Introduction
In vitro embryo production in pigs is a
crucial tool for the development of porcine models suitable for
human biomedical research due to their anatomical and physiological
similarities to human organs (1).
As a result, xenotransplantation using pig tissues and organs is
feasible (2,3). Assisted reproductive technology has
improved animal reproduction efficiency. However, polyspermy is a
major limitation of porcine in vitro fertilization (IVF),
which leads to chromosomal abnormalities in embryos (4,5).
Hence, reducing the polyspermy rate and enhancing monospermic
embryos are crucial for successful porcine IVF.
Standard IVF systems utilize hundreds of
spermatozoa, often resulting in a rise in the number of spermatozoa
that penetrate the oocyte (6).
Polyspermy is strongly linked to the initial number of capacitated
spermatozoa during the IVF process (7). One strategy to decrease the incidence
of polyspermy is to reduce the absolute number of spermatozoa, but
this results in a low oocyte penetration rate. Consequently,
certain IVF conditions which mimic the oviductal environment have
been examined to minimize polyspermy in vitro, including
shortening the co-incubation time and manipulating the IVF
equipment or culture conditions (8). These studies have effectively reduced
the incidence of polyspermy. However, the available IVF systems do
not entirely mimic in vivo conditions (9), and the percentage of monospermic
oocytes relative to the total number inseminated during the IVF
remains between 20–30% (6–8).
In mammals, the oviduct is composed of four parts;
the infundibulum, responsible for collecting ovulated oocytes; the
ampulla, where fertilization occurs; the isthmus, for
preimplantation embryo development; and the uterotubal junction,
which transits the embryos to the uterus (10). Oviductal fluid (OF) and
extracellular vesicles (EVs) secreted by the oviduct epithelial
cells (OEC; OEC-EVs) have been shown to enhance fertilization,
protect against polyspermy and sperm entry, and aid embryonic
development (11,12). EVs are nano-sized membranous
vesicles containing molecular cargo, including micro RNAs (miRNAs),
mRNAs, proteins, lipids, and metabolites, that can be easily
delivered and fused with cell plasma membranes (13,14).
They serve a crucial role in transmitting information and
organelles between living cells (15,16)
and can modify the epigenetic signature by transferring small
molecule RNAs between donor cells (17,18).
EVs are present in the female genital tract fluids and are crucial
for gamete fertilization and early preimplantation embryonic
development (19–23). Furthermore, OEC-EVs reduce
apoptosis and improve embryo quality by reducing reactive oxygen
species (ROS) (24). The first
moments of embryonic-maternal communication occur in the oviduct,
where both maternal and embryonic EVs are exchanged to prepare for
the maternal recognition of pregnancy (25).
Recently, the beneficial effects of OEC-EVs on
porcine embryos generated through parthenogenesis and cloning
technology (24) and the improved
derivation of trophoblast stem cells were reported (26). Thus, the aim of the present study
was to mimic the in vivo conditions and reduce polyspermy by
investigating the effects of porcine OEC-EVs on the interaction
between sperm and oocyte during IVF and subsequent in vitro
embryonic development. The potential effects of OEC-EVs on the
developmental competence of fertilized oocytes was explored by
examining the uptake of EVs by both sperm and oocytes, and the
effects on oocyte mitochondrial activity and cortical granule's
reaction.
Materials and methods
Chemicals
All chemicals and reagents used in this work were
purchased from MilliporeSigma unless otherwise specified.
Oocyte collection and oocyte in vitro
maturation (IVM)
Oocyte IVM was performed according to a previously
reported method (27). Briefly,
porcine ovaries and uteri including the oviducts were obtained from
a slaughterhouse in Daejeon City and transported to the laboratory
in normal saline solution containing 75 mg/ml penicillin and 50
mg/ml streptomycin. The temperature of the solution was maintained
between 34–36°C. Cumulus-oocyte complexes (COCs) were taken from
antral follicles (3–8 mm in diameter) with an 18-gauge needle
attached to a 10 ml syringe. COCs with three or more layers of
cumulus cells and homogeneous ooplasm were selected and washed
three times in HEPES-buffered Tyrode's media containing 0.05% (w/v)
polyvinyl alcohol (PVA). COCs (n=50) were in vitro matured
in 500 µl of maturation medium consisting of bicarbonate-buffered
tissue culture medium 199 (TCM-199; Gibco, Thermo Fisher
Scientific, Inc.), 10% (v/v) porcine follicular fluid, 0.91 mM
sodium pyruvate, 0.57 mM L-cysteine, 10 ng/ml epidermal growth
factor and 1 µg/ml insulin with 10 IU/ml equine chorionic
gonadotropin (eCG), 10 IU/ml human chorionic gonadotropin (hCG) and
75 µg/ml kanamycin in 4-well dishes (SPL Life Sciences). COCs were
incubated at 38.5°C in 5% CO2 in humidified air
incubator (Astec Co., Ltd.) for 22 h and then were cultured for an
additional 22 h in a hormone-free IVM medium (24).
Porcine oviductal epithelial cell
(pOEC) collection
The post-ovulatory oviducts of multiparous female
pigs (sows) were obtained from a local butcher and transported to
the laboratory according to the aforementioned method. pOECs were
isolated by mechanically scraping the oviduct with a slide and then
centrifuged three times at 700 × g for 5 min at room temperature in
10 ml of DMEM supplemented with 10% (v/v) FBS (Gibco; Thermo Fisher
Scientific, Inc.) and 1% (v/v) antibiotic-antimycotic solution
(Gibco; Thermo Fisher Scientific, Inc.). pOECs were cultivated in a
100 mm Falcon tissue culture plate with a culture medium at 39°C
and 5% CO2 in a humidified environment. On day 3 of the
initial culture, the pOEC outgrowths were examined, after which
they were grown for a further 7 days. pOECs were trypsinized in
0.05% trypsin-EDTA, washed in phosphate-buffered saline (PBS)
thrice and grown in DMEM without FBS for 48 h to obtain the
conditioned medium for isolating OEC-E (24).
EV isolation, purification, and
characterization
The supernatant was used to separate EVs using the
Total EV Isolation kit after being centrifuged at 2,000 × g for 30
min at room temperature to remove cells and cell debris from the
conditioned medium (Invitrogen; Thermo Fisher Scientific, Inc.).
