Introduction
Tumor cells use various mechanisms to escape from
host anti-tumor immune responses, such as secreting TGF-β and
IL-10. These cytokines are often detected at high levels in
malignancies and affect immune cell proliferation, activation and
differentiation (1–5). Another mechanism termed tumor
counterattack involves the expression of apoptosis-related proteins
by tumor cells also to suppress host anti-tumor immune responses.
TNF-related apoptosis-inducing ligand (TRAIL) and Fas ligand
(FasL/CD95L) are ligands of the TNF family that are associated with
specific receptors of the TNF receptor superfamily and induce
apoptosis (6). FasL overexpression
is found in various human tumor cells and FasL binds to
Fas-positive cytotoxic T lymphocytes (CTLs) (7–12).
Additionally, several experiments have observed TRAIL-mediated
apoptosis in activated CTLs that have infiltrated tumor tissue
(13–16). Thus, FasL and TRAIL expression on
tumor cells serve critical roles in inducing apoptosis in
anti-tumor immune effector cells, allowing for escape from immune
surveillance.
Receptor-binding cancer antigen expressed on SiSo
cells (RCAS1) is a tumor-associated antigen expressed in various
human carcinomas. Initially, the 22-1-1 monoclonal antibody (22-1-1
mAb) was raised against human uterine carcinoma cell line, SiSo
cells (17,18). Then, a cDNA encoding the antigen
recognized by 22-1-1 mAb was isolated and named RCAS1 (19). RCAS1 is detectable by
immunohistochemistry and levels of soluble-form RCAS1 in human
serum or cancer cell culture supernatant can be measured by
enzyme-linked immunosorbent assay (ELISA) (20). A number of reports have shown the
clinical significance of RCAS1 as a biomarker for the diagnosis and
prognosis of malignant human tumors. RCAS1 expression has been
observed in various malignant tumors and is correlated with poor
prognosis of cancer patients; serum RCAS1 levels are also found to
be significantly higher in cancer patients compared with healthy
blood donors (21–25). Soluble-form RCAS1 has been shown to
induce apoptosis in various human cell lines including normal
peripheral lymphocytes, such as T and B cells and natural killer
cells (19,26). Additionally, RCAS1 levels in
various human malignancies are correlated with the number of
apoptotic tumor infiltrating lymphocytes (TILs) surrounding the
tumor cells (23,24). To investigate the biological
functions of RCAS1, the authors previously established a
doxycycline (Dox)-induced RCAS1 overexpression model in murine
fibroblast L cells (L/ind RCAS1) and reported that RCAS1 expression
inhibited cell cycle progression via the downregulation of
cyclin D3, which subsequently induced apoptosis in L/ind RCAS1
cells (27). Additionally, it was
found that RCAS1 expression in L/ind RCAS1 cells induced
morphological changes prior to caspase-mediated apoptosis. Several
studies have shown that regulators of the actin cytoskeleton that
are associated with migratory signals are increased in invasive and
metastatic tumor cells (28–31).
These results suggest that RCAS1 might not only be involved in a
mechanism of tumor evasion from immune surveillance but could also
serve a role in tumor cell invasion and migration.
The present study clarified the biological functions
of RCAS1 by examining the signaling pathways associated with cell
morphological changes that were induced by RCAS1 expression in
L/ind RCAS1 cells, particularly those related to actin dynamics.
Additionally, it is well known that the MAPK cascades serve
critical regulatory roles in cell growth, differentiation, death
and in controlling cellular responses to stress. Therefore, the
present study also investigated the relationship between RCAS1
expression and MAPK signaling in L/ind RCAS1 cells.
Materials and methods
Cell lines and cell culture
To investigate the biological functions of RCAS1, a
doxycycline (Dox)-induced RCAS1 overexpression model was
established in murine fibroblast L cells (L/ind RCAS1) as described
in a previous report (27). The
present study used the human uterine cervical adenocarcinoma cell
line SiSo and L/ind RCAS1 cells. These cells were cultured in
Roswell Park Memorial Institute (RPMI) 1640 medium (Thermo Fisher
Scientific, Inc.) supplemented with 10% heat-inactivated fetal
bovine serum (Biowest), 100 nM non-essential amino acids (Nacalai
Tesque, Inc.) and 1 mM sodium pyruvate (Nacalai Tesque, Inc.) at
37°C in a 5% CO2 atmosphere. To induce exogenous RCAS1
expression, doxycycline (Dox; FUJIFILM Wako Pure Chemical
Corporation) was used. Dox was dissolved in dH2O at 1
mg/ml concentration and L/ind RCAS1 cells were treated with 0.5
µg/ml Dox for the indicated times.
Preparation of SiSo cell culture
supernatant containing soluble-form RCAS1
To collect the soluble-form RCAS1 from SiSo cell
culture supernatant, SiSo cells were cultured in serum-free medium.
Briefly, SiSo cells were cultured under normal conditions until
reaching 80% confluency. Then, the cell culture medium was
exchanged with fresh serum-free medium and the cells were cultured
for 48 h. The cell culture supernatants were then collected and
concentrated by ultrafiltration (Molecular weight cut-off >50
kDa). After dialysis to phosphate buffered saline (PBS), the
obtained fractions were used in subsequent experiments as the SiSo
supernatant (SiSo Sup/RCAS1+).
ELISA
Soluble-form RCAS1 in SiSo Sup was measured by
ELISA. Briefly, for precoating, 22-1-1 antibody (Medical &
Biological Laboratories Co., Ltd.) was added to each well in a
96-well plate (100 µl/well) and incubated overnight at 4°C. After
washing with PBS, Blocking One solution (Nacalai Tesque, Inc.) was
diluted 5-fold with PBS and added to each well for blocking. After
1 h of incubation at room temperature, diluted SiSo Sup was added
and incubated for 1 h at room temperature. After washing,
biotinylated 22-1-1 antibody was added and incubated for 1 h at
room temperature. After washing, peroxidase-conjugated streptavidin
(Thermo Fisher Scientific, Inc.) was added and incubated for 1 h at
room temperature. Then, peroxidase substrate solution (TMB
Microwell Peroxidase Substrate System Kirkegaard & Perry
Laboratories, Inc.) was added to each well and incubated for 20 min
at room temperature, followed by an equal volume of 1 M
H3PO4. Absorbance was measured at 450 nm
(reference wavelength: 655 nm) with a microplate reader (iMark
Microplate Reader; Bio-Rad Laboratories, Inc.).
