Introduction
Combretastatin A-4 (CA-4), a natural product
isolated from the bark of a south african tree combretum
caffrum, is a highly effective antiangiogenic agent that causes
vascular shutdown, leading to tumor death (1). CA-4 phosphate (CA-4P), a water soluble
pro-drug of CA-4, is rapidly dephosphorylated to the active
compound CA-4 and shows reversible binding kinetics to tubulin,
leading to disruption of microtubular structures (2,3).
Although CA-4P is being studied in clinical trials as a vascular
disrupting agent, cardiovascular toxicity and neurotoxicity are
dose limiting for CA-4P (4,5). These severe side-effects currently
represent the main obstacles to broad clinical application of CA-4P
(2). Thus, it is important to
develop new antitumor combination therapy with lower concentrations
of CA-4 and more specificity for tumor endothelial cells than
normal endothelial cells to avoid cardiac toxicity from endothelial
damage.
Tyrosine kinase inhibitors (TKIs) are rapidly being
integrated into the management of a variety of malignant diseases
(6). Dasatinib, a novel orally
bioactive TKI currently used to treat patients with hematologic and
solid malignancies, correlated with a combined targeting of PDGFR-β
and VEGFR/c-Src signaling pathways (7–9). The
Src kinases have multiple substrates that lead to diverse
biological effects in cancer cells, including changes in
proliferation, motility, invasion, survival and angiogenesis
(10). Dasatinib suppresses tumor
angiogenesis, invasion, and metastasis by inhibiting Src expression
(11). Cardiovascular and
hematologic toxicity are dose limiting for dasatinib; thus, it is
necessary to develop new anticancer combination therapy with lower
concentrations of dasatinib to avoid these major side-effects
(12). Dasatinib has shown
synergistic anticancer activity in combination with
chemotherapeutic agents including paclitaxel, ixabepilone and
erlotinib (12–14).
The tumor vasculature is critical to both the
survival of a solid tumor mass and its continued growth (15). Angiogenesis is a complex process
that occurs in a variety of physiologic and pathophysiologic states
and is a remodeling of an established primitive network of blood
vessels. Angiogenesis is a complex process that is essential for
growth, invasion and metastasis of tumors (16). Vascular targeting agents (VTAs)
comprise a novel class of anticancer agents which can be divided
into two groups; those that inhibit angiogenesis (angiogenesis
inhibitors) and those that target established vessels (vascular
disrupting agents) (17). Various
combinations involving an antiangiogenic agent and an antivascular
agent have shown considerable promise in preclinical models and
such combinations are currently beginning to be evaluated in
patients (18,19). Thus, we hypothesized that combining
the antiangiogenic drug dasatinib with the antivascular agent CA-4
might potentially enhance the anticancer therapeutic effects.
In the present study, we showed for the first time
that dasatinib and CA-4 in combination had substantial synergistic
antitumor efficacy against human cancer cells in vitro and
in vivo. In addition, dasatinib greatly enhanced
CA-4-mediated apoptosis in HO-8910 cells, accompanied by increased
extent of mitochondrial depolarization, cleavage of PARP and
activation of caspase cascades. Notably, our results demonstrated
that dasatinib plus CA-4 leads to increased levels of γ-H2AX
indicating increased DNA damage in HO-8910 cells. These data
suggested that the combination of dasatinib and CA-4 might be an
effective therapeutic strategy to achieve synergistic activities in
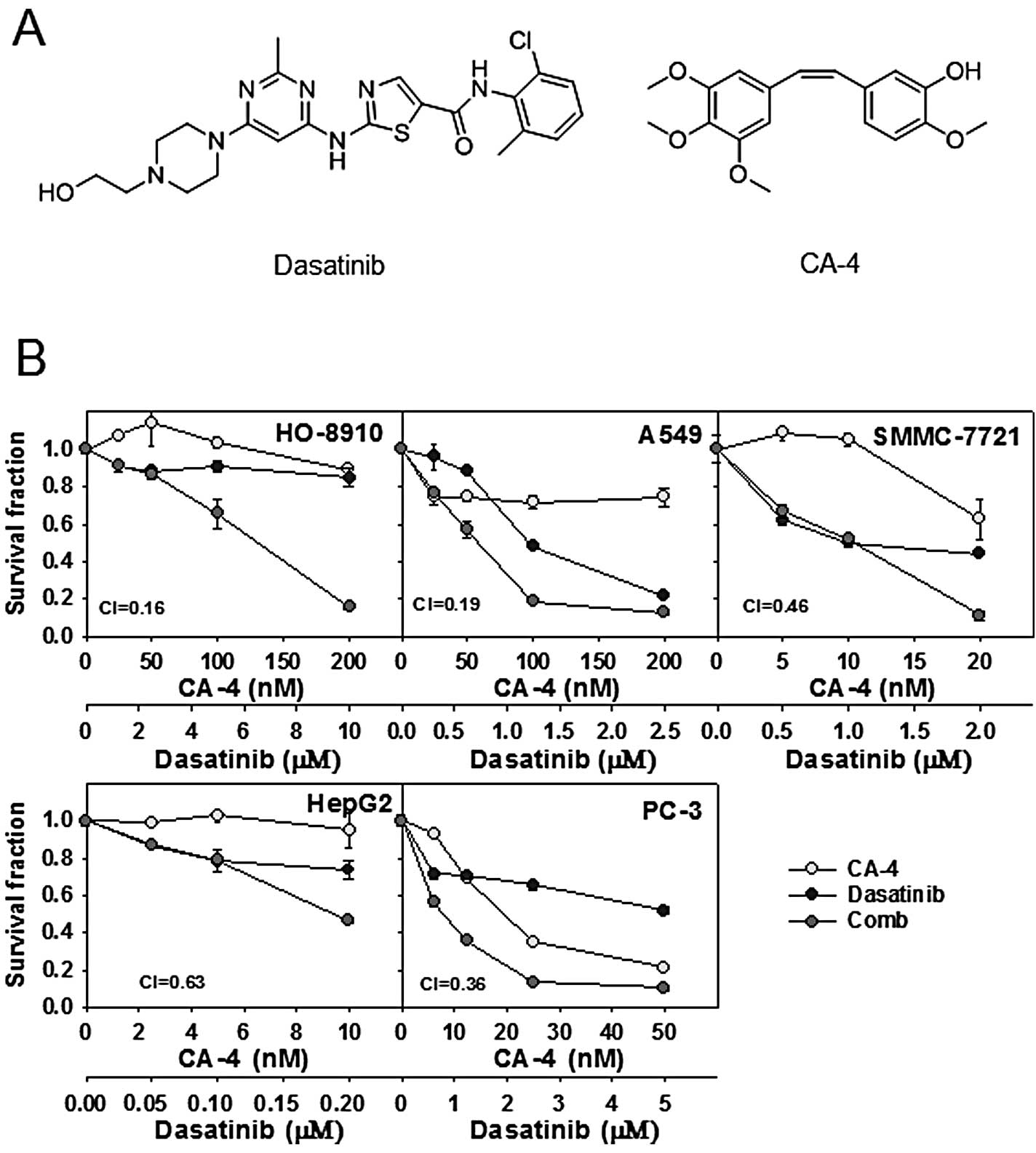
patients with solid tumors. Chemical structures of the agents are
shown in Fig. 1A.
