Introduction
Recurrence and metastasis are still the main
obstacles to improving the survival of patients with hepatocellular
carcinoma (1,2) and are associated with highly increased
morbidity and mortality worldwide. Several risk factors including
tumor size, microvessel invasion and poor differentiation, have
been demonstrated to increase the risk of recurrence and even
extrahepatic metastasis of post-resective HCC patients (1) and their identification can aid in
carrying out more positive treatment strategies, such as adjuvant
TACE. However, the heterogeneity and molecular biological
characteristics of HCC increase the complexity and difficulties of
therapy. Providing treatment according to the differential
characteristics of specific subgroups of HCC may decrease the
potential of recurrence and metastasis, and this is becoming the
future developmental trend in treatment strategy.
Chemokine receptor CXCR7, belonging to one of the
G-protein coupled seven-transmembrane receptors, has the definitive
ligand stromal cell-derived factor-1α (SDF-1α) and truncated ITAC
(CXCL11) (3,4). Accumulated research has shown
chemokine receptors to be pertinent to the progression of tumors
(5). The level of CXCR7 expression
has been demonstrated to be closely related to the invasive
activities of prostate cancer cells (6). In addition, higher expression of CXCR7
is linked to the early and metastatic recurrence in pathological
stage I non-small cell lung cancer (7). Recently, overexpression of CXCR7 was
found in the HCC cell line SMMC-7721 and tumor tissues (8). Moreover, CXCR7 can also be expressed
by endothelial cells (9). Our
previous research showed that downregulation of CXCR7 inhibited the
metastasis of HCC (10). However,
the potential merit of CXCR7 as a risk factor for extrahepatic
metastasis is unclear.
In the present study, we evaluated the predictive
role of CXCR7 as a risk factor in metastasis of post-resective
human HCC. According to the immunohistochemical staining of the
tissue microarray, high expression of CXCR7 increased the risk of
extrahepatic metastasis prominently as an independent risk factor
based on cell differentiation. Furthermore, the close relationship
between CXCR7 and osteopontin (OPN) was found and was confirmed by
immunocytochemistry and RT-PCR analysis of OPN after downregulation
of CXCR7 in a highly metastatic HCC cell line by RNA interference
(RNAi).
Materials and methods
Cell line
The highly metastatic human HCC cell line HCCLM-3
(100% lung metastatic potential) was used, which was established at
the Liver Cancer Institute of Fudan University (Shanghai, China).
The cell line was cultured in high glucose DMEM (Gibco-BRL, Grand
Island, NY, USA) supplemented with 10% fetal bovine serum (HyClone
Laboratories, Inc., Logan, UT, USA).
Patients
One hundred and sixteen patients were retrieved from
the prospectively designed database. Ethical approval was obtained
from the Zhongshan Hospital Research Ethics Committee, and informed
consent was obtained from each patient. The patients underwent
hepatectomy by the same surgical team from January 2000 to May
2004. The hepatectomy of HCC was carried out as previously
described (11). The patients were
pathologically confirmed as having HCC. Paraffin tissue sections
were stained by hematoxylin and eosin, and reviewed by two
pathologists according to the WHO histomorphologic criteria. There
were 103 male and 13 female patients with a mean age of 51.0±11.4
years (range, 18–78 years). Ninety-four patients were positive for
the hepatitis B surface antigen (HBsAg). All patients were
classified as Child-Pugh A.
Follow-up
Regular follow-up procedures in our clinic are as
follows: serum α fetoprotein (AFP) assay and liver ultrasonography
every 3 months during the first year, then every 6 months; and
magnetic resonance imaging (MRI) or computed tomography (CT)
scanning after 1 month, then every 6 months. Chest CT scanning was
regularly used to examine the lung metastasis. Lung metastasis was
confirmed by biopsy through endoscopy or pathology after partial
pulmonary resection. Until May 2009, 58 patients were found to
present with lung metastasis. Ten patients with resectable lung
metastasis received partial resection of the lung.
TMA and immunohistochemistry
Hematoxylin and eosin-stained slides were screened
for optimal tumor content and tissue adjacent to tumor (TAT) with a
distance of 2 cm. The TMA was then constructed in accordance with
standard procedures (12) based on
116 tumor tissues and 47 TATs. Two cores were taken from each
formalin-fixed, paraffin-embedded HCC samples by using punch cores
that measured 1.0 mm in diameter from the center of tumor foci and
TAT. A two-step method of immunohistochemistry including
heat-induced antigen-retrieval procedure was performed in a
standard manner. Rabbit anti-human CXCR7 IgG (Novus Biologicals,
Littleton, CO, USA) at 1:200 and rabbit anti-human osteopontin IgG
(Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 1:200 were used
as primary antibodies for detection. Detection without the primary
antibody was considered as the negative control.
