBST (0.1% Tween-20), membranes
were incubated with anti-rabbit (1:5,000) and anti-mouse secondary
antibodies (1:5,000) at room temperature for 1 h. The membranes
were rinsed again and treated with BeyoECL Moon (cat. no. P0018FS,
Beyotime Institute of Biotechnology) before bands were visualized
using the Tanon-4600 Chemiluminescence Imaging System. Finally,
blots were analyzed using ImageJ2 (version 1.53; National
Institutes of Health) to semi-quantify the protein expression
levels.
Statistical analysis
All statistical analysis was performed using
GraphPad Prism (version, 9.1.1; GraphPad Software, Inc.).
Comparisons between groups were performed using one-way ANOVA with
Dunnett's (Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7) or Tukey's post hoc test
(Fig. 8). Data are presented as
the mean ± SEM of ≥3 independent experimental repeats. P<0.05
was considered to indicate a statistically significant
difference.
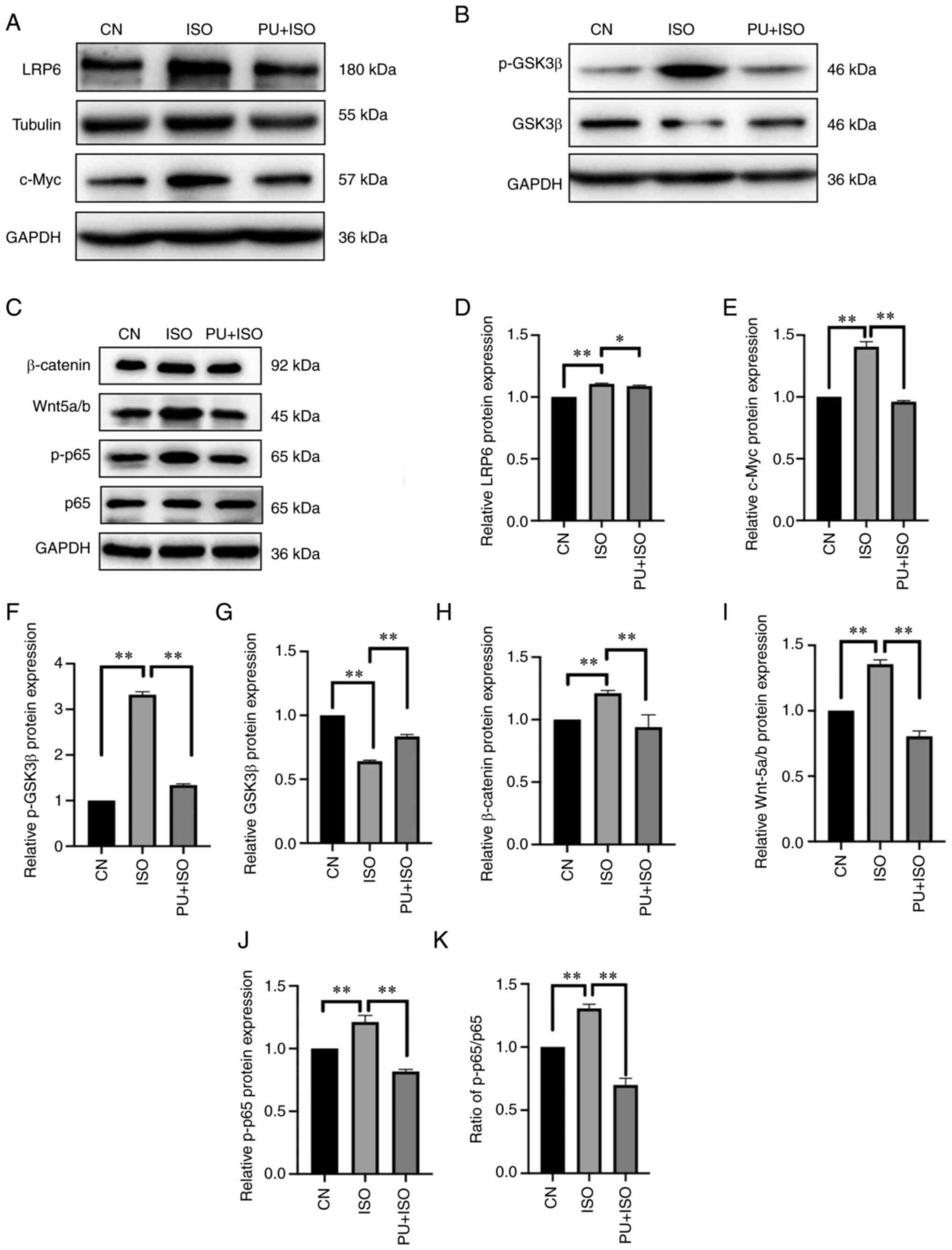 | Figure 5.Effect of PU on expression of
components in the Wnt/β-catenin and NF-κB signaling pathways. (A)
Representative western blotting images of LRP6, c-Myc, p-GSK3β and
GSK3β. (B) Representative western blotting images of p-GSK3β and
GSK3β. (C) Representative western blotting images of β-catenin,
Wnt5a/b, p-p65 and p65. (D) LRP6, (E) c-Myc, (F) p-GSK3β, (G)
GSK3β, (H) β-catenin, (I) Wnt5a/b and (J) p-p65 protein expression
levels were semi-quantified. (K) Ratio of p-p65/p-65. The blots
were analyzed using ImageJ. *P<0.05 and **P<0.01. CN,
control; ISO, isoproterenol; PU, puerarin; LRP6, low-density
lipoprotein receptor-related protein 6; p-, phosphorylated; GSK3β,
glycogen synthase kinase 3β. |
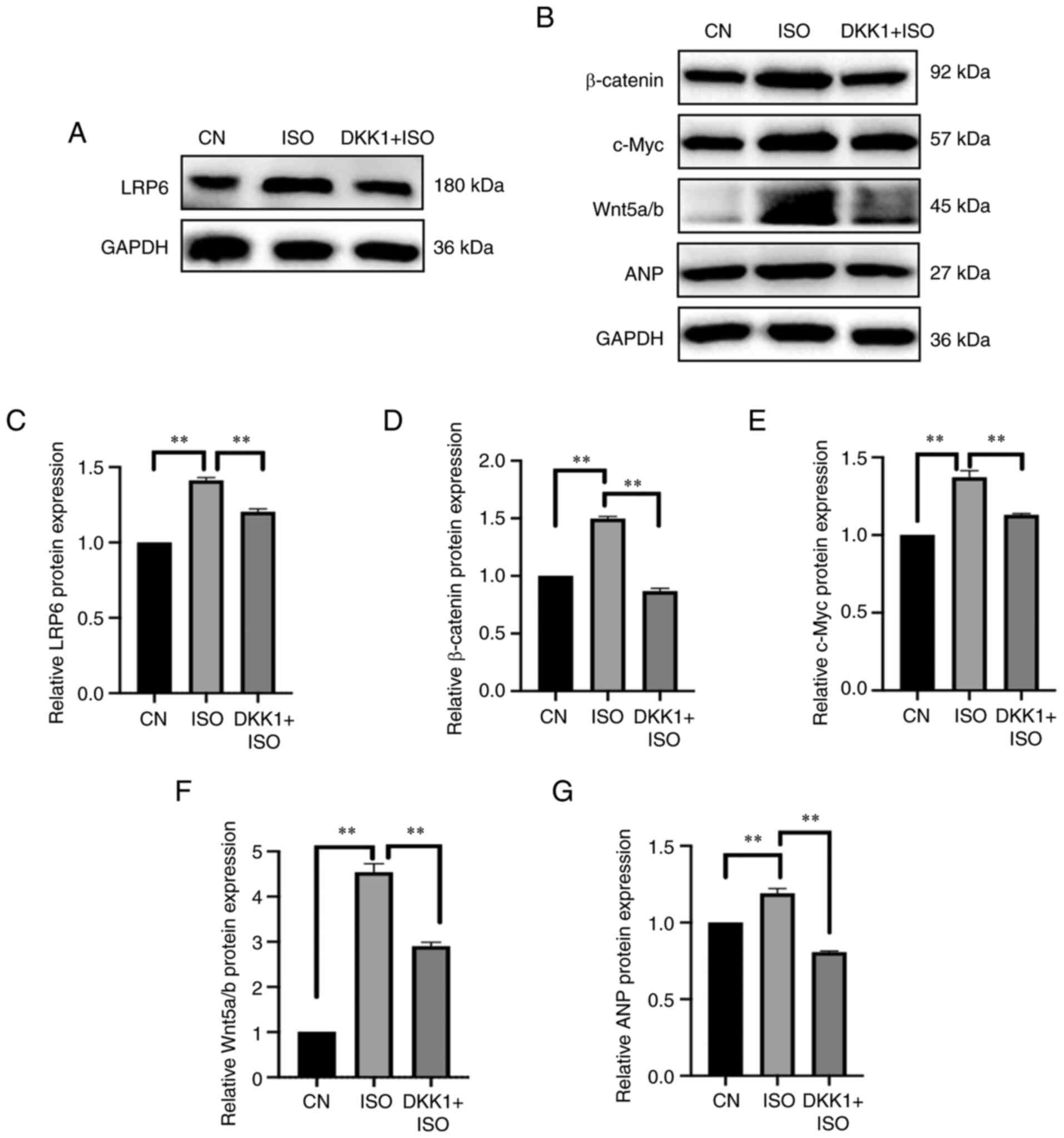 | Figure 6.Effect of DKK1 on expression of
protein in the signaling Wnt pathway. (A) Representative western
blotting images of LRP6. (B) Representative western blotting images
of β-catenin, Wnt5a/b, c-Myc and ANP. (C) LRP6, (D) β-catenin, (E)
c-Myc, (F) Wnt5a/b and (G) ANP protein expression levels were
semi-quantified. The blots were analyzed using ImageJ. **P<0.01.
