Introduction
Head and neck cancer is the eighth most common
cancer and accounts for 3% of all cancers in men in the United
States. The estimated new cases for both male and female are
~36,540 and expected deaths are 7,880 in 2010 (1). Lymph node metastasis of head and neck
squamous cell carcinoma (HNSCC) is a strong indicator of prognosis
with a 5-year survival rate of <50% for patients with unilateral
lymph node metastasis and a survival rate of <25% for patients
with bilateral metastasis. Despite the advances in combining
chemotherapy with surgery and radiotherapy, survival rates for
patients with recurrent head and neck cancer remain poor (2,3).
Therefore, identifying the crucial proteins and clarifying
mechanisms that contribute to metastasis will be significant to
provide biomarkers for prognosis and targets for treatment.
Insulin receptor substrate 1 (IRS-1) is an adaptor
protein that integrates multiple transmembrane signals from growth
factors and hormones, to regulate cell growth, survival,
differentiation and metabolism (4).
Previous studies mainly focused on its roles in metabolism, but
recently it was reported to be correlated with cancer progression
and metastasis (5). As a major
substrate of insulin and insulin-like growth factor I (IGF-I) and
mainly functioning through activating PI3K/Akt and MAPK pathway,
IRS-1 has been considered oncogenic in cancer progression. As well,
elevated expression levels and the active form of IRS-1 has been
reported in a variety of tumors, including hepatocellular
carcinomas (HCCs), pancreatic cancer, breast cancers, leiomyomas,
Wilms' tumors, rhabdomyosarcomas, liposarcomas, leiomyosarcomas and
adrenal cortical carcinomas (5,6).
However, recently Houghton et al(7) showed increased cell proliferation
after silencing of IRS1 gene expression in A549 cells
suggesting tumor suppression potential. Furthermore studies in
tumor metastasis are quite limited and controversial. IRS-1 has
been identified as both a positive and negative regulator of
metastasis in breast cancer (8–10).
While in lung cancer, IRS-1 has been confirmed to suppress tumor
metastasis (11). To date, its role
in head and neck cancer has not been identified.
The epithelial-mesenchymal transition (EMT) plays
important roles in tumor metastasis. Cells undergoing EMT lose
their epithelial characteristics and phenotype, acquire mesenchymal
properties, and thus gain enhanced motility (12). EMT is accompanied by decreased
E-cadherin expression and increased vimentin expression. Many
transcriptional factors such as snail, slug, twist and bmi1 have
been confirmed to suppress E-cadherin transcription and thus
identified as both molecular markers and inducers of EMT (13,14).
Loss of E-cadherin, not only results in loss of adherens junctions
between neighbor cells resulting in dissemination of cells from the
original tumor, but also results in releasing its cytoplasmic
binding with β-catenin. β-catenin, when translocating to and
accumulating in the nucleus, induces transcription of other
oncogenes involved in tumor progression, malignancy and metastasis
(15). Therefore, loss of
E-cadherin expression is not only the initial and essential step of
EMT, but also a potential promoter of EMT.
MicroRNAs (miRNAs) are ~22-nt non-coding RNAs that
negatively regulate gene expression at the post-transciptional
level via direct binding with the 3′ untranslated regions (UTR) of
target mRNAs (16,17). Many miRNAs have been implicated to
play important roles in tumor metastasis, such as miR-21, miR-31,
miR-125a, miR-155 and miR-373 (18,19).
Recently, miR-9 has been shown to directly target E-cadherin to
reduce its expression at post-transcription levels and thus induce
EMT (20). miR-200 family members
and miR-10b have been confirmed to indirectly regulate E-cadherin
expression and induce EMT (21,22).
In this study, we investigated the role of IRS-1 in
head and neck cancer metastasis. First, we found the expression of
IRS-1 protein in head and neck squamous cell carcinoma (HNSCC) with
lymph node metastasis decreased compared to HNSCCs without
metastasis. Moreover, we found IRS-1 mRNA expression in metastatic
lymph node tumor tissues to be decreased compared to primary tumor
tissues of HNSCCs. In a paired poorly-highly metastatic HNSCC cell
model, we identified decreased expression of IRS-1 in highly
metastatic cells. We then showed that the invasive potency of
poorly metastatic cells (which express high levels of IRS-1)
increased after downregulation of IRS-1 expression. In addition, we
found downregulation of E-cadherin and upregulation of miR-9 after
IRS-1 knockdown. These results suggest that decreased expression of
IRS-1 is correlated with HNSCC metastasis and reduction of IRS-1
promotes invasion and EMT of HNSCC cells possibly through
upregulation of miR-9.
Materials and methods
Human tissue samples
In this study, we used two cohorts of human tissue
samples from different cities of China. One cohort contained
formalin-fixed paraffin-embedded (FFPE) tumor tissue sections
collected from the Department of Oral Surgery and
Otolaryngology-Head and Neck Surgery at the Second XiangYa Hospital
of Central South University (Changsha, China) from 2006 to 2010. Of
the 108 HNSCC patients, 84 were males and 24 were females (mean
age, 56.5 years). The HNSCC histological patterns and clinical T
stages were determined according to WHO histological classification
of the HNSCC and the TNM classification of malignant tumors
(Table I). Of the patients included
in the study, 33 had local lymph node metastasis and 75 had
negative lymph nodes. No case revealed distant metastasis, so that
all of the cases were classified as M0. These specimens were
subjected to immunohistochemical (IHC) analysis.
 | Table IAnalysis of the association between
IRS-1 protein expression and HNSCC. |
Table I
Analysis of the association between
IRS-1 protein expression and HNSCC.
| IRS-1 |
|---|
|
|
|---|
| Clinicopathological
features | Positive (%) | Negative (%) |
|---|
| Age |
| ≤50 years
(n=43) | 31 (72.1) | 12 (27.9) |
| >50 years
(n=65) | 45 (69.2) | 20 (30.8) |
| Gender |
| Male (n=84) | 60 (71.4) | 24 (28.6) |
| Female (n=24) | 16 (66.7) | 8 (33.3) |
| Histological
grade |
| I (n=18) | 16 (88.9)a | 2 (11.1) |
| II (n=70) | 51(72.9)b | 19(27.1) |
| III (n=20) | 9 (45.0) | 11 (55.0) |
| Clinical stage |
| I (n=36) | 31 (86.1)c | 5 (13.9) |
| II (n=39) | 31 (79.5)d | 8 (21.5) |
| III (n=33) | 14 (42.4) | 16 (57.6) |
| Lymph node
status |
| LNM (n=33) | 22 (66.7) | 11 (33.3) |
| No LNM (n=75) | 54 (72.0) | 21 (28.0) |
The other cohort contained FFPE tumor tissues
collected from paired primary tumor and metastatic lymph node of 10
HNSCC patients at Nanjing Medical University Affiliated Nanjing
First Hospital (Nanjing, P.R. China) from 2007 to 2011. Of the 10
patients, 7 were males and 3 were females (mean age, 71.2 years).
These samples were subjected to RNA extraction and subsequent
quantitative RT-PCR analysis of IRS-1 expression.
Reagents
Rabbit polyclonal anti-insulin receptor substrate 1
antibody was purchased from Upstate Group, Inc. (Charlottesville,
VA). Mouse monoclonal anti-E-cadherin and anti-vimentin antibodies
were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz,
CA). Mouse monoclonal anti-tubulin antibody was purchased from
Sigma Chemical, Co. (St. Louis, MO). Sulforhodamine B was purchased
from Sigma Chemical, Co., dissolved in 1% (v/v) acetic acid at a
concentration of 0.4% (w/v) and stored at room temperature.
Cell lines
Paired low and high metastatic HNSCC cells, 686LN
and 686LN-M4e, have been described (23–25).