Materials and methods
Materials
CA-4 was synthesized at the Department of Chemical
and Biochemical Engineering, Zhejiang University. Dasatinib was
supplied by LC Laboratories (Woburn, MA, USA). The primary
antibodies against Mcl-1 (S-19), Bax (Δ21), Bcl-2 (C-2), PARP
(H250), pro-caspase-3 (H-277), XIAP (A-7), γ-H2AX (Ser139), and
HRP-labeled secondary anti-mouse and anti-rabbit antibodies were
purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA);
cytochrome c (136F3), cleaved-caspase-3 (D-175) were
purchased from Cell Signaling Technology (Danvers, MA, USA);
β-actin was purchased from BD Biosciences (Franklin Lakes, NJ,
USA).
Cell culture
Human ovarian cancer cell line (HO-8910), human
hepatocellular carcinoma cell line (HepG2, SMMC-7721), human
non-small cell lung cancer cell line (A549) and human prostate
cancer cell line (PC-3) were purchased from the Shanghai Institute
of Biochemistry and Cell Biology (Shanghai, China); they were
tested and authenticated for genotypes by DNA fingerprinting.
HO-8910 and HepG2 were maintained in Dulbecco’s modified Eagle’s
medium (DMEM) supplemented with 10% fetal bovine serum (FBS). A549
and PC-3 were grown in Ham’s F12 medium supplemented with 10% FBS,
and SMMC-7721 was cultured in RPMI-1640 medium supplemented with
10% FBS. All the cells were maintained in a humidified atmosphere
of 95% air plus 5% CO2 at 37°C.
Cytotoxicity assay
The antiproliferative activity of combination
treatment with dasatinib and CA-4 was measured by sulforhodamine
blue (SRB) cytotoxicity assay. Briefly, cells were fixed with 10%
TCA solution for 1 h, wells were rinsed 5 times with tap water and
then cells were stained with 0.4% SRB solution (100 μl/well) for at
least 20 min at room temperature. Wells were rinsed with 1% acetic
acid to remove unbound dye and were then left to air dry. The SRB
dye was then solubilized by placing 100 μl of unbuffered Tris-based
solution in each well, and the absorbance was measured at 515 nm
using a multi-scan spectrum. The inhibition rate of cell
proliferation was calculated for each well as (A515 control cells -
A515 treated cells)/A515 control cells × 100%.
Analysis of apoptosis by propidium iodide
(PI) staining
Cells (4×105/well) were seeded into
6-well plates and exposed to dasatinib, CA-4 or the combination for
48 h. Cells were harvested and washed with PBS, fixed with
pre-cooled 70% ethanol at 4°C overnight. Fixed cells were then
washed with PBS to remove residual ethanol, pelleted, resuspended
in 500 μl PBS containing 50 μg RNase A at 37°C and 5 μg PI in the
dark at room temperature for 30 min. For each sample,
2×104 cells were collected and analyzed using an
FACSCalibur cytometer (Becton-Dickinson, San Jose, CA, USA).
Analysis of apoptosis by DAPI
staining
Briefly, cells in 96-well plates treated with
dasatinib, CA-4 or the combination were washed twice with PBS, and
then incubated with 0.1% Triton and 0.1% DAPI. The morphology of
the cell nuclei was observed and captured using a fluorescence
microscope at excitation wavelength 350 nm.
Determination of mitochondrial membrane
depolarization
Cells (4×105/well) were exposed to
dasatinib, CA-4 or the combination for 48 h. They were then
collected and resuspended in fresh medium containing 10 μg/ml
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazol-carbocyanine
iodide (JC-1). Following incubation at 37°C for 30 min, cells were
analyzed by flow cytometry (20).
Cell lysates and western blot
analysis
Proteins were extracted with lysis buffer (50 mM
Tris-HCl, 150 mM NaCl, 1 mM EDTA, 0.1% SDS, 0.5% deoxycholic acid,
0.02% sodium azide, 1% NP-40, 2.0 μg/ml aprotinin, 1 mM
phenylmethylsulfonylfluoride). The lysates were centrifuged at
10,000 × g for 30 min at 4°C. The supernatants were transferred to
a new tube and the protein concentration was determined. To analyze
cytochrome c release from mitochondria, mitochondria was
extracted using Mitochondria/Cytosol Fractionation kit (Beyotime
Institute of Biotechnology, Haimen, China). Proteins were
fractionated on 8–15% Tris-glycine gels, and they were then
transferred to PVDF membranes (Millipore, Bedford, MA, USA) and
probed with primary antibodies (dilution range 1:500–1:1,000)
followed by horseradish peroxidase-labeled secondary antibodies at
1:5,000 dilution. Antibody binding was then detected with the use
of a chemiluminescent substrate and visualized on autoradiography
film (21).
Immunofluorescence
Cells were seeded onto 35-mm dishes and exposed to
dasatinib, CA-4 or the combination for 48 h. Subsequently, the
cells were fixed with 4% formaldehyde for 15 min. After washing
with PBS, the cells were blocked with 10% serum in PBS for 10 min
and incubated at 37°C for 2 h with γ-H2AX (1:200; Santa Cruz
Biotechnology). The cells were then washed and incubated in the
dark for 1 h at 37°C with goat anti-rabbit (FITC)-conjugated
antibodies (1:200; Earthbox, San Francisco, CA, USA). After
washing, the nuclei were counterstained with DAPI and the cells
were then washed in PBS and examined using a laser-scanning
confocal microscopy (FluoView, Olympus, Tokyo).
Plasmid transfection
pTOPO-Mcl-1 plasmid from Addgene (22) (Plasmid 21605, Addgene, Cambridge,
MA, USA) or the empty vector was transfected into cells using
Lipofectamine 2000 as recommended by the manufacturer.
Animals and antitumor activity in
vivo
Human ovarian cancer HO-8910 xenografts were
established by injecting 5×106 cells subcutaneously into
nude mice. When the tumor reached a volume of 50–150
mm3, the mice were randomized to control and treated
groups, and received vehicle (0.9% sodium chloride, i.v.
administration), CA-4 (6 mg/kg, 1% DMSO, 7% cremophor/ethanol
(3:1), and 92% sodium chloride, i.p. administration), dasatinib (5
mg/kg, i.g. administration) 4 times per week for 19 days. Mouse
weight and tumor volume were measured individually twice per week
with microcalipers until animals were sacrificed. Tumor volume (V)
was calculated as V = (length × width × high)/2. The tumor volume
at day n was expressed as RTV according to the following formula:
RTV = TVn/TV0, where TVn is the tumor volume at day n and TV0 is
the tumor volume at day 0. Therapeutic effects of treatment were
expressed in terms of T/C % using the calculation formula T/C (%) =
mean RTV of the treated group/mean RTV of the control group ×
100%.
