Introduction
Halogenated pyrimidines are well known as classic
radiosensitizers for low-linear energy transfer (LET) radiation
such as X-rays and γ-rays (1–4). They
also have strong sensitization effects for visible and ultraviolet
light (5–7). The mechanisms of bromodeoxyuridine
(BrdU)-mediated radiosensitization have been explained elsewhere
(8–12). Simply put, single-strand break
formation from BrdU-mediated radicals results in the formation of
lethal DNA double-strand breaks (DSBs). Since sensitization can be
partially reduced by adding radical scavengers such as acetone,
various reports suggest that BrdU either produces lethal DSBs or
fixes potentially lethal damage (PLD) to enhance cell killing
(13–16).
High-LET radiation has a strong effect on cell
killing when compared to low-LET radiation. Namely, it achieves a
higher relative biological effectiveness (RBE) than low-LET
radiation (17–20) by producing dense ionization and
causing complex, clustered DNA damage (21–24).
However, the complex clustered damage produced by high-LET
radiation is not fully understood (21). Radiosensitizers are typically less
effective when using high-LET radiation when compared with low-LET
radiation (25,26).
Reports have indicated that the incorporation of
halogenated pyrimidines not only increases the magnitude of
radiation-induced DNA damage, but also suppresses DNA damage repair
(2,9,16).
High-LET radiation produces ‘clustered damage’, a type of DNA
damage which is also difficult to repair (2,9,16).
LET-dependent sensitization of halogenated pyrimidines is reported
in cellular lethality (25). As LET
increases, increased clustered DNA damage is formed. At LET >100
keV/μm, the RBE declines as LET increases (27,28).
We hypothesized that the mechanism of BrdU-induced
hypersensitivity to ionizing radiation is based on the quality of
DNA DSBs. We examined the effects of combinations of high-LET heavy
ions and unifilar and bifilar BrdU substitution in Chinese hamster
ovary (CHO) cells to better understand the BrdU dependency. In this
study, we revealed that BrdU substitution followed by low-LET
radiation altered DNA damages into more complex damages similar to
those observed after high-LET radiation exposure only, while no
additional effects on cellular lethality, chromosomal aberrations
and DNA DSB formation and repair were observed following high-LET
radiation with BrdU.
Materials and methods
Cell lines and culture
Chinese Hamster ovary (CHO10B2) cells (wild-type)
were kindly supplied by Dr Joel Bedford of Colorado State
University (Fort Collins, CO, USA). Cells were grown in αMEM
(Invitrogen, Carlsbad, CA, USA) supplemented with 10%
heat-inactivated (56°C for 30 min) fetal bovine serum (FBS, Sigma,
St. Louis, MO, USA) and 1% antibiotics and antimycotics
(Invitrogen) in a humidified 5% CO2 atmosphere at 37°C.
Cell doubling time was ~12 h.
Irradiation and drug treatment
Cells were cultured in a moderately toxic
concentration of BrdU (10 μM, Sigma, St. Louis, MO, USA) for our
experiments. Log phase cells were cultured in 10 μM BrdU for 10 or
20 h before synchronization to achieve unifilar (>95%) or
bifilar (~95%) substitution, respectively. The substitution of BrdU
was confirmed by immunocytochemistry against the BrdU antibody (BD,
Franklin Lakes, NJ, USA) for unifilar and fluorescence plus Giemsa
(FPG) differential staining on metaphase chromosomes for bifilar
(29). Cell cycle synchronization
was achieved by the mitotic shake-off method (30). Two hours after shake-off, >95% of
cells were synchronized in the G1 phase before they were
exposed to ionizing radiation. Cell synchronization was confirmed
by flow cytometry. The Titan X-ray irradiator (200 kVp, 20 mA,
0.5-mm Al and 0.5-mm Cu filters; Shimadzu, Japan) yields an X-ray
dose of ~1 Gy/min at room temperature. For heavy ion exposure,
accelerated ions were irradiated using the Heavy Ion Medical
Accelerator in Chiba (HIMAC) at room temperature. Radiation
exposure was carried out in a dark environment to prevent cellular
toxicity from room light. Dosimetry and beam quality tests for
heavy ions were carried out and confirmed by operators of
Accelerator Engineering Corp. (Chiba, Japan) (31–34).
Chromosomal aberration assay
To achieve first metaphase arrest, post-irradiated
cells were treated with 0.1 μg/ml Colcemid (Sigma) 10–16 h after
irradiation. The cells were treated with 75 mM KCl for 15 min at
37°C. After hypotonic treatment, the cells were fixed with fixative
[methanol:acetic acid solution (3:1)] three times and were dropped
onto slides. The samples were stained with filtered 10% (v/v)
Giemsa solution in Gurr solution (Invitrogen). At least 30
metaphase cells were scored in at least three separate experiments.
Chromosomal aberrations were scored as dicentric, fragment, ring,
and interstitial and terminal deletion and pooled as total
chromosomal aberrations per cell.
Colony formation assay
Cells were trypsinized and plated into P-60 cell
culture dishes immediately after the ionizing radiation exposure.
