Introduction
Prostate cancer (PCa) is the most frequently
diagnosed cancer in men and the second leading cause of cancer
death among men in the United States (1). The most common site of PCa metastasis
is the bone, with bone metastases identified at autopsy in up to
90% of patients dying from PCa (2–4).
However, the cellular and molecular mechanism underlying bone
metastasis is relatively poorly understood, and more effective
therapeutic strategies are clearly required in order to oppose PCa
bone metastasis.
MicroRNAs (miRNAs) are a diverse family of small RNA
molecules that function as a crucial post-transcriptional
regulatory mechanism in various cellular functions (5,6) and
play a crucial role in tumor metastasis by regulating migration,
invasion and epithelial to mesenchymal transition (EMT) (7–10). In
PCa, a series of miRNAs were identified to regulate metastasis
including miR-21 (11), miR-221
(12), miRNA-200 (13), miR-34a (14) and let-7 (15). We have previously identified that
miR-143 and -145 repressed the ability of migration and invasion of
PC-3 cells from PCa bone metastasis, and tumor development and bone
invasion in vivo, and were negatively correlated to bone
metastasis (16). Although we have
further found that miR-143 and miR-145 may repress bone metastasis
of PCa by regulating EMT (16),
which is considered to be a crucial event in the metastatic process
(17), the exact mechanisms of
miR-143 and miR-145 regulating bone metastasis of PCa is not fully
understood.
In recent years, accumulating evidence has provided
support that a number of major cancers may be initiated by a small
subset of cancer cells with stem cell properties, referred as
cancer stem cells (CSCs), which display unlimited proliferation
potential, ability to self-renew, and capacity to generate a
progeny of differentiated cells that constitute the major tumor
population (18–20). Emerging evidence demonstrates that
CSCs might be the critical drivers of tumor progression and
metastasis (21,22). Furthermore, miRNAs also played a
pivotal role in regulating the characteristics of CSCs by
negatively regulating the expression of certain key genes in stem
cells such as CD44, Oct4, Sox2, c-Myc, and Klf4 (23). Therefore, miRNAs may play a crucial
role in repressing/promoting metastasis of cancer by regulating
CSCs. In PCa, the recent studies have showed that let-7 (15), miRNA-200 (13) and miR-34a (14) may be important in the progression
and metastasis of cancer by regulating CSCs. Because miR-145
regulated Oct4, Sox2 and Klf4, and repressed pluripotency in human
embryonic stem cells (ESCs) (24),
at the same time, CSCs may share a degree of similarity with ESCs
(25), miR-145 might regulate the
stemness factors in PCa cells. Moreover, miR-143 and miR-145 may
repress bone metastasis of PCa by regulating EMT (16), which is mechanistically linked with
stem cell signatures in PCa (13).
Thus, the above findings make us hypothesize that miR-143 and
miR-145 might regulate stem cell characteristics of PCa cells.
In this study, to test the hypothesis, we used in
vitro assays and in vivo xenograft models to examine the
effects of miR-143 and miR-145 on stem cell characteristics of PCa
bone metastasis PC-3 cells. The results demonstrated that both
miR-143 and miR-145 inhibited CSC characteristics of PC-3 cells and
suggest that they might be involved in the bone metastasis
progression of PCa by regulating CSC characteristics.
Materials and methods
Cell culture and generation of stably
transfected cell lines
The bone metastatic PCa cell line PC-3 was purchased
from American Type Culture Collection (ATCC) and maintained in F-12
culture medium (Hyclone) supplemented with 10% fetal bovine serum
(Hyclone). Stably-transfected cells were maintained in media with
the presence of puromycin (Sigma-Aldrich). Cells were grown at a
humidified atmosphere of 5% CO2 at 37˚C. The sequence of
pri-miR-143 and pri-miR-145 were cloned into pMSCV-puromycin
plasmid with restriction enzyme BglII and EcoRI (New
England Biolabs). 293FT cells were then transfected with the
aforementioned constructed plasmids combined with PIK vector or
blank pMSCV-vector as control, using the calcium phosphate method
as described previously (26).
After incubation at 37˚C for 6 h after transfection, the media were
changed and the cells were incubated overnight. To produce new
virus, the media were collected thrice a day until 293FT cells
reach to total confluence. Viruses were used to infect PC-3 cells.
Twenty-four hours after addition of viruses, infected cells were
selected by adding puromycin to growth medium. Stable cell lines
were verified by qRT-PCR. Both pMSCV and PIK plasmids were generous
gifts of Professor L.B. Song, Sun Yat-Sen University Cancer Center,
Guangzhou, China.
Cell viability assay
Cell viability was determined by
2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophen
yl)-2H-tetrazolium, monosodium salt (WST-8) assay kit (CCK-8,
Dojindo, Japan). Briefly, PC-3 cells were plated at a density of
5×103 cells/well in 96-well plates and allowed to attach
for 36 h, CCK-8 was used according to the manufacturer’s
instructions. WST-8 was added into each well for 4 hours before the
measurement. The absorbance at 450 nm was measured using a
microplate reader.
Colony formation assay
Colony formation assay was performed as previously
described (27). PC-3 cells were
plated at 300 cells as single cells onto a 65-mm Petri dish for 14
days, and colonies were stained with crystal violet. Plating
efficiency = number of colonies (≥50 cells per colony) per input
cells × 100%. To determine different colony morphologies, the
different colony morphologies were scored under a light
microscope.