Briefly, 686LN cell line was derived from lymph node metastasis of
a basal-tongue primary tumor. It has low capability for metastasis
to lymph node and lung when injected into the mylohyoid muscle near
the FOM (floor-of-mouth) of nude mice. When the primary tumor
reached a length of 2.0 cm, the cervical lymph nodes were
collected. 686LN cell lines derived from the metastatic lymph nodes
were cultured in vitro, then re-injected to another mouse as
described above for further selection. After four rounds of in
vivo selection, the highly metastatic 686LN-M4e cell line was
established, which showed 100% lymph node metastasis and 90% lung
metastasis in nude mice in vivo. 686LN-M4e also showed much
higher Matrigel invasion capability than the parental cells
(23–25). These cell lines were grown in
monolayer cultured in Dulbecco's modified Eagle's medium (DMEM)/F12
medium supplemented with 5% fetal bovine serum at 37°C in a
humidified atmosphere consisting of 5% CO2 and 95%
air.
Gene knockdown by small interfering
RNA
IRS1 SMARTpool siRNA reagent, which contains four
different targeting sequences and correlated control siRNA were
purchased from Upstate Group, Inc. Cells were seeded to 6-well
plates at 5×105 cells/well and transfected with 100
nmol/l siRNA for 24 h using Lipofectamine 2000 Transfection Reagent
from Invitrogen Life Technologies (Carlsbad, CA). After
transfection for 24 h, cells were reseeded and subjected to an
invasion assay or reseeded to 96-well plates for subsequent
Sulforhodamine B assays. Cells transfected with siRNA for 48 h were
harvested for total-RNA and whole cell protein lysates purification
and subsequent qRT-PCR and western blot analysis to evaluate
knockdown efficiency and detect other relative signals.
Invasion assay
The invasion assay was performed using Matrigel
Invasion Chambers with polyethylene terephthalate membrane on the
bottom of the chambers (8.0-μm pore size) and Matrigel Basement
Membrane Matrix from BD Biosciences (Bedford, MA) according to the
manufacturer's protocol. Briefly, 5×104 cells in 0.5 ml
serum-free medium were seeded into each invasion chamber containing
27.2 μg Matrigel Basement Membrane Matrix in 100 μl in triplicate
and incubated for 8 h. Conditional medium harvested from cultured
NIH3T3 cells was added to the bottom wells outside the chambers.
The chambers were incubated at 37°C in the incubator containing 5%
CO2 for another 24 h. Then the cells on the top side of
the chamber were removed with a cotton swab, and the cells that
invaded to the bottom side of the chamber were fixed and stained
using HEMA3 stain set from Fisher Scientific, Co., LLC (Middletown,
VA) following the manufacturer's instructions. The invasive cells
on the lower surface of the membrane were pictured and cell numbers
were counted under a microscope.
Sulforhodamine B assay
Cells after transfection with siRNA for 24 h were
reseeded into 96-well plates at 1,500 cells/well in 300 μl media.
Plates were fixed with 10% (w/v) trichloroacetic acid at 4°C each
day for 5 days. Fixed cells were washed repeatedly with distilled
water and dried. Then they were stained with 0.4% (w/v)
sulforhodamine B for 30 min at room temperature, depleted, washed
with 1% (v/v) acetic acid repeatedly and dried. Finally, the dye
was dissolved in 10 mmol/l Tris base solution for 5 min at room
temperature with agitation. Absorbance was measured at 500 nm using
μQuant™ Universal Microplate Spectrophotometer (BioTek Instruments,
Inc.).
Western blot analysis
Whole-cell protein lysis buffer containing 1%
Triton, 40 mmol/l HEPES, 120 mmol/l NaCl, 1 mmol/l EDTA, 10 mmol/l
pyrophosphate, 10 mmol/l glycerophosphate, 50 mmol/l NaF and 0.5
mmol/l Na3VO4, was stored at 4°C. Protease
inhibitors were added just before use. Cells were washed twice with
cold PBS and incubated in lysis buffer on ice for 20 min and then
centrifuged at 12,000 × g at 4°C for 20 min. Supernatant was
quantified by Bradford assay and denatured in loading buffer.
Protein lysates (30–50 μg) were separated by 10% SDS-PAGE and then
transferred to PVDF membranes using standard procedures. The
membrane was incubated with the primary antibody overnight at 4°C
and washed. After incubation in secondary antibody and wash,
membranes were subjected to Supersignal west Pico chemiluminescent
substrate from Thermo Scientific (Rockford, IL) and exposed.
Quantitative reverse
transcription-polymerase chain reaction (qRT-PCR)
Total-RNA from cells was extracted using TRIzol
reagent from Invitrogen Life Technologies according to the
manufacturer's protocol. TaqMan gene expression assays for miRNA
were purchased from Applied Biosystems Inc. (Foster, CA) and used
to quantify the relative expression of miR-9. U6 small nuclear RNA
(U6 snRNA) was treated as a normalization control. For mRNA
quantification, total-RNA was reverse transcribed following
standard procedure using M-MLV reverse transcriptase (Promega,
Madison, WI), and then subjected to quantitative PCR using Power
SYBR-Green PCR Master mix from Applied Biosystems Inc. according to
the manufacturer's instructions. Primers were described previously
and synthesized by Invitrogen Life Technologies. Forward (F) and
reverse (R) primer sequences are as follows: IRS-1, F,
5′-TGCTGGGGGTTTGGAGAATG-3′ and R, 5′-GG CACTGTTTGAAGTCCTTGACC-3′;
E-cadherin, F, 5′-CCCA CCACGTACAAGGGTC-3′ and R, 5′-CTGGGGTATTGGGG
GCATC-3′; Vimentin, F, 5′-ACCAGGTCCGTGTCCTCGT-3′ and R,
5′-CTGCCCAGGCTGTAGGTG-3′; NFκB, F, 5′-CCTG AGACAAATGGGCTACAC-3′ and
R, 5′-TTTAGGGCTTTGG TTTACACGG-3′; snail, F, 5′-GCTGCAGGACTCTAATCCA
GA-3′ and R, 5′-ATCTCCGGAGGTGGGATG-3′; GAPDH, F,
5′-ATGGGGAAGGTGAAGGTCG-3′ and R, 5′-GGGGTCAT TGATGGCAACAATA-3′
(26–31). All real-time amplifications were
measured in triplicates and performed with the ABI Prism 7300
sequence detection system from Applied Biosystems Inc. The
fold-change of miR-9, IRS-1, E-cadherin and vimentin was calculated
using the 2−ΔΔCt method.
RNA extraction from FFPE tissue
samples
Total-RNA extracted from FFPE tissues was previously
described (32). Briefly, the tumor
sections (5-μm thick) were deparaffinized with xylene and washed
three times with 100% ethanol. After drying, tissue pellets were
added by 200 μl of lysis buffer (20 mmol/l Tris-HCl, pH 8.0, 20
mmol/l EDTA, 2% sodium dodecyl sulfate) and 10 μl of proteinase K
solution(100 μg/ml). After overnight incubation at 55°C, 1 ml
TRIzol reagent from Invitrogen Life Technologies was added to the
sample and total-RNA was extracted according to the manufacturer's
protocol.
Immunohistochemistry and scores
The slides were stained with IHC using the
Ready-to-use EnVision™+ Dual Link System-HRP kit from Dako
(Carpinteria, CA). The staining conditions were adjusted according
to our laboratory experience. Briefly, each section was
deparaffinized and rehydrated, and high-temperature antigen
retrieval was achieved by heating the samples in 0.01 mol/l citrate
buffer in a domestic microwave oven at full power (750 W) for 15
min, then the samples were immersed into methanol containing 0.3%
H2O2 for 30 min at 37°C to inactivate
endogenous peroxidase. To eliminate nonspecific staining, we
incubated the slides with appropriate preimmune serum for 30 min at
room temperature, followed by incubation with primary antibody at
4°C overnight. Staining was done using the rabbit monoclonal
anti-human IRS-1 from Epitomics, Inc. (Burlingame, CA) with a 1:100
dilution. Labeled polymer-HRP was added according to the
manufacturer's instructions and incubated for 30 min. Color
reaction was developed using 3,3′-diaminobenzidine tetrachloride
(DAB) chromogen solution and all slides were counterstained with
hematoxylin. Positive control slides were included in every
experiment in addition to the internal positive controls. The
specificity of the antibody was determined with matched IgG isotype
antibody as a negative control. Moreover, a single band with
correct molecular weight in western blot analysis was assured.
