Endometrial cancer (EC) originates from the
endometrium and is one of the most common cancers in females
worldwide, accounting for 7% of all new cancer diagnoses and
leading to 4% of all estimated cancer deaths in 2022 (1). The incidence and death rates of EC
appear to have been leveling off in recent years after two decades
of increase since 1997 (1). Given
that EC mainly affects peri- and postmenopausal women, the cancer
burden of EC is likely to remain incremental due to an increase in
the adult and aging populations (2). Surgery is the primary treatment of
EC, which is accompanied by adjuvant therapies, such as
chemotherapy, followed by external beam pelvic radiotherapy and
vaginal brachytherapy. The majority of EC patients who had
undergone surgery and adjuvant therapies based on
clinicopathological characteristics, had a favorable prognosis with
a 76–95% 5-year survival rate (3).
However, since the pathogenesis of EC has not been fully
elucidated, effective treatment is deficient for advanced and
recurrent EC creating a need to explore new targets and develop new
screening methods.
The Wnt signaling pathway is a highly conserved axis
participating in various physiological and pathological processes
(4). Wnt1 was first discovered in
1982 by Dr Roel Nusse (5), after
which several other Wnt family proteins were identified, and their
functions were studied in further detail. The Wnt signaling pathway
is divided into two categories, the canonical pathway
(β-catenin-dependent) and the non-canonical pathway
(β-catenin-independent). The non-canonical pathways mediate cell
polarity and regulate intracellular levels of calcium, while the
canonical Wnt pathway is closely related to the tumorigenesis,
progression, and prognosis of certain solid tumors, including EC
(6,7).
Previous studies have confirmed that β-catenin is
the main positive mediator that activates selected genes and plays
essential roles in embryonic development, tissue homeostasis and
regeneration (4,8,9).
Previous studies that focused on the Wnt/β-catenin pathway in EC,
evaluated the role of Catenin beta 1 (CTNNB1) gene mutation, which
encodes for the β-catenin and also excessively activates the
Wnt/β-catenin pathway. CTNNB1 mutation frequently occurs in
endometrioid types of ECs (EECs) and is the most common mutation in
all early-stage and low-grade EC patients. Although these subsets
of EC patients tend to have low-risk characteristics, the presence
of CTNNB1 mutation is associated wuth worse outcomes with decreased
recurrence-free survival and overall survival (10–12).
Furthermore, other components of the Wnt/β-catenin pathway, and
their crosstalk with other signaling pathways have been determined
to occur in EC (13–17).
Non-coding RNAs (ncRNAs) are a class of functional
RNAs that play critical roles in normal cellular processes, as well
as in the pathogenesis of human diseases, including long non-coding
RNA (lncRNA), microRNA (miRNA), and circular RNA (circRNA)
(18–20). RNA-RNA interaction plays a
fundamental role at multiple levels of gene expression and
regulation (20,21). RNA transcripts containing miRNA
binding sites (also known as seed sequence) can act as a competing
endogenous RNA (ceRNA) specifically for shared miRNAs,
co-regulating with each other, and integrating ncRNAs with the
protein-coding RNA (21).
Dysregulation of a variety of ncRNAs expression and
the associated ceRNA network have been reported to engage in the
genesis and progress of various malignancies, including EC. For
example, lncRNA NEAT1 was reported to be abnormally expressed in
several cancers, and to promote cell proliferation, migration and
invasion of EC cells by sponging miR-214-3p via the
HMGA1/Wnt/β-catenin pathway (22–24).
LncRNA BMPR1B-AS1 was overexpressed in EC tissues, and exerted an
oncogenic role by competitively binding to miR-7-2-3p to modulate
the DCLK1-induced PI3K/Akt/NF-κB pathway activation (25). Also, the aberrant expression of a
series of cirRNAs has been identified as oncogenic drivers or
tumour suppressors in EC. For example, circ_0039569, circ_0007534,
circ_0005797, circ_0001610 and more were found to affect cell
proliferation, metastasis, invasion, drug-resistance and the
radiosensitivity of EC cells (26–29).
In the present review, a brief overview of the
non-canonical pathway is provided with a focus on the role of the
canonical pathway in EC. Next, the Wnt/β-catenin signaling pathway
was associated with the RNA network to further elucidate the
mechanisms of initiation and progression of EC, aiming to provide
new insights into EC prevention and intervention by utilizing
potential targets.
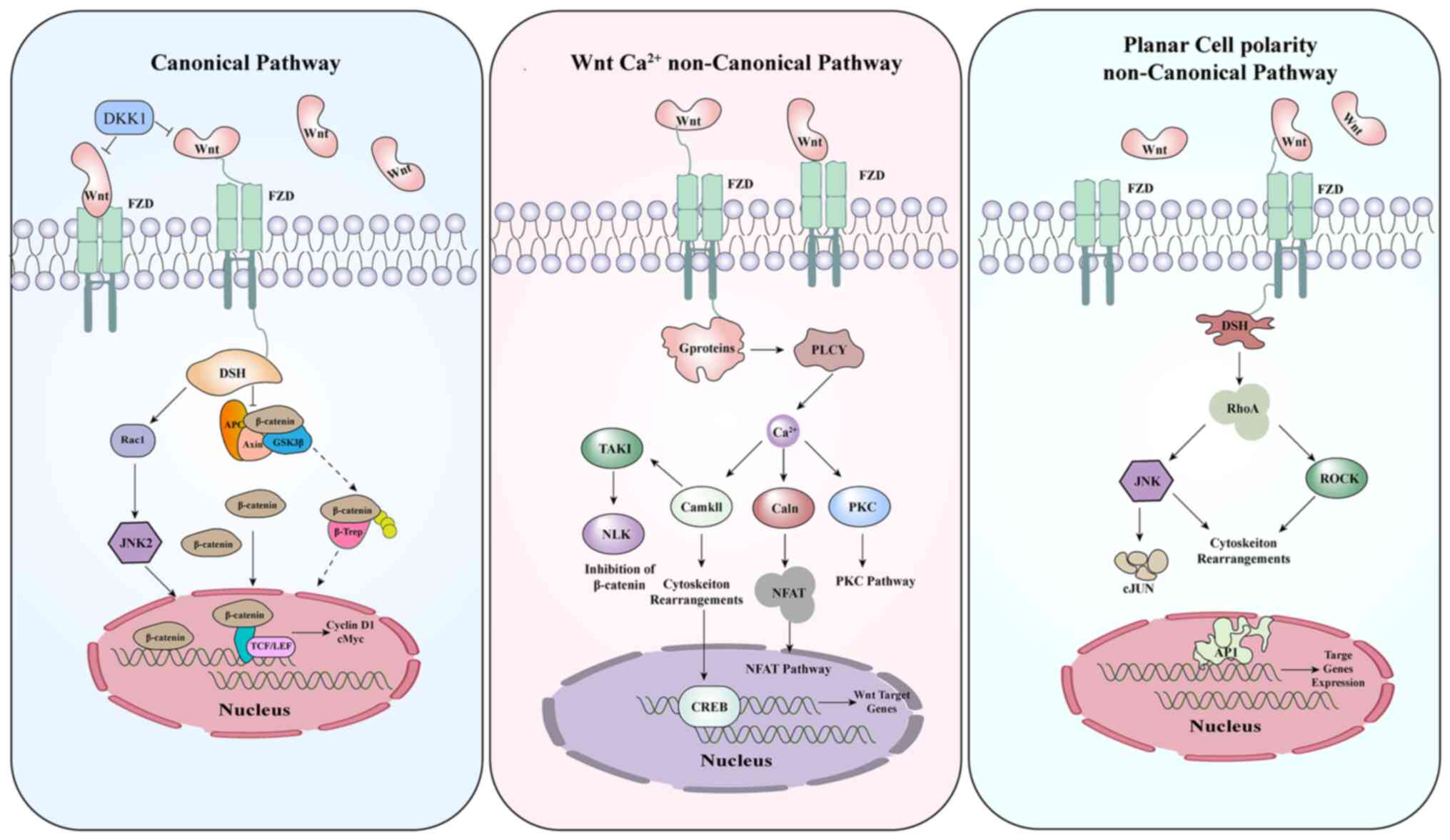
The Wnt signaling pathway is divided into two
categories, the canonical pathway (β-catenin-dependent) and the
non-canonical pathway (β-catenin-independent) (6,7)
(Fig. 1). The non-canonical
pathway regulates intracellular calcium levels and modulates cell
polarity. However, the canonical Wnt pathway has more association
with tumorigenesis, progression, and prognosis of certain solid
tumors, including EC.