DKK1, Dickkopf-1; CN, control; ISO, isoproterenol; LRP6,
low-density lipoprotein receptor-related protein 6; ANP, atrial
natriuretic peptide. |
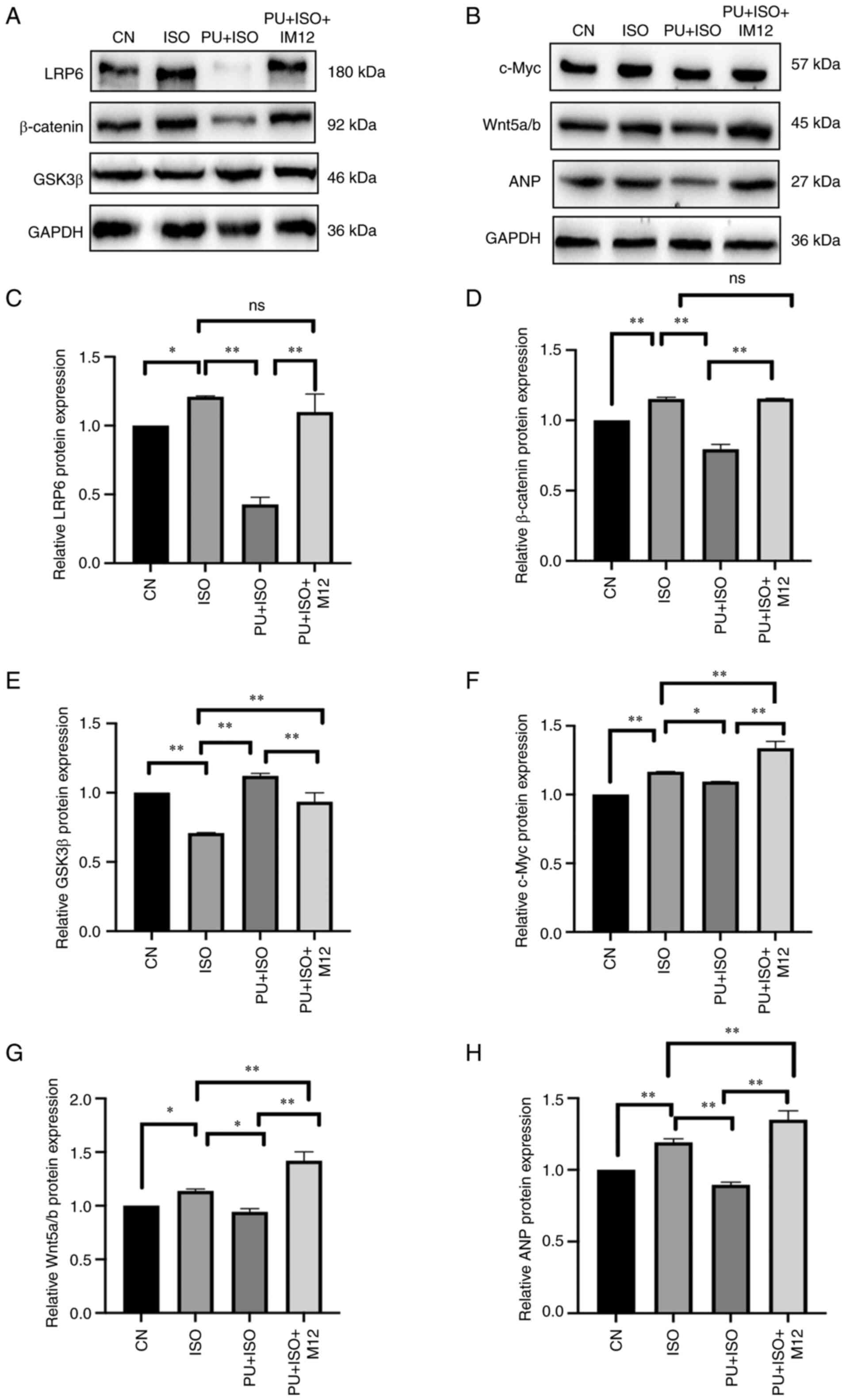 | Figure 8.Effect of ISO, PU and IM-12 on
expression of proteins in the Wnt signaling pathway. (A)
Representative western blotting images of LRP6, GSK3β and β-catenin
expression. (B) Representative western blotting images of c-Myc,
Wnt5a/b and ANP expression. (C) LRP6, (D) β-catenin, (E) GSK3β, (F)
c-Myc, (G) Wnt5a/b and (H) ANP protein expression levels were
semi-quantified. The blots were analyzed using ImageJ. *P<0.05
and **P<0.01. CN, control; ISO, isoproterenol; PU, puerarin;
LRP6, low-density lipoprotein receptor-related protein 6; GSK3β,
glycogen synthase kinase 3β; ANP, atrial natriuretic peptide; ns,
not significant. |
Results
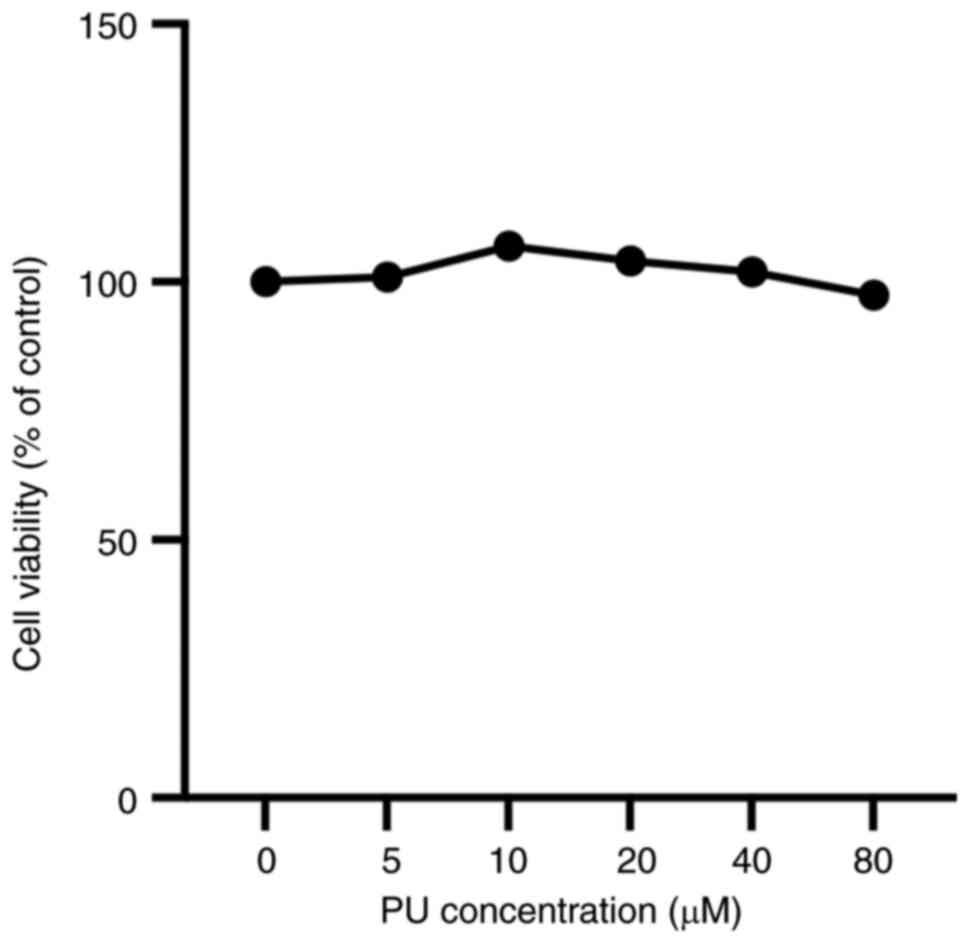
PU is not toxic to cardiomyocytes
CCK-8 assay OD values were used to assess the
effects of PU on AC16 cell viability. The results demonstrated no
significant difference in cell viability between treatment groups
(Fig. 1). These data demonstrated
that the highest concentration of PU (80 µM) had little effect on
cell viability after 6 h.