Statistical analyses
Two tailed Student’s t-tests were used to determine
the significance of differences between the experiment conditions.
Differences were considered statistically significant at P<0.05.
Combination index (18) was
well-accepted for quantifying drug synergism based on the multiple
drug-effect equation of Chou-Talalay (23,24).
For in vitro experiments, CI values were calculated for each
concentration of dasatinib, CA-4 and the combination in cell
proliferation assays using Calcusyn (Biosoft, Cambridge, UK).
Different CI values were obtained when solving the equation for
different effect levels, and mean CI values were chosen for
presentation. A CI<0.9 indicated synergism; 0.1, very strong
synergism; 0.1–0.3, strong synergism; 0.3–0.7, synergism; 0.7–0.85,
moderate synergism; 0.85–0.9, slight synergism; 0.9–1.10, additive;
and >1.10, antagonism.
Results
Cytotoxicity of the dasatinib and CA-4
combination in human cancer cell lines
Firstly, the cytotoxicity was determined using SRB
assay, after a 72-h exposure to dasatinib, CA-4 and the combination
at the indicated concentration in 5 human cancer cell lines.
Survival curves to dasatinib, CA-4, and dasatinib combined with
CA-4 are shown in Fig. 1B. CI
values were calculated using Calcusyn at the fixed-ratio
concentrations of dasatinib and CA-4 to assess combination
activity. Dasatinib plus CA-4 showed synergy in HO-8910, A549,
SMMC-7721, HepG2 and PC-3 cell lines, with the mean CI values
<0.7 (Fig. 1B).
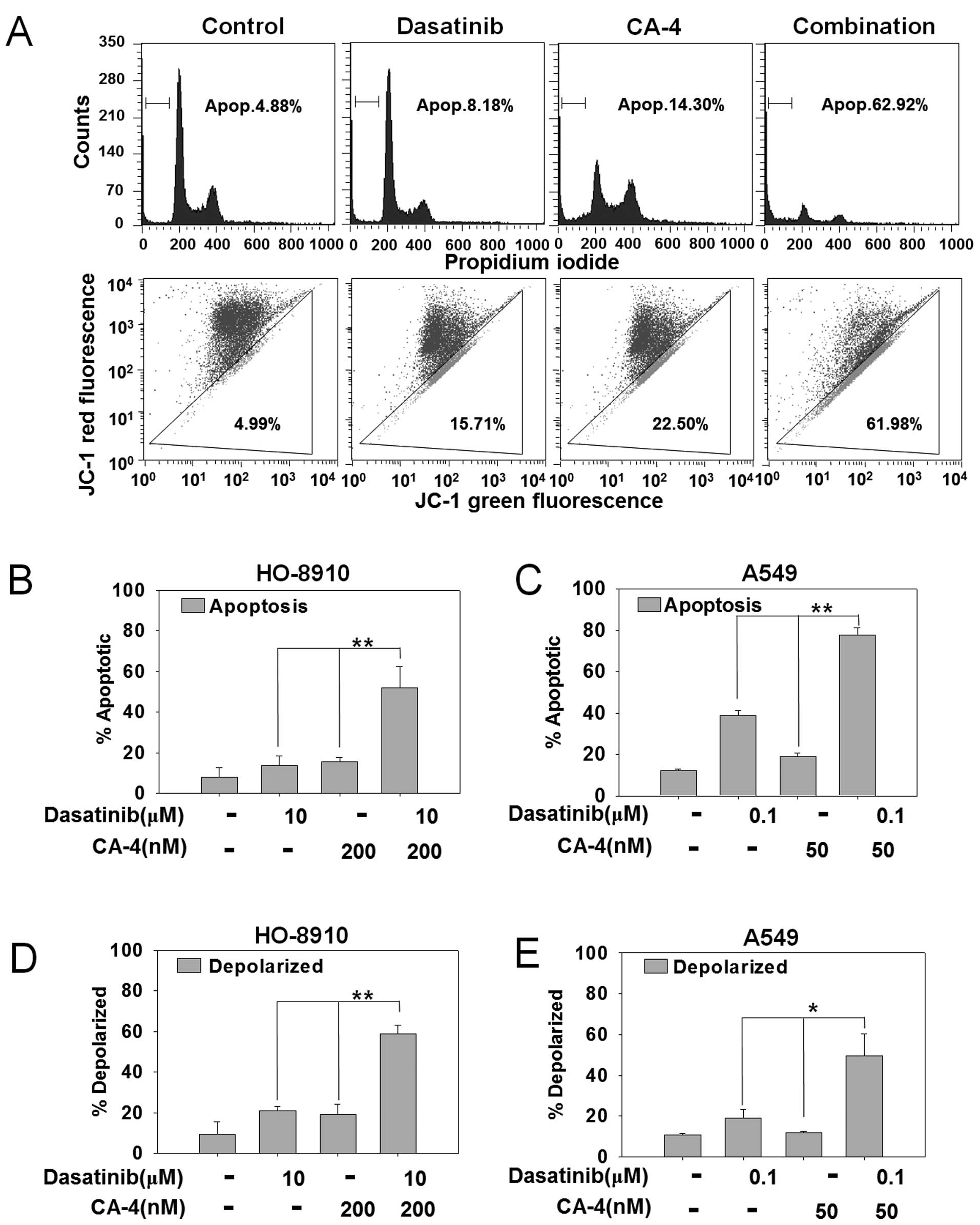
Dasatinib synergizes with CA-4 to trigger
apoptosis
To explore the mechanism of synergistic effects by
combining dasatinib and CA-4, we first detected apoptosis by PI
staining in HO-8910 and A549 cells that showed strong synergistic
effects in the cytotoxicity assay. As shown in Fig. 2A, PI staining for sub-G1 content
analysis was used to characterize the apoptosis in HO-8910 cells
treated with 10 μM dasatinib, 200 nM CA-4 or the combination for 48
h. The percentage of apoptotic cells was 4.88% in control cells,
8.16% with dasatinib, 14.30% with CA-4 and 62.92% in the
combination treatment group. The difference of apoptotic cells
between combination vs. mono-treated groups reached statistical
significant in HO-8910 and A549 cells (P<0.01) (Fig. 2B and C).
Dasatinib plus CA-4 induces apoptosis via
the mitochondrial pathway
To further confirm the combination effect of
dasatinib and CA-4 on the induction of the mitochondrial apoptosis
pathway, we next detected mitochondrial membrane potential by JC-1
staining in HO-8910 and A549 cells. HO-8910 cells were treated with
10 μM dasatinib, 200 nM CA-4 or the combination for 48 h. As
demonstrated in Fig. 2A, combined
treatment with dasatinib and CA-4 resulted in an increased
percentage of mitochondrial membrane depolarized HO-8910 cells than
either single agent (61.98% in combination-treated cells, 15.71% in
dasatinib-treated cells, 22.50% in CA-4-treated cells and 4.99% in
the control group). In addition, dasatinib + CA-4 resulted in
increased mitochondrial membrane potential than either drug alone
in HO-8910 (P<0.01) and A549 cells (P<0.05) (Fig. 2D and E). Furthermore, DAPI staining
was also performed to visualize the apoptosis by assessing
chromatin condensation. As shown in Fig. 3A, 10 μM dasatinib plus 200 nM CA-4
triggered more apoptosis than the mono-treatment in HO-8910 cells,
as indicated by the apoptotic bodies.