Scoring and categories of CXCR7
expression
The immunoassay was defined by the staining
intensity and the percentage of positive tumor cells as previously
described (13). Two pathologists
observed the results independently. For analysis of convenience, we
simplified the assay results into three expression levels as
negative, weak and strong. Based on the three expression levels,
two categories for further analysis were set up as follows:
category 1, negative and weak staining were recognized as
CXCR7neg+low and strong staining was recognized as
CXCR7high; category 2, only detectable staining of CXCR7
expression was analyzed. Weak staining was recognized as
CXCR7low and strong staining was recognized as
CXCR7high.
RNAi
Small interference RNA (siRNA) transfection of
HCCLM3 cells was performed according to the Lipofectamine 2000
protocol (14). After 24 h,
immunocytochemistry assay was performed as previously described
(15) except that 1:800 rabbit
anti-human OPN IgG (Millipore Corp., Billerica, MA, USA) was used
as the primary antibody. RT-PCR was also used to determine the mRNA
level of OPN: sense, 5′-GGACTCCATTGACTCGAACG-3′; antisense, 5′-TAA
TCTGGACTGCTTGTGGC-3′.
Statistical analysis
Pearson’s Chi-square test was used to compare
qualitative variables in the univariate analysis. When expected
sample values were <5, Fisher’s exact test was used. Twelve
potential risk factors including CXCR7 for development of lung
metastasis of HCC were all categorized variables. These included 4
clinical factors [age ≤60 years or >60 years; gender, HBsAg
status and serum AFP level ≤20 μg/l or >20 μg/l (16)], and 8 pathological factors
(cirrhosis or non-cirrhosis, tumor size ≤5 cm or >5 cm, single
or multiple tumor nodules, well or poorly encapsulated tumor, the
presence or absence of microvascular invasion, portal lymphatic
status, Edmondson grade 1/2 [well- and moderately differentiated
grades) or grade 3/4 (poorly differentiated grade) and CXCR7
staining]. Potential risk factors were selected from univariate
analysis when P<0.25. Binary logistic regression was used as
multivariate analysis to evaluate the screened risk factors.
Receiver operation curve (ROC) was used further to confirm the
predictive accuracy of the risk factors. Spearman’s rank test was
used to detect the correlation between CXCR7 and OPN. One-way ANOVA
was used for intergroup comparisons. All P-values were 2-tailed and
the statistical significance was set at 0.05. Statistical analyses
were carried out using SPSS 18.0 software (SPSS Inc., Chicago, IL,
USA).
Results
Association between high expression of
CXCR7 and extrahepatic metastasis
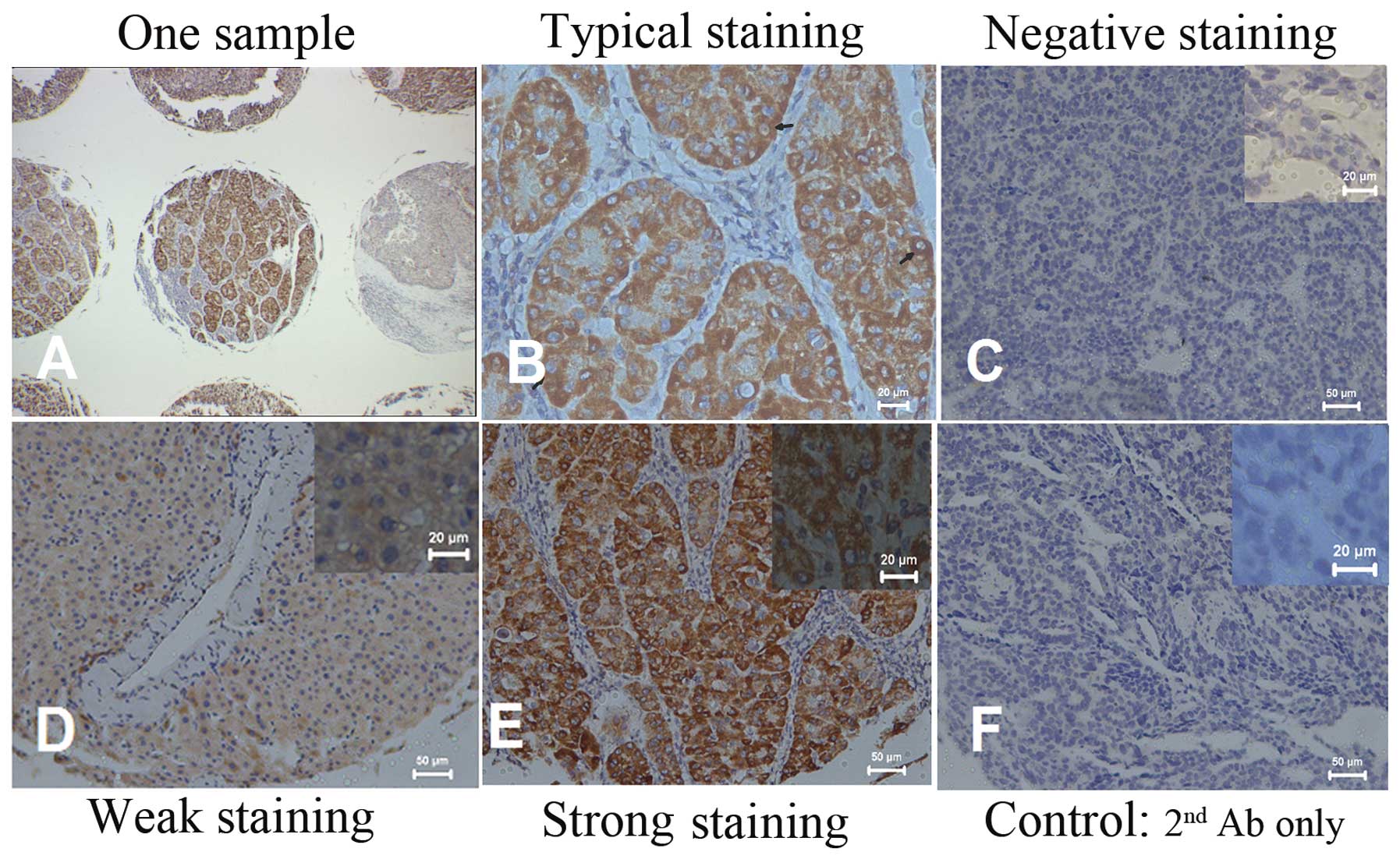
The immunopositivity for CXCR7 was mainly observed
in the membrane and cytoplasm of HCC cells. Typically, the stained
HCC cells were mostly round or oval with increased karyoplasm ratio
(Fig. 1). Differential staining
intensity of CXCR7 expression from negative to strong was observed
in 20, 45 and 51 patients, respectively (Fig. 1).
In category 1, 51.7% cases showed
CXCR7high in the patients with lung metastasis (n=58),