Approximately one week later, cells were fixed with 100% ethanol
and stained with crystal violet for colony counting. Colonies
containing >50 cells were counted as survivors. Plating
efficiency was ~75% for the control and 70% for both unifilar and
bifilar cells. RBE was calculated from doses required to achieve
10% survival fraction, and the sensitization enhancement ratio
(SER) was calculated from the doses required to achieve 37% cell
survival.
γ-H2AX formation assay
Synchronized cells were grown on chamber slides for
2 h. After irradiation of 1 Gy and various incubation times (1, 2,
or 3 h post-irradiation), the cells were fixed and stained as
previously described (35,36). Cellular imaging was accomplished
using an Olympus FV300 fluorescence confocal microscope equipped
with an Olympus Fluoview three dimensional image analysis system
(Olympus, Tokyo, Japan). The foci were scored in at least 50 cells
per data point. Three to four independent experiments were carried
out.
Statistical analysis
Statistical comparison of mean values was performed
using a t-test. Differences with a P-value of <0.05 were
considered to indicate a statistically significant result. Error
bars indicate standard error of the means. Confidence interval
values were calculated by Prism 5™ software (GraphPad, La Jolla,
CA, USA).
Results
Comparison of the BrdU
substitution-induced radiosensitization effect with X-rays and
heavy ions in a colony formation assay
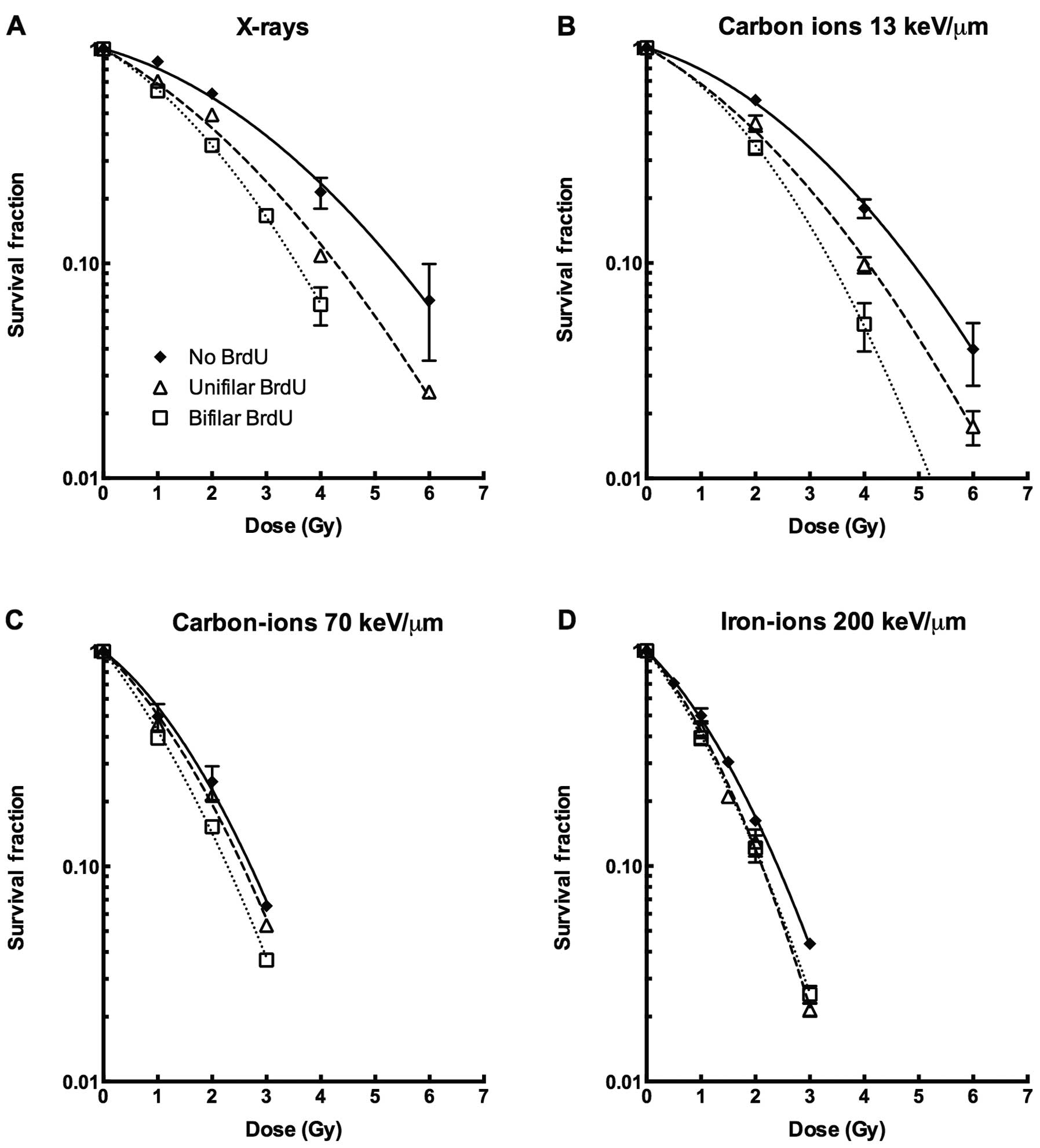
For X-rays and carbon ions with LET of 13 keV/μm,
BrdU-incorporated cells were more sensitive to ionizing radiation
when compared with the BrdU-negative controls (Fig. 1A and B). Both the initial shoulder
and slope of the survival curves were affected by BrdU
incorporation. The sensitization effect of BrdU bifilar substituted
cells was stronger than unifilar incorporation. In contrast, for
higher LET using heavy ions such as carbon ions at LET of 70 keV/μm
or iron ions at LET of 200 keV/μm, BrdU substitution did not induce
synergistic sensitization with ionizing radiation (Fig. 1C and D). The D10 value,
the dose which resulted in 10% cell survival and the
D37, value, the dose which resulted in 37% cell
survival, decreased depending on the level of BrdU incorporation
(Table I). For example, the
D10 value of 5.4 for X-irradiated unlabeled cells was
reduced to 3.6 in bifilarly labeled cells, and that of 4.9 for 13
keV/μm LET carbon ion-exposed unlabeled cells was reduced to 3.4 in
bifilarly labeled cells. For high-LET radiation, the change in
D10 value of LET 70 keV/μm carbon ions was from 2.7 to
2.3, and that of LET 200 keV/μm iron ions was from 2.4 to 2.1 for
unlabeled to bifilar BrdU substitution. Differences between sets of
D10 values were statistically significant. On the other
hand, for the D37 values of unlabeled and bifilar BrdU
substitution for high-LET carbon and iron ions, differences between
them were regarded as statistically not significant.