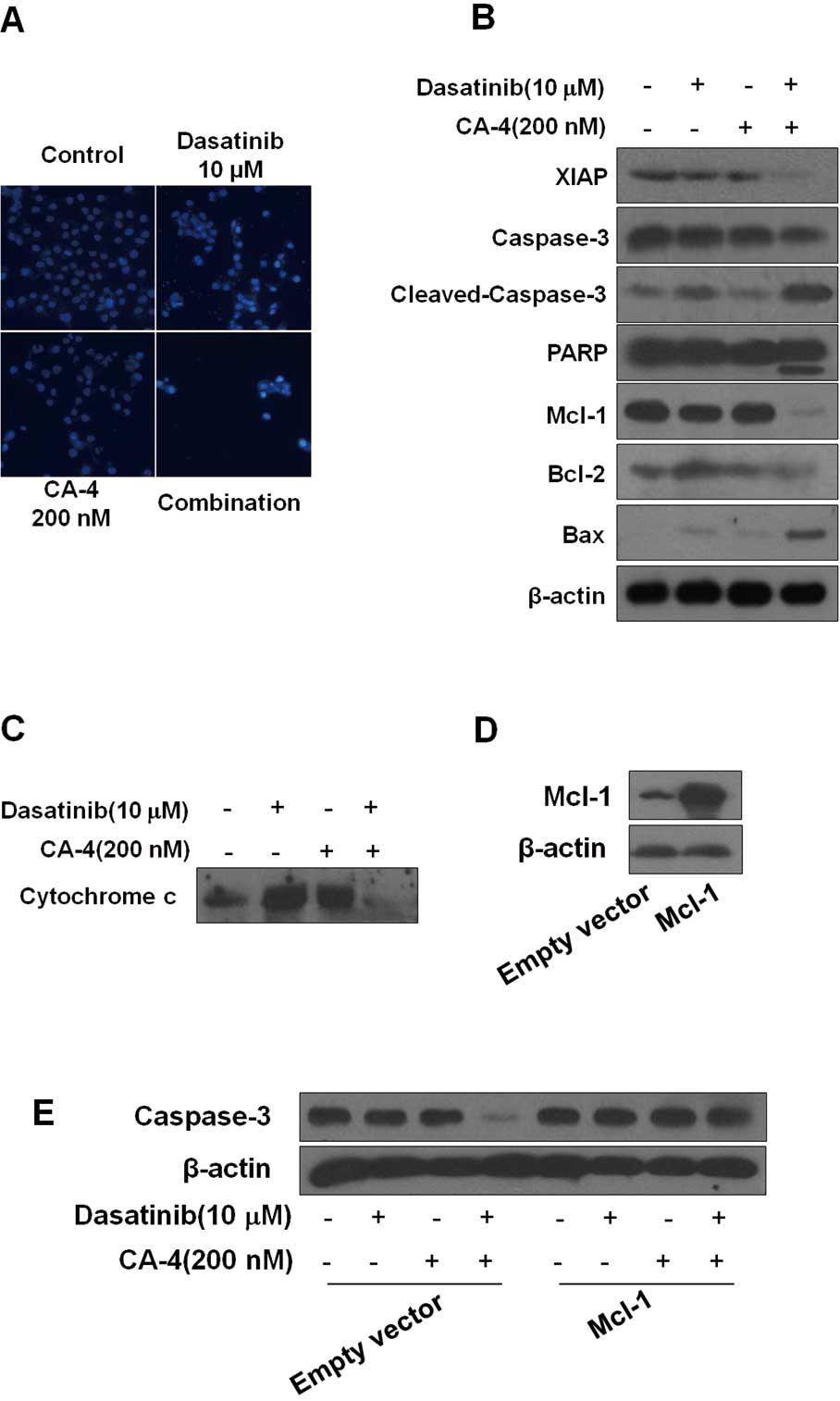 | Figure 3Dasatinib plus combretastatin A-4
(CA-4) causes activation of various apoptosis-related proteins. (A)
Dasatinib plus CA-4 induced apoptotic bodies in HO-8910 cells.
Nuclear DNA was visualized by DAPI staining. (B) HO-8910 cells were
exposed to dasatinib (10 μM), CA-4 (200 nM) or the combination for
48 h, after which protein extracts were immunoblotted with
specified antibodies for XIAP, caspase-3, cleaved-caspase-3, PARP,
Mcl-1, Bcl-2, Bax and β-actin. (C) Cytochrome c release from
mitochondria into cytosol was assessed at 48 h of incubation with
the agents. (D) HO-8910 cells were transfected with Mcl-1 plasmid
and empty vector according to the manufacturer’s recommendations.
Forty-eight hours after transfection, cell lysates were prepared
for western blot analysis. (E) The expression of caspase-3 in
HO-8910 cells that had been transfected with Mcl-1 plasmid or empty
vector and then treated with 10 μM dasatinib, either alone or in
combination with 200 nM CA-4 for 48 h, were examined. |
Dasatinib plus CA-4 combination therapy
causes mitochondrial release of proapoptotic molecules for
activation of proteases leading to substrate cleavage
Since caspase-3/PARP play a key role in the
initiation of cellular events during the apoptotic process, we next
examined the effect of dasatinib, CA-4, and the combination on the
activation of caspase-3, cleavage of PARP. As shown in Fig. 3B, we found that although dasatinib
and CA-4 had slight effect on caspase-3 and PARP, the two together
were highly effective, inducing more significant cleavage of PARP
and caspase-3. The X-linked inhibitor of apoptosis (XIAP) is the
most potent caspase inhibitor in the IAP family and inhibits
apoptotic cell death predominantly by preventing activation of
initiator caspase-9 as well as effectors caspases-3 and -7
(25). Treatment of the cells with
dasatinib plus CA-4 caused a significantly greater reduction of
XIAP than either agent used alone. Moreover, dasatinib plus CA-4
combination treatment resulted in the decrease of Bcl-2 and
increase of Bax, with an increase in the Bax/Bcl-2 ratio (Fig. 3B). To further confirm the
combination effect of dasatinib and CA-4 on the induction of the
mitochondrial apoptosis pathway, we also examined cytochrome
c release from mitochondria to cytosol. The levels of
mitochondrial cytochrome c were lower in cells treated with
dasatinib + CA-4 than in cells treated with dasatinib or CA-4 as
single agents (Fig. 3C).
Collectively, these results demonstrated that the dasatinib plus
CA-4 combination therapy caused mitochondrial release of cytochrome
c for activation of proteases leading to substrate
cleavage.
