Introduction
Hepatocellular carcinoma (HCC) is the sixth most
common solid tumor in the world and the third leading cause of
cancer-related mortality (1). The
incidence of this type of cancer has consistently increased in both
Asian and Western countries over the last 10 years (2). Most HCC patients are diagnosed when
the disease is already advanced and often accompanied by varying
degrees of liver dysfunction. As surgery proves ineffective at more
advanced stages of disease, it is imperative that safe and
effective antitumor drugs are developed. While the biological
mechanisms have not been fully elucidated (3), epidemiological and laboratory data
suggest that non-steroidal anti-inflammatory agents (NSAID) have
antitumor effects. Celecoxib is a new generation of NSAIDs that
specifically inhibit cyclooxygenase-2 (COX-2) activity and they are
currently approved by the US Food and Drug Administration (FDA) for
the treatment of arthritis.
COX-2, a key enzyme in arachidonic acid metabolism,
is overexpressed in a variety of malignant tumors, including HCC,
prostatic, colorectal carcinoma and malignant melanoma (4). COX-2 upregulation in tumor cells
correlates with the level of angiogenesis in multiple types of
tumor (5,6). Some research suggests that PGE2, which
is a product of COX-2, is responsible for activation of the
phosphatidylinositol 3-kinase (PI3K)/Akt signal transduction
pathway. Celecoxib may inhibit phosphorylation of Akt via the
COX-2-PGE2-PI3K/Akt pathway (7).
Therefore many authors have proposed COX-2 as a target for cancer
prevention and treatment.
Tumor tissue is usually accompanied by hypoxia,
which promotes HIF-1 production. HIF-1 is a heterodimeric basic
helix-loop-helix transcription factor that consists of
hypoxia-inducible factor-1α (HIF-1α) and hypoxia-inducible
factor-1β (HIF-1β) subunits (8).
HIF-1β is constitutively expressed in cells, whereas HIF-1α
stabilization can be induced by hypoxia, growth factors and
oncogenes, such as phosphatase and tensin homologue deleted from
chromosome 10 (PTEN) (9). Zundel
et al showed that PTEN suppressed HIF-1α protein
accumulation and its target gene VEGF expression (9). This process involved modulation of
Akt. HIF-1 can also be combined with COX-2 promoter-specific
hypoxia response element, thereby inducing endothelial cell
expression of COX-2. The COX-2 by mitogen activated protein kinase
pathway or PI3K pathway induced HIF-1α expression. Previous studies
revealed that HIF-1 is a downstream gene in the PI3K/AKT pathway
(8,10,11).
PI3K signaling regulates tumor growth and angiogenesis by
activating AKT and other targets, and by inducing HIF-1 and VEGF
expression. A downstream target of PI3K is the serine-threonine
kinase Akt that is activated by phosphatidylinositol-dependent
kinase 1. HIF-1 and VEGF have previously been shown to play a
crucial role in both angiogenesis and tumor growth (12,13).
Thus, our laboratory seeks to investigate HIF-1 and VEGF as
promising anticancer drug targets.
PTEN, PI3K and Akt (PTEN/PI3K/Akt) pathways have
been associated with carcinogenesis. Activated PI3K-Akt signaling
pathway may promote carcinogenesis (14), and overexpression of PI3K or Akt is
highly angiogenic (15). Hence,
PI3K/AKT signaling pathway plays an important role in regulating
the vasculature and angiogenesis. PTEN is the most common malignant
tumor suppressor gene and it is a negative regulator of PI3K-Akt
signaling pathway (16). Celecoxib
inhibits the PI3-kinase pathway and decreases the phosphorylation
of Akt in some cell lines (17),
but it remains unknown whether PTEN/PI3K/Akt/HIF-1α pathway is also
involved in celecoxib in vivo effects on HCC growth. In
recent years, celecoxib has been shown to have anti-angiogenic and
tumor growth inhibiting effects in many cancer-related animal
models (18–20). The present study focused on tumor
angiogenesis by evaluating the microvessel density (MVD) and the
expression of PI3K, P-Akt, COX-2, HIF-1α, vascular endothelial
growth factor-A (VEGF-A) and PTEN in tumor tissues in order to
investigate the molecular mechanisms through which celecoxib
inhibits tumor angiogenesis.
Materials and methods
Materials
Celecoxib was purchased from Pfizer (New York, NY,
USA) and was dissolved in dimethyl sulfoxide (DMSO) (40 mM) as a
stock solution at 4°C. DMSO was obtained from Sigma (St. Louis, MO,
USA). 5-Fluorouracil (5-FU) was purchased from Qilu Pharmaceutical
Co., Ltd. (Shandong, China) and was dissolved in normal saline (2
mg/ml) as a stock solution at 4°C. Bicinchoninic acid (BCA) protein
assay kit was also obtained from Pierce (Rockford, IL, USA).
Polyvinylidene difluoride (PVDF) membranes were from Pall Life
Sciences (Ann Arbor, MI, USA). ELISA kits were purchased from the
BlueGene Biotech Co., Ltd. Western blotting related reagents were
purchased from the Shanghai Beyotime Institute of Biotechnology,
China.
Animal models
The animal experiment was approved by the Institute
of Medicine, Shandong Academy of Medical Sciences, China. Fifty
male Kunming mice aged 5–6 weeks and weighing 18–22 g were obtained
from the Animal Experiment Center of Shandong University China.
Seven days following H22 cell injection, ascites was
extracted from H22 ascites mice under sterile
conditions. Normal saline was then added to adjust the tumor cell
concentration to 1×107/ml. Next 0.25 ml of tumor cells
were inoculated subcutaneously into the right flank of each mouse.
The mice received standard rodent chow and water ad
libitum.
Drug treatment
After the tumor reached 50–100 mm3
following tumor cell injection, the mice were randomized into five
groups with ten mice in each group. The control group took in
purified saline. The 5-FU (20 mg/kg) group was administered via
abdominal injection starting on the same day of celecoxib
administration every four days. The celecoxib high- and low-dose
group received gavage of celecoxib at 200 and 50 mg/kg once a day,
respectively. Celecoxib dose was adjusted daily based on changes in
body weight. Tumor size was measured every two days using a digital
caliper and tumor volume was calculated using the formula: (V =
W2 × L/2), where W and L are the perpendicular smaller
and large diameters, respectively. Volumes were plotted against
time. Body weight of the mice was measured every day and the
experiment lasted 3 weeks. At the end of experimentation,
retro-orbital blood was collected and the tumors were dissected and
weighed after euthanasia. Calculation of the tumor inhibitory rate
was performed using the formula: Inhibitory rate (IR) = [average
tumor weight of the control group (g) - average tumor weight of the
treatment group (g)]/average tumor weight of the control group (g)
× 100%. The tumors were immediately placed in 4% paraformaldehyde
for immunohistochemistry (IHC). Portions of each tumor were flash
frozen in liquid nitrogen and stored at −80°C.
