Introduction
PC-Spes is a herbal mixture containing extracts of
the herbs Dendranthema morifolium, Ganoderma lucidium, Glycyrrhiza
glabra, Isatis indigotica, Panax pseudo-ginseng, Rabdosia
rubescens, Scutellaria baicalensis and Serenoa repens. It has been
used for a long time by prostate cancer patients as an alternative
and/or subsidiary treatment of prostate cancer. Herbal therapy in
the treatment of benign prostatic hyperplasia as well as malignant
diseases has increased during the last years, especially in the US
(2) and there are a variety of
clinical studies about the efficiency of PC-Spes chemotherapy in
prostate cancer (3–5). In 2002, PC-Spes was recalled and
withdrawn from the US market because certain batches were
contaminated with prescription drugs. In the Netherlands, PC-Spes
was available till 2010. Previously, a growth inhibiting effect of
PC-Spes on head and neck carcinoma cell lines and primary mucosal
keratinocytes has been shown. This effect occurred consistently
through all cell lines tested, even in Paclitaxel-resistant cells
(1). Since 2010 PC-Spes is no
longer commercially available on the European market. The
succeeding herbal remedy called Prospectan is available solely as
tablets making it difficult to use for in vitro experiments.
Prostaprotect is available in Germany only as a
personal prescription formula, due to the strict German regulation
of nutritional supplements. At present there is still a discrepancy
between unique admission requirements in the EU and the single
European countries. In contrast to PC-Spes, Serenoa repens
was replaced in this formulation by an extract of Pygeum
africanum, a popular phytotherapeutic preparation, used in
Europe and USA to alleviate the symptoms of prostatic hyperplasia
(reviewed in ref. 6). Pygeum
africanum is also available as Tadenan™ capsules. It is sold as
a dietary supplement, but as well as other supplements, it is
available only in some European countries such as France and Italy.
A variety of active substances such as β-sitosterol (7), N-docosanol (8), artraric acid or
N-butylbenzene-sulfonamide (NBBS) (reviewed in ref. 9) have been isolated from Pygeum
bark extracts, most of them are growth inhibiting for prostate
carcinoma cells and mediate their effects via interaction with the
intracellular androgen receptor (AR).
The antineoplastic drug Paclitaxel is a natural
occurring diterpenoid, isolated from the pacific yew (Taxus
brevifolia) and is used as a chemotherapeutic agent for the
treatment of head and neck cancer patients either alone or in
combination therapy with other cytotoxic agents or radiotherapy.
The therapeutic effect of Paclitaxel was tested in several studies
and proved to be active in patients with squamous cell carcinoma of
the head and neck. Response rates varied from 20 to 40% (reviewed
in ref. 10).
We established a Paclitaxel resistant clonal subline
of the larynx carcinoma cell line HLaC79, (HLaC79-Clone1) and
tested the growth inhibitory/cytotoxic effects of Prostaprotect,
and of single herbal ingredients on proliferation of FADU, HLaC79
and HLaC79-Clone1 cell lines and on primary mucosal
keratinocytes.
In carcinomas in situ and tumour cell lines,
multidrug resistance is often associated with overexpression of
ATP-binding cassette transporter proteins (ABC proteins). ABC
proteins that confer drug resistance include P-glycoprotein (P-GP)
and the multidrug resistance associated proteins 1 and 2 (MRP-1,
MRP-2) as well as breast cancer resistance protein (BCRP). The
expression rates of these multidrug resistance mediating proteins
by western blot were analyzed. Since PC-Spes and Pygeum
africanum, both are growth inhibiting for prostate carcinoma
cells, partially exert their effects via interaction with the AR,
we determined expression levels of AR in the cell lines and primary
cells used in our study.
Results were compared with previous studies
concerning PC-Spes and single components of it. Results are
critically discussed with respect to convergent observations made
in prostate and head and neck cancer cells.
Materials and methods
Cell lines and cell culture
The head and neck squamous carcinoma cell line
HLaC79 was established from a lymph node metastase of a laryngeal
squamous cell carcinoma (11). The
cell line was grown with RPMI-1640 medium (Seromed, Munich,
Germany), supplemented with 10% fetal calf serum (FCS). HLaC79
cells were cultured in the presence of 10 nM Paclitaxel and a
resistant clone was isolated by selective trypsination of single
clones. The permanent HLaC79 clonal cell line HLaC79-Clone1 was
cultured in RPMI-1640 medium, supplemented with 10% FCS and 10 nM
Paclitaxel. FADU cells were grown in RPMI-1640 medium. Mucosal
keratinocytes were prepared from tonsillar tissue according to
standard protocols (12). In brief
mucosa was cut into small pieces, which were incubated overnight
with 0.2% dispase (Sigma-Aldrich, Steinheim, Germany) in Dulbecco’s
modified Eagle’s medium (DMEM; Seromed). The epithelium was
separated with sterile forceps and digested with 0.1% trypsin
(Seromed) for 20 min at 37°C. Residual trypsin was inactivated by
addition of FCS. Mucosal keratinocytes were collected by
centrifugation and cultured in defined keratinocyte serum-free
medium (Keratinocyte-SFM; Invitrogen; Karlsruhe, Germany).
Herbal plant
extracts/Paclitaxel/PC-Spes
Prostaprotect capsules (not commercially available)
and its single herbal ingredients were provided by Burg-Apotheke
Koenigstein (Koenigstein, Germany). All capsules and extracts used
for these experiments originated from one single batch. The plant
extracts or capsules mixtures were extracted in ethanol at
concentrations applied in Prostaprotect prescriptions, at 40 mg/ml
(Pygeum africanum: 50 mg/ml). 10 capsules were dissolved in
10 ml ethanol and incubated for 1 h at 37°C. Insoluble particles
were removed by low-speed centrifugation and filtration through a
22-μm filter. Aliquots were stored at −20°C. In addition aqueous
solutions of Prostaprotect and the plant extracts were prepared by
dissolving ingredients in serum-free RPMI medium. Insoluble
particles were removed by centrifugation. Paclitaxel was purchased
from Teva GmbH (Radebeul, Germany).
Cell viability and proliferation
assay
Cells were seeded at 5000 cells/well in 96-well
plates. They were treated with increasing concentrations of
Paclitaxel (10–200 nM) Prostaprotect (2–10 μl/ml) or herbal
extracts (0.2–10 μl/ml) in RPMI medium for 24 h. Controls were kept
in medium supplemented with 10 μl/ml EtOH for the ethanolic extract
analysis without drugs. Cell proliferation was measured after 48 h
by replacing the culture medium with medium containing 1 mg/ml MTT.
After 4 h of incubation, MTT-staining solution was replaced by
isopropanol and cells were incubated at 37°C for 45 min. The colour
conversion of MTT to a blue formazon dye was measured with an ELISA
reader at a wavelength of 570 nm. The amount of formazan dye is in
direct proportion to the number of metabolically active cells in
the culture. Single extracts growth curves were established in
triplicate, the mean growth curves were standardized to the
percentage of surviving cells, whereas the control cells were set
at 100%.
FACS analysis with Annexin V
antibodies
FACS analysis was performed using the Annexin V-APC
kit of BD Pharmingen (BD Biosciences, Heidelberg, Germany)
according to the kit manual. In brief, cells treated with 2 μl/ml
Pygeum africanum extract for 24 h, were harvested and washed
twice with cold PBS. Cells were then resuspended in 1X binding
buffer (0.1 M Hepes, pH 7.4, 1.4 M NaCl, 25 mM CaCl2) at
a concentration of 1×106 cells/ml. To 100 μl of this
cell suspension 5 μl Annexin V-APC and 5 μl 7-Amino-actinomycin D
(7-AAD; included in the kit) were added and incubated for 15 min in
the dark. Then 400 μl of 1X binding buffer was added. Within 1 h
FACS analysis was performed at an excitation wavelength of 650
nm.
