Introduction
All the necessary information of maintaining
cellular physiological functions is contained in nuclear DNA, which
is an essential macromolecule in eukaryotic cells (1). Enzymatic degradation of nuclear DNA
is defined as chromatinolysis (2),
which plays dual roles in regulating cellular destiny. On one hand,
degradation of damaged DNA could prevent genetic mutations and
disease occurrence. On the other hand, excessive chromatinolysis
facilitates disassembly of nucleus and makes cell death
irreversible. The mechanisms accounting for chromatinolysis remain
elusive, but previous studies have shown that gamma H2A histone
family member X (γ-H2AX) formation and nuclear translocation of
apoptosis inducing factor (AIF) are two crucial factors leading to
chromatinolysis (2,3). γ-H2AX often forms at the breaking
location of DNA double strands and serves as a platform recruiting
nucleases (4). AIF is a protein
located within the space between mitochondrial inner and outer
membranes. After translocating into nucleus and being recruited to
γ-H2AX, it could degrade DNA into oligonucleotides or smaller
molecules (2). Several compounds
have been shown to eliminate cancer cells via inducing
chromatinolysis (5–7), but it remains elusive whether
chromatinolysis is involved in regulation of ischemia-induced
neuronal death.
Maltol (3-hydroxy-2-methyl-4-pyrone) is a chemical
isolated from ginseng root via Maillard reaction (8). Although being widely used as a food
flavoring agent, it has multiple bio-activities including
anti-oxidative stress, anti-inflammatory and anti-apoptotic
(9–11). Moreover, it effectively inhibits
several pathological processes including osteoarthritis, diabetic
peripheral neuropathy and liver fibrosis (12–14).
In mice, maltol has neuroprotective effects against hypoxia-induced
damage in neurons (15,16). Similarly, it could also inhibit
neuronal death after spinal cord injury by inhibiting oxidative
stress (17). These results
indicated a potential neuroprotective effect of maltol against
ischemia-induced neuronal damage. A major factor leading to the
neuron death caused by cerebral ischemia, traumatic brain injury
and epilepsy is oxygen and glucose deprivation (OGD) (18), which is often used to investigate
the effects of natural chemicals on neuronal damage.
SH-SY5Y cells are human neuroblastoma cells with
similar properties with neurons in electrophysiology,
neurochemistry and morphology. For this reason, SH-SY5Y cells
stressed by OGD are often used as an in vitro model to
investigate neuronal injury or death caused by ischemic insults
(19). In the present study, the
effect of maltol on neuronal damage caused by ischemia was
investigated using the OGD model in SH-SY5Y cells.
Materials and methods
Reagents
Maltol and pyruvate sodium were both purchased from
MilliporeSigma. Primary antibodies against AIF (cat. no. ab32516),
phosphorylated (p)-H2AX at S139 (cat. no. ab81299), ATM (cat. no.
ab32420), p-ATM at S1981 (cat. no. 81292), catalase (cat. no.
ab209211), cystine/glutamate antiporter (xCT) (cat. no. ab175186),
mTOR (cat. no. ab134903), p-mTOR (cat. no. 109268), PKM2 (cat. no.
ab89364), p-JNK (cat. no. ab124956), TOMM20 (cat. no. ab186735) and
H2A (cat. no. ab177308) were all obtained from Abcam. Primary
antibody against β-actin was obtained from Cell Signaling
Technology, Inc.
Cell line and culture and cellular
viability assay
SH-SY5Y cells were obtained from Shanghai institute
of cell biology, Chinese Academy of Sciences (Shanghai, China). The
cells were authenticated by STR profiling. Cells were cultured in
DMEM medium (Hyclone; Cytiva) with high glucose supplemented with
10% FBS (Clark Bioscience), penicillin (100 U/ml) and streptomycin
(100 µg/ml), and maintained at 37°C and 5% CO2 in a
humid environment. MTT assay kit was used for cellular viability
examination and DMSO was used to dissolve the purple formazan. The
results were expressed as a ratio of the absorbance at 570 nm to
that in control cells.
Assay of intracellular GSH and
cysteine
The intracellular GSH levels were assayed by using
GSH assay kit (cat. no. S0052; Beyotime Institute of Biotechnology)
following the manufacturer's protocol. The result was displayed as
a ratio of the absorbance of each prepared sample at 412 nm to that
of control cells.
The intracellular cysteine levels were determined by
cysteine assay kit (cat. no. A126-1-1; Nanjing Jiancheng
Bioengineering Institute) according to the manufacturer's
instructions. The result was expressed as a ratio of the absorbance
of each prepared sample at 600 nm to that of control cells.
Measurement of reactive oxygen species
(ROS)
Intracellular ROS levels were detected by using
DCFH-DA, which was obtained from Beyotime Institute of
Biotechnology. The operation protocol was conducted according to
the manufacturer. Fluorescence was measured using a fluorescence
spectrometer (HTS 7000; PerkinElmer, Inc.) at an excitation
wavelength of 485 nm and an emission wavelength of 530 nm. The ROS
levels were displayed as arbitrary unit/mg protein, then as a ratio
to control. A fluorescence microscope (IX71; Olympus Corporation)
was used to observe and image the cells seeded on six-well plates
stained with DCFH-DA.
Neutral comet assay
Neutral comet assay was performed as previously
described (20). Briefly, SH-SY5Y
cells with or without OGD treatment were collected and suspended in
low-melting agarose. After deposited on comet slides pre-layered
with regular-melting agarose, the cells were covered with
coverslips and cooled down at 4°C for 10 min. Afterwards, the cells
were lysed in darkness at 4°C for 1 h and washed for 10 min in TBE
buffer. After electrophoresed and washed, the cells were
neutralized and stained with acridine orange for 5 min.
The slides were observed and images were captured by
using a fluorescence microscope (IX71; Olympus Corporation). ImageJ
software v1.54 (National Institutes of Health) and OpenComet 1.3
software (National Institutes of Health) were applied to measure
the cell number with DNA comets and the DNA percent content in
comet tail region (four assays, each with ~100 cells analyzed).
Gel electrophoresis and western
blotting
SH-SY5Y cells were collected and homogenized as
previously described (20). The
homogenates were centrifuged to isolate cytoplasmic, mitochondria
and nuclear fractions (21). The
protein content in each fraction was assayed with a BCA Protein
assay kit (Beyotime Institute of Biotechnology). Equal quantities
of protein (30 µg per lane) were electrophoresed on 8–12% sodium
dodecyl sulfate-polyacrylamide gels based on the molecular weight
of the target protein and transferred to PVDF membranes. The
membranes were then blocked with 5% skimmed milk in PBS for 2 h at
room temperature and incubated overnight at 4°C with primary
antibodies. Then, the membrane was incubated with horseradish
peroxidase-conjugated secondary antibody at room temperature for 2
h and washed with PBS for three times. All the primary antibodies
and secondary antibodies were diluted with PBS-T (0.05% Tween-20)
at 1:1,000. Eventually, immunoreactive proteins were visualized by
using a chemiluminescence developer (ChemiScope 5300; Clinx Science
Instrument Co., Ltd.). The loading controls in western blotting
used in the present study were as follows: β-actin was used for
cytoplasm fraction (22), Tomm20
was used for mitochondrial fraction (23) and H2AX was used for nuclear
fraction (6).