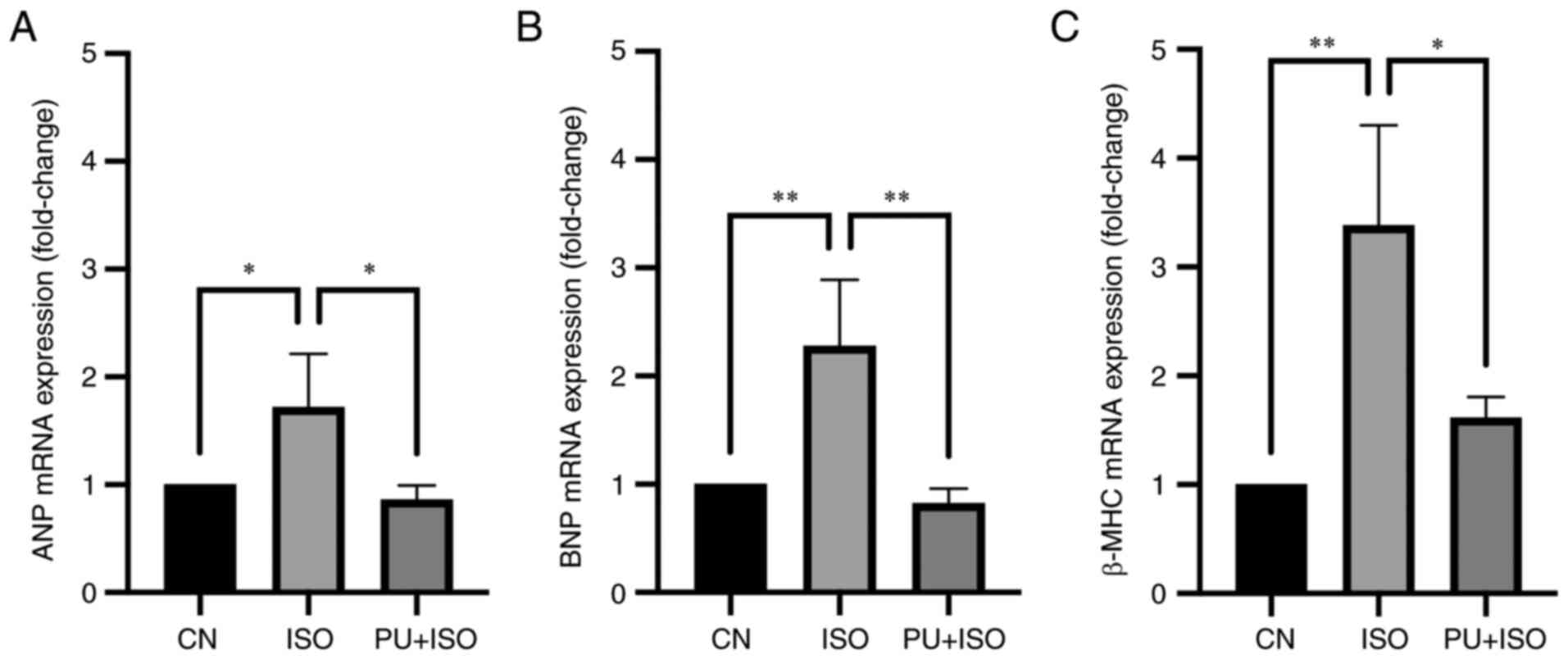
PU decreases ANP, BNP and β-MHC
transcription
The mRNA expression levels of MH markers ANP, BNP
and β-MHC were quantified using RT-qPCR. The results demonstrated
significant increases in mRNA expression levels of ANP (P<0.05;
Fig. 2A), BNP (P<0.01;
Fig. 2B) and β-MHC (P<0.01;
Fig. 2C) in the ISO group
compared with the control. By contrast, treatment with PU
significantly decreased mRNA expression levels of ANP (P<0.05),
BNP (P<0.01) and β-MHC (P<0.05) compared with the ISO group.
These results suggested that the ISO-induced MH model was
successfully established and the effect of ISO was reversed by
PU.
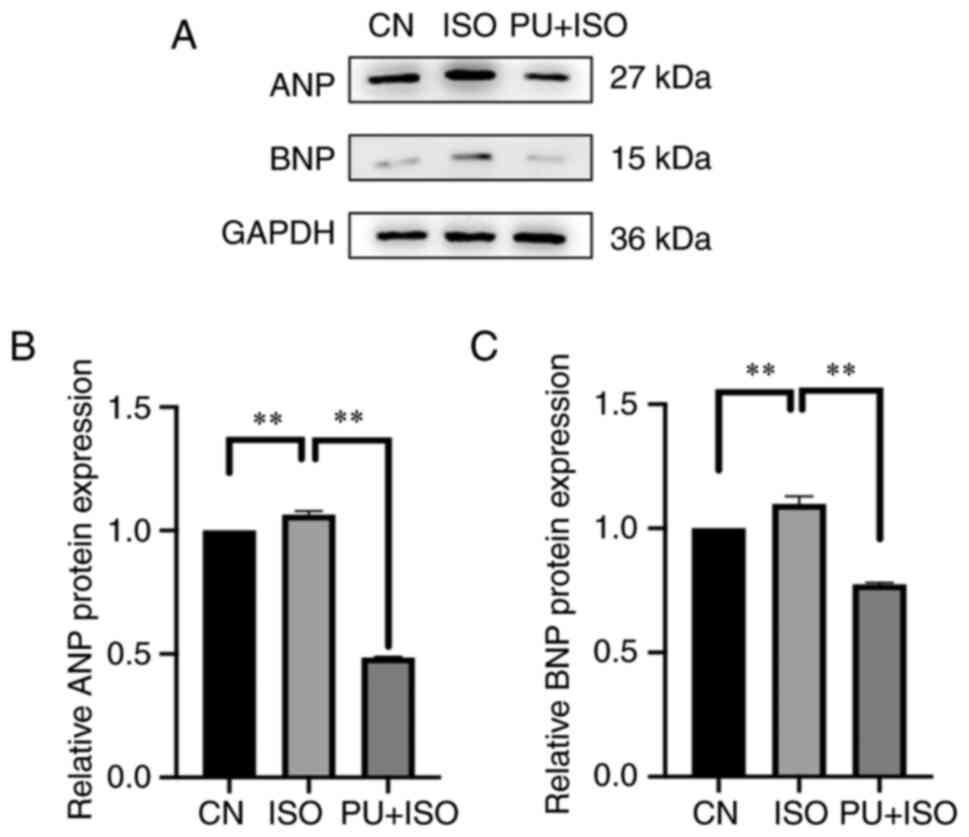
PU decreases protein expression levels
of ANP and BNP
To assess the effect of PU on ISO-induced MH,
western blotting was performed to determine protein expression
levels of ANP and BNP. Following 24 h induction with 10 µM ISO,
protein expression levels of ANP and BNP in the AC16 cardiomyocytes
were significantly higher compared with those in the control
(P<0.01; Fig. 3). However,
expression levels of ANP and BNP protein in AC16 cardiomyocytes
pretreated with PU were significantly lower compared with those in
the ISO group (P<0.01). These results demonstrated that cells
pretreated with PU were resistant to ISO-induced MH.
