Introduction
Cervical carcinoma is the second most prevalent
female cancer worldwide. Despite the generally good prognosis for
early-stage cervical cancer patients, many patients still die as a
result of metastasis and recurrence (1). How cervical cancer cells acquire the
ability to invade surrounding tissue is unclear, but
epithelial-mesenchymal transition (EMT) likely plays a role
(2). Unfortunately, little
information is available on the regulation of EMT in cervical
cancer cells and on the association of EMT program with clinical
outcome of cervical cancer patients.
EMT is an intricate process by which epithelial
cells lose their epithelial characteristics and acquire a
mesenchymal-like phenotype (3). EMT
was originally described in early embryogenesis as an essential
process for specific developmental stages that require migration
and transient dedifferentiation of embryonic epithelial cells
(4). In recent years, EMT has been
described as a relevant process in tumor progression and is not
only implicated in tumor invasion but also in other stages during
metastasis and in therapeutic resistance (5).
Nogo isoforms (Nogo-A, -B and -C) belong to the
reticulum super family of proteins (6). These three isoforms share a conserved
reticulum homology domain (RHD), which contains a 66-aa loop domain
termed Nogo-66 (7). Nogo-B/ASY is
widely expressed in many cell types and was originally
characterized as a novel human apoptosis-inducing protein (8). Nogo-B/ASY overexpression induces
apoptosis through ER stress and ER-specific signaling pathways
(9). Recent studies have shown that
Nogo-B plays a role in cell adhesion and migration (7,10,11);
the amino terminus of Nogo-B (AmNogo-B) promotes the adhesion and
migration of endothelial cells and negatively regulates
platelet-derived growth factor-induced migration in smooth muscle
cells (11).
Fibulin-5 is a recently identified fibulin family
member (12). Distinguished from
other fibulins, Fibulin-5 contains a conserved RGD motif that binds
to integrins and mediates endothelial cell adhesion (13,14).
Fibulin-5 can also suppress angiogenesis in an RGD-dependent manner
(15).
We previously reported that Nogo-B binds to
Fibulin-5 both in vitro and in vivo. In this study,
using HeLa cells as a model, we found that overexpression of Nogo-B
promotes epithelial-mesenchymal transitions in a
Fibulin-5-dependent manner. These results may contribute to a
better understanding the function of Nogo-B in tumor cell migration
and cancer metastasis.
Materials and methods
Patients and samples
We collected a total of 55 tissue specimens,
including 14 normal cervical tissues, 10 cervical intraepithelial
neoplasia (CIN) tissues, 31 cervical cancer tissues between
September 2010 and December 2011 in Tongji Hospital, Tongji Medical
College, Huazhong University of Science and Technology. Normal
cervical tissues were derived from the patients who underwent
hysterectomy due to nonmalignant disease. Cervical cancer tissues
were obtained from primary untreated patients under colposcopy.
Tumor stage and grade were determined according to the
International Federation of Gynecology and Obstetrics standards
(FIGO). Clinicopathological data for these patients are shown in
Table I. Informed consents were
obtained from all the patients.
 | Table IClinicopathological data for 55
patients providing cervical tissues. |
Table I
Clinicopathological data for 55
patients providing cervical tissues.
| Characteristic | Cancer (n=31) | CIN (n=10) | Normal (n=14) |
|---|
| Age (mean ± SD) | 47±6.2 | 39±3.6 | 41±5.9 |
| Grade |
| G1 | 11 | | |
| G2 | 12 | | |
| G3 | 8 | | |
| Stage |
| I-II | 19 | | |
| III-IV | 12 | | |
| HPV infection |
| + | 31 | 9 | 3 |
| − | 0 | 1 | 11 |
| CIN
classification |
| CIN1 | | 3 | |
| CIN2-3 | | 7 | |
This study was approved by the Ethics Committee of
Tongji Hospital, Tongji Medical College, Huazhong University of
Science and Technology. All the samples were immediately
snap-frozen and stored in liquid nitrogen (−180°C). To obtain
homogeneous and histological well-characterized samples for RNA
analyses, the nature of the tissue and its specified composition
were determined by an experienced pathologist. All cancer samples
contained at least 80% tumor tissues without necrosis. Normal
cervical samples were verified to be free of any cervical lesions.
CIN samples were verified to contain at least 80% CIN tissues.
Antibodies and reagents
Anti-Nogo-B, anti-Fibulin-5 and anti-β-actin
antibodies, horseradish peroxidase-conjugated anti-goat and
anti-rabbit IgG antibodies, Cy3-conjugated goat anti-rabbit IgG
antibodies and FITC-conjugated rabbit anti-goat IgG antibodies were
purchased from Santa Cruz Biotechnology, Inc. Anti-E-cadherin,
anti-N-cadherin and anti-vimentin antibodies were purchased from
Cell Signaling Technology.