The kit reagent was combined with the supernatant, stirred
vigorously, and incubated overnight at 2–8°C. The EV pellets were
then frozen at −80°C until future investigations. The EV pellet was
resuspended in modified Tris-buffered medium (mTBM) for
experimental purposes, and the final protein concentration was
adjusted to 50 ng/ml using a NanoDrop 2000 (Thermo Scientific;
Thermo Fisher Scientific, Inc.). The EVs were visualized using
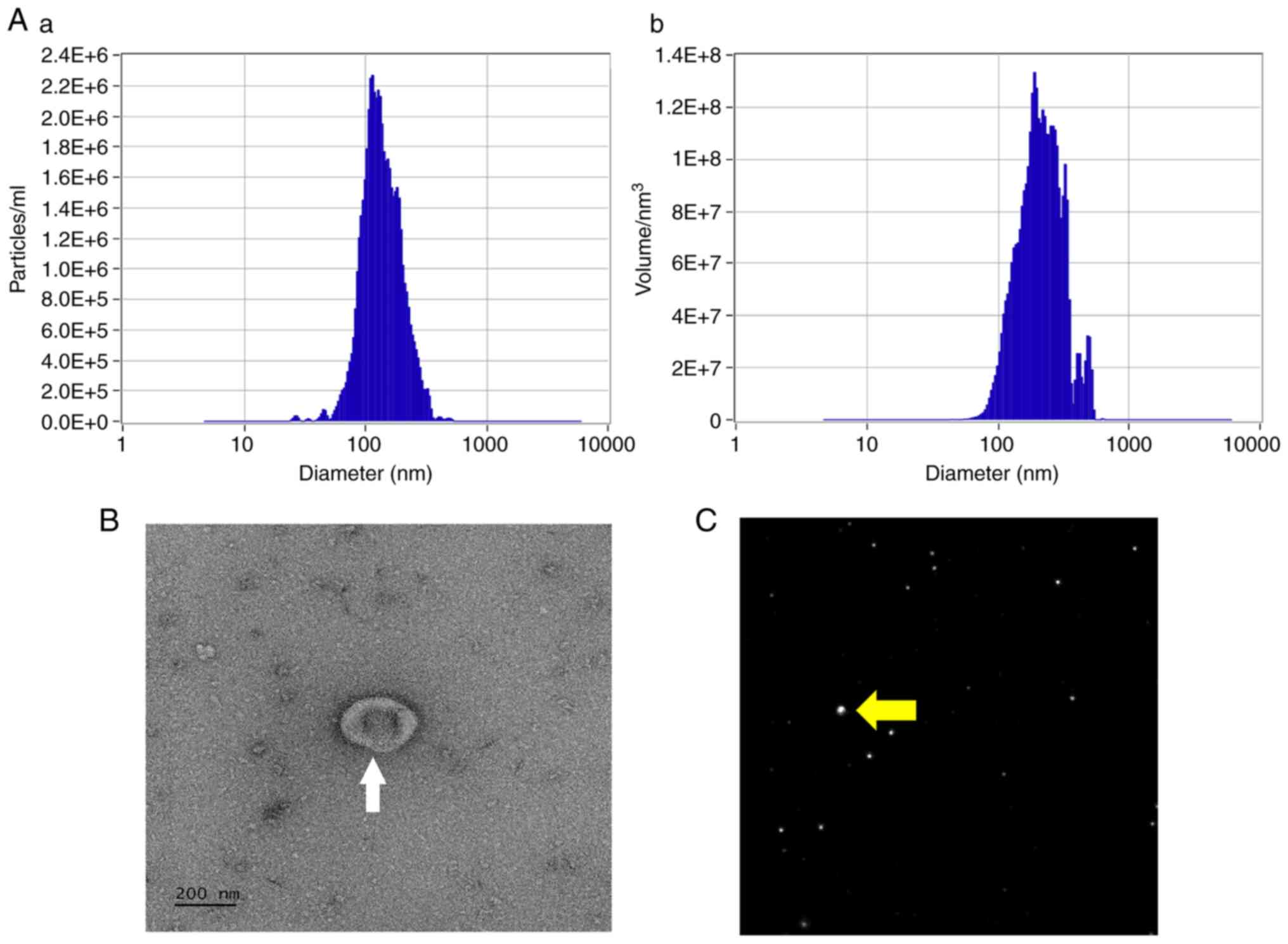
transmission electron microscopy (TEM) after negative staining with
2% uranyl acetate as described previously (24).
ZetaView nanoparticle tracking
analysis (NTA)
A ZetaView PMX 110 (Particle Metrix GmbH) instrument
was used for NTA as previously described (28). Briefly, 1 ml of diluted EVs pellet
(in 1X PBS) was loaded into the device to measure each sample at 11
different positions and two reading cycles per position. After an
automated analysis and outlier removal, the mean, median, and mode
sizes (indicated as diameter) and the concentrations were
calculated using ZetaView SP7 software (version 8.05.14; Particle
Metrix) and Microsoft Excel 365 (version 2205, Microsoft
Corporation). Device calibration was performed using 100 nm
polystyrene particles (Thermo Fisher Scientific, Inc.).
IVF and experimental groups
In vitro matured oocytes with the extruded
first polar body were washed twice with mTBM, before being cultured
in fresh droplets of mTBM (15 oocytes/50 µl) at 39°C in a
humidified atmosphere of 5% CO2. In the OEC EVs group,
mTBM contained EVs protein of 50 ng/ml (EVs-mTBM), while the
control group was EVs-free. Chilled pig semen was commercially
obtained from Darby Genetics Inc., South Korea. Oocytes were
co-incubated with 5.0×105 sperm/ml for 20 min. After
co-incubation, the attached sperm were discarded from the zona
pellucida (ZP) by gentle micro pipetting and the oocytes were
washed twice in mTBM. Oocytes were then incubated in mTBM or
EVs-mTBM without sperm for an additional 6 h in the same culture
conditions. IVF conditions with either OEC-EVs-supplemented or
plain culture mediums were compared for polyspermy, cortical
granules' reaction, and mitochondrial staining. In vitro
embryonic development was monitored after culturing the IVF oocytes
in PZM-5 medium (Functional Peptides Research Institute Co. Ltd.)
for 7 days at 38.5°C in 5% CO2 and 5% O2 in a
humidified incubator.
Penetration and polyspermy
After 18–20 h of IVF, the embryos were fixed using
3.7% (w/v) paraformaldehyde (PFA) for 30 min and washed in PBS/PVA
three times at room temperature. The embryos were stained with 10
µg/ml Hoechst 33342 in PBS/PVA for 30 min at room temperature.
After staining, the embryos were washed three times in PBS/PVA. The
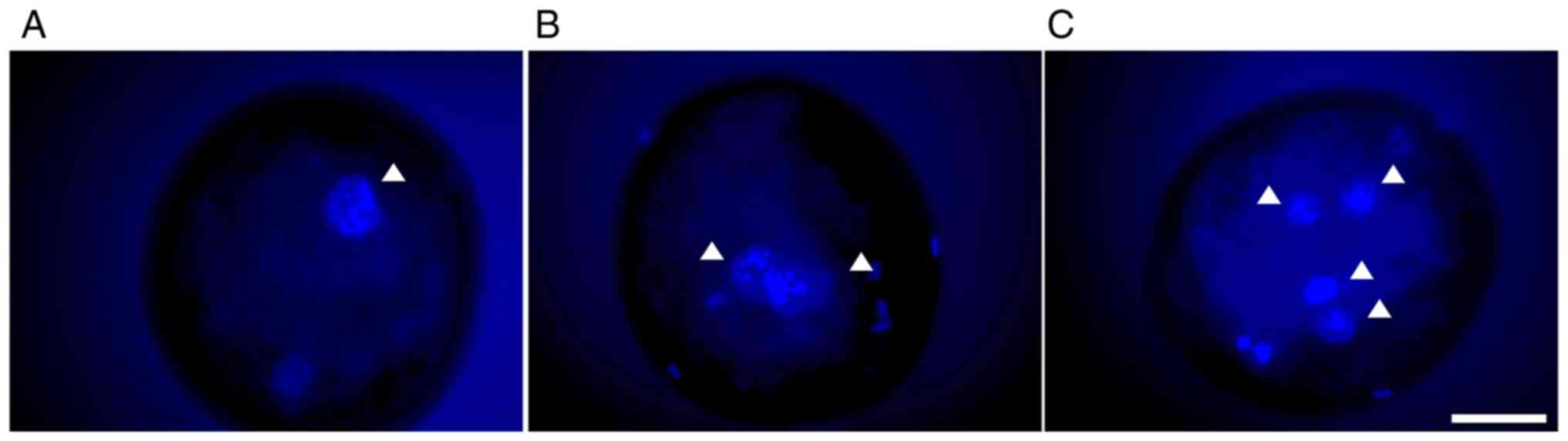
embryos were examined for penetration and pronucleus (PN) formation
using a fluorescence microscope. Embryos with one nucleus and polar
body (Fig. 1A) were considered
non-penetrated. Embryos with one female PN and one male PN in the
cytoplasm were considered to be monospermic (Fig. 1B). Embryos with more than one
nuclear staining (i.e., sperm or male PNs) were considered to be
polyspermic (Fig. 1C).