Self-renewing spheroid formation
assay
Spheroid formation assay was performed in
PC-3/miR-143, PC-3/miR-145 and PC-3/vector. Cells were plated at
400 cells/well onto 6-well polyHEMA (Sigma)-coated plates and were
grown in F12 medium (Hycolone) for 14 days supplemented with B27
(Invitrogen), 20 ng/ml EGF (Sigma), and 20 ng/ml basic FGF
(Invitrogen). After 14 days, the number of prostaspheres (tight,
spherical, non-adherent masses >100 μm in diameter) were
counted, and image of the prostaspheres were captured under inverse
microscope. Sphere formation efficiency = colonies/input cells ×
100%.
Western blot assay
Western blotting was performed as previously
described (16). Briefly, cells
were lysed with sample buffer [62.5 mmol/l Tris-HCl (pH 6.8), 2%
SDS, 10% glycerol, and 5% 2-β-mercaptoethanol]. Proteins were
resolved in SDS-polyacrylamide gel by electrophoresis and then
transferred onto Hybond-P PVDF membrane (Amersham Biosciences,
Piscataway, NJ). Antibodies used were anti-Oct4, anti-Sox2,
anti-c-Myc, and anti-Klf4 (Cell Signaling, Technology Inc.,
Beverly, MA), CD133 (Miltenyi Biotech, Auburn, CA), CD44 (Santa
Cruz Biotechnology, Santa Cruz, CA). After washing with TBS-T, the
membrane was incubated with anti-rabbit IgG secondary antibodies,
and the signals were visualized using the ECL plus western blotting
system (Amersham).
In vivo tumorigenicity assay
To determine whether miR-143 and miR-145 can inhibit
tumor development, we manipulated miR-143 and miR-145 levels in PCa
PC-3 cells and then implanted the cells into the NOD-SCID mice.
Intra-tibial injection model was used. Twelve male severe combined
immunodeficient (SCID) mice, 3–4 weeks old, were purchased from HFK
Bio-Technology, Co., Ltd. (Beijing, China). Intra-tibial injection
was performed as previously described (16). Mice were monitored weekly for tumor
growth. On week 5, hind limbs were radiographed using a Faxitron
X-ray machine (Faxitron X-ray Corp., USA) to detect the bone
lesions. Then mice were sacrificed, and tibias were collected,
decalcified and fixed in formalin for further histologic analysis.
Bone lesions were evaluated and calculated as described as
previously described (16,28). The animals were sacrificed 5 weeks
after receiving radiograph. All tumors were resected at autopsy and
sectioned for histological analysis. The animal study was approved
by the Institutional Ethical Board (IRB) in the First Affiliated
Hospital of Sun Yat-sen University.
Statistical analysis
Experimental data were expressed as mean ± standard
deviation (SD). One-way ANOVA was performed for comparing more than
two groups, and paired Student’s t-test was performed for comparing
X-ray scores. Statistical analyses were assessed using SPSS 17.0
(SPSS, Inc., Chicago, IL, USA). Statistical significance was
accepted at p<0.05.
Results
miR-143 and miR-145 inhibits cell
viability
The miR-143 and miR-145 overexpressing cell lines
(PC-3/miR-143, PC-3/miR-145) were established by retrovirus
transfection (16). Blank plasmid
transfected cells, PC-3/vector were used as control group. To test
if miR-143 and miR-145 decrease cell viability of PC-3 cells, cell
viability of PC-3/miR-143, PC-3/miR-145 and PC-3/vector was
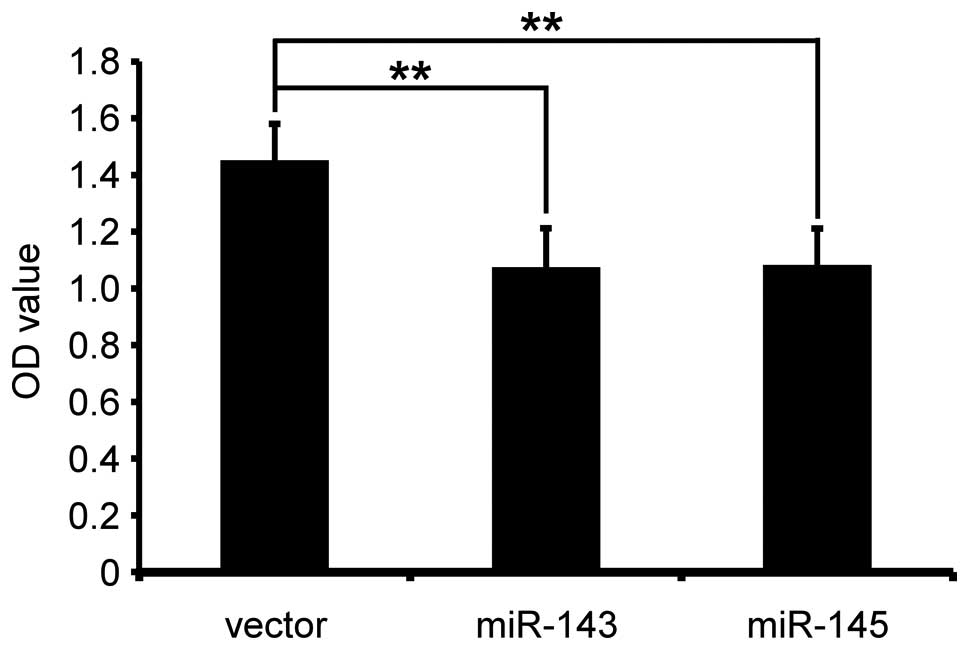
examined by CCK8 assay for 36 h. The results showed that miR-143
and miR-145 significantly reduced cell viability (PC-3/miR-143
compared with PC-3/vector, p<0.01; PC-3/miR-145, compared with
PC-3/vector, p<0.01) (Fig.