The non-canonical Wnt signaling pathway includes the
PCP pathway and calcium-dependent Wnt pathways. There are six core
components involved in this pathway: i) Frizzled (FZD), ii)
Flamingo (Fmi, also known as Stan, Celsr in vertebrates), iii)
Vang-like (Vangl), iv) Dishevelled (Dsh; Dishevelled-like (DVL) in
vertebrates), v) Prickle (Pk) and vi) Diego (Dgo; also known as
Inversin and Diversin in vertebrates) (30–34).
FZD, Celsr and Vangl are transmembrane proteins, while DVL, Pk and
Diversin are cytoplasmic proteins. Upon interacting with these
proteins, the small Rho GTPase effector molecules, c-Jun N-terminal
kinase (JNK), and Nemo-like kinase (NLK) are activated (34–37).
These processed lead to the asymmetric distribution of the PCP/Wnt
signaling pathway proteins that consequently influence the cell
polarity (34) (Fig. 1).
Several studies have confirmed that the aberrant
regulations of the PCP/Wnt signaling pathway are correlated with
developmental abnormalities and diseases including Kartagener's
syndrome, open neural tube defects, deafness, heart defects and
polycystic kidneys (38–42). Previous studies have also indicated
that the upregulation of the PCP/Wnt signaling pathway is
associated with poor prognosis in multiple cancers (43,44).
Luga et al (45) reported
that exosomes derived from breast cancer fibroblasts could activate
the Wnt11/PCP signaling, consequently promoting an invasive
behavior. As a result of this process, asymmetric distribution of
the PCP/Wnt signaling pathway proteins were observed in cancer
cells.
In addition, disruption of the Prickle1-Rictor
complex may have the ability to inhibit breast cancer migration,
while the upregulation of this complex was associated with poor
prognosis (46,47). Notably, the PCP/Wnt signaling
pathway is also highly associated with the epithelial-mesenchymal
transition (EMT), which plays a vital role in endometrial
carcinogenesis (34,48,49).
Studies have also indicated that Wnt5A and Wnt11 could initiate the
PCP/Wnt signaling pathway, while Wnt5A was reported as a tumor
suppressor in multiple cancers. Wasniewski et al (50) reported that the expression of Wnt5A
was decreased in patients with EC, thus, could be a potential
marker in EC. However, the precise role of interaction between the
PCP/Wnt signaling pathway and EMT-promoting endometrial
carcinogenesis still requires further investigation.
Unlike the PCP/Wnt signaling pathway, the
calcium-dependent Wnt signaling pathway regulates the expression of
selected gene targets by modulating intracellular calcium ion
homeostasis. It has been confirmed that the binding of Wnt5A to
Frizzled and activation of receptor tyrosine kinase orphan-like
receptor 2 (ROR2) tyrosine kinase suppresses the canonical
Wnt/β-catenin signaling pathway (51). In response to DVL and G proteins,
phospholipase C is activated, resulting in an increase in
diacylglycerol (DAG), inositol 1,4,5-triphosphate (IP3), and
intracellular calcium (34).
Calcium is a universal second messenger responsible for the
activation of calcium calmodulin-dependent protein kinase II
(CaMKII) and protein kinase C (PKC). CaMKII and PKC subsequently
activate downstream signaling molecules such as NFκB and CREB
(34). In addition, CaMKII and PKC
may play suppressive roles in regulating β-catenin (52).
Previous studies have also demonstrated that Wnt5A
initiates the calcium-dependent Wnt signaling pathway (34). Moreover, although Wnt5A may act as
a tumor suppressor in multiple cancers, Wnt5A functions as either a
proto-oncogene or a tumor suppressor depending on the cell type and
receptor availability (53–56).
Zmarzly et al (57)
reported that Wnt2, Wnt4 and Wnt5A were involved in the EMT process
and were significantly decreased in EC. In addition, Wnt5A may also
be regulated by miR-370, miR-432 and miR-200b-5p. However, the role
of calcium remains unclear and needs further investigation
(Fig. 1).
The activation of the β-catenin-dependent Wnt
signaling pathway depends on the sequential action of its
components. In brief, firstly, extracellular Wnt proteins, like
Wnt1 and Wnt3a, bind to the transmembrane coreceptors, which are
mainly comprised of FZD and low-density lipoprotein
receptor-related protein 5 or 6 (LPR5/6). With the ligation of both
segments, the DVL scaffolding protein is recruited to the plasma
membrane. Next, DVL phosphorylates LPR6 and dissociates the
‘destruction complex’, which consists of adenomatous polyposis coli
(APC), AXIN, casein kinase 1 (CK1), and glycogen synthase kinase 3
protein (GSK3), to stabilize β-catenin. Then, the cytoplasm
accumulated-β-catenin translocates to the nucleus and eventually
cooperates with the T cell-specific factor (TCF)/lymphoid
enhancer-binding factor (LEF) transcription factors to induce the
transcription of targeted genes, including CCND1, c-MYC and MMPs.
Conversely, β-catenin is sequestrated by the ‘destruction complex’
in the absence of Wnt. Subsequently, β-catenin is phosphorylated by
GSK3β and CK1α, promoting its ubiquitination and subsequent
proteasomal degradation (4,6,7,58)
(Fig. 1).
As the hyperactivation of the Wnt/β-catenin pathway
is closely associated with the tumorigenesis of EC, mutations of
CTNNB1 are linked to the carcinogenesis and progression of EC.
Therefore, mutations to CTNNB1 translate to clinicopathological and
molecular characteristics of EC (10,12,14).