whereas in the patients without lung metastasis (n=58), only 36.2%
cases showed CXCR7high. Immunopositivity for CXCR7 in
patients with lung metastasis was potentially stronger than that in
patients without metastasis [hazards ratio (HR), 1.89; 95%
confidence interval (CI), 0.90–3.97; P=0.092]. Furthermore, in
category 2, patients with lung metastasis showed significantly
strong CXCR7 staining (HR, 2.59; 95% CI, 1.13–5.92; P=0.023).
In TAT analysis, no statistical difference was noted
between patients with lung metastasis (n=23) and those without
metastasis (n=24) for CXCR7 staining (P=0.302 in category 1).
Furthermore, among 23 TATs of HCC with lung metastasis, only
microvascular invasion was found to be associated with CXCR7
staining in category 1. Tumor cells with high expression of CXCR7
in TATs of patients exhibited stronger microvascular invasion (HR,
10.67; 95% CI, 1.04–109.94; P=0.027).
Role of CXCR7 in the increased risk of
extrahepatic metastasis is correlated with cell
differentiation
In category 1, among the 58 patients with lung
metastasis, patients with CXCR7high appeared to have
better differentiated tumors (HR, 0.38; 95% CI, 0.13–1.08; P=0.067)
but increased AFP level (HR, 4.21; 95% CI, 1.15–14.39; P=0.024).
Univariate analysis indicated that 6 evaluated factors including
CXCR7 staining (P=0.092), AFP level (P=0.051), microvascular
invasion (P=0.003), Edmondson grade (P=0.012), portal lymphatic
status (P=0.061) and tumor capsule (P=0.126) were potential risk
factors. Multivariate analysis did not indicate a significant
predictive effect for CXCR7 [odds ratio (OR), 1.89; 95% CI,
0.84–4.25; P=0.125]. However, when CXCR7 staining and AFP were
maintained in the model, CXCR7 and AFP had significant effects on
the model (OR, 8.446; 95% CI, 1.24–57.45; P=0.029).
To further confirm the potential relationship
between CXCR7 and cell differentiation in category 1, we excluded
the patients for whom CXCR7 expression could not be detected, and
who stained negative from the observations in category 2. In
patients with lung metastasis and detectable CXCR7 expression
(n=46), Edmondson grade (HR, 0.26; 95% CI, 0.07–0.96; P=0.038) was
associated with CXCR7 staining. Among the 96 patients with
detectable CXCR7 expression, univariate analysis showed that 6
factors including CXCR7 staining (P=0.023), AFP level (P=0.007),
microvascular invasion (P=0.025), Edmondson grade (P=0.015), portal
lymphatic status (P=0.052) and tumor capsule (P=0.114) were
potential risk factors. Multivariate analysis showed that
microvascular invasion (OR, 3.28; 95% CI, 1.23–8.78;P=0.018),
Edmondson grade (OR, 3.87; 95% CI, 1.46–10.24; P=0.006), and AFP
(OR, 3.54; 95% CI, 1.35–9.28; P=0.010) were 3 independent risk
factors for lung metastasis. When CXCR7 staining was maintained in
the model, CXCR7 staining (OR, 4.73; 95% CI, 1.36–16.48; P=0.015)
was a dependent risk factor due to potential interaction of CXCR7
expression and Edmondson grade (OR, 0.14; 95% CI, 0.02–1.02;
P=0.053). HCC patients with CXCR7high had nearly a
5-fold higher risk to develop lung metastasis than patients with
CXCR7low.