 | Table IRelationship between the
D10 and D37 values and BrdU incorporation for
different radiation qualities. |
Table I
Relationship between the
D10 and D37 values and BrdU incorporation for
different radiation qualities.
| Radiation | D10,
D37 | No BrdU | Unifilar BrdU | Bifilar BrdU |
|---|
| X-ray | D10 | 5.4
(4.89–5.86) | 4.3
(4.08–4.45) | 3.6
(3.39–3.72) |
| D37 | 3.1
(2.49–3.58) | 2.3
(2.00–2.51) | 1.9
(1.68–2.15) |
| Carbon ions | D10 | 4.9
(4.46–5.20) | 4.1
(3.77–4.28) | 3.4
(2.98–3.66) |
| 13 keV/μm | D37 | 2.9
(2.16–3.33) | 2.2
(1.80–2.50) | 1.9
(1.48–2.30) |
| Carbon ions | D10 | 2.7
(2.47–2.96) | 2.6
(2.46–2.68) | 2.3
(2.20–2.36) |
| 70 keV/μm | D37 | 1.5
(1.13–1.74) | 1.4
(1.21–1.49) | 1.1
(1.02–1.25) |
| Iron ions | D10 | 2.4
(2.33–2.49) | 2.1
(1.99–2.23) | 2.1
(1.97–2.24) |
| 200 keV/μm | D37 | 1.3
(1.18–1.38) | 1.1
(0.95–1.29) | 1.2
(0.89–1.25) |
Comparison of BrdU substitution-induced
radiosensitization effect with X-rays and heavy ions in a
chromosomal aberration assay
As previously shown in the colony formation assay,
BrdU-mediated radiosensitization was impaired as LET increased
(Fig. 1C and D). To further
investigate the mechanism of cell killing, we analyzed first
post-irradiation metaphase chromosomes with a chromosomal
aberration assay. As predicted from the survival data, no
additional chromosomal aberrations were observed after BrdU
substitution and subsequent high-LET radiation exposure (Table II). After X-ray or LET 13 keV/μm
carbon ion exposure, BrdU substitution-mediated radiosensitization
was observed at each dose point (1 and 2 Gy) with BrdU
incorporation. For instance, bifilar incorporation at 2 Gy
increased chromosomal aberrations by 1.71- (from 0.75 to 1.33) and
1.82-fold (from 1.06 to 1.93) for X-rays and LET 13 keV/μm carbon
ions, respectively. In contrast, there was almost no
radiosensitization at any dose or incorporation time as a result of
carbon (70 keV/μm) or iron (200 keV/μm) ions.
 | Table IIChromosomal aberration assay in first
post-irradiation metaphase following irradiation at G1 phase with
BrdU substitutions. |
Table II
Chromosomal aberration assay in first
post-irradiation metaphase following irradiation at G1 phase with
BrdU substitutions.
