According to the Global Cancer Statistics 2020, the
number of new cases of renal cell carcinoma (RCC) has increased by
~100,000 over the past decade (1,2).
Among cancers of the urinary system, RCC ranks third in terms of
prevalence (3). Organ metastasis
of advanced RCC is the main cause of a poor prognosis. Since RCC is
not sensitive to conventional radiotherapy and chemotherapy
(4), the search for specific
biomarkers and individualized treatment options for RCC is an
important direction.
Epigenetic alterations are among the hallmarks of
cancer. Epigenetics has a critical role in RCC, including DNA
methylation, chromatin remodeling and histone acetylation. The main
function of the bromodomain proteins (BRD) is the acetylation
modification of histones (5). The
first BRD was discovered in 1992 (6). Although the BRD is highly conserved
structurally, BRD family proteins may be divided into eight
distinct subfamilies (I–VIII) based on their secondary structure
(5,7). Research has indicated that targeting
these BRDs may provide a novel strategy for the treatment of
metastatic RCC. The present review summarizes the roles of existing
BRD families in related pathways in renal cancer and discusses the
efficacy of existing BRD inhibitors and their prospects for
clinical application.
In eukaryotic cells, DNA is packaged into chromatin
in three processes: The binding of DNA to core histones, the
formation of nucleosome core particles and chromosome core
particles by core histones (H2A, H2B, H3 and H4) and junctional
histones (H1) (8). The functions
of histones are divided into the regulation of DNA transcription,
DNA replication and DNA repair. Of these, core histones have a
critical role in transcriptional regulation mainly through their
post-translational modifications (PTMs) (9,10).
Histone modification was the first confirmed PTM. It
was first hypothesized by Allfrey et al (11) that there was a ‘switch’ that
regulates cellular gene expression. The subsequent discovery of
histone acetyltransferase (HAT), histone deacetylase (HDAC) and the
mechanisms that control histone acetylation activity support the
hypothesis put forth by Allfrey et al (11) on the role of histone acetylation in
gene expression (12–14).
However, whether lysine acetylation controls histone
activity and how it controls gene activation and repression remain
to be fully elucidated. Until the discovery that BRD was the first
chromatin ‘reader’, it was known to exist in the nucleus as an
acetyl-lysine (Kac) binding domain and to be associated with
numerous transcriptional proteins (6,15).
Thus, histone acetylation was established as a fundamental
mechanism for regulating gene transcription. However, BRD does not
have a role in histone modification alone. Such ‘large-scale
engineering’ may require multiple histone combination modifications
or sequential initiation, with BRD acting on one or more histone
tails to activate target genes and downstream functions (16,17).
BRD-mediated lysine acetylation modifications are a major
epigenetic modification in RCC tumor metabolism. BRDs are involved
in histone modifications, transcription factor recruitment and
transcriptional regulation, and DNA damage repair in RCC as
chromatin remodeling factors (5).
Furthermore, BRDs have unique structural properties (7).
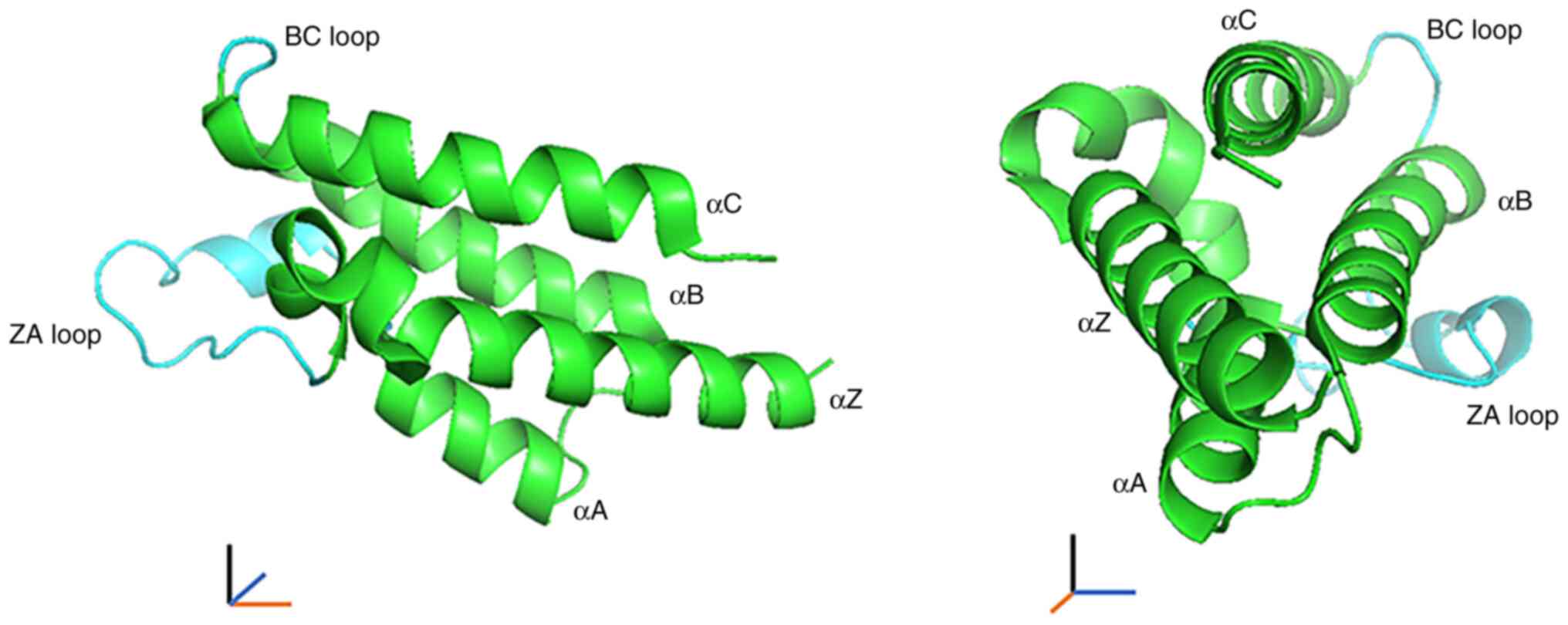
BRD consists of 110 amino acids and includes four α
helices (αZ, αA, αB and αC) with two distinct interhelical αZ-αA
(ZA) and αB-αC (BC) loop regions. The two loops in turn produce a
hydrophobic pocket that functions as a module for the recognition
of the acetyl-lysine modification (Fig. 1) (15,18).
Different BRDs have BRD modules with varying lengths of the ZA and
BC loop regions and may contain other types of action modules
(19).