Immunohistochemical staining of sections were scored
microscopically at ×400 magnification in all available tumors by
two pathologists using the qualitative scale described in the
literature with minor modifications. In scoring the results, the
intensity and the percentage of positive cells were taken into
consideration. The number of cells stained was scored as 0 (no
staining), 1+ (<25% positive cells), 2+ (>25 and <50%
positive cells) and 3+ (>50% positive cells). The intensity of
staining was scored from 1+ (weak) to 3+ (strong). The
immunoreactive score was categorized in three groups by
comprehensive evaluation of the percentage of positive cells and
staining intensity as reported previously. No staining was
considered negative (0 score), weakly, moderately and strongly
staining were considered positive (1, 2 and 3 scores,
respectively). Also, for statistical analysis, the staining results
were classified into two groups depending on the intensity and the
percentage of positive cells: ‘negative group’ (no staining, weak
intensity and in <10% positive cells); and the ‘positive group’
(strong intensity and in >10% positive cells). Chi-square test
was used for statistical analysis.
Statistical analysis
The statistical significance of differences between
different groups was analyzed with two-sided unpaired Student's
t-tests. Results were considered to be statistically significant at
p<0.05.
Results
IRS-1 expression correlates with
clinicopathological para-meters
To explore the relationship between IRS-1 expression
and metastasis of HNSCC, we detected IRS-1 expression levels in 108
HNSCC specimens using IHC methods. As shown in Table I, no correlation was found between
the expression level of IRS-1 and patient age and gender
(p>0.05). However, the positive expression rates of IRS-1 in
well and moderately-differentiated carcinoma were higher than that
in poorly-differentiated carcinoma. We also found there was a
negative correlation between the IRS-1 expression and clinical
staging (p<0.05). These results suggested that advanced clinical
stages correlated with lower IRS-1 expression. Although we did not
find any difference between the IRS-1 expression and lymph node
metastasis, we found stronger positive expression of IRS-1 in
tumors without lymph node metastasis rather than tumors with
metastasis (Table II) when we
further analyzed the positive expression intensity of IRS-1
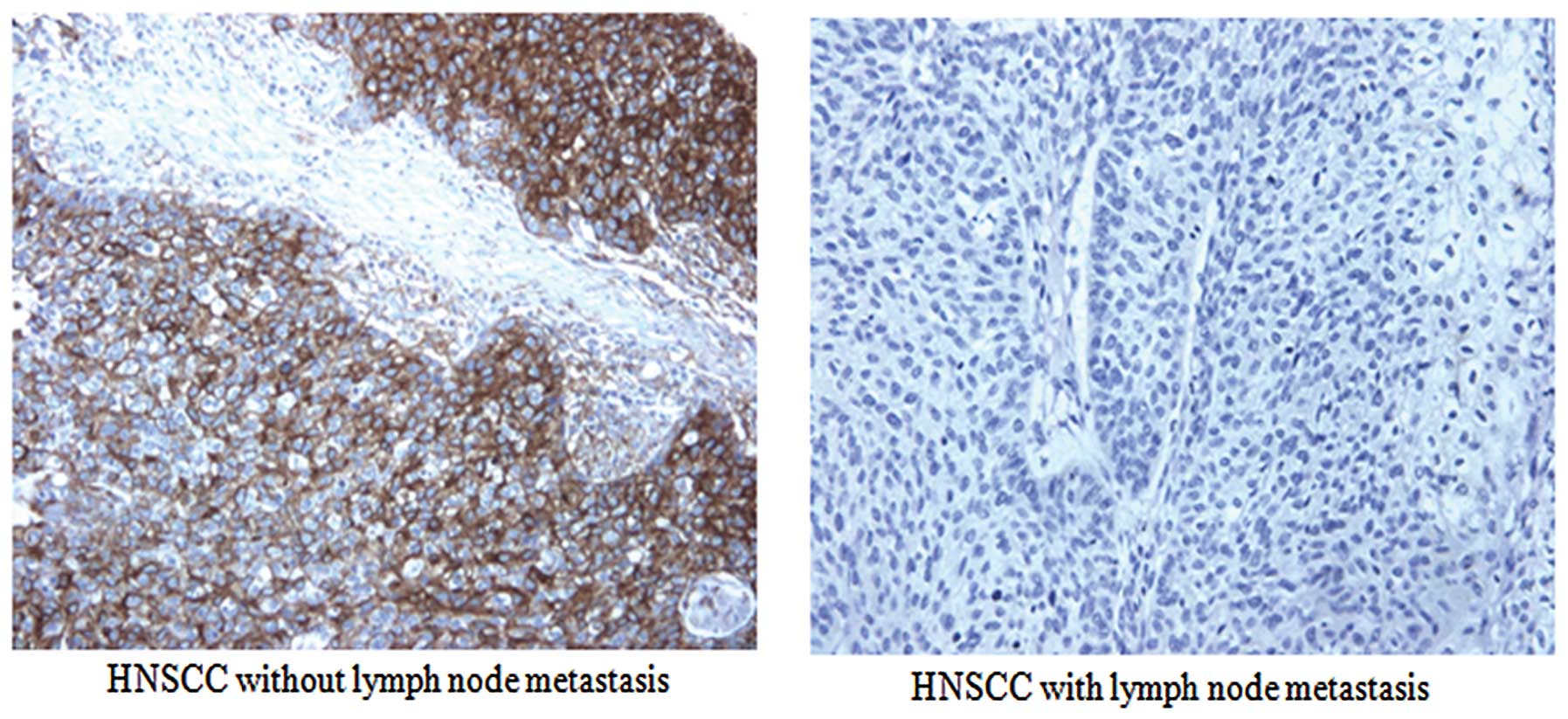
protein. Fig. 1 shows
representative staining of IRS-1 protein using IHC. These results
suggest that decreased expression levels of IRS-1 are associated
with lymph node metastasis of HNSCC.
 | Table IIAnalysis of the association between
positive expression intensity of IRS-1 protein and lymph node
metastasis of HNSCC. |
Table II
Analysis of the association between
positive expression intensity of IRS-1 protein and lymph node
metastasis of HNSCC.