| Radiation | Dose | No BrdU | Unifilar | Bifilar |
|---|
| No irradiation | 0 Gy | 0.05±0.03 | 0.11±0.05 | 0.15±0.05 |
| X-ray | 1 Gy | 0.30±0.03 | 0.48±0.03 | 0.71±0.04 |
| 2 Gy | 0.75±0.05 | 1.01±0.06 | 1.33±0.04 |
| Carbon ions | 1 Gy | 0.42±0.05 | 0.80±0.20 | 1.00±0.00 |
| 13 keV/μm | 2 Gy | 1.06±0.17 | 1.56±0.24 | 1.93±0.12 |
| Carbon ions | 1 Gy | 1.26±0.13 | 1.30±0.25 | 1.23±0.09 |
| 70 keV/μm | 2 Gy | 2.12±0.31 | 2.54±0.69 | 2.73±0.37 |
| Iron ions | 1 Gy | 1.34±0.21 | 1.26±0.26 | 1.40±0.47 |
| 200 keV/μm | 2 Gy | 2.84±0.13 | 2.68±0.79 | 2.70±0.64 |
Comparison of BrdU-induced enhancement
effect with X-rays and heavy ions in the γ-H2AX formation
assay
DNA DSBs result in chromosomal aberrations and cell
killing. To investigate the initial amount of DNA damage and DNA
repair efficiency following various radiation exposures, we
performed a γ-H2AX foci formation assay. One hour after X-ray
exposure 22.6 foci per cell for controls, 25.5 foci per cell for
unifilar substitution (13% increase), and 27.8 foci per cell for
bifilar incorporation (23% increase) were scored. At 3 h after
irradiation, the numbers of foci remaining were 9.2 for controls,
12.1 for unifilar incorporation (32% increase), and 14.5 foci for
bifilar incorporation (58% increase) (Fig. 2). For LET 13 keV/μm carbon ions, the
number of γ-H2AX foci was 18.2 and 6.6 for controls, 21.5 (18%
increase) and 9.3 (41% increase) for single BrdU incorporation, and
24.1 (52% increase) and 12.1 (83% increase) for double
incorporation at 1 and 3 h, respectively (Fig. 2). Low-LET radiation resulted in an
increased number of initial BrdU-induced DNA DSBs and an increased