Immunocytochemical staining
The SH-SY5Y cells (8×104 cells/well) were
seeded on a culture dish. After OGD, they were fixed in ethanol,
washed with PBS, and incubated with 1% Triton X-100 for 10 min at
4°C. After blocking the non-specific antibody binding sites with 5%
skimmed milk in PBS for 2 h at room temperature, the cells were
incubated overnight with anti-γ-H2AX (1:100; cat. no. ab81299;
Abcam) or anti-AIF (1:100; cat. no. ab32516; Abcam) followed by
incubation in Alexa Fluor 488-conjugated or Alexa Fluor
647-conjugated goat anti-rabbit IgG (1:200; cat. nos. A0423 or
A0468; Beyotime Institute of Biotechnology) for 1 h and then with
Heochst33258. Finally, all the cells were observed under laser
scanning confocal microscope (FV1000; Olympus Corporation).
Mitochondrial membrane potential
assay
The cells were collected and stained with JC-1 at
37°C for 20 min according to the manufacturer's instruction
(Beyotime Institute of Biotechnology). After washed with PBS, the
cells were assayed by flow cytometry (FACScan) and analyzed using
CELLquest pro software 5.1 (both from Becton-Dickinson and Company)
observed under fluorescence microscope (IX71; Olympus
Corporation).
Statistical analyses
All data was acquired from at least four independent
experiments and are expressed as the mean ± standard deviation.
Statistical analyses were performed with Microsoft Excel 2010
(Microsoft Corporation) and GraphPad Prism 6 software (GraphPad
Software, Inc.). Statistical comparisons were made using one-way
ANOVA with Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Maltol inhibits OGD-induced death and
chromatinolysis in SH-SY5Y cells
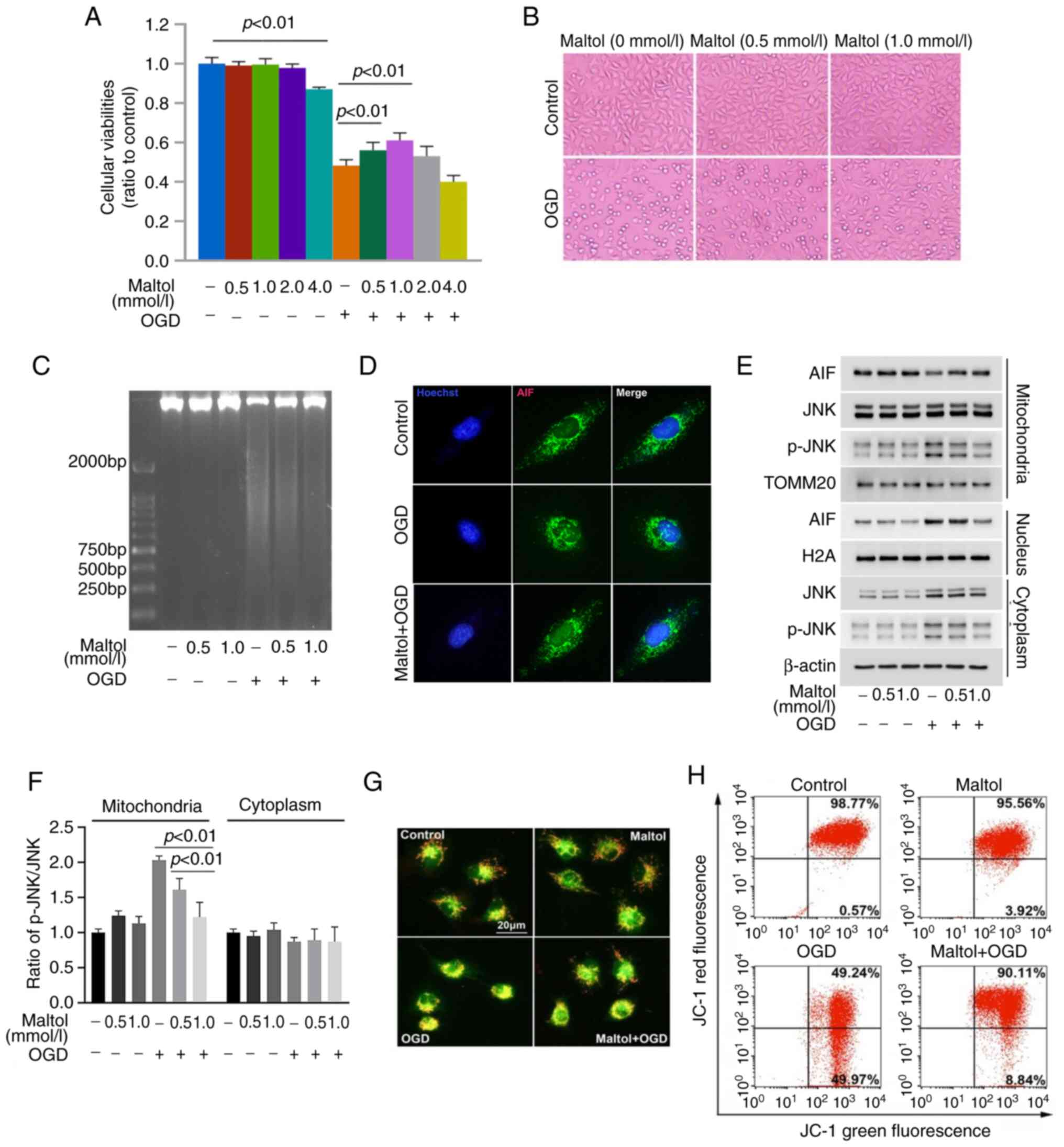
To investigate whether maltol has protective effect
on neurons stressed with OGD, MTT assay was used to examine
cellular viabilities. As previously described (24), SH-SY5Y cells were pretreated 1 h
with maltol at 0.5, 1.0, 2.0 and 4.0 mmol/l and then stressed with
OGD for 24 h. As revealed in Fig.
1A, the viability of the SH-SY5Y cells was decreased by OGD
significantly when compared with that of control cells. Light
microscopy showed that the control cells were polygonal, but
majority of the cells stressed with OGD became smaller and round
(Fig. 1B). By contrast,
OGD-induced reduction in cellular viability was apparently
prevented in the cells pretreated with 0.5 mmol/l maltol, and
further prevented when maltol dosage was increased to 1.0 mmol/l
(Fig. 1A). Pretreatment of maltol
at 4 mmol/l alone could inhibit cellular viabilities, and the
effect of maltol at 2 mmol/l was less significant than that of
maltol at 0.5 and 1.0 mmol/l (Fig.
1A). Thus, maltol at 0.5 and 1 mmol/l was used in the
subsequent studies. Morphologically, the cells with smaller size
and round shape caused by OGD were obviously inhibited in the
presence of maltol (Fig. 1B).
Therefore, the aforementioned results indicated that maltol could
effectively prevent OGD-induced injury in SH-SY5Y cells.
To clarify why maltol could exert protection against
OGD-induced damage, agarose gel electrophoresis was used to assay
its effect on chromatinolysis because chromatinolysis is a final
event leading to cell death (1).
In comparison with control cells, the DNA isolated from
OGD-stressed cells presented smear band on agarose gel after being
subjected to electrophoresis, which was obviously inhibited in the
cells pretreated with 0.5 mmol/l maltol (Fig. 1C). Notably, the inhibitory effect
of 1.0 mmol/l maltol was more obvious than that produced by 0.5
mmol/l maltol. This suggested that maltol protects SH-SY5Y cells
against OGD-induced damage via inhibiting chromatinolysis in a
dose-dependent manner.