In grade 1–2, stage I–II EECs, patients with a mutation to the
tumor harboring CTNNB1 had lower-grade tumours, lesser myometrial
invasion, a lower incidence of lymphatic/vascular space invasion,
and a lower frequency of co-TP53 mutation. While these mutations
are associated with more positive outcomes, they also increased the
risk of recurrence (59).
Another study that included 218 low-grade,
early-stage EECs confirmed that tumors with the CTNNB1 mutation are
associated with reduced disease-free survival, without impacting
overall survival (60).
Nevertheless, Kasoha et al (16) reported that patients with CTNNB1
mutations make up an aggressive subset of low-risk EECs with both
poorer progression-free survival and overall survival. Therefore,
mutations to CTNNB1 have the potential to stratify EC into a
prognostic group that requires additional therapeutic
interventions.
The levels of sensitivity and specificity of
immunohistochemical staining of β-catenin as an effective surrogate
to CTNNBI gene sequencing remains uncertain (10,13–14,61).
Individual hyperactivation of the Wnt/β-catenin pathway is
insufficient to stimulate the initiation of EC. The malignant
transformation from endometrial hyperplasia to EC only occurs when
alterations in the Wnt/β-catenin and the loss of PTEN or unopposed
estrogen are simultaneously present (15,62).
Moreover, β-catenin also serves as an adhesion protein by linking
E-cadherin and the actin cytoskeleton (63). Although the dual function of
β-catenin appears to be independent of each other, they work
together to maintain the balance of β-catenin in the cytoplasm,
cell membrane and nucleus.
The Wnt/β-catenin is also recognized as a key
regulator of EMT, by directly or indirectly regulating numerous EMT
markers, including Zeb1, Twist, Snail1 and Slug (64). In another process, the
transcription factors Twist, Snail1 and Zeb1 co-suppress E-cadherin
expression (65,66). Loss of E-cadherin and increased
Wnt/β-catenin induce EMT in carcinomas and the development of EC,
with the exact mechanism yet to be fully understood. Based on
available evidence that the aberrant Wnt/β-catenin signaling
pathway is widely involved in the progression of EC, targeting the
Wnt/β-catenin pathway is a prospective choice for late-stage and
recurrent EC patients (67,68).
ncRNAs (including lncRNAs, miRNAs and circRNAs)
consist of >90% of the human transcripts and exhibit limited
protein-coding capacity (69,70).
However, these ncRNAs mainly participate in and regulate epigenetic
modifications, cell differentiation, aging, and cell cycles by
regulating the expression of target genes expression at
post-transcriptional level (71,72).
An increasing number of studies have indicated that
aberrant expression and dysregulation of these ncRNAs are highly
linked with a variety of malignant tumors in human through several
mechanisms, including tumor autophagy, tumor resistance and tumor
immunity (73–75). Therefore, ncRNAs have dual roles as
oncogenes and tumor suppressors (76). Consequently, they have been
identified as potential biomarkers for cancers including EC
(77–79).
The Wnt signaling pathway has been proven to
function as a key pathway participating in the carcinogenesis of EC
(7,80,81).
A growing number of studies have revealed that ncRNAs could promote
or inhibit EC tumorigenesis and progression by targeting the Wnt
signaling pathway proteins (81).
To elucidate the specific role of crosstalks between ncRNAs and the
Wnt signaling pathway in EC, the available literature was
summarized. The role of ncRNAs and their target genes are listed in
Table I.
miR-15a-5p was previously reported to be
significantly decreased in human EC cells and tissues (82). Overexpression of miR-15a-5p could
inhibit the proliferation of EC cells and downregulate Cyclin D1and
p21 by binding the octamer-binding transcription factor 4 (OCT-4),
SRY-box transcription factor 2 (SOX2) and Nanog. In addition,
miR-15a-5p could inhibit Wnt3a expression by directly binding with
Wnt3a's 3′untranslated region. These mechanisms indicated that
miR-15a-5p acts as a suppressor in ECs by inhibiting the
Wnt/β-catenin signaling pathway.
More recently, lncRNA MIR210HG was found to be
upregulated in EC tissues compared with normal endometrial tissues
and was associated with poor prognosis (49). Knockdown of the MIR210HG inhibits
Wnt/β-catenin and the TGF-β pathway via the miR-337-3p/137-HMGA2
axis. Liu et al (87)
reported that lncRNA HOXB-AS1 expression was significantly higher
in EC than that in adjacent normal tissues. In addition, the
overexpression of lncRNA HOXB-AS1 promotes the proliferation,
migration and invasion of EC cells and was associated with shorter
survival. Through a certain mechanism, lncRNA HOXB-AS1 also
decreased Wnt10b, β-catenin, cyclin D1, and c-Myc expression by
targeting miR-149-3p. Wnt3a is an important member of the Wnt
family, which has been confirmed to participate in the development
and progression of multiple cancers including EC (88–90).
A recent study has indicated that lncRNA LSINCT5 promoted the
proliferation and invasion of EC cells by activating the
Wnt3a/β-catenin/c-Myc signaling pathway via HGMA2 (90).
Overexpression of miR-373 also promotes the
proliferation, migration and invasion of EC cells by directly
targeting LATS2 and upregulating β-catenin (94). Sun et al (95) reported that the expression of
miR-652 was increased in human EC tissues, which promoted their
proliferation, migration and invasion by targeting RORA (Retinoic
acid receptor-related orphan receptor A). The concurrent
overexpression of miR-652 and knockdown of RORA upregulates the
expression of β-catenin. These outcomes indicated that the
activation of the miR-652/RORA/β-catenin axis could promote EC.
A previous study indicated that lnc NEAT1 was
overexpressed in EC, which promoted the proliferation, migration
and invasion of EC cells (23).
Previous studies have indicated that lnc NEAT1 targets miR-214-3p
and miR146b-5p which are involved in EC by regulating the
Wnt/β-catenin signaling pathway (22,96).
Overexpression of lnc NEAT1 leads to a decreased amount of
miR-214-3p and miR-146b-5p, which in turn upregulates c-Myc and
MMP9. In addition, progesterone could suppress EC progression by
inhibiting c-Myc and MMP9. These results indicated that lnc NEAT1
acts as an oncogene while miR-214-3p and miR-146b-5p serve as tumor
suppressors.
It is thus evident that the Wnt/β-catenin signaling
pathway plays a vital role in the development and progression of
EC. Therapeutic agents targeting the Wnt/β-catenin signaling
pathway have gradually become a research focus (4,6,81).
Based on their varied mechanisms of action, drugs targeting the
Wnt/β-catenin signaling pathway can be divided into several
classes, including porcupine (PORCN) inhibitors, monoclonal
antibodies against FZD, FZD8 decoy receptors, CBP/β-catenin
antagonists and DKN-01 (81,97,98).
These were illustrated in Table
II.