amount of damage remaining in BrdU-incorporated cells. In contrast,
high-LET radiation resulted in no significant difference in the
number of initial BrdU-induced DNA DSBs or damage remaining in the
BrdU-incorporated cells (Fig.
2).
To estimate the effects on repair capacity resulting
from BrdU substitution, we calculated half-lives for the reduction
of γ-H2AX foci between 1 and 3 h post-irradiation (Fig. 2). Half lives for low-LET exposures
increased with the amount of BrdU substitution (from 1.54 to 2.12 h
for X-ray bifilar and 1.35 to 1.97 h for bifilar LET 13 keV/μm
carbon-ions). In contrast, high-LET exposures (iron-ions in
particular) showed no significant difference in γ-H2AX foci
half-lives with or without BrdU substitution (from 2.69 to 2.78 h,
P<0.05).
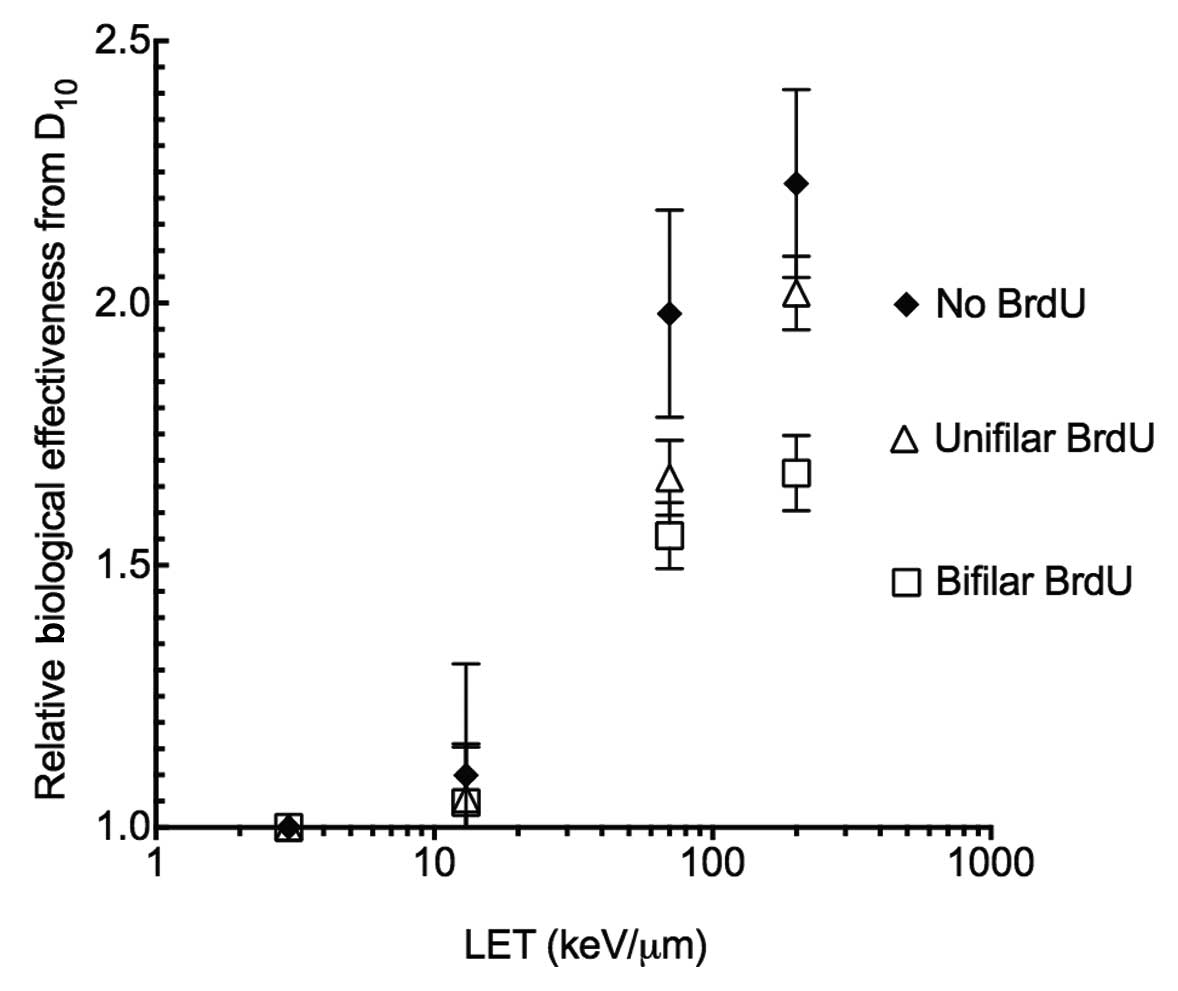
BrdU substitution effects RBE and
SER
In order to assess BrdU substitution effects on
radiosensitization, RBE and SER values were obtained. Without BrdU
substitution, CHO10B2 cells showed a maximum of an ~2.1 RBE value
at LET 200 keV/μm. The LET values were decreased when cells were
incorporated with unifilar or bifilar BrdU. The degree of reduction
was stronger for bifilar BrdU substitution than unifilar BrdU
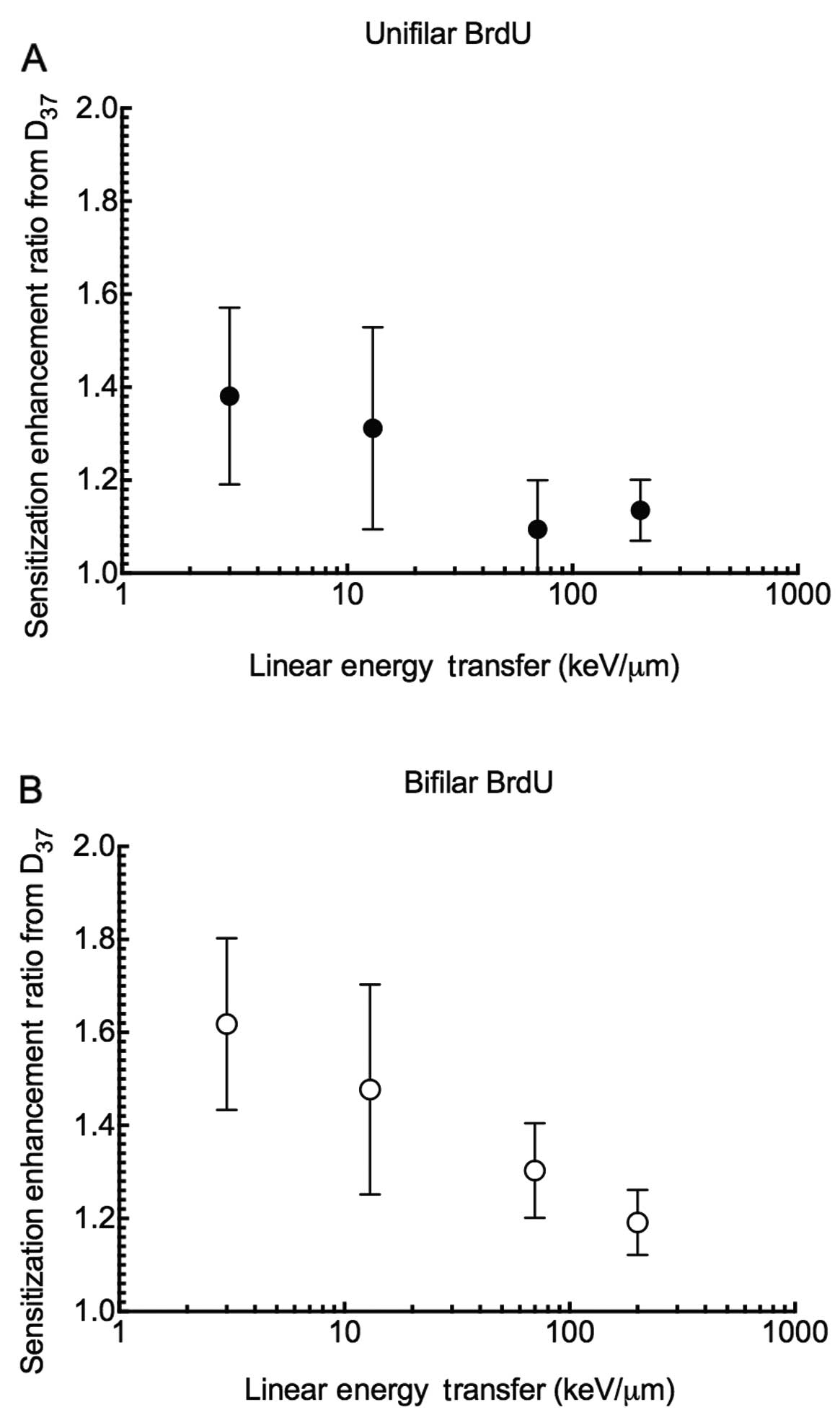
substitution (Fig. 3). SER values
showed that unifilar BrdU substitutions are ~1.4 more effective in
cell killing when compared to values without substitution with
X-ray exposure, while there was only a 1.1 increase in
effectiveness with high LET radiation such as carbon 70 keV/μm and
iron ions 200 keV/μm (Fig. 4). The
same trend was observed for bifilar substitution. The SER values
were decreased from 1.6 to 1.2.