| IRS-1 positive
expression intensity |
|---|
|
|
|---|
| Lymph node
status | +++ (%) | ++ (%) | + (%) | − (%) |
|---|
| LNM (n=33) | 2 (6.1) | 3 (9.1) | 17 (51.5) | 11 (33.3) |
| No LNM (n=75) | 4 (5.3 ) | 23 (30.7) | 27 (36.0)a | 21 (28.0) |
IRS-1 expression decreases in the
metastatic lymph node tumor tissues compared to primary tumor
tissues of HNSCC patients
To further identify relationship of IRS-1 expression
with metastasis, we compared IRS-1 mRNA expression in the primary
and metastatic lymph node tumor tissues of HNSCC patients using
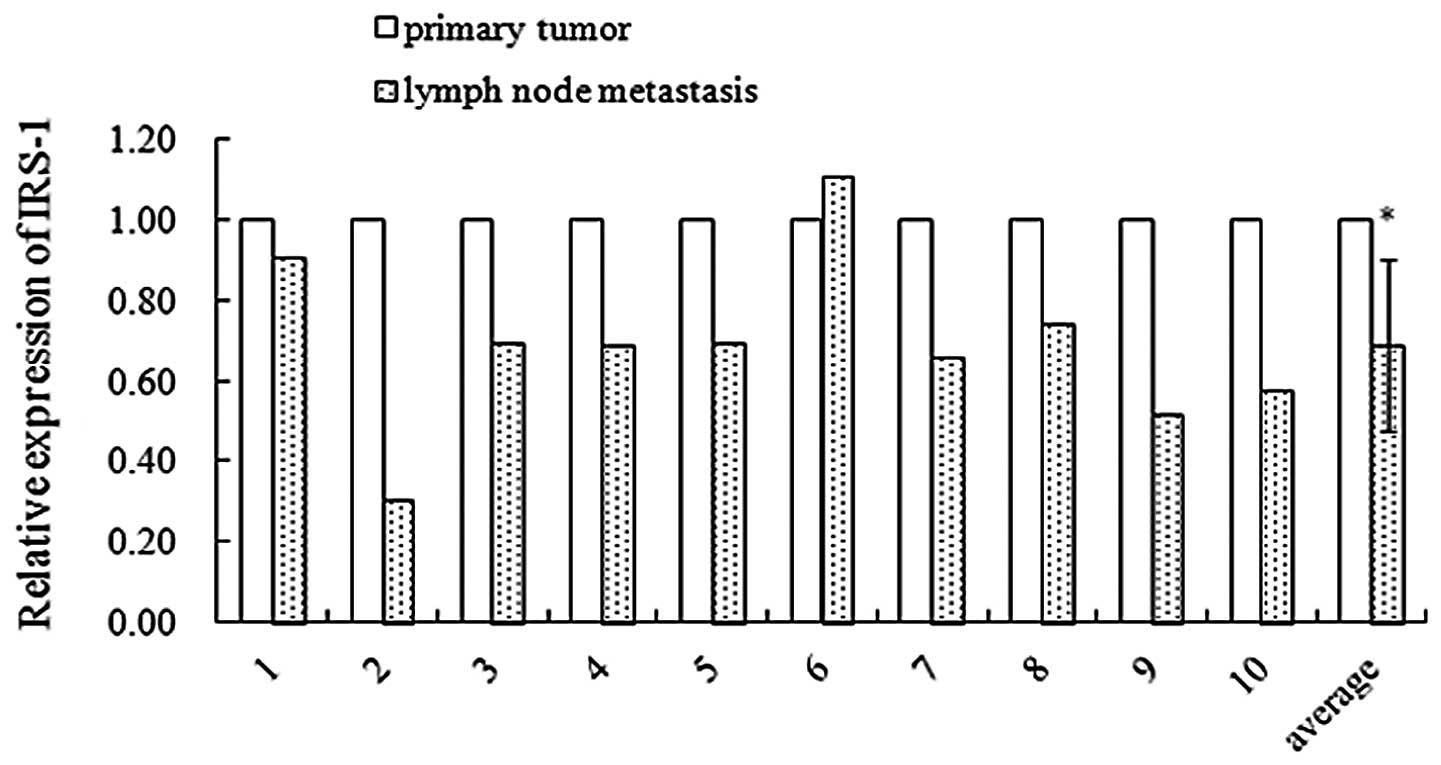
qRT-PCR assay. As shown in Fig. 2,
in 10 paired patient samples, 9 showed decreased expression of
IRS-1 mRNA in lymph node tissue samples. The average decrease rate
was 31% (p<0.05). These results suggest that IRS-1 may serve as
a suppressor of HNSCC metastasis.
IRS-1 expression is lower in highly
metastatic HNSCC cells compared to expression in its parental
poorly metastatic cells
To study the role of IRS-1 in HNSCC metastasis in
vitro, we then detected IRS-1 expression levels in paired
poorly-highly metastatic HNSCC cell lines. Highly metastatic
686LN-M4e cell lines were established from poorly metastatic 686LN
cell lines through an in vivo selection mouse model as
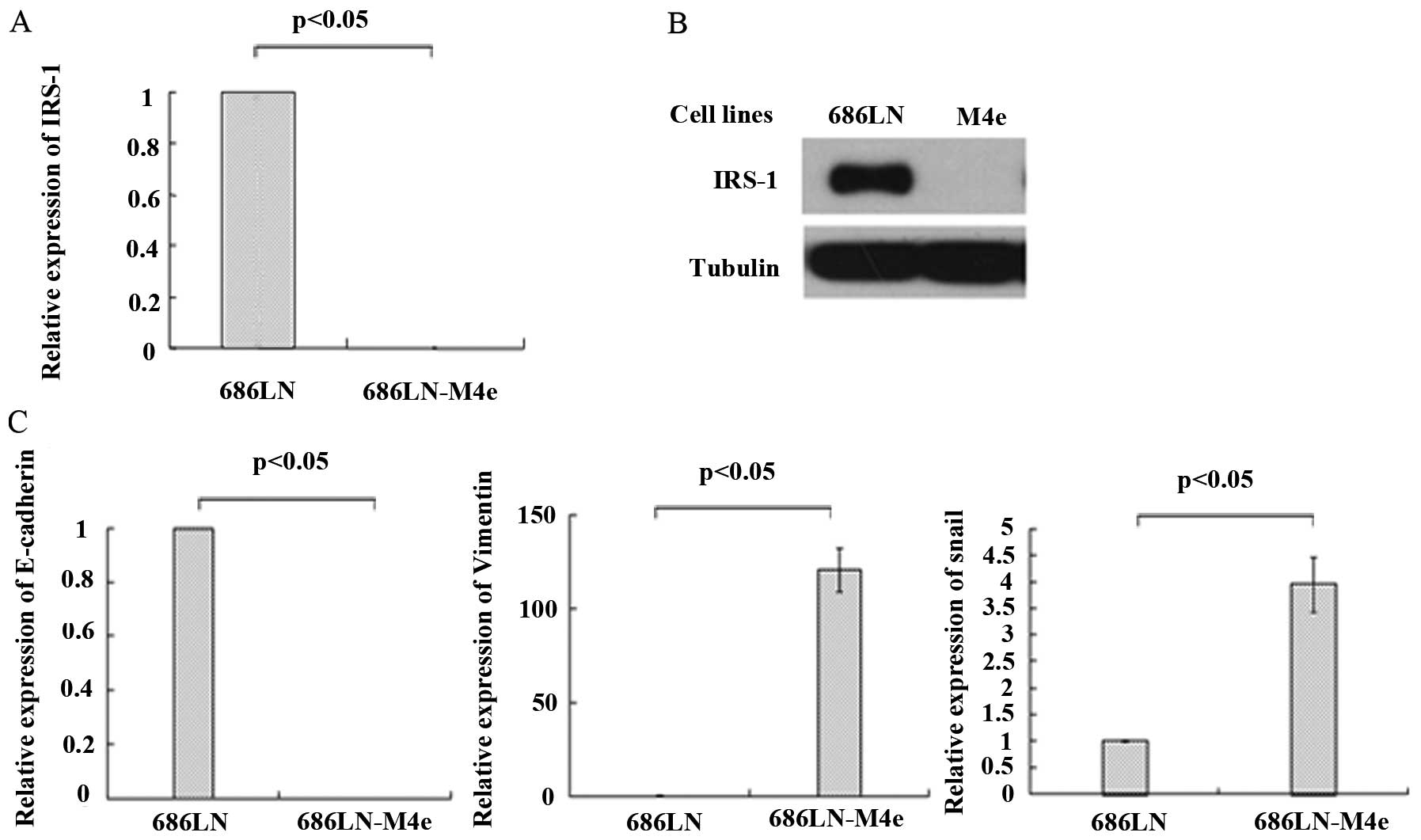
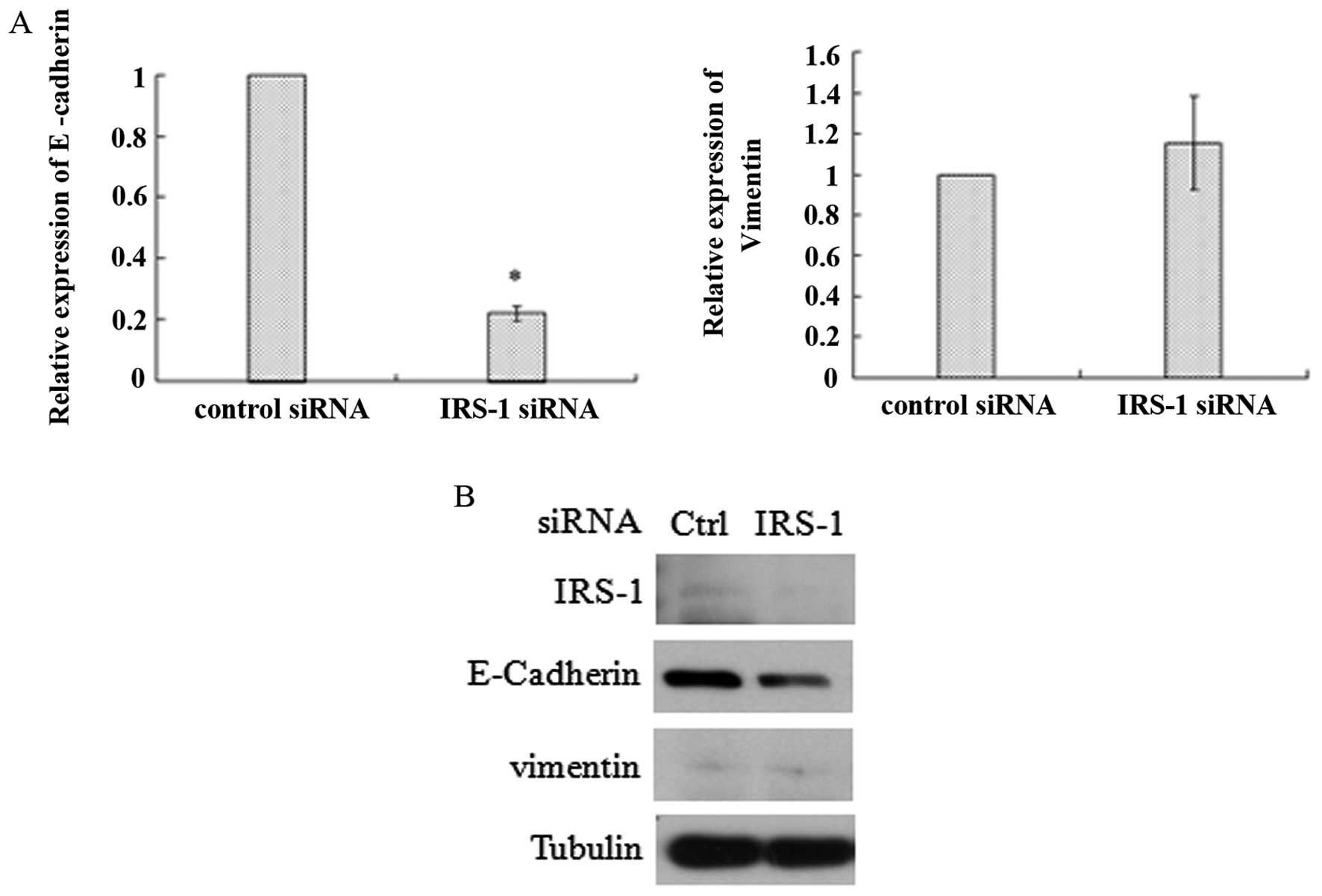
described previously (23,33). As shown in Fig. 3, both mRNA (Fig. 3A) and protein levels (Fig. 3B) of IRS-1 were significantly
decreased in highly metastatic 686LN-M4e cells when compared to
poorly metastatic 686LN cells. Using a quantitative real-time PCR
assay, the Ct value of IRS-1 products in 686LN-M4e cells was
28.54±0.10, suggesting that 686LN-M4e cells had basal levels of
IRS-1 mRNA expression. The ΔCt value (deduction of the Ct value of
