Introduction
Traditional Chinese medicine (TCM) has played a
critical role in the promotion of health, prevention of disease,
and treatment of illnesses for thousands of years in China and
other Asian countries (1,2). Many commonly used Chinese herbal
medicines are listed in the Chinese Pharmacopoeia. The dried roots
of Scutellaria baicalensis Georgi (Labiate) or S.
baicalensis is one of the most widely used herbs, and this herb
is utilized as a key ingredient in many TCM formulations (3).
Modern phytochemical and biological evaluations
demonstrated that there are four primary bioactive constituents in
S. baicalensis(4): baicalin
(baicalein-7-O-glucuronide), baicalein
(5,6,7-trihydoxyflavone), wogonoside
(wogonin-7-O-glucuronide) and wogonin
(5,7-dihydroxy-8-methoxyflavone) (Fig.
1A). These compounds are responsible for the observed
pharmacological actions (5–7), such as anti-inflammation, reduction of
total cholesterol level and blood pressure, and anti-HIV
activities. Investigators have also reported the anticancer
potential of S. baicalensis, as well as
anti-chemotherapy-induced side effects (3,8,9).
Previous studies reported that the aglycons in S.
baicalensis possessed remarkable anticancer effects (8,10). The
two major aglycons in the herb, baicalein and wogonin, can be
transformed from their glycosides, baicalin and wogonoside,
respectively (8). Special TCM
processing methods, such as high-temperature baking, steaming and
fermentation, have been used in preparing Chinese herbal medicines,
in order to reduce toxicity and adverse effects and to increase
their effectiveness (11–14). However, these approaches are not
likely to have a significant effect in promoting the transformation
from flavonoid glycosides to aglycons.
Ginseng is another very commonly used herbal
medicine worldwide. Previous studies observed that glycosidase
including cellulase markedly catalyzed certain ginsenoside
conversions to more bioactive compounds (15,16).
However, an enzymatic catalyzing method has not been reported in
preparation of S. baicalensis. In this study, using
cellulase, we performed enzyme-catalyzed transformation of the herb
to obtain aglycon-rich compounds, and their pharmacological
activities were evaluated to support the effectiveness of this
conversion. During the glycosidase-catalyzing of S.
baicalensis, variable conditions (i.e., enzyme concentration,
temperature, pH value) were used to obtain the optimal compound
conversion rates. Then, different lengths of treatment time were
used to prepare five distinct S. baicalensis extracts
(SbEs). The anticancer potential of these five SbEs have
subsequently been evaluated in connection with their aglycon
contents using human HCT-116 colon cancer cells and MCF-7 breast
cancer cells. The related anticancer mechanisms of action have also
been explored.
Materials and methods
Chemicals and reagents
HPLC grade methanol, ethanol and acetonitrile were
obtained from Tedia Co. (Fairfield, OH, USA). Milli-Q water was
supplied by a Milli-Q Water Purification System (Millipore, MA,
USA). Four flavonoid standards, i.e., baicalin, wogonoside,
baicalein and wogonin, were obtained from the National Institute
for Control of Pharmaceuticals and Biological Products (Beijing,
China). All standards were of biochemical-reagent grade and at
least 95% pure confirmed by HPLC. Cellulase (XW-G-F Cellulase,
140,000 U/g) was obtained from Novozymes Biologicals Co. (Shenyang,
China). Trypsin, McCoy’s 5A and DMEM medium, fetal bovine serum
(FBS), and penicillin-streptomycin solution (200X) were obtained
from Mediatech (Herndon, VA, USA). A CellTiter 96 Aqueous One
Solution Cell Proliferation Assay kit was obtained from Promega
(Madison, WI, USA). Propidium iodide (PI) and RNase were obtained
from Sigma (St. Louis, MO, USA). FITC Annexin V Apoptosis Detection
kit was obtained from BD Biosciences (San Diego, CA, USA).
Plant materials
The S. baicalensis root, collected from
Gansu, China, was obtained from Tianyi Drugstore Co. (Huaian,
China). The voucher samples were deposited at the Group of BioTCM
and Biocatalysis, Faculty of Life Science and Chemical Engineering,
Huaiyin Institute of Technology. Dried S. baicalensis roots
were ground with a blade-mill (FW135 Medicine Mill, Nanjing, China)
to obtain a relatively homogeneous drug powder, and then sieved
through a 100-mesh (0.15 mm) screen. The powder was dried in a
desiccator with silica gel at ambient temperature until constant
weight before extraction.
Determination of flavonoids
Ultraviolet (UV) spectra of flavonoids were recorded
on a Shimadzu UV-2401PC UV-Vis spectrophotometer (Shimadzu, Japan).
Quantitative determination was performed on an Agilent 1100 liquid
chromatographic system (Agilent Technologies, CA, USA) consisted of
a G1313A-ALS autosampler, a G1316A-Colume thermostated compartment,
a G1311A-QuatPump and a G1379A degasser, a G1315B-DAD detector, and
ChemStation software for peak identification and integration. The
separation was carried out on an Agilent Zorbax Extend-C18
reversed-phase column (4.6×250 mm, 5.0 μm). A ternary isocratic
solvent system of methanol-acetonitrile-water-acetic acid
(18:25:57:0.2, v/v) was used. The flow rate was 0.8 ml/min and the
detection wavelength was set to 275 nm. Temperature of column
compartment was maintained at 30ºC. The injection volume was 10 μl.
All tested solutions were filtered through a 0.2-μm membrane
filter. The content of the constituents were calculated using
calibration curves of four flavonoid standards.
Condition tests for cellulase-catalyzed
transformation
Cellulase was quantified accurately and dispersed in
phosphoric buffer solution to obtain 20 ml of enzyme solution with
a certain activity unit. One gram of herbal powder was added into
the enzyme solution. After adjustment of the pH value, the flask
containing the reaction mixture was fixed on a thermostatic water
bath shaker (130 rpm) for a period of treatment time at a
controlled temperature. After the treatment was finished, absolute
ethanol was added to the reaction solution and the concentration of
ethanol in the solution was 75% (v/v). The solution was sonicated
in an ultrasonic bath (Kunshan Ultrasonic Instrument Co., China)
for 30 min at 25ºC before the extract was filtered. The residues
were ultrasonically extracted twice with 20 ml of 75% ethanol. All
the filtrates were transferred into a 250 ml volumetric flask and
adjusted to the volume with 75% ethanol. A 5.0 ml of extract
solution was used for HPLC analysis. The solvent of the extract
solution was evaporated under vacuum, and then the residue was
completely dissolved in methanol. The following reaction conditions
were tested: cellulase concentration (5–30 U/g), temperature
(30–60ºC), pH value (4.0–5.0), and reaction time (0–10 h).
