Introduction
Ewing sarcoma is an aggressive primary bone cancer
with a characteristic age peak in adolescents and young adults.
State of the art treatment consists of multiagent chemotherapy and
regional tumor control by surgery and/or radiotherapy and cures
>70% of patients with localized disease and even with primary
pulmonary metastases (1). However,
the disease remains fatal in most patients with tumor dissemination
to bone and/or bone marrow (2). The
patients often show marked, even complete response to chemotherapy,
but systemic relapse almost inevitably follows (3). This pattern of relapse is suggestive
of a model of tumor growth in which rare residual cells that have
survived the intensive chemotherapy have maintained the capacity to
reinitiate the tumor. Tumorigenic potential of individual cells is
a key consideration for the design of novel immunological (4–6) and
molecularly targeted (7)
therapeutic strategies, which must target all cells contributing to
the disease to be effective. Over standard monolayer cultures,
in vitro tumor models that reflect the three-dimensional
structure of (micro)metastatic tumor growth (8,9) and
the capacity of tumor cell subsets to reinitiate the disease may be
more adequate to predict the clinical activity of novel targeting
strategies. In many malignancies, sphere formation has been
proposed to enrich for cells with stem cell-like properties
(10,11), particularly when cells were cultured
under serum-free conditions aiming to prevent cellular
differentiation (12). Indeed,
tumor spheres in Ewing sarcoma have been found to recapitulate the
histopathology of primary tumors and to activate signaling pathways
associated with in vivo tumor growth and survival (13). One study further indicated a higher
tumor-initiation capacity of sphere-cultured Ewing sarcoma cells
(14). These studies have been
limited to established tumor cell lines or cultures that have
undergone several previous passages as monolayers. Here, we
investigated the phenotypes and functional stem cell
characteristics of Ewing sarcoma spheres generated under serum-free
culture conditions from established cell lines as well as from
low-passage cell cultures newly established from tumor
biopsies.
Materials and methods
Cell lines
The Ewing sarcoma cell lines VH-64 after multiple
and after only 3 in vitro passages following isolation from
a malignant pleural effusion (VH-64-EP), and WE-68 cells were gifts
from Frans van Valen’s laboratory at the Institute of Experimental
Orthopedics of University of Muenster, Germany. TC-71 was purchased
from DSMZ (Braunschweig, Germany). Both cell lines were
characterized by the EuroBoNeT consortium (15). A-4573 and TC-32 were from the cell
line bank at Children’s Hospital Los Angeles. The identity of the
cell lines was confirmed by short tandem repeat (STR) profiling.
For standard adherent growth, tumor cells were cultured in
collagen-coated 25 cm2 tissue culture flasks (VH-64) or
in uncoated flasks (all others) in RPMI-1640 medium (Invitrogen,
Darmstadt, Germany), supplemented with 10% heat-inactivated fetal
calf serum (FCS; Thermo Fisher, Bonn, Germany) and 2 mM L-glutamine
and maintained at 37°C and 5% CO2. To generate primary
Ewing sarcoma cell cultures, biopsy material from metastatic
relapse tumors in four pediatric and young adult patients was
dissected into 1–2 mm fragments, incubated in trypsin (0.05%)/EDTA
(0.02%) solution (PAA, Cölbe, Germany), and passed through a cell
strainer. In three patients, single-cell suspensions were cultured
on collagen-coated plates, and adherent cells were re-established
in secondary culture as either spheres or monolayers. In one
patient, tumor cells obtained from a relapse biopsy were also
directly placed in sphere culture.
