Introduction
Primary breast cancer patients can be treated with
adjuvant systemic chemotherapy after surgical removal according to
classic predictive and prognostic factors, such as lymph node
status, nuclear grade, histologic grade, tumor size,
estrogen/progesterone receptor status and C-erb-B2 expression
(1). Among these factors, axillary
lymph node status is recognized as the best clinical parameter.
Based on these biomarkers, adjuvant systemic therapy is recommended
to patients. However, currently available biomarkers are relatively
inaccurate in predicting prognosis (2). Therefore, many patients may have
overtreatment or insufficient treatment. Thus, additional
biomarkers need to be established to ensure a more effective
management. Also, an easier and earlier sampling method than tissue
sampling needs to be identified. Serologic testing is an easy and
non-invasive method, and samples can be obtained repeatedly along
with the treatment phase.
The matrix metalloproteinase (MMP) family consists
of 23 zinc-dependent endopeptidases, which are all involved in the
degradation of the extracellular matrix. Based on their unique
ability to degrade the major constituent of the basement membrane,
the gelatinases MMP-2 and MMP-9 are the most important MMPs
involved in tumor invasion and metastasis (3,4). MMP-2
and MMP-9 are able to degrade type IV collagen. Type IV collagen is
abundant in basement membranes that separate epithelial cells from
the underlying stroma. An essential process in initiation of
metastasis is the degradation of the extracellular matrix (ECM),
which allows the tumor to invade local tissue, intravasate and
extravasate the blood vessels and create new metastatic formations.
This process is primarily influenced by the activity of proteinases
secreted by the tumor (5–8).
MMPs are upregulated in almost every type of cancer
and their expression is often associated with a poor prognosis for
patients (9,10). Since tissue MMPs may leach into the
blood stream and increase the circulating levels, it is believed
that MMP profile in the blood could serve as a biological marker
for disease onset, progression or monitoring. Previous studies have
shown the expression and activity of MMPs to be linked to an
advanced stage of breast cancer, increased invasion of tumor cells
and building of metastatic formations (11).
However, most reports have focused on tissue MMPs
from the primary site of breast cancer. Reports on tissue MMPs from
metastatic sites, particularly lymph nodes, are few. In order to
evaluate the clinical value of serum MMPs as a metastatic
biomarker, an association between tissue MMPs from metastatic sites
and serum MMP expression should be identified.
In the present study, we investigated the
correlation between serum and lymph node MMP expression and
axillary node metastasis.
Materials and methods
Sample collection
A total of 77 patients with invasive ductal
carcinoma without clinically apparent distant metastases and 10
patients with benign breast tumor, newly diagnosed at our
institutions between 2011 and 2012, were included in the present
study. The study design was approved by the institutional review
board of the Catholic University of Korea, College of Medicine (IRB
approval no. OC11TISI0069) and informed consent was obtained from
all patients.
Blood samples were collected from all patients
preoperatively following a standardized protocol. Serum samples
were prepared by collecting blood in empty tubes which were left at
room temperature until centrifugation. Samples were centrifuged for
10 min at 4,000 rpm, then supernatant was transferred to new tubes
and immediately stored at −80°C until enzyme-linked immunosorbent
assay (ELISA) and gelatin zymography. To ensure the reliability of
the measurements, MMP-2 and MMP-9 concentrations were determined in
parallel using the ELISA test and gelatin zymography.
We collected axillary lymph node tissue from 77
patients with breast cancer during operation. An average of 5 lymph
nodes were selected from each patient. The lymph node was bisected
along its longest axis, half of which was stored at −80°C and the
remaining half of which was embedded in paraffin and processed for
routine histology. Depending on the histologic confirmation, a
total of 56 metastatic lymph nodes and 56 non-metastatic lymph
nodes were selectively chosen for gelatin zymography. Frozen nodes
were ground by a tissue micro-dismembrator (B. Braun Melsungen AG,
Melsungen, Germany), resuspended in Tris-HEPES, pH 7.5 buffer,
homogenated on ice, centrifuged at 2,000 rpm at 4°C for 5 min. The
supernatant was collected and used for gelatin zymography
evaluation. The samples only underwent one freeze/thaw cycle before
measurements were conducted.
ELISA
We quantified the concentrations of both gelatinases
in the serum samples processed for zymography. Pro-MMP-2 and
pro-MMP-9 were determined by ELISA kits (R&D Systems,
Minneapolis, MN, USA) based on a double sandwich system whereby the
antigen is captured by a primary antibody coated on the well and
after extensive washes a secondary antibody conjugated with
horseradish peroxidase is added to immobilize the immunocomplex. In
both cases, positivity was revealed by tetramethylbenzidine and
optical density was read at 450 nm in a microtiter plate
spectrophotometer.