Western blot analysis
For western blot analysis, cells were harvested by
scraping off, and dissolved in RIPA (PBS, containing 1% NP40, 0.5%
sodium deoxycholate, 0.1 % SDS), supplemented with 10 μg/ml
phenylmethanesulfonyl fluoride (PMSF). Alternative crude membrane
fractions (13) were used for
blotting. Protein content was determined according to the method of
Lowry (14). Equal amounts of total
protein lysates were loaded on 10% SDS-polyacrylamide gels and run
at a constant current of 20 mA. Gels were blotted onto
nitrocellulose membranes according to the semidry method of
Kyhse-Andersen (15). Blots were
blocked for 1 h with TBST (10 mM Tris, 150 mM NaCl, 0.05% Tween-20,
pH 8.0), containing 5% non-fat dry milk. For detection of AR and
multidrug resistance-mediating proteins the following antibodies
were used: P-GP: Calbiochem clone C219, supplied by
Merck-Millipore, Darmstadt, Germany; Clone F4 (Sigma-Adrich);
MRP-1: Santa Cruz Biotechnology (Heidelberg, Germany); MRP-2: Santa
Cruz Biotechnology; BCRP: Alexis, supplied by Enzo Life Sciences
(Loerrach, Germany); AR: Cell Signaling, supplied by
Merck-Millipore; GAPDH: Chemicon, supplied by Merck-Millipore.
Primary antibodies were incubated overnight at 4°C,
after washing 3 times with TBST, cells were incubated with
corresponding secondary antibodies, coupled to horseradish
peroxidase for 1 h. After washing once again, detection of bound
antibody conjugates was performed with the enhanced
chemiluminescence system (ECL, Amersham Biosciences, Freiburg,
Germany), according to the manufacturer’s protocol.
Indirect immunofluorescence
Cells were grown on chamberslides. Slides were fixed
with 4% formaldehyde in phosphate. Buffered saline (PBS; 137 mM
NaCl, 2.7 mM KCl, 10 mM Na2HPO4•2
H2O, 2 mM KH2PO4, pH 7.4) for 15
min. After washing three times with PBS the fixed cells were
incubated with anti-androgen receptor antibody (Cell Signaling,
Darmstadt, Germany) for 1 h. After washing three times with PBS,
cells were incubated with a secondary goat anti-rabbit antibody
coupled to Alexafluor 488 (Invitrogen) for 1 h. After washing once
again, cell slides were mounted with anti-fade mounting medium (250
mg DABCO [1,4-diazabicyclo(2,2,2)octan] in 90% glycerol, buffered
with PBS).
Results
Expression analysis of drug resistance
proteins (P-GP, MRP-1, MRP-2 and BCRP)
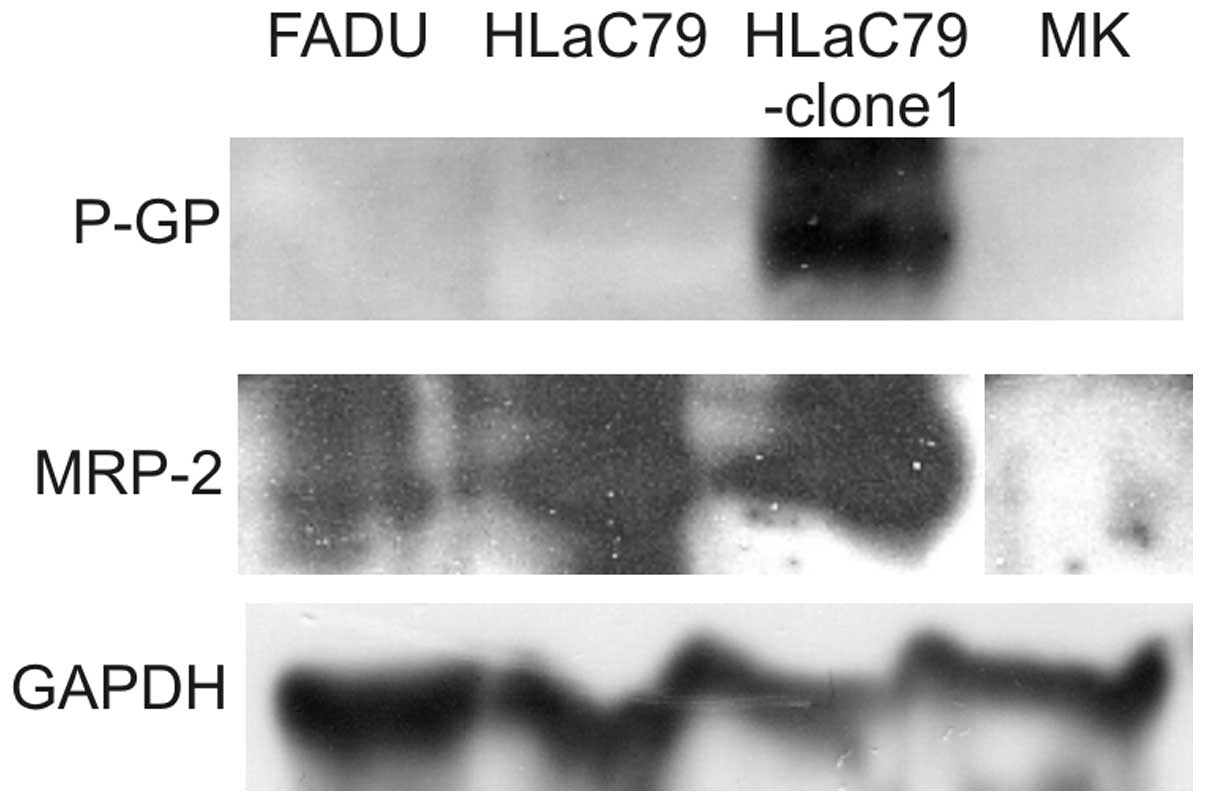
Expression of P-GP, MRP-1/2 and BCRP was tested by
western blot analysis of whole cell lysates. While P-GP was clearly
expressed in the Paclitaxel-resistant HLaC79-clone-1 subline
(Fig. 1), HLaC79, mucosal
keratinocytes as well as in FADU cells did not express P-GP. MRP-2
was detectable in all three cell lines, with HLaC79 and its
Paclitaxel resistant Clone at a similar high level. Mucosal
keratinocytes were negative for both chemoresistance markers. For
MRP-1 and BCRP no signal in any cell lysate or membrane fraction
was obtained.
Cell proliferation and viability
assay
For evaluation of cytotoxicity/growth inhibition we
exposed cell lines and primary keratinocytes to increasing
concentrations of the diluent ethanol, Paclitaxel, Prostaprotect
and herbal extracts for 24 h.
Incubation of cells with EtOH exerted only minor
cytotoxic effects (data not shown). In order to exclude possible
cytotoxic effects of the diluent, the highest concentration of 10
μl/ml EtOH was generally added. Each substance was measured in
three separate experiments in 12 wells. Results were expressed in
relation to untreated control cells (set at 100% survival
rate).