Preparation of S. baicalensis extracts
(SbEs)
After optimal conditions were obtained, a series SbE
samples were prepared for biological evaluation. Briefly, 10 g of
S. baicalensis root powder were added to 200 ml of reaction
solution containing cellulase 20 U/g. The pH value was adjusted to
4.8. The temperature was set at 50ºC. The flask containing the
reaction mixture was fixed on the shaker (130 rpm), and the
reaction was conducted for 2, 4, 6 and 8 h. Subsequent treatment
steps were the same as the above protocol. The filtrate collected
was concentrated under vacuum (<60ºC) in a rotary evaporator to
remove solvent. Then the extracts were lyophilized to obtain 2, 4,
6 and 8 h catalyzed SbE extracts: SbE2, SbE4, SbE6 and SbE8,
respectively. Another control extract SbE0, which was without
cellulase transformation, was also prepared. Before biological
evaluation, the contents of the four flavonoids in SbEs were
determined by HPLC.
Cell culture
Two cell lines, HCT-116 human colorectal carcinoma
cells and MCF-7 human breast cancer cells (ATCC, Manassas, VA, USA)
were cultured in McCoy’s 5A and DMEM Media, respectively,
supplemented with 10% fetal bovine serum and
penicillin-streptomycin (50 U/ml). Cells were maintained at 37ºC in
a humidified atmosphere, with 5% carbon dioxide and 95% air.
Cell proliferation assay using modified
trichrome strain (MTS) method
Cells were seeded in 96-well plates. After one day,
various concentrations of SbEs (dissolved in DMSO) were added to
the wells. The final concentration of DMSO was 0.5%. Controls were
exposed to culture medium containing 0.5% DMSO without drugs. All
experiments were performed in triplicate. Cell proliferation was
evaluated using an MTS assay according to the manufacturer’s
instructions. Briefly, at the end of the drug exposure period, the
medium was replaced with 100 μl of fresh medium and 20 μl of MTS
reagent (CellTiter 96 Aqueous Solution) in each well, and the plate
was returned the incubator for 1–2 h. A 60-μl aliquot of medium
from each well was transferred to an ELSA 96-well plate and its
absorbance at 490 nm was recorded. Results were expressed as a
percentage of the control (DMSO vehicles set at 100%).
Cell cycle analysis of HCT-116 cells
after staining with propidium iodide
To further evaluate the effects of SbEs on
anticancer potential, we performed cell cycle analysis of HCT-116
treated with different concentrations of SbE0 and SbE8. HCT-116
cells were seeded in 24-well tissue culture plates. On the second
day, the medium was changed, and cells were treated with different
concentrations of SbE0 or SbE8 for 48 h. After harvesting, the
cells were fixed gently by adding 80% ethanol and placing them in a
freezer for 2 h. They were then treated with 0.25% Triton X-100 for
5 min in an ice bath. The cells were resuspended in 300 μl of PBS
containing 40 μg/ml propidium iodide (PI) and 0.1 mg/ml RNase.
Cells were incubated in a dark room for 20 min at room temperature,
and then subjected to cell cycle analysis using a FACScan flow
cytometer (Becton Dickinson, Mountain View, CA, USA) and FlowJo
10.0.4 software (Tree Star, Ashland, OR, USA). For each
measurement, at least 20,000 cells were counted.
Apoptosis analysis of HCT-116 cells after
staining by Annexin V-FITC/propidium iodide
Cells were seeded in 24-well tissue culture plates.
After one day, the medium was changed, and SbE0, SbE4 and SbE8 were
added. After treatment for 48 h, cells floating in the medium were
collected. The adherent cells were detached with 0.25% trypsin.
Then, culture medium containing FBS (and floating cells) was added
to inactivate trypsin. After pipetting gently, the cells were
centrifuged for 5 min at 1500 × g. The supernatant was removed and
cells were stained with Annexin V-FITC and propidium iodide (PI)
according to the manufacturer’s instructions. Untreated cells were
used as a control for double staining. The cells were analyzed
immediately after staining using a FACScan flow cytometer. For each
measurement, at least 20,000 cells were counted.
Statistical analysis
Cell proliferation, and flow cytometry experiments
were performed in triplicate. The data are presented as mean ±
standard error (SE). A one-way ANOVA determined whether the results
had statistical significance. In some cases, a Student’s t-test was
used for comparing two groups. The level of statistical
significance was set at P<0.05.
Results
Ultraviolet spectra and HPLC chromatogram
of four flavonoids
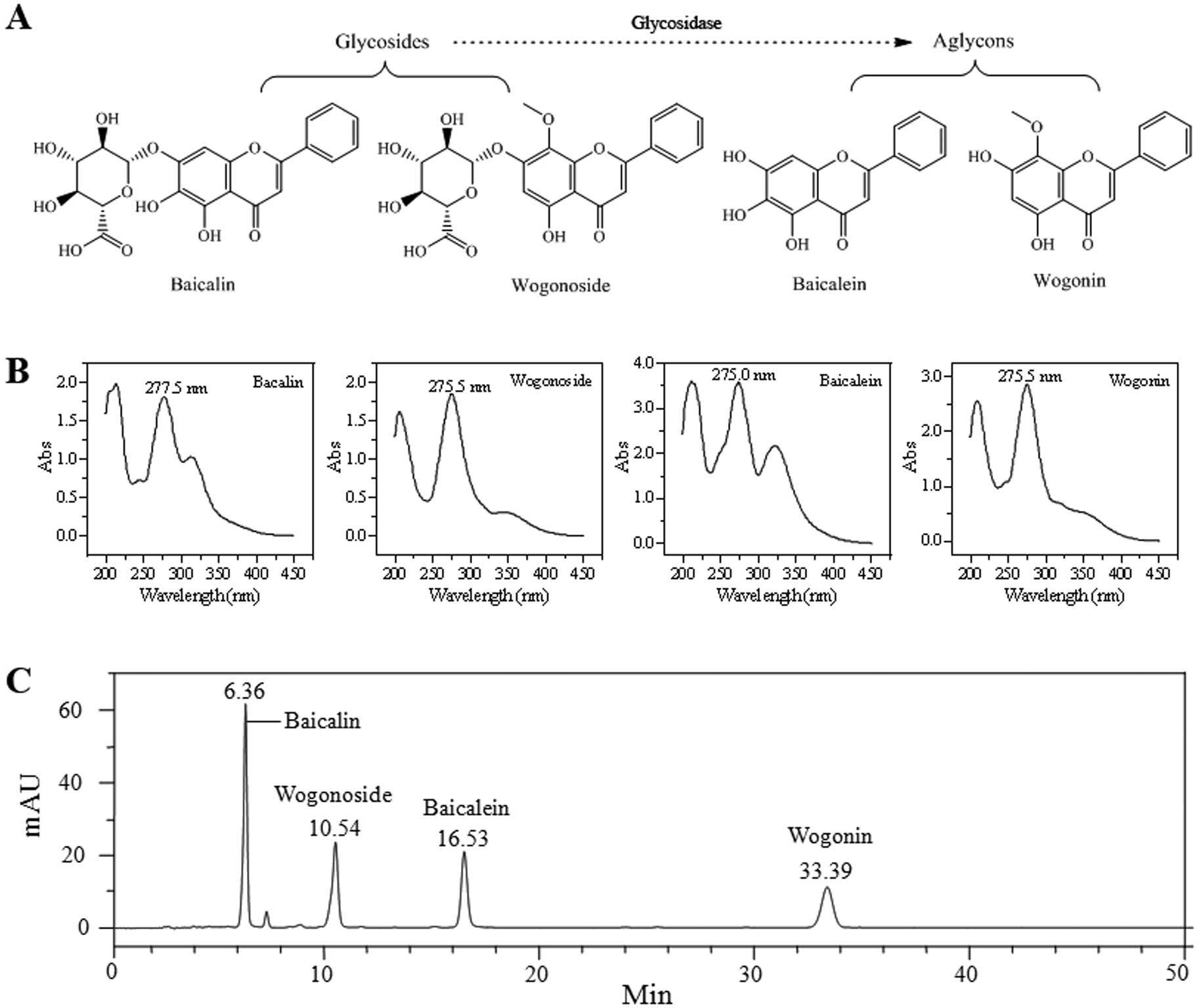
Fig. 1A shows the
chemical structures of four flavonoids (baicalin, wogonoside,
baicalein and wogonin) identified from S. baicalensis.