PORCN inhibitors (such as LGK974, ETC-159 or
CGX1321) prevent the palmitoylation of Wnt proteins, which in turn
inhibits its secretion (7). LGK974
as a drug for numerous advanced solid tumors has completed phase I
clinical trials (NCT01351103, NCT02278133). However, due to
bone-related toxicities, the efficacy and safety of LGK974 needs
further study (99–101). ETC-159, another PORCN inhibitor,
prevents the secretion and blocks function of Wnt proteins,
suggesting that ETC-159 could be an effective therapeutic agent for
EC (81,102). A phase I clinical trial to
evaluate the safety and tolerability of ETC-159 (NCT02521844) for
different solid malignancies is in progress (103).
OMP-18R5 is a monoclonal antibody against FZD and
inhibits the canonical Wnt signaling pathway. Similar to LGK974,
concerns are emerging around the bone-related safety of OMP-18R5
which has become a major obstacle for future clinical use
(NCT02050178, NCT02278133) (104–106). OMP-54F28 is composed of the IgG1
Fc and the extracellular ligand-binding FZD8 domains and exhibits
an antitumor effect in several cancers by sequestering secreted
Wnts (107,108). Several phase I clinical trials
have indicated OMP-54F28 might be an effective agent to target the
Wnt signaling pathway in advanced solid tumors, including
colorectal and pancreatic cancer (NCT01608867, NCT02092363,
NCT02050178) (109–111).
PRI-724, an inhibitor of the downstream
Wnt/β-catenin pathway, reduces the expression of
β-catenin-TCF-responsive genes by targeting the complex formation
of β-catenin and CBP (NCT01606579, NCT01764477) (81,112). Dickkopf −1(DKK-1) is a Wnt
signaling modulator overexpressed in gynecologic cancers. DKN-01 is
a humanized monoclonal antibody with DKK1 neutralizing activity.
DKN-01 was applied in multiple myeloma (NCT01457417) (113). On September 25, 2020, the Food
and Drug Administration granted accelerated approval to DKN-01 for
gastric or gastroesophageal junction adenocarcinoma. Notably, a
phase II basket study indicated a promising clinical activity of
DKN-01 in EC patients with high DKK1 expression (NCT03395080)
(114). Meanwhile, a combination
therapy consisting of DKN-01 and pembrolizumab is currently being
evaluated in clinical trials for advanced or recurrent EC
(NCT05761951).
In addition, another study indicated that
medroxyprogesterone acetate suppresses the proliferation of early
endometrial carcinoma by inactivating the Wnt/β-catenin signaling
pathway (115), followed by the
evidence that when therapy was halted, a marked recurrence was
reported (116). Preclinically,
niclosamide, salinomycin and curcumin have all been proven to
interfere with the Wnt/β-catenin signaling pathway in cancer cells
(117–119).
Based on these promising roles of ncRNAs, numerous
ncRNAs are expected to become potential therapeutic targets in the
near future (120). Clinical
trials of ncRNAs based therapies are currently underway and are
already exhibiting prospective clinical applications (120,121).
Abnormal activation of the Wnt/β-catenin signaling
pathway and β-catenin mutation contributes to the development and
progression of various cancers including gynecological cancers.
Numerous therapies that target the Wnt/β-catenin signaling are
currently tested in clinical trials in various cancers and have
demonstrated promising outcomes.
Although the molecular mechanism of the
Wnt/β-catenin signaling pathway in EC remains unclear, accumulating
evidence indicates that the crosstalk that occurs between ncRNAs
and the Wnt/β-catenin signaling pathway play significant roles in
drug resistance, metastasis and recurrence (7,81).
The present review focused on the interaction between the
lncRNA/circRNA-miRNA network and the Wnt/β-catenin signaling
pathway related proteins associated with EC. ncRNAs may serve as
potential targets for EC treatments. However, there are still a
large number of uncharacterized ncRNAs. The role of ncRNAs'
interaction with the Wnt/β-catenin signaling as targeting therapy
still needs further investigation.
Not applicable.
The present study was supported by the Joint Funds of the
National Science Foundation of China (grant no. U2004117) and the
Henan Medical Science and Technology Research Program (grant no.
LHGJ20220359).
Not applicable.
YT and RG were responsible for the concept of the
review. YT and TL were responsible for writing the manuscript. YT
and ZL made all the figures in this manuscript. ZL, MM, YL and YJ
revised the manuscript critically. All authors read and approved
the final manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xia C, Dong X, Li H, Cao M, Sun D, He S,
Yang F, Yan X, Zhang S, Li N and Chen W: Cancer statistics in China
and United States, 2022: Profiles, trends, and determinants. Chin
Med J (Engl). 135:584–590. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yen TT, Wang TL, Fader AN, Shih IM and
Gaillard S: Molecular classification and emerging targeted therapy
in endometrial cancer. Int J Gynecol Pathol. 39:26–35. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang
X, Zhou Z, Shu G and Yin G: Wnt/β-catenin signalling: Function,
biological mechanisms, and therapeutic opportunities. Signal
Transduct Target Ther. 7:32022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nusse R and Varmus HE: Many tumors induced
by the mouse mammary tumor virus contain a provirus integrated in
the same region of the host genome. Cell. 31:99–109. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu F, Yu C, Li F, Zuo Y, Wang Y, Yao L, Wu
C, Wang C and Ye L: Wnt/β-catenin signaling in cancers and targeted
therapies. Signal Transduct Target Ther. 6:3072021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McMellen A, Woodruff ER, Corr BR, Bitler
BG and Moroney MR: Wnt signaling in gynecologic malignancies. Int J
Mol Sci. 21:42722020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen Y, Chen X, Ji YR, Zhu S, Bu FT, Du
XS, Meng XM, Huang C and Li J: PLK1 regulates hepatic stellate cell
activation and liver fibrosis through Wnt/β-catenin signalling
pathway. J Cell Mol Med. 24:7405–7416. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jung YS, Jun S, Kim MJ, Lee SH, Suh HN,
Lien EM, Jung HY, Lee S, Zhang J, Yang JI, et al: TMEM9 promotes
intestinal tumorigenesis through vacuolar-ATPase-activated
Wnt/β-catenin signalling. Nat Cell Biol. 20:1421–1433. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Parrish ML, Broaddus RR and Gladden AB:
Mechanisms of mutant β-catenin in endometrial cancer progression.