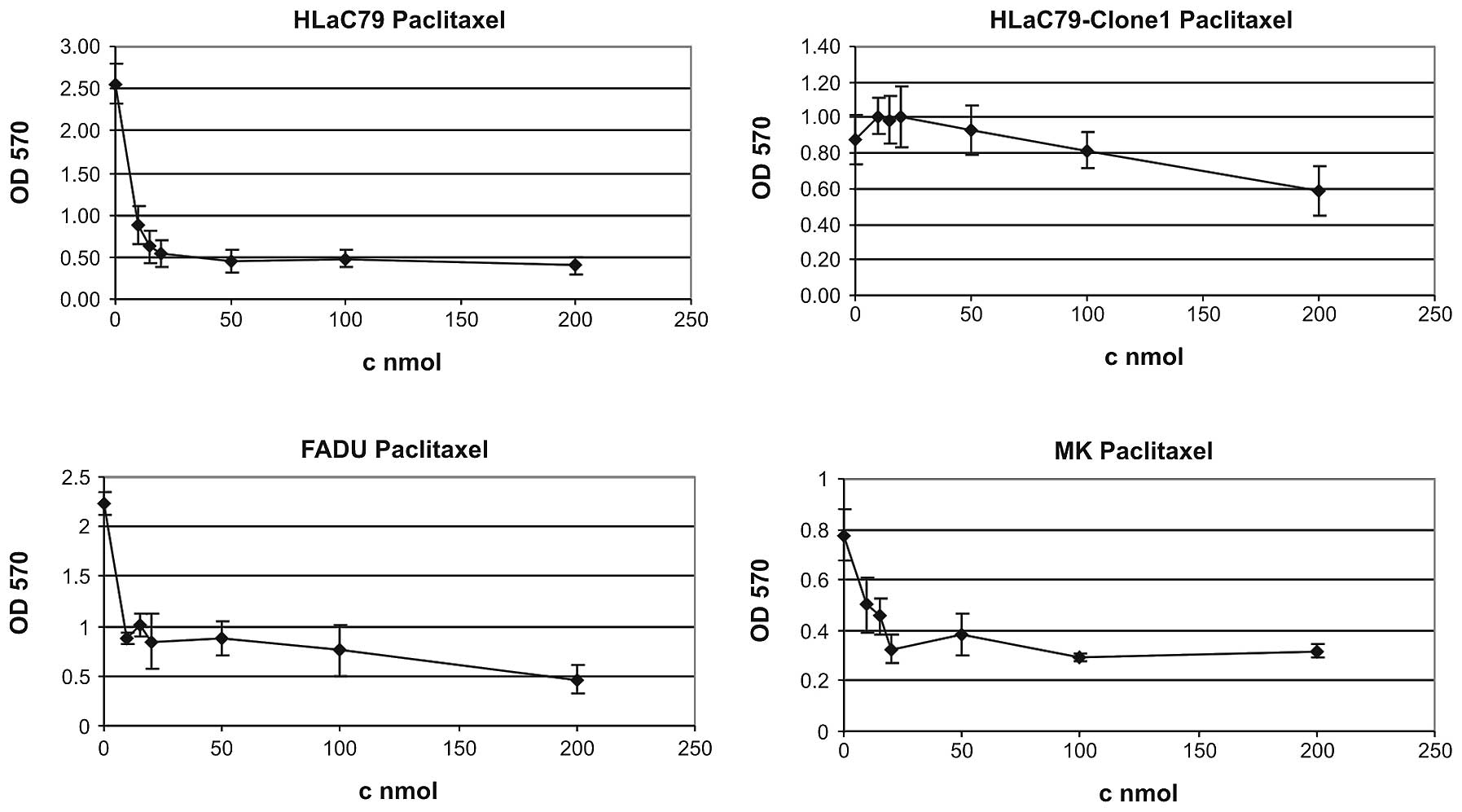
Paclitaxel and prostaprotect
The cell lines and primary cells were treated with
increasing concentrations of Paclitaxel (0–200 nm). After 48 h of
incubation cell viability and cytotoxicity of the used drugs were
measured with the MTT assay. Paclitaxel suppressed the growth of
HLaC79 cells significantly at the low dose of 10 nmol (Fig. 2, one of at least three independent
measurements for each cell type is displayed). Cell viability
decreased on average to 13.63% at 200 nmol Paclitaxel (untreated
controls set as 100% survival) in HLaC79 cells and to 20.85% in
FADU cells. HLaC79-Clone1 cells as well as slowly proliferating
primary mucosal keratinocytes in contrast showed only weak growth
inhibition up to concentrations of 200 nM Paclitaxel (mean growth
inhibition: keratinocytes 46.41%. HLaC79-Clone1 52.54% at 200 nm).
In case of highly proliferative HLaC79-Clone1 cells this can be
explained by up-regulated expression of P-GP (Fig. 1).
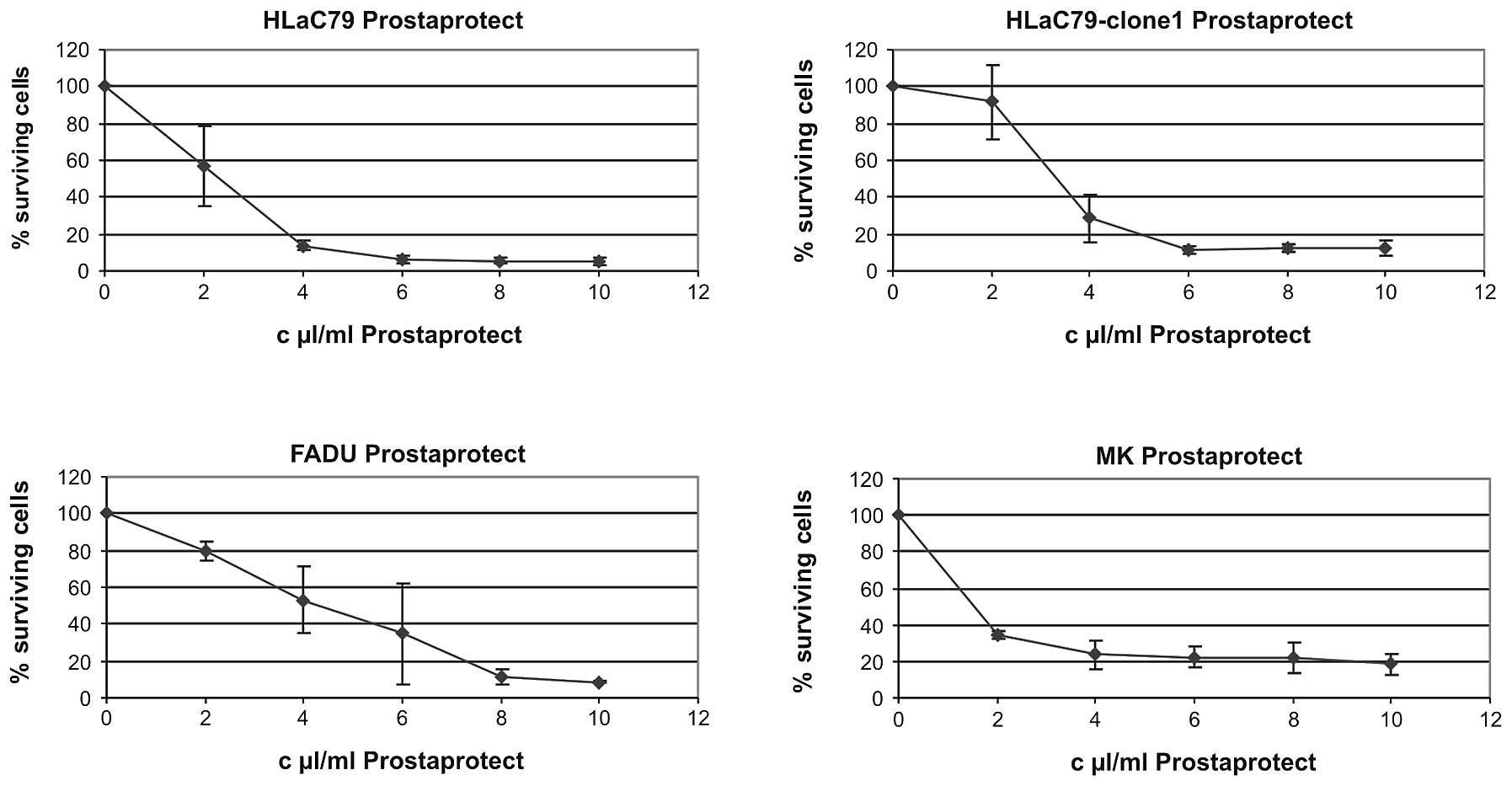
Prostaprotect proved to be strongly toxic on all
cell types (Fig. 3). The highest
concentration of 10 μl extract/ml culture medium dropped
proliferation down to 5.12% in HLaC79 cells and to 14.44% in
mucosal keratinocytes. In HLaC79-Clone1 cultures 12.09% cells
survived after 10 μl/ml prostaprotect application. In FADU cells
this treatment decreased proliferation to 8.52% of control
cells.