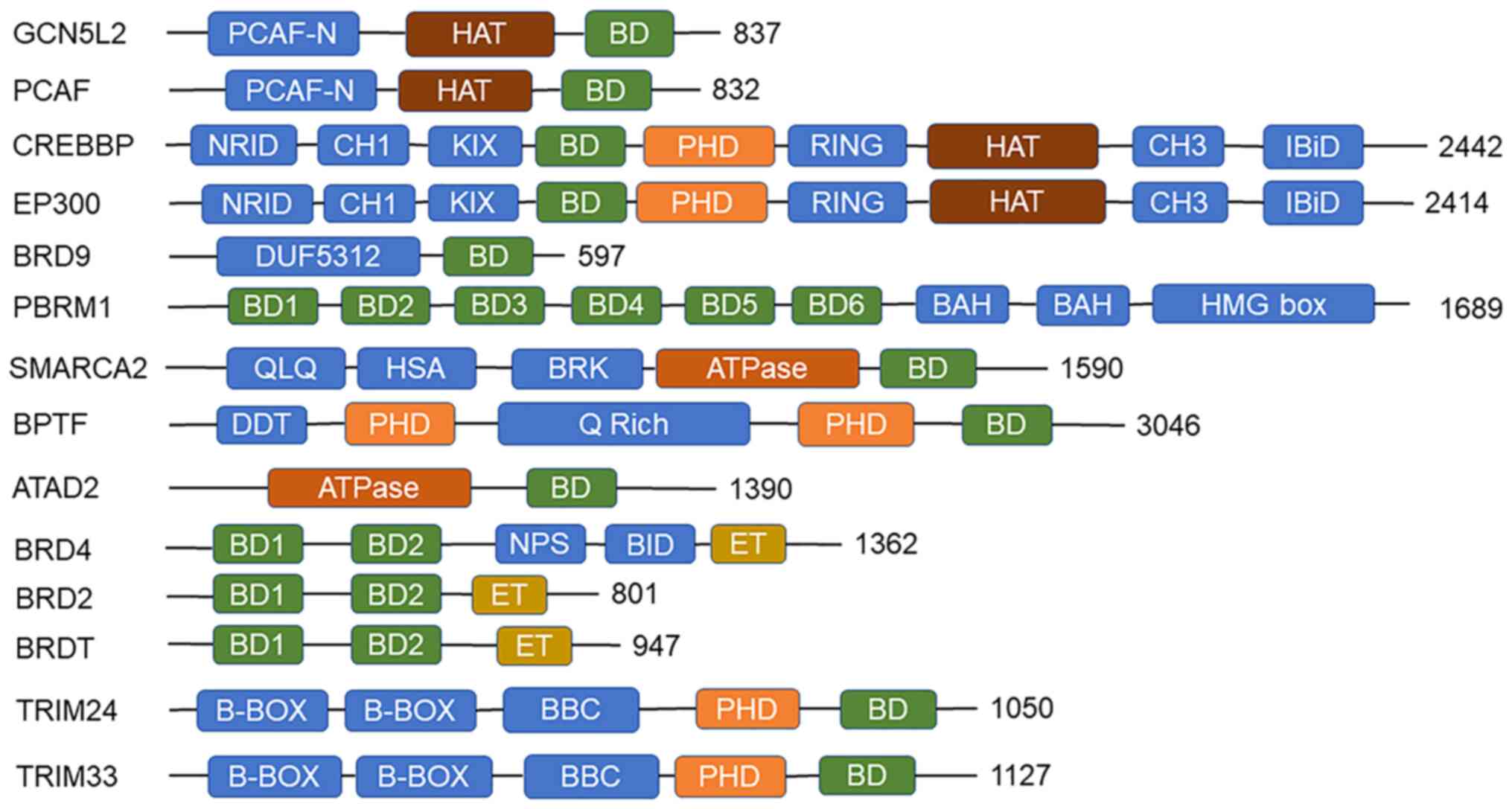
BRDs also include other domains, which combine with
BRD to perform specific functions (Fig. 2). Certain BRDs contain ATPase
structures that enhance the ability to bind Kac, contribute to the
assembly of its associated complex, facilitate the movement of the
complex along chromatin and coordinate the function of multiple
protein docking in chromatin remodeling (20). For instance, ATPase family AAA
domain containing 2 (ATAD2) of the ATPase family is directly
involved in DNA replication by being recruited to the DNA
replication site (21). In
addition, ATAD2 has been identified as a chromatin modifier that
promotes melanoma (22). Although
ATAD2 has not been extensively studied in RCC, studies have
indicated an increased risk of developing RCC in patients with
melanoma and vice versa. Both may have the same type of genomic
mutation (23). Coactivators
containing both HAT and BRD structural domains assist substrate
recruitment by enabling the HAT-mediated acetylation of multiple
lysine residues on histones and transcription factors, thereby
promoting transcriptional activation (24). ATPase-containing structures of BRDs
enhance the ability to bind Kac, contribute to the assembly of its
associated complexes, facilitate the movement of the complexes
along chromatin and coordinate multiple protein-protein functions
in chromatin remodeling. Unlike other BRDs, BRD and extraterminal
(BET) proteins have two tightly packed BRDs that specifically bind
diacetylated lysine residues (25,26),
promote chromatin opening, recruit transcription factors and
coactivators to target gene promoters and enhancers, and activate
the RNA polymerase II (Pol II) complex to promote transcriptional
elongation (27). Similarly,
tandem plant homeodomain (PHD) fingers with BRDs contribute to the
assembly and activity of their associated complexes in nucleosomes
and act on DNA replication in chromosome segregation (28,29).
BRDs are widely involved in the transcription of cancer-related
genes, and depending on the subtle differences in BRDs and various
combinations of functional groups, highly selective inhibitors may
be designed with potential clinical applications for the treatment
of metastatic cancers (30).
The development of RCC mainly includes the following
biological behaviors: Angiogenesis, proliferation and immune
regulation. In the pathogenesis of RCC, 3p deletion and von
Hippel-Lindau (VHL) gene inactivation are the most
frequently-occurring mutations (31,32).
Their co-mutation has a decisive role in the initiation of RCC.
Inactivation of VHL leads to the uncontrolled activation of
hypoxia-inducible factor (HIF) target genes that regulate
angiogenesis, glycolysis and apoptosis (4). The deletion of these tumor suppressor
genes and the activation of oncogenes eventually lead to the
development of RCC.
Different BRDs have different roles, which has led
to the consideration of different BRDs in RCC (Table I). Angiogenesis is a typical
feature of RCC and BRDs may be involved in RCC angiogenesis from
different signaling pathways (33), and may even promote angiogenesis in
RCC with a low HIF expression (34). Furthermore, the majority of the
BRDs mainly function as oncogenic proteins, promoting the
proliferation of RCC cells (35–41),
and are associated with influencing the occurrence of renal cancer
recurrence and metastasis (42).
According to the ‘braided river’ model to interpret RCC (43), the gene mutation of RCC has an
evolutionary process. Certain BRDs appear in RCC of more advanced
stages and are more likely to cause distant metastasis (39,44,45).
Finally, the formation of tumors is also inseparable from changes
in immune regulation. The immune-killing effect on the tumor is the
main error correction method of the human system. The killing of
RCC may be achieved by upregulating IFNα expression (46) and by the induction of
CD4+ T-cells (47);
polybromo 1 (PBRM1), as a protective factor, may enhance these
effects. However, E1A binding protein P300 (EP300)/CREB binding
protein (CBP) also leads to the depletion of peripheral lymphocytes
(48), and the inhibition of
peripheral inflammatory factors by BRD4 diminishes its killing
effect on tumor cells (49). On
the one hand, different or opposing roles between BRDs and
mutations in BRDs are evolutionary in RCC. These generally increase
the difficulty of RCC-specific biomarker research. On the other
hand, differential mutations in RCC allow patients to select
therapeutics with high specificity. In the following sections, the
progress of BRDs in RCC is discussed after being classified
according to their main roles.
EP300/CBP promotes cell growth and angiogenesis in
RCC through acetylation modification. EP300/CBP functions as a
co-transcription factor and forms a transcriptional complex with
proteins such as HIF1α to recruit to the promoter of VEGF (52,53),
and HIF-1α transcriptional activation or transcriptional repression
is dependent on EP300/CBP (54).
Consistent with HIF1α, HIF2α induces EP300/CBP recruitment so that
it not only binds specifically to the enhancer of HIF2α to promote
HIF2α expression for RCC cell growth, but also promotes RCC
angiogenesis by enhancing VEGF transcription (55). In addition, HIF1α and HIF2α induce
the recruitment of EP300/CBP at the promoter of telomerase reverse
transcriptase, promoting the immortalization and transformation of
renal cancer cells (56).