Maltol inhibits OGD-induced nuclear
translocation of AIF via inhibition of JNK activation
AIF that is located at mitochondria could serve as a
nuclease after translocation into nuclei and being recruited to
γ-H2AX; therefore, western blotting was used to analyze the effect
of OGD on AIF distribution. Compared with control cells,
mitochondrial AIF was apparently decreased by OGD, whereas nuclear
AIF was increased correspondingly (Fig. 1E). Consistently, confocal
microscopy revealed that AIF accumulated obviously in the nuclei of
OGD-stressed cells (Fig. 1D). This
indicated that OGD induced AIF translocation from mitochondria to
nuclei. Given that AIF release from mitochondria is decided by
mitochondrial depolarization (25), JC-1 staining combined with flow
cytometry was used to examine OGD-induced changes in mitochondrial
membrane potentials. JC-1 displays high red fluorescence after
accumulation in mitochondria, but it exists in the cytoplasm and
emits green fluorescence when the mitochondrial membrane potential
is exhausted. Both fluorescence microcopy and flow cytometry
revealed that red fluorescence decreased obviously in the cells
stressed with OGD, when compared with control cells (Fig. 1F and G). It has been reported that
activated JNK could aggravate cerebral ischemia-induced
mitochondrial depolarization by translocation to mitochondria
(26). The effect of OGD on p-JNK
distribution was examined using western blotting. Compared with
control cells, cytoplastic and mitochondrial p-JNK were obviously
increased by OGD (Fig. 1E and F).
These findings indicated that OGD induced mitochondrial
depolarization in SH-SY5Y cells. By contrast, OGD-induced depletion
of mitochondrial membrane potential, translocation of AIF from
mitochondria to nuclei as well as increased p-JNK in mitochondria
were all inhibited by maltol (Fig.
1E-H). Therefore, these data indicated that maltol inhibited
OGD-induced nuclear translocation of AIF.
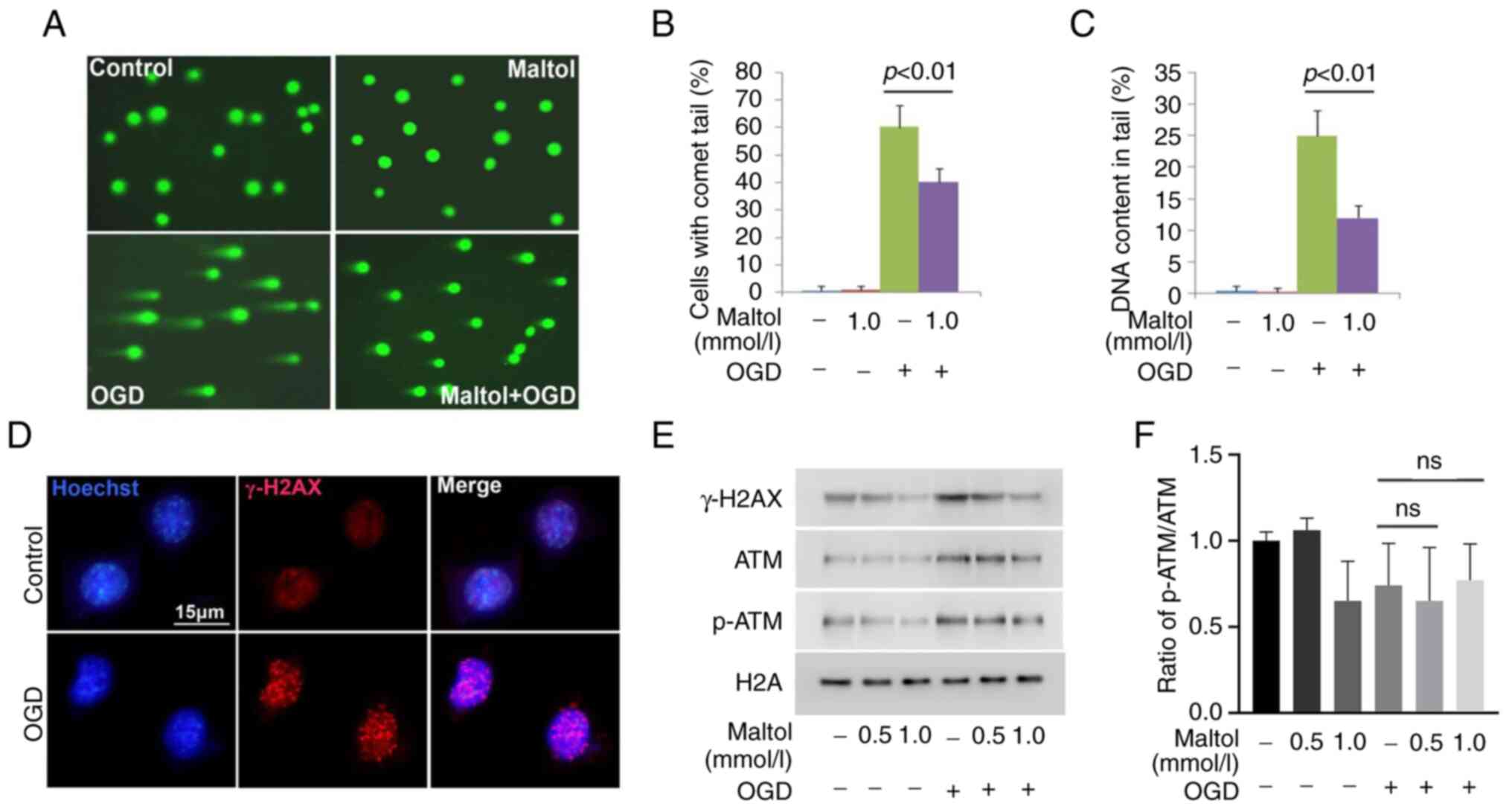
Maltol inhibits OGD-induced DNA
double-strand breaks (DSBs) in SH-SY5Y cells
To elucidate why maltol could inhibit
chromatinolysis, neutral comet assay was used to examine its effect
on DNA DSBs that is a crucial step leading to chromatinolysis. When
compared with control cells, the majority of the cells stressed
with OGD presented long comet tails (Fig. 2A). Statistical analysis proved that
the cells with comet tails and the DNA content in the tails were
both significantly improved by OGD (Fig. 2B and C). Moreover, confocal
microscopy showed that γ-H2AX foci, a prominent biomarker of DNA
DSBs, formed extensively in the nuclei of OGD-stressed cells
(Fig. 2D). Furthermore, western
blot analysis revealed that the protein level of γ-H2AX was
upregulated by OGD, when compared with control cells (Fig. 2E). Consistently, p-ATM accounting
for γ-H2AX formation was also upregulated by OGD at each indicated
time, although the ratio of p-ATM/ATM presented no statistical
difference (Fig. 2E and F). These
results indicated the OGD-induced DNA DSBs in SH-SY5Y cells. By
contrast, pretreatment with 1.0 mmol/l maltol obviously inhibited
OGD-induced increase in cells with comet tails and improvement of
DNA content in comet tails (Fig.
2A-C). Notably, maltol obviously attenuated OGD-induced
upregulation of γ-H2AX and p-ATM in a dose-dependent manner
(Fig. 2E). Therefore, these data
indicated that maltol inhibited OGD-induced DNA DSBs.