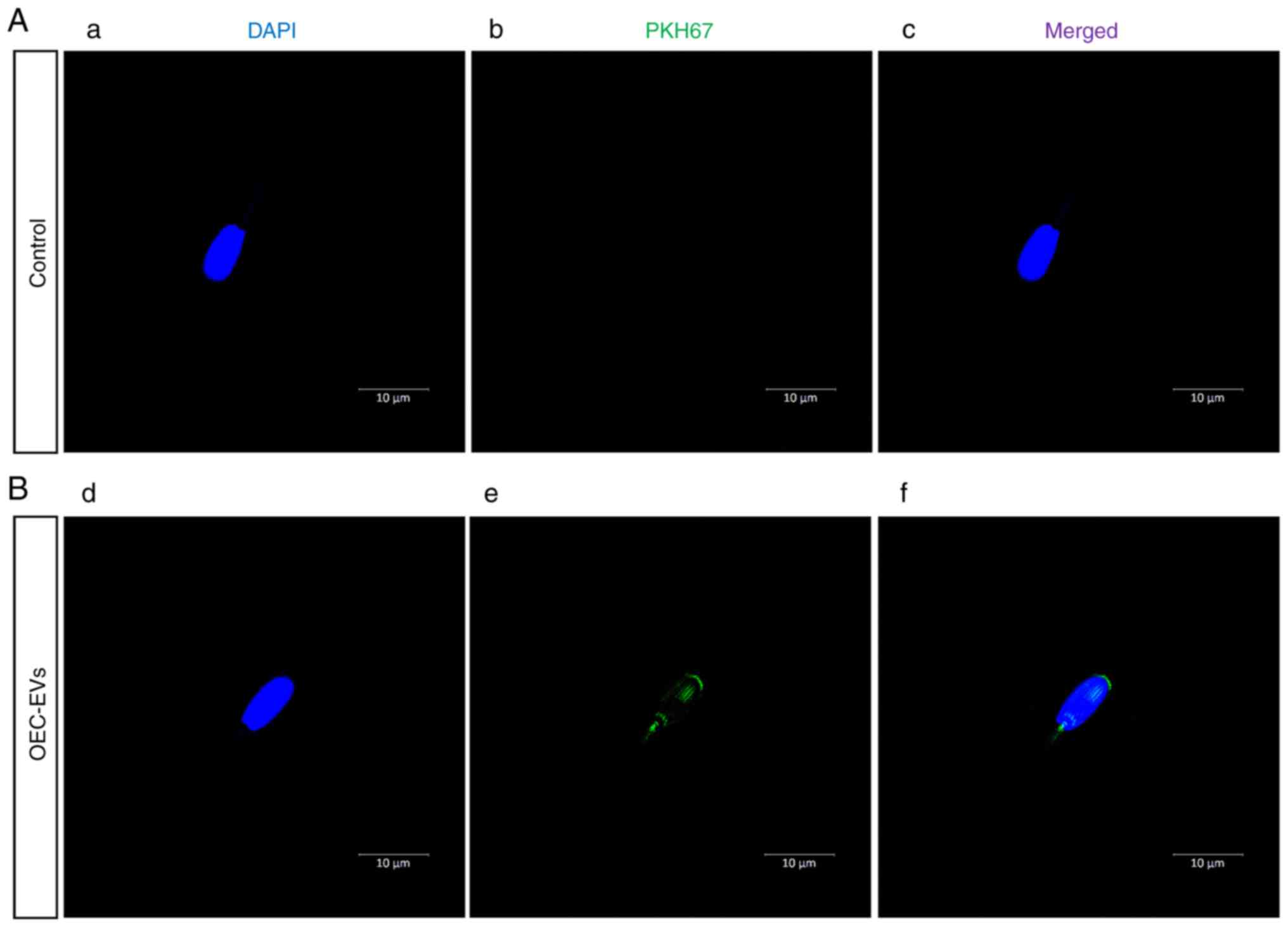
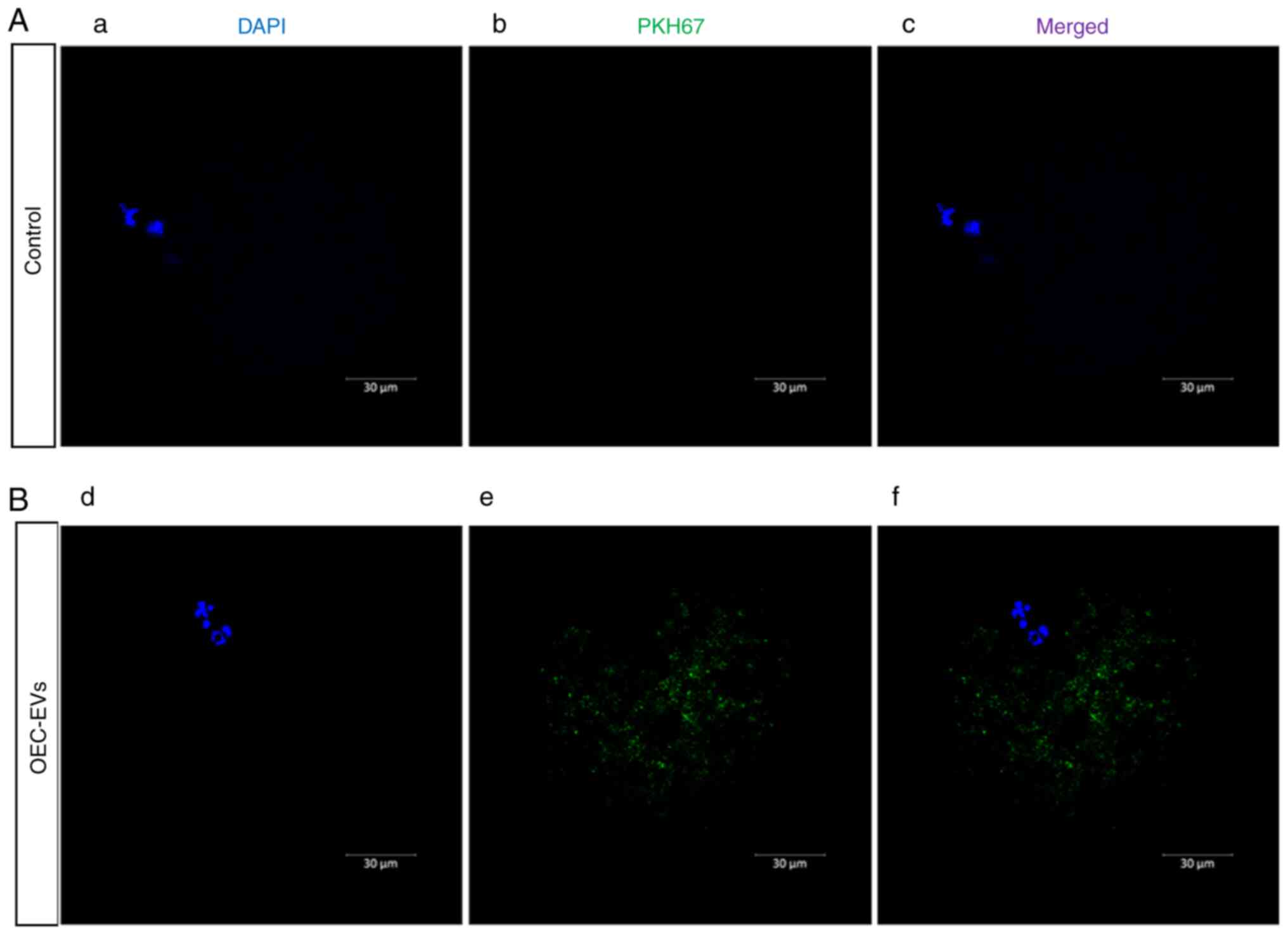
EV labeling and uptake
After OEC-EV isolation, the OEC EVs were mixed with
the lipophilic PKH67 dye according to the manufacturer's
instructions, and OEC-EVs were isolated to remove the excess free
PKH67 dye according to the manufacturer's protocol (29,30).