Stratification analyses of the
independent risk role of CXCR7 based on cell differentiation
Due to the potential relationship between highly
expressed CXCR7 and relatively good differentiation of tumor cells,
we further analyzed the risk predictive role of CXCR7 in patients
with Edmondson grade 1/2 (n=73). None of the clinicopathological
factors were associated with the expression of CXCR7 staining in
category 1 (data not shown). In univariate analysis, 6 factors
including CXCR7 staining (P=0.017), AFP level (P=0.003),
microvascular invasion (P=0.008), number of tumor nodules
(P=0.044), portal lymphatic status (P=0.025) and tumor capsule
(P=0.134) were potential risk factors. Multivariate analysis showed
that CXCR7 staining (OR, 3.4; 95% CI, 1.07–10.84; P=0.038),
microvascular invasion (OR, 4.07; 95% CI, 1.23–13.48; P=0.021) and
AFP (OR, 8.18; 95% CI, 1.81–36.92; P=0.006) were 3 independent risk
factors for lung metastasis of patients with well and moderately
differentiated HCC. HCC patients with CXCR7high had a
3.4-fold higher risk for developing lung metastasis than patients
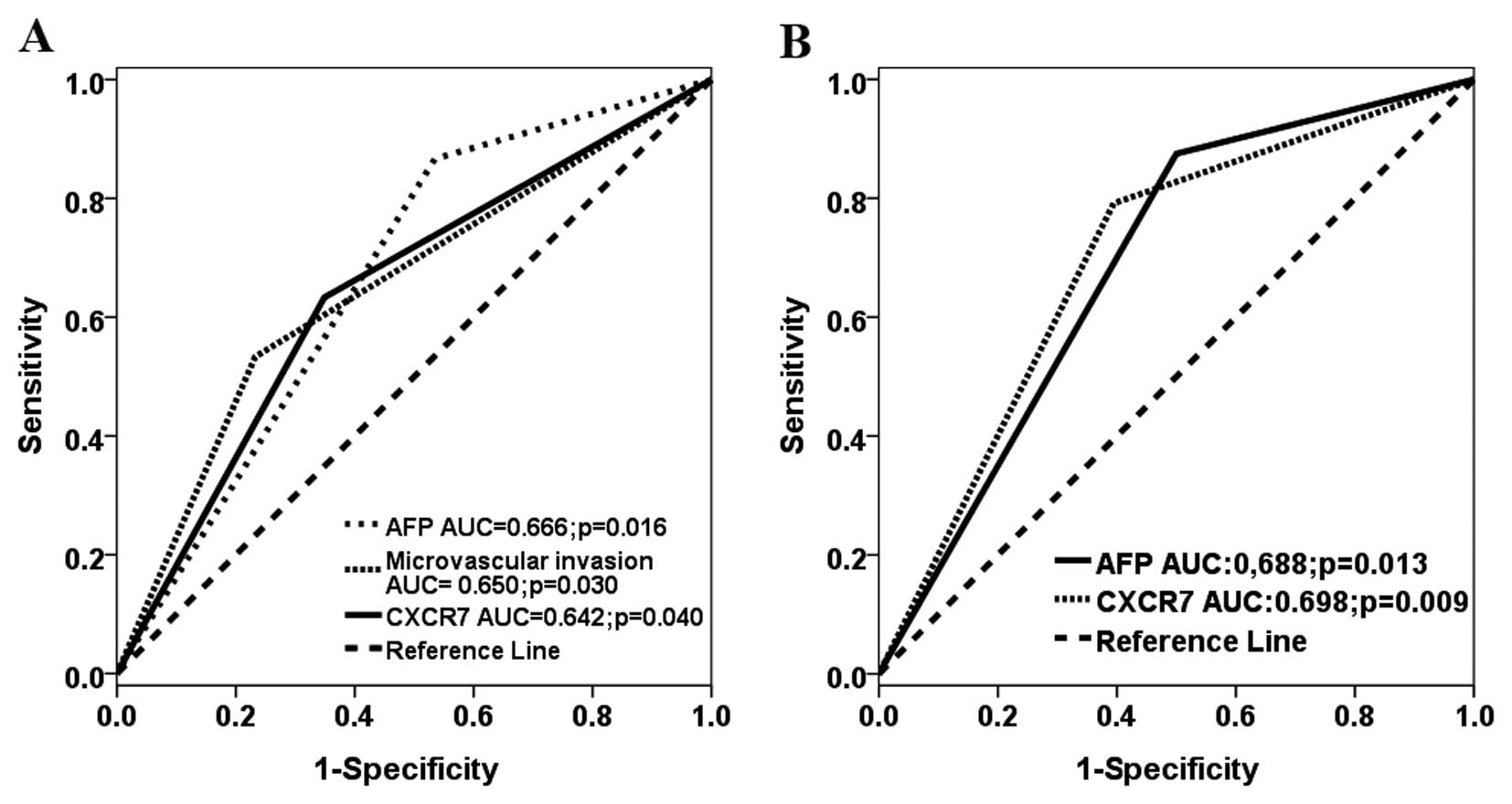
with CXCR7neg+low. In the ROC analysis, three factors
predicted the development of lung metastasis, with AUC of 0.642
(95% CI, 0.512–0.772; P=0.040) for CXCR7 staining; AUC of 0.666
(95% CI, 0.541–0.790; P=0.016) for AFP and AUC of 0.650 (95% CI,
0.519–0.781; P=0.030) for microvascular invasion (Fig. 2A) In addition, we also analyzed the
potential risk factors in 43 patients with poorly differentiated
tumors (Edmondson grade 3/4). Univariate analysis showed that no
potential risk factor was associated with lung metastasis except
for microvascular invasion (P=0.065).