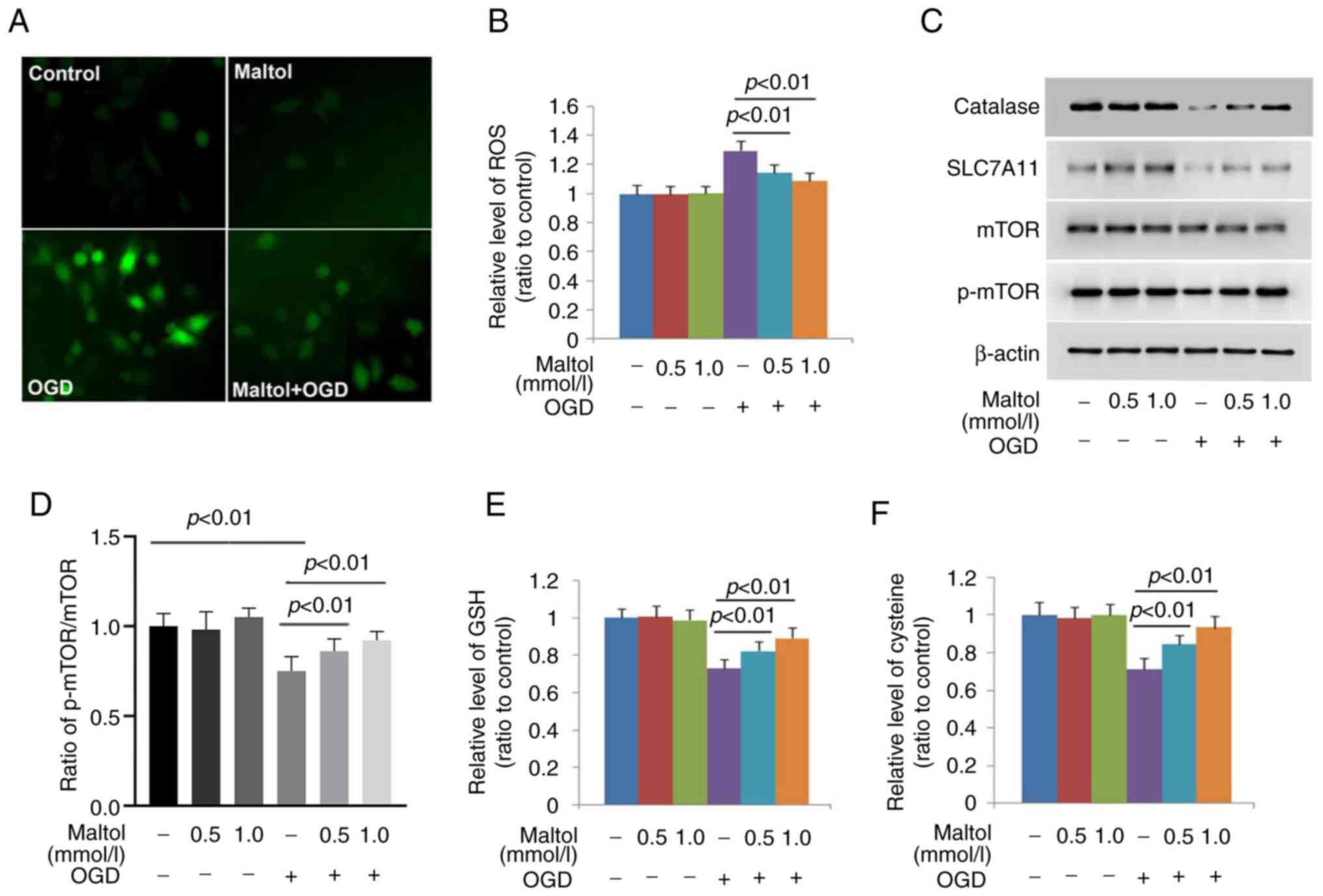
Maltol inhibits OGD-induced ROS via
maintaining catalase, xCT, GSH and cysteine levels
Because oxidative stress characterized by
intracellular accumulation of ROS is a factor leading to nuclear
translocation of AIF and DNA DSBs and OGD could induce oxidative
stress (6,24), DCFH-DA (a probe for ROS) was used
to examine whether maltol could inhibit OGD-induced ROS. As
revealed by fluorescence microscopy, the green fluorescence
exhibited by DCFH-DA was markedly brighter in OGD-stressed cells
than that in control cells, which was obviously attenuated in the
cells pretreated with 1.0 mmol/l maltol (Fig. 3A). Statistical analysis proved as
well that the increased fluorescence intensity caused by OGD could
be significantly inhibited by 0.5 mmol/l maltol, and further
inhibited when maltol dosage was improved to 1.0 mmol/l (Fig. 3B). These results indicated that
maltol inhibited OGD-induced ROS in a dose-dependent manner.
To address why maltol could prevent OGD-induced ROS,
its effect was examined on catalase (which can catalyze reduction
of hydrogen peroxide into water and oxygen) and GSH (an
intracellular antioxidant synthesized from cysteine) (27). As revealed by western blotting,
maltol exerted inhibitory effect on OGD-induced downregulation of
catalase, which became more apparent when maltol dosage was
increased from 0.5 to 1.0 mmol/l (Fig.
3C and D). Although GSH and cysteine were both depleted by OGD,
their levels were maintained dose-dependently by maltol (Fig. 3E and F). Since cysteine is
converted from cystine, the effect of maltol on the protein level
of SLC7A11 was investigated. As a light chain of xCT accounting for
transporting extracellular cystine into cells, SLC7A11 level is
also regulated by activated mTOR (28). It was revealed that maltol
inhibited OGD-induced downregulation of SLC7A11 and p-mTOR in a
dose-dependent manner (Fig. 3C and
D). Therefore, the aforementioned results indicated that maltol
prevented OGD-induced ROS via inhibiting depletion of GSH and
cysteine, downregulation of catalase and SLC7A11, and inactivation
of mTOR.