Ultraviolet spectroscopy of these four flavonoids is shown in
Fig. 1B. The maximum absorption
wavelength (λmax) of the four flavonoids was
277.5, 275.5, 275.0 and 275.5 nm, respectively. Thus, for the HPLC
analysis, 275 nm was a suitable wavelength for the determination of
the four flavonoids. Fig. 1C is the
HPLC chromatogram recorded at 275 nm. The four flavonoids were well
separated under the established HPLC condition, and this HPLC
method can be utilized to accurately measure these compound
contents in the test samples.
Optimization of conditions for cellulase
catalyzed deglycosylation
To determine the optimal conditions of cellulase in
catalyzing flavonoids from S. baicalensis, variable
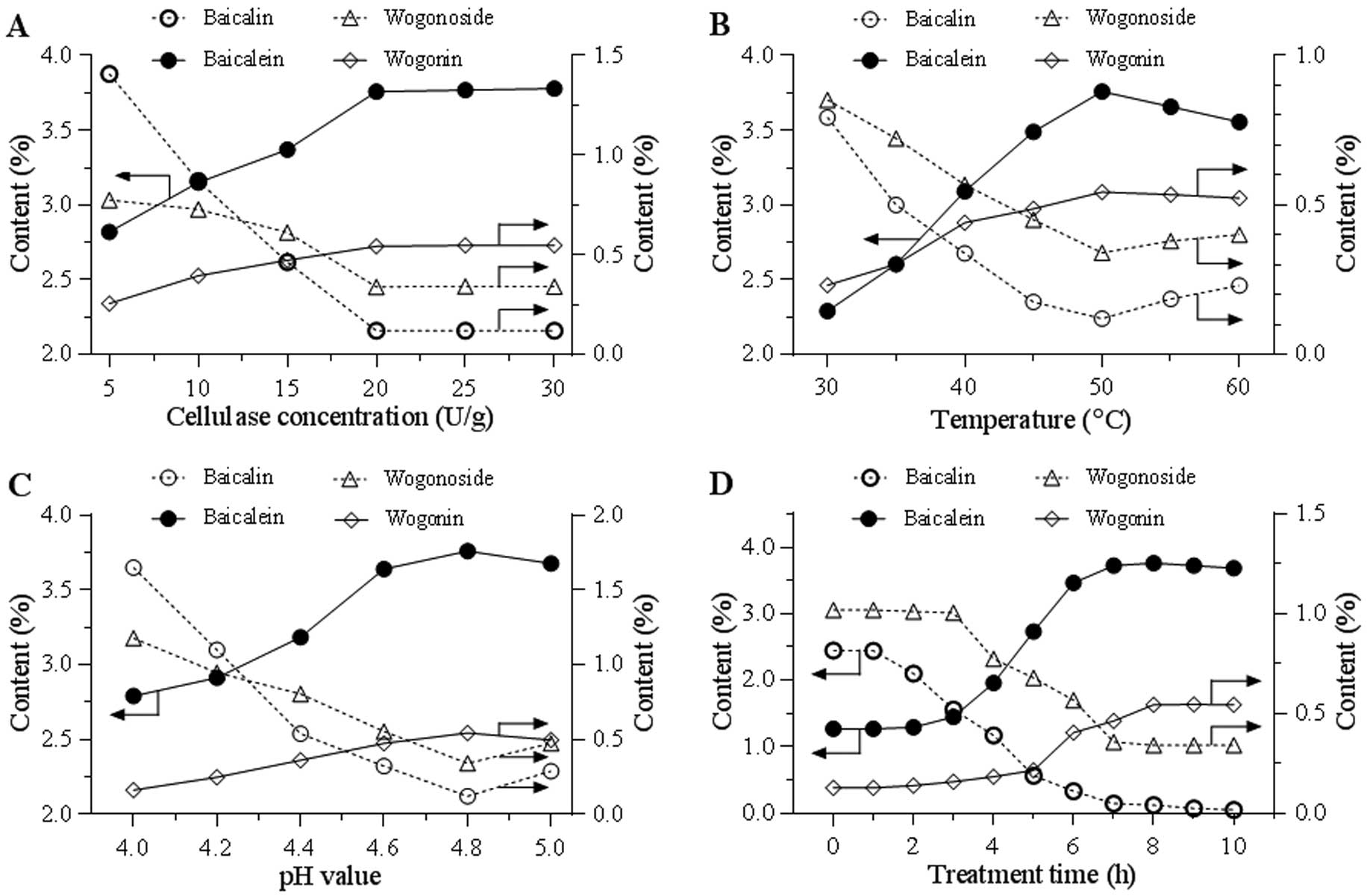
conditions were tested. Fig. 2A
indicates the effects of cellulase concentrations from 5–30 U/g.
Along with an increase of the enzyme concentration, an increase of
the two aglycon contents and a decrease of the two glycoside
contents were observed. Using the selected cellulase concentration
of 20 U/g, the effect of temperature (30–60ºC) on transformation
was tested. Fig. 2B indicates that
50ºC was the optimal reaction temperature. Using the selected
cellulase concentration and temperature, Fig. 2C indicates the effects of pH value
from 4.0–5.0, and a pH of 4.8 was selected for further evaluation.