GAPDH) was 14.44±0.10 in 686LN-M4e cells, while it was 5.03±0.19 in
686LN cells, suggesting a much lower mRNA expression of IRS-1 in
686LN-M4e cells. A western blot analysis showed that protein levels
of IRS-1 were undetectable in 686LN-M4e cells compared to 686LN
cells. Thus, IRS-1 expression levels are decreased both at mRNA and
protein levels in highly metastatic cell lines of HNSCC, suggesting
IRS-1 to be a suppressor of head and neck cancer metastasis.
In addition, previous studies by Zhang et
al(23) showed decreased
E-cadherin and increased vimentin expression in 686LN-M4e cells
compared with its parental 686LN cells, which suggested that highly
metastatic cells gained EMT features. In this study, we detected
E-cadherin and vimentin expression levels in these paired
metastatic cell lines. As shown in Fig.
3C, E-cadherin mRNA expression was low and vimentin was high in
686LN-M4e cells, consistent with previous studies. We also detected
increased expression of snail in 686LN-M4e cells, which is a well
known transcriptional suppressor of E-cadherin. These findings
further suggest EMT in promoting HNSCC metastasis.
Downregulation of IRS-1 expression
promotes invasion of 686LN cells
Poorly metastatic 686LN cells have been previously
shown to be less invasive than paired highly metastatic 686LN-M4e
cells (24); therefore, to study
the function of IRS-1 in HNSCC metastatsis, we first detected the
impact of downregulating IRS-1 on invasive ability of 686LN cells
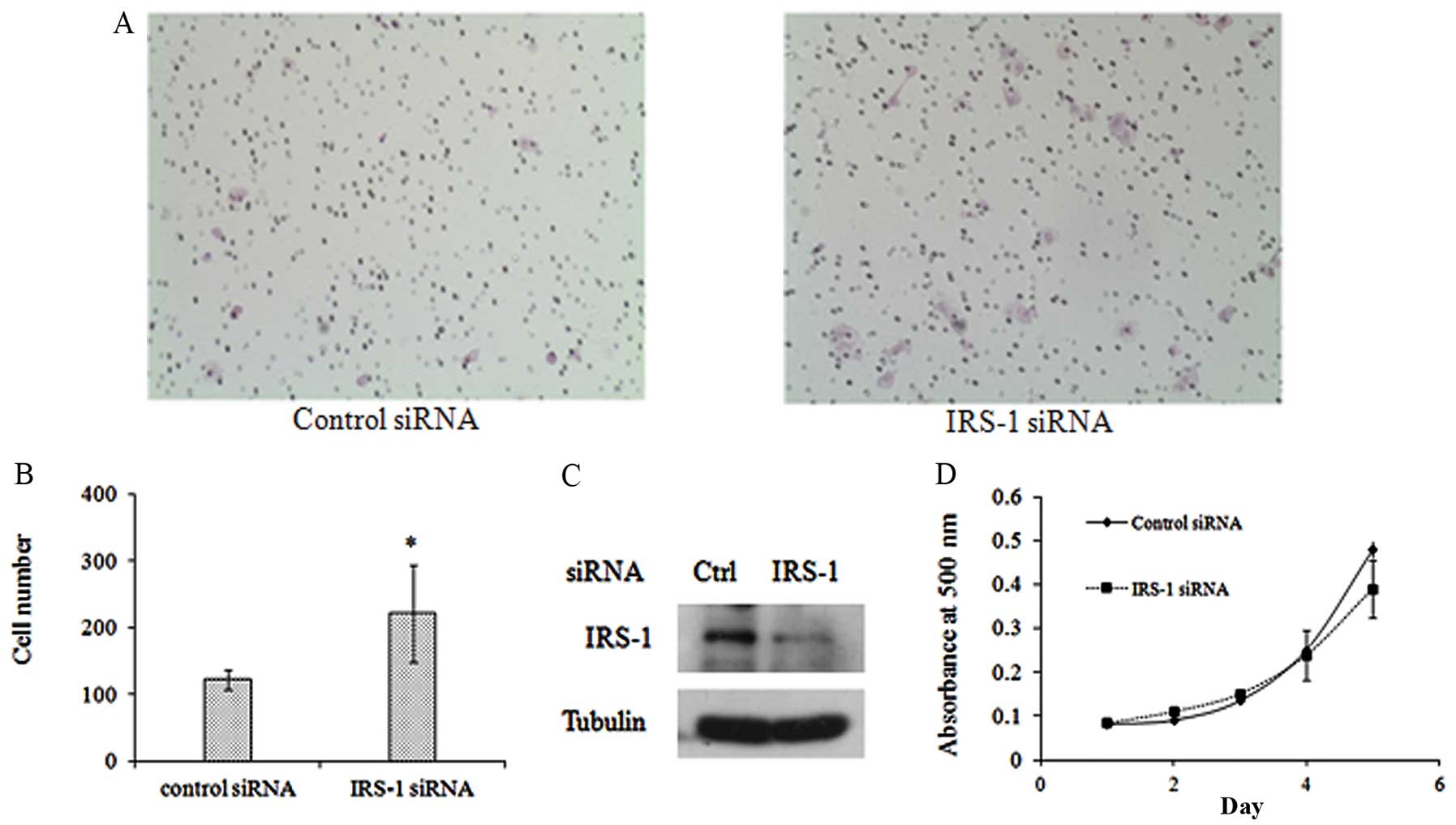
(higher expression levels of IRS-1). As shown in Fig. 4A, increased cell numbers invading to
the bottom side of the membrane after transfection with IRS-1 siRNA
was observed as compared to control siRNA transfection.