1).
miR-143 and miR-145 inhibits colony
formation in PC-3
To determine efficiency of miR-143 and miR-145
inhibiting colony-forming of PC-3 in vitro, colony-forming
assay was performed in PC-3/miR-143, PC-3/miR-145 and PC-3/vector.
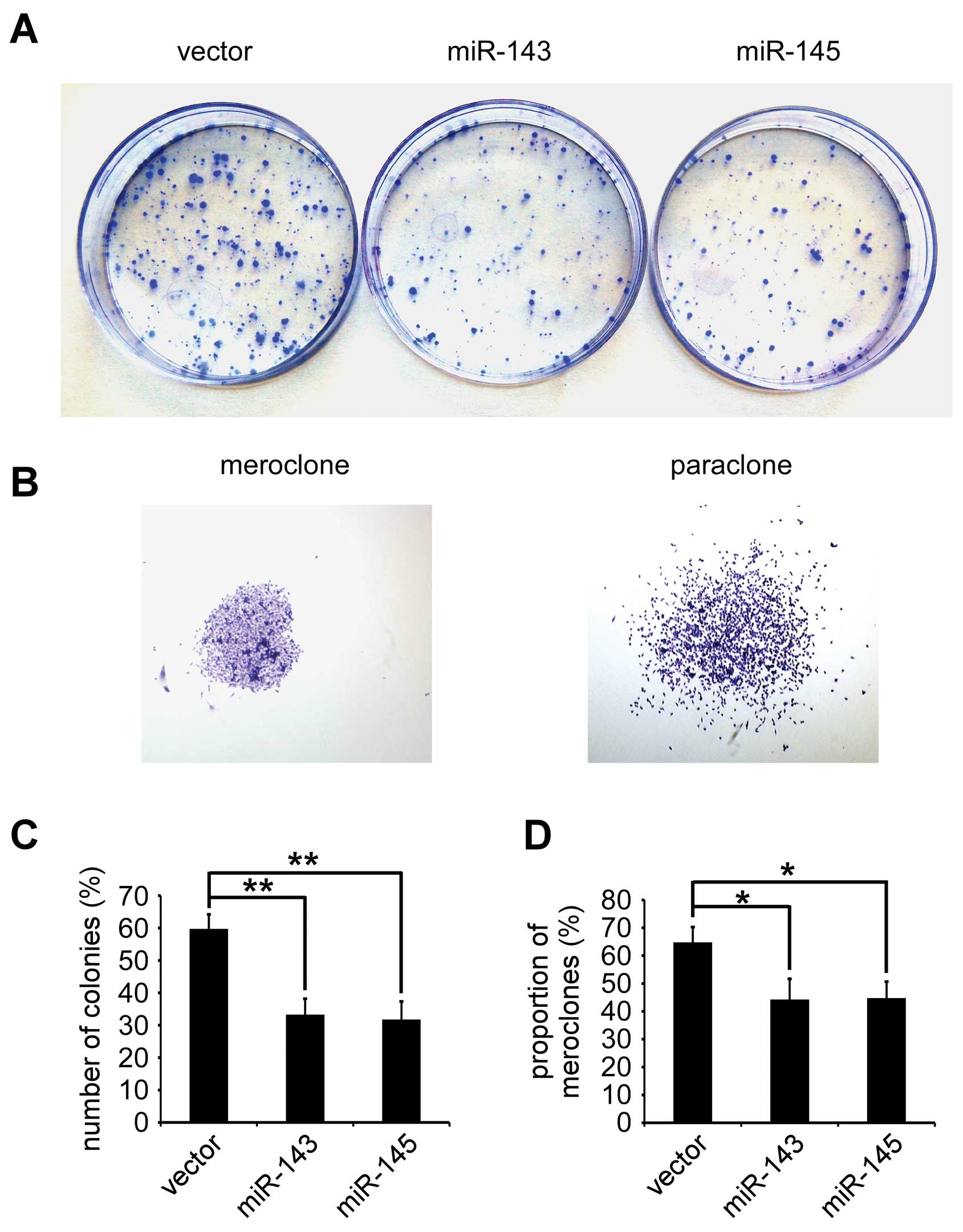
The number of colonies (% plating efficiency) were 33.25±4.92% in
PC-3/miR-143, 31.75±5.56% in PC-3/miR-145, and 59.75±4.42% in
PC-3/vector, and significantly decreased in PC-3/miR-143 and
PC-3/miR-145 compared with PC-3/vector (p<0.01, respectively)
(Fig. 2A and C). Colonies with
different morphologies in vitro are classified as
holoclones, meroclones, and paraclones (27). Holoclones are generally more round
and tightly packed, and paraclones are irregular in composition and
often contain more elongated or flattened cells, and meroclones are
an intermediate phenotype. We only found meroclones and paraclones
in PC-3 cells (Fig. 2B). The
proportion of meroclones was 44.25±7.46% in PC-3/miR-143,
44.75±5.90% in PC-3/miR-145, and 64.75±5.50% in PC-3/vector the
miR-143 and miR-145 significantly decreased the proportion of
meroclones of PC-3 cells (p<0.05) (Fig. 2D).