Gelatin zymography
Gelatin zymography was performed as follows: gels
(SDS-PAGE, 10%) were co-polymerized with gelatin (1 mg/ml) (G9382,
Sigma Chemical Co., St. Louis, MO, USA). Ten microliters of
gel-loading buffer and same amount of sample were loaded onto the
wells of the gel. We used HT1080 cell line (Korean Cell Line Bank,
Seoul, Korea) conditioned medium as standard control. This
conditioned medium is a well known standard for detecting MMP-2 and
MMP-9 activity (12). The values of
MMP activity were reported as a percentage of active form of total
form. To activate latent MMPs (pro-MMP), all samples were incubated
with 1 mmol/l APMA (Sigma) for 3 h at 37°C before loading to gel.
Electrophoresis was carried out using the minigel slab apparatus
(Mini-PROTEAN; Bio-Rad Laboratories, Hercules, CA, USA) at a
constant voltage of 125 V, until the dye reached the bottom of the
gel. After electrophoresis, gels were washed in renaturing buffer
[2.5% Triton X-100 in 50 mM Tris-HCl (pH 7.5)] for 1 h at room
temperature with gentle agitation. Then, the zymograms were
incubated for 18 h at 37°C in developing buffer [5 mM
CaCl2, 3 mM NaN3 in 50 mM Tris-HCl (pH 7.5)].
Gels were then stained with Coomassie blue and destained with 30%
methanol and 10% acetic acid. Gels were acquired and photographed
by gel imaging system (Molecular Imager® Gel DocTM XR;
Bio-Rad Laboratories), and the area of destained band was then
measured in pixels for band intensity with an image analysis
software system (ImageJ 1.45s; National Institutes of Health,
Bethesda, MD, USA) for quantification. These data were normalized
to the value of a standard band (HT1080) and were represented as
the fold-change over control values.
Statistical analysis
All statistical analyses were performed by SPSS for
Windows v. 19.0 (SPSS Inc., Chicago, IL, USA). ELISA data were
found not to be normally distributed and were therefore analyzed by
the non-parametric Mann-Whitney U test. Gelatin zymography data
were found to be normally distributed and were analyzed with the
unpaired Student’s t-test. Pearson’s correlation coefficient