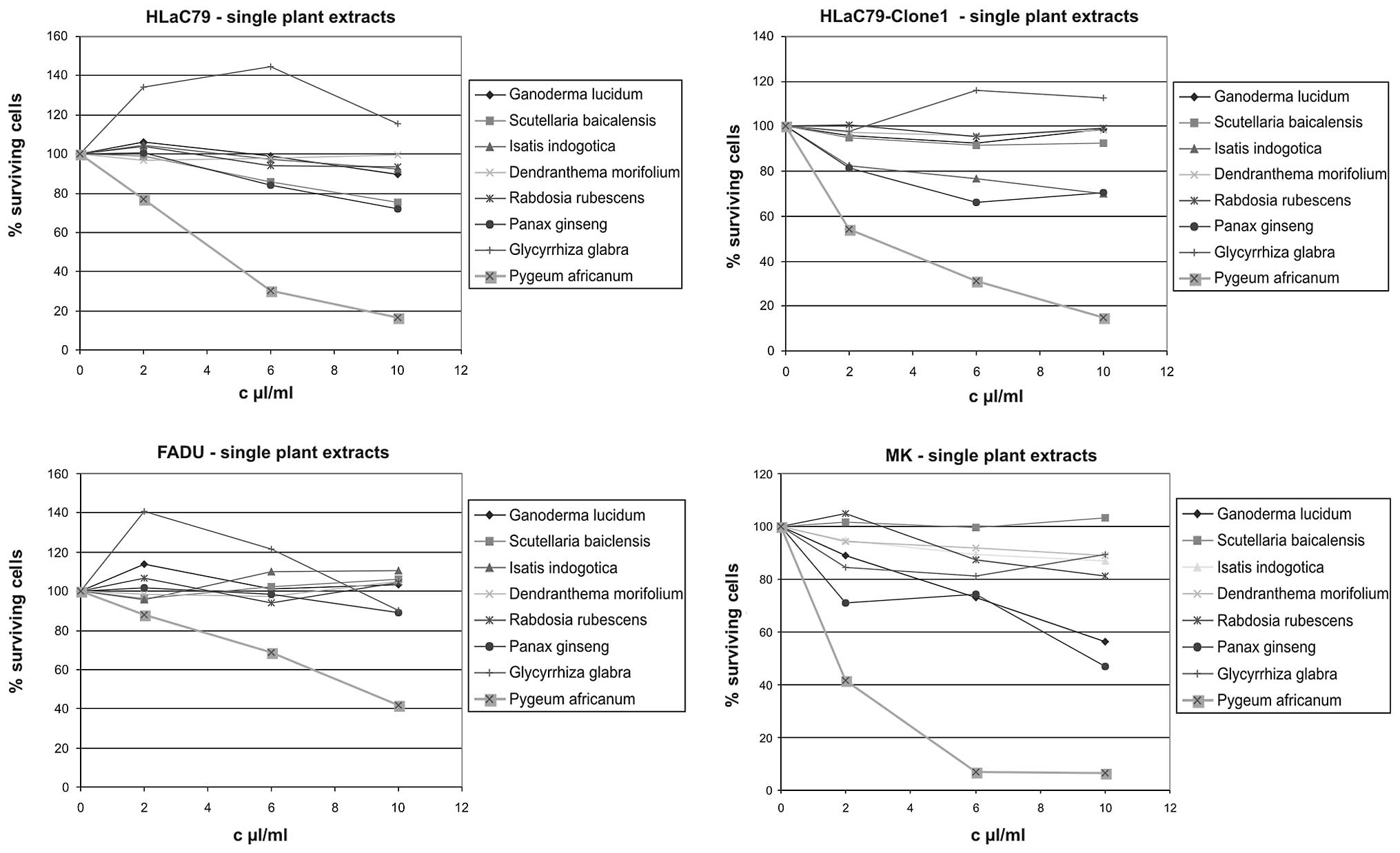
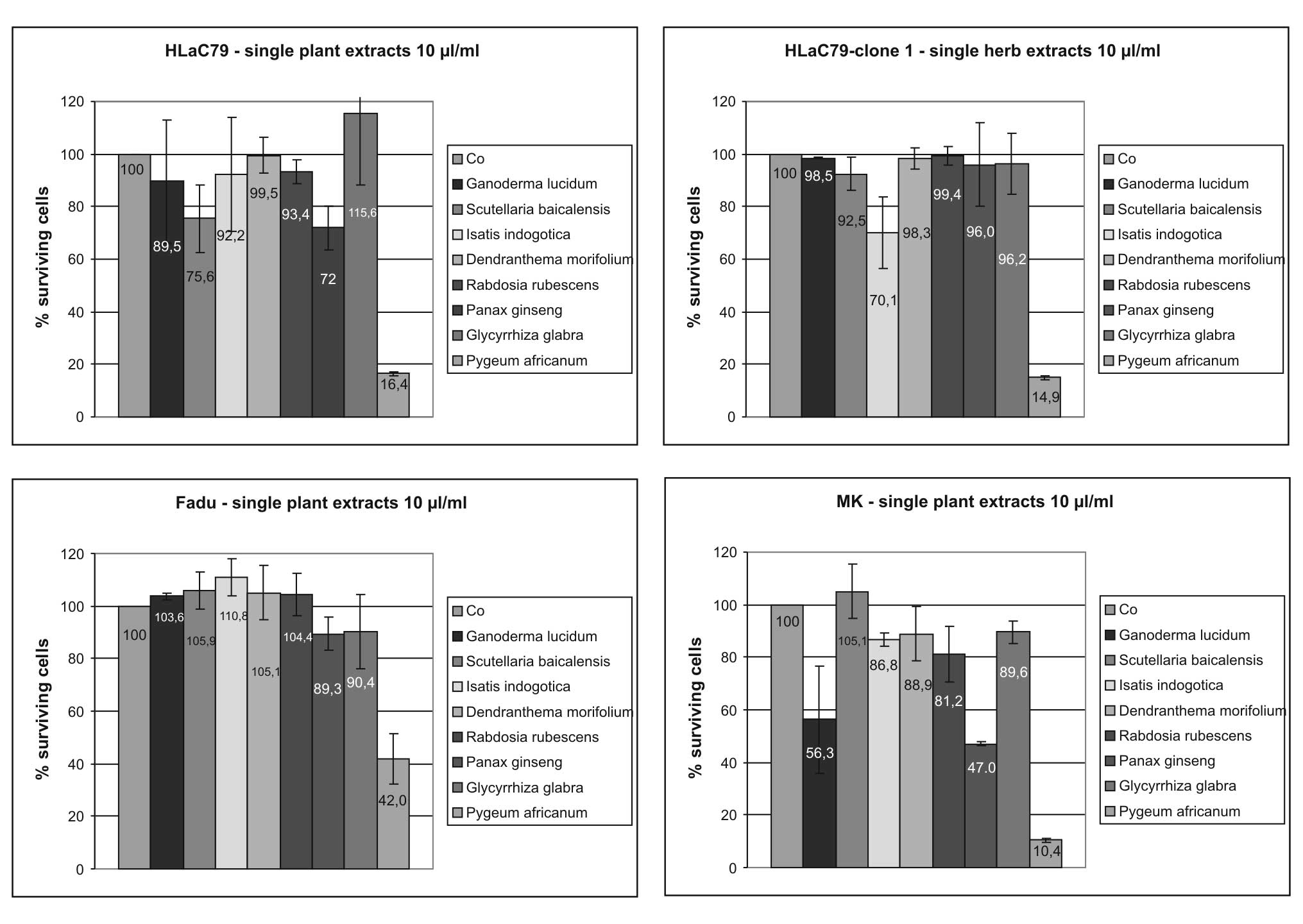
Single plant extracts
Growth inhibiting properties of single herbal
ingredients of Prostaprotect were tested using extract
concentrations adapted to those used in the capsules. Growth curves
in Fig. 4 were fitted by setting
OD570 values of untreated control cells as 100% survival, which
allows a direct comparison of individual extract concentrations in
one diagram. Growth inhibition rates in percent of control cells at
the highest extract concentration of 10 μl/ml for each herb are
summarized in Fig. 5.