Real-time PCR
Total RNA from tissues and cells was isolated by
using the AxyPrep Multisource Total RNA miniprep kit (Axygen) and
reverse transcribed by PrimeScript RT Master Mix Perfect Real-time
(Takara). The resulting cDNA samples were amplified by real-time
PCR using gene-specific primer sets in conjunction with the SYBR
Premix Ex Taq (Takara) in an Mx3000P instrument. The qPCR was
performed with the following conditions: activation at 95°C for 5
min followed by 40 cycles of denaturation at 94°C for 15 sec,
amplification at 60°C for 30 sec, elongation at 72°C for 30 sec. In
the last, a cycle of solubility curve was added to examine the
amplification quality. For all RT-PCR analyses, GAPDH mRNA was used
to normalize the RNA inputs. The primer sequences used to amplify
the genes are listed in Table
II.
 | Table IIPrimer sets for real-time PCR. |
Table II
Primer sets for real-time PCR.
| Gene | Primer
sequence |
|---|
| Nogo-B | F:
5′-GCAGTGTTGATGTGGGTATTT-3′ |
| R:
5′-CTGTGCCTGATGCCGTTC-3′ |
| E-Cadherin | F:
5′-CAGGTCTCCTCATGGCTTTGC-3′ |
| R:
5′-CTTCCGAAAAGAAGGCTGTCC-3′ |
| N-Cadherin | F:
5′-ATGCCCAAGACAAAGAAACC-3′ |
| R:
5′-CTGTGCTTGGCAAGTTGTCT-3′ |
| Vimentin | F:
5′-GATGCGTGAGATGGAAGAGA-3′ |
| R:
5′-ATTTCAACGCCTCCAAGAAG-3′ |
| Slug | F:
5′-ATTTCAACGCCTCCAAGAAG-3′ |
| R:
5′-CGAGGTGAGGATCTCTGGTT-3′ |
| Snail | F:
5′-GAAGATGCACATCCGAAGC-3′ |
| R:
5′-GGAGAATGGCTTCTCACCAG-3′ |
| TWIST1 | F:
5′-CGGACAAGCTGAGCAAGAT-3′ |
| R:
5′-GGACCTGGTACAGGAAGTCG-3′ |
| ZEB1 | F:
5′-AACGGAAACCAGGATGAAAG-3′ |
| R:
5′-TTGTCACACAGGTCACATGC-3′ |
| ZEB2 | F:
5′-CAAGTTCAAGTGCACGGAGT-3′ |
| R:
5′-GTTTGGGCATTCGTAAGGTT-3′ |
Cell culture and transfection
Human cervix epithelial adenocarcinoma HeLa cells
were cultured in a humidified 5% CO2 incubator at 37°C
in DMEM supplemented with 10% fetal bovine serum (FBS). DNA
transfections were performed using the FuGene HD Transfection
Reagent (Roche), and RNA transfections were performed using the
X-tremeGene siRNA Transfection Reagent (Roche). Stable HeLa cells
overexpressing Nogo-B were constructed by transfection and selected
with puromycin. HeLa cells stably overexpressing the empty vector
were used as a negative control and are referred to as Blank.
RNA interference
The pSilencer 3.0 recombinant vectors were
constructed by inserting 64-mer synthetic DNA oligonucleotides that
encode two 19-nt reverse complements with homology to a portion of
the target gene. For the three Nogo-B siRNA constructs, siA1:
(5′-GTTTGCAGTGTTGATGTGG-3′), siA2: (5′-GTCCCTGGATTGAAGCGCAA-3′) and
siA3: (5′-ATCAGATGAAGGCCACCCA-3′), the respective target sequences
are nt 903–921, nt 1092–1110 and nt 741–759. The siRNAs against
Fibulin-5 (sc-43121) were purchased from Santa Cruz Biotechnology,
Inc. and a scrambled siRNA was used as a control.
Immunofluorescence
HeLa cells were fixed in 4% paraformaldehyde for 15
min at 25°C, followed by permeabilization in 1% Triton X-100. The
cells were then washed with PBS and blocked with 0.5% BSA for 1 h
at 25°C. Then, the cells were incubated with primary antibody at
4°C overnight, washed three times with PBS, incubated with
secondary antibody for 45 min at room temperature, washed five
times with PBS and mounted on a slide with 50% glycerol. Confocal
images were captured with a Leica TCS SP2 AOBS MP.
In vitro cell migration and invasion
assays
Cell invasion and migration assays were performed as
described (16). Briefly, the cells
were starved with serum-free DMEM for 24 h before performing the
assays, and the cells were then added in DMEM with 0.1% FBS to the
upper chamber of a Transwell plate (Costar) containing a membrane
with 8-μm pores. For the cell migration assays, the cells were
allowed to migrate for 12 h into the lower chamber containing DMEM
with 10% FBS. The cell invasion assays were similarly performed,
except that cells were cultured on inserts with Matrigel (BD
Biosciences) and allowed to invade for 24 h. Finally, the cells
that invaded the synthetic basement membranes were fixed and
stained with crystal violet.
In vitro wound healing assays
HeLa cells (2×105) were plated in 35-mm
cell culture dishes in 1.5 ml of DMEM. When the cells grew to
approximately 85% confluence, the wounds were made by scratching
the monolayer culture using a sterile 200-μl micropipette tip. The
cells were then washed four times with 2 ml of PBS (warmed to 37°C)
for ~30 sec per wash to remove any floating cells; 1.5 ml of
culture medium was then added. The wounded regions were marked on
the outer bottom surface of the plate. Several selected fields
(n=4) from each wound and plate (time 0) were photographed using an
inverted light microscope. The culture plates were placed back in
the humidified cell incubator and incubated at 37°C, 5%
CO2 for up to 18–24 h. The same wound fields from each
plate were photographed every 6–8 h until the wound was completely
closed.
Statistics
The data are presented as the mean ± SD. SPSS 17.0
software (SPSS Inc., Chicago, IL, USA) was used for all statistical
analyses with P<0.05 considered statistically significant.