Fig. 2D shows the effects of
treatment time from 0 to 10 h. The content curves of the two
aglycons and two glycosides were obtained and they clearly indicate
that treatment time significantly affected the flavonoid
conversion. Overall, the optimized catalyzing conditions are: 20
U/g of enzyme, pH 4.8, treatment at 50ºC for 8 h.
Preparation of cellulase catalyzed S.
baicalensis extracts (SbEs) for bioactivity assay
Based on Fig. 2D
observation, at fixed conditions (cellulase 20 U/g, pH 4.8 and
50ºC), different lengths of time were used to treat S.
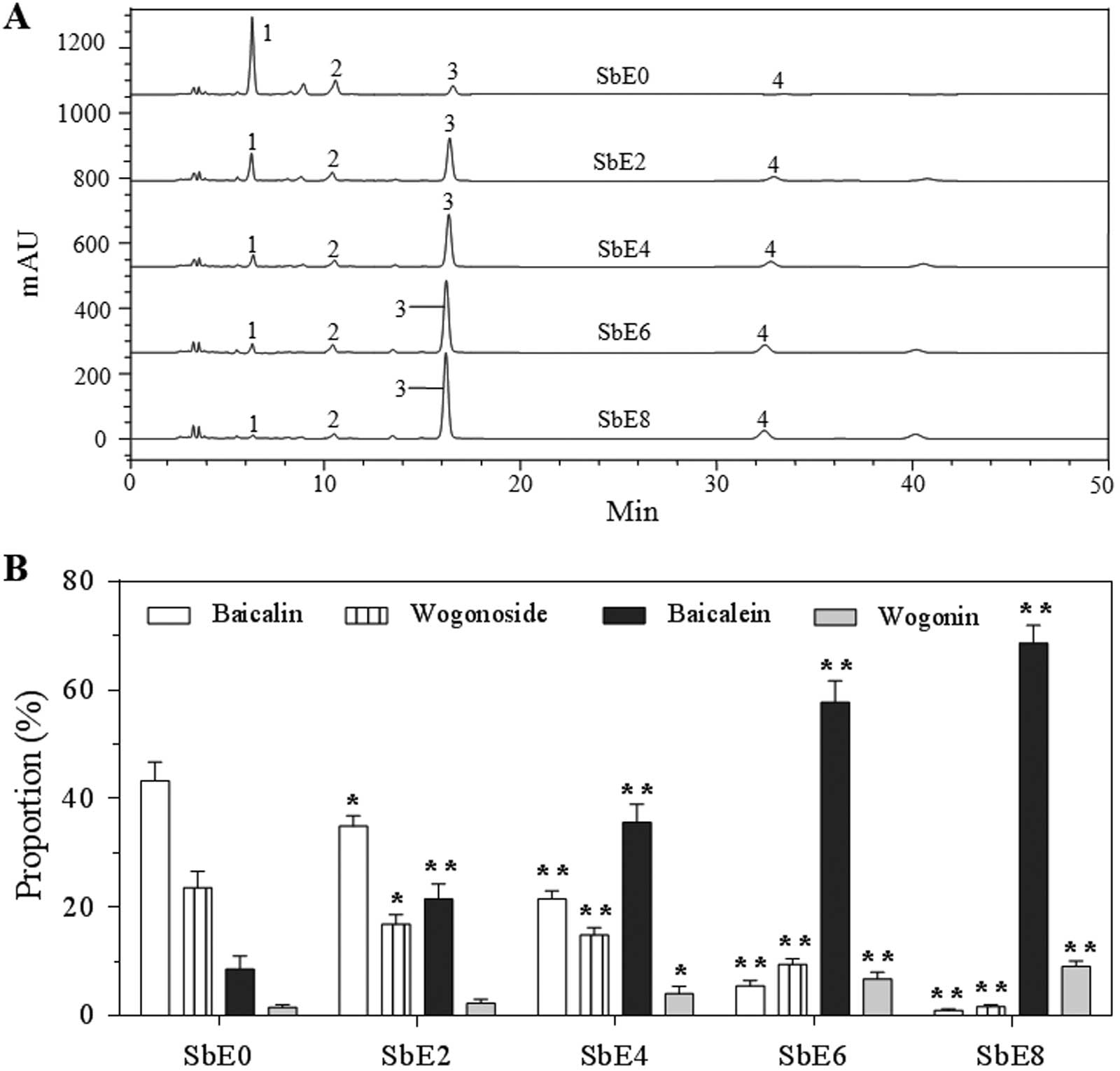
baicalensis. Fig. 3A shows HPLC
chromatograms of five SbE sample treatments for 0, 2, 4, 6 and 8 h
(SbE0, SbE2, SbE4, SbE6 and SbE8). Significantly different
proportions of the four flavonoids in the five SbEs are shown in
Fig. 3B. For SbE0, the content of
the two glycosides was highest, and the content of the two aglycons
was the lowest. However, for SbE8, the content of the two
glycosides was the lowest, and the content of the two aglycons was
the highest. The proportion of baicalin, wogonoside, baicalein and
wogonin, was 43.3, 23.6, 8.5 and 1.6% in SbE0; 34.9, 16.8, 21.5 and
2.3% in SbE2; 21.5, 14.9, 35.6 and 4.1% in SbE4; 5.5, 9.4, 57.7 and
6.7% in SbE6; and 1.0, 1.7, 68.6 and 9.1% in SbE8, respectively.
The results indicate that the proportion of the four flavonoids in
the SbEs was significantly changed after cellulase treatment.