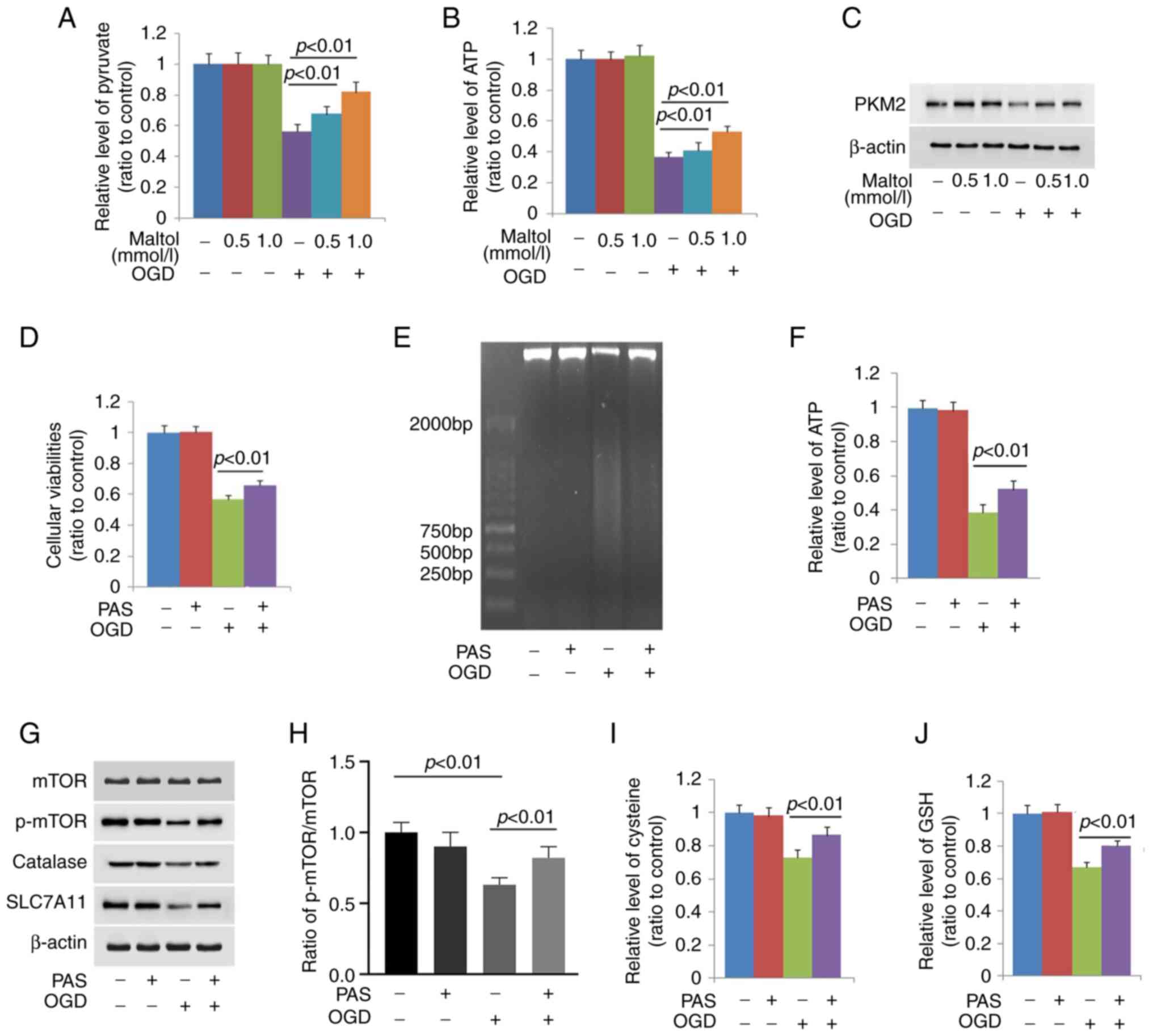
Maltol inhibits OGD-induced mTOR
inactivation by maintaining pyruvate level
To address why maltol prevented OGD-induced mTOR
inactivation, its effect on OGD-induced changes in glycolysis
function was investigated, considering that glycolysis dysfunction
leads to mTOR inactivation (29).
It was found that OGD induced obvious depletion of ATP and pyruvate
(Fig. 4A and B). Moreover, western
blotting showed as well that PKM2 was obviously downregulated by
OGD (Fig. 4C). These indicated
that OGD induced glycolysis dysfunction.
Given that pyruvate could be used to generate ATP
after entering TCA (30), the
SH-SY5Y cells were pretreated with exterior pyruvate at 10 mmol/l
for 1 h and then stressed with OGD for 24 h. It was found that
supplement of exterior pyruvate not only inhibited OGD-induced
death in SH-SY5Y cells, but also reversed ATP depletion (Fig. 4D and F). Consistently, pyruvate
apparently inhibited OGD-induced downregulation of p-mTOR (Fig. 4G and H). This indicated that
pyruvate depletion exacerbated OGD-induced mTOR inactivation.
Moreover, OGD-induced downregulation of xCT and depletion of
cysteine and GSH were all prevented by pyruvate (Fig. 4G, I and J). The smear band on
agarose gel presented by the DNA isolated from OGD-stressed cells
was obviously inhibited by pyruvate (Fig. 4E). The aforementioned results
indicated that pyruvate depletion contributed to OGD-induced mTOR
inactivation.
Notably, it was revealed that the depleted pyruvate
and ATP and downregulated PKM2 caused by OGD were all prevented by
maltol in a dose-dependent manner (Fig. 4A-C). These indicated that maltol
inhibited pyruvate depletion caused by OGD by maintaining
glycolysis function.
Discussion
In summary, it was demonstrated in the present study
that maltol protected SH-SY5Y cells against OGD-induced
chromatinolysis by inhibiting DNA DSBs and nuclear translocation of
AIF. Then, it was found that maltol attenuated OGD-induced ROS,
which could lead to DNA DSBs and nuclear translocation of AIF.
Mechanistically, it was revealed that maltol attenuated ROS via two
pathways; one was inhibiting depletion of GSH and cysteine by
maintaining xCT level and the other was abrogating catalase
downregulation. In addition, it was identified that maltol
alleviated OGD-induced mTOR inactivation via inhibiting pyruvate
depletion. Considering that activated mTOR could promote xCT
expression and inhibit catalase degradation by autophagy, the
present data suggested that ROS-dependent chromatinolysis induced
by OGD was inhibited by maltol via preventing glycolysis
dysfunction-dependent inactivation of mTOR.
As a crucial step leading to cell death,
chromatinolysis is involved in regulation of apoptosis and
necroptosis. It was reported that it not only contributes to
staurosporine-induced apoptosis in Jurkat cells, but also regulates
shikonin-triggered glioma cell necroptosis (5,31).
DNA was cleaved into fragments of ~180–200 bp by activated
endonucleases such as caspase-activated DNase and endonuclease G
during the process of apoptosis (5), but was cleaved randomly under the
condition of necroptosis (5). This
explains why the nuclear DNA extracted from necroptotic cells
presents continuous smear bands after electrophoresis on agarose
gel, whereas the DNA extracted from apoptotic ones displays ladder
bands (5). It was reported that
both brain ischemia and OGD induce neuronal death via necroptosis;
despite that, it remains elusive whether chromatinolysis plays a
role during the process of neuronal death caused by brain ischemia
or OGD (32,33). Previously, it was reported that OGD
could induce damages in mitochondria, endoplasmic reticulum and
disrupt in cell membrane (34–36).
In the present study, it was identified that OGD could induce
chromatinolysis given that the nuclear DNA extracted from the cells
stressed by OGD presented smear band on agarose gel. By contrast,
pretreatment with maltol obviously inhibited the smear band. Thus,
maltol protected SH-SY5Y cells against OGD stress via inhibition of
chromatinolysis.
Nuclear translocation of AIF from mitochondria is a
crucial event causing chromatinolysis in both apoptotic and
necroptotic cells (2,31). Within nuclei, AIF is recruited to
λ-H2AX on damaged DNA and performs as a nuclease to degrade DNA
(2). It is also required for
nuclear recruitment of macrophage migration inhibitory factor
(MIF), which exacerbates chromatinolysis via cleaving genomic DNA
into large fragments (37). Either
cerebral ischemia or OGD was reported to induce neuronal damage via
causing AIF translocation from mitochondria to nuclei (38,39).
Further study showed that activated JNK could exacerbate cerebral
ischemia-induced mitochondrial depolarization by translocation to
mitochondria (26). Different with
previous reports showing that ginsenoside Rb1 and baicalein exerted
protection on neuronal mitochondria against ischemic insults
(38,39), maltol could enhance
PINK1/Parkin-mediated autophagic removal of damaged mitochondria
(33). The data of the present
study proved that maltol effectively inhibited OGD-induced
mitochondria depolarization, nuclear translocation of AIF and
mitochondrial accumulation of p-JNK. Thus, maltol prevented
OGD-induced nuclear translocation of AIF by inhibition of JNK
activation, which further attenuated OGD-induced
chromatinolysis.