Results
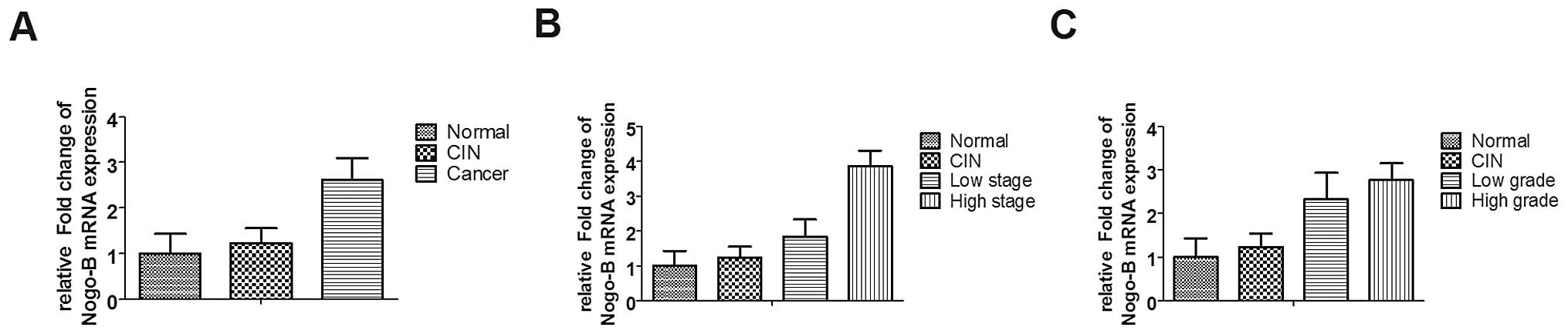
Expression pattern of Nogo-B in cervical
tissue samples
Samples including 14 normal cervical tissues, 10
cervical intraepithelial neoplasia (CIN) tissues, 31 cervical
cancer tissues were analyzed by real-time PCR. Data were organized
and presented as mean ± SD according to tumor stage (T1–T2/T3–T4)
or grade (G1/G2–G3), and Nogo-B mRNA expression in each group were
normalized to normal cervical tissue group (Fig. 1). We used one-way ANOVA statistics
to analyse variance between normal tissues, CIN tissues and cancer
tissues. The results showed that the expression of Nogo-B increased
in cervical cancer (Fig. 1A,
P=0.0438), and the increased expression of Nogo-B was correlated
with tumor stage (Fig. 1B,
P=0.0005) and grade (Fig. 1C,
P=0.0109).
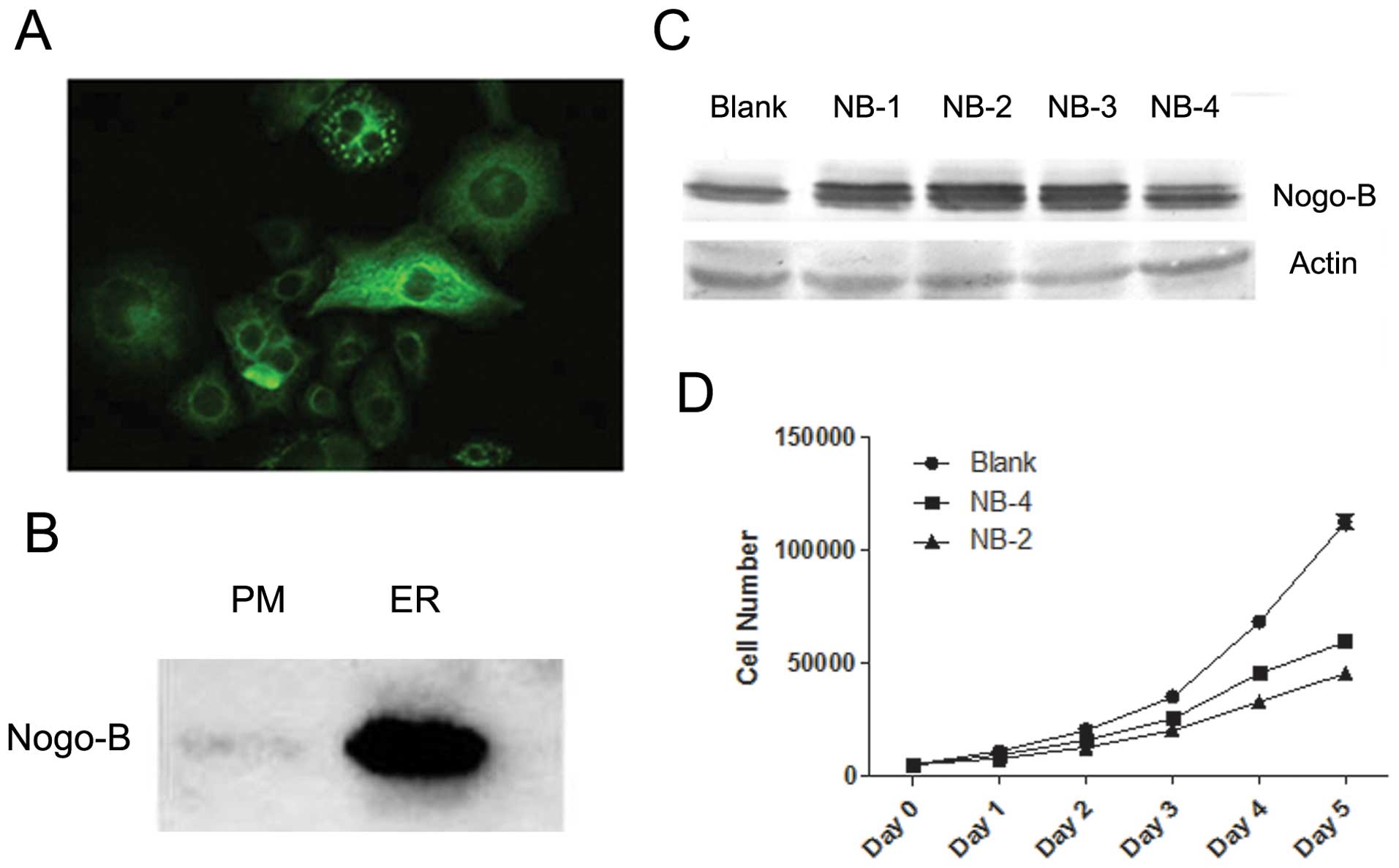
Overexpression of Nogo-B inhibits HeLa
cell proliferation
The function of a protein is always related to its
distribution. Thus, we first determined the cellular localization
of endogenous Nogo-B in HeLa cells using immunofluorescence. The
results showed that Nogo-B, similar to other RTN family members, is
mainly distributed in the cytoplasm and especially in the
endoplasmic reticulum (ER) (Fig.