EVs were then supplemented with sperm or oocytes for 6 h to monitor
their uptake using a confocal microscope. For negative control
staining, the plain conditioned medium was mixed with PKH67 and
processed through the same EV labeling procedure.
Cortical granule (CG) staining
Three times, in vitro matured oocytes, were
washed with PBS containing 0.1% (PBS/PVA). The OEC EVs group
oocytes were treated for 20 min with OEC EVs in mTBM and the
control group was kept in mTBM without EVs. The oocytes were then
fixed using 3.7% (w/v) PFA at room temperature for 30 min, followed
by three 10 min washes in PBS/PVA per oocyte. This was followed by
a 1% Triton X-100 treatment for 30 min and three 10-min washes in
PBS/PVA. Oocytes were then cultured for 30 min at room temperature
in FITC-labeled Peanut Agglutinin (PNA) diluted 1:500 in PBS in a
dark box. Following staining, the oocytes were washed for 10 min in
PBS/PVA three times. Finally, the nuclei were stained with DAPI for
10 min, mounted on slides, and images were acquired using a DMi8
confocal microscope (Leica Microsystems GmbH).
Active mitochondria staining
After 18–20 h, the IVF embryos were washed with
PBS/PVA three times and were then cultivated for 30 min at 37°C in
500 nM MitoTracker Red CMXRos (cat. no. M7512; Invitrogen; Thermo
Fisher Scientific, Inc.) in a dark box. After staining, the embryo
was washed three times for 10 min in PBS/PVA. Each embryo was fixed
in 3.7% PFA at room temperature for 30 min, followed by three 10
min washes in PBS/PVA. Finally, the nuclei were stained using DAPI
for 10 min at room temperature, mounted on slides, and images were
acquired using a DMi8 confocal microscope (Leica Microsystems
GmbH).
Statistical analysis
Lieven's test and Kolmogorov-Smirnov test were used
to confirm the homogeneity of variance and the normality of
distribution, respectively. At least 10–20 replicates were
performed per experiment. For data normalization, the mean
expression level value was calculated from the control group, and
the means were normalized to an arbitrary unit (1-fold) by dividing
the values against the control group. The image intensities were
analyzed using ImageJ (version 1.53k; National Institutes of
Health). Data were analyzed using unpaired Student's t-test using
SPSS software (SPSS 22.0; IBM Corp). P<0.05 was considered to
indicate a statistically significant difference.
Results
Porcine OEC culture and EV
characterization
OEC confluent cells were achieved as reported
previously (24) and the
expression of oviduct epithelial cells' specific gene expression
[oviduct-specific glycoprotein (OVGP1)] and proteomics were
performed for further confirmation. OEC-EVs were isolated,
visualized, and characterized using TEM and NTA, the mean diameter
of the isolated EVs was 146.2±57.2 nm, and the mean concentration
was 5.3×109 particles/ml (Fig. 1A-C).
Effects of OEC-EVs on sperm
penetration and polyspermy
Sperm penetration into the oocyte was detected 18–20
h after IVF. Overall, non-penetration and non-fertilized oocytes
(Fig. 2A), penetrated and
monospermic fertilized embryos (Fig.
2B), and penetrated and polyspermic abnormally fertilized
embryos (Fig. 2C) were observed.
The penetration rate did not differ between the OEC-EV and control
groups (74.4±1.5 vs. 74.2±2.3, P=0.27). However, the polyspermy
rate was significantly lower in the OEC-EV group when compared with
the control group (32.9±2.5 vs. 43.8±3.1; P=0.0026; Table I).
 | Table I.Effect of OEC-EVs during penetration
and polyspermy after in vitro fertilization. |
Table I.
Effect of OEC-EVs during penetration
and polyspermy after in vitro fertilization.
| Group | Total number | Penetration (% ±
SD) | Polyspermy (% ±
SD) |
|---|
| Control | 203 | 151 (74.4±4.2) | 90 (43.8±8.7) |
| OEC-EVs | 209 | 155 (74.2±6.6) | 67
(32.9±7.0)a |
Effects of OEC-EVs on IVF embryo
development
The cleavage rate differed significantly between the
OEC-EV and the control groups (67.6±2.5 vs. 57.3±1.9; P=0.0028).
Furthermore, the OEC-EV group had significantly more embryos than
the control group (16.4±1.2 vs. 10.2±0.8; P=0.0001; Table II).
 | Table II.Effect of OEC-EVs on the development
of the in vitro fertilized embryo. |
Table II.
Effect of OEC-EVs on the development
of the in vitro fertilized embryo.
| Group | Total number | Cleaved (% ±
SD) | Developed to Bl (%
± SD) |
|---|
| Control | 293 | 168 (57.3±6.2) | 30 (10.2±2.8) |
| OEC-EVs | 318 | 215
(67.6±8.4)a | 52
(16.4±4.0)a |
Effects of OEC-EVs on sperm and oocyte
communication
In the OEC-EV group, it was observed that OEC-EVs
attached to the heads and tails of sperm (Fig. 3) and OEC-EVs that entered the
oocyte through the cell membrane (Fig.
4), which was not observed in the control group.