Mcl-1 overexpression rescues cells from
synergistic killing by the combination of dasatinib and CA-4
We found that Mcl-1 expression was markedly
downregulated in dasatinib plus CA-4 combination-treated cells
compared with single agent alone, indicating that Mcl-1 might be
involved in the synergistic effect of the combination treatment
(Fig. 3B). To further analyze
whether Mcl-1 downregulation was required for dasatinib plus CA-4
combination treatment-induced apoptosis, we performed Mcl-1
overexpression experiments. We successfully increased the protein
expression of Mcl-1 in HO-8910 cells by transfecting with
pTOPO-Mcl-1 plasmid (Fig. 3D). The
decrease of pro-caspase-3 caused by dasatinib plus CA-4 was also
increased due to the elevated levels of Mcl-1 in the cells
(Fig. 3E). Our observations
indicate that the downregulation of Mcl-1 may contribute to the
synergistic effect of dasatinib and CA-4.
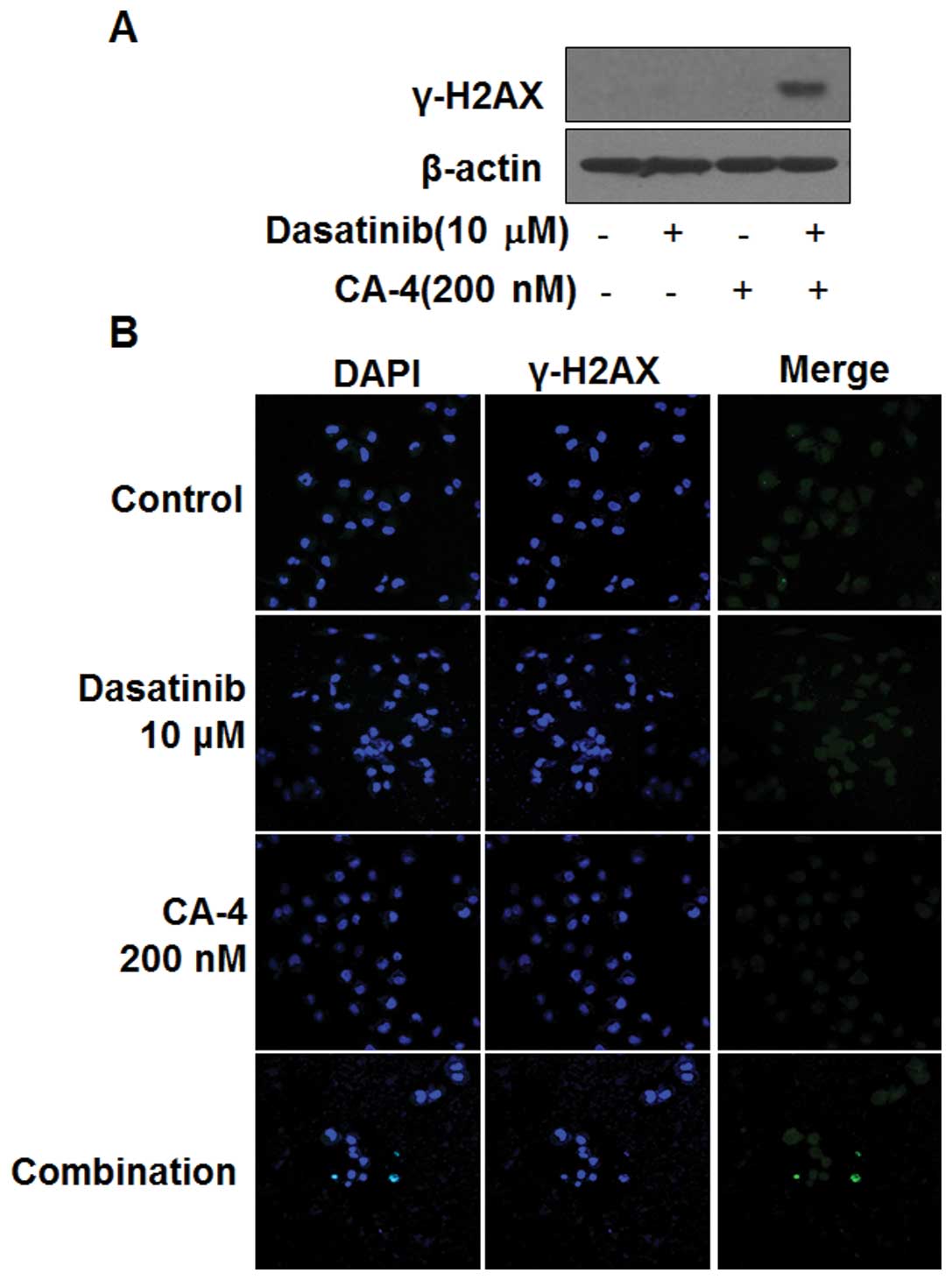
Dasatinib in combination with CA-4
induces DNA damage
The induction of phosphorylated H2AX (γ-H2AX) is a
marker of DNA damage. Next, we determined whether dasatinib in
combination with CA-4 could induce DNA damage in HO-8910 cells. As
shown in Fig. 4A, the induction of
γ-H2AX in HO-8910 cells was observed by western blot analysis, only
in the combination setting after 6 h, indicating DNA damage might
be involved in the synergistic effect of dasatinib and CA-4.
Furthermore, immunofluorescence results showed the number of
γ-H2AX-positive cells was significantly increased in HO-8910 cells
treated with dasatinib and CA-4, but not in the mono-treatment
groups (Fig. 4B).
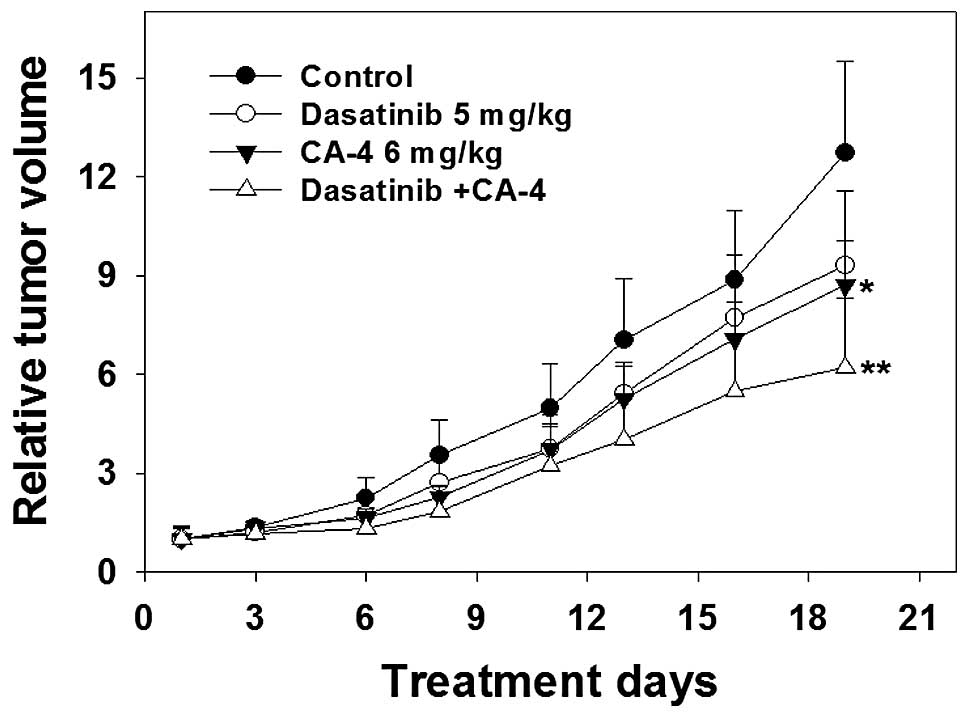
The antitumor activity of dasatinib and
CA-4 combination therapy against human HO-8910 xenografts
To further characterize the anticancer efficacy of
combination treatment, the in vivo activity of dasatinib and
CA-4 was tested on an ovarian cancer HO-8910 xenograft model in
nude mice. As shown in Fig. 5 and
Table I, the i.g. administration of
dasatinib at a dose of 5 mg/kg four times every week for 19 days
produced slight antitumor effect in mean RTV compared with that of
the control group (mean RTV, CA-4 vs. control: 9.3 vs. 12.7;
P>0.05). However, with the dosage of 6 mg/kg four times every
week for 19 days, CA-4 exerted a moderate tumor growth inhibitory
effect (mean RTV, CA-4 vs. control: 8.7 vs. 12.7; P<0.05). As
expected, dasatinib plus CA-4 caused marked tumor growth inhibition
(T/C value, 48.8%), significantly greater than CA-4- (T/C value,
68.5%) or dasatinib treatment alone (T/C value, 73.2%). The RTV of
the combination group was markedly decreased compared to that of
the control group (P<0.01); notably, compared with the dasatinib
or the CA-4 group, the combination group exerted significantly more
potent antitumor activities against the HO-8910 xenograft in nude
mice.