miR-143 and miR-145 inhibit tumor
spheroid formation
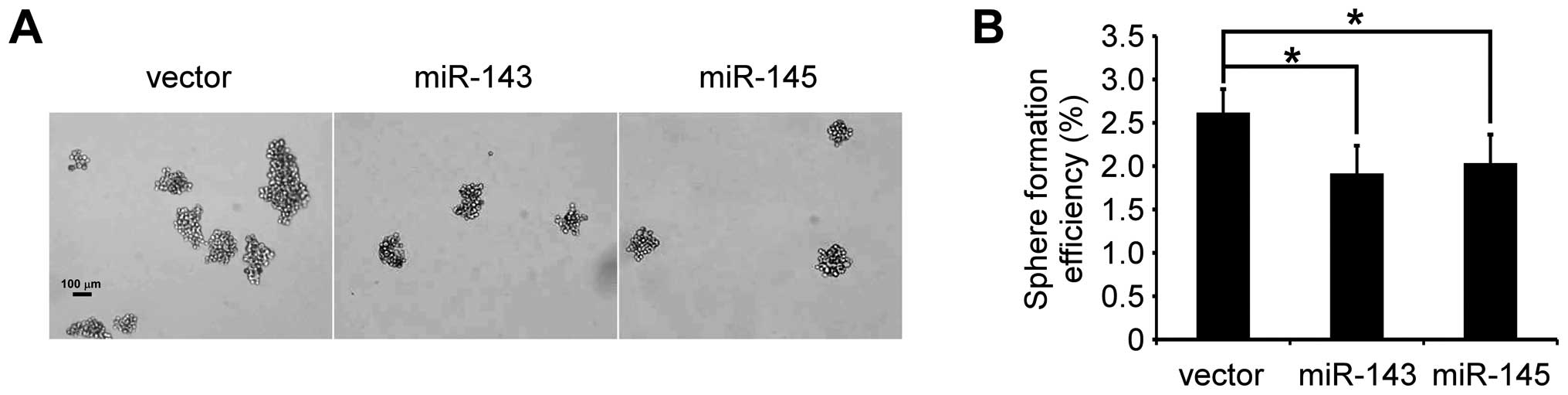
The ability to grow as non-adherent spheroids in the
sphere medium has been widely used to assess the self-renewal
capability of CSCs and is one of the characteristics of prostate
CSCs (20,29). To confirm that miR-143 and miR-145
can inhibit the self-renewal capability of PC-3 cells, prostasphere
formation of PC-3 cells was studied. As shown in Fig. 3, after culturing for 14 days under
non-adherent conditions, there were prostaspheres in all the three
kinds of cells. The spheroid formation efficiency was 2.618±0.27%
in PC-3/vector, 1.915±0.32% in PC-3/miR-143 and 2.034±0.33% in
PC-3/miR-145, confirming the presence of the self-renewal cells in
PC-3/miR-143, PC-3/miR-145 and PC-3/vector. Further, both miR-143
and miR-145 suppressed significantly prostasphere formation
(p<0.05, respectively). This result indicated that miR-143 and
miR-145 repressed CSCs properties of PC-3 cells.
miR-143 and miR-145 inhibit CSC marker
and stemness factor expression
Because CD133 and CD44 have been described as
prostate CSC markers based on clinical investigations and in
vitro studies of prostate cancer cell lines (14,30–32),
we first investigated if miR-143 and miR-145 repressed the
expression in PC-3 cells. The expression of CD133 and CD44 was
examined by western blotting. As shown in Fig. 4, overexpression of miR-143 and
miR-145 repressed the expression of CD133 and CD44. Furthermore,
since transcription factors Oct-4, Sox-2, c-Myc and KLF4 are the