Antiproliferative effect of five SbEs on
HCT-116 and MCF-7 cells
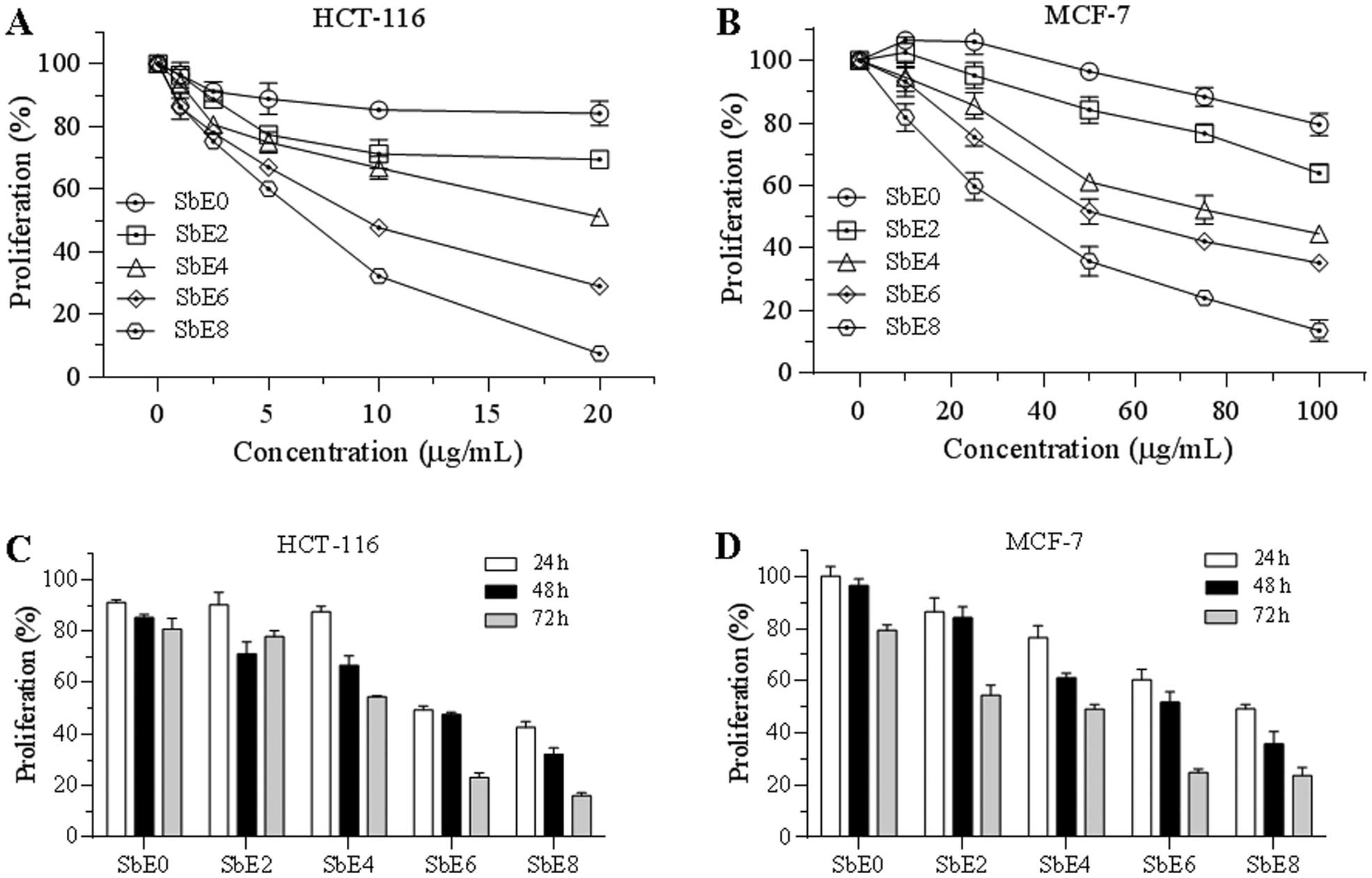
Effects of cellulase-catalyzed extracts on cancer
cell growth inhibition were assayed. As shown in Fig. 4, after treatment with 20 μg/ml for
48 h, SbE0 inhibited HCT-116 cell growth by 15.9%, while SbE8
inhibited cell growth by 92.6%. Antiproliferative potential of
other SbEs were between SbE0 and SbE8 (Fig. 4A). Sensitivity of MCF-7 cells by
SbEs was lower than that of HCT-116. At 100 μg/ml, SbE0 inhibited
MCF-7 cell growth by 20.5%, while SbE2, SbE4, SbE6 and SbE8
inhibited cell growth by 26.1, 55.4, 64.8 and 86.4%, respectively
(Fig. 4B). Of note, at some lower
concentrations, SbE0 and SbE2 actually improved MCF-7 cell growth.
The IC50 (50% inhibitory concentration) of SbE8 for
HCT-116 and MCF-7 cells was ~5 and 30 μg/ml, respectively.
Incubation time also influenced antiproliferative effects of SbEs
on cancer cells. In treatment with 10 μg/ml of SbE8 for 24, 48 and
72 h, HCT-116 cell growth was inhibited by 57, 68 and 84%,
respectively (Fig. 4C). Similar
results were also observed on MCF-7 cells (Fig. 4D), suggesting that SbEs inhibit
cancer cell proliferation in a time-dependent manner. In addition,
SbE8 had the strongest antiproliferative effects on both cancer
cell lines. These results demonstrated that 8 h of cellulase
catalyzing significantly increased the antiproliferative potential
of S. baicalensis.
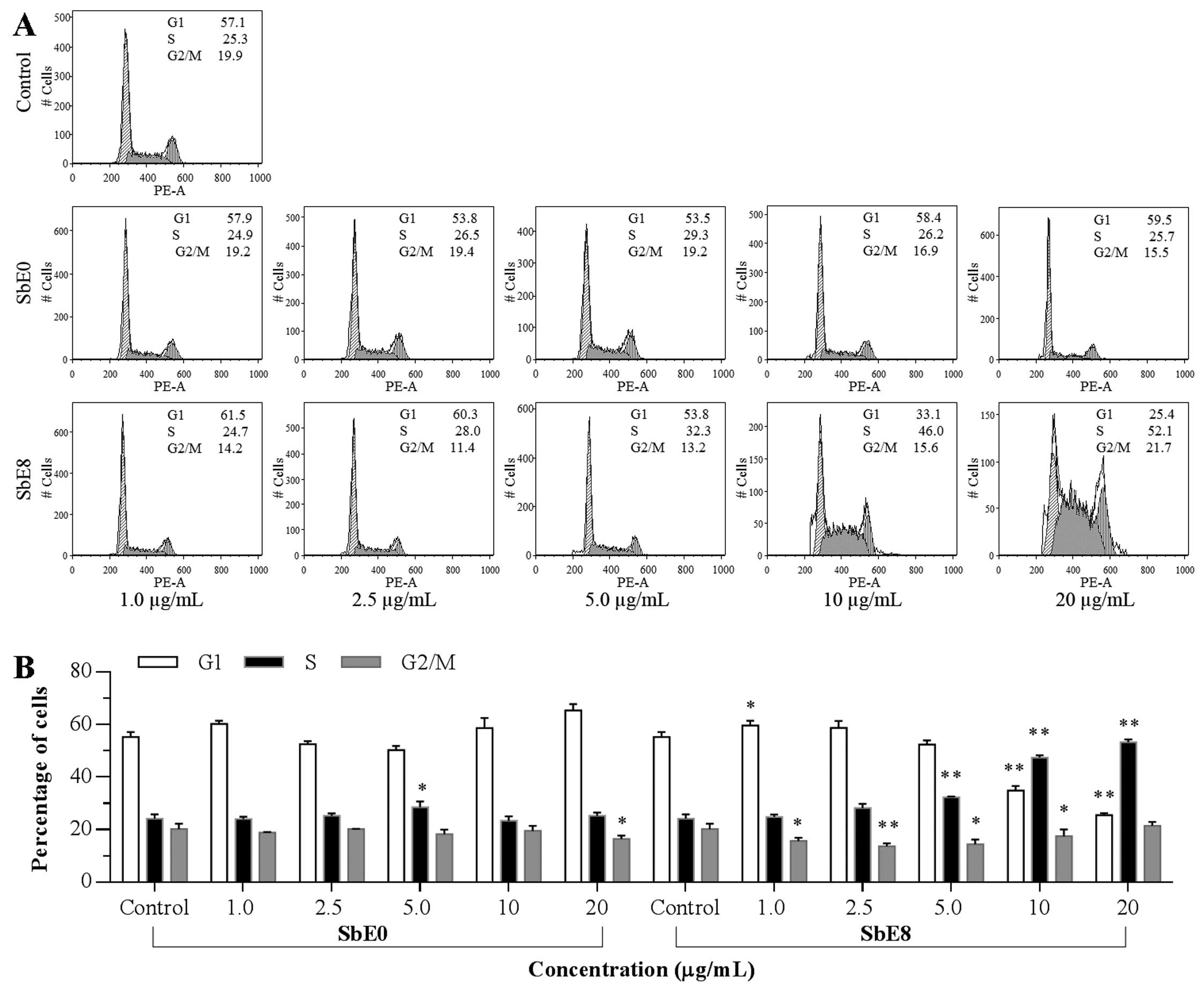
Effect of SbE0 and SbE8 on HCT-116 cell
cycle
To explore the potential mechanism by which
cellulase-catalyzed extract inhibits HCT-116 cell growth, the cell
cycle profile was assayed by flow cytometry. As shown in Fig. 5, compared to the control, SbE0 did
not change the cell cycle profile obviously, whereas significant
changes were observed with SbE8. Although treatment with SbE8 at 1
and 2.5 μg/ml did not change the cell cycle profile, when the
treatment concentration was increased, the cell cycle profile was
changed. Compared to the control (57.1% of G-phase, 25.3% of
S-phase, and 19.9% of G2/M-phase), the percentage of G1-, S- and
G2/M-phase cells after treatment with SbE8 for 48 h were 33.1, 46.0
and 15.6% at 10 μg/ml; and 25.4, 52.1 and 21.7% at 20 μg/ml,
respectively. These results indicate that SbE8 significantly
induced cell cycle arrest in the S-phase.