Sphere culture and analysis
Tumor cells from monolayer cultures were seeded at
2,000 cells/well (1 cell/μl, cell lines) and/or 10,000 cells/well
(5 cells/μl, primary cell cultures) in ultra-low attachment 6-well
plates (Costar, Corning, NY, USA). The serum-free sphere culture
medium was adjusted from a published report (12) and consisted of DMEM/F12 (1:1)
supplemented with 4% B27 (reagents from Invitrogen), 20 ng/ml
recombinant human epidermal growth factor (rhEGF; Strathmann
Biotech, Hamburg, Germany), 20 ng/ml leukaemia inhibitory factor
(LIF; 20 ng/ml), and 10 IE/ml (5 μg/ml) heparin (Roche, Mannheim,
Germany). rhEGF was added once on day 4 or 5. Maximum perpendicular
sphere diameters were quantified with an inverted microscope using
an eyepiece reticle with scales (Carl Zeiss, Goettingen, Germany).
Spheres of various diameters were resuspended, and total numbers of
viable cells were quantified by microscopy and trypan blue
exclusion. Simple linear regression analysis was performed to
establish the relationship between sphere diameters and cell
counts.
Flow cytometry
Ewing sarcoma cells were stained with
fluorescein-conjugated mAbs directed against CD117 (clone 104D2),
CD99 (clone TÜ12), (BD Pharmingen, Heidelberg, Germany), CD133
(clone AC133; Miltenyi Biotec, Bergisch Gladbach, Germany), and
CD57 (clone NC1; Beckman Coulter, Krefeld, Germany). For each
sample, 20,000 cells were analyzed with FACSCalibur and BD
CellQuest software or with FACSCanto and FACSDiva software.
Relative fluorescence intensities (RFI) were calculated by dividing
median fluorescence intensities of mAb-stained cells by those
obtained with isotype antibodies or in the absence of antibody.
Side population (SP) cell assay
Ewing sarcoma cells were resuspended from either
monolayer or sphere cultures in culture medium. Viable cells
(0.2–0.3×106) were incubated with the fluorescent dye
Hoechst 33342 at a concentration of 2 μg/ml at 37°C and 5%
CO2 for 90 min. Verapamil (50 μM) (Sigma-Aldrich,
Munich, Germany) was added to parallel samples to prevent Hoechst
exclusion from SP. After staining, the cells were pelleted at 4°C,
and the pellets were resuspended in ice-cold Hank’s balanced salt
solution (HBSS) with 2% FCS, 10 mM HEPES and 2 μg/ml propidium
iodide (PI). For each sample, 15,000–25,000 cells were analyzed
using a BD FACSAria II and FACSDiva Software. Hoechst 33342 was
excited at 375 nm, and fluorescence emission was detected using
450DF20 (blue) and LP670 (far-red) filters. Non-viable cells were
excluded on the basis of PI uptake.
Mouse model
Mouse experiments were approved by the animal care
committee of the local government (Bezirksregierung Muenster, Az.
87–51.04.2010.A117). Ewing sarcoma cells growing as spheres in
serum-free medium and cells growing as monolayers under standard
growth conditions were resuspended using cell dissociation solution
(0.04 M Tris-HCl, 1 mM EDTA, 0.15 M NaCl, pH 7.4). Equal numbers of
cells from each cell preparation were injected subcutaneously into
the right and left flank, respectively, of eight to 12-week old NOD
scid γ (NSG) mice (Charles River Laboratories, Sulzfeld, Germany),
and tumor growth was monitored twice a week by caliper
quantification of diameters.
Statistical analysis
Data were analyzed descriptively using
box-and-whisker plots. The box corresponds to the 25th/75th
percentile. Whiskers were drawn from the ends of the box to the 5th
and 95th percentile of data values. The Mann-Whitney U test was
used to compare mean values in Fig.