Front Oncol. 12:10093452022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ledinek Z, Sobocan M and Knez J: The Role
of CTNNB1 in endometrial cancer. Dis Markers. 2022:14424412022.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moroney MR, Woodruff E, Qamar L, Bradford
AP, Wolsky R, Bitler BG and Corr BR: Inhibiting Wnt/beta-catenin in
CTNNB1-mutated endometrial cancer. Mol Carcinog. 60:511–523. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pijnenborg JM, Kisters N, van Engeland M,
Dunselman GA, de Haan J, de Goeij AF and Groothuis PG: APC,
beta-catenin, and E-cadherin and the development of recurrent
endometrial carcinoma. Int J Gynecol Cancer. 14:947–956. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moreno-Bueno G, Hardisson D, Sanchez C,
Sarrio D, Cassia R, Garcia-Rostan G, Prat J, Guo M, Herman JG,
Matias-Guiu X, et al: Abnormalities of the APC/beta-catenin pathway
in endometrial cancer. Oncogene. 21:7981–7990. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
van der Zee M, Jia Y, Wang Y,
Heijmans-Antonissen C, Ewing PC, Franken P, Demayo FJ, Lydon JP,
Burger CW, Fodde R and Blok LJ: Alterations in Wnt-beta-catenin and
Pten signalling play distinct roles in endometrial cancer
initiation and progression. J Pathol. 230:48–58. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kasoha M, Dernektsi C, Seibold A, Bohle
RM, Takacs Z, Ioan-Iulian I, Solomayer EF and Juhasz-Boss I:
Crosstalk of estrogen receptors and Wnt/β-catenin signaling in
endometrial cancer. J Cancer Res Clin Oncol. 146:315–327. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen JJ, Xiao ZJ, Meng X, Wang Y, Yu MK,
Huang WQ, Sun X, Chen H, Duan YG, Jiang X, et al: MRP4 sustains
Wnt/beta-catenin signaling for pregnancy, endometriosis and
endometrial cancer. Theranostics. 9:5049–5064. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matsui M and Corey DR: Non-coding RNAs as
drug targets. Nat Rev Drug Discov. 16:167–179. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fitzgerald JB, George J and Christenson
LK: Non-coding RNA in ovarian development and disease. Adv Exp Med
Biol. 886:79–93. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Anastasiadou E, Jacob LS and Slack FJ:
Non-coding RNA networks in cancer. Nat Rev Cancer. 18:5–18. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tay Y, Rinn J and Pandolfi PP: The
multilayered complexity of ceRNA crosstalk and competition. Nature.
505:344–352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang J, Zhao X, Guo Z, Ma X, Song Y and
Guo Y: Regulation of NEAT1/miR-214-3p on the growth, migration and
invasion of endometrial carcinoma cells. Arch Gynecol Obstet.
295:1469–1475. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Z, Wei D, Yang C, Sun H, Lu T and Kuang
D: Overexpression of long noncoding RNA, NEAT1 promotes cell
proliferation, invasion and migration in endometrial endometrioid
adenocarcinoma. Biomed Pharmacother. 84:244–251. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun C, Li S, Zhang F, Xi Y, Wang L, Bi Y
and Li D: Long non-coding RNA NEAT1 promotes non-small cell lung
cancer progression through regulation of miR-377-3p-E2F3 pathway.
Oncotarget. 7:51784–51814. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lai T, Qiu H, Si L, Zhen Y, Chu D and Guo
R: Long noncoding RNA BMPR1B-AS1 facilitates endometrial cancer
cell proliferation and metastasis by sponging miR-7-2-3p to
modulate the DCLK1/Akt/NF-κB pathway. Cell Cycle. 21:1599–1618.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou Y, Pan A, Zhang Y and Li X:
Hsa_circ_0039569 facilitates the progression of endometrial
carcinoma by targeting the miR-197/high mobility group protein A1
axis. Bioengineered. 13:4212–4225. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yi H, Han Y and Li S: Oncogenic circular
RNA circ_0007534 contributes to paclitaxel resistance in
endometrial cancer by sponging miR-625 and promoting ZEB2
expression. Front Oncol. 12:9854702022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu Y, Yuan H and He T: Downregulated
circular RNA hsa_circ_0005797 inhibits endometrial cancer by
modulating microRNA-298/Catenin delta 1 signaling. Bioengineered.
13:4634–4645. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gu X, Shi Y, Dong M, Jiang L, Yang J and
Liu Z: Exosomal transfer of tumor-associated macrophage-derived
hsa_circ_0001610 reduces radiosensitivity in endometrial cancer.
Cell Death Dis. 12:8182021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Adler PN, Krasnow RE and Liu J: Tissue
polarity points from cells that have higher Frizzled levels towards
cells that have lower Frizzled levels. Curr Biol. 7:940–949. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wolff T and Rubin GM: Strabismus, a novel
gene that regulates tissue polarity and cell fate decisions in
Drosophila. Development. 125:1149–1159. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Theisen H, Purcell J, Bennett M, Kansagara
D, Syed A and Marsh JL: dishevelled is required during wingless
signaling to establish both cell polarity and cell identity.
Development. 120:347–360. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gubb D, Green C, Huen D, Coulson D,
Johnson G, Tree D, Collier S and Roote J: The balance between
isoforms of the prickle LIM domain protein is critical for planar
polarity in Drosophila imaginal discs. Genes Dev. 13:2315–2327.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Taciak B, Pruszynska I, Kiraga L, Bialasek
M and Krol M: Wnt signaling pathway in development and cancer. J
Physiol Pharmacol. 69((2))2018.PubMed/NCBI
|
|
35
|
Humphries AC and Mlodzik M: From
instruction to output: Wnt/PCP signaling in development and cancer.
Curr Opin Cell Biol. 51:110–116. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Minegishi K, Hashimoto M, Ajima R, Takaoka
K, Shinohara K, Ikawa Y, Nishimura H, Mcmahon AP, Willert K, Okada
Y, et al: A Wnt5 activity asymmetry and intercellular signaling via
PCP proteins polarize node cells for left-right symmetry breaking.
Dev Cell. 40:439–452.e4. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Katoh M: WNT/PCP signaling pathway and
human cancer (review). Oncol Rep. 14:1583–1588. 2005.PubMed/NCBI
|
|
38
|
Simons M and Mlodzik M: Planar cell
polarity signaling: From fly development to human disease. Annu Rev
Genet. 42:517–540. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Curtin JA, Quint E, Tsipouri V, Arkell RM,
Cattanach B, Copp AJ, Henderson DJ, Spurr N, Stanier P, Fisher EM,
et al: Mutation of Celsr1 disrupts planar polarity of inner ear
hair cells and causes severe neural tube defects in the mouse. Curr
Biol. 13:1129–1133. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Simons M and Walz G: Polycystic kidney
disease: Cell division without a c(l)ue? Kidney Int. 70:854–864.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Garriock RJ, D'Agostino SL, Pilcher KC and
Krieg PA: Wnt11-R, a protein closely related to mammalian Wnt11, is
required for heart morphogenesis in Xenopus. Dev Biol. 279:179–192.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pennekamp P, Menchen T, Dworniczak B and
Hamada H: Situs inversus and ciliary abnormalities: 20 years later,
what is the connection? Cilia. 4:12015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hong CF, Chen WY and Wu CW: Upregulation
of Wnt signaling under hypoxia promotes lung cancer progression.
Oncol Rep. 38:1706–1714. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kurayoshi M, Oue N, Yamamoto H, Kishida M,
Inoue A, Asahara T, Yasui W and Kikuchi A: Expression of Wnt-5a is
correlated with aggressiveness of gastric cancer by stimulating
cell migration and invasion. Cancer Res. 66:10439–10448. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Luga V, Zhang L, Viloria-Petit AM,
Ogunjimi AA, Inanlou MR, Chiu E, Buchanan M, Hosein AN, Basik M and
Wrana JL: Exosomes mediate stromal mobilization of autocrine
Wnt-PCP signaling in breast cancer cell migration. Cell.