2A). In addition, we observed a low level of Nogo-B that was
localized to the plasma membrane (PM) (Fig. 2A). Western blotting confirmed these
observations; we detected endogenous Nogo-B expression both in the
ER and on the PM (Fig. 2B).
To further investigate the effect of Nogo-B, we
established several stable cell lines by overexpressing Nogo-B in
HeLa cells (Fig. 2C). Among these,
the NB-2 and NB-4 cell lines were selected to use in subsequent
assays because they highly and moderately overexpressed Nogo-B,
respectively, relative to the original HeLa cells.
During the establishment of Nogo-B stable
transfectants, we had noted that the highly expressing Nogo-B HeLa
cell lines grew more slowly with respect to the original HeLa cell
line. Thus, we used the MTT assay to determine the proliferative
rate of these HeLa cell lines. The results confirmed our
observation: cells overexpressing Nogo-B had decreased cell
proliferation (Fig. 2D). Duplicate
proliferation assays demonstrated a similar effect. The average
proliferation rates of NB-2 and NB-4 were only 1.64±0.15% and
1.57±0.08%, respectively, which were both lower than the
proliferation rate of Blank cells (1.87±0.21).
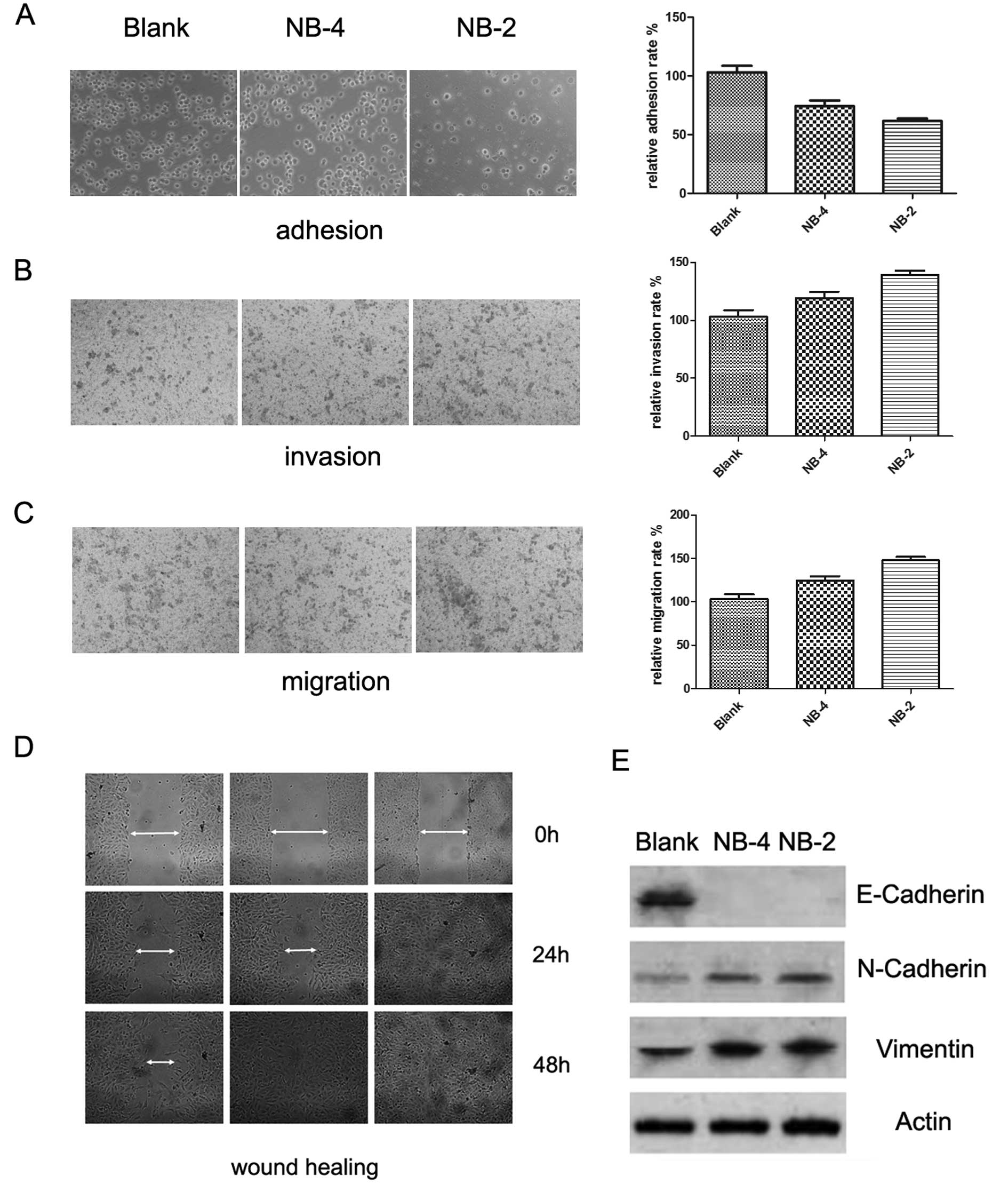
Nogo-B promotes EMT in HeLa cells
We previously reported that Nogo-B could regulate
cancer cell motility, and EMT has been described as a relevant
process in tumor metastasis. Thus, we examined the effect of Nogo-B
on EMT. We compared the cell adhesion, migration and invasion