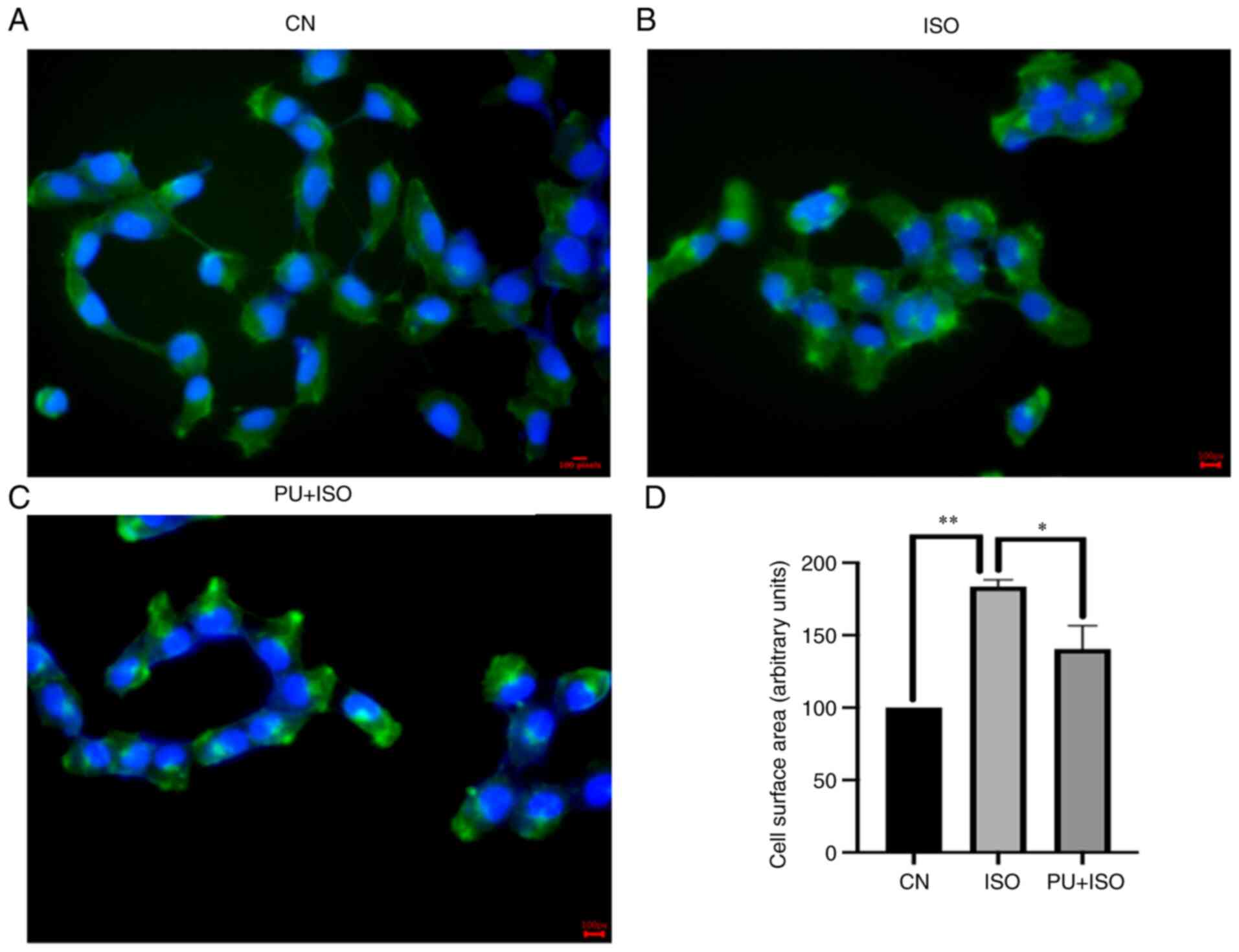
ISO-treated AC16 cell surface area
decreases significantly following PU pretreatment
Changes in AC16 myocardial cell area were observed
using fluorescence microscopy. The surface area of AC16 cells in
the ISO group significantly increased compared with those in the
control (P<0.01; Fig. 4A, B and
D). However, PU pretreatment significantly decreased surface
area of AC16 cells compared with those in the ISO group (P<0.05;
Fig. 4C). These results suggested
that MH was prevented by PU pretreatment.
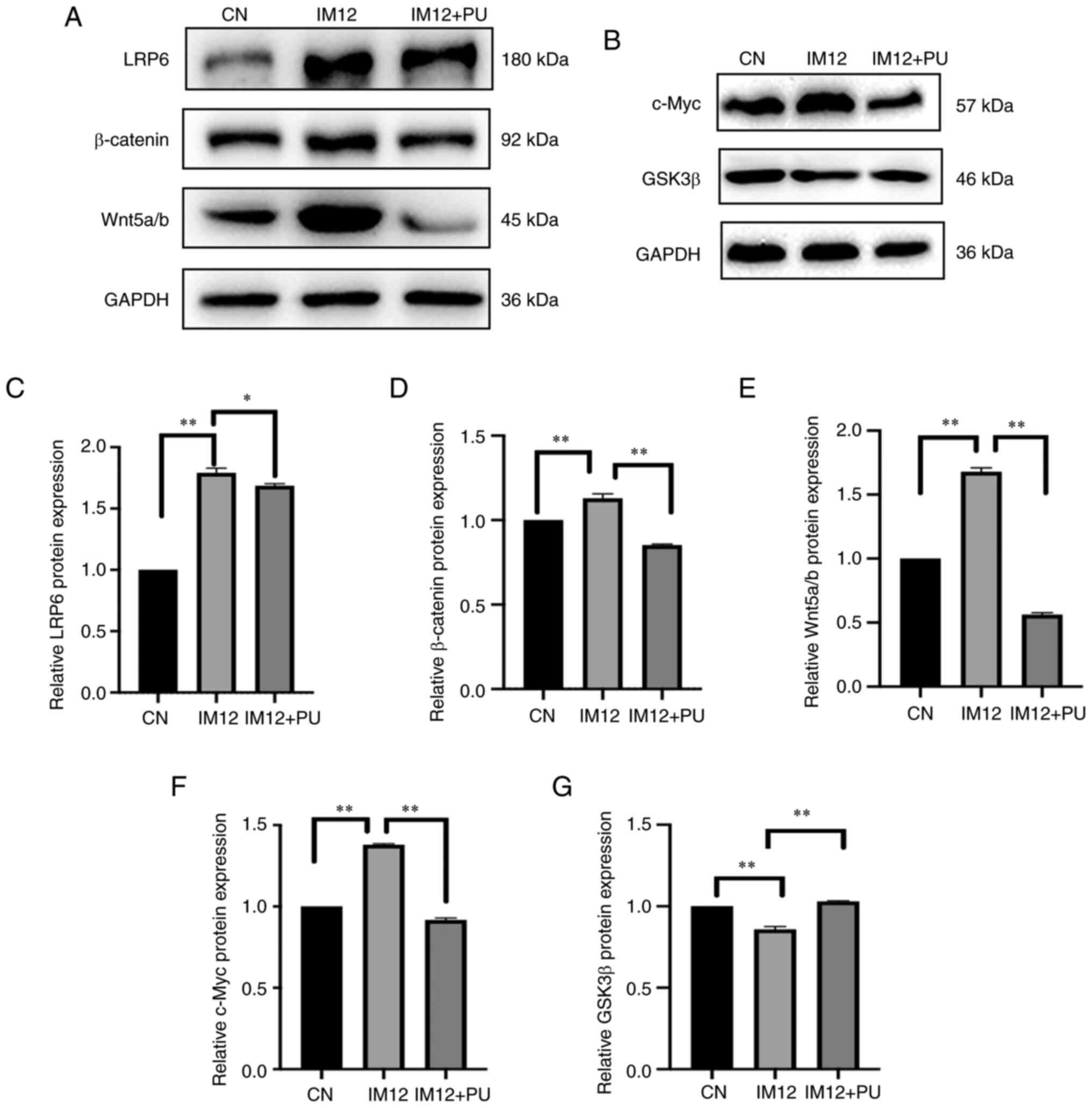
PU inhibits the Wnt/β-catenin
signaling pathway
To determine the potential anti-MH mechanism of PU,
core components in the Wnt/β-catenin and NF-κB signaling pathways
were assessed using western blotting. The protein expression levels
of LRP6, β-catenin, Wnt-5a/b and c-Myc and phosphorylation levels
of p65 and GSK3β, significantly increased in the ISO group compared
with the control group (P<0.01; Fig. 5A-F and H-K). However, the
expression of GSK3β was significantly decreased by ISO compared
with the control group (P<0.01; Fig. 5G). There was a significant
decrease in the expression of LRP6 (P<0.05), β-catenin
(P<0.01), Wnt-5a/b (P<0.01) and c-Myc (P<0.01) as well as
phosphorylation levels of p65 (P<0.01) and GSK3β (P<0.01) in
the AC16 cells pretreated with PU compared with the ISO group.
Furthermore, expression of GSK3β (P<0.01) was significantly
increased by PU pretreatment compared with the ISO group. These
results suggested that the protective effects of PU on MH may be
mediated by Wnt and NF-κB signaling pathway activity.
PU serves as an inhibitor of the Wnt
signaling pathway by antagonizing its activator
A Wnt inhibitor and activator were used to verify if
PU regulated the Wnt signaling pathway. DKK1 is an inhibitor of the
Wnt signaling pathway that has been reported to regulate embryonic
development (44). Wnt signaling
transduction is blocked by DKK1 binding, which induces LRP6
internalization (45).