Quantification of cell numbers showed that the increase was
statistically significant (p<0.05) (Fig. 4B). Western blot analysis confirmed
the knockdown efficiency of IRS-1 as shown in Fig. 4C. These results suggest that
downregulation of IRS-1 expression enhances the invasive potency of
686LN cells.
IRS-1 has been demonstrated to facilitate activation
of a number of intracellular signaling molecules involved in cell
growth, survival and transformation. Additionally, IRS-1 was
reported to be correlated with cancer development and progression.
Moreover, expression of IRS-1 or its active form-tyrosine
phosphorylated IRS-1 was shown to be elevated in certain types of
tumor tissues compared to normal tissues (5,9).
Therefore, we performed a sulforhodamine B assay to evaluate the
effect of IRS-1 on the growth of 686LN cells. Fig. 4D shows the growth curve of 686LN
cells transfected with IRS-1 siRNA or control siRNA. Even though on
Day 5, viability of cells with IRS-1 knockdown decreased slightly,
the alteration is minor and not statistically significant
(p>0.05). These results suggest that IRS-1 knockdown did not
affect viability of 686LN cells.
Downregulation of IRS-1 expression
induces epithelial-mesenchymal transition
EMT is well-known to promote migration and invasion
of many cancer cell types and plays crucial roles in cancer
metastasis. E-cadherin downregulation has been considered as not
only a marker of EMT, but also as a promoter of EMT. To further
confirm the function of IRS-1 and clarify its mechanism, we
detected critical markers of EMT. As shown in Fig. 5, downregulation of IRS-1 resulted in
significant decrease of E-cadherin in both mRNA and protein levels
and thus indicated EMT. But another EMT marker-vimentin did not
increase as we had expected. Since previous findings have also
shown distinctive E-cadherin expression in metastatic and
nonmetastatic HNSCC human tissues (3), our findings suggest that knockdown of
IRS-1 which induced loss of E-cadherin may account for enhanced
cell invasive potency and EMT.
IRS-1 knockdown increases miR-9
expression in 686LN cells
It has been recently reported that miR-9 is the only
miRNA that directly targets the 3′ UTR of E-cadherin and induces
its downregulation (20).
Therefore, we further detected the effects of IRS-1 knockdown on
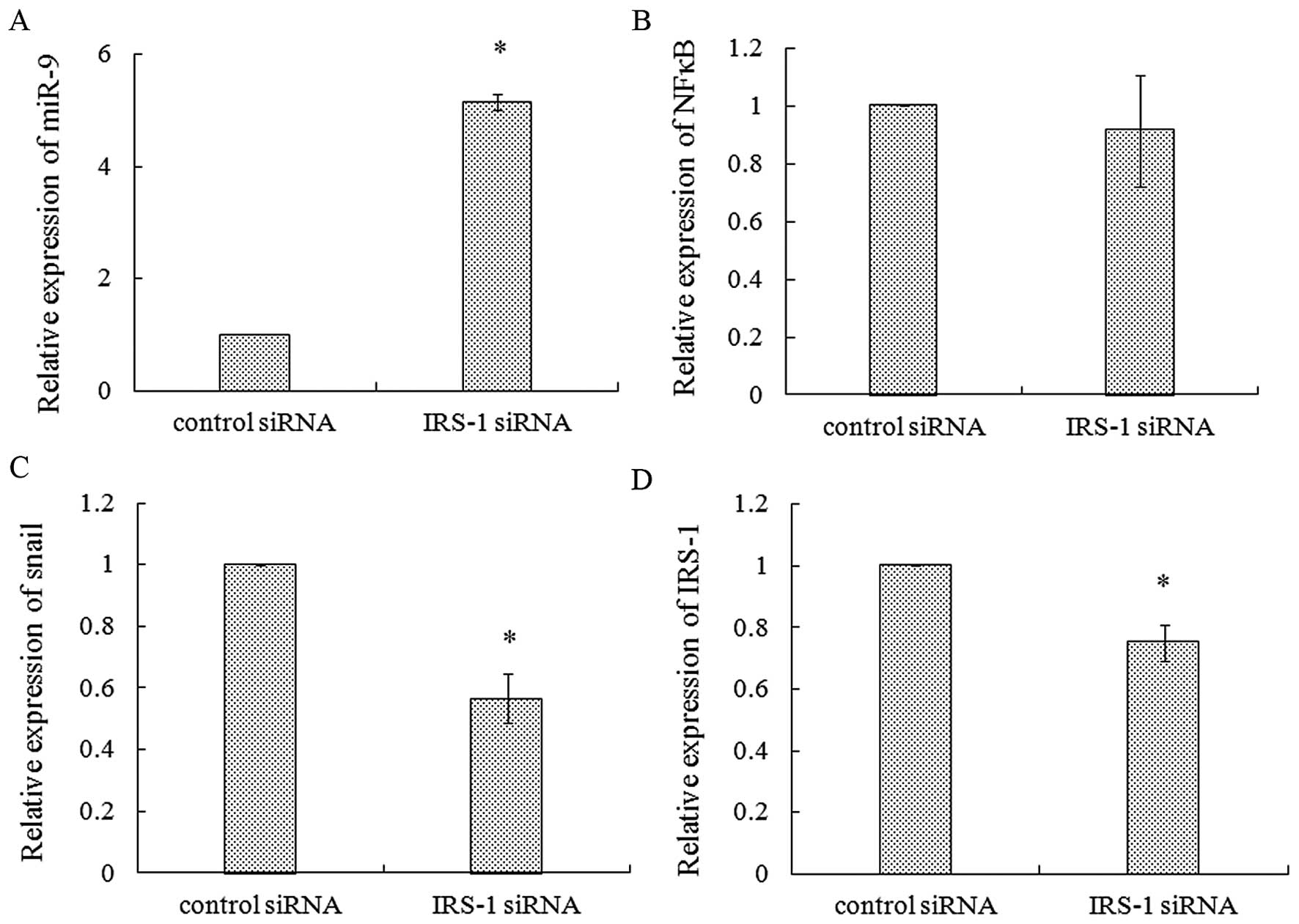
miR-9 expression in 686LN cells. Our results showed that miR-9
increased 5.15-fold (Fig. 6A) after
IRS-1 siRNA transfection for 48 h as compared with control siRNA,
and the parallel expression of E-cadherin decreased significantly
(Fig. 5A). NFκB has been reported
as another target gene of miR-9 in gastric cancer (34). NFκB has been demonstrated to play
important roles in cell growth, survival, apoptosis and motility,
thus promoting cancer progression, malignancy and metastasis
(35). Therefore, we also evaluated
NFκB expression after IRS-1 siRNA knockdown. As shown in Fig. 6B, no alteration of NFκB was
observed. In addition, we found decreased expression of snail in
686LN cells with IRS-1 knockdown, suggesting snail may not be
involved in E-cadherin downregulation (Fig. 6C). Fig.