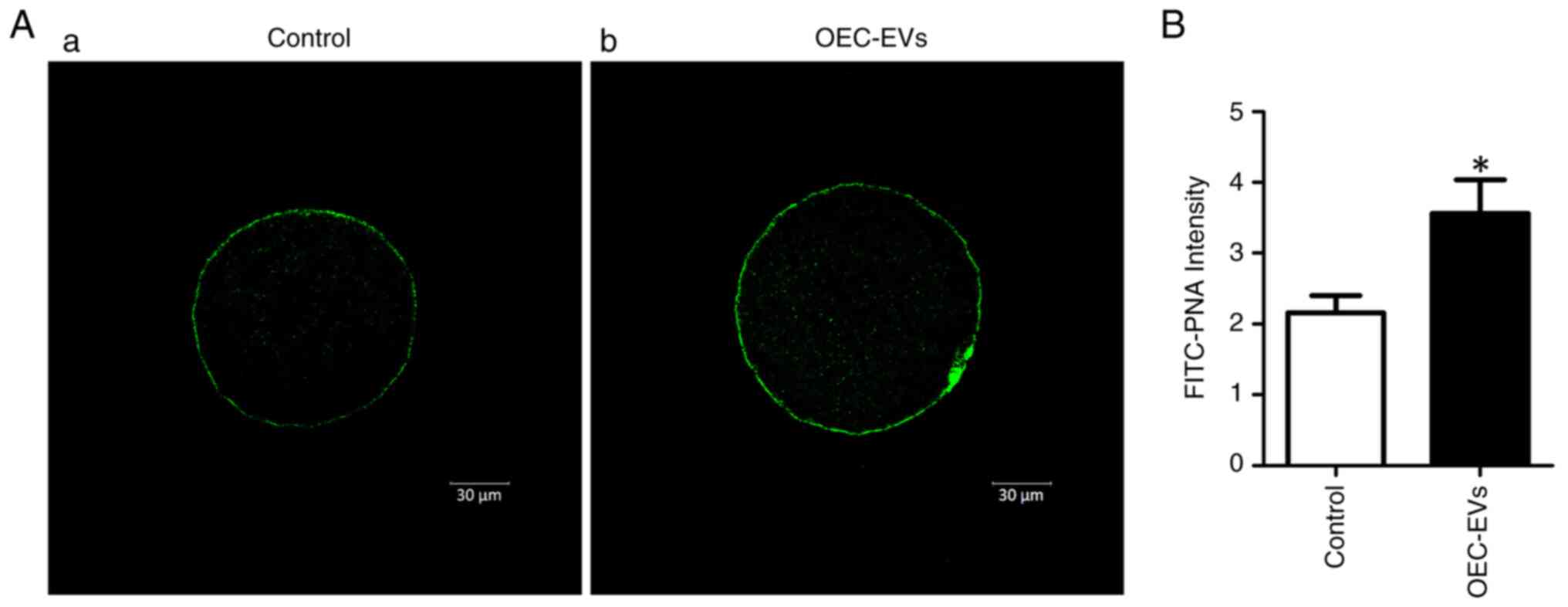
Effects of OEC-EVs on CGs
The CG distribution in the control and OEC-EV groups
after FITC-PNA staining were presented (Fig. 5A). The OEC-EV group had
significantly higher fluorescence intensity values than those in
the control group (3.56±0.47 vs. 2.15±0.24; P<0.05; Fig. 5B).
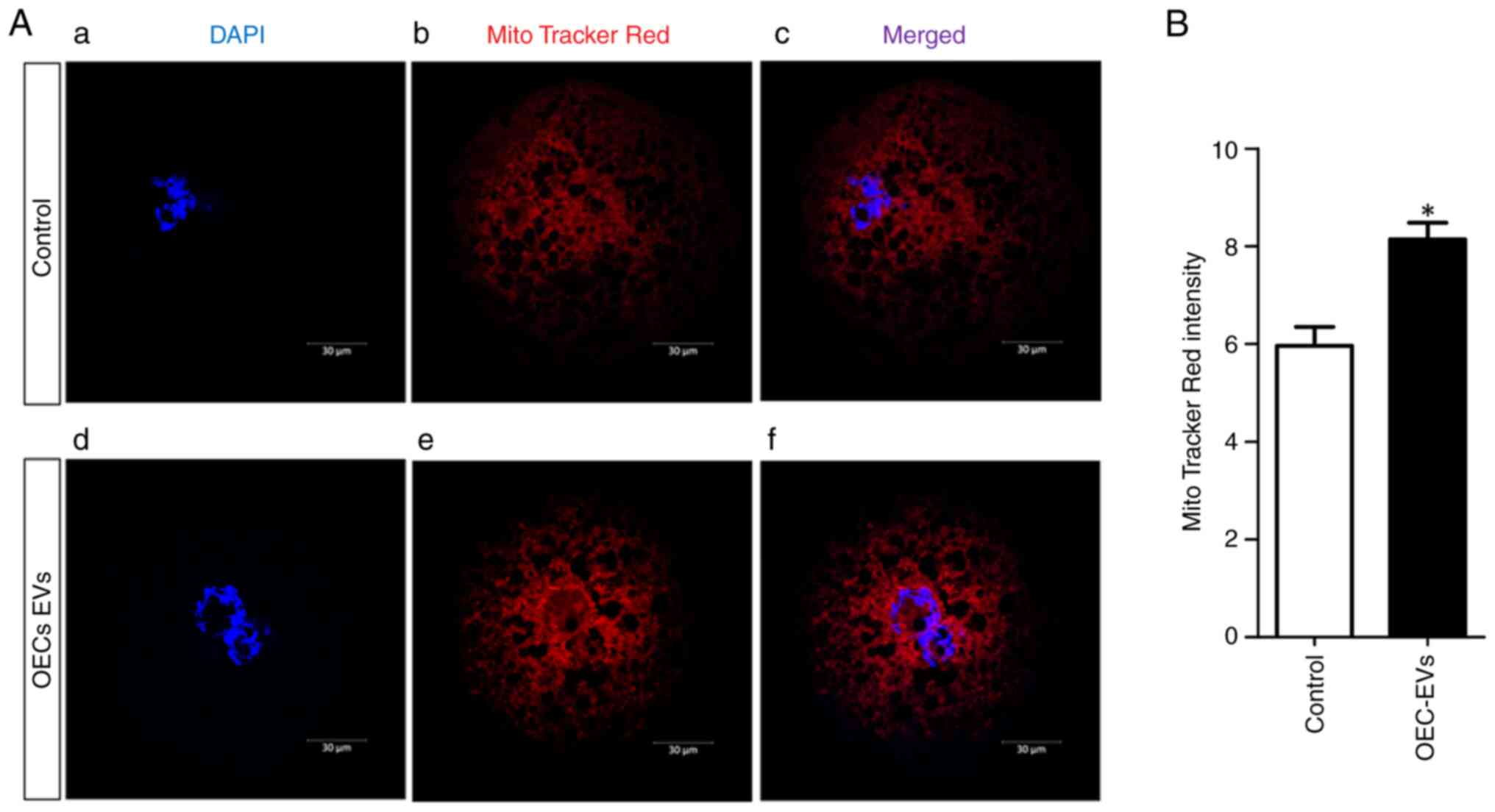
Effects of OEC-EVs on mitochondrial
activity
Mitochondrial activity was detected in both control
and OEC-EV-supplemented groups using MitoTracker Red staining
(Fig. 6A). The fluorescence values
of active mitochondria were higher in the OEC-EV group compared
with those in the control group (8.14±0.34 vs. 5.96±0.38;
P=0.00007; Fig. 6B).