DNA DSBs is another factor leading to
chromatinolysis. Upon occurrence of DNA DSBs, ATM is activated and
then generates λ-H2AX which could serve as a platform to recruit
nucleases (40). It has been
reported that both cerebral ischemia and OGD could trigger DNA DSBs
in neuron (41–43). Besides ion radiation, ROS are
regarded as a crucial factor leading to DNA DSBs because bioactive
macromolecules including proteins, lipids and nucleic acids are
easily attacked by ROS (44).
Moreover, ROS is a common pathological feature of cerebral
ischemia, traumatic brain injury and epilepsy (44). Previous studies showed that
pretreatment with maltol could effectively rescue hydrogen
peroxide-induced death in human SH-SY5Y cells and rat retinal
neuronal cells (45,46). In the present study, it was found
that maltol markedly prevented OGD-induced λ-H2AX formation and
phosphorylation of ATM, as well as effectively inhibited ROS
accumulation. Another study revealed that maltol not only
upregulates the expression of Nrf2, but also promotes its
translocation into nuclei (12).
Within nuclei, Nrf2 acts as a transcription factor accounting for
upregulating the expression of inducible antioxidant enzymes
(47). Thus, inhibition of
ROS-dependent DNA DSBs is the other pathway via which maltol
prevented OGD-induced chromatinolysis.
ROS resulting from disrupted balance between their
generation and clearance could lead to DNA DSBs and nuclear
translocation of AIF (6). The ROS
induced by transient ischemia or GOD was closely associated with
downregulation of catalase and depletion of GSH (48–50).
Dioscin attenuated OGD-induced oxidative stress in hippocampal
neurons via reversing catalase downregulation and GSH depletion
(49). Cysteine that is converted
from cystine appears to play a crucial role in regulation of
intracellular ROS levels. A previous study showed that cysteine
exerts inhibitory effect on intracellular
H2O2 via two pathways. One is to be used for
synthesizing GSH which is then used by GPX4 to reduce
H2O2, and the other is to inhibit superoxide
generation by mitochondrial complex III (51). In the present study, it was
identified that OGD induced downregulation of catalase and
depletion of GSH and cysteine in SH-SY5Y cells, which were all
significantly prevented by pretreatment with maltol. Therefore,
maltol prevented OGD-induced ROS via maintaining GSH and cysteine
levels.
Downregulation of SLC7A11 is a pathway via which
ischemia improves neuronal ROS levels (52). It was found that inhibition of
SLC7A11 resulted in ROS accumulation by depletion of GSH and
cysteine. As a specific inhibitor of SLC7A11, erastin was reported
to induce death in primary spinal cord neurons via improving
intracellular ROS (53). Previous
studies showed that the protein level of SLC7A11 could be regulated
by p53 and mTOR, both of which play opposite role in regulation of
SLC7A11 expression. Activated p53 could suppress SLC7A11 expression
directly, but activated mTOR upregulates SLC7A11 at transcriptional
level via mediating Oct1 signaling (54). Because catalase and Δ133p53 (an
inhibitor of full-length p53) are both selective substrates of
autophagy, mTOR inactivation could promote catalase removal and p53
activation via autophagic pathway (55). Thus, mTOR inactivation exacerbates
downregulation of SLC7A11 and catalase. In the present study, it
was found that maltol obviously restored OGD-induced mTOR
inactivation. Consistently, another study showed as well that
maltol not only could restore mTOR activity via the PI3K/Akt
pathway, but also could inhibit p53 activation (56). Thus, maltol prevented OGD-induced
downregulation of SLC7A11 and catalase via maintaining mTOR
activity.
mTOR signaling is often inactivated upon lack of
energy supply (54). Previous
studies showed that pyruvate plays an important role in regulating
mTOR activity. After being produced primarily by glycolysis,
pyruvate enters into tricarboxylic acid cycle and then is used to
generate ATP to supply energy (57). Additionally, pyruvate is also an
interior ROS scavenger, which is supported by the finding that
pyruvate effectively protected neuronal cells against challenge by
hydrogen peroxide (58). Thus,
pyruvate depletion could lead to energy failure and oxidative
stress, both of which could inactivate mTOR. Reversely, mTOR
inactivation could result in pyruvate depletion. It was recently
reported that suppression of mTOR by deoxy-shikonin inhibited
glycolysis and depleted pyruvate in acute myeloid leukemia cells
(59). Therefore, pyruvate
depletion and mTOR inactivation could exacerbate mutually. In the
present study, it was identified that supplement of exterior
pyruvate not only inhibited OGD-induced ATP depletion, but also
prevented p-mTOR downregulation. Correspondingly, the downregulated
catalase and SLC7A11 and the depleted GSH and cysteine were all
abrogated by exterior pyruvate. Thus, pyruvate depletion due to
glycolysis dysfunction contributed to OGD-induced inactivation of
mTOR. Notably, maltol significantly prevented OGD-triggered
depletion of pyruvate. Thus, maltol maintained pyruvate level in
the cells stressed by OGD.
In conclusion, the present study demonstrated that
maltol effectively inhibited OGD-induced chromatinolysis via
maintaining pyruvate level in SH-SY5Y cells and may be a potential
medicine for cerebral ischemia.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Nature and
Science Foundation of China (grant nos. 81972346 and 82173027), the
Scientific Research Foundation of Jilin (grant no. 20200201405JC),
the Achievement Transformation Fund of the First Hospital of Jilin
University (grant no. CGZHYD202012-028) and the Development and
Reform Fund of Jilin (grant no. 2014G074).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SZ conceptualized the study and provided
methodology. XZ provided resources and performed visualization. XW
conducted investigation, prepared the original draft and wrote the
manuscript. CL conducted investigation. XZ, CH and TL performed
validation, formal analysis and data curation. TL conducted
validation and visualization. PG conceptualized and supervised the
study, performed visualization, wrote, reviewed and edited the
manuscript. All authors read and approved the final manuscript. SZ
and PG confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kawane K, Motani K and Nagata S: DNA
degradation and its defects. Cold Spring Harb Perspect Biol.
6:a0163942014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Artus C, Boujrad H, Bouharrour A, Brunelle
MN, Hoos S, Yuste VJ, Lenormand P, Rousselle JC, Namane A, England
P, et al: AIF promotes chromatinolysis and caspase-independent
programmed necrosis by interacting with histone H2AX. Embo J.
29:1585–1599. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baritaud M, Cabon L, Delavallée L,
Galán-Malo P, Gilles ME, Brunelle-Navas MN and Susin SA:
AIF-mediated caspase-independent necroptosis requires ATM and
DNA-PK-induced histone H2AX Ser139 phosphorylation. Cell Death Dis.