analysis was calculated to determine the association between the
serum and tissue levels of MMP-2 and MMP-9. In all cases,
differences were considered statistically significant for values of
P<0.05.
Results
Patient characteristics
The patient population consisted of 77 patients with
primary breast cancer aged between 27–79 years (52.03±9.5 years)
and 10 patients with benign breast tumor aged between 20–53 years
(35.1±13.72 years). All breast cancer types were invasive ductal
carcinoma and the pathology of the benign tumor group was only
fibroadenoma. The clinicopathological characteristics of the breast
cancer patients area listed in Table
I.
 | Table ICharacteristics of breast cancer
patients (N=77). |
Table I
Characteristics of breast cancer
patients (N=77).
| N | % |
|---|
| Age (years) |
| Mean ± SD | 52.03±9.50 | |
| T stage |
| T1 | 31 | 40.2 |
| T2 | 41 | 53.2 |
| T3 | 5 | 6.4 |
| N stage |
| N0 | 42 | 54.5 |
| N1 | 24 | 31.1 |
| N2 | 5 | 6.4 |
| N3 | 6 | 7.7 |
| Stage |
| I | 23 | 29.8 |
| IIA | 27 | 35.0 |
| IIB | 15 | 19.4 |
| III | 12 | 15.5 |
| Histologic grade |
| G1 | 34 | 44.1 |
| G2 | 34 | 44.1 |
| G3 | 9 | 11.6 |
| Nuclear grade |
| N1 | 5 | 6.4 |
| N2 | 39 | 50.6 |
| N3 | 33 | 42.8 |
| Estrogen
receptor |
| Negative | 32 | 41.5 |
| Positive | 45 | 58.4 |
| Progesterone
receptor |
| Negative | 41 | 53.2 |
| Positive | 36 | 46.7 |
| HER-2 |
| Negative | 55 | 71.4 |
| Positive | 22 | 59.4 |
Serum levels and activities of MMP-2 and
MMP-9
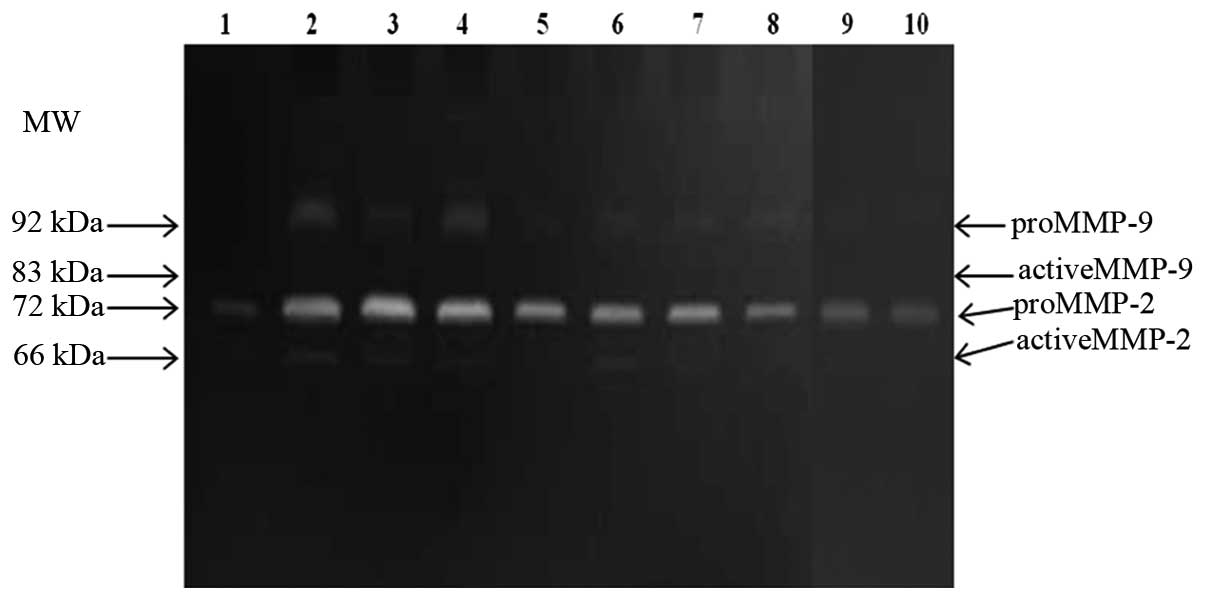
The gelatin zymography assay was optimized to
quantify MMP-2 and MMP-9 in both latent and active form in serum
sample preparations (Fig. 1). A
statistically significant difference in MMP-2 and MMP-9 levels was
noted between the breast cancer and benign breast tumor patient
group [MMP-2, mean 2.76 vs. 1.57 (x fold-change), P<0.001;
MMP-9, mean 7.26 vs. 2.45 (x fold-change), P<0.001]. The
activity of MMPs was measured as the ratio (%) of active form to
total form; active and total form of MMPs were quantified by the