Discussion
The results of the present study indicated that
OEC-EVs support successful porcine IVF by enhancing oocyte
mitochondrial activity, reducing polyspermy, and supporting the CG
reaction. Polyspermic oocyte penetration is a common reason for
decreased IVF success and low blastocyst rates in porcine species
(4,9,31).
Additionally, previous studies have reported that oxidative stress
can alter CG distribution (32–34).
First, the effects of EVs on polyspermy were
investigated. Amongst other things, oocytes prevent polyspermy by
producing CGs that contain enzymes to harden the ZP and prevent
subsequent spermatozoa fusion. Recently, EVs have emerged as an
important component of several biological fluids, serving a crucial
role in communication among different reproductive cells (35). The present study demonstrated that
OEC-EVs significantly increased CG content (Fig. 5), which supported previous findings
(12,36–38).
Coy et al (38) and Batista
et al (37) reported that
incubation of oviductal fluid with porcine IVM oocytes caused ZP
hardening, increased ZP resistance to proteolytic digestion, and
reduced sperm binding to ZP, subsequently decreasing the polyspermy
incidence. The protein cargo contents of OEC-EVs, specifically
OVGP1 (22), could be related to
increasing monospermy by masking the protease-cleaving sites of ZP
proteins and causing ZP hardening (39–41).
Furthermore, the molecular mechanisms regarding CG formation and
release are not well-known. Nonetheless, six genes have been
associated with CG distribution after IVM: Rho/Rac guanine
nucleotide exchange factor 2, microtubule-associated protein 1B,
C-X-C motif chemokine ligand 12, fibronectin 1, DAB adaptor protein
2, and SRY-box transcription factor 9. However, the expression of
these genes decreased after IVM (42). The main component of CG is
metalloproteinase ovastacin (43),
and it is hypothesized that the OEC-EV contents indirectly enhance
ovastacin mRNA expression. Importantly, evidence suggests that EVs
penetrate the ZP and release their cargo contents inside the
ooplasm (36,44), similar to what was observed in the
present study (Fig. 4).
The effects of OEC-EVs on oocyte mitochondrial
activity were investigated to identify possible mechanisms related
to EVs and to improve porcine IVF success. The results of the
present study demonstrated that EV treatment increased
mitochondrial activity. A previous study suggested that
fertilization levels are related to oocyte quality, particularly
mitochondrial activity (45). The
number and distribution of active mitochondria in an oocyte serve a
crucial role in regulating the fertilization of sperm by providing
ATP and Ca2+ ion responses (46). Abnormal changes in mitochondria,
lipid droplets, calcium release after electro-activation, and the
adenosine triphosphate (ATP) and glutathione content in oocytes
during aging may result in poor developmental competence of
parthenotes (47). OEC-EVs
enhanced mitochondrial activity (Fig.
6), providing a better ATP environment for nuclear
reprogramming and good conditions for chromosome expansion into
chromatin (48,49). Therefore, EVs may initiate several
activities in the cytoplasm, which allow the egg to acquire energy
and reach a mature state. In vivo and in vitro,
oviduct EVs have large amounts of protein related to ATP, adenyl
ribonucleotide, purine nucleotide binding, glucose and hexose
catabolic processes, and glycolysis (22). Thus, it was hypothesized that
OEC-EVs increase mitochondrial activity (16,50)
and help provide the large quantity of ATP required for successful
fertilization.
Furthermore, the results of the present study
indicated that OEC-EVs mediate sperm-oocyte interaction within an
in vivo environment. Fallopian tube-secreted EVs contribute
to improving the developmental competence and the fertilization of
oocytes by reducing the ROS levels, eliciting anti-apoptotic
effects, improving lipid metabolism, and increasing the expression
of reprogramming-related genes (11,24).
Many studies have presented isolation, characterization, and
microRNA analyses of oviduct EVs in various species (12,25,35,36,51).
Oviduct EV characterization identified proteins with critical roles
in the gamete/embryo-oviduct interactions, such as OVGP1, heat
shock protein (HSP) 90, HSPA8, HSP70, gelsolin and ezrin (22). In addition, spermatozoa undergo
several modifications in the female reproductive tract for the
capacitation process to acquire a complete fertilizing ability
(52,53). Porcine oviduct EVs attach to sperm
membranes (54) and participate in
the maintenance of sperm viability and reducing motility, functions
associated with the oviduct sperm reservoir (36,55).
Oviduct EVs also regulate plasma membrane Ca2+-ATPase 1
to promote sperm motility (7).
Moreover, proteins and miRNAs in OEC-EVs may enhance cross-talk in
oocyte fertilization (53,56). Ferraz et al (57) reported that oviductal EV
supplementation to fresh epididymal spermatozoa enhanced sperm
motility and functions. Furthermore, Al-Dossary et al
(58) demonstrated that oviductal
EVs primarily enhance sperm motility, viability, capacitation, and
acrosome reactions during oviduct transit. Therefore, it is
hypothesized that OEC-EVs affect sperm penetration and capacitation
via the molecular cargo contents, such as OVGP1, myosin heavy chain
9, valosin-containing protein, and annexin A5, or at least in part.
Previous studies have observed interactions with these proteins,
which are responsible for regulating sperm functions, membrane
scaffold/trafficking processes, fertilization, and acrosome
reaction (12,22,38,40).
In conclusion, it can be hypothesized that the
primary reason for the lower quality of IVF embryos compared to
in vivo embryos is due to differences between synthetic
medium and pure natural biological secretions. Furthermore, it is
suspected that bioactive EVs are a crucial missing link in
artificial synthesis. Therefore, it is important to maintain a
state as close to that found in vivo as possible to increase
the success rate of embryos in vitro. Results of the present
study support these hypotheses, as they demonstrated that the
addition of OEC-EVs improved porcine IVF by enhancing CG
distribution, increasing mitochondrial activity and reducing
polyspermy.
Acknowledgements
The authors would like to thank Mr Steve Park at
With Instrument Company (Seoul, Republic of Korea) for performing
the ZetaView NTA analysis.
Funding
This work was supported by the Ministry of Science, Information,
and Communication Technology (MSIT) through the National Research
Foundation of Korea (NRF) (grant nos. 2021R1A2C2009294,
2022R1I1A1A01065412) and the Brain Pool program (grant no.
2021H1D3A2A02040098).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XF conceived the study. XF and JC designed the
study. XF, BMT, SB, CS, GS, DZ, IMS, SL, XSC, and JC performed the
experiments. XF and IMS performed data analysis. XF, IMS, SL, and
JC wrote the manuscript. XF and JC confirm the authenticity of all
the raw data. All authors read and approved the final version of
the manuscript.