ELISA assays
We detected the levels of P-Akt, COX-2 and PTEN in
the serum using double antibody sandwich method and the serum
levels of PI3K, HIF-1α and VEGF-A using the competition law. We
adhered strictly to the ELISA kit instructions, and measured after
termination the color OD values of the standard curve, calculating
the concentration of the sample. All assays were performed in
triplicate.
Histology and immunohistochemistry
Tumor tissue was fixed overnight in 4%
paraformaldehyde, followed by paraffin infiltration and embedding.
Paraffin-embedded tumor samples were processed into tissue array
blocks, which were cut into 4-μm sections for hematoxylin and eosin
(H&E) and immunohistochemical staining. Sections were de-waxed
in xylene, rehydrated through graded concentrations of ethanol and
rinsed in distilled water. Sections were subjected to heat-induced
epitope retrieval in 10 mM citrate buffer (pH 6.0) for 15 min, and
then cooled to room temperature prior to treatment with 3% hydrogen
peroxide in absolute methanol (to inactivate endogenous peroxidase
activity). Sections were then washed 3× with PBS followed by
dropwise addition of the first antibody and subsequent incubation
overnight under 4°C. The tissue was incubated at room temperature
for 45 min. Sections were then washed and sequentially incubated
with a secondary antibody. The tissue was incubated at room
temperature for 1 h, and then washed and colored with DAB for 15
min and finally counterstained with hematoxylin. Tumor angiogenesis
was evaluated by MVD, which was analyzed with anti-mouse CD34
monoclonal antibody (Beijing Biosynthesis Biotechnology Co., Ltd.)
against CD34 expressed in the endothelial cells of microvessels.
The microvessel count was carried out in accordance with the method
of Weidner et al (21).
Initially, we selected 3 dense microvessel fields separately at the
original magnification ×40 and ×100, and the numbers of
CD34-stained cells were then counted at the original magnification
×400 and averaged for statistical analysis.
Western blotting
The expression profiles for PI3K, total Akt, P-Akt,
COX-2, HIF-1α, VEGF-A and PTEN were determined by western blot
assay. The protein concentration was determined with the BCA kit.
The samples were boiled, sheared, and clarified by centrifugation
and stored at −20°C. Equal quantities (20 μg) of protein were
loaded onto 12% SDS-polyacrylamide electrophoresis gel and resolved
proteins were electrotransferred to nitrocellulose filter.
Membranes were blocked with 5% skim milk in TBST (1 M Tris-buffer
saline, pH 7.4, 5M NaCl, 0.1% Tween-20) buffer for 1 h before
primary antibody addition. Western blot analyses were carried out
using the appropriate antibody [Akt, PI3K and P-Akt (Cell
Signaling, Danvers, MA, USA); COX-2, HIF-1α, VEGF-A and PTEN
(Beijing Biosynthesis Biotechnology Co., Ltd.)]. The membranes were
then developed using the ECL plus chemiluminescence detection
system. The band intensities were analyzed by ImageJ software
(Wayne Rasband National Institutes of Health, Bethesda, MD, USA)
and normalized to total Akt or β-actin (Cell Signaling).
Statistical analysis
The descriptive statistics are provided with means ±
SD. A repeated-measure ANOVA test was used to assess dose-dependent
effects of celecoxib on tumor tissue. Data was analyzed using an
ANOVA pairwise comparison method (SNK methods) and the Pearson’s
analysis of correlation method. A P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of celecoxib on H22
hepatoma tumor growth
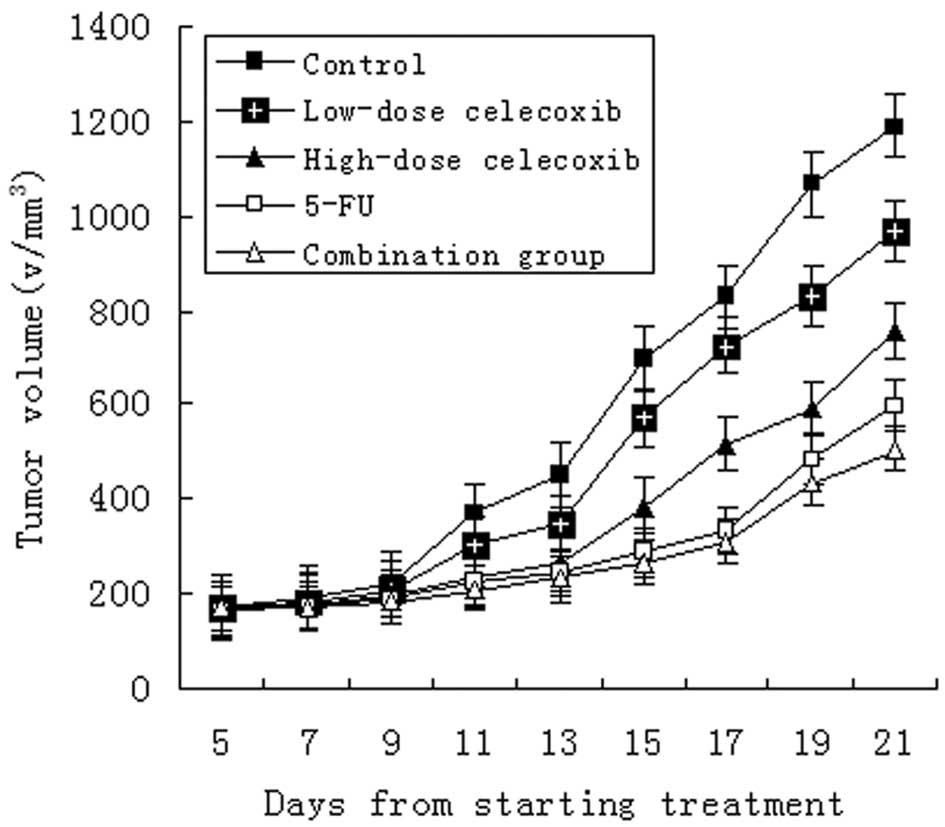
Tumor dimensions increased in all groups. Compared
to controls, treatments with 5-FU (20 mg/kg) alone, either
high-dose (200 mg/kg) or low-dose (50 mg/kg) celecoxib alone, and a
combination of 5-FU (20 mg/kg) and celecoxib (50 mg/kg) were found
to markedly inhibit the tumor growth (Fig. 1). The inhibitory rate was 65.8,
49.3, 37.0 and 79.5%, respectively (Table I, Fig.
1; P<0.05 for each comparison). The inhibitory effect was
stronger in the high-dose celecoxib groups, 5-FU groups and the
combination groups (P<0.01). Although the inhibitory rates of
the high-dose and low-dose celecoxib groups were lower, the mice in
both groups were in good condition and increased in body weight
following the experiment (Table I).