151:1542–1556. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Daulat AM, Bertucci F, Audebert S, Serge
A, Finetti P, Josselin E, Castellano R, Birnbaum D, Angers S and
Borg JP: PRICKLE1 Contributes to Cancer Cell Dissemination through
Its Interaction with mTORC2. Dev Cell. 37:311–325. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang L, Luga V, Armitage SK, Musiol M,
Won A, Yip CM, Plotnikov SV and Wrana JL: A lateral signalling
pathway coordinates shape volatility during cell migration. Nat
Commun. 7:117142016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yang N, Chen H, Huang Y, Song X, Yang P,
Zhang S, Yan W, Li N and Feng Z: The role and significance of wnt5a
in regulating epithelial-mesenchymal transition in endometrioid
adenocarcinoma. Cancer Manag Res. 13:6527–6535. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ma J, Kong FF, Yang D, Yang H, Wang C,
Cong R and Ma XX: lncRNA MIR210HG promotes the progression of
endometrial cancer by sponging miR-337-3p/137 via the
HMGA2-TGF-β/Wnt pathway. Mol Ther Nucleic Acids. 24:905–922. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wasniewski T, Kiezun J, Krazinski BE,
Kowalczyk AE, Szostak B, Wierzbicki PM and Kiewisz J: WNT5A gene
and protein expression in endometrial cancer. Folia Histochem
Cytobiol. 57:84–93. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mikels A, Minami Y and Nusse R: Ror2
receptor requires tyrosine kinase activity to mediate Wnt5A
signaling. J Biol Chem. 284:30167–30176. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Nakano K, Chihara Y, Kobayashi S, Iwanaga
M, Utsunomiya A, Watanabe T and Uchimaru K: Overexpression of
aberrant Wnt5a and its effect on acquisition of malignant
phenotypes in adult T-cell leukemia/lymphoma (ATL) cells. Sci Rep.
11:41142021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Pukrop T, Klemm F, Hagemann T, Gradl D,
Schulz M, Siemes S, Trumper L and Binder C: Wnt 5a signaling is
critical for macrophage-induced invasion of breast cancer cell
lines. Proc Natl Acad Sci USA. 103:5454–5459. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang Q, Symes AJ, Kane CA, Freeman A,
Nariculam J, Munson P, Thrasivoulou C, Masters JR and Ahmed A: A
novel role for Wnt/Ca2+ signaling in actin cytoskeleton remodeling
and cell motility in prostate cancer. PLoS One. 5:e104562010.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Macleod RJ, Hayes M and Pacheco I: Wnt5a
secretion stimulated by the extracellular calcium-sensing receptor
inhibits defective Wnt signaling in colon cancer cells. Am J
Physiol Gastrointest Liver Physiol. 293:G403–G411. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kremenevskaja N, von Wasielewski R, Rao
AS, Schofl C, Andersson T and Brabant G: Wnt-5a has tumor
suppressor activity in thyroid carcinoma. Oncogene. 24:2144–2154.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zmarzly N, Hermyt E, Kruszniewska-Rajs C,
Gola J, Witek A, Mazurek U, Ostenda A and Boron D: Expression
Profile of EMT-related Genes and miRNAs involved in signal
transduction via the Wnt pathway and cadherins in endometrial
cancer. Curr Pharm Biotechnol. 22:1663–1671. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tewari D, Bawari S, Sharma S, Deliberto LK
and Bishayee A: Targeting the crosstalk between canonical
Wnt/β-catenin and inflammatory signaling cascades: A novel strategy
for cancer prevention and therapy. Pharmacol Ther. 227:1078762021.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kurnit KC, Kim GN, Fellman BM, Urbauer DL,
Mills GB, Zhang W and Broaddus RR: CTNNB1 (beta-catenin) mutation
identifies low grade, early stage endometrial cancer patients at
increased risk of recurrence. Mod Pathol. 30:1032–1041. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ruz-Caracuel I, Lopez-Janeiro A,
Heredia-Soto V, Ramon-Patino JL, Yebenes L, Berjon A, Hernandez A,
Gallego A, Ruiz P, Redondo A, et al: Clinicopathological features
and prognostic significance of CTNNB1 mutation in low-grade,
early-stage endometrial endometrioid carcinoma. Virchows Arch.
479:1167–1176. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Costigan DC, Dong F, Nucci MR and Howitt
BE: Clinicopathologic and immunohistochemical correlates of CTNNB1
mutated endometrial endometrioid carcinoma. Int J Gynecol Pathol.
39:119–127. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Goad J, Ko YA, Kumar M, Jamaluddin MFB and
Tanwar PS: Oestrogen fuels the growth of endometrial hyperplastic
lesions initiated by overactive Wnt/β-catenin signalling.
Carcinogenesis. 39:1105–1116. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Valenta T, Hausmann G and Basler K: The
many faces and functions of β-catenin. EMBO J. 31:2714–2736. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Heuberger J and Birchmeier W: Interplay of
cadherin-mediated cell adhesion and canonical Wnt signaling. Cold
Spring Harb Perspect Biol. 2:a29152010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Barrallo-Gimeno A and Nieto MA: The Snail
genes as inducers of cell movement and survival: Implications in
development and cancer. Development. 132:3151–3161. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Conacci-Sorrell M, Simcha I, Ben-Yedidia
T, Blechman J, Savagner P and Ben-Ze'ev A: Autoregulation of
E-cadherin expression by cadherin-cadherin interactions: The roles
of beta-catenin signaling, Slug, and MAPK. J Cell Biol.
163:847–857. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhang Y and Wang X: Targeting the
Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol.
13:1652020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Krishnamurthy N and Kurzrock R: Targeting
the Wnt/beta-catenin pathway in cancer: Update on effectors and
inhibitors. Cancer Treat Rev. 62:50–60. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhou Y, Zhu Y, Xie Y and Ma X: The role of
long Non-coding RNAs in immunotherapy resistance. Front Oncol.
9:12922019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wilusz JE, Sunwoo H and Spector DL: Long
noncoding RNAs: Functional surprises from the RNA world. Genes Dev.
23:1494–1504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Heuston EF, Lemon KT and Arceci RJ: The
beginning of the road for Non-Coding RNAs in normal hematopoiesis
and hematologic malignancies. Front Genet. 2:942011. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhang S, Shen S, Yang Z, Kong X, Liu F and
Zhen Z: Coding and Non-coding RNAs: Molecular basis of
forest-insect outbreaks. Front Cell Dev Biol. 8:3692020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Mendell JT: Targeting a long Noncoding RNA
in breast cancer. N Engl J Med. 374:2287–2289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Karimzadeh MR, Pourdavoud P, Ehtesham N,
Qadbeigi M, Asl MM, Alani B, Mosallaei M and Pakzad B: Regulation
of DNA methylation machinery by epi-miRNAs in human cancer:
Emerging new targets in cancer therapy. Cancer Gene Ther.