Discussion
In order to investigate the reduced synergistic
effects of a combination of high-LET radiation and BrdU
incorporation, we compared the damage resulting from low- or
high-LET radiation exposure with and without BrdU substitution with
several endpoints. We showed that BrdU substitution mediated
radiosensitization effects on low-LET photon radiation and particle
radiation but similar cellular lethality was observed after
high-LET radiation (Fig. 1).
Linstadt et al(25) proved
that the extent of radiosensitization caused by IdU, a halogenated
pyrimidine, decreased as the LET increased. In addition, they found
very small sensitization enhancements for the distal peak (82
keV/μm) and Bragg peak (183 keV/μm) of a Neon ion beam and no
radiosensitization was observed for an extremely high-LET Lanthanum
ion beam with 1000 keV/μm (25).
Our study was consistent with these results as carbon ions with LET
70 keV/μm and iron ions with LET 200 keV/μm following unifilar or
bifilar BrdU incorporation yielded very weak radiosensitization for
cellular lethality. Fig. 3 shows
the RBE values calculated from D10 with BrdU
substitution (Table I). Smaller RBE
values were noted after high-LET radiation exposure with BrdU
substitution. Among the same BrdU substituted cells, high-LET and
high RBE advantage was lost. The SER values for high-LET were
smaller when cells were incorporated with more BrdU (Fig. 4A and B). When halogenated pyrimidine
is used for clinical practice, it is worthy to note that
enhancement of cell killing would be smaller using carbon ion
radiotherapy than that expected in X-ray radiotherapy. Normal
tissues incorporated with BrdU would be severely sensitized
following low-LET exposure. Therefore, the dose to patients should
be reduced to avoid adverse side effects.
Fig. 2 shows that
initial (1 h post-irradiation) DNA damage observed as
phosphorylated H2AX foci was increased with BrdU incorporation
degrees only for low-LET radiation but not high-LET radiation.
Therefore, we assume that initial DNA damages are increased with
BrdU substitution for low-LET radiation but not for high-LET
radiation. This result was consistent with other reports (4,15). As
a result of more initial damage and slower repair, there were
additional γ-H2AX foci in BrdU substituted cells after low-LET
radiation. γ-H2AX foci are excellent markers for DNA double-strand
breaks. H2AX is phosphorylated ~2 mega bases from a DSB site
(35,36). We could not exclude the possibility
that additional DSBs were produced by BrdU sensitization following
high-LET radiation within short range to be recognized as a single
focus.
In order to evaluate DNA repair kinetics, we
calculated the half-life of γ-H2AX foci from 1 to 3 h after
irradiation (Fig. 2). Many DNA DSB
repair deficient mutants were found to have slower kinetics of DSB
repair and γ-H2AX foci disappearance (37–39).
Half-lives of γ-H2AX foci were affected by BrdU substitutions for
low-LET radiation, but not for high-LET radiation (Fig. 2). The results coincide with other
studies, suggesting that slower repair is one of the possible
mechanisms for BrdU-induced radiosensitization (2,13,16,40).
In contrast, one study found that halogenated pyrimidines did not
effect the repair of PLD for low-LET radiation (X-rays and neon
ions with LET 38 keV/μm) and sublethal damage repair (25).
We clearly observed that the combination of BrdU and
high-LET radiation does not increase the half-life of γ-H2AX foci
disappearance any more than high-LET radiation alone. This suggests
that the BrdU substitution did not modify DNA DSBs produced by
high-LET radiation or such modifications were naturally formed by
high-LET radiation. Multiple publications suggest that high-LET
radiation produces dense ionization in their tracks and produce
multiple damages near DNA double-strand breaks and form clustered
and complex damage (4,12,22,41).
These damages are very difficult to repair and it results in high
lethality per absorbed physical dose. BrdU-mediated free radicals
form lesions such as single-strand breaks, double-strand breaks,
and complex double-strand breaks (12). We assumed that BrdU could not
contribute any biological response once high-LET produced enough
dense ionization on their target. LET >100 keV/μm constitutes an
overdose as excess ionizing events do not efficiently produce DSBs
(41–43).
These results indicate that there was no detectable
difference in the amount of DNA double-strand break formation and
no detectable effects for repair kinetics for high-LET radiation
with or without BrdU incorporation. These results were directly
correlated to no differences in the frequency of chromosomal
aberrations in metaphase chromosomes and cellular lethality for
high LET radiation with or without BrdU substitution (Fig. 1, Table
II).
BrdU and other halogenated pyrimidines appear not to
be practical sensitizers to combine with carbon ion radiotherapy
due to the severe sensitization to normal tissue and the small
sensitization to cancer at higher LET radiation. But LET for the
spread out Bragg peak for carbon ion radiotherapy contains a wide
range of LET (32,34,44).
In this range we would expect some sensitization for tumor control
but the SER would not be as high as that for low-LET radiation such
as photon and proton radiation.
Acknowledgments
We thank Dr Jamie Bush and Mr. Charles Yurkon of
Colorado State University for proofreading our manuscript. This
study was partially supported by the Japan Society for the
Promotion of Science Grant in Aid (Young Scientists B19710056 to
T.K.)and by start-up funds from Colorado State University and Dr
Akiko M. Ueno Radiation Biology Research Fund. This study was part
of a Research Project with Heavy Ions at the NIRS (National
Institute of Radiological Sciences)-HIMAC.