6D shows significant decrease of IRS-1 after siRNA transfection
in the experiments aforementioned. These results suggested the
important role of miR-9 mediated downregulation of E-cadherin in
IRS-1 knockdown-induced invasion of 686LN cells.
Discussion
Metastasis is responsible for the high morbidity of
head and neck cancer patients. Thus, accurate diagnosis and
effective novel therapeutic regimens of metastasis are pivotal for
treatment of HNSCC. In this study, we found IRS-1 expression to be
decreased in the metastatic lymph node tumor tissues of HNSCC
patients compared with the primary tumor tissues. Additionally, we
identified decreased expression of IRS-1 in highly metastatic HNSCC
cells (686LN-M4e) compared with poorly metastatic cells (686LN).
These results indicate that IRS-1 downregulation may be a predictor
for metastasis of HNSCC. In the other cohort analyzed by IHC, even
though the positive staining rate for IRS-1 protein had no
difference between HNSCCs with and without lymph node metastasis,
we found decreased positive staining intensity of IRS-1 protein in
HNSCCs with lymph node metastasis. These results further suggest
that the low expression of IRS-1 may predict metastasis of HNSCC.
Barnes et al(36) have shown
increased expression of insulin-like growth factor type I receptor
(IGF-IR) both in cell lines and tumors of head and neck cancer.
Even though IRS-1 is the major mediator of IGF-IR activation, its
expression levels and activity have not been detected and are
unknown in head and neck cancer. In the present study, we detected
the IRS-1 expression in 108 HNSCCs using IHC and found that IRS-1
expression was negatively correlated with histological grade and
clinical stages. These results suggest IRS-1 may be developed as a
new predictive marker for progression and metastasis of HNSCC. This
may help clinicians to predict the biological behavior of tumors,
specifically in small biopsy specimens, and may provide more
accurate staging systems and means of monitoring patients with this
type of cancer (37).
IRS-1, as a positive effector of growth factors and
hormones, has been shown to facilitate the activation of a number
of intracellular signaling molecules, especially PI3K and MAPK,
involved in cell metabolism, growth, survival, transformation and
motility, thus IRS-1 is considered tumorigenic. Studies have also
demonstrated that IRS-1 can be both tumor promoter and suppressor
(5). Currently, studies involving
the role of IRS-1 in tumor transformation and metastasis are
controversial. In breast cancer, prostate cancer and neuroblastoma,
IRS-1 has been confirmed to induce cell motility and
transformation, suggesting it as a positive regulator (4,5). But
in other studies in breast cancer, Ma et al(10) and Gibson et al(38) have demonstrated that suppression of
IRS-1 promotes mammary tumor metastasis using an IRS-1 knockout
mouse model. In lung cancer, Shi et al(11) showed that IRS-1 suppresses cell
migration, invasion and EMT. In this study, for the first time, we
showed that in HNSCC cells, downregulation of IRS-1 enhances
invasive potency and induces EMT. Recent findings by Houghton et
al(7) demonstrated that
silencing IRS-1 expression increases proliferation of A549 cells,
suggesting it as a tumor suppressor in lung cancer. This is
consistent with studies by Shi et al(11) that IRS-1 is a metastatic suppressor.
Therefore, the IRS-1 impact may depend on cancer types.
When cells undergo EMT, they lose the features of
epithelial cells and acquire features of mesenchymal cells.
Therefore, hallmarks of epithelial cells such as E-cadherin
decrease and hallmarks of mesenchymal cells including vimentin and
fibronection increase. Among them, loss of E-cadherin expression is
the critical step of EMT in tumor metastasis. Other markers of EMT
also include elevated transcriptional factors such as snail, slug,
twist, bmi1 and SIP1 that suppress E-cadherin expression (12–14).
In this study, we detected a significant decrease of E-cadherin
after IRS-1 knockdown, but we did not observe an increase in
vimentin expression. EMT is a multi-step and complicated process;
therefore, the alterations of markers are sequential events. It is
possible that elevation of vimentin may be present in the later
stages of IRS-1 induced EMT, since 686LN-M4e cells have extremely
higher levels of vimentin as compared with their parental 686LN
cells (Fig. 4A). We could not rule
out other possibilities, such as other markers than vimentin that
may play essential roles for IRS-1 induced EMT of head and neck
cancer cells. Since loss of E-cadherin is not only the most common
indicator of EMT, but also a strong promoter of EMT, our results
suggest that IRS-1 may suppress EMT.
In recent years, miRNA has emerged as a new class of
gene expression regulators that are predicted to be involved in 30%
of human gene regulation. Deregulation of miRNAs has been confirmed
in many kinds of disease including cancer. Moreover, different
deregulated miRNA expression profiles may serve as predictive
markers for cancer types and subtypes, drug sensitivity and
different therapeutic strategies (16,17,19).
Overexpressed miRNAs in tumors function through downregulating
oncogene expression, while decreased miRNAs in tumors contribute
through upregulating expression of tumor suppressors. MiR-9 has
been suggested as both an oncogene and a tumor suppressor,
depending on different cancer types. MiR-9 acts as a putative tumor
suppressor gene in recurrent ovarian cancer, gastric cancer and
medulloblastoma. Its predicted target genes include RAB34, TrkC,
CDX2 and FOXO1 (19,39,40).
Wan et al(34) demonstrated
that miR-9 suppresses gastric cancer through direct targeting of
NFκB. Since NFκB plays important roles in tumor metastasis by
increasing VEGF and MMPs transcription, we also investigated its
expression (35). Our results
showed that NFκB was not affected by IRS-1 knockdown. In addition,
upregulation of miR-9 would result in downregulation of NFκB
expression and its downstream signals in metastasis; therefore,
IRS-1- induced upregulation of miR-9 does not seem likely to
function through NFκB. We also found downregulation of snail by
IRS-1 knockdown, which suggested that decreased E-cadherin
expression may not be through snail-mediated transcriptional
regulation. Recently, miR-9 has been confirmed to directly target
E-cadherin in breast cancer, and thus promotes EMT and tumor
metastasis (20). In this study, we
identified upregulation of miR-9 and correlated downregulation of
E-cadherin after IRS-1 knockdown; thus, our results suggest that
miR-9 mediated suppression of E-cadherin is involved in the IRS-1
suppressive impact on tumor metastasis.
In summary, we identified negative correlation of
IRS-1 expression with tumor metastasis both in tissue samples and
cell lines. Furthermore, we showed that downregulation of IRS-1
expression by siRNA promotes poorly metastatic HNSCC cells invasive
potency and induces EMT possibly through upregulation of miR-9
expression and subsequent loss of E-cadherin. Our findings indicate
that IRS-1 may have a negative impact on the survival of HNSCC
patients and may serve as a new potential target for cancer
therapy.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 30873099, 81172004 and
81102458). We thank Dr Zhuo Chen for providing 686LN and 686LN-M4e
cell lines (Georgia, USA). We thank Dr Heath Elrod for editing the
article.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2
|
Som PM: Detection of metastasis in
cervical lymph nodes: CT and MR criteria and differential
diagnosis. AJR Am J Roentgenol. 158:961–969. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Muller S, Su L, Tighiouart M, et al:
Distinctive E-cadherin and epidermal growth factor receptor
expression in metastatic and nonmetastatic head and neck squamous
cell carcinoma: predictive and prognostic correlation. Cancer.