After cells were pretreated with DKK1, protein
expression levels of components in the Wnt signaling pathway, such
as LRP6, β-catenin, Wnt5a/b and c-Myc, were significantly
suppressed compared with ISO group (P<0.01; Fig. 6A-G); this effect was similar to
the aforementioned effect mediated by PU. Furthermore, expression
of ANP, a cardiac hypertrophy marker, was significantly decreased
by DKK1 pretreatment (P<0.01; Fig.
6G), which suggested that inhibition of the Wnt signaling
pathway may be beneficial for the treatment of MH.
IM-12 enhances Wnt signaling, primarily by
inhibiting GSK-3β (46). In the
present study, IM-12 significantly decreased GSK3β expression
compared with the control (P<0.01; Fig. 7G). The protein expression levels
of LRP6, β-catenin, Wnt-5a/b and c-Myc were significantly increased
by IM-12 compared with the control (P<0.01; Fig. 7A-F). However, protein expression
levels of LRP6 (P<0.05), β-catenin (P<0.01), Wnt-5a/b
(P<0.01) and c-Myc (P<0.01) were significantly decreased by
PU pretreatment. These results suggested that PU suppressed
IM-12-induced activation of the Wnt/β-catenin signaling
pathway.
The effect of IM-12 on expression of markers in the
Wnt signaling pathway and ISO-induced MH in the presence of PU
pretreatment were assessed (Fig.
8). Following treatment with IM-12, PU-induced inhibition of
Wnt signaling pathway protein expression was partially reversed.
With the exception of protein expression of GSK3β, which was
increased by PU but significantly reversed by IM-12 compared with
the PU + ISO group, expression levels of all proteins in the PU +
ISO + IM12 group were significantly increased compared with PU
group (P<0.01). Moreover, the inhibitory effects of PU on the MH
marker ANP was partially but significantly reversed by IM-12
treatment compared with the PU + ISO group (P<0.01). These
results demonstrated that the protective effects of PU on MH may be
mediated by inhibition of the Wnt signaling pathway.
Discussion
Despite therapeutic advances, there is a lack of
effective therapeutic treatment strategies for MH. Pathological
cardiac hypertrophy is induced by various factors, including
pressure (47) and volume
overload (48) and pharmaceutical
induction, such as induction using angiotensin II (49) and ISO (50). ISO is a β-receptor agonist that
causes acute myocardial contraction, ischemia, hypoxia and cause
damage to myocardial cells due to production of a large number of
peroxynitrite and oxygen free radicals in rats (51). Prabhu et al (52) reported that activities of
myocardial mitochondrial cathepsin-D and β-glucuronidase are
significantly decreased by high-dose ISO and that ISO decreases
stability of the myocardial mitochondrial membrane. ISO is widely
used to induce MH (50,53). There is evidence demonstrating
that ISO stimulation leads to MH (53). ISO activates MAPK in
cardiomyocytes and phosphorylates Raf/MEK/ERK signaling pathway
components (54). The increase of
p-ERK1/2 protein expression levels is a downstream effect of
ISO-induced protein kinase C ε activation, which leads to MH
(55). A previous study reported
that chronic infusion of ISO results in compensatory cardiac
hypertrophy associated with protein kinase A activity and
N-terminal cleaved histone deacetylase production (56). The mechanism of ISO-induced
myocardial injury may be associated with oxidative stress, calcium
overload, inflammation and lipid peroxidation (57). It has been confirmed that in
ISO-induced MH, ISO treatment markedly increases reactive oxygen
species levels in cardiomyocytes (58), activates the NF-κB signaling
pathway in AC16 cells and induces an inflammatory response
(59). Further studies are
required to determine the mechanism underlying ISO-induced MH.
Based on a previous study (60),
10 µM ISO was chosen as the induction concentration for the present
study.
In failing human hearts, there is a significant
increase in both mRNA and protein expression of ANP, BNP and β-MHC
(61); whereas expression of
these three genes is normally stable in normal cardiomyocytes
(62,63). A previous study reported that PU
decreases mRNA expression levels of MH markers ANP and BNP in a
dose-dependent manner, with 40 µM being the optimal concentration
(64). Combined with the results
from the present CCK-8 assay, 40 µM was selected as the
concentration to induce MH for subsequent experimentation in the
present study. It has been reported that the early stage of MH is
characterized by the increase of the surface area of cardiomyocytes
(65). Changes in cell surface
area (quantified and analyzed using immunofluorescence) directly
reflect progression of cardiac hypertrophy; this method is used to
assess MH. In a previous study, H9c2 cells were stained with
crystal violet and surface area was observed as a measure of MH to
confirm that the surface area of CD38 knockdown cells was not
enlarged (66). Another study
used wheat germ agglutinin staining to evaluate the size of
cardiomyocytes from heart sections (67). A previous study used surface area
of cells stained with gentian violet to assess MH (68). Studies have also aimed to use cell
surface area to assess the role of miR-339-5p in cardiomyocyte
hypertrophy (69) and determine
the therapeutic potential of pyrroloquinoline quinone for
ISO-induced cardiac hypertrophy (59). In the present study, pretreatment
with PU was demonstrated to exert protective effects against
ISO-induced MH, demonstrated by decreased area of cardiomyocytes
and expression of markers of cardiac hypertrophy. Therefore, PU may
be a promising clinical agent for the prevention and treatment of
MH, although its mechanism remains to be fully elucidated.
The signal transduction mechanism underlying MH is
complex. Inflammation (70) and
oxidative stress (71) have both
been previously demonstrated to cause alterations leading to MH and
heart failure. A previous systematic study of Wnt on a genome-wide
level reported an association between MH and the Wnt expression
profile (72). The Wnt signaling
pathway has also been reported to be associated with cardiac
disease; excessive stimulation of Wnt signaling is detrimental to
cardiovascular pathology (42).
PU has been reported to demonstrate anti-inflammatory and
antioxidant activities (73).
However, the potential effects of PU on MH and the Wnt signaling
pathway in cardiomyocytes remain poorly understood. Therefore, the
present study aimed to explore if PU demonstrate effects against MH
in an in vitro model, with specific focus on the
Wnt/β-catenin signaling pathway.
The Wnt signal transduction pathway is divided into
classical and non-classical branches (74). The present study verified the
expression of associated proteins and demonstrated that PU
inhibited ISO-induced MH by blocking the classical branch of the
Wnt/β-catenin signaling pathway. Wnt signaling is typically
activated by the membrane receptor complex consisting of frizzled
(Fzd), LRP5/6 and disheveled (Dvl) (75). Following activation of the Wnt
receptor, Fzd binds to Wnt protein and LRP5/6 to form a receptor
complex (76). Dvl is recruited
by the receptor complex and activated via phosphorylation before
binding to the β-catenin degradation complex, which separates
β-catenin from the degradation complex to promote stabilization and
aggregation before β-catenin translocates into the nucleus
(77). β-catenin is a core
component of the Wnt/β-catenin signaling pathway and is reported to
be upregulated in cardiomyocytes from patients with acute
myocardial infarction and heart tissue of spontaneously
hypertensive rats (78). However,
GSK3β mediates both β-catenin-dependent and -independent cascades
(79). A previous study reported
that GSK3β exerts negative regulatory effects on the Wnt signaling
pathway (80). In the nucleus,
β-catenin binds to T cell factor/lymphoid enhancer factor (Tcf/Lef)
to activate transcription of Wnt target genes (81). c-Myc and Tcf/Lef are downstream
targets of the Wnt signaling pathway; a previous study reported
that activity of these targets is inhibited by low expression of
β-catenin (82).
In the present study, western blotting was used to
assess expression of core proteins in the Wnt signaling pathway;
expression levels of these proteins in the ISO group was
significantly increased but significantly reversed by PU
pretreatment. However, GSK3β protein expression levels exhibited an
opposite trend, which is likely due to its negative regulatory
mechanism of Wnt; following activation of Wnt signaling, activity
of GSK3β is hampered (83). A
previous study reported that decreased expression of GSK3β in
cardiac fibroblasts that led to adverse ventricular remodeling
(84). Therefore, inhibition of
GSK3β may increase the activity of Wnt signaling, thereby leading
to cardiac hypertrophy. PU induces expression of GSK3β, as
demonstrated in the present study, and serves a protective role.
Furthermore, numerous studies have demonstrated that the
Wnt/β-catenin and NF-κB signaling pathways exhibit crosstalk and
this interaction is enhanced by the inflammatory response (85,86). In the present study, it was
demonstrated that, in the ISO group, the protein expression of
Wnt5a/b and β-catenin and the phosphorylation levels of p65 were
significantly increased compared with the control but significantly
decreased by pretreatment with PU. These observations are in
accordance with those in previous studies which suggested that
there may be synergy between the two signaling pathways during the
development of MH (87,88). However, more evidence is needed to
support the synergistic effect.