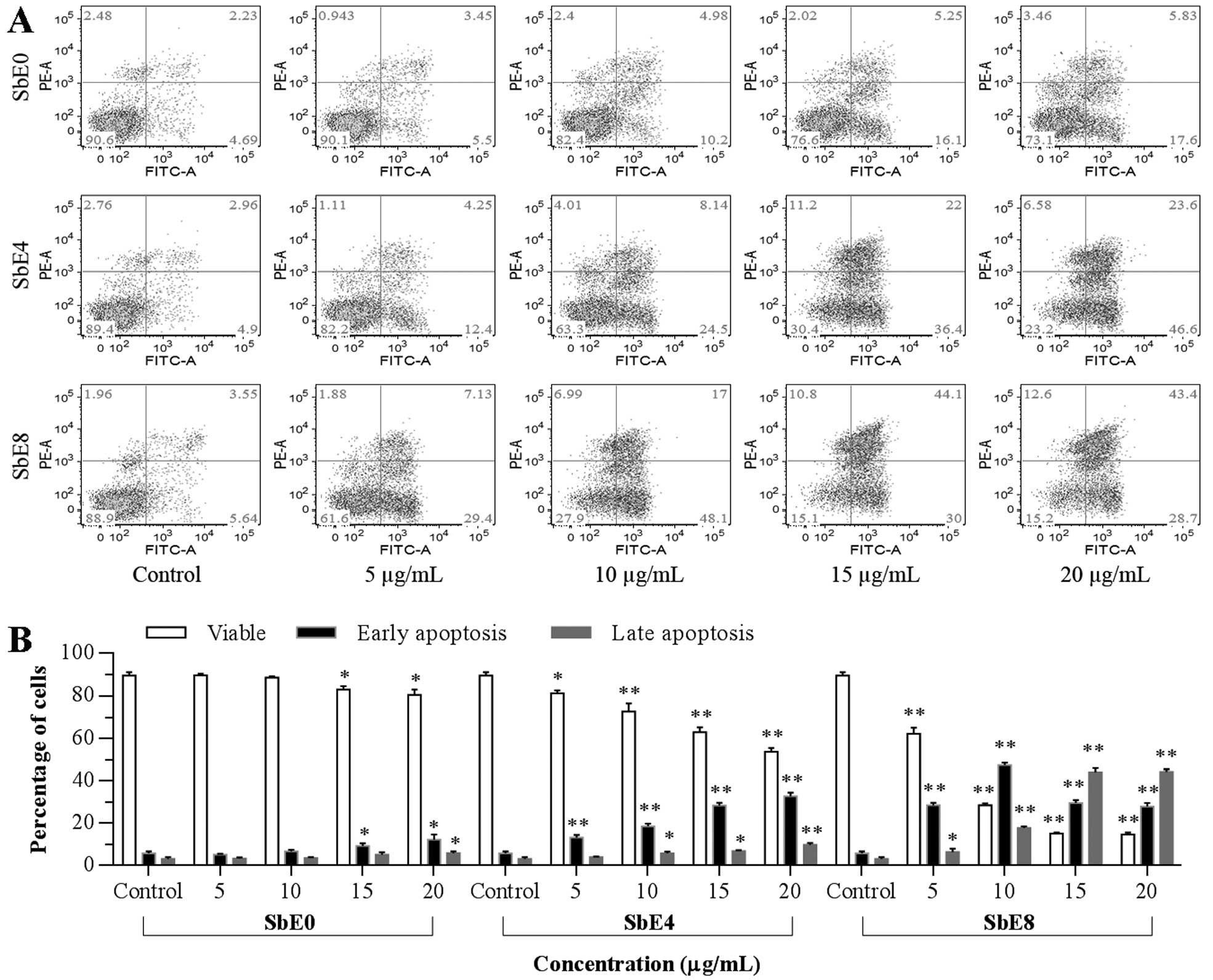
Apoptotic effect of SbE0, SbE4 and SbE8
on HCT-116 cells
The cell proliferation assay results suggested that
the cancer cell growth inhibitory capacity of SbEs is related to
the cellulase pretreatment time (Fig.
4). To further explore the mechanisms of actions of SbEs, we
carried out an apoptotic assay. As shown in Fig. 6, compared to the control (early
apoptosis 4.7–5.6%, late apoptosis 2.2–3.6%), after 48 h of
treatment with 5 and 10 μg/ml of extracts, SbE0 did not increase
early and late apoptosis while SbE4 moderately increased the early
apoptosis to 17.6%, whereas SbE8 quickly increased the early
apoptosis to 48.1%. At the same time, we observed SbE4 and SbE8
significantly increased late apoptosis. This result suggests that
the antiproliferative effect of SbE4 and SbE8 was mediated by the
induction of apoptosis, and induced apoptosis in a dose-dependent
manner. Furthermore, it was shown that apoptotic induction
potential was positively correlated with the cellulase treatment
time.