capabilities between the Blank, NB-4 and NB-2 HeLa cells, which had
different expression levels of the Nogo-B protein. The results
showed that Nogo-B enhanced cell migration (Fig. 3B) and invasion (Fig. 3C) but inhibited the adhesion of HeLa
cells (Fig. 3A) in a dose-dependent
manner. This was especially true in the wound healing assay where
we observed that HeLa cells had a random migration pattern
(Fig. 3D), which has been described
as novel mesenchymal behavior. Then, we analyzed the expression of
EMT-related markers by western blotting. E-cadherin, an epithelial
marker, was significantly downregulated in Nogo-B overexpressing
cells compared with the empty vector-transfected cells. In
contrast, the proteins N-cadherin and vimentin, two mesenchymal
markers, were upregulated in Nogo-B overexpressing cells compared
with the control cells (Fig. 3E).
Altogether, these data suggest that Nogo-B promotes EMT in HeLa
cells.
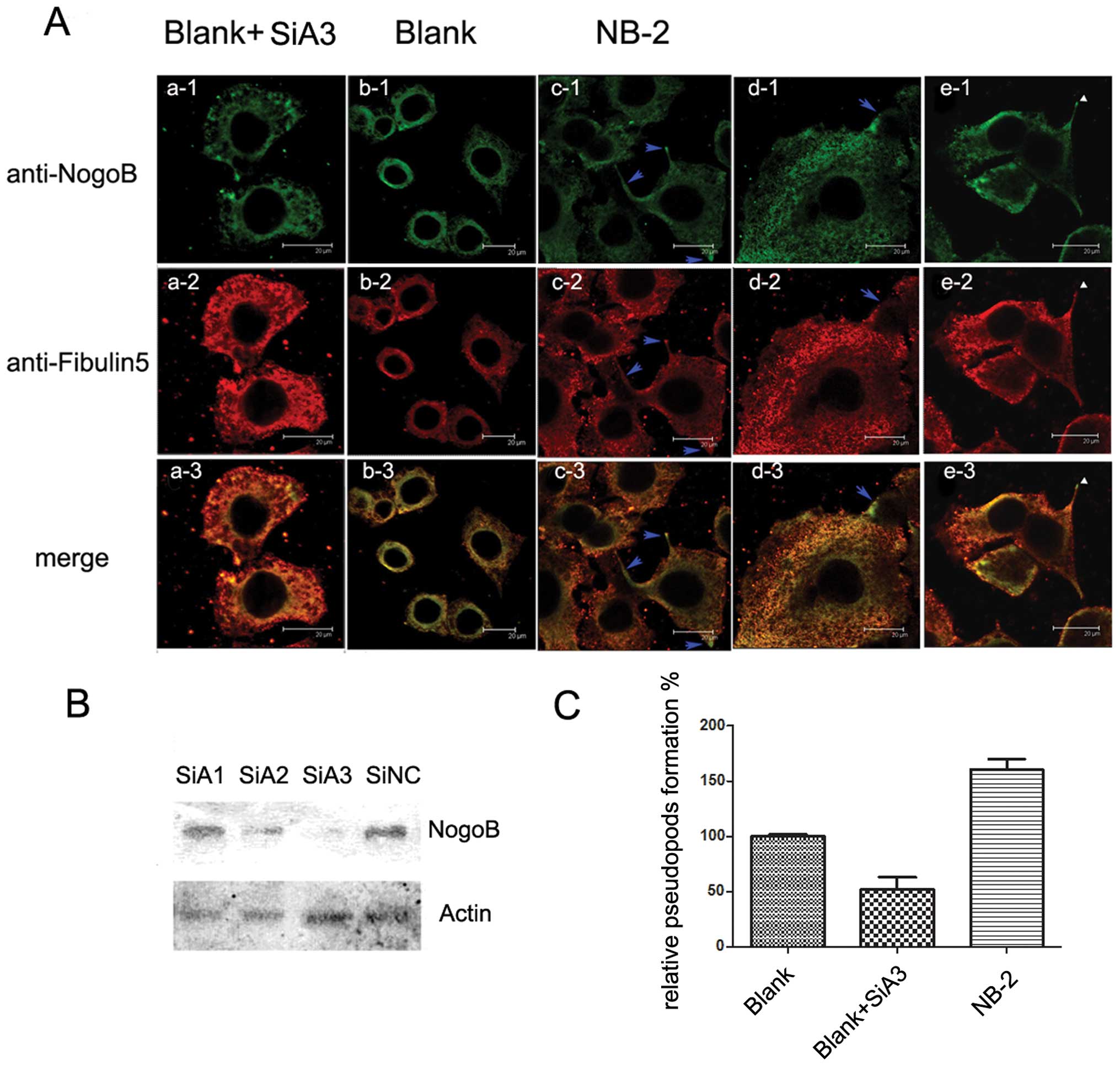
Nogo-B and Fibulin-5 co-localize in
pseudopods and enhance pseudopod formation
Due to the difference in cell behavior between the
high and low Nogo-B overexpressing HeLa cells and the Blank HeLa
cells, we investigated the other changes between the Nogo-B
overexpressing cells and the Blank cells. We compared the
morphological images of these three cell lines and found that the
Nogo-B overexpressing cell lines formed more pseudopods than did
the Blank cells. The number of pseudopod cells formed increased
with the increase of Nogo-B expression (Fig. 4C).