This suggests that celecoxib not only inhibited the growth of
H22 hepatocarcinoma, but reduced the tumor’s consumption
of the body resources.
 | Table IInhibitory effect of celecoxib on
H22 hepatocarcinoma (mean ± SEM, n=10). |
Table I
Inhibitory effect of celecoxib on
H22 hepatocarcinoma (mean ± SEM, n=10).
| Body weight (g) | | |
|---|
|
| | |
|---|
| Group | Before
experiment | After experiment | Tumor weight (g) | IR (%) |
|---|
| Control | 22.78 | 25.56 | 0.73±0.18 | _ |
| Low-dose
celecoxib | 22.94 | 24.61 | 0.46±0.05a | 37.0 |
| High-dose
celecoxib | 23.96 | 25.21 | 0.37±0.04b | 49.3 |
| 5-FU | 23.49 | 23.86 | 0.25±0.06b | 65.8 |
| 5-FU +
celecoxib | 23.68 | 24.59 | 0.15±0.04b | 79.5 |
The levels of PTEN, PI3K, P-Akt, COX-2,
HIF-1α and VEGF-A in serum after treatment with celecoxib
The levels of PI3K, P-Akt, COX-2, HIF-1α and VEGF-A
in the serum of mice treated with celecoxib high- and low-dose,
5-FU, and combination groups were significantly lower than the
tumor-bearing control group. In the treatment groups, the levels of
PTEN in serum were significantly higher than those of the
tumor-bearing control group (Table
II; P<0.05 for each comparison). In addition, celecoxib at
each concentration was significantly different between groups
(P<0.01).
 | Table IIDetermination of PI3K, P-Akt, COX-2,
HIF-1α, VEGF-A and PTEN in the serum of mice after treatment (mean
± SEM, n=10). |
Table II
Determination of PI3K, P-Akt, COX-2,
HIF-1α, VEGF-A and PTEN in the serum of mice after treatment (mean
± SEM, n=10).
| Group | PTEN (ng/ml) | PI3K (ng/ml) | P-Akt (ng/ml) | COX-2 (ng/ml) | HIF-1 (ng/ml) | VEGF-A (PG/ml) |
|---|
| Control | 0.03±0.01 | 1.87±0.10 | 4.57±0.19 | 0.98±0.01 | 4.56±0.25 | 157.4±11.28 |
| Low-dose
celecoxib | 0.37±0.07a | 1.25±0.06a | 3.47±0.09a | 0.92±0.01a | 3.49±0.27a | 123.8±14.15a |
| High-dose
celecoxib | 0.51±0.04a | 0.10±0.04a | 2.52±0.12a | 0.87±0.02a | 2.39±0.18a | 100.0±4.85a |
| 5-FU | 0.82±0.03a | 0.75±0.05a | 1.57±0.10a | 0.77±0.02a | 1.66±0.19a | 69.4±5.55a |
| 5-FU +
celecoxib | 1.51±0.32a | 0.51±0.03a | 0.83±0.03a | 0.61±0.04a | 0.81±0.12a | 50.2±1.40a |
Pathological, morphometric and MVD
analysis of H22 hepatocarcinoma after treatment
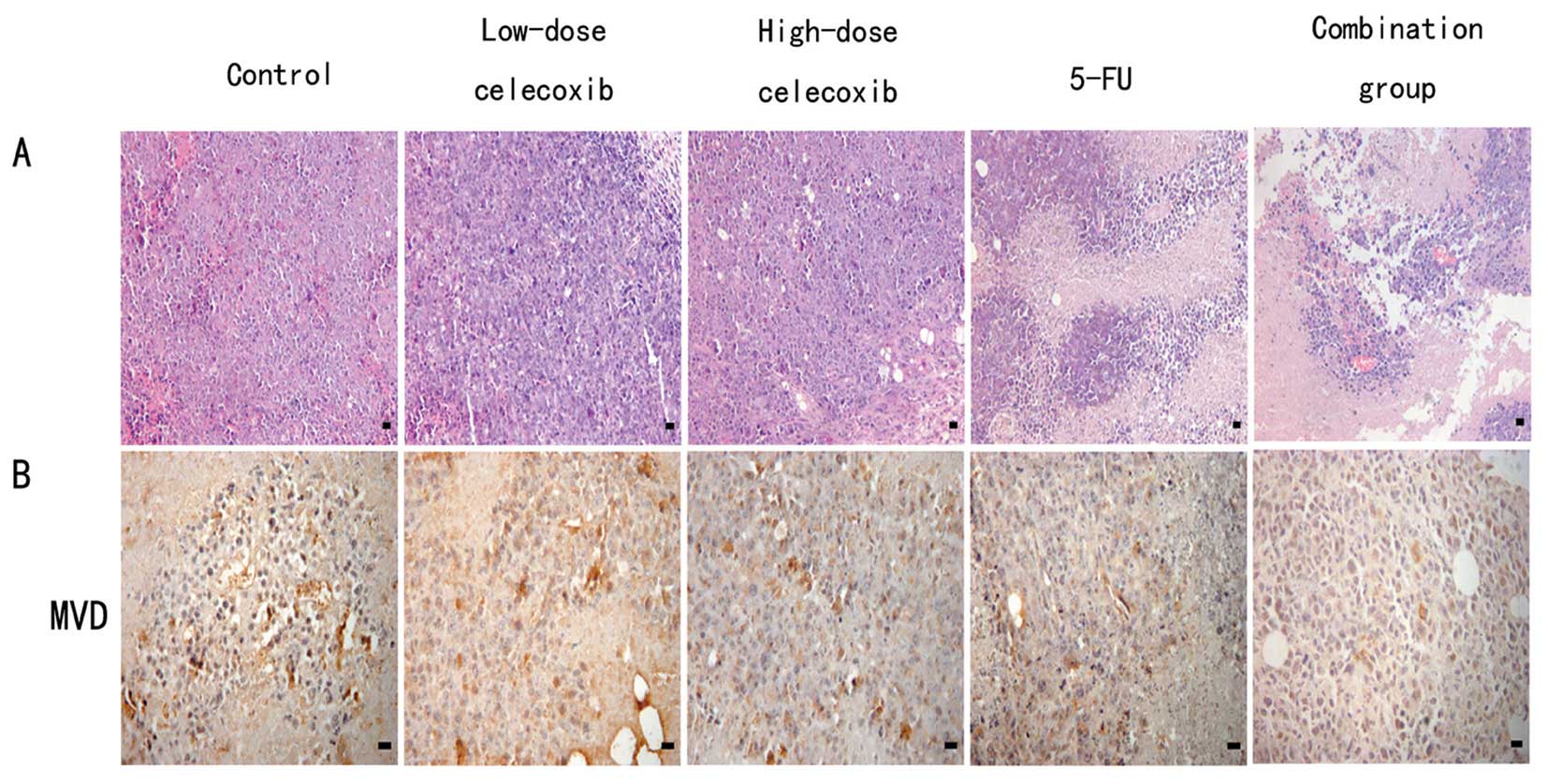
H&E staining showed that H22
hepatocarcinoma cells demonstrated flaky or nested irregular
growth. In the control group, tumor angiogenesis richness, rare
nuclear pyknosis, nuclear karyorrhexis and other morphological
changes of apoptosis were shown. Celecoxib high- and low-dose,
5-FU, and combination groups showed multiple large patchy necrosis
areas. Most of the tumor cells exhibited morphological changes
characteristic of apoptotic processes such as nuclear pyknosis and
karyorrhexis, which were significantly lower in the MVD than the
control groups (Fig. 2A).