Discussion
The aims of processing Chinese medicinal herbs are
to reduce or even eliminate the botanically-related toxicity and
side effects and/or to elevate their therapeutic activities. In
addition, some processing procedures can modify a given herb’s
medicinal character based on the TCM theory, making it more
compatible with other herbs for a TCM formulation (17,18).
In our previous studies, we observed that heat-processed American
ginseng and Panax notoginseng converted their ginsenoside
profile and increased the anticancer potential. However, the
steaming method did not markedly affect the S. baicalensis
composition profile, and more importantly, the high temperature
could even degrade some flavonoid structures (19,20).
In the present study, we utilized cellulase to
catalyze flavonoids in S. baicalensis. Our HPLC analysis
showed that, at optimized conditions obtained in our study, there
was a very significant transformation from two glycosides, baicalin
and wogonoside, to two aglycons, baicalein and wogonin,
respectively. The anticancer potential was further tested using
five different SbE extracts. These five extracts received variable
lengths of treatment time with cellulase, and different levels of
baicalein and wogonin were shown in HPLC chromatograms (Fig. 3). Subsequently, human colon cancer
and breast cancer cells were used to evaluate antiproliferative
potential of these five SbEs, and our data showed that the higher
the aglycon content, the stronger the antiproliferation activities
(Fig. 4). This observation is
consistent with our previous study (8).
Many studies have demonstrated anticancer effects of
baicalein (10,21,22).
We also observed that baicalein could protect against
chemotherapeutic agent-induced cardiotoxicity by attenuation of
oxidant injury and JNK activation (9). To further increase the bioactivity of
a botanical compound, we previously prepared derivatives of
protopanaxadiol (PPD), a ginseng compound, for enhanced anticancer
activity (23). Attempts have also
been made for novel synthetic baicalein derivatives against human
tumor cells (24), and the
structure modification could be one of interesting directions in
future baicalein research.
Compared to numerous baicalein anticancer
investigations, evaluation of wogonin has received less attention.
Recently, however, data showed that both baicalein and wogonin had
antitumor actions in human HT-29 human colorectal cancer cells
(25). The functional role of
wogonin in anti-angiogenesis has also been reported. Wogonin
suppressed IL-6-induced vascular endothelial growth factor (VEGF)
by modulating the IL-6R/JAK1/STAT3 signaling pathway, suggesting
that the compound provided a therapeutic option in the treatment of
the related pathological angiogenesis (26). In our future studies, it would be
helpful to compare the bioactivity of baicalein and wogonin using
the same experimental conditions.
Similar to most Chinese herbal medicines, S.
baicalensis is ingested orally. In the present study, we
evaluated the herbal anticancer potential using in vitro
human cancer cell lines for initial antiproliferation comparison of
five SbEs with variable aglycon concentrations. The next step
should be to use in vivo cancer models to further verify our
observations. For in vivo long-term antitumor observation,
it would be desirable to mix the botanical extract or compound(s)
with animal chow, a safe and practical method for drug
delivery.
Two important issues are related to herbal oral
administration. First, the test compound bioavailability and
pharmacokinetic data should be obtained, which have been examined
previously using both baicalin and baicalein (27,28).
Secondly, intestinal microbiota play a key role in botanical
compound biotransformation (29,30).
As a result, the bioactivity of a given compound could be increased
or reduced due to the effects of intestinal microbiota. We
previously observed that after oral ginseng administration, ginseng
saponins were metabolized extensively by intestinal microbiota
(14,31,32).
Thus, in understanding the pharmacological actions of S.
baicalensis, a great deal of attention should be paid to
compound metabolites via gut microbiome.
Cellulase refers to a group of enzymes which, acting
together, hydrolyze cellulose and other compounds. The cellulase
contain different enzymes, such as endoglucanase,
cellobiohydrolase, β-glucosidase and other glycosidases (33,34).
In future studies, which enzyme in cellulase played the key role in
the conversion from glycoside to aglycon remains to be
elucidated.
In conclusion, for the first time, we utilized
cellulase, a group of glycosidase, to convert two glycosides in
S. baicalensis into two aglycons, baicalein and wogonin.
Using optimal conditions identified in our study, glycosidase
catalyzing very markedly increased the content of the two aglycons.
Comparison studies using five distinct SbEs showed that the higher
the aglycons, the better the anticancer activity. Further studies,
including the use of in vivo animal cancer models, should be
conducted. However, the present study demonstrated that using
glycosidase to catalyze S. baicalensis offers a promising
approach to increasing the herb’s anticancer potential.
Acknowledgements
This work was supported in part by the NIH/NCCAM
grants K01 AT005362 and P01 AT 004418, the Natural Science
Foundation of Jiangsu Province (BK2008194), Jiangsu Overseas
Research and Training Program for University Prominent Young and
Middle-aged Teachers and Presidents, Science and Technology Project
of Department of Traditional Chinese Medicines in Jiangsu Province
(LZ11163).
References
|
1
|
Bochorakova H, Paulova H, Slanina J, Musil
P and Taborska E: Main flavonoids in the root of Scutellaria
baicalensis cultivated in Europe and their comparative
antiradical properties. Phytother Res. 17:640–644. 2003.PubMed/NCBI
|
|
2
|
Wang CZ, Mehendale SR, Calway T and Yuan
CS: Botanical flavonoids on coronary heart disease. Am J Chin Med.
39:661–671. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lam W, Bussom S, Guan FL, Jiang ZL, Zhang
W, Gullen EA, Liu SH and Cheng YC: The four-herb Chinese medicine
PHY906 reduces chemotherapy-induced gastrointestinal toxicity. Sci
Transl Med. 2:45ra592010.PubMed/NCBI
|
|
4
|
Makino T, Hishida A, Goda Y and Mizukami
H: Comparison of the major flavonoid content of S-baicalensis,
S-lateriflora, and their commercial products. J Nat Med.
62:294–299. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu JA, Attele AS, Zhang L and Yuan CS:
Anti-HIV activity of medicinal herbs: Usage and potential
development. Am J Chin Med. 29:69–81. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chou TC, Chang LP, Li CY, Wong CS and Yang
SP: The antiinflammatory and analgesic effects of baicalin in
carrageenan-evoked thermal hyperalgesia. Anesth Analg.
97:1724–1729. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bergeron C, Gafner S, Clausen E and
Carrier DJ: Comparison of the chemical composition of extracts from
Scutellaria lateriflora using accelerated solvent extraction
and supercritical fluid extraction versus standard hot water or 70%
ethanol extraction. J Agric Food Chem. 53:3076–3080.
2005.PubMed/NCBI
|
|
8
|
Wang CZ, Li XL, Wang QF, Mehendale SR and
Yuan CS: Selective fraction of Scutellaria baicalensis and
its chemopreventive effects on MCF-7 human breast cancer cells.