2. P<0.05 was considered to indicate a statistically
significant difference. Statistical analyses were performed using
PASW statistics 18 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Ewing sarcoma cells grow as spheres when
cultured under anchorage-independent serum-free conditions
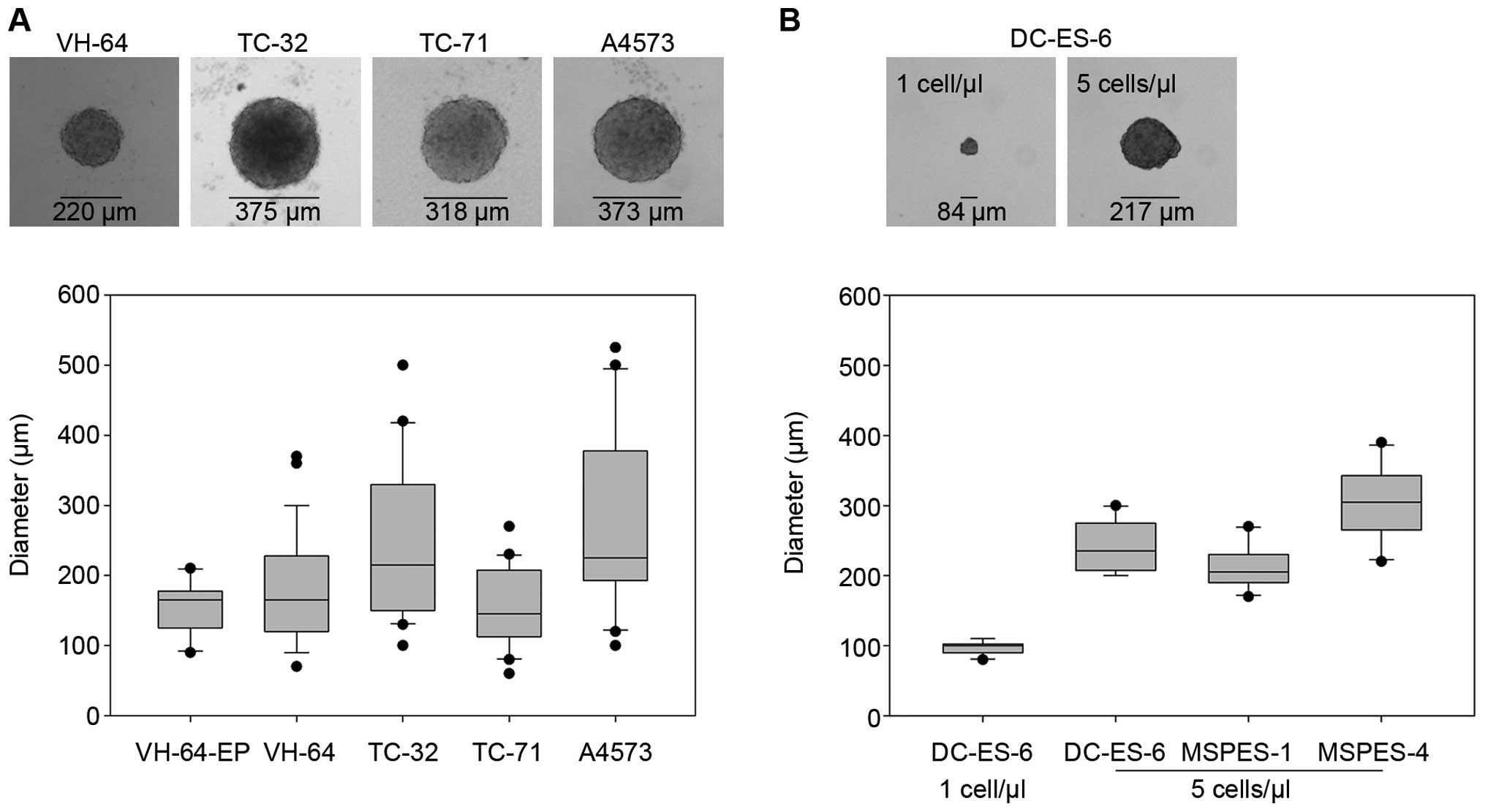
Ewing sarcoma cells from five established cell lines
were plated at 1 cell/μl in serum-free medium with minimal
supplements. Macroscopically visible spheres, corresponding to
>120 μm diameters, reliably appeared within 8–9 days in four of
the cell lines (VH-64, TC-32, TC-71 and A4573) (Fig. 1A), while WE-68 cells failed to form
spheres in four individual experiments. Median sphere diameters
varied between individual cell lines. Early passages of VH-64 cells
(VH-64-EP) seeded at 1 cell/μl medium grew as spheres with
comparable median diameters to the established cell line (VH-64).