Immunodepletion of soluble-form RCAS1
from SiSo Sup
Immunodepletion of soluble-form RCAS1 from SiSo Sup
(SiSo Sup/RCAS1−) was performed by the following method.
First, 22-1-1 antibody was used to prepare anti-RCAS1 beads by
mixing 22-1-1 antibody and Pierce Protein L magnetic beads (Thermo
Fisher Scientific, Inc.) and incubating overnight at 4°C. After
incubation, SiSo Sup was added to the solution containing
antibody-Protein L complexes and incubated for 1 h at room
temperature. After incubation, RCAS1-antibody-Protein L complexes
were removed with a magnetic stand SiSo Sup/RCAS1− was
collected. After immunodepletion, SiSo Sup/RCAS1− was
used in ELISA.
Cytotoxicity of doxycycline to L
cells
Cytotoxity test of Dox to L cells was performed by
WST-8 assay. Cells (2.5×103) were seeded in a 96-well
plate and incubated overnight at 4°C. The culture medium was then
replaced with fresh medium containing each concentration of Dox for
48 h. At the final 4-h, the cells were incubated with WST-8 reagent
(FUJIFILM Wako Pure Chemical Corporation). Absorbance was measured
using a iMark microplate reader (Bio-Rad Laboratories, Inc.) at 450
nm with a reference wavelength of 630 nm.
Time-lapse observation of cell
morphological changes
L/ind RCAS1 cells (3×104) were seeded in
a glass-bottom dish (Matsunami Glass Ind., Ltd.) and incubated
overnight at 4°C. After adding SiSo Sup or Dox, the cultured cells
were observed by time-lapse imaging with a EVOS FL cell imaging
system (Thermo Fisher Scientific, Inc.).
Confocal immunofluorescence
microscopy
To observe actin stress fibers in L/ind RCAS1 cells,
immunofluorescence staining was performed. L/ind RCAS1 cells
(1×105) were seeded in a 35-mm poly-L-lysine coated
glass-bottom dish and cultured for 48 h. After Dox induction, the
cells were washed twice with cold PBS and fixed with 4%
paraformaldehyde for 20 min at room temperature. The fixed cells
were permeabilized with 0.1% Triton X-100 in PBS for 5 min at room
temperature. After blocking with Blocking One solution (Nacalai
Tesque, Inc.) for 1 h at room temperature, the cells were incubated
with anti-RCAS1 antibody (ProteinTech Group, Inc.) in PBS overnight
at 4°C. After washing with PBS, the cells were incubated with
fluorescein isothiocyanate (FITC)-conjugated goat-anti mouse IgG
(Beckman Coulter, Inc.) and 100 nM rhodamine phalloidin
(Cytoskeleton, Inc.) for 2 h at room temperature. After washing
with PBS, the stained cells were observed with a confocal
microscope (LSM710; Zeiss AG).
Measurement of cell index by the
real-time cell-monitoring analysis (RTCA) system
Cell index (CI) was acquired by the iCELLigence
system (ACEA Biosciences, Inc.) as the RTCA system. All monitoring
was performed at 37°C with regulated CO2 content (5%).
E-plates (culture plates for the iCELLigence system) containing 100
µl culture medium per well were equilibrated to 37°C and CI was set
to zero under these conditions. L/ind RCAS1 cells (2×104
cells/well) were added in 50 µl culture medium. After 24 h
incubation at 37°C, Dox was added to each well. The CI was
monitored in real-time for 48 h after cell seeding.
Analysis of the F-actin/G-actin
ratio
Briefly, 1×105 cells were seeded in a
35-mm dish and cultured for 48 h. After Dox induction, the cells
were homogenized in 200 µl of F-actin stabilization buffer [50 mM
PIPES (pH 6.9), 50 mM NaCl, 5 mM MgCl2, 5 mM EGTA, 5%
glycerol, 0.1% Triton X-100, 0.1% Nonidet P-40, 0.1% Tween-20, 0.1%
2-mercaptoethanol, 0.001% antifoam C, 1 mM ATP, 10 µM pepstatin A,
15 µM leupeptin, 10 mM benzamidine, 4 µM tosyl arginine methyl
ester]. The supernatant of the protein extract was collected after
centrifugation at 100,000 × g for 1 h at 37°C. The pellet was
resuspended in ice-cold distilled H2O plus 10 µM
cytochalasin D and then incubated on ice for 1 h to dissociate
F-actin. The resuspended pellet was gently mixed every 15 min.
Supernatant of the resuspended pellet was collected after
centrifugation at 5,000 × g for 2 min at 4°C. Equal volumes of the
first (G-actin) and second (F-actin) supernatants were subjected to
immunoblot analysis using anti-β-actin antibody (Merck KGaA).
Western blot analysis
Following Dox induction, L/ind RCAS1 cells were
lysed in CelLytic-M (Merck) containing a protease inhibitor
cocktail (Nacalai Tesque, Inc.) and phosphatase inhibitors (5 mM
NaF, 1 mM Na3VO4). Cell lysates were
centrifuged at 12,000 × g for 30 min at 4°C and supernatants were
collected. Protein concentrations of each sample were measured
using the BCA protein assay kit (Thermo Fisher Scientific, Inc.).
Equal amounts of protein (10 µg/lane) were electrophoresed on a
5–20% gradient SDS-Polyacrylamide gel electrophoresis (SDS-PAGE)
gel and transferred onto PVDF membranes. The membranes were
incubated with Blocking One solution (Nacalai Tesque, Inc.)
containing phosphatase inhibitors for 1 h at room temperature,
followed by incubation with the following primary antibodies for 1
h at room temperature: Anti-RCAS1 (cat. no. 66170-1; Proteintech
Group, Ltd.; 1:5,000), anti-cofilin and anti-phosphorylated
(p-)cofilin (cat. no. CK6040; ECM Biosciences; 1:2,000); anti-p38,
anti-p-p38, anti-extracellular signal-regulated kinase (ERK) 1/2
and anti-p-ERK 1/2 (cat. nos. 9913 and 9926 Cell Signaling
Technology, Inc.; 1:5,000). After washing with TBS −0.05% Tween,
the membranes were incubated with peroxidase-conjugated goat
anti-mouse IgG or peroxidase-conjugated goat anti-rabbit IgG (cat.
nos. 7074 and 7076; Cell Signaling Technology, Inc.; 1:10,000) as
secondary antibodies for 1 h at room temperature. Immunoreactive
proteins were visualized using ImmunoStar LD (FUJIFILM Wako Pure
Chemical Corporation). Images were captured using a Multi
ImagerIIMultiBox (Bio Tools Inc.). Protein contents were compared
with the corresponding GAPDH controls and normalized For
quantification of blots, AlphaEaseFC Software (version 4.0.1; Alpha
Innotech) was used.