The most toxic plant extract in the Prostaprotect
mixture proved to be Pygeum africanum bark extract, dropping
cell survival to 16.40% (HLaC79), 14.31% (HLaC79-Clone1), 10.42
(mucosal keratinocytes) and 42.01% (FADU; Fig. 5) at 10 μl/ml. Primary mucosal
keratinocytes proved to be selectively sensitive towards high
concentrations of Panax ginseng and Ganoderma lucidum
extracts (56.32% cell survival for Ganoderma lucidum and
46.99 % cell survival for Panax ginseng at 10 μl/ml applied
extract concentration; Figs. 4 and
5).
We observed a remarkable growth stimulation at lower
concentrations (2–6 μl/ml) of Glycyrrhiza glabra extract in the
carcinoma cell lines, but not in primary mucosa cells (Fig. 4). It has to be pointed out, however,
that the concentration of licorice extract used in these
experiments is >10-fold higher than the concentration used in
PC-Spes (40 mg/ml vs. 3.2 mg/ml in PC-Spes). Aqueous solutions of
herbal extracts revealed no acute cytotoxicity on cell cultures,
even at high concentrations (data not shown).
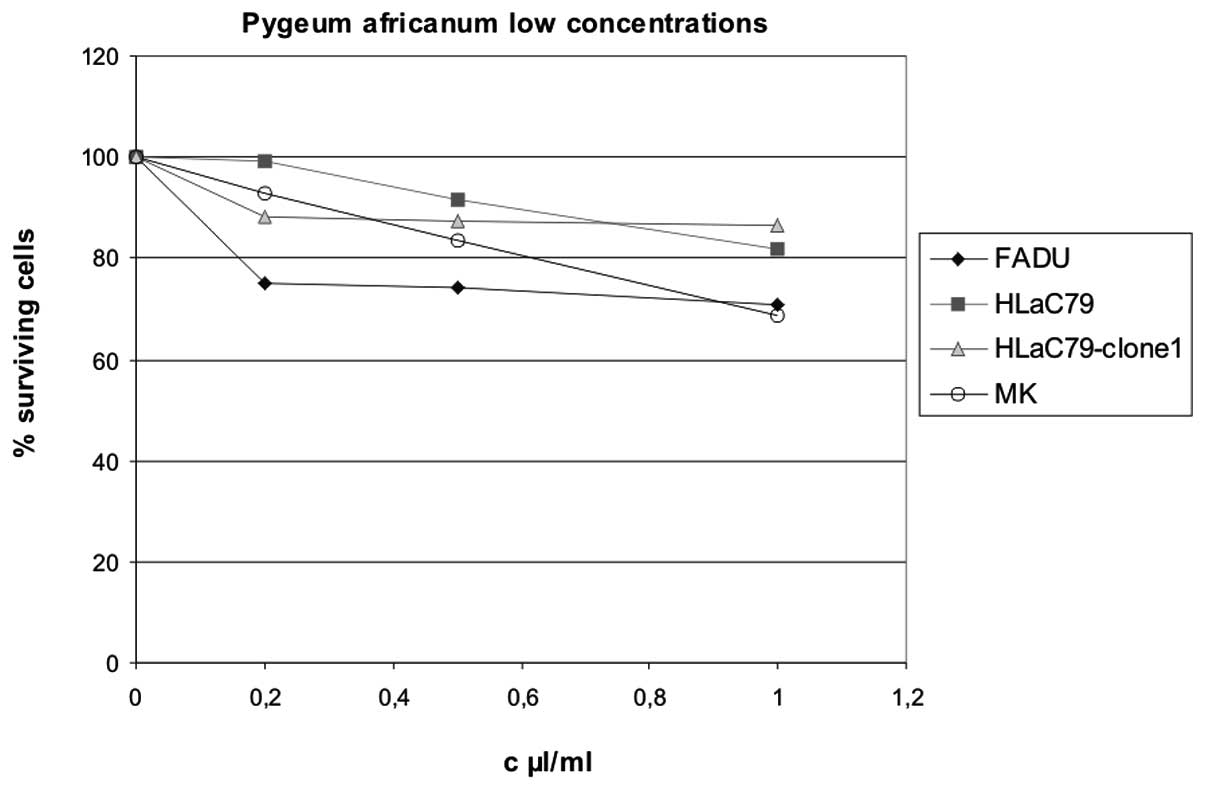
Comparison of our experimental design with
previously published Pygeum studies revealed a wide
variation of extract concentrations used for in vitro
experiments, ranging from 10 μg/ml (16) to 750 μg/ml culture medium (7). According to the given formulation in
Prostaprotect, we applied concentrations between 100 and 500 μg/ml
Pygeum extract for treatment of cell cultures. To cover the
different concentrations used in literature so far, we tested
Pygeum africanum extract at lower concentrations from 10–50
μg/ml culture medium. Results are displayed in Fig. 6. At low concentrations up to 1 μl/ml
(50 μg/ml) Pygeum africanum extract exerted only a weak
growth inhibition throughout carcinoma cell lines and primary
mucosal keratinocytes.