Our previous study indicated that Nogo-B could
interact with Fibulin-5 and increase Fibulin-5 secretion.
Therefore, to further characterize the stimulation of Nogo-B
overexpression on the behavior of HeLa cells, we performed a double
immunostaining of Nogo-B and Fibulin-5 in the Nogo-B overexpressing
cell line (NB-2), control cell line (Blank) and Blank cells
transfected with SiA3 (a siRNA against Nogo-B, showed in Fig. 4B). The images of Nogo-B/Fibulin-5
staining revealed that, in NB-2 cells, Nogo-B and Fibulin-5 tended
to accumulate in pseudopods (Fig.
4Ac1-e3). In contrast, the distribution of Nogo-B and Fibulin-5
in Blank cells was almost the same with a slight gathering around
the nucleus (Fig. 4Ab1-b3).
Additionally, in Blank cells transfected with SiA3, the ER that
contained Nogo-B became porous (Fig.
4Aa1) and incomplete, and the cells became rounded. Moreover,
the secretion of Fibulin-5 was inhibited, and Fibulin-5 accumulated
in the cytoplasm (Fig. 4Aa2). We
can also observe that the immunostaining of Nogo-B accumulated in
different types of pseudopods, including filopodium (Fig. 4Ac1) and lamellipodium (Fig. 4Ad1). The enrichment of Nogo-B and
Fibulin-5 in these pseudopods was much higher than that of the
surrounding areas (blue arrow).
Of note, in Fig.
4Ae1-e3, we found that Nogo-B was present and that Fibulin-5
was absent from the tops of some pseudopods (white triangles),
which may be evidence that Nogo-B functions as a carrier to bring
Fibulin-5 to the membrane.
Downregulation of Fibulin-5 blocks EMT
induced by Nogo-B
Since Fibulin-5 plays an important role in cell
motility and is involved in cancer metastasis, we proposed that the
EMT induced by overexpression of Nogo-B may be through its binding
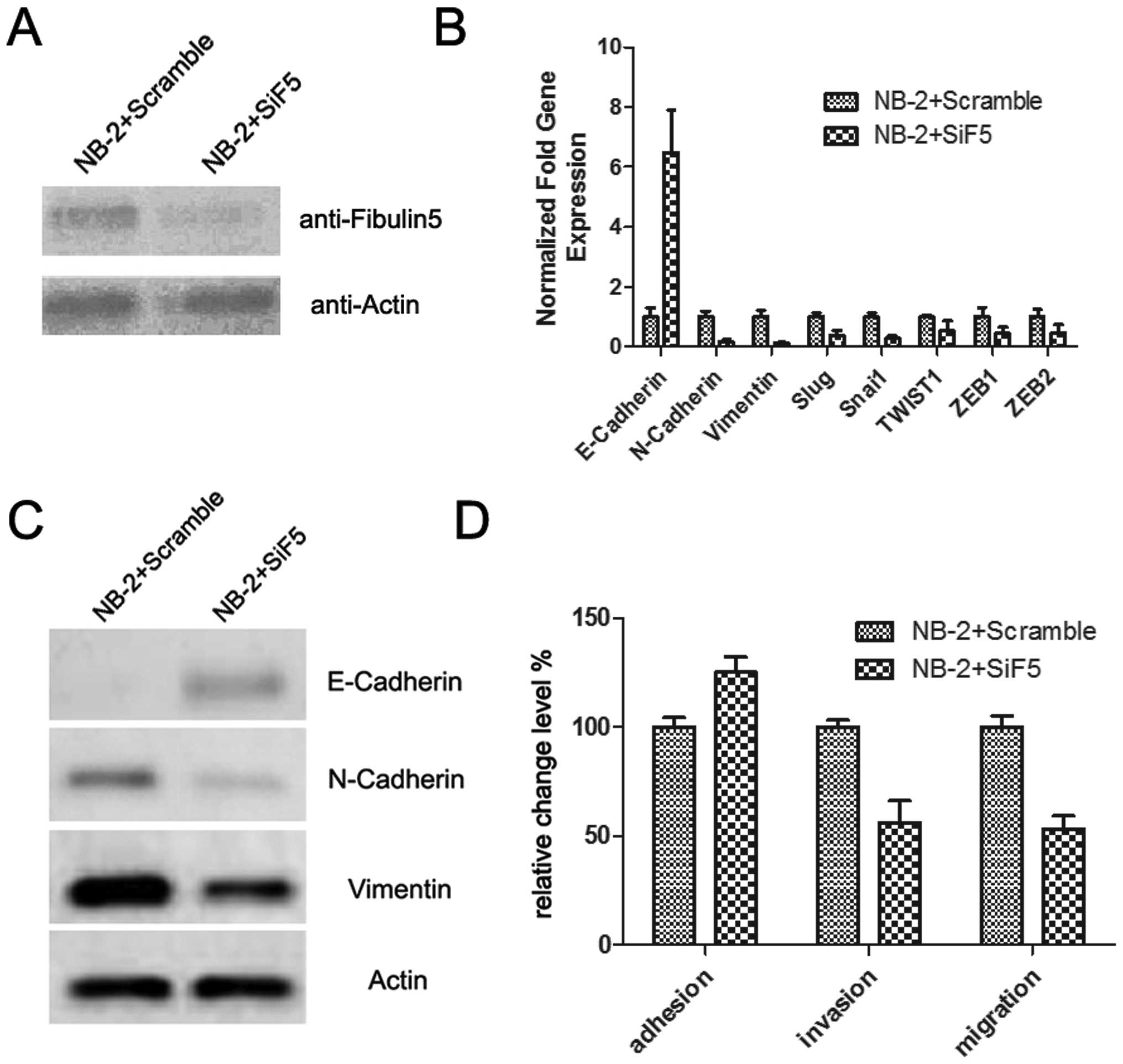
with Fibulin-5. Thus, we introduced a siRNA against Fibulin-5
(SiF5), which significantly downregulated the expression of the
Fibulin-5 protein (Fig. 5A). Then,
we examined the effect of SiF5 on EMT and cell motility in NB-2
cells that had the highest Nogo-B expression. As expected, compared
with NB-2 cells transfected with a scrambled RNA, the migration and
invasion levels of NB-2 cells transfected with SiF5 were
downregulated, while the adhesion level was upregulated (Fig. 5D).