28:157–174. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Li C, Liu H, Wei R, Liu Z, Chen H, Guan X,
Zhao Z, Wang X and Jiang Z: LncRNA EGOT/miR-211-5p affected
radiosensitivity of rectal cancer by competitively regulating
ErbB4. Onco Targets Ther. 14:2867–2878. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Hong BS, Ryu HS, Kim N, Kim J, Lee E, Moon
H, Kim KH, Jin MS, Kwon NH, Kim S, et al: Tumor Suppressor
miRNA-204-5p Regulates Growth, Metastasis, and Immune
Microenvironment Remodeling in Breast Cancer. Cancer Res.
79:1520–1534. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Huang Y, Liu G, Ma H, Tian Y, Huang C, Liu
F, Jia Y and Jiang D: Plasma lncRNA FEZF1-AS1 as a potential
biomarker for diagnosis of non-small-cell lung carcinoma. Medicine
(Baltimore). 99:e210192020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Liao Y, Cao W, Zhang K, Zhou Y, Xu X, Zhao
X, Yang X, Wang J, Zhao S, Zhang S, et al: Bioinformatic and
integrated analysis identifies an lncRNA-miRNA-mRNA interaction
mechanism in gastric adenocarcinoma. Genes Genomics. 43:613–622.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Piergentili R, Zaami S, Cavaliere AF,
Signore F, Scambia G, Mattei A, Marinelli E, Gulia C and Perelli F:
Non-Coding RNAs as prognostic markers for endometrial cancer. Int J
Mol Sci. 22:31512021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Liu D, Song Z, Wang X and Ouyang L:
Ubiquitin C-Terminal Hydrolase L5 (UCHL5) accelerates the growth of
endometrial cancer via activating the Wnt/β-catenin signaling
pathway. Front Oncol. 10:8652020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Fatima I, Barman S, Rai R, Thiel KWW and
Chandra V: Targeting Wnt signaling in endometrial cancer. Cancers
(Basel). 13:23512021. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wang ZM, Wan XH, Sang GY, Zhao JD, Zhu QY
and Wang DM: miR-15a-5p suppresses endometrial cancer cell growth
via Wnt/β-catenin signaling pathway by inhibiting WNT3A. Eur Rev
Med Pharmacol Sci. 21:4810–4818. 2017.PubMed/NCBI
|
|
83
|
Li Y, Liu J, Piao J, Ou J and Zhu X:
Circ_0109046 promotes the malignancy of endometrial carcinoma cells
through the microRNA-105/SOX9/Wnt/β-catenin axis. IUBMB Life.
73:159–176. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Shang JC, Yu GZ, Ji ZW, Wang XQ and Xia L:
MiR-105 inhibits gastric cancer cells metastasis,
epithelial-mesenchymal transition by targeting SOX9. Eur Rev Med
Pharmacol Sci. 23:6160–6169. 2019.PubMed/NCBI
|
|
85
|
Xu H, Gong Z, Shen Y, Fang Y and Zhong S:
Circular RNA expression in extracellular vesicles isolated from
serum of patients with endometrial cancer. Epigenomics. 10:187–197.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Shen Q, He T and Yuan H: Hsa_circ_0002577
promotes endometrial carcinoma progression via regulating
miR-197/CTNND1 axis and activating Wnt/β-catenin pathway. Cell
Cycle. 18:1229–1240. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Liu D, Qiu M, Jiang L and Liu K: Long
Noncoding RNA HOXB-AS1 is upregulated in endometrial carcinoma and
sponged miR-149-3p to Upregulate Wnt10b. Technol Cancer Res Treat.
19:15330338209674622020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wang H and Xie Y: BRD7-Mediated miR-3148
inhibits progression of cervical cancer by targeting
Wnt3a/β-catenin pathway. Reprod Sci. 27:877–887. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Shen G, Gao Q, Liu F, Zhang Y, Dai M, Zhao
T, Cheng M, Xu T, Jin P, Yin W, et al: The Wnt3a/β-catenin/TCF7L2
signaling axis reduces the sensitivity of HER2-positive epithelial
ovarian cancer to trastuzumab. Biochem Biophys Res Commun.
526:685–691. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Jiang H, Li Y, Li J, Zhang X, Niu G, Chen
S and Yao S: Long noncoding RNA LSINCT5 promotes endometrial
carcinoma cell proliferation, cycle, and invasion by promoting the
Wnt/β-catenin signaling pathway via HMGA2. Ther Adv Med Oncol.
11:17588359198746492019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Park SA, Kim LK, Kim YT, Heo TH and Kim
HJ: Long non-coding RNA steroid receptor activator promotes the
progression of endometrial cancer via Wnt/β-catenin signaling
pathway. Int J Biol Sci. 16:99–115. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Wang H, Yang Q, Li J, Chen W, Jin X and
Wang Y: MicroRNA-15a-5p inhibits endometrial carcinoma
proliferation, invasion and migration via downregulation of VEGFA
and inhibition of the Wnt/β-catenin signaling pathway. Oncol Lett.
21:3102021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Chen P, Xing T, Wang Q, Liu A, Liu H, Hu
Y, Ji Y, Song Y and Wang D: MicroRNA-202 inhibits cell migration
and invasion through targeting FGF2 and inactivating
Wnt/beta-catenin signaling in endometrial carcinoma. Biosci Rep.
39:BSR201906802019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Li Y, Sun D, Gao J, Shi Z, Chi P, Meng Y,
Zou C and Wang Y: MicroRNA-373 promotes the development of
endometrial cancer by targeting LATS2 and activating the
Wnt/beta-Catenin pathway. J Cell Biochem. 120:8611–8618. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Sun X, Dongol S, Qiu C, Xu Y, Sun C, Zhang
Z, Yang X, Zhang Q and Kong B: miR-652 promotes tumor proliferation
and metastasis by targeting RORA in endometrial cancer. Mol Cancer
Res. 16:1927–1939. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Huang X, Zhong R, He X, Deng Q, Peng X, Li
J and Luo X: Investigations on the mechanism of progesterone in
inhibiting endometrial cancer cell cycle and viability via
regulation of long noncoding RNA NEAT1/microRNA-146b-5p mediated
Wnt/β-catenin signaling. IUBMB Life. 71:223–234. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Jung YS and Park JI: Wnt signaling in
cancer: Therapeutic targeting of Wnt signaling beyond β-catenin and
the destruction complex. Exp Mol Med. 52:183–191. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Werner J, Boonekamp KE, Zhan T and Boutros
M: The roles of secreted Wnt ligands in cancer. Int J Mol Sci.
24:53492023. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Doo DW, Meza-Perez S, Londono AI,
Goldsberry WN, Katre AA, Boone JD, Moore DJ, Hudson CT, Betella I,
Mccaw TR, et al: Inhibition of the Wnt/β-catenin pathway enhances
antitumor immunity in ovarian cancer. Ther Adv Med Oncol.