Furthermore, EP300/CBP binding to H3AcK18 enhances RCC cell
viability, adhesion and invasiveness (35).
EP300/CBP also promotes RCC progression by promoting
macrophage infiltration through the RNA-binding motif protein 15
(RBM15)-C-X-C motif chemokine ligand 11 signaling axis (57). In addition, EP300/CBP upregulation
is also associated with T-cell dysfunction. EP300/CBP reduces
tumor-infiltrating lymphocytes by downregulating immune checkpoint
gene expression via binding to serine and arginine-rich splicing
factor 2 and cause immune escape of RCC cells (48).
EP300/CBP is structurally and functionally complex;
however, it currently only has two related inhibitors in RCC.
Inhibitors targeting the BRD binding of EP300/CBP include C646,
which effectively inhibits EP300/CBP (57). HBS1, a high-affinity ligand of
cysteine-histidine-rich region 1, functions as an antibody to
HIF1α, but is able to block the binding of HIF1α to the CH300
structural domain of EP300/CBP without affecting normal cell
function (58).
Normally, BRD4 is inhibited by caspase-3, leading to
the pyroptosis of RCC cells. When BRD4 is activated, it inhibits
the production of the peripheral inflammatory factors, IL-1β and
IL-18, by cells. This allows immune evasion of RCC from IL-1β and
IL-18 (49), promotes RCC cell
proliferation and epithelial-mesenchymal transition (EMT) and
inhibits tumor killing by peripheral T-cells (62). Another study demonstrated that BRD4
acetylation modified the histones of B-cell lymphoma-2 (BCL2) and
C-MYC to increase their expression (36). MYC is a heterogeneous gene fragment
frequently activated in cancer, and targeting BRD4 may inhibit its
expression (63). This suggests
that BRD4 upregulation is closely related to RCC; the inhibition of
BRD4 gene expression and the enhancement of peripheral inflammatory
factors may become a novel treatment strategy for metastatic
RCC.
Pharmacological studies on kidney cancer cells have
revealed that BRD4 and the PI3K/mTOR pathway complement each other
to promote the proliferation of kidney cancer cells. VS-5584, a
dual inhibitor of PI3K/mTOR, may effectively inhibit the
proliferation and survival of RCC cells (65), but leads to increased BRD4
expression, resulting in enhanced tumor drug resistance. By
contrast, the simultaneous inhibition of BRD4 and PI3K/mTOR
significantly inhibits tumor cell survival and does not increase
tumor drug resistance. Furthermore, the dual inhibitor of mTORC1/2
(Palomid 529) has been found to be more effective in BRD4-negative
RCC cells than normal RCC cells (66), demonstrating that BRD4 and the
PI3K/mTOR pathway have a complementary effect on each other. By
contrast, SF2523 is a dual inhibitor of BRD4 and PI3K-AKT; SF2523
is more effective than the PI3K inhibitor and JQ1 in killing RCC
cells (67). Furthermore, the BET
inhibitor OTX015 exerts a therapeutic effect on cancer patients
with deletion of BRCA1-associated protein 1 (BAP1), a ubiquitin
carboxy-terminal hydrolase (68);
patients with a BAP1 deletion in RCC tend to have poor prognosis
(69).
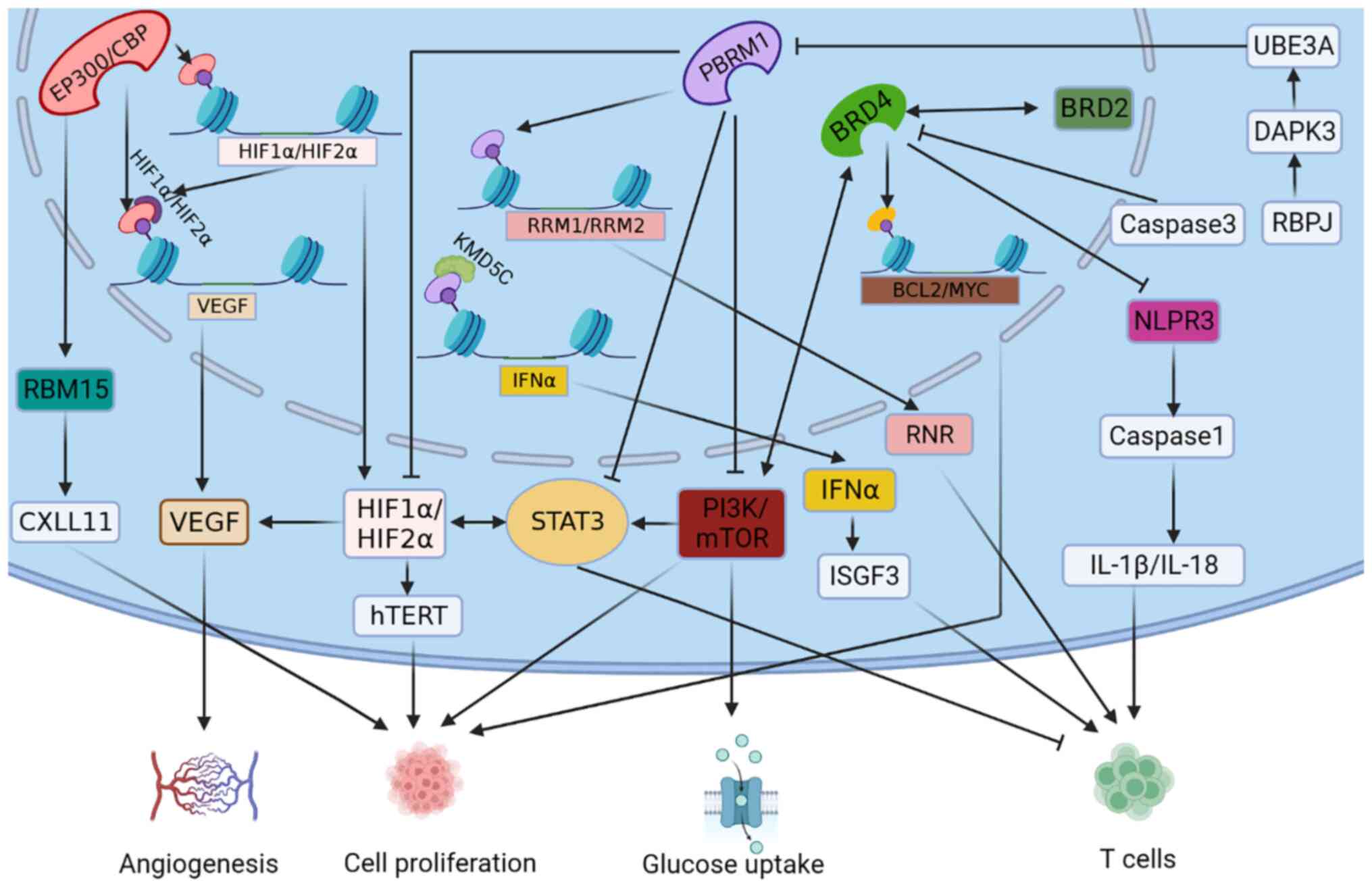
The aforementioned inhibitors exert potent effects
by inhibiting BRD4 or when BRD4 is inhibited (Fig. 3). The inhibition of BET family
members has great therapeutic potential in the treatment of RCC.
Currently, clinical resistance to BET inhibitors limits their
application; however, synergistic antitumor effects have been
observed when used in combination with other tumor suppressors
(70). Therefore, the design of
BET BRDs dual-target inhibitors and their combination is a
reasonable strategy, which may be used to enhance the efficacy of
cancer therapy and reduce drug resistance. However, extensive
animal studies are still required to verify the efficacy and
toxicology prior to clinical application.