References
|
1
|
Mitchell JB, Russo A, Cook JA, Straus KL
and Glatstein E: Radiobiology and clinical application of
halogenated pyrimidine radiosensitizers. Int J Radiat Biol.
56:827–836. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Franken NA, Van Bree CV, Kipp JB and
Barendsen GW: Modification of potentially lethal damage in
irradiated Chinese hamster V79 cells after incorporation of
halogenated pyrimidines. Int J Radiat Biol. 72:101–109. 1997.
View Article : Google Scholar
|
|
3
|
McLaughlin PW, Lawrence TS, Seabury H, et
al: Bromodeoxyuridine-mediated radiosensitization in human glioma:
the effect of concentration, duration, and fluoropyrimidine
modulation. Int J Radiat Oncol Biol Phys. 30:601–607. 1994.
View Article : Google Scholar
|
|
4
|
Kinsella TJ, Dobson PP, Mitchell JB and
Fornace AJ Jr: Enhancement of X ray-induced DNA damage by
pre-treatment with halogenated pyrimidine analogs. Int J Radiat
Oncol Biol Phys. 13:733–739. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zeng Y and Wang Y: UVB-induced formation
of intrastrand cross-link products of DNA in MCF-7 cells treated
with 5-bromo-2′-deoxyuridine. Biochemistry. 46:8189–8195.
2007.PubMed/NCBI
|
|
6
|
Zeng Y and Wang Y: Sequence-dependent
formation of intrastrand crosslink products from the UVB
irradiation of duplex DNA containing a 5-bromo-2′-deoxyuridine or
5-bromo-2′-deoxycytidine. Nucleic Acids Res. 34:6521–6529.
2006.PubMed/NCBI
|
|
7
|
Puck TT and Kao FT: Genetics of somatic
mammalian cells. V Treatment with 5-bromodeoxyuridine and visible
light for isolation of nutritionally deficient mutants. Proc Natl
Acad Sci USA. 58:1227–1234. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iliakis G, Pantelias G and Kurtzman S:
Mechanism of radiosensitization by halogenated pyrimidines: effect
of BrdU on cell killing and interphase chromosome breakage in
radiation-sensitive cells. Radiat Res. 125:56–64. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Webb CF, Jones GD, Ward JF, Moyer DJ,
Aguilera JA and Ling LL: Mechanisms of radiosensitization in
bromodeoxyuridine-substituted cells. Int J Radiat Biol. 64:695–705.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zimbrick JD, Ward JF and Myers LS Jr:
Studies on the chemical basis of cellular radiosensitization by
5-bromouracil substitution in DNA. I Pulse- and steady-state
radiolysis of 5-bromouracil and thymine. Int J Radiat Biol Relat
Stud Phys Chem Med. 16:505–523. 1969. View Article : Google Scholar
|
|
11
|
Zimbrick JD, Ward JF and Myers LS Jr:
Studies on the chemical basis of cellular radiosensitization by
5-bromouracil substitution in DNA. II Pulse- and steady state
radiolysis of bromouracil-substituted and -unsubstituted DNA. Int J
Radiat Biol Relat Stud Phys Chem Med. 16:525–534. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Watanabe R and Nikjoo H: Modelling the
effect of incorporated halogenated pyrimidine on radiation-induced
DNA strand breaks. Int J Radiat Biol. 78:953–966. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jones GD, Ward JF, Limoli CL, Moyer DJ and
Aguilera JA: Mechanisms of radiosensitization in
iododeoxyuridine-substituted cells. Int J Radiat Biol. 67:647–653.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Iliakis G, Wang Y, Pantelias GE and
Metzger L: Mechanism of radiosensitization by halogenated
pyrimidines: effect of BrdU on repair of DNA breaks, interphase
chromatin breaks, and potentially lethal damage in plateau-phase
CHO cells. Radiat Res. 129:202–211. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iliakis G, Kurtzman S, Pantelias G and
Okayasu R: Mechanism of radiosensitization by halogenated
pyrimidines: effect of BrdU on radiation induction of DNA and
chromosome damage and its correlation with cell killing. Radiat
Res. 119:286–304. 1989. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Iliakis G and Kurtzman S: Mechanism of
radiosensitization by halogenated pyrimidines: bromodeoxyuridine
and beta-arabinofuranosyladenine affect similar subsets of
radiation-induced potentially lethal lesions in plateau-phase
Chinese hamster ovary cells. Radiat Res. 127:45–51. 1991.