 | Table IIn vivo efficacy of dasatinib
in combination with CA-4 against HO-8910 xenografts. |
Table I
In vivo efficacy of dasatinib
in combination with CA-4 against HO-8910 xenografts.
| No. of animals | Body weight
(g) | | |
|---|
|
|
| | |
|---|
| Group | Start | End | Start | End | Mean RTV | T/C (100%) |
|---|
| Control | 10 | 10 | 20.6±0.6 | 23.4±0.7 | 12.7 | - |
| Dasatinib (5
mg/kg) | 10 | 10 | 20.7±1.5 | 22.7±1.1 | 9.3 | 73.2 |
| CA-4 (6 mg/kg) | 10 | 10 | 20.2±1.4 | 21.7±1.1 | 8.7a | 68.5 |
| Combination | 10 | 10 | 20.5±0.6 | 20.5±0.6 | 6.2b,c,d | 48.8 |
Discussion
Dasatinib, an orally active, small molecule
targeting agent that inhibits multiple members of the SRC family
and other tyrosine kinases, such as PDGFR, is initially approved
for use in Ph+ acute lymphoblastic leukemia and is currently
undergoing clinical trials in a variety of solid tumor types
(26). Dasatinib reduces
angiogenesis and metastasis through inhibiting Src family kinases
(27). Inhibitors of angiogenesis
interfere with new vessel formation and therefore have a
preventative action, with little effect on the existing tumor blood
vessels, and are likely to be of particular benefit in early-stage
cancer therapy (28).
Vascular-disrupting agents target the established tumor blood
vessels, resulting in tumor ischemia and necrosis (29). CA-4 is the lead compound as a
vascular-disrupting agent and is currently in Phase II/III clinical
trials against a range of malignancies, in combination with
conventional chemotherapeutic agents and radiotherapy (30,31).
Furthermore, CA-4 displays minimal toxicity profile at low dose,
indicating its potential as a candidate for drug combinations in
the solid tumor therapy (32,33).
Since both the initiation of new vessel formation and the integrity
of the existing blood vessel network are critical to tumor growth
and survival, various combinations involving an antiangiogenic
agent and an antivascular agent could cause significantly more
effective antitumor responses in various preclinical models
(34). Thus, combining dasatinib
with CA-4 may be a logical way to potentially enhance response
rates and prolong survival times for patients by targeting tumor
blood vessels.
The results of the present report, for the first
time, indicated that the synergistic anticancer effects in
vitro and in vivo achieved by dasatinib plus CA-4 were
observed in human cancer cells and a xenograft nude mice model. CI
values and the significant decline of the survival curves in the
combination group strongly demonstrated that dasatinib potentiated
the CA-4-imposed cytotoxicity in 5 human solid tumor cell lines,
including ovarian cancer (HO-8910), hepatocellular carcinoma
(HepG2, SMMC-7721), non-small cell lung cancer (A549) and prostate
cancer (PC-3). Furthermore, the strong synergistic effect was also
validated on the HO-8910 xenograft nude mice model. As single
agents, dasatinib and CA-4 merely displayed insignificant
activities against the HO-8910 xenograft model, respectively; by
contrast, the coadministration of dasatinib and CA-4 clearly
arrested tumor growth by 51.2%. Moreover, the combination of
dasatinib and CA-4 markedly improved the antitumor capacities in
vivo without increasing toxicities, as indicated by the nearly
constant body weights in the combination-treated group on day 19.
As we reported, the combination of dasatinib and CA-4 might be an
effective therapeutic strategy to achieve synergistic antitumor
effects in patients with solid tumors.
Our data showed that synergism in vitro
achieved by the combination of dasatinib and CA-4 was accompanied
by enhanced apoptosis. Activation of caspase-3 leads to the
cleavage of PARP, the presence of cleaved PARP is one of the most
used diagnostic tools for the detection of apoptosis in several
cell types (35). From western blot
analysis, marked increases in cleaved-PARP and caspase-3 were
observed in HO-8910 cells following dasatinib and CA-4 combination
treatment. IAPs also play an important role during the apoptotic
process; XIAP is the best known member of this family, and
correlates with resistance to chemotherapy (36). Our data showed that the synergistic
effect on apoptosis obtained with the dasatinib/CA-4 co-treatment
was accompanied by a large decrease in antiapoptotic XIAP protein.
Mitochondria play a key role in apoptotic cell death by releasing
the effector proteins, including cytochrome c and
Smac/DIABLO, from the mitochondrial intermembrane space (IMS)
(37). Our results showed that loss
of mitochondrial membrane potential was significantly greater with
dasatinib plus CA-4 than with either drug alone, suggesting that
combination therapy might activate the mitochondrial-mediated
apoptosis pathway in HO-8910 cells. In addition, we observed that
the combination of dasatinib and CA-4 enhanced cytochrome c
release from mitochondria into the cytosol in HO-8910 cells. IMS
protein release can result from the mitochondrial outer membrane
permeabilization (MOMP), which is thought to be regulated by
proteins of the Bcl-2 family (38).