BRDT functions as a chromatin remodeling factor in
recognizing acetylated histones and recruiting transcriptional
complexes (71). It interacts with
eukaryotic translation initiation factor 4E-binding protein 1
(eIF4EBP1) to promote C-MYC transcription and RCC progression. BRDT
inhibitor PLX51107 exerts an inhibitory effect on RCC cells;
however, eIF4EBP1 overexpression hinders its inhibitory effect
(72). Therefore, dual inhibitors
of both proteins or a combination of both inhibitors may be
required for clinical application in RCC. The initiation of BET
family proteins in RCC does not appear to proceed via a sole
mechanism, i.e. not only in terms of the binding of BETs to other
proteins, but also among BETs (64), which complement each other while
interacting.
BPTF contains two PHD fingers and a BRD that
regulates gene transcription by interacting with the MYC-associated
zinc finger protein, ZF87/MAZ (73). BPTF is a subunit of the ISWI
chromatin remodeling complex, NURF, and has a crucial role in
chromatin remodeling as a transcription factor (74). The increased expression of BPTF
causes glycolytic reprogramming and distant lung metastasis in RCC.
Its inhibitor, AU1, is effective in inhibiting the metastatic
potential of patient-derived cells, metastatic RCC-derived
organoids, as well as in situ xenograft models of metastatic
RCC, suggesting that BPTF may be applied as an initial therapeutic
marker for metastatic RCC (44).
BRD9 is highly expressed in HIF2α-deficient RCC.
SOX17 recruits BRD9 to upregulate genes in RCC pathogenesis,
including VEGFR2 (34). There is a
negative correlation between the expression of BRD9 and HIF2α, and
the expression of related genes is upregulated by BRD9 to target
the Notch1-Hes1 signaling pathway to promote the proliferation,
migration and invasion of RCC cells (39). A previous study demonstrated that
BRD9 inhibitor (I-BRD9, also known as GSK602) effectively inhibited
tumor growth in vitro and in vivo. It also prolonged
the survival of RCC mice compared to sunitinib (34). Due to HIF activation in RCC caused
by VHL inactivation, targeting HIF2α and applying anti-angiogenic
targeted drugs are considered mainstream therapeutic approaches for
metastatic RCC (78). A lower
expression of HIF2α in RCC was indicated to be associated with a
lower survival rate and prognosis. Hence, the search for novel drug
targets in HIF2α-deficient RCC may be promising. BRD9 is expected
to be a valuable target for the treatment of HIF2α-deficient
RCC.
PBRM1 is frequently mutated in human tumors and
PRBM1 comprises 1,689 amino acids. PBRM1 is characterized by a
C-terminal high migration pattern, including two bromine-associated
homologous structural domains and six bromine structural domains
(79). These bromine structural
domains combine with acetylated residues at the histone tail
(80), and each bromine domain has
a different affinity for the specific acetylated peptides on the
histones and may coordinate with the exact pattern of acetylated
lysine residues in the nucleosome (81,82).
Previous studies suggested that RCC is characterized by partial
loss of chromosome 3p, while PBRM1 resides on 3p and has
deletion mutations at a relatively high frequency, just second only
to VHL in RCC, accounting for ~30–40% of RCC cases (79,80,83).
As PBRM1 is a tumor suppressor, the deletion mutation of
PBRM1 combined with VHL deletion produces stable
tumor models (84,85).
RCC develops its mutations differently and at
different time-points. In certain RCC cell lines, PBRM1 may remain
present following the deletion of VHL. The deletion mutation
or suppressed state of PBRM1 may be caused by other factors. For
instance, recombination signal binding protein for immunoglobulin
kappa J region (RBPJ) promotes death-associated protein kinase 3
binding to ubiquitin-protein ligase E3A (UBE3A), destabilizing
PBRM1 in RCC cells and thereby reducing the protective effect of
PBRM1 on normal renal tissue. UBE3A knockdown increased the
sensitivity of CDK inhibitors, and RBPJ inhibitors modulate CDK4/6
inhibitor drug sensitivity to improve tumor suppression (86). By inhibiting PBRM1 upstream as
described above, the presence of PBRM1 has been found to exert
protective effects on normal kidney tissue, suggesting that it
inhibits the action of common oncogenic factors in RRC. However,
following the knockdown of PBRM1, it has been found that PBRM1
deletion did not allow for the inhibition of the HIF1/STAT3
signaling pathway in the kidney, and PBRM1 deletion attenuated the
activity of the negative regulator of mTORC1, TSC1, facilitating
mTORC1 activation and leading to advanced RCC (84,87).
In addition, deletion of PBRM1 enhanced the hypoxic response,
leading to increased induction of HIF1α and HIF2α, which promoted
angiogenesis and cell proliferation in kidney cancer (88,89).
The simultaneous knockdown of both PBRM1 and VHL genes in RCC cell
lines also resulted in the activation of HIF and in the
upregulation of PI3K signaling, promoting glucose uptake and
adhesion in RCC cells (Fig. 3)
(90,91).
PBRM1 deficiency is not only involved in
angiogenesis following the induction of hypoxia, but also allows
tumor cells to acquire the related functions of immune evasion.
PBRM1 recruits lysine demethylase 5C to upregulate IFNα gene
expression and acts on IFN-stimulated gene factor 3 to inhibit RCC
progression (46). Furthermore,
PBRM1 has been found to bind directly to the RRM1 and RRM2 sites
and promote the infiltration of CD4 T-cells in the peripheral
tissues of RCC (47). As with
anti-vascular targeting agents, anti-programmed cell death 1 agents
are associated with improved clinical prognosis in patients with
PBRM1-deficient mutated RCC (92). This suggests that
PBRM1-deficient RCC is sensitive to targeted drugs and
immune agents.
SWI/SNF-related, matrix-associated, actin-dependent
regulator of chromatin subfamily A member 2 (SMARCA2)S
mutations are highly associated with VHL inactivation, an elevated
tumor grade and a relatively poorer prognosis (93,94).
RCC cells in the G2/M phase are promoted to undergo apoptosis and
cell cycle arrest by SMARCA2 mutations, not only by
SMARCA2 deletion but also by epigenetic silencing of
SMARCA2. Therefore, the inhibition of SMARCA2 transcription
has a carcinogenic role (95), and
the restoration of SMARCA2 expression by a HDAC3 inhibitor
(RGFP966) effectively inhibited tumor progression in RCC (96). The restoration of the expression of
tumor suppressor genes may thus be an effective strategy for the
treatment of RCC.
GCN5L2 was the first histone acetyltransferase
identified with an N-terminal structural domain, a conserved HAT
structural domain and a C-terminal bromine structural domain
(97). The aforementioned three
structural domains are also found in PCAF, with only one molecular
weight difference between the two (Fig. 2). The two proteins have
approximately the same function, although PCAF can be methylated
(98). Furthermore, both have
opposite effects on RCC outcomes. The overexpression of GCN5L2
upregulates monocarboxylate transporter 1 (MCT1), thus promoting
glycolysis in RCC cells. The proliferation of RCC cells with a high
GCN5L2 expression may be significantly inhibited by an MCT1
inhibitor (AZD3965) (99).
PCAF is known as GCN5-related N-acetyltransferase in
the lysine acetyltransferase family (100). As previously mentioned, PCAF (the
EP300/CBP-associated factor) is a tumor suppressor gene in RCC
(101). NADPH oxidase (NOX)4
functions as a mitochondrial energy sensor and the derived reactive
oxygen species inhibit the acetylation of PCAF, promote metabolic
reprogramming and enhance drug resistance in RCC cells (102). Promoting PACF expression or
inhibiting NOX to reduce drug resistance in RCC may be a novel
therapeutic approach.