View Article : Google Scholar
|
|
17
|
Blakely EA and Kronenberg A: Heavy-ion
radiobiology: new approaches to delineate mechanisms underlying
enhanced biological effectiveness. Radiat Res. 150:S126–S145. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eguchi-Kasai K, Murakami M, Itsukaichi H,
et al: Repair of DNA double-strand breaks and cell killing by
charged particles. Adv Space Res. 22:543–549. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsujii H, Mizoe JE, Kamada T, et al:
Overview of clinical experiences on carbon ion radiotherapy at
NIRS. Radiother Oncol. 73(Suppl 2): S41–S49. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jakel O: The relative biological
effectiveness of proton and ion beams. Z Med Phys. 18:276–285.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Prise KM, Pinto M, Newman HC and Michael
BD: A review of studies of ionizing radiation-induced double-strand
break clustering. Radiat Res. 156:572–576. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fakir H, Sachs RK, Stenerlow B and Hofmann
W: Clusters of DNA double-strand breaks induced by different doses
of nitrogen ions for various LETs: experimental measurements and
theoretical analyses. Radiat Res. 166:917–927. 2006. View Article : Google Scholar
|
|
23
|
Hada M and Georgakilas AG: Formation of
clustered DNA damage after high-LET irradiation: a review. J Radiat
Res. 49:203–210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pinto M, Prise KM and Michael BD: Evidence
for complexity at the nanometer scale of radiation-induced DNA DSBs
as a determinant of rejoining kinetics. Radiat Res. 164:73–85.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Linstadt D, Blakely E, Phillips TL and
Castro JR: Radiosensitization produced by iododeoxyuridine with
high linear energy transfer heavy ion beams. Int J Radiat Oncol
Biol Phys. 15:703–710. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oliveira NG, Castro M, Rodrigues AS, et
al: Wortmannin enhances the induction of micronuclei by low and
high LET radiation. Mutagenesis. 18:37–44. 2003. View Article : Google Scholar
|
|
27
|
Skarsgard LD: Radiobiology with heavy
charged particles: a historical review. Phys Med. 14(Suppl 1):
1–19. 1998.PubMed/NCBI
|
|
28
|
Hamada N, Imaoka T, Masunaga S, et al:
Recent advances in the biology of heavy-ion cancer therapy. J
Radiat Res. 51:365–383. 2010. View Article : Google Scholar
|
|
29
|
Nagasawa H and Little JB: Effect of tumor
promoters, protease inhibitors, and repair processes on
X-ray-induced sister chromatid exchanges in mouse cells. Proc Natl
Acad Sci USA. 76:1943–1947. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Terasima T and Tolmach LJ: Changes in
X-ray sensitivity of HeLa cells during the division cycle. Nature.
190:1210–1211. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kanai T, Endo M, Minohara S, et al:
Biophysical characteristics of HIMAC clinical irradiation system
for heavy-ion radiation therapy. Int J Radiat Oncol Biol Phys.
44:201–210. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kanai T, Matsufuji N, Miyamoto T, et al:
Examination of GyE system for HIMAC carbon therapy. Int J Radiat
Oncol Biol Phys. 64:650–656. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Matsufuji N, Fukumura A, Komori M, Kanai T
and Kohno T: Influence of fragment reaction of relativistic heavy
charged particles on heavy-ion radiotherapy. Phys Med Biol.
48:1605–1623. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Matsufuji N, Kanai T, Kanematsu N, et al:
Specification of carbon ion dose at the National Institute of
Radiological Sciences (NIRS). J Radiat Res. 48(Suppl A): A81–A86.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rogakou EP, Boon C, Redon C and Bonner WM:
Megabase chromatin domains involved in DNA double-strand breaks in
vivo. J Cell Biol. 146:905–915. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rogakou EP, Boon C and Bonner WM:
Formation of a novel histone derivative, H2AX phosphorylated on
serine-139, is an immediate cellular response to non-lethal and
lethal amounts of ionizing radiation, and is also found during
apoptosis and in germ cells. Mol Biol Cell. 8:1858. 1997.
|
|
37
|
Banath JP, MacPhail SH and Olive PL:
Radiation sensitivity, H2AX phosphorylation, and kinetics of repair
of DNA strand breaks in irradiated cervical cancer cell lines.
Cancer Res. 64:7144–7149. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kuhne M, Riballo E, Rief N, Rothkamm K,
Jeggo PA and Lobrich M: A double-strand break repair defect in
ATM-deficient cells contributes to radiosensitivity. Cancer Res.
64:500–508. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kato TA, Nagasawa H, Weil MM, Little JB
and Bedford JS: Levels of gamma-H2AX foci after low-dose-rate
irradiation reveal a DNA DSB rejoining defect in cells from human
ATM heterozygotes in two at families and in another apparently
normal individual. Radiat Res. 166:443–453. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang Y, Pantelias GE and Iliakis G:
Mechanism of radiosensitization by halogenated pyrimidines: the
contribution of excess DNA and chromosome damage in BrdU
radiosensitization may be minimal in plateau-phase cells. Int J
Radiat Biol. 66:133–142. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Prise KM, Folkard M, Newman HC and Michael
BD: Effect of radiation quality on lesion complexity in cellular
DNA. Int J Radiat Biol. 66:537–542. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chapman JD, Doern SD, Reuvers AP, et al:
Radioprotection by DMSO of mammalian cells exposed to X-rays and to
heavy charged-particle beams. Radiat Environ Biophys. 16:29–41.
1979. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang TC, Craise LM, Mei MT and Tobias CA:
Neoplastic cell transformation by heavy charged particles. Radiat
Res Suppl. 8:S177–S187. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fukumura A, Tsujii H, Kamada T, et al:
Carbon-ion radiotherapy: clinical aspects and related dosimetry.
Radiat Prot Dosimetry. 137:149–155. 2009. View Article : Google Scholar : PubMed/NCBI
|