In category 2, stratification analysis of cell
differentiation, or Edmondson grade 1/2 (n=62) further confirmed
the risk predictive role of CXCR7 in category 1. In univariate
analysis, 6 factors including CXCR7 staining (P=0.002) were
potential risk factors (Table I).
Multivariate analysis showed that CXCR7 staining (OR, 6.40; 95% CI,
1.64–24.92; P=0.007) and AFP (OR, 9.32; 95% CI, 1.76–49.36;
P=0.009) were 2 independent risk factors for lung metastasis of
patients with Edmondson grade 1/2. Patients with
CXCR7high had a 6.4-fold higher risk of developing lung
metastasis than patients with CXCR7low based on the
differentiation of the tumor cells. In the ROC analysis, both
factors accurately predicted the occurrence of lung metastasis,
with AUC of 0.698 (95% CI, 0.565–0.832; P=0.009) for CXCR7 staining
and AUC of 0.688 (95% CI, 0.555–0.820; P=0.013) for AFP (Fig. 2B).
 | Table IUnivariate analysis of risk factors
for metastasis in category 2 based on Edmondson grade 1/2. |
Table I
Univariate analysis of risk factors
for metastasis in category 2 based on Edmondson grade 1/2.
| No. of patients | |
|---|
|
| |
|---|
| Variable | With lung metastasis
(n=24) | Without lung
metastasis (n=38) | P-value |
|---|
| Age (years) |
| ≤60 | 17 | 30 | 0.467 |
| >60 | 7 | 8 | |
| Gendera |
| Male | 23 | 33 | 0.391 |
| Female | 1 | 5 | |
| HBsAg |
| Negative | 6 | 5 | 0.234 |
| Positive | 18 | 33 | |
| Cirrhosis |
| Absent | 20 | 27 | 0.271 |
| Present | 4 | 11 | |
| AFP (μg/l) |
| ≤20 | 3 | 19 | 0.003 |
| >20 | 21 | 19 | |
| Tumor size (cm) |
| ≤5 | 6 | 15 | 0.241 |
| >5 | 18 | 23 | |
| No. of tumor
nodules |
| Single | 14 | 31 | 0.046 |
| Multiple | 10 | 7 | |
| Tumor capsule |
| Well capsulated | 12 | 26 | 0.147 |
| Poorly
capsulated | 12 | 12 | |
| Microvascular
invasion |
| Negative | 12 | 28 | 0.058 |
| Positive | 12 | 10 | |
| Portal lymphatic
statusa |
| No | 21 | 38 | 0.054 |
| Yes | 3 | 0 | |
| CXCR7 staining |
| Low | 5 | 23 | 0.002 |
| High | 19 | 15 | |
Risk predictive role of CXCR7 is related
to OPN expression
Since OPN has been generally accepted as a factor
closely related to the metastasis of HCC (17,18),