113:97–107. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dearth RK, Cui X, Kim HJ, Hadsell DL and
Lee AV: Oncogenic transformation by the signaling adaptor proteins
insulin receptor substrate (IRS)-1 and IRS-2. Cell Cycle.
6:705–713. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Metz HE and Houghton AM: Insulin receptor
substrate regulation of phosphoinositide 3-kinase. Clin Cancer Res.
17:206–211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chang Q, Li Y, White MF, Fletcher JA and
Xiao S: Constitutive activation of insulin receptor substrate 1 is
a frequent event in human tumors: therapeutic implications. Cancer
Res. 62:6035–6038. 2002.
|
|
7
|
Houghton AM, Rzymkiewicz DM, Ji H, et al:
Neutrophil elastase-mediated degradation of IRS-1 accelerates lung
tumor growth. Nat Med. 16:219–223. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koda M, Sulkowska M, Kanczuga-Koda L and
Sulkowski S: Expression of insulin receptor substrate 1 in primary
breast cancer and lymph node metastases. J Clin Pathol. 58:645–649.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dearth RK, Cui X, Kim HJ, et al: Mammary
tumorigenesis and metastasis caused by overexpression of insulin
receptor substrate 1 (IRS-1) or IRS-2. Mol Cell Biol. 26:9302–9314.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma Z, Gibson SL, Byrne MA, Zhang J, White
MF and Shaw LM: Suppression of insulin receptor substrate 1 (IRS-1)
promotes mammary tumor metastasis. Mol Cell Biol. 26:9338–9351.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi J, Wang DM, Wang CM, et al: Insulin
receptor substrate-1 suppresses transforming growth
factor-beta1-mediated epithelial-mesenchymal transition. Cancer
Res. 69:7180–7187. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vernon AE and LaBonne C: Tumor metastasis:
a new twist on epithelial-mesenchymal transitions. Curr Biol.
14:R719–R721. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Martin A and Cano A: Tumorigenesis: Twist1
links EMT to self-renewal. Nat Cell Biol. 12:924–925. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McCarthy N: Metastasis: Twisting BMI1. Nat
Rev Cancer. 10:6662010. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: an alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eulalio A, Huntzinger E and Izaurralde E:
Getting to the root of miRNA-mediated gene silencing. Cell.
132:9–14. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ventura A and Jacks T: MicroRNAs and
cancer: short RNAs go a long way. Cell. 136:586–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Croce CM and Calin GA: miRNAs, cancer, and
stem cell division. Cell. 122:6–7. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nicoloso MS, Spizzo R, Shimizu M, Rossi S
and Calin GA: MicroRNAs--the micro steering wheel of tumour
metastases. Nat Rev Cancer. 9:293–302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma L, Young J, Prabhala H, et al: miR-9, a
MYC/MYCN-activated microRNA, regulates E-cadherin and cancer
metastasis. Nat Cell Biol. 12:247–256. 2010.PubMed/NCBI
|
|
21
|
Burk U, Schubert J, Wellner U, et al: A
reciprocal repression between ZEB1 and members of the miR-200
family promotes EMT and invasion in cancer cells. EMBO Rep.
9:582–589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wellner U, Schubert J, Burk UC, et al: The
EMT-activator ZEB1 promotes tumorigenicity by repressing
stemness-inhibiting microRNAs. Nat Cell Biol. 11:1487–1495. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang X, Su L, Pirani AA, et al:
Understanding metastatic SCCHN cells from unique genotypes to
phenotypes with the aid of an animal model and DNA microarray
analysis. Clin Exp Metastasis. 23:209–222. 2006. View Article : Google Scholar
|
|
24
|
Zhang X, Liu Y, Gilcrease MZ, et al: A
lymph node metastatic mouse model reveals alterations of
metastasis-related gene expression in metastatic human oral
carcinoma sublines selected from a poorly metastatic parental cell
line. Cancer. 95:1663–1672. 2002. View Article : Google Scholar
|
|
25
|
Zhang H, Su L, Muller S, et al:
Restoration of caveolin-1 expression suppresses growth and
metastasis of head and neck squamous cell carcinoma. Br J Cancer.
99:1684–1694. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cantarini MC, de la Monte SM, Pang M, et
al: Aspartyl-asparagyl beta hydroxylase over-expression in human
hepatoma is linked to activation of insulin-like growth factor and
notch signaling mechanisms. Hepatology. 44:446–457. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gregory PA, Bert AG, Paterson EL, et al:
The miR-200 family and miR-205 regulate epithelial to mesenchymal
transition by targeting ZEB1 and SIP1. Nat Cell Biol. 10:593–601.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bazzoni F, Rossato M, Fabbri M, et al:
Induction and regulatory function of miR-9 in human monocytes and
neutrophils exposed to proinflammatory signals. Proc Natl Acad Sci
USA. 106:5282–5287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dubrovska A, Kim S, Salamone RJ, et al:
The role of PTEN/Akt/PI3K signaling in the maintenance and
viability of prostate cancer stem-like cell populations. Proc Natl
Acad Sci USA. 106:268–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tiede S, Kloepper JE, Ernst N, Poeggeler
B, Kruse C and Paus R: Nestin in human skin: exclusive expression
in intramesenchymal skin compartments and regulation by leptin. J
Invest Dermatol. 129:2711–2720. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shao M, Cao L, Shen C, et al:
Epithelial-to-mesenchymal transition and ovarian tumor progression
induced by tissue transglutaminase. Cancer Res. 69:9192–9201. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tsuji S, Hisaoka M, Morimitsu Y, et al:
Detection of SYT-SSX fusion transcripts in synovial sarcoma by
reverse transcription-polymerase chain reaction using archival
paraffin-embedded tissues. Am J Pathol. 153:1807–1812. 1998.
View Article : Google Scholar
|
|
33
|
Chen Z, Zhang K, Zhang X, et al:
Comparison of gene expression between metastatic derivatives and
their poorly metastatic parental cells implicates crucial
tumor-environment interaction in metastasis of head and neck
squamous cell carcinoma. Clin Exp Metastasis. 20:335–342. 2003.
View Article : Google Scholar
|
|
34
|
Wan HY, Guo LM, Liu T, Liu M, Li X and
Tang H: Regulation of the transcription factor NF-kappaB1 by
microRNA-9 in human gastric adenocarcinoma. Mol Cancer. 9:162010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Garg A and Aggarwal BB: Nuclear
transcription factor-kappaB as a target for cancer drug
development. Leukemia. 16:1053–1068. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Barnes CJ, Ohshiro K, Rayala SK, El-Naggar
AK and Kumar R: Insulin-like growth factor receptor as a
therapeutic target in head and neck cancer. Clin Cancer Res.
13:4291–4299. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Forastiere A, Koch W, Trotti A and
Sidransky D: Head and neck cancer. N Engl J Med. 345:1890–1900.
2001. View Article : Google Scholar
|
|
38
|
Gibson SL, Ma Z and Shaw LM: Divergent
roles for IRS-1 and IRS-2 in breast cancer metastasis. Cell Cycle.
6:631–637. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Myatt SS, Wang J, Monteiro LJ, et al:
Definition of microRNAs that repress expression of the tumor
suppressor gene FOXO1 in endometrial cancer. Cancer Res.
70:367–377. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rotkrua P, Akiyama Y, Hashimoto Y, Otsubo
T and Yuasa Y: MiR-9 downregulates CDX2 expression in gastric
cancer cells. Int J Cancer. 129:2611–2620. 2011. View Article : Google Scholar : PubMed/NCBI
|