We also analyzed the mRNA and protein expression of
EMT-related markers. The results showed that after SiF5
transfection, E-cadherin was visibly upregulated, while other
mesenchymal markers, such as N-cadherin and vimentin, or EMT
inducers, such as Snail, TWIST1, ZEB1 and ZEB2, were downregulated.
These results suggest that the EMT procedure induced by Nogo-B
overexpression had been blocked by Fibulin-5 downregulation. Thus,
Nogo-B may promote EMT through Fibulin-5.
Discussion
Uterine cervical cancer is one of the most common
cancers in women worldwide, with an estimated global incidence of
470,000 new cases and approximately 233,000 deaths per year
(17,18). Cervical cancer is mainly contributed
by the presence of high-risk human papillomavirus (HPV) oncogene
expression (19). The viral protein
products of HPV DNA interact with the anti-oncogenic function of
the retinoblastoma and the p53 proteins and inactivate these tumor
suppressors in normal keratinocytes. However, not all of those
infected by HPV develop cervical cancers; it indicates that factors
other than HPV viral proteins also contribute to the progression to
cervical cancer (20).
The process of EMT is essential for certain
morphogenetic movements within the embryo and is strongly
associated with the pathological process of tumor invasion
(3,4). During carcinoma invasion, EMT provides
tumor cells with the ability to dissociate from one another and
degrade and to actively migrate into the basal membrane and invade
the adjacent connective tissues (21,22).
Cells undergoing EMT exhibit multiple phenotypic changes, such as
the loss of apical-basal polarity, concomitantly with the
acquisition of a motile behavior and a profound reorganization of
the cytoskeleton (23,24). Among these changes, the loss of
expression or function of the E-cadherin cell-cell adhesion
molecule has emerged as an important event for the local invasion
of epithelial tumor cells, suggesting that E-cadherin is an
invasion-suppressor gene (25).
Different growth factors and cytokines have also been implicated in
the process of EMT in both epithelial cell systems and embryonic
development, such as transforming growth factor-β (TGF-β), which
was first described as an inducer of EMT in normal mammary
epithelial cells (MECs) (26) and
is now recognized as a master regulator of EMT in a variety of cell
types and tissues (27).
In this study, we first characterized that Nogo-B is
associated with cervical cancer progression. Then we successfully
identified Nogo-B as an inducer of EMT in HeLa cells.
Overexpressing Nogo-B drastically downregulated the expression of
E-cadherin while upregulating mesenchymal markers, such as
N-cadherin and vimentin. Furthermore, overexpression of Nogo-B
could also inhibit cell adhesion while promoting cell migration and
invasion. Nogo-B was originally identified as an apoptosis-inducing
protein through multiple pathways (8,9,28);
recent studies on Nogo-B identified its function in cell motility.
Nogo-B promotes vascular cell adhesion, stimulates endothelial cell
migration and attenuates PDGF-induced smooth muscle migration
(29). We previously found that
Nogo-B mediates HeLa cell adhesion and motility (16). All of these results indicate that
Nogo-B has multiple functions in different cells.
Pseudopods are cellular extensions used for
movement. The direction and trajectory of cell movement depend on
how the cells extend these pseudopods. In the absence of external
or internal signals, pseudopods are not formed at random (30). Our observations showed that Nogo-B
and Fibulin-5 preferred to accumulate in pseudopods, while the
number of cells with pseudopods increased with an increase in
Nogo-B expression.
Our previous studies showed that Fibulin-5 interacts
with Nogo-B, while Fibulin-5 has also been reported to initiate and
enhance epithelial-mesenchymal transition (EMT) and EMT induced by
TGF-β in mammary epithelial cells (31). Thus, we wondered whether Nogo-B
promotes EMT via Fibulin-5. We introduced a siRNA that could
effectively reduce the expression of Fibulin-5. These experiments
showed that the EMT procedure induced by Nogo-B overexpression had
been visibly blocked by the siRNA. Thus, our results suggested that
Nogo-B may promote EMT partially through Fibulin-5. Although we
cannot yet assess the complete signaling cascade of Nogo-B in EMT,
our study may enrich the understanding of the pathways that
regulate tumor cell movements and guide us to develop antagonists
of metastasis as cervical cancer therapeutics.