To establish the capacity of early tumor cell cultures to form
spheres under serum-free conditions, we initiated parallel
monolayer and anchorage-independent sphere cultures of tumor cells
from biopsy material. Tumor biopsies were obtained from patients
with disseminated Ewing sarcomas at either primary diagnosis or at
relapse (Table I). The identity of
the cultures was confirmed by the presence of the t(11;22)
translocation by RT-PCR analysis and by homogeneous expression of
CD99 by flow cytometry. Re-establishment of tumor cells from one
initial monolayer passage generated small, microscopically visible
spheres (Fig. 1B). Seeding of tumor
cells at higher densities of 5 cells/μl resulted in larger spheres
with comparable sizes to those derived from established cell lines
(Fig. 1B).
 | Table IClinical and molecular
characteristics of Ewing sarcoma patients whose biopsies were used
to generate primary cell cultures. |
Table I
Clinical and molecular
characteristics of Ewing sarcoma patients whose biopsies were used
to generate primary cell cultures.
| Patient | Age at first
diagnosis (years) | Primary tumor
localization | Current biopsy | Translocation | Clinical
outcome |
|---|
| MSPES-1 | 15 | Localized,
femur | Disseminated
relapse (soft tissue metastasis) | t(11;22) | DOD 36 mo after
diagnosis |
| MSPES-4 | 20 | Pelvis + pulmonary
metastases | Disseminated
relapse (liver metastasis) | t(11;22) | PD 22 mo after
diagnosis |
| DC-ES-6 | 9 | Pelvis + bone
metastases | Progressive disease
(vertebral column) | t(11;22) | DOD 9 mo after
diagnosis |
| MSPES-5 | 11 | Multifocal bone +
pulmonary metastases | Pulmonary
relapse | t(21;22) | PD 16 mo after
diagnosis |
Ewing sarcoma spheres and monolayers
share a similar phenotype
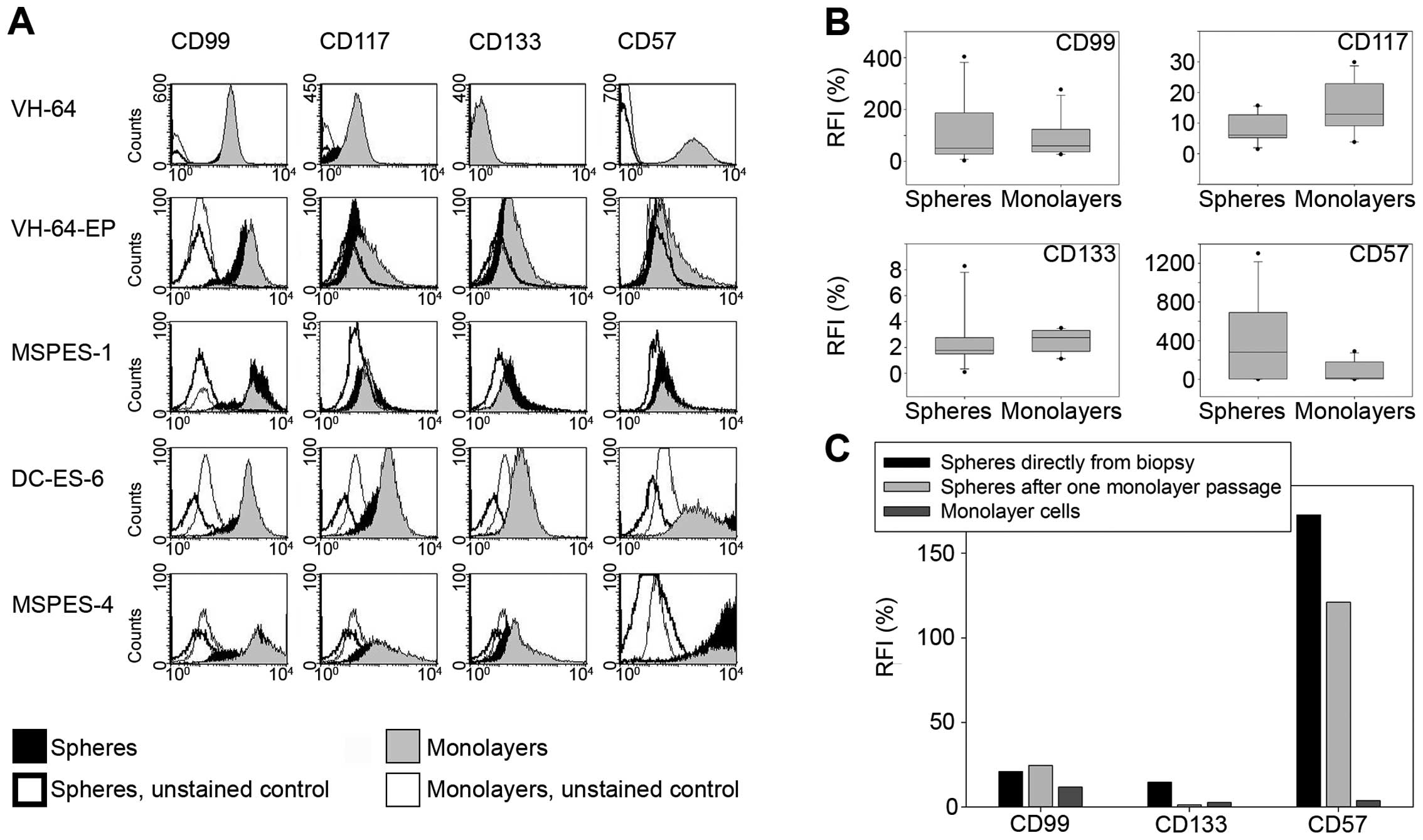
To investigate whether monolayer vs. serum-free