The Bcl-2 protein family includes proapoptotic members, such as
Bax, Bad and Bcl-Xs, and antiapoptotic members, such as Bcl-2,
Bcl-XL and Mcl-1. Antiapoptotic members act as repressors of
apoptosis by blocking the release of cytochrome c, whereas
proapoptotic members act as promoters (39). The final action is dependent on the
balance between Bcl-2 and Bax, and the increase of Bax/Bcl-2 ratio
can induce the release of cytochrome c(40). Our results demonstrated a marked
increase of the Bax/Bcl-2 ratio in HO-8910 cells treated with
dasatinib plus CA-4, indicating that the regulation of Bcl-2
protein family expression plays an important role in
combination-induced apoptosis. Mcl-1, primarily localized to the
outer mitochondrial membrane, has been suggested to function as an
antiapoptotic factor by suppressing cytochrome c release
from mitochondria via heterodimerization with and neutralization of
effector proapoptotic Bcl-2 family members. Mcl-1 expression was
not altered in either the dasatinib or the CA-4 treatment group;
however, dasatinib plus CA-4 treatment significantly downregulated
the expression of Mcl-1 in HO-8910 cells, indicating that Mcl-1
might be involved in the synergistic effect of combination
treatment. Moreover, elevated expression of Mcl-1 led to a reduced
apoptosis induced by dasatinib plus CA-4, highlighting that
downregulated Mcl-1 was necessary for the potentiating effect of
dasatinib to CA-4-triggered apoptosis.
Among the different forms of complex DNA damage,
double-strand breaks (DSBs) are considered to be among the most
lethal forms of DNA damage, severely compromising genomic stability
(41). H2AX moved to the center of
cellular responses to DNA damage after the discovery that it
becomes locally phosphorylated on Ser139, to generate γ-H2AX, in
the vicinity of DSBs (42).
Quantitation of γ-H2AX is one of the earliest events in the DNA
damage signaling and repair (43,44).
To maintain genomic stability following DNA damage, multicellular
organisms activate checkpoints that induce cell cycle arrest or
apoptosis (45). A number of
anticancer drugs exert their effect by causing DSBs and subsequent
apoptosis induction, such as DNA replication inhibitors,
crosslinking agents and topoisomerase inhibitors (46). In our study, a clear increase in
γ-H2AX expression was observed in the dasatinib + CA-4 group
compared with the mono-treatment groups. Furthermore, in HO-8910
cells, the number of γ-H2AX-positive cells increased in the
combination treatment group. These results indicated that dasatinib
plus CA-4 might induce DSBs in HO-8910 cells, thereby activating
apoptosis pathways, and finally resulting in the synergism of these
two drugs.
In conclusion, we presented evidence showing
enhanced therapeutic activity of dasatinib when combined with CA-4
in vitro and in vivo. The enhanced apoptosis induced
by dasatinib plus CA-4 was accompanied by the larger extent of DNA
damage, mitochondrial depolarization, caspase-3 activation and PARP
cleavage in HO-8910 cells. Additional preclinical studies, such as
antivascular efficacy, are required to systematically evaluate the
applicability of this combination as a therapeutic strategy for
cancer patients. As we reported, the combination of dasatinib and
CA-4 might be an effective therapeutic strategy to achieve
synergistic antitumor effects.
Acknowledgements
The authors thank Mr. Yong-zhou Hu of Zhejiang
University for providing CA-4 for this study. The authors
acknowledge the financial support from the Teachers Research Fund
of Zhejiang University City College (J-12019, J-12021), the
Zhejiang Provincial Foundation of National Science (Y2100682,
LQ12H31001), the Science Research Foundation of Zhejiang Health
Bureau (2012KYA068, 2012KYB066), the Zhejiang Provincial Program
for the Cultivation of High-level Innovative Health talents
(2010-190-4), the Student Research Fund of Zhejiang University City
College (XZ2012562091, X2012562098) and the Scientific Research
Fund of Zhejiang Provincial Education Department (Y201120633).
References
|
1
|
Jin X, Yang YD, Chen K, et al: HDMCP
uncouples yeast mitochondrial respiration and alleviates steatosis
in L02 and hepG2 cells by decreasing ATP and
H2O2 levels: a novel mechanism for NAFLD. J
Hepatol. 50:1019–1028. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Simoni D, Romagnoli R, Baruchello R, et
al: Novel A-ring and B-ring modified combretastatin A-4 (CA-4)
analogues endowed with interesting cytotoxic activity. J Med Chem.
51:6211–6215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tian CH, Zhao JF, Xu YP, et al:
Involvement of Bombyx mori nucleopolyhedrovirus ORF41 (Bm41)
in BV production and ODV envelopment. Virology. 387:184–192.
2009.PubMed/NCBI
|
|
4
|
Nagaiah G and Remick SC: Combretastatin A4
phosphate: a novel vascular disrupting agent. Future Oncol.
6:1219–1228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cooney MM, Radivoyevitch T, Dowlati A, et
al: Cardiovascular safety profile of combretastatin a4 phosphate in
a single-dose phase I study in patients with advanced cancer. Clin
Cancer Res. 10:96–100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Su C, He P, Yan RJ, Zhao CB and Zhang C:
Study of the orientation-controlled damping temperature based on
selective distribution of oligo-phenol in acrylate
rubber/chlorinated butyl rubber blends. Polymer Composites.
33:860–865. 2012. View
Article : Google Scholar
|
|
7
|
Ablikim M, Achasov MN, Alberto D, et al:
Search for ηc′ decays into vector meson pairs. Physical
Review D. 84:0911022011.
|
|
8
|
Li J, Li J, Li S, et al: Ameliorative
effect of grape seed proanthocyanidin extract on
thioacetamide-induced mouse hepatic fibrosis. Toxicol Lett.
213:353–360. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang CL, Wang H and Yan J: Leptospirosis
prevalence in Chinese populations in the last two decades. Microbes
Infect. 14:317–323. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bao YY, Xue J, Wu WJ, Wang Y, Lv ZY and
Zhang CX: An immune-induced Reeler protein is involved in the
Bombyx mori melanization cascade. Insect Biochem Mol Biol.
41:696–706. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang XF, Zhang CH, Yang B, Lv N, Pan QF
and Ye GX: Aggregation mechanism of Ag atoms deposited on liquid
surfaces. J Phys Soc Jpn. 80:104603–104604. 2011. View Article : Google Scholar
|
|
12
|
Li GL, Roy B, Li XH, et al: Quantification
of silkworm coactivator of MBF1 mRNA by SYBR Green I real-time
RT-PCR reveals tissue- and stage-specific transcription levels. Mol
Biol Rep. 36:1217–1223. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang CQ, Liu YH, Ma XY, Feng Z and Ma ZH:
Characterization of sensitivity of Rhizoctonia solani,
causing rice sheath blight, to mepronil and boscalid. Crop
Protection. 28:381–386. 2009.
|
|
14
|
Ma TY, Yan M, Zhang CS, Pei YM and Jiang
CB: Stress influences on magnetization and magnetostriction in
magnetically annealed Tb0.36Dy0.64(Fe0.85Co0.15)(2) polycrystals. J
Appl Phys. 105:0939152009. View Article : Google Scholar
|
|
15
|
Dark GG, Hill SA, Prise VE, Tozer GM,
Pettit GR and Chaplin DJ: Combretastatin A-4, an agent that
displays potent and selective toxicity toward tumor vasculature.