area of destained band on the gel. No such differences were found
in MMP-2 and MMP-9 activity between the two groups.
In terms of node positivity, serum MMP-2 levels were
significantly higher in node-positive patients compared to
node-negative patients [mean 3.47 vs. 2.23 (x fold-change);
P<0.001], and higher serum MMP-9 levels were found in
node-positive patients compared to node negative patients,
significantly [mean 8.44 vs. 6.17 (x fold-change); P=0.047). No
such differences were found in the activity of MMP-2 and MMP-9
between the two groups (Table
II).
 | Table IISerum zymogram results of MMP-2 and
MMP-9 in benign tumor, breast cancer, node positive and node
negative groups. |
Table II
Serum zymogram results of MMP-2 and
MMP-9 in benign tumor, breast cancer, node positive and node
negative groups.
| Benign tumor
(n=10) | Breast cancer
(n=77) | P-valuea | Node (+)(n=35) | Node (−) (n=42) | P-valueb |
|---|
| MMP-2 (x
fold-change) | 1.57 | 2.76 | <0.001 | 3.47 | 2.23 | <0.001 |
| MMP-2 activity
(%) | 8.2 | 12.5 | 0.480 | 13.1 | 12.0 | 0.808 |
| MMP-9 (x
fold-change) | 2.45 | 7.26 | <0.001 | 8.44 | 6.17 | 0.047 |
| MMP-9 activity
(%) | 81.6 | 85.8 | 0.222 | 84.0 | 78.4 | 0.128 |
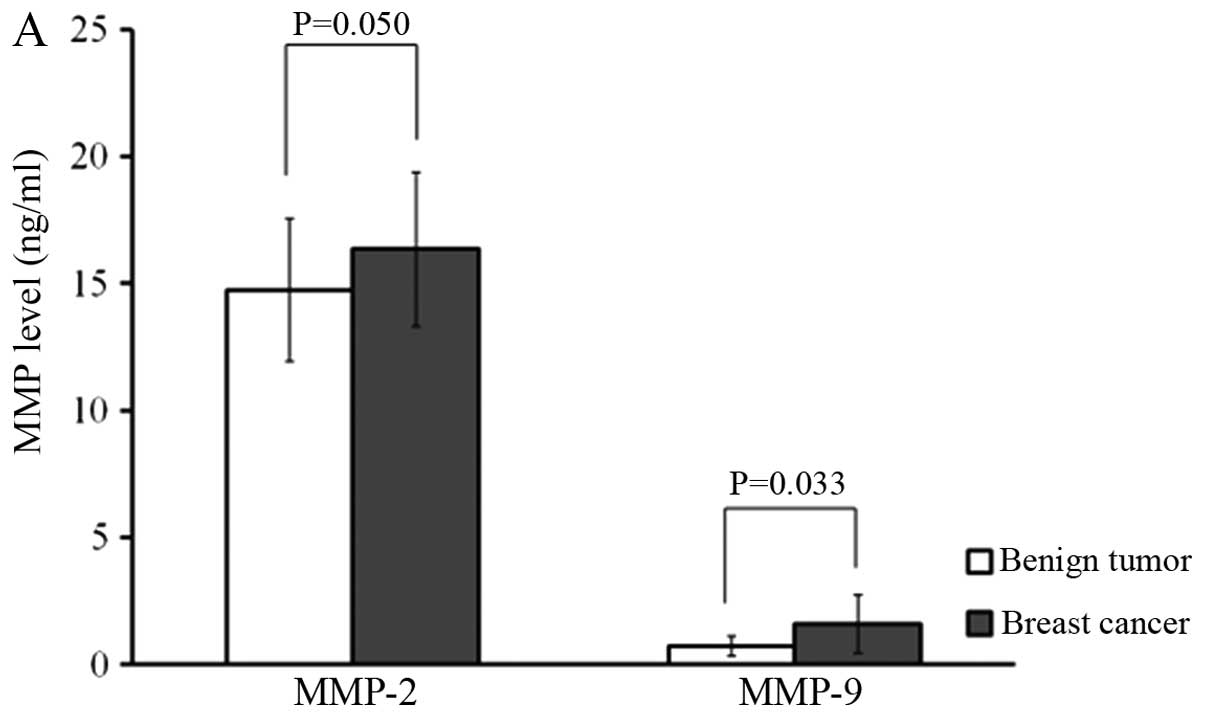
To ensure the reliability of the measurements, MMP-2
and MMP-9 concentrations were determined in parallel using the
ELISA test and gelatin zymography. MMP-2 (16.35±3.03 vs. 14.73±2.82
ng/ml; P=0.050) and MMP-9 levels (1.59±1.44 vs. 0.73±0.39 ng/ml;
P=0.033) determined by ELISA were significantly more concentrated
in breast cancer patients than in benign breast tumor patients. In
terms of node positivity, MMP-2 (17.76±2.82 vs. 15.14±2.71 ng/ml;
P=0.004) and MMP-9 (1.75±0.93 vs. 1.46±1.77 ng/ml; P=0.044) levels
were significantly higher in node-positive patients than in
node-negative patients (Fig. 2).
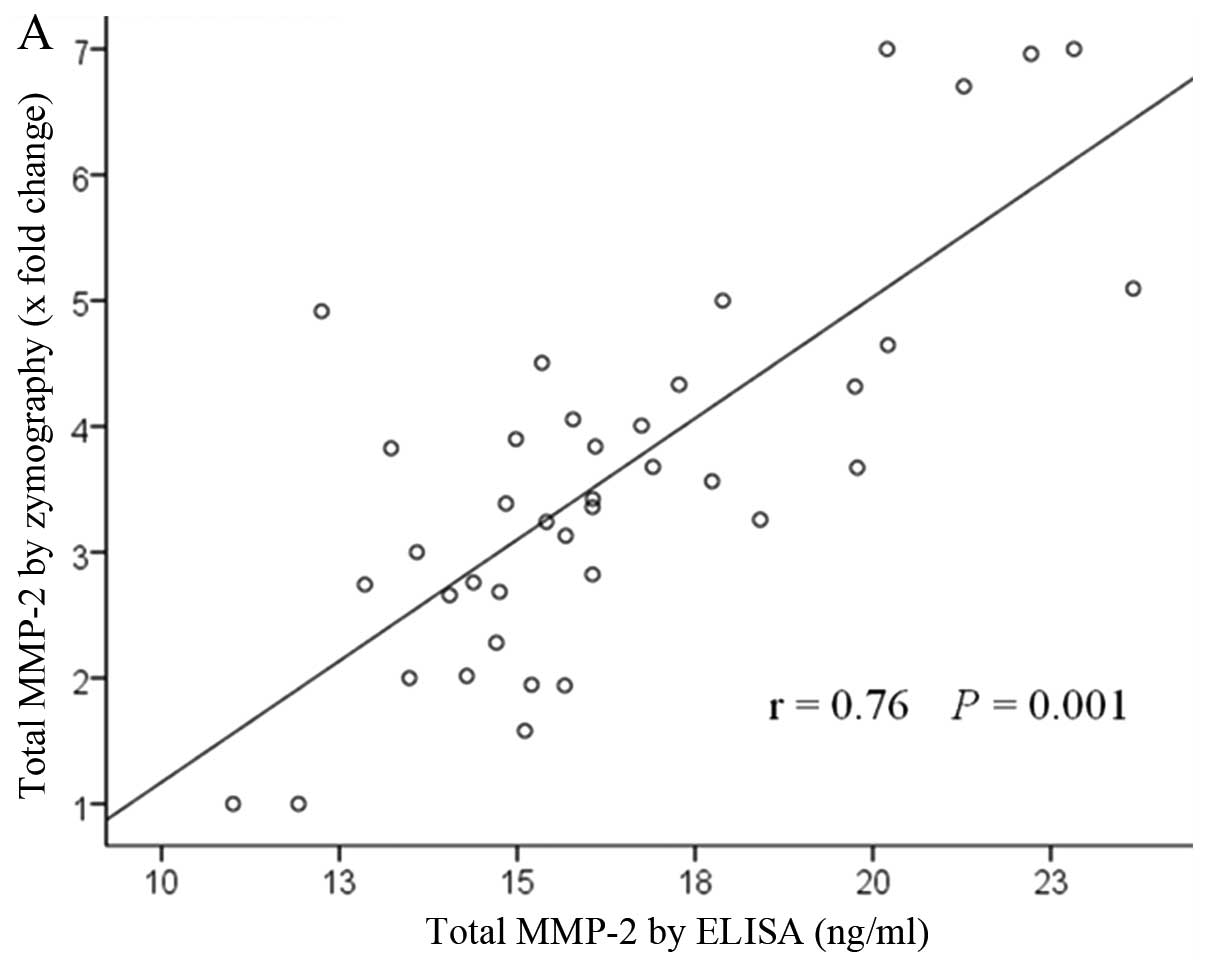
Pearson correlation coefficient analyses revealed a correlation
between ELISA and gelatin zymography results for both MMP-2 and
MMP-9 in each sample (r=0.76, P=0.001 and r=0.81, P=0.001) as shown
in Fig. 3.