Statistical analysis
All experiments were performed in triplicate and
results are represented as mean ± standard deviation. Statistical
significance was evaluated using one-way ANOVA followed by Tukey
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Cell morphological changes induced by
soluble-form RCAS1
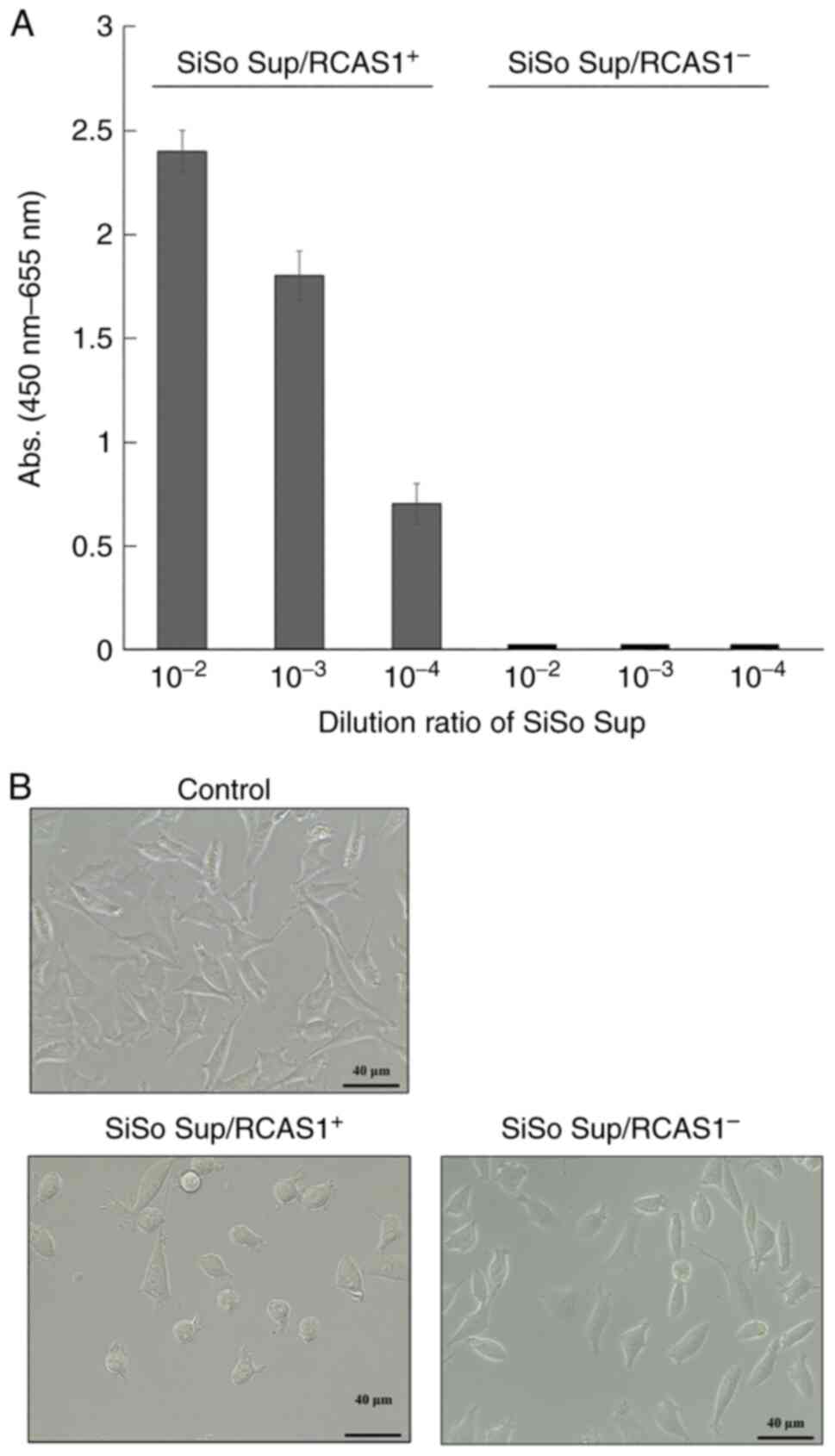
SiSo Sup/RCAS1+ and SiSo
Sup/RCAS1−, which was the fraction that had soluble-form
RCAS1 removed by immunodepletion, were prepared. The effect of
soluble-form RCAS1 against wild-type L cells expressing the RCAS1
receptor was examined. After adding each SiSo Sup preparation
(RCAS1+/RCAS1−) at a 1/100 v/v ratio, L cells
were cultured for 24 h. Soluble-form RCAS1 in SiSo
Sup/RCAS1+ was detected by ELISA (Fig. 1A) and cell morphological changes
(round shape) of L cells were observed after incubation with SiSo
Sup/RCAS1+ (Figs. 1B
and S1). Conversely, RCAS1 was
not detected in SiSo Sup/RCAS1− and the cell
morphological changes were not induced (Fig. 1A and B). These results suggested
that the cell morphological changes induced in L cells by SiSo Sup
required RCAS1.