key stemness factors and are required for maintaining self-renewal
and pluripotency of stem cells (24,33),
we sought to determine whether miR-143 and miR-145 regulate the
expression of these stemness factors. As shown in Fig. 4, overexpression of miR-143 and
miR-145 downregulated the expression of Oct4, c-Myc and Klf4, but
Sox2 was not detected in PC-3/miR-143, PC-3/miR-145 and
PC-3/vector. These results suggested that miR-143 and miR-145 might
modulate CSCs properties in PC-3 cells by regulating CD133, CD44,
Oct4, c-Myc and Klf4.
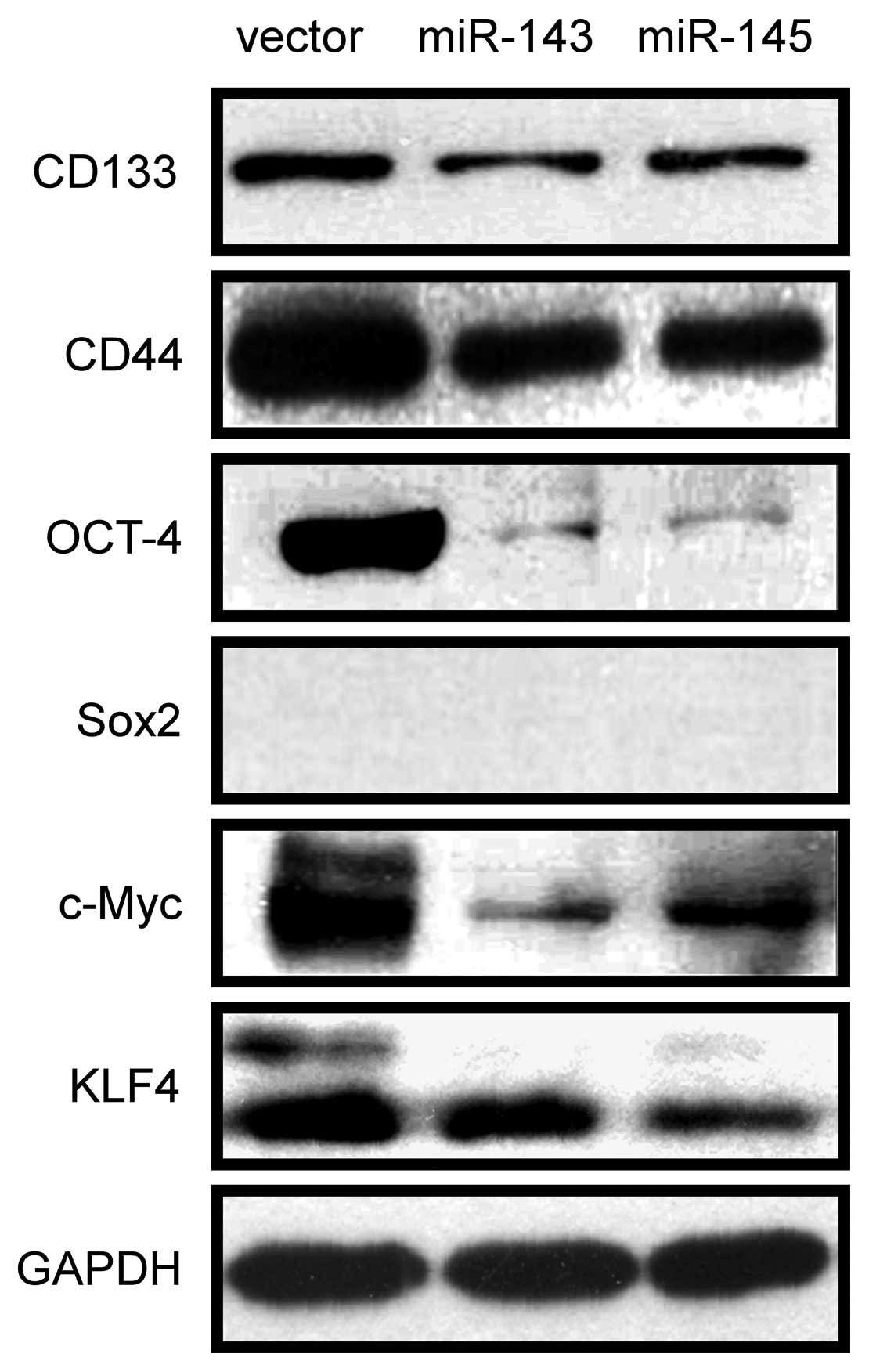 | Figure 4miR-143 and miR-145 inhibit CSC
marker and stemness factor expression. CD133, CD44, OCT4, SOX2,
C-MYC and KLF4 were detected by western blotting in PC-3/miR-143,
PC-3/miR-145 and PC-3/vector. Downregulation of CD133, CD44, OCT4,
C-MYC and KLF4CD133, KLF4 was observed in PC-3/miR-143,
PC-3/miR-145 compared with PC-3/vector, but SOX2 was not detected
in PC-3 cells. |
miR-143 and miR-145 inhibit
tumorigenicity in vivo
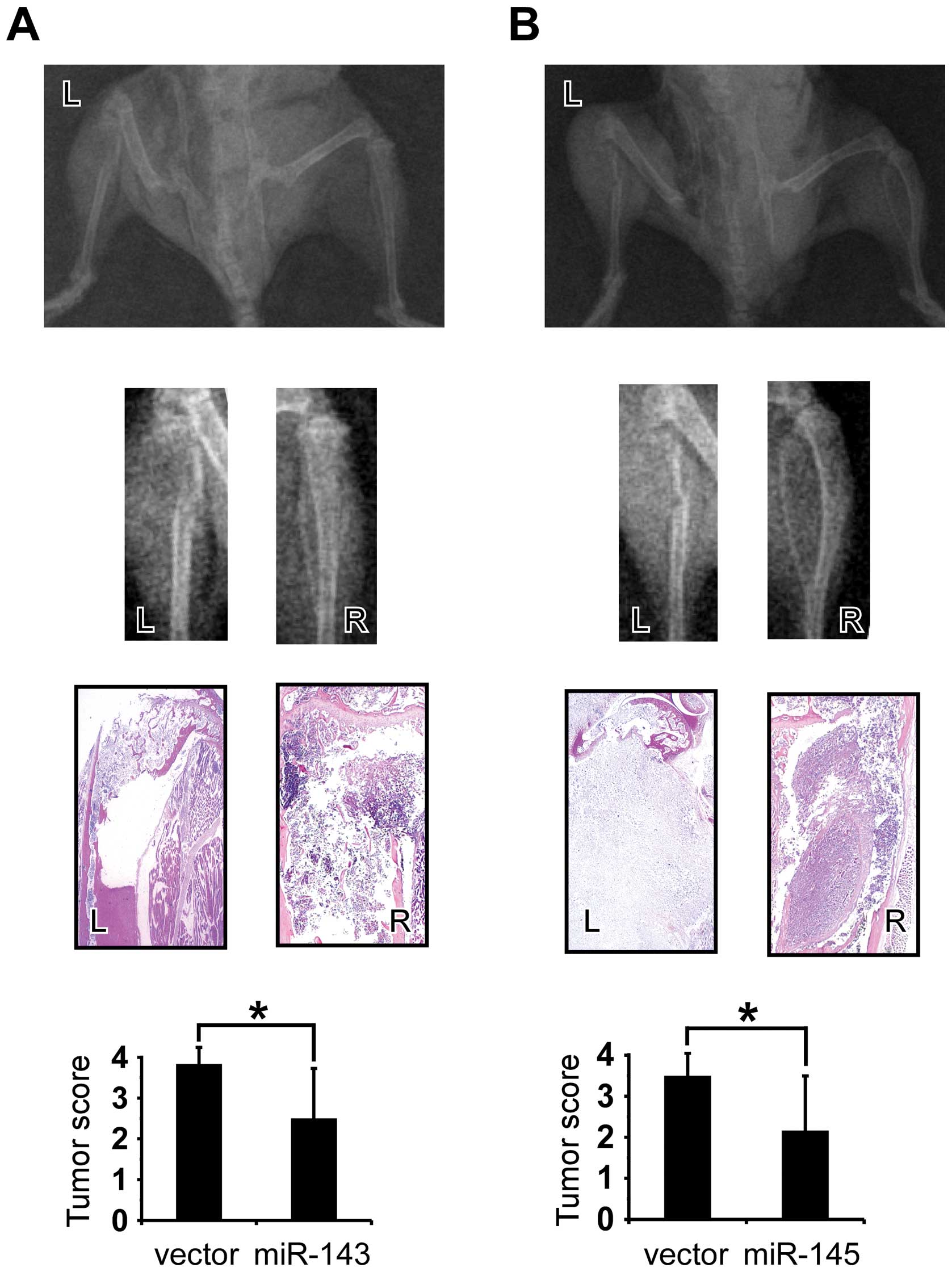
To determine if miR-143 and miR-145 repressed
tumorigenicity in vivo, male SCID mice were inoculated with
PC-3/miR-143, PC-3/miR-145 and PC-3/vector cells through the
intra-tibial route. Five weeks after inoculation, as showed in
Fig. 5A and B, skeletal lesions in
the left tibias were obviously larger than those in the right
tibias, which mean that PC-3/miR-143 and PC-3/miR-145 had less
skeletal invasion and tumorigenicity ability than PC-3/vector.