Cells positive for CD34 were stained brown.
Microvessel distribution is shown in Fig. 2B. The MVD of the control group,
5-FU, high-dose (200 mg/kg) and low-dose (50 mg/kg) celecoxib, and
combination of 5-FU with celecoxib groups were 10.32±4.13,
3.87±1.63, 5.65±3.96, 7.63±3.12 and 1.68±1.23, respectively. The
5-FU alone, high- and low-dose celecoxib and combination groups all
demonstrated inhibition of MVD in comparison to the control group
(P<0.05 for each comparison), which suggests that celecoxib
inhibits angiogenesis.
Expression of PTEN, PI3K, P-Akt, COX-2,
HIF-1α and VEGF-A in H22 hepatocarcinoma tumors
Based on immunohistochemical staining, PI3K, P-Akt,
COX-2, VEGF-A, PTEN and CD34 were expressed in the cytoplasm or
membrane of tumor cells. HIF-1α was expressed in the nucleus and
cytoplasm of tumor cells. Cells positive for PI3K, P-Akt, COX-2,
HIF-1α, VEGF-A and PTEN were stained brown (Fig. 3). The expression of PI3K, P-Akt,
COX-2, HIF-1 and VEGF-A in the control group was markedly higher
than that in the other treatment groups. The expression of PTEN in
the treatment groups was higher than that in the control group,
especially in the combination, 5-FU, and high-dose celecoxib
groups. Gray scale intensity variants of PI3K, P-Akt, COX-2,
HIF-1α, VEGF-A and PTEN immunoreactivity were evaluated by Leica
Qwin V3 software. Sections were evaluated in each of 5 randomly
selected positive regions at the original magnification ×200.
Fig. 4 indicates an inverse
relationship between the gray scale intensity and the protein
expression. Higher gray scale intensity indicates weaker protein
expression, and lower intensity indicates stronger protein
expression. Treatment with combination group, 5-FU group, and
high-dose and low-dose celecoxib group resulted in a reduction in
PI3K, P-Akt, COX-2, HIF-1α and VEGF-A expression. PI3K, P-Akt,
COX-2, HIF-1α and VEGF-A expression decreased significantly in both
the 5-FU alone and combination groups when compared with the other
treatment groups showing a dose-dependency on high-dose and
low-dose celecoxib. In each comparison, there was a significant
difference (P<0.05). In addition, celecoxib at each
concentration was significantly different between groups
(P<0.01). PI3K and P-Akt, COX-2, HIF-1α, VEGF-A expression were
positively correlated (r=0.965, P<0.01; r=0.965, P<0.01;
r=0.946, P<0.01; r=0.957, P<0.01). P-Akt and COX-2, HIF-1α,
VEGF-A expression were positively correlated (r=0.959, P<0.01;
r=0.958, P<0.01; r=0.963, P<0.01). COX-2 and HIF-1α, VEGF-A
expression were positively correlated (r=0.972, P<0.01; r=0.977,
P<0.01). HIF-1α and VEGF-A expression were positively correlated
(r=0.954, P<0.01). PTEN expression increased significantly in
both the 5-FU alone and combination groups when compared with the
other treatment groups showing a dose-dependency on high-dose and
low-dose celecoxib. In each comparison, there was a significant
difference (P<0.05). In addition, at each of the celecoxib
concentrations there was a significant difference between groups
(P<0.01). PTEN and PI3K, P-Akt, COX-2, HIF-1α, VEGF-A expression
were negatively correlated (r=−0.969, P<0.01; r=−0.961,
P<0.01; r=−0.974, P<0.01; r=−0.951, P<0.01; r=−0.974,
P<0.01).
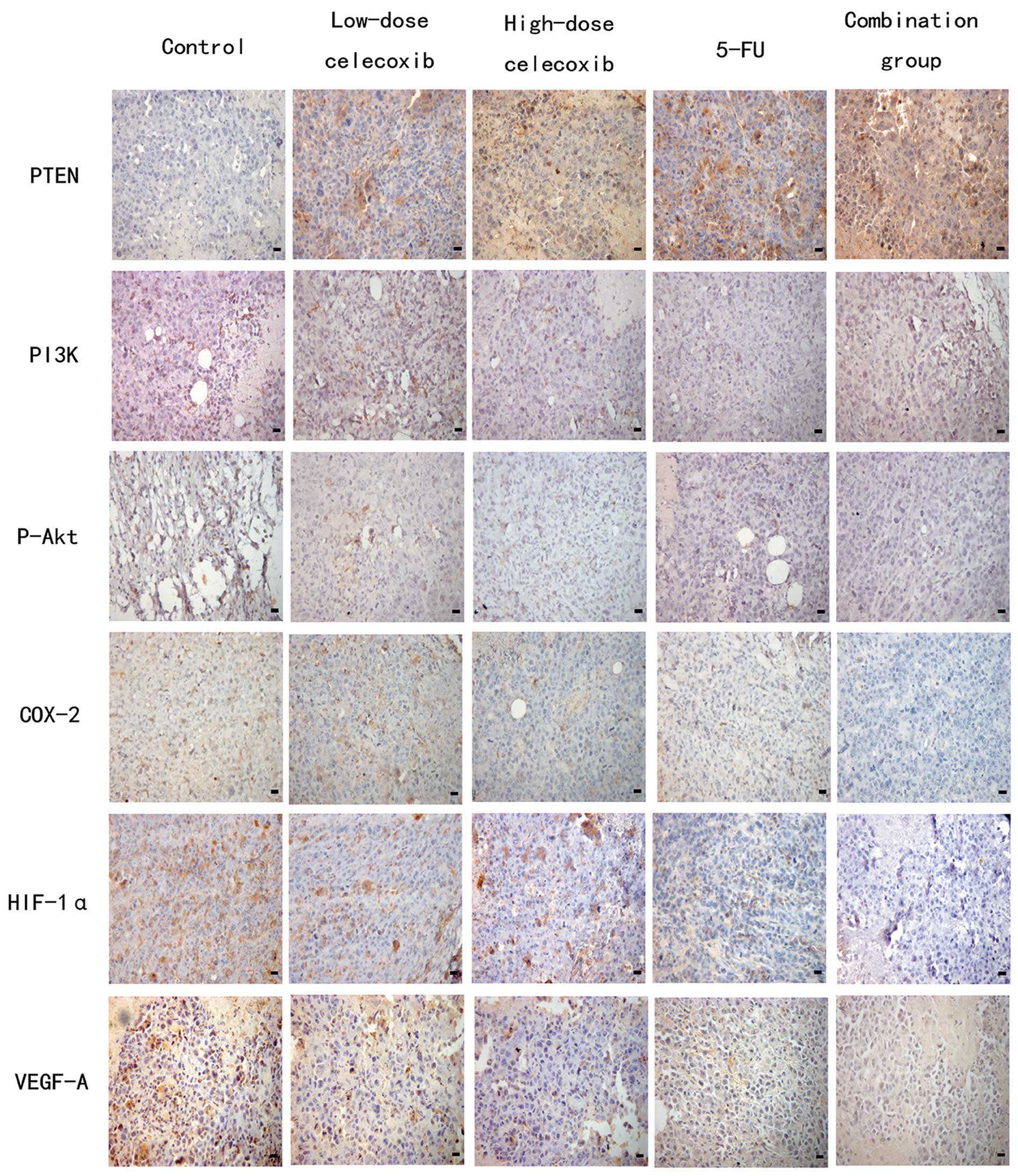 | Figure 3Effects of celecoxib on the expression
of PTEN, PI3K, P-Akt, COX-2, HIF-1α and VEGF-A in H22
hepatocarcinoma tissue were detected by immunohistochemistry.