The TRIM family is a class of proteins with E3
ubiquitin ligase activity. Its members include a number of key
biological processes, including autophagy, carcinogenic,
intracellular signaling, protein ubiquitination and innate immunity
(103). TRIM24 and TRIM33 form
chromatin remodeling complexes with heterochromatin protein 1 and
HDAC, with chromatin remodeling, mainly of different molecular
weights (Fig. 2) (104). However, the two have distinct
roles in the progression of RCC. In RCC, the transcription of
TRIM24 is regulated by bone morphogenetic protein (BMP)8A, and the
upregulation of TRIM24 by BMP8A enhances the Wnt-regulated Wnt
signaling pathway, thus promoting proliferation, invasion, and
metastasis and drug resistance (40,45).
In contrast to TRIM24, TRIM33 functions as a tumor
suppressor in RCC and its overexpression inhibits β-linked proteins
on the Wnt signaling pathway, which reduces the expression of
cyclin D1 and C-MYC. Consequently, TRIM33 inhibits the growth of
RCC and leads to the upregulation of E-calmodulin expression and
the downregulation of N-calmodulin to reduce the EMT potential of
RCC cells (103). In addition,
the overexpression of TRIM33 significantly inhibits TGFβ-induced
Smad activation, inhibiting RCC progression (105).
Large-scale studies have identified mutations in
BRDs that cause dysfunction and lead to cancer development. BRDs
are intimately involved in the regulation of gene transcription;
therefore, the inhibition of BRD structural proteins is considered
a strategy for targeting oncogenic transcription factors that have
long been considered attractive drug targets, but cannot be
directly regulated with small molecule inhibitors. This can also be
tailored to the nature of the tumor, with different transcriptional
repression. For instance, BET inhibition has been shown to block
the association of MYC with BRD4 and MYC transcription (61,63).
In anti-angiogenesis, the transcriptional expression of HIF, VEGF
and VEGFA can be reduced by targeted inhibition of BRDs (33,34).
In immunotherapy, immune checkpoints can be suppressed by targeting
and inhibiting BRDs or activating BRDs to restore the
transcriptional activity of antitumor factors (46–48).
As presented in Table II, a large
number of related drugs (Fig. S1)
have been applied to basic studies on RCC and these have exhibited
improved tumor-suppressive effects by directly inhibiting BRD or
its downstream functional molecules. Targeting BRD structural
proteins holds promise in clinical practice; however, to date, at
least to the best of our knowledge, no clinical trials have been
performed on RCC.
RCC is a common disease of the urinary system;
however, its development process is highly complex, while research
on BRDs has provided certain insight. BRDs are fully involved in
the proliferation, angiogenesis and immune regulation of RCC, and
supplement the signaling pathways of these cellular behaviors.
Inhibiting the expression of these oncogenes or restoring the
expression of those tumor suppressor genes may exert a notable
inhibitory effect on RCC and may provide a potential solution for
RCC resistance to VEGF-targeted drugs. Of note, the aforementioned
studies on BRD molecules have revealed information regarding the
promotion or inhibition of RCC; however, the specific underlying
mechanisms remain to be fully elucidated and further in-depth
research is required, as clinical trials on related inhibitors are
lacking.
Not applicable.
The present study was supported by the National Natural Science
Foundation of China (grant no. 81860456).
Data sharing is not applicable to this article, as
no new data were generated or analyzed during the current
study.
QW and HL wrote the manuscript. KL, WC and XZ
acquired the data. JX and JG interpreted the data. JZ and XZ made
substantial contributions to conception and design. All authors
contributed to the article and approved the submitted version. All
authors have read and agreed to the published version of the
manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hsieh JJ, Purdue MP, Signoretti S, Swanton
C, Albiges L, Schmidinger M, Heng DY, Larkin J and Ficarra V: Renal
cell carcinoma. Nat Rev Dis Primers. 3:170092017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fujisawa T and Filippakopoulos P:
Functions of bromodomain-containing proteins and their roles in
homeostasis and cancer. Nat Rev Mol Cell Biol. 18:246–262. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Haynes SR, Dollard C, Winston F, Beck S,
Trowsdale J and Dawid IB: The bromodomain: A conserved sequence
found in human, Drosophila and yeast proteins. Nucleic Acids Res.
20:26031992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boyson SP, Gao C, Quinn K, Boyd J,
Paculova H, Frietze S and Glass KC: Functional roles of bromodomain
proteins in cancer. Cancers (Basel). 13:36062021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fyodorov DV, Zhou BR, Skoultchi AI and Bai
Y: Emerging roles of linker histones in regulating chromatin
structure and function. Nat Rev Mol Cell Biol. 19:192–206. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Allis CD and Jenuwein T: The molecular
hallmarks of epigenetic control. Nat Rev Genet. 17:487–500. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Izzo A and Schneider R: The role of linker
histone H1 modifications in the regulation of gene expression and
chromatin dynamics. Biochim Biophys Acta. 1859:486–495. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Allfrey VG, Faulkner R and Mirsky AE:
Acetylation and methylation of histones and their possible role in
the regulation of rna synthesis. Proc Natl Acad Sci USA.
51:786–794. 1964. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brownell JE, Zhou J, Ranalli T, Kobayashi
R, Edmondson DG, Roth SY and Allis CD: Tetrahymena histone
acetyltransferase A: A homolog to yeast Gcn5p linking histone
acetylation to gene activation. Cell. 84:843–851. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuo MH, Brownell JE, Sobel RE, Ranalli TA,
Cook RG, Edmondson DG, Roth SY and Allis CD: Transcription-linked
acetylation by Gcn5p of histones H3 and H4 at specific lysines.
Nature. 383:269–272. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Taunton J, Hassig CA and Schreiber SL: A
mammalian histone deacetylase related to the yeast transcriptional
regulator Rpd3p. Science. 272:408–411. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dhalluin C, Carlson JE, Zeng L, He C,
Aggarwal AK and Zhou MM: Structure and ligand of a histone
acetyltransferase bromodomain. Nature. 399:491–496. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Strahl BD and Allis CD: The language of
covalent histone modifications. Nature. 403:41–45. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tan M, Luo H, Lee S, Jin F, Yang JS,
Montellier E, Buchou T, Cheng Z, Rousseaux S, Rajagopal N, et al:
Identification of 67 histone marks and histone lysine crotonylation
as a new type of histone modification. Cell. 146:1016–1028. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jeanmougin F, Wurtz JM, Le Douarin B,
Chambon P and Losson R: The bromodomain revisited. Trends Biochem
Sci. 22:151–153. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Romero FA, Taylor AM, Crawford TD, Tsui V,
Côté A and Magnuson S: Disrupting acetyl-lysine recognition:
Progress in the development of bromodomain inhibitors. J Med Chem.