H&E-staining was performed as histological confirmation. The
extent and areas of skeletal lesions were assessed by X-ray scores,
and PC-3/miR-143 and PC-3/miR-145 showed significantly less ability
in forming tumors and bone invasion compared with PC-3/vector
(p<0.05, respectively). The results suggested that miR-143 and
miR-145 could repress bone invasion and tumorigenicity.
Discussion
In this study, we found that miR-143 and miR-145
inhibited the cell viability, suppressed colony formation and
repressed tumor sphere formation of PC-3 cells from PCa bone
metastasis. Furthermore, miR-143 and miR-145 repressed expression
of CSC markers and stemness factors including CD133, CD44, Oct4,
c-Myc and Klf4 in PC-3 cells. Both inhibited bone invasion and
tumorigenicity of PC-3 cells in NOD-SCID mice tibia. These findings
demonstrate that miR-143 and miR-145 negatively regulate the CSCs
properties of PC-3 cells from PCa bone metastasis. Importantly,
CSCs may be the critical drivers of tumor progression and
metastasis (21,22). Thus, our results suggest that
miR-143 and miR-145 might play a significant role in the bone
metastasis progression of PCa by regulating CSC
characteristics.
Emerging evidence suggests that miRNAs may function
as the regulators of CSC characteristics in many studies (13–16,23,34).
In PCa, let-7 inhibited self-renewal and clonogenic capacity of
cancer cells by directly targeting EZH2 (15). The miR-34a was established as an
important negative regulator of CD44+ PCa cells
(putative CSCs) and is involved in PCa development and metastasis
(14). The role of miR-34a in
controlling CSC characteristics appears to be important by directly
repressing CD44 expression in PCa (14). In this study, we found miR-143 and
miR-145 also can repressed the expression of CD44, which is
speculated as one of miR-143 and miR-145 putative targets (miRWalk)
and is the most common of CSC markers (32). Previously, CD44+ PCa
cells were shown to have the stem-like properties of increased
tumorigenic, clonogenic, and metastatic potential (30). Although CD44 does not seem to belong
to the stemness genes, such as Oct4 and Klf4, that are central for
maintaining stem cell characteristics, CD44 can contribute to the
activation of stem cell regulatory genes and can be a target of
these genes (32). More
importantly, a recent study has demonstrated that the
transcriptional reprogramming led by nuclear CD44 has an active
role in transforming cancer cells to a CSC-like phenotype (17). Therefore, our finding suggested that
miR-143 and miR-145 may possess a similar function with miR-34a in
controlling CSC characteristics of PCa, and regulate metastasis of
PCa by targeting CD44.
Previously, it was found that miR-145 directly
targets the 3′UTRs of the stemness factors Oct4, Sox2, and Klf4 in
ESCs (24) and the stemness factor
c-Myc also is a direct target for miR-145 (35). Yang et al (36) and Chiou et al (37) demonstrated that miR145 repressed
CSCs characteristics by targeting Oct4 and Sox2 in
glioblastoma-CD133+ and lung adenocarcinoma-associated
CSCs. Our results showed that miR-145 repressed the expression of
Oct4, c-Myc and Klf4 of PC-3 cells. Thus, miR-145 may regulate CSC
characteristics of PC-3 cells, at least in part, by directly
targeting Oct4, c-Myc, and Klf4. In this study, the results also
showed that miR-143 play a similar role to miR-145 regulating CSC
characteristics of PC-3 cells and repressed the expression of Oct4,
c-Myc, and Klf4. However, how miR-143 regulate the stemness factors
and the exact mechanism of miR-143 regulation of CSC
characteristics need to be further explored. We did not detect the
expression of Sox2 in PC-3 cells by western blot analysis. The
transcripts for Sox2 were not detected in the PC-3 cells by reverse
transcription (RT)-PCR (38). Thus,
Sox2 may not be the factor regulated by miR-143 and miR-145 in PC-3
cells.
CSCs and EMT-type cells have been proposed to play
critical roles in cancer metastasis as demonstrated in several
human malignancies (39). Recent
evidence has demonstrated that the EMT can generate cancer cells
with properties of stem cells (13,40–42).
This important finding implies a direct link between EMT and cancer
stem cells. Thus, the discovery of molecular knowledge related to
CSC characteristics and EMT in PCa is important. Previously it was
found that miR-200 and let-7 played a critical role in linking EMT
phenotype with stem cell signatures by regulating the expression of
Lin28B and Notch1 (13). In this
study, we found that miR-143 and miR-145 regulated CSC
characteristics of PCa. Importantly, our previous study found that
overexpression of miR-143 and miR-145 repressed EMT of PC-3 cells
of PCa (16). Therefore, miR-143
and miR-145 might be the new links between the characteristics of
cancer stem-like cells and EMT in PC-3 cells.