Tissue levels of MMP-2 and MMP-9 in the
lymph node
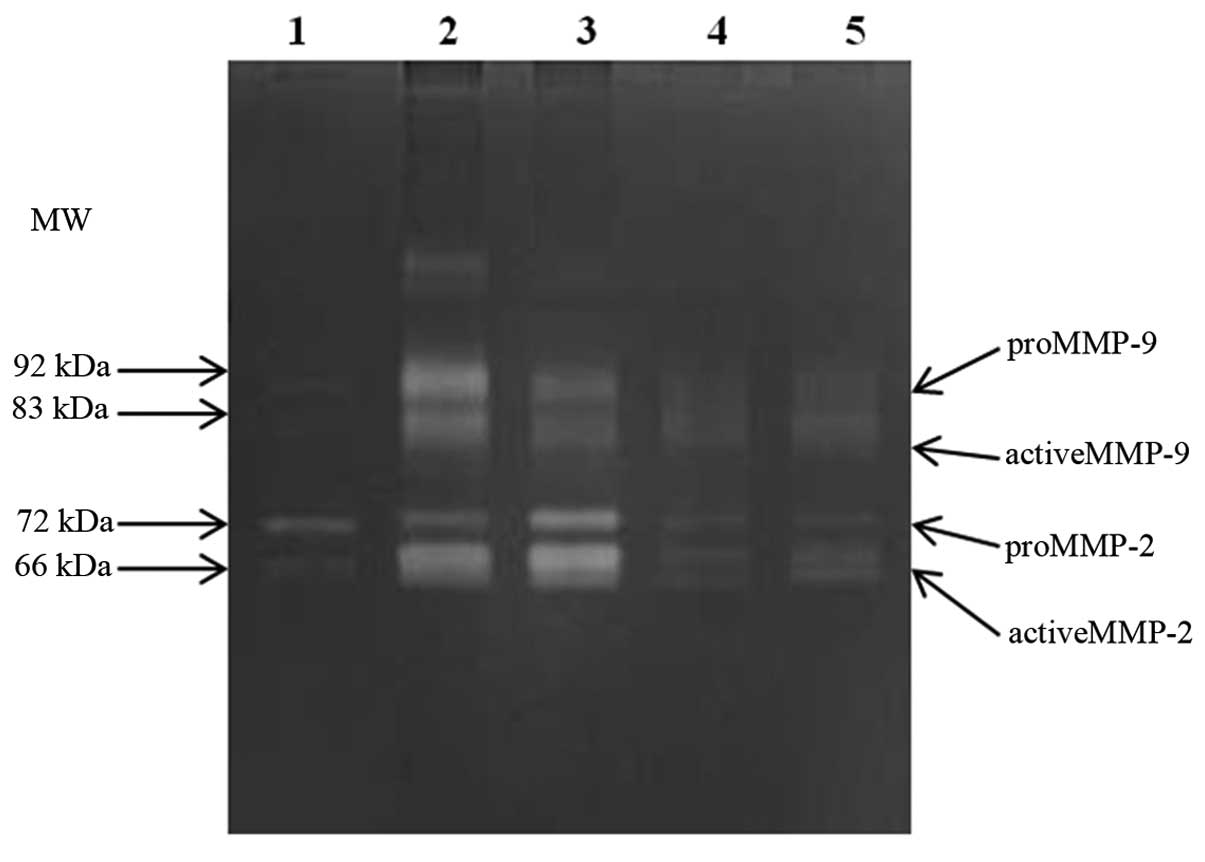
Quantitative analysis of the tissue samples from
lymph nodes was carried out by gelatin zymography (Fig. 4). The total forms of MMP-2 and MMP-9
in the lymph nodes were significantly higher in the metastatic than
in the non-metastatic node group [MMP-2, mean 3.79 vs. 0.95 (x
fold-change), P=0.050; MMP-9, mean 30.42 vs. 7.98 (x fold-change),
P=0.048]. In the metastatic node group, a proportion of the active
MMP-9 was significantly higher than in the non-metastatic node
group (mean 58.6 vs. 42.7%; P=0.001), whereas MMP-2 activities did
not show a significant difference (Table III).