we explored the potential association between the expression of
CXCR7 and OPN. In this analysis, expression of OPN was divided into
two groups: OPNhigh and OPNlow. Following
stratification of category 1 based on cell differentiation
(Edmondson grade 1/2) (n=73), CXCR7 correlated well and positively
with expression of OPN (Spearman’s ρ=0.240; P=0.019). Furthermore,
stratification analysis of category 2 (n=62) confirmed the positive
and high correlation between CXCR7 and OPN (Spearman’s ρ=0.453;
P<0.001). There was a significantly statistical difference
between CXCR7 and OPN expression (Fisher’s exact test, P=0.000). In
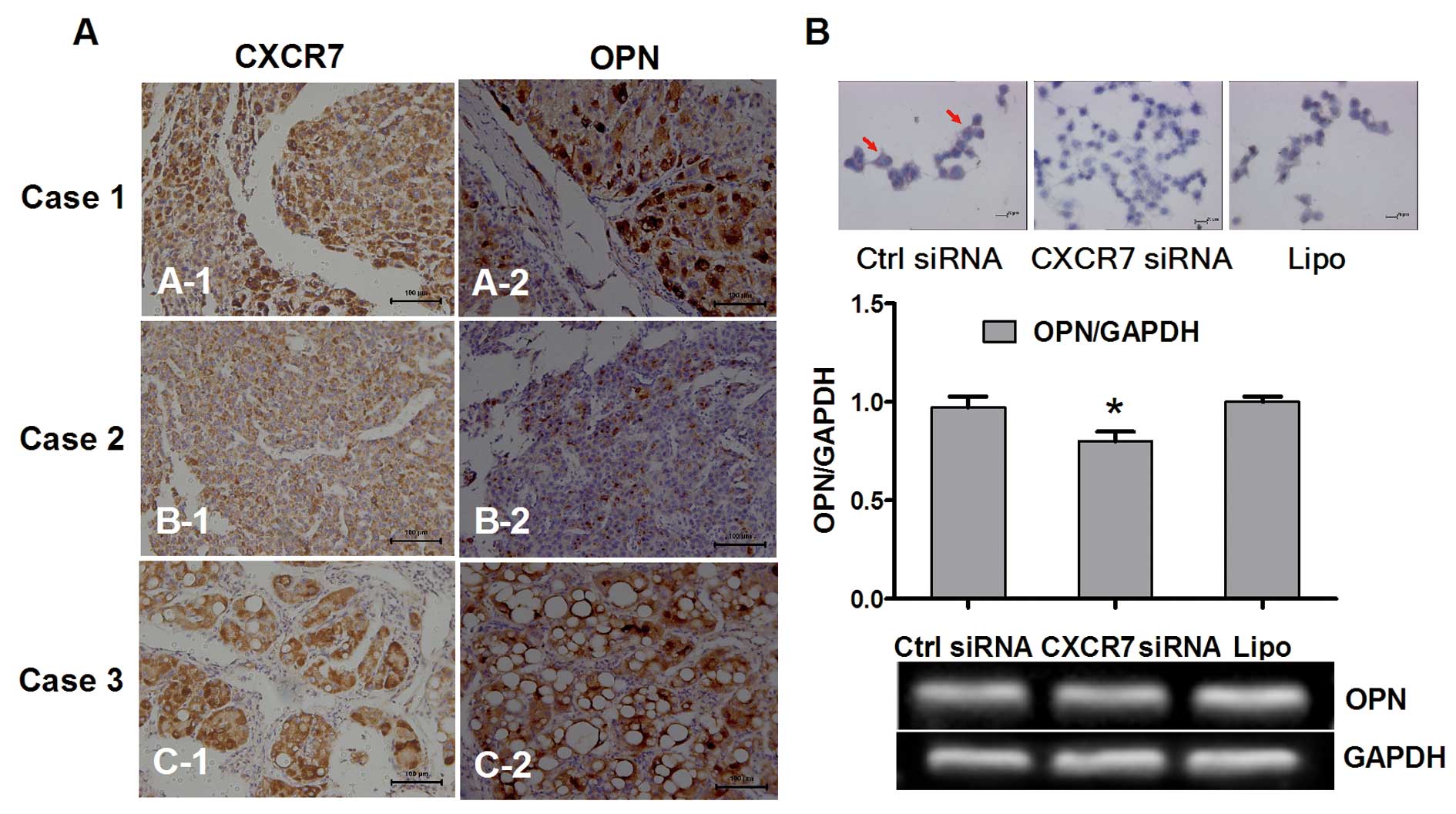
addition, analyses based on immunostaining indicated that the
distribution and strength of CXCR7 were consistently and closely
associated with OPN in HCCs with well or moderate differentiation
(Fig. 3A).
To confirm the potential regulatory role of CXCR7 in
the expression of OPN, we detected the change in expression of OPN
following downregulation of expression of CXCR7 in a highly
metastatic HCC cell line. Both immunocytochemistry and RT-PCR found
decreased expression of OPN following RNAi of CXCR7 (Fig. 3B).
Discussion
In the present study, we found that high expression
of CXCR7 increased the risk of metastasis in HCC patients receiving
hepatectomy, particularly when tumor cells were well and moderately
differentiated. Stratification analysis based on Edmondson grade
1/2 indicated that high expression of CXCR7 was a strong
independent risk factor for metastasis. ROC analysis justified the
predictive role of CXCR7. The results of category 2 further
confirmed the results of category 1. These findings indicated that
CXCR7 was a valuable risk factor for predicting metastasis in
patients with relatively well-differentiated HCC. Moreover,
accumulation of chromosomal changes has been found to be associated
with metastatic behavior, and that LOH on 16q is a useful
prognostic indicator for metastasis after curative resection of HCC
(19). In addition, HBsAg was shown
to have a predictive role in extrahepatic metastasis after hepatic
resection in patients with large hepatocellular carcinoma (20).