3:e3902012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pilch DR, Sedelnikova OA, Redon C, Celeste
A, Nussenzweig A and Bonner WM: Characteristics of gamma-H2AX foci
at DNA double-strand breaks sites. Biochem Cell Biol. 81:123–129.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ding Y, He C, Lu S, Wang X, Wang C, Wang
L, Zhang J, Piao M, Chi G, Luo Y, et al: MLKL contributes to
shikonin-induced glioma cell necroptosis via promotion of
chromatinolysis. Cancer Lett. 467:58–71. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He C, Lu S, Wang XZ, Wang CC, Wang L,
Liang SP, Luo TF, Wang ZC, Piao MH, Chi GF and Ge PF: FOXO3a
protects glioma cells against temozolomide-induced DNA double
strand breaks via promotion of BNIP3-mediated mitophagy. Acta
Pharmacol Sin. 42:1324–1337. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Berdelle N, Nikolova T, Quiros S, Efferth
T and Kaina B: Artesunate induces oxidative DNA damage, sustained
DNA double-strand breaks, and the ATM/ATR damage response in cancer
cells. Mol Cancer Ther. 10:2224–2233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han Y, Xu Q, Hu JN, Han XY, Li W and Zhao
LC: Maltol, a food flavoring agent, attenuates acute
alcohol-induced oxidative damage in mice. Nutrients. 7:682–696.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Murakami K, Ishida K, Watakabe K,
Tsubouchi R, Haneda M and Yoshino M: Prooxidant action of maltol:
Role of transition metals in the generation of reactive oxygen
species and enhanced formation of 8-hydroxy-2′-deoxyguanosine
formation in DNA. Biometals. 19:253–257. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu W, Wang Z, Hou JG, Zhou YD, He YF,
Jiang S, Wang YP, Ren S and Li W: The liver protection effects of
maltol, a flavoring agent, on carbon tetrachloride-induced acute
liver injury in mice via inhibiting apoptosis and inflammatory
response. Molecules. 23:21202018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Z, Hao W, Hu J, Mi X, Han Y, Ren S,
Jiang S, Wang Y, Li X and Li W: Maltol Improves APAP-Induced
hepatotoxicity by inhibiting oxidative stress and inflammation
response via NF-κB and PI3K/Akt signal pathways. Antioxidants
(Basel). 8:3952019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu DC, Wang YH, Lin JH, Miao ZM, Xu JJ
and Wu YS: Maltol inhibits the progression of osteoarthritis via
the nuclear factor-erythroid 2-related factor-2/heme oxygenase-1
signal pathway in vitro and in vivo. Food Funct. 12:1327–1337.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo N, Li C, Liu Q, Liu S, Huan Y, Wang X,
Bai G, Yang M, Sun S, Xu C and Shen Z: Maltol, a food flavor
enhancer, attenuates diabetic peripheral neuropathy in
streptozotocin-induced diabetic rats. Food Funct. 9:6287–6297.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mi XJ, Hou JG, Jiang S, Liu Z, Tang S, Liu
XX, Wang YP, Chen C, Wang Z and Li W: Maltol mitigates
thioacetamide-induced liver fibrosis through TGF-β1-mediated
activation of PI3K/Akt signaling pathway. J Agric Food Chem.
67:1392–1401. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hong S, Iizuka Y, Lee T, Kim CY and Seong
GJ: Neuroprotective and neurite outgrowth effects of maltol on
retinal ganglion cells under oxidative stress. Mol Vis.
20:1456–1462. 2014.PubMed/NCBI
|
|
16
|
Kim YB, Oh SH, Sok DE and Kim MR:
Neuroprotective effect of maltol against oxidative stress in brain
of mice challenged with kainic acid. Nutr Neurosci. 7:33–39. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mao Y, Du J, Chen X, Al Mamun A, Cao L,
Yang Y, Mubwandarikwa J, Zaeem M, Zhang W, Chen Y, et al: Maltol
promotes mitophagy and inhibits oxidative stress via the
Nrf2/PINK1/Parkin pathway after spinal cord injury. Oxid Med Cell
Longev. 2022:13376302022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Badiola N, Penas C, Miñano-Molina A,
Barneda-Zahonero B, Fadó R, Sánchez-Opazo G, Comella JX, Sabriá J,
Zhu C, Blomgren K, et al: Induction of ER stress in response to
oxygen-glucose deprivation of cortical cultures involves the
activation of the PERK and IRE-1 pathways and of caspase-12. Cell
Death Dis. 2:e1492011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee HJ, Lyu da H, Koo U, Lee SJ, Hong SS,
Kim K, Kim KH, Lee D and Mar W: Inhibitory effect of
2-arylbenzofurans from the Mori Cortex Radicis (Moraceae) on oxygen
glucose deprivation (OGD)-induced cell death of SH-SY5Y cells. Arch
Pharm Res. 34:1373–1380. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou Z, Lu B, Wang C, Wang Z, Luo T, Piao
M, Meng F, Chi G, Luo Y and Ge P: RIP1 and RIP3 contribute to
shikonin-induced DNA double-strand breaks in glioma cells via
increase of intracellular reactive oxygen species. Cancer Lett.
390:77–90. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang C, He C, Lu S, Wang X, Wang L, Liang
S, Wang X, Piao M, Cui J, Chi G and Ge P: Autophagy activated by
silibinin contributes to glioma cell death via induction of
oxidative stress-mediated BNIP3-dependent nuclear translocation of
AIF. Cell Death Dis. 11:6302020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Begum H, Murugesan P and Tangutur AD:
Western blotting: A powerful staple in scientific and biomedical
research. Biotechniques. 73:58–69. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang Y, Wen Q, Huang J, Luo M, Xiao Y, Mo
R and Wang J: Manganese (II) chloride leads to dopaminergic
neurotoxicity by promoting mitophagy through BNIP3-mediated
oxidative stress in SH-SY5Y cells. Cell Mol Biol Lett. 26:232021.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang HF, Wang ZQ, Ding Y, Piao MH, Feng
CS, Chi GF, Luo YN and Ge PF: Endoplasmic reticulum stress
regulates oxygen-glucose deprivation-induced parthanatos in human
SH-SY5Y cells via improvement of intracellular ROS. CNS Neurosci
Ther. 24:29–38. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Andrabi SA, Dawson TM and Dawson VL:
Mitochondrial and nuclear cross talk in cell death: Parthanatos.
Ann N Y Acad Sci. 1147:233–241. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xia P, Zhang F, Yuan Y, Chen C, Huang Y,
Li L, Wang E, Guo Q and Ye Z: ALDH 2 conferred neuroprotection on
cerebral ischemic injury by alleviating mitochondria-related
apoptosis through JNK/caspase-3 signing pathway. Int J Biol Sci.
16:1303–1323. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ali SS, Ahsan H, Zia MK, Siddiqui T and
Khan FH: Understanding oxidants and antioxidants: Classical team
with new players. J Food Biochem. 44:e131452020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li C, Chen H, Lan Z, He S, Chen R, Wang F,
Liu Z, Li K, Cheng L, Liu Y, et al: mTOR-dependent upregulation of
xCT blocks melanin synthesis and promotes tumorigenesis. Cell Death
Differ. 26:2015–2028. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dey P, Kundu A, Sachan R, Park JH, Ahn MY,
Yoon K, Lee J, Kim ND, Kim IS, Lee BM, et al: PKM2 knockdown
induces autophagic cell death via AKT/mTOR pathway in human
prostate cancer cells. Cell Physiol Biochem. 52:1535–1552.
2019.PubMed/NCBI
|
|
30
|
Li XB, Gu JD and Zhou QH: Review of
aerobic glycolysis and its key enzymes-new targets for lung cancer
therapy. Thorac Cancer. 6:17–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Candé C, Vahsen N, Kouranti I, Schmitt E,
Daugas E, Spahr C, Luban J, Kroemer RT, Giordanetto F, Garrido C,
et al: AIF and cyclophilin A cooperate in apoptosis-associated
chromatinolysis. Oncogene. 23:1514–1521. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li J, Zhang J, Zhang Y, Wang Z, Song Y,
Wei S, He M, You S, Jia J and Cheng J: TRAF2 protects against
cerebral ischemia-induced brain injury by suppressing necroptosis.