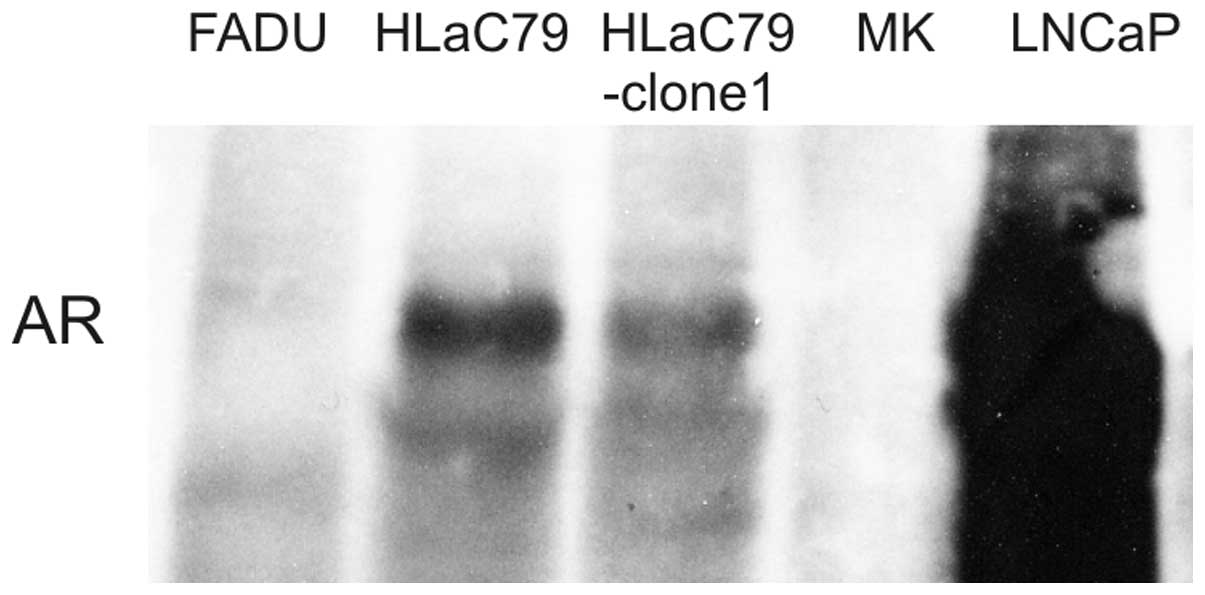
Expression of AR
To reveal an eventual association between AR
expression and toxicity of Pygeum africanum extract AR
expression was analyzed by western blotting and immunofluorescence
staining. HLaC79 and HLaC79-Clone1 both showed positive reaction
with AR-antibodies. Expression of AR appeared weak in comparison to
cell lysates of the prostatic carcinoma cell line LNCaP, used as a
positive control. FADU cells and primary keratinocytes did not
express AR (Fig. 7).
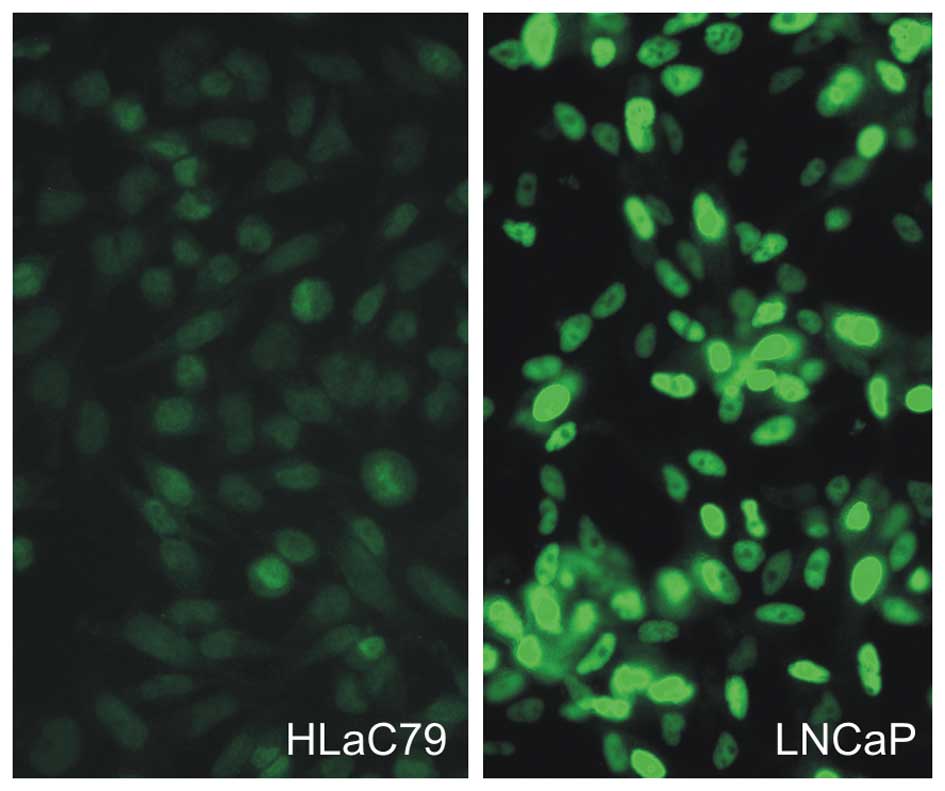
In order to exclude clonal or aberrant expression of
AR in our cell lines we performed immunofluorescence staining.
Antibody staining showed a weak but specific nuclear staining
throughout the population of HLaC79 and HLaC79-Clone1 cells (HLaC79
Fig. 8). FADU cells and mucosal
keratinocytes were negative for AR staining.
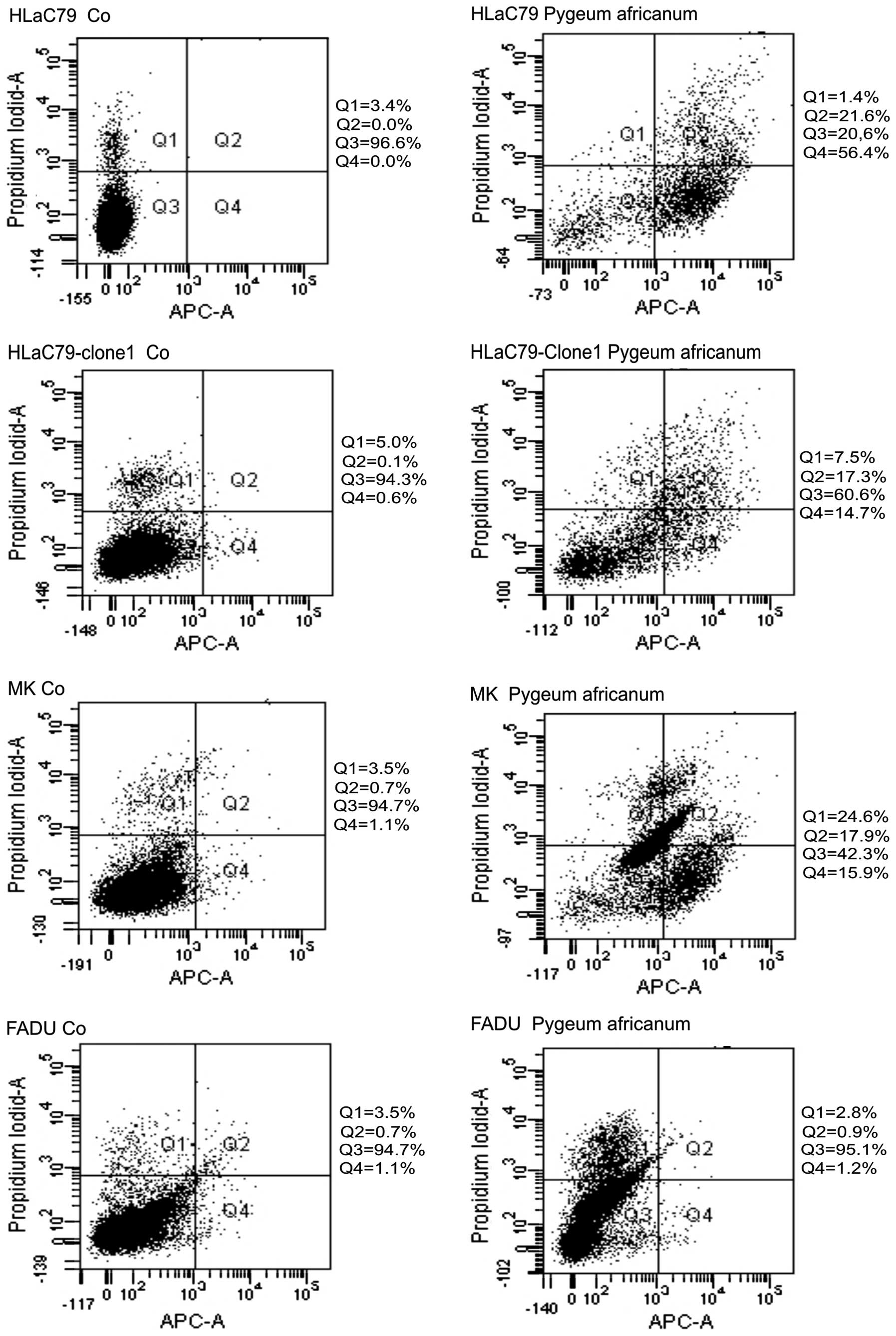
Apoptosis - FACS analysis with Annexin V
antibodies
FACS analysis with the Annexin V-APC kit was carried
out for Pygeum africanum, the herbal extract acting most
toxic in our cell lines and primary cells. Pygeum africanum
extract significantly increased apoptotic cell fractions after 24 h
incubation in both the Paclitaxel-sensitive cell line HLaC79 and
the Paclitaxel-resistant cell line HLaC79-Clone1 (Fig. 9: HLaC79-Clone1 14.7% apoptotic
fraction; HLaC79 56.4%). HLaC79 and HLaC79-Clone1 differed in
sensitivity, which might be caused by the increased detoxification
capacity of HLaC79-Clone1 cells. In FADU cells, however, a low
concentration of Pygeum extract was not able to
significantly trigger apoptosis (1.2%; Fig. 9).
Discussion
In advanced laryngeal and hypopharyngeal cancer the
chemotherapeutic agent Paclitaxel is commonly used for chemotherapy
in order to preserve laryngeal and/or pharyngeal structures.
Although Paclitaxel generally seems to be a powerful agent, it
failed to reach a local-regional tumour control in 12% of patients
according to a previously published study (10). Chemotherapeutic failure may be
related either to inherited resistance against the drug or/and the
acquirement of resistance during the therapy. Drug resistance is
mostly a multifactorial procedure, in the case of Paclitaxel
several mechanisms have been described. One mechanism is the
overexpression of multidrug resistance proteins, such as
P-glycoprotein (P-GP) (coded by the multidrug resistance gene 1,
MDR-1, P-GP), multidrug resistance-associated proteins (such as
MRP1 and MRP2) or breast cancer resistance protein (BCRP). P-GP
overexpression in Paclitaxel-resistant HLaC79-Clone1 cells was
confirmed.
Considering single components combined in the
Prostaprotect prescription, we observed a growth stimulating effect
of licorice extract in head and neck cancer cell lines. In
contrast, Hsieh et al(17)
observed a clear anti-mitogenic effect of Glycyrrhiza extract on
prostate carcinoma cell lines. The Glycyrrhiza extract used in our
study was over 10-fold higher concentrated than those used by Hsieh
et al(17). Kimura et
al(18) described a growth
stimulating effect of Glycyrrhizin and some analogues on primary
hepatocytes acting via binding to EGF receptors. Molarities of the
single substances used in the above mentioned study can’t be
related to our extracts, but tyrosin phosphorylation of EGF
receptors, which are overexpressed in 90% of head and neck
carcinomas (19) might also occur
in head and neck cancer cell lines.
In the Prostaprotect mixture Pygeum africanum
turned out to be the major toxic component. Pygeum
africanum, also available as Tadenan capsules is sold as a
dietary supplement, used to treat prostatic hyperplasia, has been
shown to hold a variety of active substances such as β-sitosterol