 | Table IIITissue zymogram results of MMP-2 and
MMP-9 in metastatic node and non-metastatic node groups. |
Table III
Tissue zymogram results of MMP-2 and
MMP-9 in metastatic node and non-metastatic node groups.
| Metastatic node
(n=56) | Non-metastatic node
(n=56) | P-valuea |
|---|
| MMP-2 (x
fold-change) | 3.79 | 0.95 | 0.05 |
| MMP-2 activity
(%) | 67.5 | 74.8 | 0.676 |
| MMP-9 (x
fold-change) | 30.42 | 7.98 | 0.048 |
| MMP-9 activity
(%) | 58.6 | 42.7 | 0.001 |
Correlation of zymographic results of
MMP-2 and MMP-9 levels between the serum and lymph node
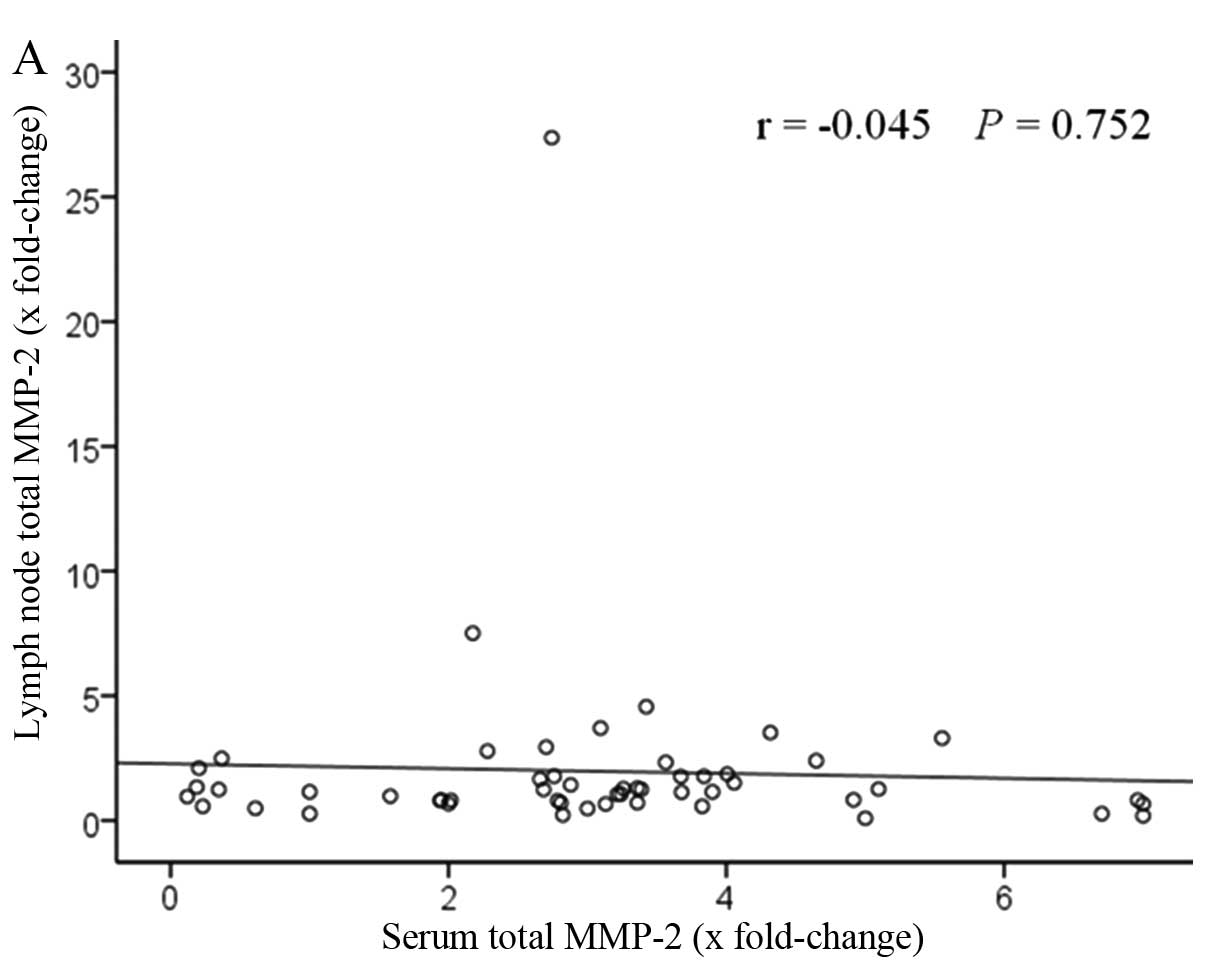
Fig. 5 shows the
correlation of MMP-2 and MMP-9 levels between the serum and lymph
node. The patients with elevated serum levels had high lymph node
levels. A positive correlation was found between serum total MMP-9
levels and lymph node levels, significantly (r=0.34, P=0.011). By
contrast, no statistical difference was observed with total MMP-2
levels (r=−0.045, P=0.752).