In our study, morphologically typical holoclones
were not detected in PC-3 cells. The results of Pfeiffer and
Schalken (27) are in agreement
with ours. However, other studies showed that the number of
holoclones formed by PC-3 cells was composed of approximately 10%
of all clones (43,44). The main reason for this phenomenon
is that higher plating density was adopted in our and Pfeiffer and
Schalken studies compared with previous report in which PC3 cells
were plated under diluted conditions (~1 cell per well) (44,45).
Moreover, it was demonstrated earlier that PC-3 cells had an
intrinsic impaired cell-cell adhesion because of the E-cadherin and
associated α-catenin were frequently reduced or absent in this
cells, thus, they were not able to form tightly packed colonies
(45). Additionally, continuous and
rapid change of different colony types made the colonies difficult
to distinguish from each other (27,45).
The definition of the three colony morphologies differs somewhat
from each other, and there are no strict borderlines between the
colony types, which makes the grading fairly subjective (27).
In conclusion, we have demonstrated, for the first
time, that miR-143 and miR-145 inhibit CSC properties of PC-3 cells
from PCa bone metastasis. Our findings suggest that miR-143 and
miR-145 might inhibit the bone metastasis progression of PCa by
repressing CSC characteristics, and might hold significant promise
as a new class of molecular therapy for human PCa bone metastasis,
potentially by modulating cancer stem cells.
Acknowledgements
We thank Dr Wenjian Wang, Dr Longjuan Zhang and Dr
Wen Li from the Surgical Laboratory at The First Affiliated
Hospital of Sun Yat-sen University for their excellent technical
help. We also thank NSFC-Guangdong Joint funding, China (No.
u0732001); Science and Technology planning project of Guangdong
Province, China (No. 2008B030301037), Science and Technology
Planning Project of Guangzhou, China (11C22060772) and Science and
Technology Planning Project of Zhuhai, China (2009) for supporting
this study.
Abbreviations:
|
PCa
|
prostate cancer
|
|
miRNA
|
microRNAs
|
|
CSCs
|
cancer stem cells
|
|
EMT
|
epithelial to mesenchymal
transition
|
|
ESCs
|
embryonic stem cells
|
References
|
1
|
Landis SH, Murray T, Bolden S and Wingo
PA: Cancer statistics, 1999. CA Cancer J Clin. 49:8–31. 1999.
View Article : Google Scholar
|
|
2
|
Bubendorf L, Schopfer A, Wagner U, et al:
Metastatic patterns of prostate cancer: an autopsy study of 1,589
patients. Hum Pathol. 31:578–583. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shah RB, Mehra R, Chinnaiyan AM, et al:
Androgen-independent prostate cancer is a heterogeneous group of
diseases: lessons from a rapid autopsy program. Cancer Res.
64:9209–9216. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rana A, Chisholm GD, Khan M, Sekharjit SS,
Merrick MV and Elton RA: Patterns of bone metastasis and their
prognostic significance in patients with carcinoma of the prostate.
Br J Urol. 72:933–936. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Inui M, Martello G and Piccolo S: MicroRNA
control of signal transduction. Nat Rev Mol Cell Biol. 11:252–263.
2010. View
Article : Google Scholar
|
|
6
|
Schickel R, Boyerinas B, Park SM and Peter
ME: MicroRNAs: key players in the immune system, differentiation,
tumorigenesis and cell death. Oncogene. 27:5959–5974. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baranwal S and Alahari SK: miRNA control
of tumor cell invasion and metastasis. Int J Cancer. 126:1283–1290.
2010.PubMed/NCBI
|
|
8
|
Garzon R, Calin GA and Croce CM: MicroRNAs
in cancer. Annu Rev Med. 60:167–179. 2009. View Article : Google Scholar
|
|
9
|
Khew-Goodall Y and Goodall GJ:
Myc-modulated miR-9 makes more metastases. Nat Cell Biol.
12:209–211. 2010.PubMed/NCBI
|
|
10
|
Nicoloso MS, Spizzo R, Shimizu M, Rossi S
and Calin GA: MicroRNAs - the micro steering wheel of tumour
metastases. Nat Rev Cancer. 9:293–302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li T, Li D, Sha J, Sun P and Huang Y:
MicroRNA-21 directly targets MARCKS and promotes apoptosis
resistance and invasion in prostate cancer cells. Biochem Biophys
Res Commun. 383:280–285. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Spahn M, Kneitz S, Scholz CJ, et al:
Expression of microRNA-221 is progressively reduced in aggressive
prostate cancer and metastasis and predicts clinical recurrence.
Int J Cancer. 127:394–403. 2010.PubMed/NCBI
|
|
13
|
Kong D, Banerjee S, Ahmad A, et al:
Epithelial to mesenchymal transition is mechanistically linked with
stem cell signatures in prostate cancer cells. PLoS One.
5:e124452010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu C, Kelnar K, Liu B, et al: The
microRNA miR-34a inhibits prostate cancer stem cells and metastasis
by directly repressing CD44. Nat Med. 17:211–215. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kong D, Heath E, Chen W, et al: Loss of
let-7 up-regulates EZH2 in prostate cancer consistent with the
acquisition of cancer stem cell signatures that are attenuated by
BR-DIM. PLoS One. 7:e337292012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Peng X, Guo W, Liu T, et al:
Identification of miRs-143 and -145 that is associated with bone
metastasis of prostate cancer and involved in the regulation of
EMT. PLoS One. 6:e203412011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Su YJ, Lai HM, Chang YW, Chen GY and Lee
JL: Direct reprogramming of stem cell properties in colon cancer
cells by CD44. EMBO J. 30:3186–3199. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Clarke MF, Dick JE, Dirks PB, et al:
Cancer stem cells - perspectives on current status and future
directions: AACR Workshop on cancer stem cells. Cancer Res.