Phytomedicine. 17:63–68. 2010.
|
|
9
|
Chang WT, Li J, Haung HH, Liu HP, Han M,
Ramachandran S, Li CQ, Sharp WW, Hamann KJ, Yuan CS, Vanden Hoek TL
and Shao ZH: Baicalein protects against doxorubicin-induced
cardiotoxicity by attenuation of mitochondrial oxidant injury and
JNK activation. J Cell Biochem. 112:2873–2881. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shieh DE, Cheng HY, Yen MH, Chiang LC and
Lin CC: Baicalin-induced apoptosis is mediated by Bcl-2-dependent,
but not p53-dependent, pathway in human leukemia cell lines. Am J
Chin Med. 34:245–261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cai BC, Qin KM, Wu H, Cai H, Lu TL and
Zhang XD: Chemical mechanism during chinese medicine processing.
Prog Chem. 24:637–649. 2012.
|
|
12
|
Wu J, Hong B, Wang J, Wang X, Niu SJ and
Zhao CJ: The comparative research on constituents of Radix
aconiti and its processing by HPLC quadrupole TOF-MS. Biomed
Chromatogr. 26:1301–1307. 2012.
|
|
13
|
Sun S, Wang CZ, Tong R, Li XL, Fishbein A,
Wang Q, He TC, Du W and Yuan CS: Effects of steaming the root of
Panax notoginseng on chemical composition and anticancer
activities. Food Chem. 118:307–314. 2010.
|
|
14
|
Wang D, Liao PY, Zhu HT, Chen KK, Xu M,
Zhang YJ and Yang CR: The processing of Panax notoginseng
and the transformation of its saponin components. Food Chem.
132:1808–1813. 2012.
|
|
15
|
Ko S, Suzuki Y, Suzuki K, Choi K and Cho
B: Marked production of ginsenosides Rd, F-2, Rg(3), and compound K
by enzymatic method. Chem Pharm Bull. 55:1522–1527. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chang KH, Jee HS, Lee NK, Park SH, Lee NW
and Paik HD: Optimization of the enzymatic production of
20(S)-ginsenoside Rg(3) from white ginseng extract using response
surface methodology. N Biotechnol. 26:181–186. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zou JL and Huang KM: The Chinese Materia
Medica. The English-Chinese Encyclopedia of Practical TCM. Xu XC:
1st edition. Higher Education Press; Beijing: 1994
|
|
18
|
Qu JF, Zhang SH and Xie R: The Chinese
Materia Medica. A Practical English-Chinese Library of Traditional
Chinese Medicine. Zhang EQ: 1st edition. Publishing House of
Shanghai College of Traditional Chinese Medicine; Shanghai:
1990
|
|
19
|
Patras A, Brunton NP, O’Donnell C and
Tiwari BK: Effect of thermal processing on anthocyanin stability in
foods; mechanisms and kinetics of degradation. Trends Food Sci
Technol. 21:3–11. 2010. View Article : Google Scholar
|
|
20
|
Ross CF, Hoye C and Fernandez-Plotka VC:
Influence of heating on the polyphenolic content and antioxidant
activity of grape seed flour. J Food Sci. 76:C884–C890. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chiu YW, Lin TH, Huang WS, Teng CY, Liou
YS, Kuo WH, Lin WL, Huang HI, Tung JN, Huang CY, Liu JY, Wang WH,
Hwang JM and Kuo HC: Baicalein inhibits the migration and invasive
properties of human hepatoma cells. Toxicol Appl Pharmacol.
255:316–326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Donald G, Hertzer K and Eibl G: Baicalein
- an intriguing therapeutic phytochemical in pancreatic cancer.
Curr Drug Targets. 13:1772–1776. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Du GJ, Dai Q, Williams S, Wang CZ and Yuan
CS: Synthesis of protopanaxadiol derivatives and evaluation of
their anticancer activities. Anticancer Drugs. 22:35–45. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ding D, Zhang B, Meng T, Ma Y, Wang X,
Peng H and Shen J: Novel synthetic baicalein derivatives caused
apoptosis and activated AMP-activated protein kinase in human tumor
cells. Org Biomol Chem. 9:7287–7291. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim SJ, Kim HJ, Kim HR, Lee SH, Cho SD,
Choi CS, Nam JS and Jung JY: Antitumor actions of baicalein and
wogonin in HT-29 human colorectal cancer cells. Mol Med Rep.
6:1443–1449. 2012.PubMed/NCBI
|
|
26
|
Lin CM, Chen YH, Ong JR, Ma HP, Shyu KG
and Bai KJ: Functional role of wogonin in anti-angiogenesis. Am J
Chin Med. 40:415–427. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Akao T, Kawabata K, Yanagisawa E, Ishihara
K, Mizuhara Y, Wakui Y, Sakashita Y and Kobashi K: Baicalin, the
predominant flavone glucuronide of scutellariae radix, is absorbed
from the rat gastrointestinal tract as the aglycone and restored to
its original form. J Pharm Pharmacol. 52:1563–1568. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lai MY, Hsiu SL, Tsai SY, Hou YC and Chao
PD: Comparison of metabolic pharmacokinetics of baicalin and
baicalein in rats. J Pharm Pharmacol. 55:205–209. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yim JS, Kim YS, Moon SK, Cho KH, Bae HS,
Kim JJ, Park EK and Kim DH: Metabolic activities of ginsenoside
Rb1, baicalin, glycyrrhizin and geniposide to their bioactive
compounds by human intestinal microflora. Biol Pharm Bull.
27:1580–1583. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim YS, Kim JJ, Cho KH, Jung WS, Moon SK,
Park EK and Kim DH: Biotransformation of ginsenoside Rb1, crocin,
amygdalin, geniposide, puerarin, ginsenoside Re, hesperidin,
poncirin, glycyrrhizin, and baicalin by human fecal microflora and
its relation to cytotoxicity against tumor cells. J Microbiol
Biotechnol. 18:1109–1114. 2008.
|
|
31
|
Wang HY, Qi LW, Wang CZ and Li P:
Bioactivity enhancement of herbal supplements by intestinal
microbiota focusing on ginsenosides. Am J Chin Med. 39:1103–1115.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang CZ, Calway T and Yuan CS: Herbal
medicines as adjuvants for cancer therapeutics. Am J Chin Med.
40:657–669. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mosier NS, Hall P, Ladisch CM and Ladisch
MR: Reaction kinetics, molecular action, and mechanisms of
cellulolytic proteins. Adv Biochem Eng Biotechnol. 65:23–40.
1999.PubMed/NCBI
|
|
34
|
Puri M, Sharma D and Barrow CJ:
Enzyme-assisted extraction of bioactives from plants. Trends
Biotechnol. 30:37–44. 2012. View Article : Google Scholar : PubMed/NCBI
|