Acknowledgements
This study was supported by National Natural Science
Fund of China (No. 39880031), National Natural Science Fund key
Project of China (No. C03031906), National 863 plans projects of
China (No. 2007AA02Z156).
References
|
1
|
zur Hausen H: Human papillomaviruses in
the pathogenesis of anogenital cancer. Virology. 184:9–13.
1991.PubMed/NCBI
|
|
2
|
Lee MY, Chou CY, Tang MJ and Shen MR:
Epithelial-mesenchymal transition in cervical cancer: correlation
with tumor progression, epidermal growth factor receptor
overexpression, and snail up-regulation. Clin Cancer Res.
14:4743–4750. 2008. View Article : Google Scholar
|
|
3
|
Hay ED: An overview of
epithelio-mesenchymal transformation. Acta Anat. 154:8–20. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: at the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oertle T, Merkler D and Schwab ME: Do
cancer cells die because of Nogo-B? Oncogene. 22:1390–1399. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Su Z, Cao L, Zhu Y, et al: Nogo enhances
the adhesion of olfactory ensheathing cells and inhibits their
migration. J Cell Sci. 120:1877–1887. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qi B, Qi Y, Watari A, et al: Pro-apoptotic
ASY/Nogo-B protein associates with ASYIP. J Cell Physiol.
196:312–318. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kuang E, Wan Q, Li X, Xu H, Zou T and Qi
Y: ER stress triggers apoptosis induced by Nogo-B/ASY
overexpression. Exp Cell Res. 312:1983–1988. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liao H, Duka T, Teng FY, et al: Nogo-66
and myelin-associated glycoprotein (MAG) inhibit the adhesion and
migration of Nogo-66 receptor expressing human glioma cells. J
Neurochem. 90:1156–1162. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miao RQ, Gao Y, Harrison KD, et al:
Identification of a receptor necessary for Nogo-B stimulated
chemotaxis and morphogenesis of endothelial cells. Proc Natl Acad
Sci USA. 103:10997–11002. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Albig AR and Schiemann WP: Fibulin-5
function during tumorigenesis. Future Oncol. 1:23–35. 2005.
View Article : Google Scholar
|
|
13
|
Nakamura T, Ruiz-Lozano P, Lindner V, et
al: DANCE, a novel secreted RGD protein expressed in developing,
atherosclerotic, and balloon-injured arteries. J Biol Chem.
274:22476–22483. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakamura T, Lozano PR, Ikeda Y, et al:
Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature.
415:171–175. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Albig AR, Neil JR and Schiemann WP:
Fibulins 3 and 5 antagonize tumor angiogenesis in vivo. Cancer Res.
66:2621–2629. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou S, Xiao W, Wan Q, et al: Nogo-B
mediates HeLa cell adhesion and motility through binding of
Fibulin-5. Biochem Biophys Res Commun. 398:247–253. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Matsukura T and Sugase M: Pitfalls in the
epidemiologic classification of human papillomavirus types
associated with cervical cancer using polymerase chain reaction:
driver and passenger. Int J Gynecol Cancer. 18:1042–1050. 2008.
View Article : Google Scholar
|
|
18
|
Munoz N, Bosch FX, de Sanjose S, et al:
Epidemiologic classification of human papillomavirus types
associated with cervical cancer. N Engl J Med. 348:518–527. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
zur Hausen H: Papillomaviruses and cancer:
from basic studies to clinical application. Nat Rev Cancer.
2:342–350. 2002.PubMed/NCBI
|
|
20
|
Care A, Catalucci D, Felicetti F, et al:
MicroRNA-133 controls cardiac hypertrophy. Nat Med. 13:613–618.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liang X: EMT: new signals from the
invasive front. Oral Oncol. 47:686–687. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Christofori G: New signals from the
invasive front. Nature. 441:444–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: an alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Christofori G and Semb H: The role of the
cell-adhesion molecule E-cadherin as a tumour-suppressor gene.
Trends Biochem Sci. 24:73–76. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miettinen PJ, Ebner R, Lopez AR and
Derynck R: TGF-beta induced transdifferentiation of mammary
epithelial cells to mesenchymal cells: involvement of type I
receptors. J Cell Biol. 127:2021–2036. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zavadil J and Bottinger EP: TGF-beta and
epithelial-to-mesenchymal transitions. Oncogene. 24:5764–5774.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Q, Qi B, Oka K, et al: Link of a new
type of apoptosis-inducing gene ASY/Nogo-B to human cancer.
Oncogene. 20:3929–3936. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Acevedo L, Yu J, Erdjument-Bromage H, et
al: A new role for Nogo as a regulator of vascular remodeling. Nat
Med. 10:382–388. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Van Haastert PJ: How cells use pseudopods
for persistent movement and navigation. Sci Signal.
4:62011.PubMed/NCBI
|
|
31
|
Lee YH, Albig AR, Regner M, Schiemann BJ
and Schiemann WP: Fibulin-5 initiates epithelial-mesenchymal
transition (EMT) and enhances EMT induced by TGF-beta in mammary
epithelial cells via a MMP-dependent mechanism. Carcinogenesis.
29:2243–2251. 2008. View Article : Google Scholar : PubMed/NCBI
|