66:9339–9344. 2006. View Article : Google Scholar
|
|
19
|
Sun S and Wang Z: ALDH high adenoid cystic
carcinoma cells display cancer stem cell properties and are
responsible for mediating metastasis. Biochem Biophys Res Commun.
396:843–848. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Monteiro J and Fodde R: Cancer stemness
and metastasis: therapeutic consequences and perspectives. Eur J
Cancer. 46:1198–1203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hatfield S and Ruohola-Baker H: microRNA
and stem cell function. Cell Tissue Res. 331:57–66. 2008.
View Article : Google Scholar
|
|
24
|
Xu N, Papagiannakopoulos T, Pan G, Thomson
JA and Kosik KS: MicroRNA-145 regulates OCT-4, SOX2, and KLF4 and
represses pluripotency in human embryonic stem cells. Cell.
137:647–658. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wong DJ, Segal E and Chang HY: Stemness,
cancer and cancer stem cells. Cell Cycle. 7:3622–3624. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang S, Balch C, Chan MW, et al:
Identification and characterization of ovarian cancer-initiating
cells from primary human tumors. Cancer Res. 68:4311–4320. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pfeiffer MJ and Schalken JA: Stem cell
characteristics in prostate cancer cell lines. Eur Urol.
57:246–254. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang M, Burton DW, Geller J, et al: The
bisphosphonate olpadronate inhibits skeletal prostate cancer
progression in a green fluorescent protein nude mouse model. Clin
Cancer Res. 12:2602–2606. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bisson I and Prowse DM: WNT signaling
regulates self-renewal and differentiation of prostate cancer cells
with stem cell characteristics. Cell Res. 19:683–697. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Patrawala L, Calhoun T,
Schneider-Broussard R, et al: Highly purified CD44+
prostate cancer cells from xenograft human tumors are enriched in
tumorigenic and metastatic progenitor cells. Oncogene.
25:1696–1708. 2006.PubMed/NCBI
|
|
31
|
Trerotola M, Rathore S, Goel HL, et al:
CD133, Trop-2 and alpha2beta1 integrin surface receptors as markers
of putative human prostate cancer stem cells. Am J Transl Res.
2:135–144. 2010.PubMed/NCBI
|
|
32
|
Zöller M: CD44: can a cancer-initiating
cell profit from an abundantly expressed molecule? Nat Rev Cancer.
11:254–267. 2011.PubMed/NCBI
|
|
33
|
Schoenhals M, Kassambara A, De Vos J, Hose
D, Moreaux J and Klein B: Embryonic stem cell markers expression in
cancers. Biochem Biophys Res Commun. 383:157–162. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu C and Tang DG: MicroRNA regulation of
cancer stem cells. Cancer Res. 71:5950–5954. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sachdeva M, Zhu S, Wu F, et al: p53
represses c-Myc through induction of the tumor suppressor miR-145.
Proc Natl Aca Sci USA. 106:3207–3212. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang YP, Chien Y, Chiou GY, Cherng JY,
Wang ML, Lo WL, et al: Inhibition of cancer stem cell-like
properties and reduced chemoradioresistance of glioblastoma using
microRNA145 with cationic polyurethane-short branch PEI.
Biomaterials. 33:1462–1476. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chiou GY, Cherng JY, Hsu HS, et al:
Cationic polyurethanes-short branch PEI-mediated delivery of Mir145
inhibited epithelial-mesenchymal transdifferentiation and cancer
stem-like properties and in lung adenocarcinoma. J Control Release.
159:240–250. 2012. View Article : Google Scholar
|
|
38
|
Lee MY, Lu A and Gudas LJ: Transcriptional
regulation of Rex1 (zfp42) in normal prostate epithelial cells and
prostate cancer cells. J Cell Physiol. 224:17–27. 2010.PubMed/NCBI
|
|
39
|
Hayashida T, Jinno H, Kitagawa Y and
Kitajima M: Cooperation of cancer stem cell properties and
epithelial-mesenchymal transition in the establishment of breast
cancer metastasis. J Oncol. 2011:5914272011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mani SA, Guo W, Liao MJ, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Morel AP, Lièvre M, Thomas C, Hinkal G,
Ansieau S and Puisieux A: Generation of breast cancer stem cells
through epithelial-mesenchymal transition. PLoS One. 3:e28882008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pirozzi G, Tirino V, Camerlingo R, et al:
Epithelial to mesenchymal transition by TGFβ-1 induction increases
stemness characteristics in primary non small cell lung cancer cell
line. PLoS One. 6:e215482011.
|
|
43
|
Li H, Chen X, Calhoun-Davis T, Claypool K
and Tang DG: PC3 human prostate carcinoma cell holoclones contain
self-renewing tumor-initiating cells. Cancer Res. 68:1820–1825.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang K and Waxman DJ: PC3 prostate
tumor-initiating cells with molecular profile
FAM65Bhigh/MFI2low/LEF1low
increase tumor angiogenesis. Mol Cancer. 9:3192010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Morton RA, Ewing CM, Nagafuchi A, Tsukita
S and Isaacs WB: Reduction of E-cadherin levels and deletion of the
alpha-catenin gene in human prostate cancer cells. Cancer Res.
53:3585–3590. 1993.PubMed/NCBI
|