Original magnification, ×400. PTEN, phosphatase and tensin
homologue deleted from chromosome 10; PI3K, phosphatidylinositol
3-kinase; P-Akt, phospho-Akt; COX-2, cyclooxygenase-2; HIF-1α,
hypoxia-inducible factor-1α; VEGF-A, vascular endothelial growth
factor-A; 5-FU, 5-fluorouracil. |
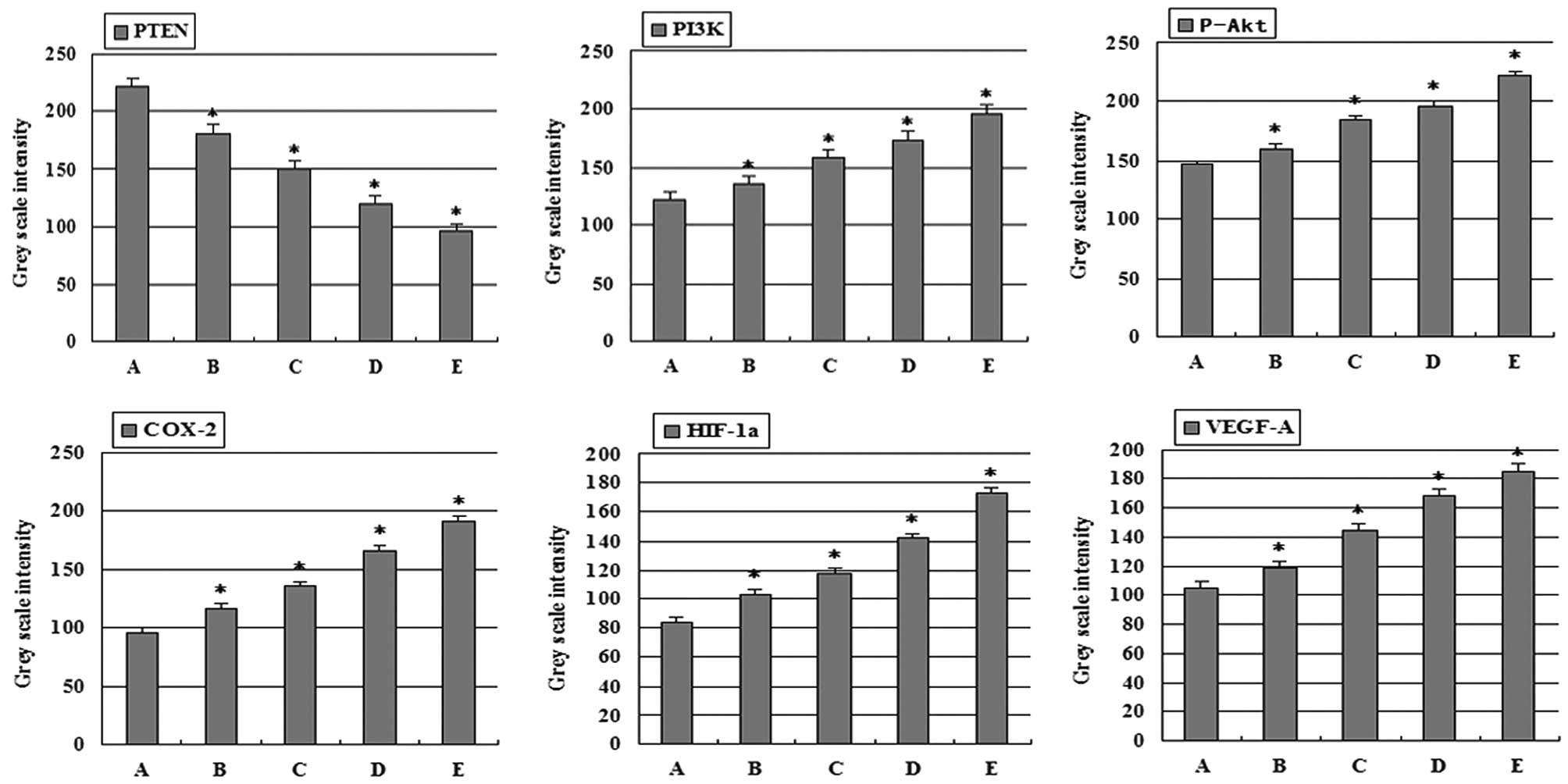 | Figure 4Gray scale intensity variants were
evaluated by Leica Qwin V3 software for PTEN, PI3K, P-Akt, COX-2,
HIF-1α and VEGF-A in H22 hepatocarcinoma tissue.
Sections in each of 5 randomly selected positive regions (original
magnification, ×200). Higher gray scale intensity represents weaker
protein expression, and lower, stronger protein expression.
*P<0.05, significantly different vs. control. (A)
Control group; (B) low-dose celecoxib (50 mg/kg); (C) high-dose
celecoxib (200 mg/kg); (D) 5-FU (20 mg/kg); (E) combination
treatment with 5-FU (20 mg/kg) and celecoxib (50 mg/kg). PTEN,
phosphatase and tensin homologue deleted from chromosome 10; PI3K,
phosphatidylinositol 3-kinase; P-Akt, phospho-Akt; COX-2,
cyclooxygenase-2; HIF-1α, hypoxia-inducible factor-1α; VEGF-A,
vascular endothelial growth factor-A; 5-FU, 5-fluorouracil. |
Effect of celecoxib treatment on PTEN,
PI3K, P-Akt, COX-2, HIF-1α and VEGF-A protein expression as
assessed by western blot analysis
PI3K, COX-2, HIF-1α, VEGF-A and PTEN expression was
normalized to β-actin expression by band intensity. P-Akt protein
expression was normalized to total Akt expression by band
intensity. As shown in Fig. 5,
PI3K, P-Akt, COX-2, HIF-1α and VEGF-A expression was reduced in the
high-dose and low-dose celecoxib, 5-FU and combination groups. PTEN
expression was increased significantly in treatment groups when
compared to the control group. Band intensities were analyzed by
ImageJ software. PI3K, P-Akt, COX-2, HIF-1α and VEGF-A expression
decreased significantly in the H22 hepatocarcinoma
tissue treated with 5-FU alone or with the combination with
celecoxib. This decreased expression showed a dose-dependent trend
in the high-dose and low-dose celecoxib groups. In addition,
celecoxib at each concentration showed a significant difference
between groups (P<0.05). Furthermore, PI3K and P-Akt, COX-2,
HIF-1α, VEGF-A expression were positively correlated (r=0.989,
P<0.05; r=0.978, P<0.01; r=0.975, P<0.05; r=0.993,
P<0.05). P-Akt and COX-2, HIF-1α, VEGF-A expression were
positively correlated (r=0.990, P<0.05; r=0.990, P<0.05;
r=0.983, P<0.05). COX-2 and HIF-1α, VEGF-A expression were
positively correlated (r=0.989, P<0.05; r=0.969, P<0.05).