Cell Death Dis. 10:3282019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tang MB, Li YS, Li SH, Cheng Y, Zhang S,
Luo HY, Mao CY, Hu ZW, Schisler JC, Shi CH, et al: Anisomycin
prevents OGD-induced necroptosis by regulating the E3 ligase CHIP.
Sci Rep. 8:63792018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Park YH, Broyles HV, He S, McGrady NR, Li
L and Yorio T: Involvement of AMPA receptor and its flip and flop
isoforms in retinal ganglion cell death following oxygen/glucose
deprivation. Invest Ophthalmol Vis Sci. 57:508–526. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li HQ, Xia SN, Xu SY, Liu PY, Gu Y, Bao
XY, Xu Y and Cao X: γ-Glutamylcysteine alleviates ischemic
stroke-induced neuronal apoptosis by inhibiting ROS-Mediated
endoplasmic reticulum stress. Oxid Med Cell Longev.
2021:29610792021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qu Y, Tang J, Wang H, Li S, Zhao F, Zhang
L, Richard Lu Q and Mu D: RIPK3 interactions with MLKL and CaMKII
mediate oligodendrocytes death in the developing brain. Cell Death
Dis. 8:e26292017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Y, An R, Umanah GK, Park H, Nambiar
K, Eacker SM, Kim B, Bao L, Harraz MM, Chang C, et al: A nuclease
that mediates cell death induced by DNA damage and poly(ADP-ribose)
polymerase-1. Science. 354:aad68722016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li WH, Yang YL, Cheng X, Liu M, Zhang SS,
Wang YH and Du GH: Baicalein attenuates caspase-independent cells
death via inhibiting PARP-1 activation and AIF nuclear
translocation in cerebral ischemia/reperfusion rats. Apoptosis.
25:354–369. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liang J, Yu Y, Wang B, Lu B, Zhang J,
Zhang H and Ge P: Ginsenoside Rb1 attenuates oxygen-glucose
deprivation-induced apoptosis in SH-SY5Y cells via protection of
mitochondria and inhibition of AIF and cytochrome c release.
Molecules. 18:12777–12792. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee JH and Paull TT: Cellular functions of
the protein kinase ATM and their relevance to human disease. Nat
Rev Mol Cell Biol. 22:796–814. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sánchez-Morán I, Rodríguez C, Lapresa R,
Agulla J, Sobrino T, Castillo J, Bolaños JP and Almeida A: Nuclear
WRAP53 promotes neuronal survival and functional recovery after
stroke. Sci Adv. 6:eabc57022020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cheng J, Fan YQ, Jiang HX, Chen SF, Chen
J, Liao XY, Zou YY, Lan HY, Cui Y, Chen ZB, et al: Transcranial
direct-current stimulation protects against cerebral
ischemia-reperfusion injury through regulating Cezanne-dependent
signaling. Exp Neurol. 345:1138182021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kihara S, Shiraishi T, Nakagawa S, Toda K
and Tabuchi K: Visualization of DNA double strand breaks in the
gerbil hippocampal CA1 following transient ischemia. Neurosci Lett.
175:133–136. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Floyd RA and Carney JM: Free radical
damage to protein and DNA: Mechanisms involved and relevant
observations on brain undergoing oxidative stress. Ann Neurol. 32
(Suppl 1):S22–S27. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang Y, Wang J, Xu C, Pan H and Zhang Z:
Maltol inhibits apoptosis of human neuroblastoma cells induced by
hydrogen peroxide. J Biochem Mol Biol. 39:145–149. 2006.PubMed/NCBI
|
|
46
|
Song Y, Hong S, Iizuka Y, Kim CY and Seong
GJ: The neuroprotective effect of maltol against oxidative stress
on rat retinal neuronal cells. Korean J Ophthalmol. 29:58–65. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang H, Davies KJA and Forman HJ:
Oxidative stress response and Nrf2 signaling in aging. Free Radic
Biol Med. 88:314–336. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dai Y, Zhang H, Zhang J and Yan M:
Isoquercetin attenuates oxidative stress and neuronal apoptosis
after ischemia/reperfusion injury via Nrf2-mediated inhibition of
the NOX4/ROS/NF-κB pathway. Chem Biol Interact. 284:32–40. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu A, Zhang W, Wang S, Wang Y and Hong J:
HMGB-1/RAGE signaling inhibition by dioscin attenuates hippocampal
neuron damage induced by oxygen-glucose deprivation/reperfusion.
Exp Ther Med. 20:2312020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Armogida M, Spalloni A, Amantea D, Nutini
M, Petrelli F, Longone P, Bagetta G, Nisticò R and Mercuri NB: The
protective role of catalase against cerebral ischemia in vitro and
in vivo. Int J Immunopathol Pharmacol. 24:735–747. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Rhee SG and Kil IS: Mitochondrial
H2O2 signaling is controlled by the concerted
action of peroxiredoxin III and sulfiredoxin: Linking mitochondrial
function to circadian rhythm. Free Radic Biol Med. 99:120–127.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Fu C, Wu Y, Liu S, Luo C, Lu Y, Liu M,
Wang L, Zhang Y and Liu X: Rehmannioside A improves cognitive
impairment and alleviates ferroptosis via activating PI3K/AKT/Nrf2
and SLC7A11/GPX4 signaling pathway after ischemia. J
Ethnopharmacol. 289:1150212022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wei N, Lu T, Yang L, Dong Y and Liu X:
Lipoxin A4 protects primary spinal cord neurons from
Erastin-induced ferroptosis by activating the Akt/Nrf2/HO-1
signaling pathway. FEBS Open Bio. 11:2118–2126. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Woo Y, Lee HJ, Kim J, Kang SG, Moon S, Han
JA, Jung YM and Jung YJ: Rapamycin Promotes ROS-Mediated cell death
via functional inhibition of xCT Expression in Melanoma Under
γ-Irradiation. Front Oncol. 11:6654202021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Horikawa I, Fujita K, Jenkins LM, Hiyoshi
Y, Mondal AM, Vojtesek B, Lane DP, Appella E and Harris CC:
Autophagic degradation of the inhibitory p53 isoform Δ133p53α as a
regulatory mechanism for p53-mediated senescence. Nat Commun.
5:47062014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Mi XJ, Hou JG, Wang Z, Han Y, Ren S, Hu
JN, Chen C and Li W: The protective effects of maltol on
cisplatin-induced nephrotoxicity through the AMPK-mediated PI3K/Akt
and p53 signaling pathways. Sci Rep. 8:159222018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wang X, Perez E, Liu R, Yan LJ, Mallet RT
and Yang SH: Pyruvate protects mitochondria from oxidative stress
in human neuroblastoma SK-N-SH cells. Brain Res. 1132:1–9. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zeng J, Yang GY, Ying W, Kelly M, Hirai K,
James TL, Swanson RA and Litt L: Pyruvate improves recovery after
PARP-1-associated energy failure induced by oxidative stress in
neonatal rat cerebrocortical slices. J Cereb Blood Flow Metab.
27:304–315. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wu H, Zhao H and Chen L: Deoxyshikonin
inhibits viability and glycolysis by suppressing the Akt/mTOR
pathway in acute myeloid leukemia cells. Front Oncol. 10:12532020.
View Article : Google Scholar : PubMed/NCBI
|