RCAS1 levels in L/ind RCAS1 cells and
morphological changes
Previously it was found that RCAS1 expression
induced cell morphological changes prior to caspase-mediated
apoptosis in L/ind RCAS1 cells (27). To elucidate the mechanism of these
cell morphological changes, L/ind RCAS1 cells were used to analyze
the relationship between RCAS1 expression and cell morphological
changes. L/ind RCAS1 cells, which transformed with a tetracycline
induced rcas1 gene expression system, were established
previously (27). This cell line
expressed RCAS1 protein by Dox induction. For this experiment,
L/ind RCAS1 cells were treated with 0.5 µg/ml Dox over a course of
time. The present study confirmed no cytotoxity at this
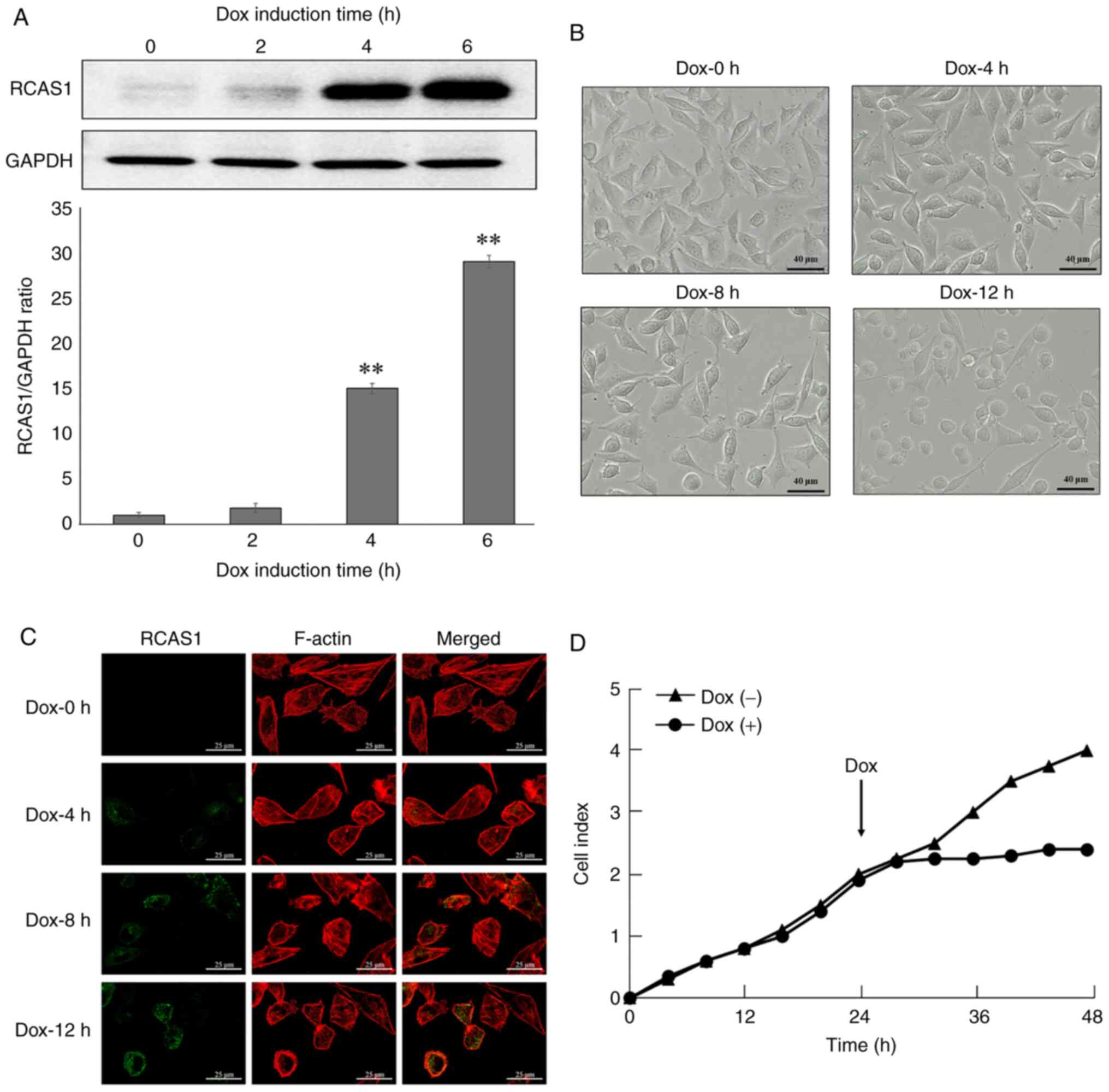
concentration of Dox against L cells (Fig. S2). RCAS1 expression in L/ind RCAS1
cells was measured by western blotting using an anti-RCAS1
antibody. RCAS1 was detected at 4 h after Dox induction and
increased in a time-dependent manner (Fig. 2A). After Dox stimulation, cell
motility was initially decreased and a rounded morphology which is
one of the characteristics of morphological changes in adherent
cells was induced in contrast to the characteristic fibroblastic
morphology of control cells (Dox −0 h; Fig. 2B). To further investigate the cell
morphological changes induced by RCAS1 expression, the conformation
of actin stress fibers in L/ind RCAS1 cells was examined by
immunofluorescence using rhodamine phalloidin and anti-RCAS1
antibody. In control cells (Dox-0 h), actin stress fibers were
spread throughout the cytosol. By contrast, the actin stress fibers
had disappeared in RCAS1-expressing cells, which was correlated
with increased RCAS1 expression (Fig.
2C). These results suggested that RCAS1 expression might induce
morphological changes in L/ind RCAS1 cells including the
disappearance of actin stress fibers. To quantify the cell
morphology, CI was analyzed by RTCA system. The CI measurement
provides quantitative information about the biological status of
adherent cells. In fact, the meaning of CI is the number of
survival cell on the surface of E-plate. These data include the
cell number, viability and morphology as a real-time profile. RTCA
system showed that the CI of Dox treated cells was lower than
control cells (Fig. 2D).