Generally, poor differentiation indicates a more
invasive character of tumor cells. In the present study, however,
more than half of the patients (73/116) had well or moderate
differentiation, and lung metastasis occurred in 30 patients in
this group. Although well differentiation is generally associated
with a favorable prognosis, pathological factors such as CXCR7 were
still used to stratify patients with respect to lung metastasis
after resection in this study. Based on CXCR7 expression, we may
recognize and distinguish the patients prone to lung metastasis
from patients with relatively well-differentiated HCC and intervene
in advance. Therefore, this group of patients may acquire survival
benefits.
Why does high expression of CXCR7 have a strong
predictive role for lung metastasis in patients with relative
well-differentiated HCC but not in patients with
poor-differentiated HCC? One of the potential reasons is the
heterogeneity of HCC (21,22). Similarly to our study, previous
research has shown that although early HCC is generally associated
with a favorable prognosis, pathologic factors can still be used to
stratify patients with respect to survival after resection
(23). In addition, histological
heterogeneity was found in small but established HCC, which was
accompanied by increased proliferative activity and p53 protein
overexpression. The expression of β-catenin protein also has
heterogeneous distribution, even in the same histological grade
region of the same tumor (24).
Therefore, some HCC cells may acquire a new differentiation
character such as high expression of CXCR7, which promotes the
directional chemotaxis and metastasis of tumor cells to lung. On
the other hand, apart from stimulating metastasis, research
suggests other functions for CXCR7 such as its involvement in
differentiation and development (25,26).
In poorly differentiated tumors, an embryonic stem cell-like gene
expression signature suggests that CXCR7 is epigenetically
controlled by SUZ12.
In the present study, we also found an association
between CXCR7 and OPN in stratification analyses based on cell
differentiation, which may be pertinent to the increased risk of
extrahepatic metastasis. Furthermore, in this study, knockdown of
CXCR7 in a highly metastatic HCC cell line induced the
downregulation of OPN which was demonstrated by immunocytochemistry
and mRNA detection. Our findings are similar to research on the
role of CXCR7 in the early development of osteoarthritis, which
found that overexpression of CXCR7 upregulated the expression of
OPN 9-fold (27). Recent research
has shown that CXCR7 participates in the non-classic pathway by
activating mitogen-activated protein kinases through β-arrestin,
(28) which may cross-talk with the
Wnt signaling pathway. It is well known that OPN is the usual
target of Wnt signaling in tumor progression (29,30).
The activated transmembrane CXCR7 may cross-talk with Wnt signaling
through β-arrestin, and upregulate the expression of OPN, which
further stimulates the metastatic ability of tumor cells. However,
the exact mechanism deserves future investigation.
In conclusion, high expression of CXCR7 increases
the risk of post-operative metastasis in patients with relatively
well-differentiated HCC. The potential for early occurence of lung
metastasis can be predicted in advance based on CXCR7 detection.
This group of patients may acquire a survival benefit from the
early detection and prevention of lung metastasis.
Acknowledgements
This study was supported by the State Key Basic
Research Program Grant of the Ministry of Science and Technology,
China (no. 2004CB518708), the Youth Fund from Zhongshan Hospital,
and the Youth Backbone Fund from Fudan University (B-233).
References
|
1
|
Zhou XD: Recurrence and metastasis of
hepatocellular carcinoma: progress and prospects. Hepatobiliary
Pancreat Dis Int. 1:35–41. 2002.PubMed/NCBI
|
|
2
|
Tang Z, Zhou X, Lin Z, et al: Surgical
treatment of hepatocellular carcinoma and related basic research
with special reference to recurrence and metastasis. Chin Med J.
112:887–891. 1999.PubMed/NCBI
|
|
3
|
Balabanian K, Lagane B, Infantino S, et
al: The chemokine SDF-1/CXCL12 binds to and signals through the
orphan receptor RDC1 in T lymphocytes. J Biol Chem.
280:35760–35766. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burns JM, Summers BC, Wang Y, et al: A
novel chemokine receptor for SDF-1 and I-TAC involved in cell
survival, cell adhesion, and tumor development. J Exp Med.
203:2201–2213. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ben-Baruch A: Organ selectivity in
metastasis: regulation by chemokines and their receptors. Clin Exp
Metastasis. 25:345–356. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang J, Shiozawa Y, Wang Y, et al: The
role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in
prostate cancer. J Biol Chem. 283:4283–4294. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iwakiri S, Mino N, Takahashi T, et al:
Higher expression of chemokine receptor CXCR7 is linked to early
and metastatic recurrence in pathological stage I non small cell
lung cancer. Cancer. 115:2580–2593. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zheng K, Li HY, Su XL, et al: Chemokine
receptor CXCR7 regulates the invasion, angiogenesis and tumor
growth of human hepatocellular carcinoma cells. J Exp Clin Cancer
Res. 29:312010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Monnier J, Boissan M, L’Helgoualc’h A, et
al: CXCR7 is up-regulated in human and murine hepatocellular
carcinoma and is specifically expressed by endothelial cells. Eur J
Cancer. 48:138–148. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xue TC, Chen RX, Han D, et al:
Down-regulation of CXCR7 inhibits the growth and lung metastasis of
human hepatocellular carcinoma cells with highly metastatic
potential. Exp Ther Med. 3:117–123. 2012.PubMed/NCBI
|
|
11
|
Sun HC, Zhuang PY, Qin LX, et al:
Incidence and prognostic values of lymph node metastasis in
operable hepatocellular carcinoma and evaluation of routine
complete lymphadenectomy. J Surg Oncol. 96:37–45. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Simon R, Mirlacher M and Sauter G: Tissue
microarrays. Methods Mol Med. 97:377–389. 2004.