To verify whether inhibition of the Wnt signaling
pathway ameliorated ISO-induced MH, DKK1, an inhibitor of the Wnt
signaling pathway, was used. DKK1 is a member of the DKK family
that is reported to antagonize the Wnt/β-catenin signaling pathway
by decreasing β-catenin and increasing OCT4 expression (89). The results of the present study
demonstrated that the DKK1 group exhibited trends in the expression
of protein in the Wnt signaling pathway that were comparable to
those follow PU pretreatment, in that PU exerted inhibitory effects
on Wnt signaling comparable with those mediated by the known Wnt
inhibitor. IM-12, an activator of the Wnt signaling pathway, has
been reported to increase expression of β-catenin and downstream
proteins while suppressing expression of GSK-3β (46). The present study assessed whether
IM-12 reversed the inhibitory effects of PU on ISO-induced MH, thus
confirming whether PU exerted its effects via inhibition of the Wnt
signaling pathway. The present study demonstrated that PU inhibited
expression of Wnt signaling pathway proteins induced by IM-12 and
increased expression of GSK-3β. Furthermore, IM-12, the specific
activator of the Wnt signaling pathway, was demonstrated to
eliminate the protective effects of PU on MH. In conclusion, PU
served a cardioprotective role partially via inhibition of the Wnt
signaling pathway.
However, in the present study only core proteins in
the Wnt signaling pathway were assessed; therefore the detailed
underlying molecular mechanism in the upstream regulation of
Wnt/β-catenin signaling during MH was not fully elucidated. Since
Wnt signaling induces nuclear localization of β-catenin (90), it is important to determine the
localization and expression of β-catenin. These limitations of the
present study require addressing in future. In future studies, the
inhibitor-like mechanism of PU and its effects on other disease
caused by aberrant activation of the Wnt signaling pathway,
including colon cancer, hepatocellular carcinoma and pancreatic,
lung and ovarian cancer (91),
should be explored.
In summary, the present study demonstrated that
pretreatment of AC16 cells with PU attenuated ISO-induced MH. The
underlying mechanism was associated with inhibition of protein
expression in the Wnt/β-catenin signaling pathway and NF-κB
activity. These results suggested that PU may be a potential agent
for treating MH. Based on the importance of the Wnt signaling
pathway for initiation, maintenance, progression and relapse of MH,
PU may serve an inhibitor-like role for the treatment of
cardiovascular and associated disease caused by hyperactivation of
the Wnt signaling pathway. Furthermore, the present study may
provide a novel direction for development and use of agents for MH
treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by Shanghai Special Project of
Biomedical Science and Technology (grant no. 21S11901700).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW performed experiments, analyzed data and wrote
the manuscript. KH performed experiments and data analysis. LM
designed the study, analyzed data and revised the manuscript. LW
performed data analysis. YY participated in discussions and
analyzed data. YL designed the study and obtained funding. XW, KH,
LM and YL confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for participation
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Roth GA, Mensah GA, Johnson CO, Addolorato
G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ,
Benziger CP, et al: Global burden of cardiovascular diseases and
risk factors, 1990–2019: Update from the GBD 2019 study. J Am Coll
Cardiol. 76:2982–3021. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cho YS, Moon SC, Ryu KS and Ryu KH: A
study on clinical and healthcare recommending service based on
cardiovascula disease pattern analysis. Int J Biosci Biotechnol.
8:287–294. 2016.
|
|
3
|
Nalban N, Sangaraju R, Alavala S, Mir SM,
Jerald MK and Sistla R: Arbutin attenuates isoproterenol-induced
cardiac hypertrophy by inhibiting TLR-4/NF-κB pathway in mice.
Cardiovasc Toxicol. 20:235–248. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roth GA, Mensah GA and Fuster V: The
global burden of cardiovascular diseases and risks: A compass for
global action. J Am Coll Cardiol. 76:2980–2981. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oh T, Kim D, Lee S, Won C, Kim S, Yang JS,
Yu J, Kim B and Lee J: Machine learning-based diagnosis and risk
factor analysis of cardiocerebrovascular disease based on KNHANES.
Sci Rep. 12:22502022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Leong DP, Joseph PG, McKee M, Anand SS,
Teo KK, Schwalm JD and Yusuf S: Reducing the global burden of
cardiovascular disease, part 2: Prevention and treatment of
cardiovascular disease. Circ Res. 121:695–710. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Van Camp G: Cardiovascular disease
prevention. Acta Clin Belg. 69:407–411. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao Y, Jia WW, Ren S, Xiao W, Li GW, Jin
L and Lin Y: Difluoromethylornithine attenuates
isoproterenol-induced cardiac hypertrophy by regulating apoptosis,
autophagy and the mitochondria-associated membranes pathway. Exp
Ther Med. 22:8702021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gallo S, Vitacolonna A, Bonzano A,
Comoglio P and Crepaldi T: ERK: A key player in the pathophysiology
of cardiac hypertrophy. Int J Mol Sci. 20:21642019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ellison GM, Waring CD, Vicinanza C and
Torella D: Physiological cardiac remodelling in response to
endurance exercise training: Cellular and molecular mechanisms.
Heart. 98:5–10. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Selvetella G, Hirsch E, Notte A, Tarone G
and Lembo G: Adaptive and maladaptive hypertrophic pathways: Points
of convergence and divergence. Cardiovasc Res. 63:373–380. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shimizu I and Minamino T: Physiological
and pathological cardiac hypertrophy. J Mol Cell Cardiol.
97:245–262. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kurosawa Y, Kojima K, Kato M, Ohashi R,
Minami K and Narita H: Protective action of angiotensin converting
enzyme inhibitors on cardiac hypertrophy in the aortic-banded rat.
Jpn Heart J. 40:645–654. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
A Romero C, Mathew S, Wasinski B, Reed B,
Brody A, Dawood R, Twiner MJ, McNaughton CD, Fridman R, Flack JM,
et al: Angiotensin-converting enzyme inhibitors increase
anti-fibrotic biomarkers in African Americans with left ventricular
hypertrophy. J Clin Hypertens (Greenwich). 23:1008–1016. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y, Shen HJ, Wang XQ, Liu HQ, Zheng LY
and Luo JD: EndophilinA2 protects against angiotensin II-induced
cardiac hypertrophy by inhibiting angiotensin II type 1 receptor
trafficking in neonatal rat cardiomyocytes. J Cell Biochem.
119:8290–8303. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Walsh-Wilkinson É, Drolet MC, Le Houillier
C, Roy ÈM, Arsenault M and Couet J: Sex differences in the response
to angiotensin II receptor blockade in a rat model of eccentric
cardiac hypertrophy. PeerJ. 7:e74612019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chang CS, Tsai PJ, Sung JM, Chen JY, Ho
LC, Pandya K, Maeda N and Tsai YS: Diuretics prevent
thiazolidinedione-induced cardiac hypertrophy without compromising
insulin-sensitizing effects in mice. Am J Pathol. 184:442–453.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Okura T, Miyoshi K, Irita J, Enomoto D,
Jotoku M, Nagao T, Watanabe K, Matsuokan H, Ashihara T, Higaki J,
et al: Comparison of the effect of combination therapy with an
angiotensin II receptor blocker and either a low-dose diuretic or
calcium channel blocker on cardiac hypertrophy in patients with
hypertension. Clin Exp Hypertens. 35:563–569. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang X, Zhang MC and Wang CT: Loss of
LRRC25 accelerates pathological cardiac hypertrophy through
promoting fibrosis and inflammation regulated by TGF-β1. Biochem
Biophys Res Commun. 506:137–144. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zang Y, Wan J, Zhang Z, Huang S, Liu X and
Zhang W: An updated role of astragaloside IV in heart failure.
Biomed Pharmacother. 126:1100122020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma Y, Kang R and Liu X: Research progress
in prevention and cure of fibrosis by traditional Chinese medicine.
Mod Appl Sci. 2:127–132. 2008. View Article : Google Scholar
|
|
22
|
Yang QY, Chen KJ, Lu S and Sun HR:
Research progress on mechanism of action of Radix Astragalus in the
treatment of heart failure. Chin J Integr Med. 18:235–240. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Karmazyn M and Gan XT: Treatment of the
cardiac hypertrophic response and heart failure with ginseng,
ginsenosides, and ginseng-related products. Can J Physiol
Pharmacol. 95:1170–1176. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang J, Wang HX, Zhang YJ, Yang YH, Lu ML,
Zhang J, Li ST, Zhang SP and Li G: Astragaloside IV attenuates
inflammatory cytokines by inhibiting TLR4/NF-кB signaling pathway
in isoproterenol-induced myocardial hypertrophy. J Ethnopharmacol.