Effect of RCAS1 expression on the
F/G-actin ratio and cofilin phosphorylation
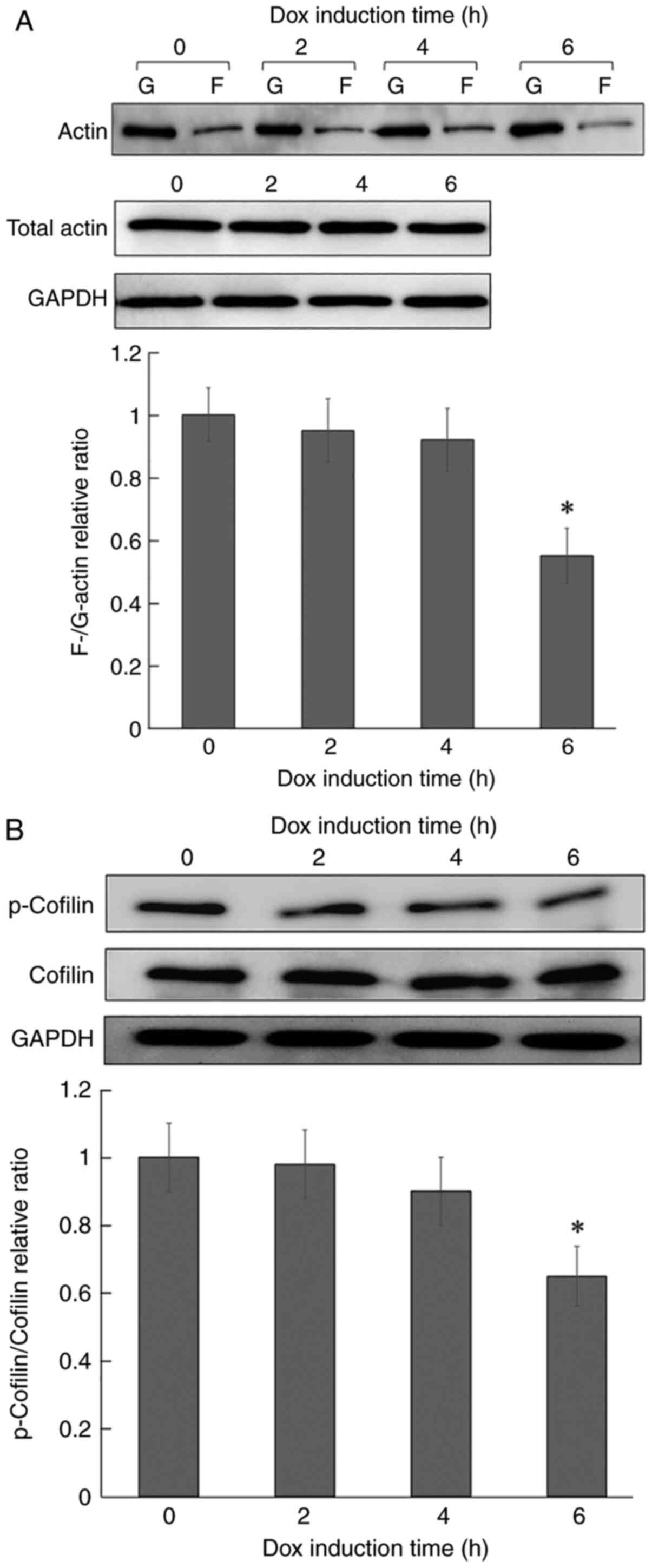
To confirm the disappearance of actin stress fibers
in L/ind RCAS1 cells, actin dynamics were examined by measuring the
F/G-actin ratio and cofilin phosphorylation in L/ind RCAS1 cells.
The F/G-actin ratio was significantly decreased to ~50% of the
level in control cells at 6 h post-induction (Fig. 3A). On the other hand, the total
amount of actin was unchanged. Subsequently, it was determined that
cofilin phosphorylation, which binds to actin and regulates its
polymerization and depolymerization, was significantly decreased in
a time-dependent manner following Dox stimulation (Fig. 3B). These results suggested that the
disappearance of actin stress fibers following RCAS1 expression
might be due to increased actin depolymerization by cofilin.
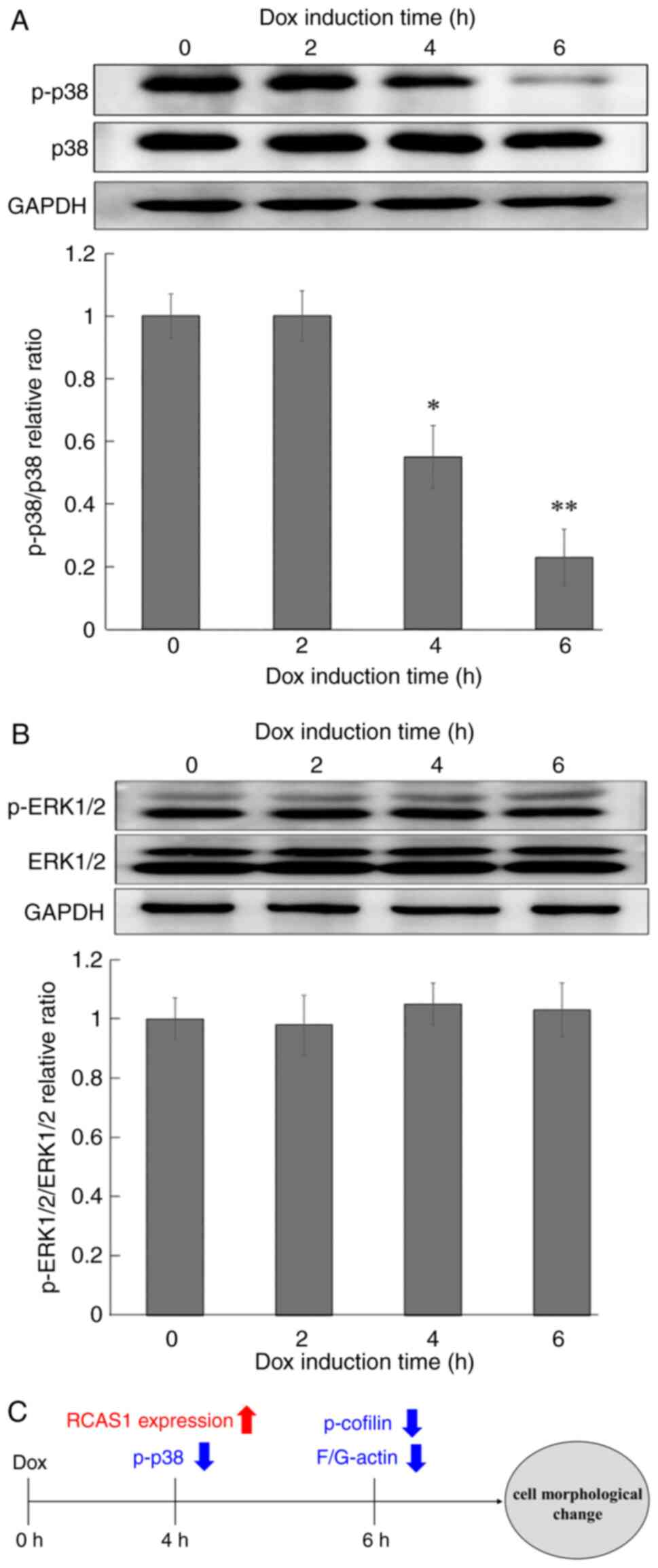
Effect of RCAS1 expression on MAPK
phosphorylation
MAPK signaling is upstream of the cofilin
phosphorylation pathway. To examine the effect of RCAS1 on MAPK
expression and phosphorylation, western blotting analysis we
performed, which revealed that p38 MAPK phosphorylation was
significantly decreased and in a time-dependent manner following
RCAS1 expression (Fig. 4A). By
contrast, ERK1/2 expression and phosphorylation were unchanged by
RCAS1 expression (Fig. 4B).