Ethical approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brevini TAL, Antonini S, Cillo F, Crestan
M and Gandolfi F: Porcine embryonic stem cells: Facts, challenges
and hopes. Theriogenology. 68 (Suppl 1):S206–S213. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Martinez CA, Cambra JM, Maside C, Cuello
C, Roca J, Martinez EA, Parrilla I and Gil MA: High pre-freezing
sperm dilution improves monospermy without affecting the
penetration rate in porcine IVF. Theriogenology. 131:162–168. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tanihara F, Hirata M, Nguyen NT, Le QA,
Hirano T and Otoi T: Effects of concentration of CRISPR/Cas9
components on genetic mosaicism in cytoplasmic microinjected
porcine embryos. J Reprod Dev. 65:209–214. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gil MA, Cuello C, Parrilla I, Vazquez JM,
Roca J and Martinez EA: Advances in swine in vitro embryo
production technologies. Reprod Domest Anim. 45 (Suppl 2):S40–S48.
2010. View Article : Google Scholar
|
|
5
|
Grupen CG: The evolution of porcine embryo
in vitro production. Theriogenology. 81:24–37. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nguyen HT, Dang-Nguyen TQ, Somfai T, Men
NT, Viet Linh N, Xuan Nguyen B, Noguchi J, Kaneko H and Kikuchi K:
Selection based on morphological features of porcine embryos
produced by in vitro fertilization: Timing of early cleavages and
the effect of polyspermy. Anim Sci J. 91:e134012020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nguyen HT, Dang-Nguyen TQ, Somfai T, Men
NT, Beck-Woerner B, Viet Linh N, Xuan Nguyen B, Noguchi J, Kaneko H
and Kikuchi K: Excess polyspermy reduces the ability of porcine
oocytes to promote male pronuclear formation after in vitro
fertilization. Anim Sci J. 92:e136502021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Romar R, Cánovas S, Matás C, Gadea J and
Coy P: Pig in vitro fertilization: Where are we and where do we go?
Theriogenology. 137:113–121. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Romero-Aguirregomezcorta J, Soriano-Úbeda
C and Matás C: Involvement of nitric oxide during in vitro oocyte
maturation, sperm capacitation and in vitro fertilization in pig.
Res Vet Sci. 134:150–158. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li S and Winuthayanon W: Oviduct: Roles in
fertilization and early embryo development. J Endocrinol.
232:R1–R26. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Harris EA, Stephens KK and Winuthayanon W:
Extracellular vesicles and the oviduct function. Int J Mol Sci.
21:82802020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alcântara-Neto AS, Fernandez-Rufete M,
Corbin E, Tsikis G, Uzbekov R, Garanina AS, Coy P, Almiñana C and
Mermillod P: Oviduct fluid extracellular vesicles regulate
polyspermy during porcine in vitro fertilisation. Reprod Fertil
Dev. 32:409–418. 2020. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim KM, Abdelmohsen K, Mustapic M,
Kapogiannis D and Gorospe M: RNA in extracellular vesicles. Wiley
Interdiscip Rev RNA. 8:10.1002/wrna.1413. 2017. View Article : Google Scholar
|
|
14
|
Jeppesen DK, Fenix AM, Franklin JL,
Higginbotham JN, Zhang Q, Zimmerman LJ, Liebler DC, Ping J, Liu Q,
Evans R, et al: Reassessment of exosome composition. Cell.
177:428–445.e18. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mathieu M, Martin-Jaular L, Lavieu G and
Théry C: Specificities of secretion and uptake of exosomes and
other extracellular vesicles for cell-to-cell communication. Nat
Cell Biol. 21:9–17. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
D'Souza A, Burch A, Dave KM, Sreeram A,
Reynolds MJ, Dobbins DX, Kamte YS, Zhao W, Sabatelle C, Joy GM, et
al: Microvesicles transfer mitochondria and increase mitochondrial
function in brain endothelial cells. J Control Release.
338:505–526. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Willis GR, Reis M, Gheinani AH,
Fernandez-Gonzalez A, Taglauer ES, Yeung V, Liu X, Ericsson M, Haas
E, Mitsialis SA and Kourembanas S: Extracellular vesicles protect
the neonatal lung from hyperoxic injury through the epigenetic and
transcriptomic reprogramming of myeloid cells. Am J Respir Crit
Care Med. 204:1418–1432. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mannavola F, D'Oronzo S, Cives M, Stucci
LS, Ranieri G, Silvestris F and Tucci M: Extracellular vesicles and
epigenetic modifications are hallmarks of melanoma progression. Int
J Mol Sci. 21:522019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bridi A, Perecin F and Silveira JCD:
Extracellular vesicles mediated early embryo-maternal interactions.
Int J Mol Sci. 21:11632020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Almiñana C and Bauersachs S: Extracellular
vesicles in the oviduct: Progress, challenges and implications for
the reproductive success. Bioengineering (Basel). 6:322019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Almiñana C and Bauersachs S: Extracellular
vesicles: Multi-signal messengers in the gametes/embryo-oviduct
cross-talk. Theriogenology. 150:59–69. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Almiñana C, Corbin E, Tsikis G,
Alcântara-Neto AS, Labas V, Reynaud K, Galio L, Uzbekov R, Garanina
AS, Druart X and Mermillod P: Oviduct extracellular vesicles
protein content and their role during oviduct-embryo cross-talk.
Reproduction. 154:153–168. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Saadeldin IM, Oh HJ and Lee BC:
Embryonic-maternal cross-talk via exosomes: Potential implications.
Stem Cells Cloning. 8:103–107. 2015.PubMed/NCBI
|
|
24
|
Fang X, Tanga BM, Bang S, Seong G,
Saadeldin IM, Lee S and Cho J: Oviduct epithelial cells-derived
extracellular vesicles improve preimplantation developmental
competence of in vitro produced porcine parthenogenetic and cloned
embryos. Mol Reprod Dev. 89:54–65. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mazzarella R, Bastos NM, Bridi A, Del
Collado M, Andrade GM, Pinzon J, Prado CM, Silva LA, Meirelles FV,
Pugliesi G, et al: Changes in oviductal cells and small
extracellular vesicles mirnas in pregnant cows. Front Vet Sci.
8:6397522021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fang X, Tanga BM, Bang S, Seo C, Kim H,
Saadeldin IM, Lee S and Cho J: Oviduct epithelial cell-derived
extracellular vesicles improve porcine trophoblast outgrowth. Vet
Sci. 9:6092022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Roy PK, Qamar AY, Tanga BM, Bang S, Seong
G, Fang X, Kim G, Edirisinghe SL, De Zoysa M, Kang DH, et al:
Modified spirulina maxima pectin nanoparticles improve the
developmental competence of in vitro matured porcine oocytes.
Animals (Basel). 11:24832021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mehdiani A, Maier A, Pinto A, Barth M,
Akhyari P and Lichtenberg A: An innovative method for exosome
quantification and size measurement. J Vis Exp.
509742015.PubMed/NCBI
|
|
29
|
Simonsen JB: Pitfalls associated with
lipophilic fluorophore staining of extracellular vesicles for
uptake studies. J Extracell Vesicles. 8:15822372019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takov K, Yellon DM and Davidson SM:
Confounding factors in vesicle uptake studies using fluorescent
lipophilic membrane dyes. J Extracell Vesicles. 6:13887312017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wheeler MB, Clark SG and Beebe DJ:
Developments in in vitro technologies for swine embryo production.