150:1062–1070. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu ZH, Liu HB and Wang J: Astragaloside
IV protects against the pathological cardiac hypertrophy in mice.
Biomed Pharmacother. 97:1468–1478. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guo J, Gan XT, Haist JV, Rajapurohitam V,
Zeidan A, Faruq NS and Karmazyn M: Ginseng inhibits cardiomyocyte
hypertrophy and heart failure via NHE-1 inhibition and attenuation
of calcineurin activation. Circ Heart Fail. 4:79–88. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qin N, Gong QH, Wei LW, Wu Q and Huang XN:
Total ginsenosides inhibit the right ventricular hypertrophy
induced by monocrotaline in rats. Biol Pharm Bull. 31:1530–1535.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bu L, Dai O, Zhou F, Liu F, Chen JF, Peng
C and Xiong L: Traditional Chinese medicine formulas, extracts, and
compounds promote angiogenesis. Biomed Pharmacother.
132:1108552020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Luo J, Xu H and Chen K: Systematic review
of compound danshen dropping pill: A chinese patent medicine for
acute myocardial infarction. Evid Based Complement Alternat Med.
2013:8080762013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tu Y: Artemisinin-A gift from traditional
Chinese medicine to the world (nobel lecture). Angew Chem Int Ed
Engl. 55:10210–10226. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang R, Yuan BC, Ma YS, Zhou S and Liu Y:
The anti-inflammatory activity of licorice, a widely used Chinese
herb. Pharm Biol. 55:5–18. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mu F, Duan J, Bian H, Zhai X, Shang P, Lin
R, Zhao M, Hu D, Yin Y, Wen A and Xi M: Metabonomic strategy for
the evaluation of Chinese medicine Salvia miltiorrhiza and
Dalbergia odorifera interfering with myocardial
ischemia/reperfusion injury in rats. Rejuvenation Res. 20:263–277.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang S, Zhang S, Wang S, Gao P and Dai L:
A comprehensive review on Pueraria: Insights on its
chemistry and medicinal value. Biomed Pharmacother. 131:1107342020.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hou N, Huang Y, Cai SA, Yuan WC, Li LR,
Liu XW, Zhao GJ, Qiu XX, Li AQ, Cheng CF, et al: Puerarin
ameliorated pressure overload-induced cardiac hypertrophy in
ovariectomized rats through activation of the PPARα/PGC-1 pathway.
Acta Pharmacol Sin. 42:55–67. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yuan G, Shi S, Jia Q, Shi J, Shi S, Zhang
X, Shou X, Zhu X and Hu Y: Use of network pharmacology to explore
the mechanism of Gegen (Puerariae lobatae Radix) in the
treatment of type 2 diabetes mellitus associated with
hyperlipidemia. Evid Based Complement Alternat Med.
2021:66334022021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou YX, Zhang H and Peng C: Puerarin: A
review of pharmacological effects. Phytother Res. 28:961–975. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu J, Zhang HJ, Ji BP, Cai SB, Wang RJ,
Zhou F, Yang JS and Liu HJ: A diet formula of Puerariae radix,
Lycium barbarum, Crataegus pinnatifida, and Polygonati rhizoma
alleviates insulin resistance and hepatic steatosis in CD-1 mice
and HepG2 cells. Food Funct. 5:1038–1049. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu B, Wu Z, Li Y, Ou C, Huang Z, Zhang J,
Liu P, Luo C and Chen M: Puerarin prevents cardiac hypertrophy
induced by pressure overload through activation of autophagy.
Biochem Biophys Res Commun. 464:908–915. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gao W, Guo N, Zhao S, Chen Z, Zhang W, Yan
F, Liao H and Chi K: Carboxypeptidase A4 promotes cardiomyocyte
hypertrophy through activating PI3K-AKT-mTOR signaling. Biosci Rep.
40:BSR202006692020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Foulquier S, Daskalopoulos EP, Lluri G,
Hermans KCM, Deb A and Blankesteijn WM: WNT signaling in cardiac
and vascular disease. Pharmacol Rev. 70:68–141. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Weeks KL, Bernardo BC, Ooi JYY, Patterson
NL and McMullen JR: The IGF1-PI3K-Akt signaling pathway in
mediating exercise-induced cardiac hypertrophy and protection. Adv
Exp Med Biol. 1000:187–210. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fan J, Qiu L, Shu H, Ma B, Hagenmueller M,
Riffel JH, Meryer S, Zhang M, Hardt SE, Wang L, et al: Recombinant
frizzled1 protein attenuated cardiac hypertrophy after myocardial
infarction via the canonical Wnt signaling pathway. Oncotarget.
9:3069–3080. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lieven O, Knobloch J and Rüther U: The
regulation of Dkk1 expression during embryonic development. Dev
Biol. 340:256–268. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li Y, Lu W, King TD, Liu CC, Bijur GN and
Bu G: Dkk1 stabilizes Wnt co-receptor LRP6: Implication for Wnt
ligand-induced LRP6 down-regulation. PLoS One. 5:e110142010.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang T, Duan YM, Fu Q, Liu T, Yu JC, Sui
ZY, Huang L and Wen GQ: IM-12 activates the Wnt-β-catenin signaling
pathway and attenuates rtPA-induced hemorrhagic transformation in
rats after acute ischemic stroke. Biochem Cell Biol. 97:702–708.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cheng Y, Shen A, Wu X, Shen Z, Chen X, Li
J, Liu L, Lin X, Wu M, Chen Y, et al: Qingda granule attenuates
angiotensin II-induced cardiac hypertrophy and apoptosis and
modulates the PI3K/AKT pathway. Biomed Pharmacother.
133:1110222021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Guo Y, Yu ZY, Wu J, Gong H, Kesteven S,
Iismaa SE, Chan AY, Holman S, Pinto S, Pironet A, et al: The
Ca2+-activated cation channel TRPM4 is a positive
regulator of pressure overload-induced cardiac hypertrophy. Elife.
10:e665822021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Schnelle M, Chong M, Zoccarato A, Elkenani
M, Sawyer GJ, Hasenfuss G, Ludwig C and Shah AM: In vivo
[U-13C]glucose labeling to assess heart metabolism in
murine models of pressure and volume overload. Am J Physiol Heart
Circ Physiol. 319:H422–H431. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ma X, Song Y, Chen C, Fu Y, Shen Q, Li Z
and Zhang Y: Distinct actions of intermittent and sustained
β-adrenoceptor stimulation on cardiac remodeling. Sci China Life
Sci. 54:493–501. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ribeiro DA, Buttros JB, Oshima C,
Bergamaschi CT and Campos RR: Ascorbic acid prevents acute
myocardial infarction induced by isoproterenol in rats: Role of
inducible nitric oxide synthase production. J Mol Histol.
40:99–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Prabhu S, Narayan S and Devi CS: Mechanism
of protective action of mangiferin on suppression of inflammatory
response and lysosomal instability in rat model of myocardial
infarction. Phytother Res. 23:756–760. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Xu H, Wang Z, Chen M, Zhao W, Tao T, Ma L,
Ni Y and Li W: YTHDF2 alleviates cardiac hypertrophy via regulating
Myh7 mRNA decoy. Cell Biosci. 11:1322021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhang GX, Kimura S, Murao K, Yu X, Obata
K, Matsuyoshi H and Takaki M: Effects of angiotensin type I
receptor blockade on the cardiac Raf/MEK/ERK cascade activated via
adrenergic receptors. J Pharmacol Sci. 113:224–233. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li L, Cai H, Liu H and Guo T: β-Adrenergic
stimulation activates protein kinase Cε and induces extracellular
signal-regulated kinase phosphorylation and cardiomyocyte
hypertrophy. Mol Med Rep. 11:4373–4380. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Werhahn SM, Kreusser JS, Hagenmüller M,
Beckendorf J, Diemert N, Hoffmann S, Schultz JH, Backs J and
Dewenter M: Adaptive versus maladaptive cardiac remodelling in
response to sustained β-adrenergic stimulation in a new ‘ISO on/off
model’. PLoS One. 16:e02489332021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Garg M and Khanna D: Exploration of
pharmacological interventions to prevent isoproterenol-induced
myocardial infarction in experimental models. Ther Adv Cardiovasc
Dis. 8:155–169. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Liu BY, Li L, Liu GL, Ding W, Chang WG, Xu
T, Ji XY, Zheng XX, Zhang J and Wang JX: Baicalein attenuates
cardiac hypertrophy in mice via suppressing oxidative stress and
activating autophagy in cardiomyocytes. Acta Pharmacol Sin.
42:701–714. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wen J, Shen J, Zhou Y, Zhao X, Dai Z and
Jin Y: Pyrroloquinoline quinone attenuates isoproterenol
hydrochloride-induced cardiac hypertrophy in AC16 cells by
inhibiting the NF-κB signaling pathway. Int J Mol Med. 45:873–885.