|
|
13
|
Lugli A, Spichtin H, Maurer R, et al:
EphB2 expression across 138 human tumor types in a tissue
microarray: high levels of expression in gastrointestinal cancers.
Clin Cancer Res. 11:6450–6458. 2005. View Article : Google Scholar
|
|
14
|
Dalby B, Cates S, Harris A, et al:
Advanced transfection with Lipofectamine 2000 reagent: primary
neurons, siRNA, and high-throughput applications. Methods.
33:95–103. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brooks SA: Basic immunocytochemistry for
light microscopy. Methods Mol Biol. 878:1–30. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Trevisani F, D’Intino PE, Morselli-Labate
AM, et al: Serum alpha-fetoprotein for diagnosis of hepatocellular
carcinoma in patients with chronic liver disease: influence of
HBsAg and anti-HCV status. J Hepatol. 34:570–575. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen RX, Xia YH, Cui JF, Xue TC and Ye SL:
Osteopontin, a single marker for predicting the prognosis of
patients with tumor-node-metastasis stage I hepatocellular
carcinoma after surgical resection. J Gastroenterol Hepatol.
25:1435–1442. 2010. View Article : Google Scholar
|
|
18
|
Pan HW, Ou YH, Peng SY, et al:
Overexpression of osteopontin is associated with intrahepatic
metastasis, early recurrence, and poorer prognosis of surgically
resected hepatocellular carcinoma. Cancer. 98:119–127. 2003.
View Article : Google Scholar
|
|
19
|
Nishida N, Fukuda Y, Komeda T, et al:
Prognostic impact of multiple allelic losses on metastatic
recurrence in hepatocellular carcinoma after curative resection.
Oncology. 62:141–148. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sasaki A, Kai S, Endo Y, et al: Hepatitis
B virus infection predicts extrahepatic metastasis after hepatic
resection in patients with large hepatocellular carcinoma. Ann Surg
Oncol. 14:3181–3187. 2007. View Article : Google Scholar
|
|
21
|
Emile JF, Lemoine A, Azoulay D, Debuire B,
Bismuth H and Reynes M: Histological, genomic and clinical
heterogeneity of clear cell hepatocellular carcinoma.
Histopathology. 38:225–231. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Unsal H, Yakicier C, Marcais C, et al:
Genetic heterogeneity of hepatocellular carcinoma. Proc Natl Acad
Sci USA. 91:822–826. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nathan H, Schulick RD, Choti MA and Pawlik
TM: Predictors of survival after resection of early hepatocellular
carcinoma. Ann Surg. 249:799–805. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
An FQ, Matsuda M, Fujii H, et al: Tumor
heterogeneity in small hepatocellular carcinoma: analysis of tumor
cell proliferation, expression and mutation of p53 and β-catenin.
Int J Cancer. 93:468–474. 2001.
|
|
25
|
Ben-Porath I, Thomson MW, Carey VJ, et al:
An embryonic stem cell-like gene expression signature in poorly
differentiated aggressive human tumors. Nat Genet. 40:499–507.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee TI, Jenner RG, Boyer LA, et al:
Control of developmental regulators by Polycomb in human embryonic
stem cells. Cell. 125:301–313. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jones SW, Brockbank SM, Mobbs ML, et al:
The orphan G-protein coupled receptor RDC1: evidence for a role in
chondrocyte hypertrophy and articular cartilage matrix turnover.
Osteoarthritis Cartilage. 14:597–608. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rajagopal S, Kim J, Ahn S, et al:
Beta-arrestin- but not G protein-mediated signaling by the’decoy’
receptor CXCR7. Proc Natl Acad Sci USA. 107:628–632. 2010.
|
|
29
|
Ravindranath A, Yuen HF, Chan KK, et al:
Wnt-β-catenin-Tcf-4 signalling-modulated invasiveness is dependent
on osteopontin expression in breast cancer. Br J Cancer.
105:542–551. 2011.
|
|
30
|
Rohde F, Rimkus C, Friederichs J, et al:
Expression of osteopontin, a target gene of de-regulated Wnt
signaling, predicts survival in colon cancer. Int J Cancer.
121:1717–1723. 2007. View Article : Google Scholar : PubMed/NCBI
|