HIF-1α and VEGF-A expression were positively correlated (r=0.961,
P<0.01). PTEN expression also showed a dose-dependent trend in
the high-dose and low-dose celecoxib groups. In addition, celecoxib
at each concentration was significantly different between groups
(P<0.01). Furthermore, PTEN and PI3K, P-Akt, COX-2, HIF-1α,
VEGF-A expression were negatively correlated (r=−0.996, P<0.01;
r=−0.987, P<0.05; r=−0.977, P<0.01; r=−0.970, P<0.05;
r=−0.993, P<0.05).
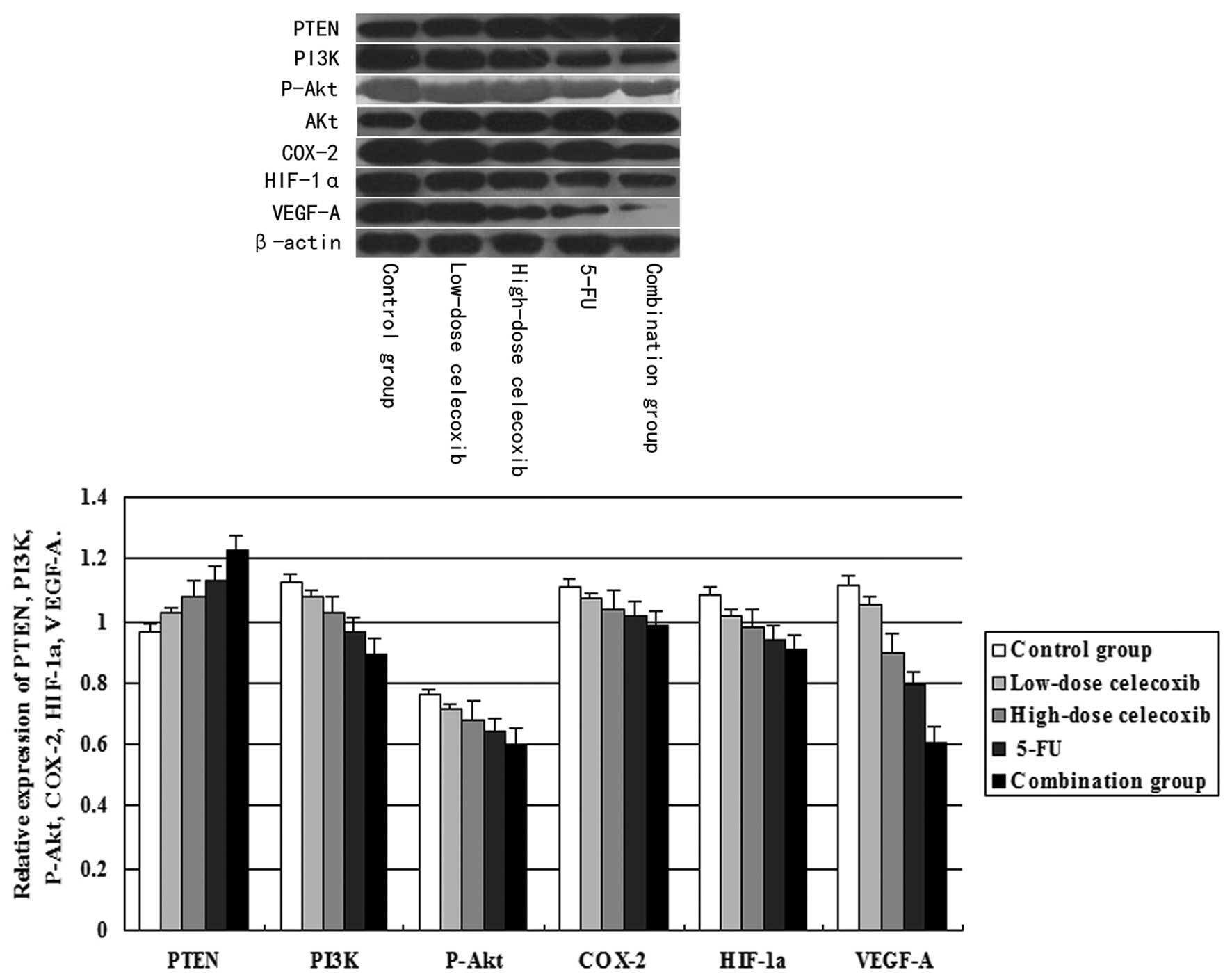 | Figure 5Effects of celecoxib on the expression
of PTEN, PI3K, P-Akt, COX-2, HIF-1α and VEGF-A in H22
hepatocarcinoma tissue were detected by western blot analysis. The
relative expression of PTEN, PI3K, P-Akt, COX-2, HIF-1α and VEGF-A
was analyzed by ImageJ software. Columns in the histograms
represent the mean of 5 separate experiments. In each comparison,
there was a significant difference (P<0.05). PTEN, phosphatase
and tensin homologue deleted from chromosome 10; PI3K,
phosphatidylinositol 3-kinase; P-Akt, phospho-Akt; COX-2,
cyclooxygenase-2; HIF-1α, hypoxia-inducible factor-1α; VEGF-A,
vascular endothelial growth factor-A; 5-FU, 5-fluorouracil. |
Discussion
The liver is a highly vascular organ that depends on
angiogenesis for cellular regeneration. In HCC, angiogenesis relies
on autocrine and paracrine interactions between tumor cells and
vascular endothelial cells (22).
Thus, the development of new anti-angiogenic drugs has become an
important strategy for cancer treatment. In the present study, we
showed that celecoxib-mediated H22 hepatocarcinoma
angiogenesis and tumor growth inhibition in vivo involves
PTEN/PI3K/AKT/HIF-1α signaling pathways. Studies have shown that
HIF-1 expression is necessary for tumor growth in certain tumor
cell lines, such as hepatomas. Therefore, decreased HIF-1α
expression is associated with slower cell growth and tumor
angiogenesis (12,23). Inhibition of COX-2 has been shown to
be a promising antitumor and antiangiogenic strategy in several
types of tumor (24,25). Inhibition of VEGF-A expression also
has a marked effect on tumor growth. Additionally, the interruption
of the PI3K/AKT pathway inhibits tumor growth and tumor
angiogenesis in vivo (26).