Decreased p38 phosphorylation was observed almost at the same time
that RCAS1 expression was induced (Fig. 4A), suggesting that p38 might be
closely involved in the RCAS1 signaling pathway.
Discussion
RCAS1 is expressed on the cell surface and in the
cytoplasm of various cancer cells. It has been reported that
soluble-form RCAS1 secreted from tumor cells acts as a ligand for a
putative RCAS1 receptor (RCAS1-R) and induces cell growth
inhibition and apoptosis (19).
Immune cells such as T and B lymphocytes and NK cells express
RCAS1-R and undergo cell growth inhibition and apoptosis following
RCAS1 stimulation (19).
Therefore, RCAS1 expression is thought to be related to tumor
evasion from the immunosurveillance and it has been suggested that
RCAS1 may serve an important role in tumor malignancy. In fact,
RCAS1 expression is related to malignant characteristics, such as
tumor size, invasion depth, clinical stage and poor overall
survival. Thus, a number of clinical studies have reported the
clinical importance of RCAS1 (20–26).
By contrast, there have been few basic studies of the biological
functions of RCAS1.
In the present study, it was shown that cell
morphological changes (rounded shape) of L cells were observed
after incubation with SiSo Sup/RCAS1+. By contrast, the cell
morphological changes were not induced by SiSo Sup/RCAS1-. These
results suggested that the cell morphological changes induced in L
cells by SiSo Sup required RCAS1. However, the concentration of
soluble-form RCAS1 was unknown in the present study. Therefore, it
is planned to confirm the relationship between soluble-form RCAS1
and cell morphological changes by conducting studies using the
recombinant RCAS1 protein.
Next, L/ind RCAS1 cells were used to investigate the
biological functions of RCAS1. The initial results showed that cell
morphological changes were observed 8 h following Dox induction.
Our previous study showed that caspase-3 activation, which is a
hallmark of apoptosis, was observed at 12 h following Dox induction
(27). In cells undergoing
apoptosis, loss of adhesion and cell morphological changes are
often observed via the activated caspase cascade (32–35).
However, loss of adhesion and subsequent cell detachment
accompanied by cell morphological changes can cause cell cycle
arrest and a form of apoptosis termed anoikis (36–39).
This suggests that apoptosis is not the trigger for RCAS1-induced
cell morphological changes.
To uncover the mechanism through which RCAS1 induces
cell morphological changes, the actin cytoskeleton was analyzed in
L/ind RCAS1 cells. Actin is one of the major cytoskeletal proteins
in eukaryotic cells and exists in cells in two forms, the globular
(G-actin) and filamentous (F-actin) forms. F-actin is formed by
polymerization of G-actin and the relative F/G-actin ratio is
determined by the amounts of monomeric actin. F-actin is the major
component of the actin cytoskeleton and critical for various
cellular functions including the maintenance and regulation of cell
morphology, adhesion, motility and intracellular and extracellular
networks (40,41). In the present study, cytoskeleton
imaging showed that actin stress fibers had disappeared together
with increased RCAS1 expression. To support this finding, the
F-/G-actin ratio was decreased in time-dependent following Dox
induction. The polymerization or depolymerization of actin is
regulated by various molecules. Cofilin is known to be a central
regulator of actin dynamics that induces actin depolymerization and
is inactivated by phosphorylation of serine 3 (42). Thus, the phosphorylation state of
cofilin following RCAS1 expression was examined. As a result,
cofilin phosphorylation was found to be significantly decreased at
6 h of Dox induction. These results suggested that the cell
morphological changes induced by RCAS1 might be due to increased
actin depolymerization by cofilin. Since the relationship between
p38 MAP kinase and actin is more widely known (43,44),
the present study focused on the analysis of actin dynamics.
Meanwhile, it is clear that myosin is also involved in cell
morphological changes. Therefore, the relationship between RCAS1
expression and myosin network will be analyzed in the future.
To further analyze the RCAS1 signaling pathway, the
effect of RCAS1 on MAPKs we examined. The MAPK family can be
divided into three groups: Jun N-terminus kinase (JNK), ERK and
p38. MAPKs serve well-known roles in cell proliferation,
differentiation, oncogenesis, cell death and inflammation in
eukaryotes (43,44). Studies have shown that p38
signaling is connected with actin polymerization. It is reported
that VEGF-A induces actin reorganization and migration of
endothelial cells through a p38 MAPK pathway (45). The p38 signaling pathway might
regulate cytoskeleton rearrangements in LPS-induced macrophages
(46). The present study showed
that RCAS1 expression significantly decreased p38 phosphorylation
levels. Markedly, the downregulation of p38 phosphorylation was
detected at 4 h of Dox induction. This decrease in p38
phosphorylation was observed almost simultaneously with RCAS1
expression, suggesting that p38 might be a key molecule in the
RCAS1 signaling pathway (Fig.
4C).
It is well known that p38 phosphorylation is
controlled by MAP 2K and MAP 3K. To clarify the RCAS1 signal
pathway, the association between RCAS1 expression and intracellular
changes of MAP 2K and MAP 3K will be examined in the future. Also,
in RCAS1 signal pathway, the relationship between p38 and actin
dynamics remains to be elucidated. LIM kinases are regulated by
several upstream signaling pathways to influence the architecture
of the actin cytoskeleton by regulating the activity of the cofilin
family. The p38 signal pathway is widely known as one of the
upstream signals that regulate the activation of LIM kinase
(45). Thus, the relationship p38
and LIM kinases in L/ind RCAS1 needs to be clarified in the future.
In addition, fibroblast not tumor cells were used in this study. To
clarify the RCAS1 function in tumor cells, it is necessary to
investigate using tumor cells. It is planned to perform RCAS1
knockdown experiments using SiSo (human uterine carcinoma cell
line) and MCF-7 (human breast cancer cells) which express RCAS1
originally. The effect of RCAS1 siRNA on intracellular molecular
change in these cells will be investigated in the future.