Cancer Res. 57:1829–1834. 1997.PubMed/NCBI
|
|
16
|
Gay LJ and Felding-Habermann B:
Contribution of platelets to tumour metastasis. Nat Rev Cancer.
11:123–134. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Assifi MM and Hines OJ: Anti-angiogenic
agents in pancreatic cancer: a review. Anticancer Agents Med Chem.
11:464–469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pasquier E, Ciccolini J, Carre M, et al:
Propranolol potentiates the anti-angiogenic effects and anti-tumor
efficacy of chemotherapy agents: implication in breast cancer
treatment. Oncotarget. 2:797–809. 2011.PubMed/NCBI
|
|
19
|
Fang ZN, Yang B, Chen MG, Zhang CH, Xie JP
and Ye GX: Growth and morphology of ultra-thin Al films on liquid
substrates studied by atomic force microscopy. Thin Solid Films.
517:3408–3411. 2009. View Article : Google Scholar
|
|
20
|
Cao J, Xu D, Wang D, et al: ROS-driven Akt
dephosphorylation at Ser-473 is involved in 4-HPR-mediated
apoptosis in NB4 cells. Free Radic Biol Med. 47:536–547. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu XW, Su Y, Zhu H, et al:
HIF-1α-dependent autophagy protects HeLa cells from fenretinide
(4-HPR)-induced apoptosis in hypoxia. Pharmacol Res. 62:416–425.
2010.
|
|
22
|
Wang C, Wang JF, Chen C, et al: Learning
to extract web news title in template independent way. Rough Sets
and Knowledge Technology Proceedings Gold Coast. Springer; Berlin:
pp. 192–199. 2009, View Article : Google Scholar
|
|
23
|
Chou TC and Talalay P: Generalized
equations for the analysis of inhibitions of Michaelis-Menten and
higher-order kinetic systems with two or more mutually exclusive
and nonexclusive inhibitors. Eur J Biochem. 115:207–216. 1981.
View Article : Google Scholar
|
|
24
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: the combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Salvesen GS and Duckett CS: IAP proteins:
blocking the road to death’s door. Nat Rev Mol Cell Biol.
3:401–410. 2002.
|
|
26
|
Zhang CL, Feng LF, Hoppe S and Hu GH:
Residence time distribution: an old concept in chemical engineering
and a new application in polymer processing. AIChE J. 55:279–283.
2009. View Article : Google Scholar
|
|
27
|
Zhang CL, Chen WQ and Yang JS:
Electrically forced vibration of a rectangular piezoelectric plate
of monoclinic crystals. Int J Appl Electrom Mechanics. 31:207–218.
2009.
|
|
28
|
Osusky KL, Hallahan DE, Fu A, Ye F, Shyr Y
and Geng L: The receptor tyrosine kinase inhibitor SU11248 impedes
endothelial cell migration, tubule formation, and blood vessel
formation in vivo, but has little effect on existing tumor vessels.
Angiogenesis. 7:225–233. 2004. View Article : Google Scholar
|
|
29
|
Wu D, Zhu DS, He Y, Zhang CQ and Feng L:
Nondestructive detection of grey mold of eggplant based on ground
multi-spectral imaging sensor. Guang Pu Xue Yu Guang Pu Fen Xi.
28:1496–1500. 2008.(In Chinese).
|
|
30
|
Tozer GM, Kanthou C, Lewis G, Prise VE,
Vojnovic B and Hill SA: Tumour vascular disrupting agents:
combating treatment resistance. Br J Radiol. 81:S12–S20. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu R, Ding W, Liu T, et al: XN05, a novel
synthesized microtubule inhibitor, exhibits potent activity against
human carcinoma cells in vitro. Cancer Lett. 285:13–22. 2009.
View Article : Google Scholar
|
|
32
|
Busk M, Bohn AB, Skals M, Wang T and
Horsman MR: Combretastatin-induced hypertension and the
consequences for its combination with other therapies. Vascul
Pharmacol. 54:13–17. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhu H, Zhang J, Xue N, Hu Y, Yang B and He
Q: Novel combretastatin A-4 derivative XN0502 induces cell cycle
arrest and apoptosis in A549 cells. Invest New Drugs. 28:493–501.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Siemann DW, Chaplin DJ and Horsman MR:
Vascular-targeting therapies for treatment of malignant disease.
Cancer. 100:2491–2499. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Narula J, Pandey P, Arbustini E, et al:
Apoptosis in heart failure: release of cytochrome c from
mitochondria and activation of caspase-3 in human cardiomyopathy.
Proc Natl Acad Sci USA. 96:8144–8149. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kashkar H: X-linked inhibitor of
apoptosis: a chemoresistance factor or a hollow promise. Clin
Cancer Res. 16:4496–4502. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhao GL, Li H, Zhang CS and Han GR:
Preparation of TiO2 films under low temperature
condition on the glass substrate. Rare Metal Mat Eng. 37:148–150.
2008.
|
|
38
|
Xu HJ, Yang ZN, Zhao JF, et al: Bombyx
mori nucleopolyhedrovirus ORF56 encodes an occlusion-derived
virus protein and is not essential for budded virus production. J
Gen Virol. 89:1212–1219. 2008. View Article : Google Scholar
|
|
39
|
Ablikim M, Bai JZ, Ban Y, et al:
Determination of the psi (3770), psi (4040), psi (4160) and psi
(4415) resonance parameters. Physics Lett B. 660:315–319. 2008.
View Article : Google Scholar
|
|
40
|
Saxena P and Tesar PJ: Vascular
obstruction related to mediastinal fibrosis: an interesting
clinical entity. J Thorac Cardiovasc Surg. 134:1379–1380. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kinner A, Wu W, Staudt C and Iliakis G:
γ-H2AX in recognition and signaling of DNA double-strand breaks in
the context of chromatin. Nucleic Acids Res. 36:5678–5694.
2008.
|
|
42
|
Mah LJ, El-Osta A and Karagiannis TC:
γH2AX: a sensitive molecular marker of DNA damage and repair.
Leukemia. 24:679–686. 2010.
|
|
43
|
Smart DJ, Ahmedi KP, Harvey JS and Lynch
AM: Genotoxicity screening via the γH2AX by flow assay. Mutat Res.
715:25–31. 2011.
|
|
44
|
Zhang DY, Pan Y, Zhang C, et al:
Wnt/β-catenin signaling induces the aging of mesenchymal stem cells
through promoting the ROS production. Mol Cell Biochem. 374:13–20.
2012.
|
|
45
|
Gartner A, Milstein S, Ahmed S, Hodgkin J
and Hengartner MO: A conserved checkpoint pathway mediates DNA
damage - induced apoptosis and cell cycle arrest in C.
elegans. Mol Cell. 5:435–443. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kawanishi S and Hiraku Y: Amplification of
anticancer drug-induced DNA damage and apoptosis by DNA-binding
compounds. Curr Med Chem Anticancer Agents. 4:415–419. 2004.
View Article : Google Scholar : PubMed/NCBI
|