In the present study, celecoxib effectively inhibited the
expression of PI3K, P-Akt, COX-2, HIF-1α and VEGF-A. The inhibitory
rates of the 5-FU, high-dose (200 mg/kg) and low-dose (50 mg/kg)
celecoxib, and combination of 5-FU with celecoxib groups were 65.8,
49.3, 37.0 and 79.5%, respectively. Furthermore, we found that
celecoxib enhanced the antitumor effect of 5-FU, which is
consistent with the results of previous studies (27,28).
HIF-1α is one of the most important regulatory
molecules that respond to hypoxia for cell survival and
angiogenesis (29). HIF-1α
activates the transcription of many genes, including COX-2 and VEGF
by binding to the hypoxia response element (HRE) in the COX-2 and
VEGF promoter. HIF-1α, COX-2 and VEGF-A expression is strongly
associated with cancer progression and angiogenesis. To identify
and characterize how celecoxib inhibited the overexpression of
VEGF-A, we cultured low-dose and high-dose celecoxib groups to
analyze the expression of related proteins such as COX-2 and HIF-1α
by ELISA, immunohistochemistry and western blotting. The expression
of HIF-1α, COX-2, and VEGF-A decreased in a celecoxib
dose-dependent manner. Often coupled with the rapid growth of the
tumor cells is the shortage of oxygen and nutrients. During
hypoxia, HIF-1α can activate the expression of downstream signaling
proteins such as VEGF-A and COX-2 and play a key role in tumor
avoidance of the associated adverse effects on cell survival. There
is a very strong correlation between VEGF-A expression and blood
vessel density in many tumor types. In the present study, HIF-1α,
COX-2 and VEGF-A had weak expression in the high-dose and low-dose
celecoxib group. Additionally, tumor tissue in the high-dose
celecoxib group showed decreased MVD reinforcing the theory that
celecoxib effectively inhibited HIF-1α, COX-2 and VEGF-A protein.
Moreover, it follows that the inhibition of HIF-1α, COX-2 and
VEGF-A may play a major role in celecoxib-inhibited angiogenesis.
Thus, these results strongly suggest that celecoxib is a potential
anti-angiogenic agent.
The PI3K/Akt signaling pathway is activated in the
majority of human types of cancer (30). The activation of the PI3K/AKT/mTOR
signaling pathway in endothelial cells promotes their survival when
cultured in vitro (31) and
in the tumor vasculature in vivo (32). It is likely that celecoxib also
inhibits angiogenesis by modulating the PI3K/AKT/HIF-1 pathway. In
the present study, we found that celecoxib downregulated PI3K,
P-Akt and HIF-1α expression in a dose-dependent manner. Thus,
celecoxib may inhibit H22 hepatocarcinoma growth and
angiogenesis through PI3K, P-Akt and HIF-1α expression. Studies
have shown that HIF-1α expression and activity are regulated by
major signal transduction pathways including those involving PI3K
(11,33). Therefore, one possibility that would
account for the decreased levels of HIF-1α protein is the decreased
PI3K/Akt signaling in H22 hepatocarcinoma. However,
studies investigating the role of PI3K signaling in HIF-1α
expression were contradictory based on the cell lines used. PI3K
and Akt activity was observed to be required for HIF-1α expression
in prostate cancer cells (11,34),
while its inhibition in 1c1c7 mouse hepatocytes did not affect
HIF-1α expression (35). Therefore,
further research is needed to ascertain the pathway responsible for
the inhibitory effect of celecoxib on angiogenesis.
PTEN is the most common malignant tumor suppressor
gene. PTEN is a phosphatase that opposes the action of PI3K,
thereby reducing the level of activated (phosphorylated) AKT.
PTEN-deficient endothelial cells display increased angiogenesis and
tumorigenesis (36). In the present
study, celecoxib effectively inhibited the expression of PI3K and
P-Akt while it increased the expression of PTEN. It is possible
that the induction of PTEN may play a major role in
celecoxib-inhibited angiogenesis. In our study, tumor-bearing mice
treated with celecoxib had slight to mild side-effects. This was
possibly due to the short treatment duration. Celecoxib is indeed
safer than most other chemotherapeutic agents; however, the dose
for cancer treatment remains to be optimized. In addition, it would
be beneficial to investigate with a broader scope by conducting
further studies in a variety of tumor models.
In conclusion, we demonstrated that celecoxib can
inhibit tumor angiogenesis by reducing the production of PI3K,
P-Akt, COX-2, HIF-1α and VEGF-A, and increasing the production of
PTEN in a dose-dependent manner. This finding provides an
explanation as to why celecoxib inhibits the tumor angiogenesis of
H22 hepatocarcinoma in vivo. We also found that
celecoxib synergistically enhanced the antitumor effect of 5-FU.
Collectively, these data suggest that celecoxib inhibited
H22 hepatocarcinoma angiogenesis and tumor growth in
vivo involves PTEN/PI3K/AKT/HIF-1 signaling pathways. This
yields potential insight into the mechanism of celecoxib-inhibited
angiogenesis. Our study has important clinical implications and may
potentially lead to therapeutic treatment options for HCC and other
types of cancer.
Acknowledgements
This study was supported by funding from the
National Natural Science Foundation of China (nos. 81073102 and
30873408).
References
|
1
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar
|
|
2
|
Nordenstedt H, White DL and El-Serag HB:
The changing pattern of epidemiology in hepatocellular carcinoma.
Dig Liver Dis. 42(Suppl 3): S206–S214. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vinogradova Y, Hippisley-Cox J, Coupland C
and Logan RF: Risk of colorectal cancer in patients prescribed
statins, nonsteroidal anti-inflammatory drugs, and cyclooxygenase-2
inhibitors: nested case-control study. Gastroenterology.
133:393–402. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cui W, Yu CH and Hu KQ: In vitro and in
vivo effects and mechanisms of celecoxib-induced growth inhibition
of human hepatocellular carcinoma cells. Clin Cancer Res.
11:8213–8221. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim HS, Youm HR, Lee JS, Min KW, Chung JH
and Park CS: Correlation between cyclooxygenase-2 and tumor
angiogenesis in non-small cell lung cancer. Lung Cancer.
42:163–170. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shi H, Xu JM, Hu NZ and Xie HJ: Prognostic
significance of expression of cyclooxygenase-2 and vascular
endothelial growth factor in human gastric carcinoma. World J
Gastroenterol. 9:1421–1426. 2003.PubMed/NCBI
|
|
7
|
Yasumaru M, Tsuji S, Tsujii M, et al:
Inhibition of angiotensin II activity enhanced the antitumor effect
of cyclooxygenase-2 inhibitors via insulin-like growth factor I
receptor pathway. Cancer Res. 63:6726–6734. 2003.PubMed/NCBI
|
|
8
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar
|
|
9
|
Zundel W, Schindler C, Haas-Kogan D, et
al: Loss of PTEN facilitates HIF-1-mediated gene expression.