12:17588359209137982020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Rodon J, Argiles G, Connolly RM,
Vaishampayan U, de Jonge M, Garralda E, Giannakis M, Smith DC,
Dobson JR, McLaughlin ME, et al: Phase 1 study of single-agent
WNT974, a first-in-class Porcupine inhibitor, in patients with
advanced solid tumours. Br J Cancer. 125:28–37. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Tabernero J, Van Cutsem E, Garralda E, Tai
D, De Braud F, Geva R, van Bussel MTJ, Fiorella Dotti K, Elez E, de
Miguel MJ, et al: A Phase Ib/II Study of WNT974 + Encorafenib +
cetuximab in patients with BRAF V600E-Mutant KRAS wild-type
metastatic colorectal cancer. Oncologist. 28:230–238. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Madan B, Ke Z, Harmston N, Ho SY, Frois
AO, Alam J, Jeyaraj DA, Pendharkar V, Ghosh K, Virshup IH, et al:
Wnt addiction of genetically defined cancers reversed by PORCN
inhibition. Oncogene. 35:2197–2207. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
He B, You L, Uematsu K, Xu Z, Lee AY,
Matsangou M, Mccormick F and Jablons DM: A monoclonal antibody
against Wnt-1 induces apoptosis in human cancer cells. Neoplasia.
6:7–14. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Davis SL, Cardin DB, Shahda S, Lenz HJ,
Dotan E, O'Neil BH, Kapoun AM, Stagg RJ, Berlin J, Messersmith WA
and Cohen SJ: A phase 1b dose escalation study of Wnt pathway
inhibitor vantictumab in combination with nab-paclitaxel and
gemcitabine in patients with previously untreated metastatic
pancreatic cancer. Invest New Drugs. 38:821–830. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Diamond JR, Becerra C, Richards D, Mita A,
Osborne C, O'Shaughnessy J, Zhang C, Henner R, Kapoun AM, Xu L, et
al: Phase Ib clinical trial of the anti-frizzled antibody
vantictumab (OMP-18R5) plus paclitaxel in patients with locally
advanced or metastatic HER2-negative breast cancer. Breast Cancer
Res Treat. 184:53–62. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Smith DC, Rosen LS, Chugh R, Goldman JW,
Xu L, Kapoun A, Brachmann RK, Dupont J, Stagg RJ, Tolcher AW, et
al: First-in-human evaluation of the human monoclonal antibody
vantictumab (OMP-18R5; anti-Frizzled) targeting the WNT pathway in
a phase I study for patients with advanced solid tumors. J Clin
Oncol. 31 (Suppl 15):25402013. View Article : Google Scholar
|
|
107
|
Le PN, Mcdermott JD and Jimeno A:
Targeting the Wnt pathway in human cancers: Therapeutic targeting
with a focus on OMP-54F28. Pharmacol Ther. 146:1–11. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Fischer MM, Cancilla B, Yeung VP,
Cattaruzza F, Chartier C, Murriel CL, Cain J, Tam R, Cheng CY,
Evans JW, et al: WNT antagonists exhibit unique combinatorial
antitumor activity with taxanes by potentiating mitotic cell death.
Sci Adv. 3:e17000902017. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Jimeno A, Gordon M, Chugh R, Messersmith
W, Mendelson D, Dupont J, Stagg R, Kapoun AM, Xu L, Uttamsingh S,
et al: A First-in-Human Phase I study of the anticancer stem cell
agent ipafricept (OMP-54F28), a decoy receptor for Wnt ligands, in
patients with advanced solid tumors. Clin Cancer Res. 23:7490–7497.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Moore KN, Gunderson CC, Sabbatini P,
McMeekin DS, Mantia-Smaldone G, Burger RA, Morgan MA, Kapoun AM,
Brachmann RK, Stagg R, et al: A phase 1b dose escalation study of
ipafricept (OMP54F28) in combination with paclitaxel and
carboplatin in patients with recurrent platinum-sensitive ovarian
cancer. Gynecol Oncol. 154:294–301. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Dotan E, Cardin DB, Lenz HJ, Messersmith
W, O'Neil B, Cohen SJ, Denlinger CS, Shahda S, Astsaturov I, Kapoun
AM, et al: Phase Ib Study of Wnt Inhibitor Ipafricept with
Gemcitabine and nab-paclitaxel in patients with previously
untreated stage IV pancreatic cancer. Clin Cancer Res.
26:5348–5357. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Emami KH, Nguyen C, Ma H, Kim DH, Jeong
KW, Eguchi M, Moon RT, Teo JL, Kim HY, Moon SH, et al: A small
molecule inhibitor of beta-catenin/CREB-binding protein
transcription [corrected]. Proc Natl Acad Sci USA. 101:12682–12687.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Pozzi S, Fulciniti M, Yan H, Vallet S, Eda
H, Patel K, Santo L, Cirstea D, Hideshima T, Schirtzinge L, et al:
In vivo and in vitro effects of a novel anti-Dkk1 neutralizing
antibody in multiple myeloma. Bone. 53:487–496. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Arend R, Dholakia J, Castro C, Matulonis
U, Hamilton E, Jackson CG, Lybarger K, Goodman HM, Duska LR, Mahdi
H, et al: DKK1 is a predictive biomarker for response to DKN-01:
Results of a phase 2 basket study in women with recurrent
endometrial carcinoma. Gynecol Oncol. 172:82–91. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Wang Y, Hanifi-Moghaddam P, Hanekamp EE,
Kloosterboer HJ, Franken P, Veldscholte J, van Doorn HC, Ewing PC,
Kim JJ, Grootegoed JA, et al: Progesterone inhibition of
Wnt/beta-catenin signaling in normal endometrium and endometrial
cancer. Clin Cancer Res. 15:5784–5793. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Yahata T, Fujita K, Aoki Y and Tanaka K:
Long-term conservative therapy for endometrial adenocarcinoma in
young women. Hum Reprod. 21:1070–1075. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Arend RC, Londono-Joshi AI, Samant RS, Li
Y, Conner M, Hidalgo B, Alvarez RD, Landen CN, Straughn JM and
Buchsbaum DJ: Inhibition of Wnt/β-catenin pathway by niclosamide: A
therapeutic target for ovarian cancer. Gynecol Oncol. 134:112–120.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Kusunoki S, Kato K, Tabu K, Inagaki T,
Okabe H, Kaneda H, Suga S, Terao Y, Taga T and Takeda S: The
inhibitory effect of salinomycin on the proliferation, migration
and invasion of human endometrial cancer stem-like cells. Gynecol
Oncol. 129:598–605. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Feng W, Yang CX, Zhang L, Fang Y and Yan
M: Curcumin promotes the apoptosis of human endometrial carcinoma
cells by downregulating the expression of androgen receptor through
Wnt signal pathway. Eur J Gynaecol Oncol. 35:718–723.
2014.PubMed/NCBI
|
|
120
|
Wang WT, Han C, Sun YM, Chen TQ and Chen
YQ: Noncoding RNAs in cancer therapy resistance and targeted drug
development. J Hematol Oncol. 12:552019. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Kelnar K, Peltier HJ, Leatherbury N,
Stoudemire J and Bader AG: Quantification of therapeutic miRNA
mimics in whole blood from nonhuman primates. Anal Chem.
86:1534–1542. 2014. View Article : Google Scholar : PubMed/NCBI
|