In conclusion, the present study showed that RCAS1
expression induced a downregulation of p38 phosphorylation,
followed by cytoskeletal rearrangements. Several studies have shown
that molecules linked to migration signals and the cytoskeleton are
upregulated in invasive and metastatic tumor cells (28,41).
Additionally, it has been suggested that the p38 signaling pathway
is involved not only in proliferation, metastasis and migration,
but also in poor response to chemotherapy (47–49).
It is hoped that the findings of the present study will contribute
to the analysis of the biological functions of RCAS1 and can be
applied to future tumor treatments that target RCAS1.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Professor Tomoyo
Kawakubo-Yasukochi (OBT Research Center, Faculty of Dental Science,
Kyushu University, Fukuoka, Japan) and Dr Manabu Nakashima
(formerly of the Department of Immunological and Molecular
Pharmacology, Faculty of Pharmaceutical Sciences, Fukuoka
University, Fukuoka, Japan) for technical assistance and valuable
discussions of the present study.
Funding
The present study was supported by JSPS KAKENHI grant number
JP19K07764.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TN and MHa designed the project, performed the
experiments, analyzed the data and prepared the manuscript. TN and
MHa confirm the authenticity of all the raw data. MHo and DI
designed the project. DI reviewed and edited the manuscript. All
authors read and approved the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fontana A, Frei K, Bodmer S, Hofer E,
Schreier MH, Palladino MA Jr and Zinkernagel RM: Transforming
growth factor-beta inhibits the generation of cytotoxic T cells in
virus-infected mice. J Immunol. 143:3230–3234. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li MO, Wan YY, Sanjabi S, Robertson AK and
Flavell RA: Transforming growth factor-beta regulation of immune
responses. Annu Rev Immunol. 24:99–146. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kriegel MA, Li MO, Sanjabi S, Wan YY and
Flavell RA: Transforming growth factor-beta: Recent advances on its
role in immune tolerance. Curr Rheumatol Rep. 8:138–144. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pisa P, Halapi E, Pisa EK, Gerdin E,
Hising C, Bucht A, Gerdin B and Kiessling R: Selective expression
of interleukin 10, interferon gamma, and granulocyte-macrophage
colony-stimulating factor in ovarian cancer biopsies. Proc Natl
Acad Sci USA. 89:7708–7712. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
De Smedt T, Van Mechelen M, De Becker G,
Urbain J, Leo O and Moser M: Effect of interleukin-10 on dendritic
cell maturation and function. Eur J Immunol. 27:1229–1235. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reed JC: Apoptosis-targeted therapies for
cancer. Cancer Cell. 3:17–22. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Andreola G, Rivoltini L, Castelli C, Huber
V, Perego P, Deho P, Squarcina P, Accomero P, Lozupone P, Lugini L,
et al: Induction of lymphocyte apoptosis by tumor cell secretion of
FasL-bearing microvesicles. J Exp Med. 195:1303–1316. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Okada K, Komuta K, Hashimoto S, Matsuzaki
S, Kanematsu T and Koji T: Frequency of apoptosis of
tumor-infiltrating lymphocytes induced by fas counterattack in
human colorectal carcinoma and its correlation with prognosis. Clin
Cancer Res. 6:3560–3564. 2000.PubMed/NCBI
|
|
9
|
Reimer T, Herrnring C, Koczan D, Richter
D, Gerber B, Kabelitz D, Friese K and Thiesen HJ: FasL:Fas ratio-a
prognostic factor in breast carcinomas. Cancer Res. 60:822–828.
2002.PubMed/NCBI
|
|
10
|
Bennett MW, O'Connell J, O'Sullivan GC,
Brady C, Roche D, Collins JK and Shanahan F: The Fas counterattack
in vivo: Apoptotic depletion of tumor-infiltrating lymphocytes
associated with Fas ligand expression by human esophageal
carcinoma. J Immunol. 160:5669–5675. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
O'Connell J, Bennett MW, O'Sullivan GC,
Collins JK and Shanahan F: The Fas counterattack: Cancer as a site
of immune privilege. Immunol Today. 120:46–52. 1999. View Article : Google Scholar
|
|
12
|
Rivoltini L, Carrabba M, Huber V, Castelli
C, Novellino L, Dalerba P, Mortarini R, Arancia G, Anichini A, Fais
S and Parmiani G: Immunity to cancer: Attack and escape in T
lymphocyte-tumor cell interaction. Immunol Rev. 188:97–113. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huber V, Fais S, Iero M, Lugini L, Canese
P, Squarcina P, Zaccheddu A, Colone M, Arancia G, Gentile M, et al:
Human colorectal cancer cells induce T-cell death through release
of proapoptotic microvesicles: Role in immune escape.
Gastroenterology. 128:1796–1804. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Giovarelli M, Musiani P, Garotta G, Ebner
R, Di Carlo E, Kim Y, Cappello P, Rigamonti L, Bernabei P, Novelli
F, et al: A ‘stealth effect’: Adenocarcinoma cells engineered to
express TRAIL elude tumor-specific and allogeneic T cell reactions.
J Immunol. 163:4886–4893. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koyama S, Koike N and Adachi S: Expression
of TNF-related apoptosis-inducing ligand (TRAIL) and its receptors
in gastric carcinoma and tumor-infiltrating lymphocytes: A possible
mechanism of immune evasion of the tumor. J Cancer Res Clin Oncol.
128:73–79. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kassouf N and Thornhill MH: Oral cancer
cell lines can use multiple ligands, including Fas-L, TRAIL and
TNF-alpha, to induce apoptosis in Jurkat T cells: Possible
mechanisms for immune escape by head and neck cancers. Oral Oncol.
44:672–682. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sonoda K, Nakashima M, Saito T, Amada S,
Kamura T, Nakano H and Watanabe T: Establishment of a new human
uterine cervical adenocarcinoma cell-line, siso, and its reactivity
to anticancer reagents. Int J Oncol. 6:1099–1104. 1995.PubMed/NCBI
|
|
18
|
Sonoda K, Nakashima M, Kaku T, Kamura T,
Nakano H and Watanabe T: A novel tumor-associated antigen expressed
in human uterine and ovarian carcinomas. Cancer. 77:1501–1509.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakashima M, Sonoda K and Watanabe T:
Inhibition of cell growth and induction of apoptotic cell death by
the human tumor-associated antigen RCAS1. Nat Med. 5:938–942. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Enjoji M, Nakamuta M, Noguchi K, Sugimoto
R, Kotoh K, Nawata H, Nakashima M and Watanabe T: RCAS1 expression
in immune-mediated liver diseases. J Clin Gastroenterol.