59:1271–1298. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mashtalir N, D'Avino AR, Michel BC, Luo J,
Pan J, Otto JE, Zullow HJ, McKenzie ZM, Kubiak RL, St Pierre R, et
al: Modular organization and assembly of SWI/SNF family chromatin
remodeling complexes. Cell. 175:1272–1288.e20. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Koo SJ, Fernández-Montalván AE, Badock V,
Holton SJ, von Ahsen O, Toedling J, Vittori S, Bradner JE and
Gorjánácz M: ATAD2 is an epigenetic reader of newly synthesized
histone marks during DNA replication. Oncotarget. 7:70323–70335.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baggiolini A, Callahan SJ, Montal E, Weiss
JM, Trieu T, Tagore MM, Tischfield SE, Walsh RM, Suresh S, Fan Y,
et al: Developmental chromatin programs determine oncogenic
competence in melanoma. Science. 373:eabc10482021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maubec E, Chaudru V, Mohamdi H, Grange F,
Patard JJ, Dalle S, Crickx B, Paillerets BB, Demenais F and Avril
MF: Characteristics of the coexistence of melanoma and renal cell
carcinoma. Cancer. 116:5716–5724. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dancy BM and Cole PA: Protein lysine
acetylation by p300/CBP. Chem Rev. 115:2419–2452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morinière J, Rousseaux S, Steuerwald U,
Soler-López M, Curtet S, Vitte AL, Govin J, Gaucher J, Sadoul K,
Hart DJ, et al: Cooperative binding of two acetylation marks on a
histone tail by a single bromodomain. Nature. 461:664–668. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shi J, Wang Y, Zeng L, Wu Y, Deng J, Zhang
Q, Lin Y, Li J, Kang T, Tao M, et al: Disrupting the interaction of
BRD4 with diacetylated Twist suppresses tumorigenesis in basal-like
breast cancer. Cancer Cell. 25:210–225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hnisz D, Abraham BJ, Lee TI, Lau A,
Saint-André V, Sigova AA, Hoke HA and Young RA: Super-enhancers in
the control of cell identity and disease. Cell. 155:934–947. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thompson PJ, Norton KA, Niri FH, Dawe CE
and McDermid HE: CECR2 is involved in spermatogenesis and forms a
complex with SNF2H in the testis. J Mol Biol. 415:793–806. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xiao A, Li H, Shechter D, Ahn SH, Fabrizio
LA, Erdjument-Bromage H, Ishibe-Murakami S, Wang B, Tempst P,
Hofmann K, et al: WSTF regulates the H2A.X DNA damage response via
a novel tyrosine kinase activity. Nature. 457:57–62. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Filippakopoulos P, Qi J, Picaud S, Shen Y,
Smith WB, Fedorov O, Morse EM, Keates T, Hickman TT, Felletar I, et
al: Selective inhibition of BET bromodomains. Nature.
468:1067–1073. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Szücs S, Müller-Brechlin R, DeRiese W and
Kovacs G: Deletion 3p: The only chromosome loss in a primary renal
cell carcinoma. Cancer Genet Cytogenet. 26:369–373. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Latif F, Tory K, Gnarra J, Yao M, Duh FM,
Orcutt ML, Stackhouse T, Kuzmin I, Modi W, Geil L, et al:
Identification of the von Hippel-Lindau disease tumor suppressor
gene. Science. 260:1317–1320. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Grabmaier K, A de Weijert MC, Verhaegh GW,
Schalken JA and Oosterwijk E: Strict regulation of CAIX(G250/MN) by
HIF-1alpha in clear cell renal cell carcinoma. Oncogene.
23:5624–5631. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang C, Chen L, Lou W, Su J, Huang J, Liu
A, Xu Y, He H, Gao Y, Xu D and Li Q: Aberrant activation of m6A
demethylase FTO renders HIF2αlow/− clear cell renal cell
carcinoma sensitive to BRD9 inhibitors. Sci Transl Med.
13:eabf60452021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cocco E, Leo M, Canzonetta C, Di Vito S,
Mai A, Rotili D, Di Napoli A, Vecchione A, De Nunzio C, Filetici P
and Stoppacciaro A: KAT3B-p300 and H3AcK18/H3AcK14 levels are
prognostic markers for kidney ccRCC tumor aggressiveness and target
of KAT inhibitor CPTH2. Clin Epigenetics. 10:442018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu X, Liu D, Gao X, Xie F, Tao D, Xiao X,
Wang L, Jiang G and Zeng F: Inhibition of BRD4 suppresses cell
proliferation and induces apoptosis in renal cell carcinoma. Cell
Physiol Biochem. 41:1947–1956. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen W, Zhang H, Chen Z, Jiang H, Liao L,
Fan S, Xing J, Xie Y, Chen S, Ding H, et al: Development and
evaluation of a novel series of Nitroxoline-derived BET inhibitors
with antitumor activity in renal cell carcinoma. Oncogenesis.
7:832018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ji S, Su X, Zhang H, Han Z, Zhao Y and Liu
Q: MicroRNA-372 functions as a tumor suppressor in cell invasion,
migration and epithelial-mesenchymal transition by targeting ATAD2
in renal cell carcinoma. Oncol Lett. 17:2400–2408. 2019.PubMed/NCBI
|
|
39
|
Lou W, Gao K, Xu C and Li Q:
Bromodomain-containing protein 9 is a prognostic biomarker
associated with immune infiltrates and promotes tumor malignancy
through activating notch signaling pathway in negative HIF-2α clear
cell renal cell carcinoma. IUBMB Life. 73:1334–1347. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jiang T, Mao H, Chen Q, Cao L, He Y, Gao
X, Chen W and Zhang H: Trim24 prompts tumor progression via
inducing EMT in renal cell carcinoma. Open Med (Wars).
15:1153–1162. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jin W, Miao Q, Wang M, Zhang Y, Ma Y,
Huang Y, Wu H, Lin Y, Hu B and Pan J: A rare case of adrenal gland
abscess due to anaerobes detected by metagenomic next-generation
sequencing. Ann Transl Med. 8:2472020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Aurilio G, Santoni M, Massari F,
Cimadamore A, Rizzo A, Mollica V, Verri E, Battelli N and Montironi
R: Metabolomic profiling in renal cell carcinoma patients: News and
views. Cancers (Basel). 13:52292021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hsieh JJ and Cheng EH: A braided cancer
river connects tumor heterogeneity and precision medicine. Clin
Transl Med. 5:422016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang C, Chen L, Liu Y, Huang J, Liu A, Xu
Y, Shen Y, He H and Xu D: Downregulated METTL14 accumulates BPTF
that reinforces super-enhancers and distal lung metastasis via
glycolytic reprogramming in renal cell carcinoma. Theranostics.