Discussion
MMPs have been reported to be involved in metastasis
in several types of cancer including breast cancer (13–19).
Several authors have reported clinical correlations between MMP
levels in the serum and in the tissue from primary lesions of
breast cancer. However, these studies did not include metastatic
lesions, particularly the lymph node. Limited studies have assessed
the tissue level of MMPs in the lymph node (20).
The aim of the present study was to investigate the
clinical value of MMP-2 and MMP-9 levels in the sera and axillary
lymph node of breast cancer patients. In the sera, the results of
the present study showed a statistically significant difference in
MMP-2 and MMP-9 levels between the breast cancer and benign breast
tumor patient group. Furthermore, higher levels of both gelatinases
were noted in the serum of node-positive patients than
node-negative patients among breast cancer patients. These results
are similar to those found in the current literature (21,22).
In the axillary lymph node tissue, the results of the present study
showed higher levels of total MMP-9, total MMP-2 and MMP-9
activities in metastatic lymph nodes than in non-metastatic lymph
nodes.
We assessed the correlation between high serum
levels of both gelatinases with high tissue levels from a single
patient. Serum levels of both gelatinases seem to be indicators of
axillary lymph node metastasis and high malignant potential if they
are positively correlated with high levels measured in the lymph
node. The present study suggests that higher levels of total MMP-9
measured in the serum and lymph node are associated with node
metastasis, and that measurements of the two clinical parameters
from a single patient have a positive correlation. These data are
important from two perspectives; firstly, this biological marker
could be used to diagnose axillary lymph node metastasis
preoperatively in addition to established imaging test and, thus,
minimize the extent of surgical removal in axilla to lower surgical
complication rate. Secondly, it could be useful to predict disease
progression, survival and metastasis.
Notably, MMP-9 activity and MMP-2 activity in the
serum and tissue showed no significant deferences between the
metastatic and non-metastatic node group. We assume the reason is
that it is difficult to quantify active form and determine actual
activity of MMPs. In the first place, gelatin zymography does not
identify activity of MMPs inhibited by tissue inhibitors of
metalloproteinases (TIMPs) or α2-macroglobulin.
Secondly, the zymographical method contains the SDS in the sample
buffer that could have an effect on the activity of the MMPs and
the dissolution of complexes of MMPs with TIMPs. In addition, serum
sample contains neutrophils and macrophages that produce and
secrete various MMPs (23), that
might complicate the analysis of activity of MMPs.
Nevertheless, from the results of the present study
that MMP-9 in breast cancer patient’s serum correlates with that in
their axillary lymph node, MMP-9 may be a useful biomarker. It has
been reported that high levels of plasma activity of MMP-9 could be
adequate in describing the aggressive biological behavior of breast
tumors as it was useful as a prognostic factor (24).
We analyzed the correlation of serum MMP levels with
clinical parameters to relate our data with disease features.
Univariate analysis revealed no correlation between both
gelatinases and established prognostic factors for breast cancer,
such as tumor size, histologic grade, nuclear grade, tumor staging,
c-erbB-2 expression, estrogen receptor concentration and
progesterone receptor concentration (data not shown). They may
result from the limited number of samples and patients in the
present study and further studies are required to validate the
association between our results and clinical parameters.
In conclusion, the present study demonstrated a
correlation between serum and lymph node MMP-9 levels in breast
cancer patients, which suggests a potential to use MMP-9 as a
predictor of breast cancer development, progression and axillary
node metastasis.
Acknowledgements
The present research was supported by the Korea
Breast Cancer Foundation.
References
|
1
|
Goldhirsch A, Glick JH, Gelber RD, Coates
AS, Thurlimann B and Senn HJ: Meeting highlights: international
expert consensus on the primary therapy of early breast cancer
2005. Ann Oncol. 16:1569–1583. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thomssen C and Janicke F: Do we need
better prognostic factors in node-negative breast cancer? Eur J
Cancer. 36:293–298. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Talvensaari-Mattila A, Paakko P, Hoyhtya
M, Blanco-Sequeiros G and Turpeenniemi-Hujanen T: Matrix
metalloproteinase-2 immunoreactive protein: a marker of
aggressiveness in breast carcinoma. Cancer. 83:1153–1162. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li HC, Cao DC, Liu Y, et al: Prognostic
value of matrix metalloproteinases (MMP-2 and MMP-9) in patients
with lymph node-negative breast carcinoma. Breast Cancer Res Treat.