Genes Dev. 14:391–396. 2000.
|
|
10
|
Hudson CC, Liu M, Chiang GG, et al:
Regulation of hypoxia-inducible factor 1α expression and function
by the mammalian target of rapamycin. Mol Cell Biol. 22:7004–7014.
2002.
|
|
11
|
Zhong H, Chiles K, Feldser D, et al:
Modulation of hypoxia-inducible factor 1α expression by the
epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP
pathway in human prostate cancer cells: implications for tumor
angiogenesis and therapeutics. Cancer Res. 60:1541–1545. 2000.
|
|
12
|
Carbajo-Pescador S, Ordoñez R, Benet M,
Jover R, García-Palomo A, Mauriz JL and González-Gallego J:
Inhibition of VEGF expression through blockade of Hif1α and STAT3
signalling mediates the anti-angiogenic effect of melatonin in
HepG2 liver cancer cells. Br J Cancer. 109:83–91. 2013.
|
|
13
|
De Francesco EM, Lappano R, Santolla MF,
Marsico S, Caruso A and Maggiolini M: HIF-1α/GPER signaling
mediates the expression of VEGF induced by hypoxia in breast cancer
associated fibroblasts (CAFs). Breast Cancer Res. 15:R642013.
|
|
14
|
Lawlor MA and Alessi DR: PKB/Akt: a key
mediator of cell proliferation, survival and insulin responses? J
Cell Sci. 114:2903–2910. 2001.PubMed/NCBI
|
|
15
|
Jiang BH, Zheng JZ, Aoki M and Vogt PK:
Phosphatidylinositol 3-kinase signaling mediates angiogenesis and
expression of vascular endothelial growth factor in endothelial
cells. Proc Natl Acad Sci USA. 97:1749–1753. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Osaki M, Oshimura M and Ito H: PI3K-Akt
pathway: its functions and alterations in human cancer. Apoptosis.
9:667–676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kulp SK, Yang YT, Hung CC, et al:
3-Phosphoinositide-dependent protein kinase-1/Akt signaling
represents a major cyclooxygenase-2-independent target for
celecoxib in prostate cancer cells. Cancer Res. 64:1444–1451. 2004.
View Article : Google Scholar
|
|
18
|
Basu GD, Pathangey LB, Tinder TL, Lagioia
M, Gendler SJ and Mukherjee P: Cyclooxygenase-2 inhibitor induces
apoptosis in breast cancer cells in an in vivo model of spontaneous
metastatic breast cancer. Mol Cancer Res. 2:632–642.
2004.PubMed/NCBI
|
|
19
|
Leahy KM, Ornberg RL, Wang Y, Zweifel BS,
Koki AT and Masferrer JL: Cyclooxygenase-2 inhibition by celecoxib
reduces proliferation and induces apoptosis in angiogenic
endothelial cells in vivo. Cancer Res. 62:625–631. 2002.PubMed/NCBI
|
|
20
|
Ragel BT, Jensen RL, Gillespie DL,
Prescott SM and Couldwell WT: Celecoxib inhibits meningioma tumor
growth in a mouse xenograft model. Cancer. 109:588–597. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Weidner N, Carroll PR, Flax J, Blumenfeld
W and Folkman J: Tumor angiogenesis correlates with metastasis in
invasive prostate carcinoma. Am J Pathol. 143:401–409.
1993.PubMed/NCBI
|
|
22
|
Whittaker S, Marais R and Zhu AX: The role
of signaling pathways in the development and treatment of
hepatocellular carcinoma. Oncogene. 29:4989–5005. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang FZ, Peng-Jiao, Yang NN, et al:
PF-04691502 triggers cell cycle arrest, apoptosis and inhibits the
angiogenesis in hepatocellular carcinoma cells. Toxicol Lett.
220:150–156. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xin X, Majumder M, Girish GV, Mohindra V,
Maruyama T and Lala PK: Targeting COX-2 and EP4 to control tumor
growth, angiogenesis, lymphangiogenesis and metastasis to the lungs
and lymph nodes in a breast cancer model. Lab Invest. 92:1115–1128.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma JX, Sun YL, Wang YQ, Wu HY, Jin J and
Yu XF: Triptolide induces apoptosis and inhibits the growth and
angiogenesis of human pancreatic cancer cells by downregulating
COX-2 and VEGF. Oncol Res. 20:359–368. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fang J, Zhou Q, Liu LZ, Xia C, Hu X, Shi X
and Jiang BH: Apigenin inhibits tumor angiogenesis through
decreasing HIF-1α and VEGF expression. Carcinogenesis. 28:858–864.
2007.PubMed/NCBI
|
|
27
|
Bassiouny AR, Zaky A and Neenaa HM:
Synergistic effect of celecoxib on 5-fluorouracil-induced apoptosis
in hepatocellular carcinoma patients. Ann Hepatol. 9:410–418.
2010.PubMed/NCBI
|
|
28
|
Chow LW, Tung SY, Ng TY, et al: Concurrent
celecoxib with 5-fluorouracil/epirubicin/cyclophosphamide followed
by docetaxel for stages II – III invasive breast cancer: the
OOTR-N001 study. Expert Opin Investig Drugs. 22:299–307.
2013.PubMed/NCBI
|
|
29
|
Harris AL: Hypoxia - a key regulatory
factor in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yuan TL and Cantley LC: PI3K pathway
alterations in cancer: variations on a theme. Oncogene.
27:5497–5510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Olsson AK, Dimberg A, Kreuger J and
Claesson-Welsh L: VEGF receptor signalling? in control of vascular
function. Nat Rev Mol Cell Biol. 7:359–371. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Benjamin LE and Keshet E: Conditional
switching of vascular endothelial growth factor (VEGF) expression
in tumors: induction of endothelial cell shedding and regression of
hemangioblastoma-like vessels by VEGF withdrawal. Proc Natl Acad
Sci USA. 94:8761–8766. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jiang BH, Jiang G, Zheng JZ, Lu Z, Hunter
T and Vogt PK: Phosphatidylinositol 3-kinase signaling controls
levels of hypoxia-inducible factor 1. Cell Growth Differ.
12:363–369. 2001.PubMed/NCBI
|
|
34
|
Yin F, Giuliano AE, Law RE and Van Herle
AJ: Apigenin inhibits growth and induces G2/M arrest by modulating
cyclin-CDK regulators and ERK MAP kinase activation in breast
carcinoma cells. Anticancer Res. 21:413–420. 2001.PubMed/NCBI
|
|
35
|
Arsham AM, Plas DR, Thompson CB and Simon
MC: Phosphatidylinositol 3-kinase/Akt signaling is neither required
for hypoxic stabilization of HIF-1α nor sufficient for
HIF-1-dependent target gene transcription. J Biol Chem.
277:15162–15170. 2002.
|
|
36
|
Hamada K, Sasaki T, Koni PA, et al: The
PTEN/PI3K pathway governs normal vascular development and tumor
angiogenesis. Genes Dev. 19:2054–2065. 2005. View Article : Google Scholar : PubMed/NCBI
|