11:3676–3693. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yu YP, Cai LC, Wang XY, Cheng SY, Zhang
DM, Jian WG, Wang TD, Yang JK, Yang KB and Zhang C: BMP8A promotes
survival and drug resistance via Nrf2/TRIM24 signaling pathway in
clear cell renal cell carcinoma. Cancer Sci. 111:1555–1566. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liao L, Liu ZZ, Langbein L, Cai W, Cho EA,
Na J, Niu X, Jiang W, Zhong Z, Cai WL, et al: Multiple tumor
suppressors regulate a HIF-dependent negative feedback loop via
ISGF3 in human clear cell renal cancer. Elife. 7:e379252018.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Szarkowska J, Cwiek P, Szymanski M,
Rusetska N, Jancewicz I, Stachowiak M, Swiatek M, Luba M,
Konopinski R, Kubala S, et al: RRM2 gene expression depends on
BAF180 subunit of SWISNF chromatin remodeling complex and
correlates with abundance of tumor infiltrating lymphocytes in
ccRCC. Am J Cancer Res. 11:5965–5978. 2021.PubMed/NCBI
|
|
48
|
Wang Z, Li K, Chen W, Wang X, Huang Y,
Wang W, Wu W, Cai Z and Huang W: Modulation of SRSF2 expression
reverses the exhaustion of TILs via the epigenetic regulation of
immune checkpoint molecules. Cell Mol Life Sci. 77:3441–3452. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tan YF, Wang M, Chen ZY, Wang L and Liu
XH: Inhibition of BRD4 prevents proliferation and
epithelial-mesenchymal transition in renal cell carcinoma via NLRP3
inflammasome-induced pyroptosis. Cell Death Dis. 11:2392020.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Arany Z, Huang LE, Eckner R, Bhattacharya
S, Jiang C, Goldberg MA, Bunn HF and Livingston DM: An essential
role for p300/CBP in the cellular response to hypoxia. Proc Natl
Acad Sci USA. 93:12969–12973. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yang XJ, Ogryzko VV, Nishikawa J, Howard
BH and Nakatani Y: A p300/CBP-associated factor that competes with
the adenoviral oncoprotein E1A. Nature. 382:319–324. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Pawlus MR, Wang L and Hu CJ: STAT3 and
HIF1α cooperatively activate HIF1 target genes in MDA-MB-231 and
RCC4 cells. Oncogene. 33:1670–1679. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jung JE, Lee HG, Cho IH, Chung DH, Yoon
SH, Yang YM, Lee JW, Choi S, Park JW, Ye SK and Chung MH: STAT3 is
a potential modulator of HIF-1-mediated VEGF expression in human
renal carcinoma cells. FASEB J. 19:1296–1298. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lee SH, Kang JH, Ha JS, Lee JS, Oh SJ,
Choi HJ, Song J and Kim SY: Transglutaminase 2-mediated p53
depletion promotes angiogenesis by increasing HIF-1α-p300 binding
in renal cell carcinoma. Int J Mol Sci. 21:50422020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Datta K, Li J, Bhattacharya R, Gasparian
L, Wang E and Mukhopadhyay D: Protein kinase C zeta transactivates
hypoxia-inducible factor alpha by promoting its association with
p300 in renal cancer. Cancer Res. 64:456–462. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lou F, Chen X, Jalink M, Zhu Q, Ge N, Zhao
S, Fang X, Fan Y, Björkholm M, Liu Z and Xu D: The opposing effect
of hypoxia-inducible factor-2alpha on expression of telomerase
reverse transcriptase. Mol Cancer Res. 5:793–800. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zeng X, Chen K, Li L, Tian J, Ruan W, Hu
Z, Peng D and Chen Z: Epigenetic activation of RBM15 promotes clear
cell renal cell carcinoma growth, metastasis and macrophage
infiltration by regulating the m6A modification of CXCL11. Free
Radic Biol Med. 184:135–147. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kushal S, Lao BB, Henchey LK, Dubey R,
Mesallati H, Traaseth NJ, Olenyuk BZ and Arora PS: Protein domain
mimetics as in vivo modulators of hypoxia-inducible factor
signaling. Proc Natl Acad Sci USA. 110:15602–15607. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Latif AL, Newcombe A, Li S, Gilroy K,
Robertson NA, Lei X, Stewart HJS, Cole J, Terradas MT, Rishi L, et
al: BRD4-mediated repression of p53 is a target for combination
therapy in AML. Nat Commun. 12:2412021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Civenni G, Bosotti R, Timpanaro A, Vàzquez
R, Merulla J, Pandit S, Rossi S, Albino D, Allegrini S, Mitra A, et
al: Epigenetic control of mitochondrial fission enables
self-renewal of stem-like tumor cells in human prostate cancer.
Cell Metab. 30:303–318.e6. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Weissman JD, Singh AK, Devaiah BN, Schuck
P, LaRue RC and Singer DS: The intrinsic kinase activity of BRD4
spans its BD2-B-BID domains. J Biol Chem. 297:1013262021.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Tewary P, Brooks AD, Xu YM, Wijeratne EMK,
Babyak AL, Back TC, Chari R, Evans CN, Henrich CJ, Meyer TJ, et al:
Small-molecule natural product physachenolide C potentiates
immunotherapy efficacy by targeting BET proteins. Cancer Res.
81:3374–3386. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Delmore JE, Issa GC, Lemieux ME, Rahl PB,
Shi J, Jacobs HM, Kastritis E, Gilpatrick T, Paranal RM, Qi J, et
al: BET bromodomain inhibition as a therapeutic strategy to target
c-Myc. Cell. 146:904–917. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sakaguchi T, Yoshino H, Sugita S, Miyamoto
K, Yonemori M, Osako Y, Meguro-Horike M, Horike SI, Nakagawa M and
Enokida H: Bromodomain protein BRD4 inhibitor JQ1 regulates
potential prognostic molecules in advanced renal cell carcinoma.
Oncotarget. 9:23003–23017. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Xu M, Xu L, Wang Y, Dai G, Xue B, Liu YY
and Zhu J and Zhu J: BRD4 inhibition sensitizes renal cell
carcinoma cells to the PI3K/mTOR dual inhibitor VS-5584. Aging
(Albany NY). 12:19147–19158. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Xing ZY, Wang Y, Cheng L, Chen J, He XZ
and Xing W: Bromodomain-containing protein 4 (BRD4) inhibition
sensitizes palomid 529-induced anti-renal cell carcinoma cell
activity in vitro and in vivo. Cell Physiol Biochem. 50:640–653.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhu H, Mao JH, Wang Y, Gu DH, Pan XD, Shan
Y and Zheng B: Dual inhibition of BRD4 and PI3K-AKT by SF2523
suppresses human renal cell carcinoma cell growth. Oncotarget.
8:98471–98481. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Xu YY, Ren ZL, Liu XL, Zhang GM, Huang SS,
Shi WH, Ye LX, Luo X, Liu SW, Li YL and Yu L: BAP1 loss augments
sensitivity to BET inhibitors in cancer cells. Acta Pharmacol Sin.
43:1803–1815. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Hsieh JJ, Chen D, Wang PI, Marker M,
Redzematovic A, Chen YB, Selcuklu SD, Weinhold N, Bouvier N,
Huberman KH, et al: Genomic biomarkers of a randomized trial
comparing first-line everolimus and sunitinib in patients with
metastatic renal cell carcinoma. Eur Urol. 71:405–414. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Feng L, Wang G, Chen Y, He G, Liu B, Liu
J, Chiang CM and Ouyang L: Dual-target inhibitors of bromodomain
and extra-terminal proteins in cancer: A review from medicinal
chemistry perspectives. Med Res Rev. 42:710–743. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Pivot-Pajot C, Caron C, Govin J, Vion A,
Rousseaux S and Khochbin S: Acetylation-dependent chromatin
reorganization by BRDT, a testis-specific bromodomain-containing
protein. Mol Cell Biol. 23:5354–5365. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wan P, Chen Z, Zhong W, Jiang H, Huang Z,
Peng D, He Q and Chen N: BRDT is a novel regulator of eIF4EBP1 in
renal cell carcinoma. Oncol Rep. 44:2475–2486. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zahid H, Olson NM and Pomerantz WCK:
Opportunity knocks for uncovering the new function of an
understudied nucleosome remodeling complex member, the bromodomain
PHD finger transcription factor, BPTF. Curr Opin Chem Biol.
63:57–67. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wysocka J, Swigut T, Xiao H, Milne TA,
Kwon SY, Landry J, Kauer M, Tackett AJ, Chait BT, Badenhorst P, et
al: A PHD finger of NURF couples histone H3 lysine 4 trimethylation
with chromatin remodelling. Nature. 442:86–90. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Nayak A, Dutta M and Roychowdhury A:
Emerging oncogene ATAD2: Signaling cascades and therapeutic
initiatives. Life Sci. 276:1193222021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Liu H, Wen Q, Yan S, Zeng W, Zou Y, Liu Q,
Zhang G, Zou J and Zou X: Tumor-promoting ATAD2 and its preclinical
challenges. Biomolecules. 12:10402022. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Chen D, Maruschke M, Hakenberg O,
Zimmermann W, Stief CG and Buchner A: TOP2A, HELLS, ATAD2, and TET3
are novel prognostic markers in renal cell carcinoma. Urology.