(7), N-docosanol (8), artraric acid or
N-butylbenzene-sulfonamide (NBBS) (reviewed in ref. 9). All these substances have been isolated
from Pygeum bark extracts, most of them are growth
inhibiting for prostate carcinoma cells and mediate their effects
via interaction with the intracellular androgen receptor (AR).
Shenouda et al(7) showed a
growth inhibiting effect of Pygeum extract on AR-dependent
LNCaP as well as AR-independent growing PC3 prostate carcinoma cell
lines. However, they did not observe any toxic effect on
AR-negative CaCO2 colon cancer cells at very high
concentrations and concluded a clear action of Pygeum
extract via the AR.
The role of AR in the development of laryngeal
cancer is still controversial. A number of publications are
available concerning AR expression in head and neck carcinoma
tissue, expression rates ranging between 0% (20) and 68.3% (21). Even in normal adjacent tissue no
common expression rates for AR are available. While Chen et
al(21) observed 0% AR
expression in normal mucosa, Nehse et al(22) report even higher AR expression in
mucosa than in tumour tissue. These controversial results are at
least partially caused by the different detection methods used,
such as in situ hybridization, or RT-PCR for measuring mRNA
transcription, immunohistochemistry and receptor assays for
determination of protein expression or activity.
In the present study AR expression was examined on
protein level, using western blot detection and immunofluorescence
staining and revealed a weak AR expression signal in HLaC79 and
HLaC79-Clone 1 cells. Nevertheless we observed strong toxicity of
Pygeum extract on all cell lines, AR-positive or -negative,
when used in concentrations adapted to Prostaprotect
concentrations. Using lower concentrations of Pygeum extract
gained a closer look on cellular changes. All three cell lines
survived quantitatively. There was no striking difference between
AR-positive and AR-negative cell lines in the MTT assay caused by
treatment with Pygeum extract, but apoptosis was more
pronounced in AR-positive HLaC79 and HLaC79-Clone1 cells. On the
other hand there is a tremendous difference in Pygeum
sensitivity between vulnerable HLaC79 cells and p-GP expressing
HLaC79-Clone1 cells. Furthermore, we observed that Pygeum
extract at low doses massively triggered apoptosis in primary
keratinocytes, although these cells were clearly AR-negative.
The discrepancy to previous studies is probably
based on two major problems: first the diversity of extracts used
for experiments is a tremendous black box. There is no standard
formulation available, except Tadenan capsules, which are no longer
available in most European countries presumably because of art
protection constraints (Phytolab Inc., personal communication).
Besides Tadenan is not useful for in vitro investigations
because peanut oil is the major solvent in the capsules. A variety
of undefined Pygeum capsules, powders and tablets
circulating at the European market are sold via internet shops.
Extracts and concentrations used for investigations are not
comparable. The second problem is the lack of a holistic
consideration in studies. Most studies have concentrated on cause
and effect of drugs applied to cells with one certain aspect
focused on, for example the role of the AR. Considering the system
of a cell in its entirety, however, includes also a view to the
capacity of drug detoxification, growth rates, genetic
constellation etc. How tightly the diverse cellular mechanisms are
linked has been shown for example by Fedoruk et al(23) who demonstrated, that P-GP increases
the efflux of dihydrotestosterone (DHT) from cells and is able to
reduce androgen responsive gene activity in prostate cancer cells.