Reprod Fertil Dev. 16:15–25. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim JW, Park HJ, Yang SG and Koo DB:
Anti-oxidative effects of exogenous ganglioside GD1a and GT1b on
embryonic developmental competence in pigs. J Anim Reprod
Biotechnol. 35:347–356. 2020. View Article : Google Scholar
|
|
33
|
Park SH, Jeong PS, Joo YE, Kang HG, Kim
MJ, Lee S, Song BS, Kim SU, Cho SK and Sim BW: Luteolin
orchestrates porcine oocyte meiotic progression by maintaining
organelle dynamics under oxidative stress. Front Cell Dev Biol.
9:6898262021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiao X, Ding Z, Meng F, Zhang X, Wang Y,
Chen F, Duan Z, Wu D, Zhang S, Miao Y and Huo L: The toxic effects
of Fluorene-9-bisphenol on porcine oocyte in vitro maturation.
Environ Toxicol. 35:152–158. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Saadeldin IM, Gad A and Mermillod P:
Editorial: Biofluid extracellular vesicles and their involvement in
animal reproductive physiology. Front Vet Sci. 8:7471382021.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Alcântara-Neto AS, Schmaltz L, Caldas E,
Blache MC, Mermillod P and Almiñana C: Porcine oviductal
extracellular vesicles interact with gametes and regulate sperm
motility and survival. Theriogenology. 155:240–255. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Batista RITP, Moro LN, Corbin E, Alminana
C, Souza-Fabjan JMG, de Figueirêdo Freitas VJ and Mermillod P:
Combination of oviduct fluid and heparin to improve monospermic
zygotes production during porcine in vitro fertilization.
Theriogenology. 86:495–502. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Coy P, Lloyd R, Romar R, Satake N, Matas
C, Gadea J and Holt WV: Effects of porcine pre-ovulatory oviductal
fluid on boar sperm function. Theriogenology. 74:632–642. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Coy P and Avilés M: What controls
polyspermy in mammals, the oviduct or the oocyte? Biol Rev Camb
Philos Soc. 85:593–605. 2010.PubMed/NCBI
|
|
40
|
Coy P, Cánovas S, Mondéjar I, Saavedra MD,
Romar R, Grullón L, Matás C and Avilés M: Oviduct-specific
glycoprotein and heparin modulate sperm-zona pellucida interaction
during fertilization and contribute to the control of polyspermy.
Proc Natl Acad Sci USA. 105:15809–15814. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Avilés M, Coy P and Rizos D: The oviduct:
A key organ for the success of early reproductive events. Anim
Front. 5:25–31. 2015. View Article : Google Scholar
|
|
42
|
Kulus M, Kranc W, Jeseta M,
Sujka-Kordowska P, Konwerska A, Ciesiółka S, Celichowski P,
Moncrieff L, Kocherova I, Józkowiak M, et al: Cortical granule
distribution and expression pattern of genes regulating cellular
component size, morphogenesis, and potential to differentiation are
related to oocyte developmental competence and maturational
capacity in vivo and in vitro. Genes (Basel). 11:8152020.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Körschgen H, Kuske M, Karmilin K,
Yiallouros I, Balbach M, Floehr J, Wachten D, Jahnen-Dechent W and
Stöcker W: Intracellular activation of ovastacin mediates
pre-fertilization hardening of the zona pellucida. Mol Hum Reprod.
23:607–616. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Saadeldin IM, Kim SJ, Choi YB and Lee BC:
Improvement of cloned embryos development by co-culturing with
parthenotes: A possible role of exosomes/microvesicles for embryos
paracrine communication. Cell Reprogram. 16:223–234. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Babayev E and Seli E: Oocyte mitochondrial
function and reproduction. Curr Opin Obstet Gynecol. 27:175–181.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Niu YJ, Zhou W, Nie ZW, Shin KT and Cui
XS: Melatonin enhances mitochondrial biogenesis and protects
against rotenone-induced mitochondrial deficiency in early porcine
embryos. J Pineal Res. 68:e126272020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hao ZD, Liu S, Wu Y, Wan PC, Cui MS, Chen
H and Zeng SM: Abnormal changes in mitochondria, lipid droplets,
ATP and glutathione content, and Ca(2+) release after
electro-activation contribute to poor developmental competence of
porcine oocyte during in vitro ageing. Reprod Fertil Dev.
21:323–332. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Clapier CR, Iwasa J, Cairns BR and
Peterson CL: Mechanisms of action and regulation of ATP-dependent
chromatin-remodelling complexes. Nat Rev Mol Cell Biol. 18:407–422.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Singhal N, Graumann J, Wu G, Araúzo-Bravo
MJ, Han DW, Greber B, Gentile L, Mann M and Schöler HR:
Chromatin-remodeling components of the BAF complex facilitate
reprogramming. Cell. 141:943–955. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Russell AE, Jun S, Sarkar S, Geldenhuys
WJ, Lewis SE, Rellick SL and Simpkins JW: Extracellular vesicles
secreted in response to cytokine exposure increase mitochondrial
oxygen consumption in recipient cells. Front Cell Neurosci.
13:512019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Machtinger R, Laurent LC and Baccarelli
AA: Extracellular vesicles: Roles in gamete maturation,
fertilization and embryo implantation. Hum Reprod Update.
22:182–193. 2016.PubMed/NCBI
|
|
52
|
Qamar AY, Fang X, Bang S, Mahiddine FY,
Kim MJ and Cho J: The interplay between exosomes and spermatozoa.
Alzahrani FA and Saadeldin IM: Role of Exosomes in Biological
Communication Systems. Springer; Singapore: pp. 115–139. 2021
|
|
53
|
Saadeldin IM: Extracellular vesicles
mediate the embryonic-maternal paracrine communication. Alzahrani
FA and Saadeldin IM: Role of Exosomes in Biological Communication
Systems. Springer; Singapore: pp. 77–97. 2021, View Article : Google Scholar
|
|
54
|
Du J, Shen J, Wang Y, Pan C, Pang W, Diao
H and Dong W: Boar seminal plasma exosomes maintain sperm function
by infiltrating into the sperm membrane. Oncotarget. 7:58832–58847.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kumaresan A, Johannisson A, Humblot P and
Bergqvist AS: Effect of bovine oviductal fluid on motility,
tyrosine phosphorylation, and acrosome reaction in cryopreserved
bull spermatozoa. Theriogenology. 124:48–56. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bathala P, Fereshteh Z, Li K, Al-Dossary
AA, Galileo DS and Martin-DeLeon PA: Oviductal extracellular
vesicles (oviductosomes, OVS) are conserved in humans: Murine OVS
play a pivotal role in sperm capacitation and fertility. Mol Hum
Reprsod. 24:143–157. 2018.PubMed/NCBI
|
|
57
|
Ferraz MAMM, Carothers A, Dahal R, Noonan
MJ and Songsasen N: Oviductal extracellular vesicles interact with
the spermatozoon's head and mid-piece and improves its motility and
fertilizing ability in the domestic cat. Sci Rep. 9:94842019.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Al-Dossary AA, Strehler EE and
Martin-DeLeon PA: Expression and secretion of plasma membrane
Ca2+-ATPase 4a (PMCA4a) during murine estrus: association with
oviductal exosomes and uptake in sperm. PLoS One. 8:e801812013.
View Article : Google Scholar : PubMed/NCBI
|