102:265.e1–265.e7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Hsieh JJ, Le VH, Oyama T, Ricketts CJ, Ho
TH and Cheng EH: Chromosome 3p loss-orchestrated VHL, HIF, and
epigenetic deregulation in clear cell renal cell carcinoma. J Clin
Oncol. Oct 29–2018.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Jonasch E, Walker CL and Rathmell WK:
Clear cell renal cell carcinoma ontogeny and mechanisms of
lethality. Nat Rev Nephrol. 17:245–261. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Carril-Ajuria L, Santos M, Roldán-Romero
JM, Rodriguez-Antona C and de Velasco G: Prognostic and predictive
value of PBRM1 in clear cell renal cell carcinoma. Cancers (Basel).
12:162019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Thompson M: Polybromo-1: The chromatin
targeting subunit of the PBAF complex. Biochimie. 91:309–319. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Brownlee PM, Chambers AL, Oliver AW and
Downs JA: Cancer and the bromodomains of BAF180. Biochem Soc Trans.
40:364–369. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Peña-Llopis S, Vega-Rubín-de-Celis S, Liao
A, Leng N, Pavía-Jiménez A, Wang S, Yamasaki T, Zhrebker L,
Sivanand S, Spence P, et al: BAP1 loss defines a new class of renal
cell carcinoma. Nat Genet. 44:751–759. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Espana-Agusti J, Warren A, Chew SK, Adams
DJ and Matakidou A: Loss of PBRM1 rescues VHL dependent replication
stress to promote renal carcinogenesis. Nat Commun. 8:20262017.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Gu YF, Cohn S, Christie A, McKenzie T,
Wolff N, Do QN, Madhuranthakam AJ, Pedrosa I, Wang T, Dey A, et al:
Modeling renal cell carcinoma in mice: Bap1 and Pbrm1 inactivation
drive tumor grade. Cancer Discov. 7:900–917. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Liu W, Zhang B, Zhang D, Guo F, Ye K, Zhu
L and Jin X: The RBPJ/DAPK3/UBE3A signaling axis induces PBRM1
degradation to modulate the sensitivity of renal cell carcinoma to
CDK4/6 inhibitors. Cell Death Dis. 13:2952022. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Nargund AM, Pham CG, Dong Y, Wang PI,
Osmangeyoglu HU, Xie Y, Aras O, Han S, Oyama T, Takeda S, et al:
The SWI/SNF protein PBRM1 restrains VHL-loss-driven clear cell
renal cell carcinoma. Cell Rep. 18:2893–2906. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Gao W, Li W, Xiao T, Liu XS and Kaelin WG
Jr: Inactivation of the PBRM1 tumor suppressor gene amplifies the
HIF-response in VHL-/- clear cell renal carcinoma. Proc Natl Acad
Sci USA. 114:1027–1032. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Zhou M, Leung JY, Gessner KH, Hepperla AJ,
Simon JM, Davis IJ and Kim WY: PBRM1 inactivation promotes
upregulation of human endogenous retroviruses in a HIF-dependent
manner. Cancer Immunol Res. 10:285–290. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Chowdhury B, Porter EG, Stewart JC,
Ferreira CR, Schipma MJ and Dykhuizen EC: PBRM1 regulates the
expression of genes involved in metabolism and cell adhesion in
renal clear cell carcinoma. PLoS One. 11:e01537182016. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Porter EG, Dhiman A, Chowdhury B, Carter
BC, Lin H, Stewart JC, Kazemian M, Wendt MK and Dykhuizen EC: PBRM1
regulates stress response in epithelial cells. iScience.
15:196–210. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Braun DA, Hou Y, Bakouny Z, Ficial M,
Sant' Angelo M, Forman J, Ross-Macdonald P, Berger AC, Jegede OA,
Elagina L, et al: Interplay of somatic alterations and immune
infiltration modulates response to PD-1 blockade in advanced clear
cell renal cell carcinoma. Nat Med. 26:909–918. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Xia QY, Rao Q, Cheng L, Shen Q, Shi SS, Li
L, Liu B, Zhang J, Wang YF, Shi QL, et al: Loss of BRM expression
is a frequently observed event in poorly differentiated clear cell
renal cell carcinoma. Histopathology. 64:847–862. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Guerrero-Martinez JA and Reyes JC: High
expression of SMARCA4 or SMARCA2 is frequently associated with an
opposite prognosis in cancer. Sci Rep. 8:20432018. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Fang R, Xia Q, Sun J, Ng HZ, Liang Y, Wang
X, Wang X, Ma H, Zhou X, Cheng Y and Rao Q: Hypermethylation of BRM
promoter plays oncogenic roles in development of clear cell renal
cell carcinoma. J Cancer. 10:5256–5263. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Fang R, Pan R, Wang X, Liang Y, Wang X, Ma
H, Zhou X, Xia Q and Rao Q: Inactivation of BRM/SMARCA2 sensitizes
clear cell renal cell carcinoma to histone deacetylase complex
inhibitors. Pathol Res Pract. 216:1528672020. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Schuetz A, Bernstein G, Dong A, Antoshenko
T, Wu H, Loppnau P, Bochkarev A and Plotnikov AN: Crystal structure
of a binary complex between human GCN5 histone acetyltransferase
domain and acetyl coenzyme A. Proteins. 68:403–407. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Ononye OE and Downey M: Posttranslational
regulation of the GCN5 and PCAF acetyltransferases. PLoS Genet.
18:e10103522022. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Guo Y, Liu B, Liu Y, Sun W, Gao W, Mao S
and Chen L: Oncogenic chromatin modifier KAT2A activates MCT1 to
drive the glycolytic process and tumor progression in renal cell
carcinoma. Front Cell Dev Biol. 9:6907962021. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Vetting MW, de Carvalho LP, Yu M, Hegde
SS, Magnet S, Roderick SL and Blanchard JS: Structure and functions
of the GNAT superfamily of acetyltransferases. Arch Biochem
Biophys. 433:212–226. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Lu J, Qian C, Ji Y, Bao Q and Lu B: Gene
signature associated with bromodomain genes predicts the prognosis
of kidney renal clear cell carcinoma. Front Genet. 12:6439352021.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Shanmugasundaram K, Nayak BK, Friedrichs
WE, Kaushik D, Rodriguez R and Block K: NOX4 functions as a
mitochondrial energetic sensor coupling cancer metabolic
reprogramming to drug resistance. Nat Commun. 8:9972017. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Xu Y, Wu G, Zhang J, Li J, Ruan N, Zhang
J, Zhang Z, Chen Y, Zhang Q and Xia Q: TRIM33 overexpression
inhibits the progression of clear cell renal cell carcinoma in vivo
and in vitro. Biomed Res Int. 2020:84092392020.PubMed/NCBI
|
|
104
|
Lu K, Pan Y, Huang Z, Liang H, Ding ZY and
Zhang B: TRIM proteins in hepatocellular carcinoma. J Biomed Sci.
29:692022. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Jingushi K, Ueda Y, Kitae K, Hase H, Egawa
H, Ohshio I, Kawakami R, Kashiwagi Y, Tsukada Y, Kobayashi T, et
al: miR-629 targets TRIM33 to promote TGFβ/Smad signaling and
metastatic phenotypes in ccRCC. Mol Cancer Res. 13:565–574. 2015.
View Article : Google Scholar : PubMed/NCBI
|