2020.PubMed/NCBI
|
|
60
|
Zhao Y, Jiang Y, Chen Y, Zhang F, Zhang X,
Zhu L and Yao X: Dissection of mechanisms of Chinese medicinal
formula Si-Miao-Yong-an decoction protects against cardiac
hypertrophy and fibrosis in isoprenaline-induced heart failure. J
Ethnopharmacol. 248:1120502020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhang C, Wang Y, Ge Z, Lin J, Liu J, Yuan
X and Lin Z: GDF11 attenuated ANG II-induced hypertrophic
cardiomyopathy and expression of ANP, BNP and beta-MHC through
down-regulating CCL11 in mice. Curr Mol Med. 18:661–671. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Cameron VA, Rademaker MT, Ellmers LJ,
Espiner EA, Nicholls MG and Richards AM: Atrial (ANP) and brain
natriuretic peptide (BNP) expression after myocardial infarction in
sheep: ANP is synthesized by fibroblasts infiltrating the infarct.
Endocrinology. 141:4690–4697. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Edwards JG: Cardiac MHC gene expression:
More complexity and a step forward. Am J Physiol Heart Circ
Physiol. 294:H14–H15. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yuan Y, Zong J, Zhou H, Bian ZY, Deng W,
Dai J, Gan HW, Yang Z, Li H and Tang QZ: Puerarin attenuates
pressure overload-induced cardiac hypertrophy. J Cardiol. 63:73–81.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yeh YL, Tsai HI, Cheng SM, Pai P, Ho TJ,
Chen RJ, Lai CH, Huang PJ, Padma VV and Huang CY: Mechanism of
Taiwan Mingjian Oolong tea to inhibit isoproterenol-induced
hypertrophy and apoptosis in cardiomyoblasts. Am J Chin Med.
44:77–86. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Guan XH, Hong X, Zhao N, Liu XH, Xiao YF,
Chen TT, Deng LB, Wang XL, Wang JB, Ji GJ, et al: CD38 promotes
angiotensin II-induced cardiac hypertrophy. J Cell Mol Med.
21:1492–1502. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hu H, Jiang M, Cao Y, Zhang Z, Jiang B,
Tian F, Feng J, Dou Y, Gorospe M, Zheng M, et al: HuR regulates
phospholamban expression in isoproterenol-induced cardiac
remodelling. Cardiovasc Res. 116:944–955. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Huo S, Shi W, Ma H, Yan D, Luo P, Guo J,
Li C, Lin J, Zhang C, Li S, et al: Alleviation of inflammation and
oxidative stress in pressure overload-induced cardiac remodeling
and heart failure via IL-6/STAT3 inhibition by raloxifene. Oxid Med
Cell Longev. 2021:66990542021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Bi X, Zhang Y, Yu Y, Yuan J, Xu S, Liu F,
Ye J and Liu P: MiRNA-339-5p promotes isoproterenol-induced
cardiomyocyte hypertrophy by targeting VCP to activate the mTOR
signaling. Cell Biol Int. 46:288–299. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Han B, Xu J, Shi X, Zheng Z, Shi F, Jiang
F and Han J: DL-3-n-butylphthalide attenuates myocardial
hypertrophy by targeting gasdermin D and inhibiting gasdermin D
mediated inflammation. Front Pharmacol. 12:6881402021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Shah AK, Bhullar SK, Elimban V and Dhalla
NS: Oxidative stress as a mechanism for functional alterations in
cardiac hypertrophy and heart failure. Antioxidants (Basel).
10:9312021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Gai Z, Wang Y, Tian L, Gong G and Zhao J:
Whole genome level analysis of the Wnt and DIX gene families in
mice and their coordination relationship in regulating cardiac
hypertrophy. Front Genet. 12:6089362021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Qin H, Zhang Y, Wang R, Du X, Li L and Du
H: Puerarin suppresses Na+-K+-ATPase-mediated systemic inflammation
and CD36 expression, and alleviates cardiac lipotoxicity in vitro
and in vivo. J Cardiovasc Pharmacol. 68:465–472. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Moon RT, Kohn AD, De Ferrari GV and Kaykas
A: WNT and beta-catenin signalling: Diseases and therapies. Nat Rev
Genet. 5:691–701. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Agostino M and Pohl SÖ: The structural
biology of canonical Wnt signalling. Biochem Soc Trans.
48:1765–1780. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Hua Y, Yang Y, Li Q, He X, Zhu W, Wang J
and Gan X: Oligomerization of Frizzled and LRP5/6 protein initiates
intracellular signaling for the canonical WNT/β-catenin pathway. J
Biol Chem. 293:19710–19724. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Gao C and Chen YG: Dishevelled: The hub of
Wnt signaling. Cell Signal. 22:717–727. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zeng KW, Wang JK, Wang LC, Guo Q, Liu TT,
Wang FJ, Feng N, Zhang XW, Liao LX, Zhao MM, et al: Small molecule
induces mitochondrial fusion for neuroprotection via targeting CK2
without affecting its conventional kinase activity. Signal
Transduct Target Ther. 6:712021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Ríos JA, Godoy JA and Inestrosa NC: Wnt3a
ligand facilitates autophagy in hippocampal neurons by modulating a
novel GSK-3β-AMPK axis. Cell Commun Signal. 16:152018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Barker N, Morin PJ and Clevers H: The
Yin-Yang of TCF/beta-catenin signaling. Adv Cancer Res. 77:1–24.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Piazza F, Manni S, Tubi LQ, Montini B,
Pavan L, Colpo A, Gnoato M, Cabrelle A, Adami F, Zambello R, et al:
Glycogen synthase kinase-3 regulates multiple myeloma cell growth
and bortezomib-induced cell death. BMC Cancer. 10:5262010.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Guo Y, Gupte M, Umbarkar P, Singh AP, Sui
JY, Force T and Lal H: Entanglement of GSK-3β, β-catenin and TGF-β1
signaling network to regulate myocardial fibrosis. J Mol Cell
Cardiol. 110:109–120. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Guan X, He Y, Wei Z, Shi C, Li Y, Zhao R,
Pan L, Han Y, Hou T and Yang J: Crosstalk between Wnt/β-catenin
signaling and NF-κB signaling contributes to apical periodontitis.
Int Immunopharmacol. 98:1078432021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Jia D, Yang W, Li L, Liu H, Tan Y, Ooi S,
Chi L, Filion LG, Figeys D and Wang L: β-Catenin and NF-κB
co-activation triggered by TLR3 stimulation facilitates stem
cell-like phenotypes in breast cancer. Cell Death Differ.
22:298–310. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Shang S, Hua F and Hu ZW: The regulation
of β-catenin activity and function in cancer: Therapeutic
opportunities. Oncotarget. 8:33972–33989. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Gitau SC, Li X, Zhao D, Guo Z, Liang H,
Qian M, Lv L, Li T, Xu B, Wang Z, et al: Acetyl salicylic acid
attenuates cardiac hypertrophy through Wnt signaling. Front Med.
9:444–456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Olsen NT, Dimaano VL, Fritz-Hansen T,
Sogaard P, Chakir K, Eskesen K, Steenbergen C, Kass DA and Abraham
TP: Hypertrophy signaling pathways in experimental chronic aortic
regurgitation. J Cardiovasc Transl Res. 6:852–860. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Liu JJ, Shentu LM, Ma N, Wang LY, Zhang
GM, Sun Y, Wang Y, Li J and Mu YL: Inhibition of NF-κB and
Wnt/β-catenin/GSK3β signaling pathways ameliorates cardiomyocyte
hypertrophy and fibrosis in streptozotocin (STZ)-induced type 1
diabetic rats. Curr Med Sci. 40:35–47. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Ou L, Fang L, Tang H, Qiao H, Zhang X and
Wang Z: Dickkopf Wnt signaling pathway inhibitor 1 regulates the
differentiation of mouse embryonic stem cells in vitro and
in vivo. Mol Med Rep. 13:720–730. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Kim S, Song G, Lee T, Kim M, Kim J, Kwon
H, Kim J, Jeong W, Lee U, Na C, et al: PARsylated transcription
factor EB (TFEB) regulates the expression of a subset of Wnt target
genes by forming a complex with β-catenin-TCF/LEF1. Cell Death
Differ. 28:2555–2570. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zhang L, Guo Z, Wang Y, Geng J and Han S:
The protective effect of kaempferol on heart via the regulation of
Nrf2, NF-κβ, and PI3K/Akt/GSK-3β signaling pathways in
isoproterenol-induced heart failure in diabetic rats. Drug Dev Res.
80:294–309. 2019. View Article : Google Scholar : PubMed/NCBI
|