34:286–287. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Enjoji M, Nakashima M, Yamaguchi K, Kotoh
K and Nakamuta M: Significance of RCAS1 antigen in hepatocellular,
cholangiocellular and pancreatic carcinomas. J Gastroenterol
Hepatol. 20:1143–1148. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Giaginis C, Giagini A and Theocharis S:
Receptor-binding cancer antigen expressed on SiSo cells (RCAS1): A
novel biomarker in the diagnosis and prognosis of human neoplasia.
Histol Histopathol. 24:761–776. 2009.PubMed/NCBI
|
|
23
|
Dutsch-Wicherek M: RCAS1, MT, and vimentin
as potential markers of tumor microenvironment remodeling. Am J
Reprod Immunol. 63:181–188. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sonoda K: Novel therapeutic strategies to
target RCAS1, which induces apoptosis via ectodomain shedding.
Histol Histopathol. 26:1475–1486. 2011.PubMed/NCBI
|
|
25
|
Szubert S, Dziobek K and Wicherek L: High
post-treatment serum soluble receptor-binding cancer antigen
expressed on SiSo cells (sRCAS1) levels is associated with poor
survival of patients with cervical cancer. J Obset Gynaecol Res.
46:499–506. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sonoda K, Miyamoto S, Nakashima M and Wake
N: Receptor-binding cancer antigen expressed on SiSo cells induces
apoptosis via ectodomain shedding. Exp Cell Res. 316:1795–1803.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nishinakagawa T, Fujii S, Nozaki T, Maeda
T, Machida K, Enjoji M and Nakashima M: Analysis of cell cycle
arrest and apoptosis induced by RCAS1. Int J Mol Med. 25:717–722.
2010.PubMed/NCBI
|
|
28
|
Yamaguchi H and Condeelis J: Regulation of
the actin cytoskeleton in cancer cell migration and invasion.
Biochim Biophys Acta. 1773:642–652. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Condeelis J, Singer R and Segall JE: The
great escape: When cancer cells hijack the genes for chemotaxis and
motility. Annu Rev Cell Dev Biol. 21:695–718. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sahai E: Mechanisms of cancer cell
invasion. Curr Opin Genet Dev. 15:87–96. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yamaguchi H, Wyckoff J and Condeelis J:
Cell migration in tumors. Curr Opin Cell Biol. 17:559–564. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim B, van Golen CM and Feldman EL:
Insulin-like growth factor I induces preferential degradation of
insulin receptor substrate-2 through the phosphatidylinositol
3-kinase pathway in human neuroblastoma cells. Endocrinology.
146:5350–5357. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lavastre V, Chiasson S, Cavalli H and
Girard D: Viscum album agglutinin-I induces apoptosis and
degradation of cytoskeletal proteins via caspases in human
leukaemia eosinophil AML14.3D10 cells: Differences with purified
human eosinophils. Br J Haematol. 130:527–535. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lavastre V, Pelletier M, Saller R,
Hostanska K and Girard D: Mechanisms involved in spontaneous and
Viscum album agglutinin-I-induced human neutrophil apoptosis:
Viscum album agglutinin-I accelerates the loss of antiapoptotic
Mcl-1 expression and the degradation of cytoskeletal paxillin and
vimentin proteins via caspases. J Immunol. 168:1419–1427. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Levkau B, Herren B, Koyama H, Ross R and
Raines EW: Caspase-mediated cleavage of focal adhesion kinase
pp125FAK and disassembly of focal adhesions in human endothelial
cell apoptosis. J Exp Med. 187:579–586. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Frisch SM and Francis H: Disruption of
epithelial cell-matrix interactions induces apoptosis. J Cell Biol.
124:619–626. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Frisch SM, Vuori K, Ruoslahti E and
Chan-Hui PY: Control of adhesion-dependent cell survival by focal
adhesion kinase. J Cell Biol. 134:793–799. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu WB, Peng HC and Huang TF: Disintegrin
causes proteolysis of beta-catenin and apoptosis of endothelial
cells. Involvement of cell-cell and cell-ECM interactions in
regulating cell viability. Exp Cell Res. 286:115–127. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhao JH, Reiske H and Guan JL: Regulation
of the cell cycle by focal adhesion kinase. J Cell Biol.
143:1997–2008. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Milligan RA, Whittaker D and Safer D:
Molecular structure of F-actin and location of surface binding
sites. Nature. 348:217–221. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dos Remedios CG, Chhabra D, Kekic M,
Dedova IV, Tsubakihara M, Berry DA and Nosworthy NJ: Actin binding
proteins: Regulation of cytoskeletal microfilaments. Physiol Rev.
83:433–473. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Moon A and Drubin DG: The ADF/cofilin
proteins: Stimulus-responsive modulators of actin dynamics. Mol
Biol Cell. 6:1423–1431. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Qi M and Elion EA: MAP kinase pathways. J
Cell Sci. 118:3569–3572. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
p38 protein kinases. Science. 298:1911–1912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kobayashi M, Nishita M, Mishima T, Ohashi
K and Mizuno K: MAPKAPK-2-mediated LIM-kinase activation is
critical for VEGF-induced actin remodeling and cell migration. EMBO
J. 25:713–726. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bian H, Li F, Wang W, Zhao Q, Gao S, Ma J,
Li X, Ren W, Qin C and Qi J: MAPK/p38 regulation of cytoskeleton
rearrangement accelerates induction of macrophage activation by
TLR4, but not TLR3. Int J Mol Med. 40:1495–1503. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Koul HK, Pal M and Koul S: Role of p38 MAP
kinase signal transduction in solid tumors. Genes Cancer.
4:342–359. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pereira L, Igea A, Canovas B, Dolado I and
Nebreda AR: Inhibition of p38 MAPK sensitizes tumour cells to
cisplatin-induced apoptosis mediated by reactive oxygen species and
JNK. EMBO Mol Med. 5:1759–1774. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bakour N, Moriarty F, Moore G, Robson T
and Annett SL: Prognostic significance of glucocorticoid receptor
expression in cancer: A systematic review and meta-analysis.
Cancers (Basel). 13:16492021. View Article : Google Scholar : PubMed/NCBI
|