88:75–85. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giambernardi TA, Grant GM, Taylor GP, et
al: Overview of matrix metalloproteinase expression in cultured
human cells. Matrix Biol. 16:483–496. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Iwasaki M, Nishikawa A, Fujimoto T, et al:
Anti-invasive effect of MMI-166, a new selective matrix
metalloproteinase inhibitor, in cervical carcinoma cell lines.
Gynecol Oncol. 85:103–107. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kato Y, Yamashita T and Ishikawa M:
Relationship between expression of matrix metalloproteinase-2 and
matrix metalloproteinase-9 and invasion ability of cervical cancer
cells. Oncol Rep. 9:565–569. 2002.PubMed/NCBI
|
|
8
|
McMasters KM, Giuliano AE, Ross MI, et al:
Sentinel-lymph-node biopsy for breast cancer - not yet the standard
of care. N Engl J Med. 339:990–995. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Curran S, Dundas SR, Buxton J, Leeman MF,
Ramsay R and Murray GI: Matrix metalloproteinase/tissue inhibitors
of matrix metalloproteinase phenotype identifies poor prognosis
colorectal cancers. Clin Cancer Res. 10:8229–8234. 2004. View Article : Google Scholar
|
|
10
|
Forget MA, Desrosiers RR and Beliveau R:
Physiological roles of matrix metalloproteinases: implications for
tumor growth and metastasis. Can J Physiol Pharmacol. 77:465–480.
1999. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Duffy MJ, Maguire TM, Hill A, McDermott E
and O’Higgins N: Metalloproteinases: role in breast carcinogenesis,
invasion and metastasis. Breast Cancer Res. 2:252–257. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Giannelli G, Bergamini C, Fransvea E,
Marinosci F, Quaranta V and Antonaci S: Human hepatocellular
carcinoma (HCC) cells require both alpha3beta1 integrin and matrix
metalloproteinases activity for migration and invasion. Lab Invest.
81:613–627. 2001. View Article : Google Scholar
|
|
13
|
Vasala K, Paakko P and
Turpeenniemi-Hujanen T: Matrix metalloproteinase-2 immunoreactive
protein as a prognostic marker in bladder cancer. Urology.
62:952–957. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gerhards S, Jung K, Koenig F, et al:
Excretion of matrix metalloproteinases 2 and 9 in urine is
associated with a high stage and grade of bladder carcinoma.
Urology. 57:675–679. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liabakk NB, Talbot I, Smith RA, Wilkinson
K and Balkwill F: Matrix metalloprotease 2 (MMP-2) and matrix
metalloprotease 9 (MMP-9) type IV collagenases in colorectal
cancer. Cancer Res. 56:190–196. 1996.
|
|
16
|
Liotta LA, Tryggvason K, Garbisa S, Hart
I, Foltz CM and Shafie S: Metastatic potential correlates with
enzymatic degradation of basement membrane collagen. Nature.
284:67–68. 1980. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Davies B, Miles DW, Happerfield LC, et al:
Activity of type IV collagenases in benign and malignant breast
disease. Br J Cancer. 67:1126–1131. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brown PD, Bloxidge RE, Anderson E and
Howell A: Expression of activated gelatinase in human invasive
breast carcinoma. Clin Exp Metastasis. 11:183–189. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Di Carlo A, Terracciano D, Mariano A and
Macchia V: Matrix metalloproteinase-2 and matrix
metalloproteinase-9 type IV collagenases in serum of patients with
pleural effusions. Int J Oncol. 26:1363–1368. 2005.PubMed/NCBI
|
|
20
|
Daniele A, Zito AF, Giannelli G, et al:
Expression of metalloproteinases MMP-2 and MMP-9 in sentinel lymph
node and serum of patients with metastatic and non-metastatic
breast cancer. Anticancer Res. 30:3521–3527. 2010.PubMed/NCBI
|
|
21
|
Hanemaaijer R, Verheijen JH, Maguire TM,
et al: Increased gelatinase-A and gelatinase-B activities in
malignant vs. benign breast tumors. Int J Cancer. 86:204–207. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zucker S, Hymowitz M, Conner C, et al:
Measurement of matrix metalloproteinases and tissue inhibitors of
metalloproteinases in blood and tissues. Clinical and experimental
applications. Ann NY Acad Sci. 878:212–227. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kjeldsen L, Bjerrum OW, Hovgaard D,
Johnsen AH, Sehested M and Borregaard N: Human neutrophil
gelatinase: a marker for circulating blood neutrophils.
Purification and quantitation by enzyme linked immunosorbent assay.
Eur J Haematol. 49:180–191. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ranuncolo SM, Armanasco E, Cresta C, Bal
De Kier Joffe E and Puricelli L: Plasma MMP-9 (92 kDa-MMP) activity
is useful in the follow-up and in the assessment of prognosis in
breast cancer patients. Int J Cancer. 106:745–751. 2003. View Article : Google Scholar : PubMed/NCBI
|