This cross-functional features are especially important, when
herbal mixtures such as PC-Spes are used for studies, with
components influencing different cellular functions such as AR
expression (17) or P-GP activity
and/or expression (reviewed in ref. 24).
All other components used for formulation of the
Prostaprotect mixture exerted only minor cytotoxicity on cell lines
and primary cells. Solely the extract of Panax ginseng
inhibited the growth of mucosal keratinocytes quantitatively. This
is in contrast to the study of Hsieh et al(17), who described strong toxic effects of
Glycyrrhiza, Isatis, Scutellaria, Dendranthema, Rabdosia, Ganoderma
and Panax on prostate carcinoma cell lines even at the
concentration of 5 μl/ml medium. One reason for the discrepancy
between the studies might be the different cellular systems but
again, the problem of diversity of extracts used for treatment of
cells exists.
In summary, we demonstrated that individual herbs
such as Pygeum africanum extract used for treatment of
prostatic diseases might also achieve growth inhibition in head and
neck cancer cells, even if these cells are resistant to Paclitaxel.
The growth inhibiting effect seems to be affected both by
detoxification capacity of cells, as well as the expression of AR.
The role of the AR in development and course of head and neck
cancer remains to be revealed. Furthermore, it should be
reconsidered as to which combinations of natural compounds make
sense for practical use. Nevertheless, it seems possible, that
combinations of purified herbal compounds may be used in
combination with conventional anticancer therapy, to achieve
synergistic activities.
Acknowledgements
We are grateful to Dr Jürgen Arnhold for providing
information about Prostaprotect prescription and composition. We
would like to thank Dr Petrus Tas for critical reading of the
manuscript.
References
|
1
|
Schmidt M, Polednik C, Gruensfelder P,
Roller J and Hagen R: The effects of PC-Spes on chemosensitive and
chemoresistant head and neck cancer cells and primary mucosal
keratinocytes. Oncol Rep. 21:1297–1305. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DiPaola RS, Zhang H, Lambert GH, Meeker R,
Licitra E, Rafi MM, Zhu BT, Spaulding H, Goodin S, Toledano MB,
Hait WN and Gallo MA: Clinical and biologic activity of an
estrogenic herbal combination (PC-Spes) in prostate cancer. N Engl
J Med. 339:785–791. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
De la Taille A, Buttyan R, Hayek O,
Bagiella E, Shabsigh A, Burchardt M, Burchardt T, Chopin DK and
Katz AE: Herbal therapy PC-Spes: in vitro effects and evaluation of
its efficacy in 69 patients with prostate cancer. J Urol.
164:1229–1234. 2000.PubMed/NCBI
|
|
4
|
Moyad MA, Pienta KJ and Montie JE: Use of
PC-Spes, a commercially available supplement for prostate cancer,
in a patient with hormone-naive disease. Urology. 54:319–324. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Small EJ, Frohlich MW, Bok R, Shinohara K,
Grossfeld G, Rozenblat Z, Kelly WK, Corry M and Reese DM:
Prospective trial of the herbal supplement PC-Spes in patients with
progressive prostate cancer. J Clin Oncol. 18:3595–3603.
2000.PubMed/NCBI
|
|
6
|
Dedhia RC and McVary KT: Phytotherapy for
lower urinary tract symptoms secondary to benign prostatic
hyperplasia. J Urol. 179:2119–2125. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shenouda NS, Sakla MS, Newton LG, et al:
Phytosterol Pygeum africanum regulates prostate cancer in
vitro and in vivo. Endocrine. 31:72–81. 2007.
|
|
8
|
Pierini N, Citti F, Di Marzio S, Pozzato C
and Quercia V: Identification and determination of N-docosanol in
the bark extract of Pygeum africanum and in patent medicines
containing it. Boll Chim Farm. 121:27–34. 1982.(In Italian).
|
|
9
|
Roell D and Baniahmad A: The natural
compounds atraric acid and N-butylbenzene-sulfonamide as
antagonists of the human androgen receptor and inhibitors of
prostate cancer cell growth. Mol Cell Endocrinol. 332:1–8. 2011.
View Article : Google Scholar
|
|
10
|
Pfreundner L, Hoppe F, Willner J, Preisler
V, Bratengeier K, Hagen R, Helms J and Flentje M: Induction
chemotherapy with paclitaxel and cisplatin and CT-based 3D
radiotherapy in patients with advanced laryngeal and hypopharyngeal
carcinomas - a possibility for organ preservation. Radiother Oncol.
68:163–170. 2003. View Article : Google Scholar
|
|
11
|
Zenner HP, Lehner W and Herrmann IF:
Establishment of carcinoma cell lines from larynx and submandibular
gland. Arch Otorhinolaryngol. 225:269–277. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Imaizumi F, Asahina I, Moriyama T, Ishii M
and Omura K: Cultured mucosal cell sheet with a double layer of
keratinocytes and fibroblasts on a collagen membrane. Tissue Eng.
10:657–664. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Limtrakul P, Anuchapreeda S and Buddhasukh
D: Modulation of human multidrug-resistance MDR-1 gene by natural
curcuminoids. BMC Cancer. 4:132004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. J
Biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
15
|
Kyhse-Andersen J: Electroblotting of
multiple gels: a simple apparatus without tank for rapid transfer
of proteins from polyacrylamide to nitrocellulose. J Biochem
Biophys Methods. 10:203–210. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yablonsky F, Nicolas V, Riffaud JP and
Bellamy F: Antiproliferative effect of Pygeum africanum
extract on rat prostatic fibroblasts. J Urol. 157:2381–2387.
1997.
|
|
17
|
Hsieh TC and Wu JM: Mechanism of action of
herbal supplement PC-SPES: elucidation of effects of individual
herbs of PC-SPES on proliferation and prostate specific gene
expression in androgen-dependent LNCaP cells. Int J Oncol.
20:583–588. 2002.PubMed/NCBI
|
|
18
|
Kimura M, Inoue H, Hirabayashi K, Natsume
H and Ogihara M: Glycyrrhizin and some analogues induce growth of
primary cultured adult rat hepatocytes via epidermal growth factor
receptors. Eur J Pharmacol. 431:151–161. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Grandis JR and Tweardy DJ: Elevated levels
of transforming growth factor alpha and epidermal growth factor
receptor messenger RNA are early markers of carcinogenesis in head
and neck cancer. Cancer Res. 53:3579–3584. 1993.PubMed/NCBI
|
|
20
|
Bianchini C, Pastore A, Pelucchi S, et al:
Sex hormone receptor levels in laryngeal carcinoma: a comparison
between protein and RNA evaluations. Eur Arch Otorhinolaryngol.
265:1089–1094. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen B, Wang J, Li W and Ji W: Expression
of androgen receptor and estrogen receptor in carcinoma of larynx.
Lin Chuang Er Bi Yan Hou Ke Za Zhi. 20:649–651. 2006.(In
Chinese).
|
|
22
|
Nehse G and Tunn S: Androgen and
progesterone receptors in oral carcinoma. J Craniomaxillofac Surg.
22:114–119. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fedoruk MN, Gimenez-Bonafe P, Guns ES,
Mayer LD and Nelson CC: P-glycoprotein increases the efflux of the
androgen dihydrotestosterone and reduces androgen responsive gene
activity in prostate tumor cells. Prostate. 59:77–90. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Izzo AA and Ernst E: Interactions between
herbal medicines and prescribed drugs: a systematic review. Drugs.
61:2163–2175. 2001. View Article : Google Scholar : PubMed/NCBI
|