sphere culture conditions affect the cellular phenotype of Ewing
sarcoma cells, we compared surface expression of various cellular
and differentiation markers, including markers previously
associated with functional stem cell characteristics (Fig. 2A and B). The MIC2 gene product CD99,
which is used as a diagnostic marker of Ewing sarcoma, was strongly
expressed by all cell lines, and expression was unaffected by the
type of cell culture. Moreover, the tyrosine kinase CD117 (c-KIT),
which was found to have a role in survival and metastasis of Ewing
sarcoma (16), did not vary between
monolayers and spheres. CD133 has been proposed as a marker of
tumor-initiating cells (17). In
our system, CD133 expression did not vary between spheres and
monolayers. CD57 (HNK-1) is a marker for neural crest stem cells
and was previously associated with sphere growth and tumorigenicity
in Ewing sarcoma (14). Cell
surface density of CD57 was highly variable among spheres but
median expression did not significantly differ from monolayer
cultures. To exclude that primary passaging as monolayer cultures
on collagen-coated plates had affected the characteristics of Ewing
sarcoma spheres, we initiated spheres directly from fresh tumor
dissociates of a relapse biopsy in an additional patient (MSPES-5)
and compared the phenotype to MSPES-5 spheres generated via one
monolayer passage. While CD57 expression in this cell line was
substantially higher on spheres compared to monolayers, no
difference was observed between the two types of spheres (Fig. 2C). Thus, a single monolayer passage
in serum-containing medium did not affect the phenotype of
subsequent sphere cultures compared to spheres directly initiated
from biopsy material. Taken together, phenotyping did not reveal
any consistent and significant differences between spheres and
monolayers.
Sphere cultures from Ewing sarcoma cells
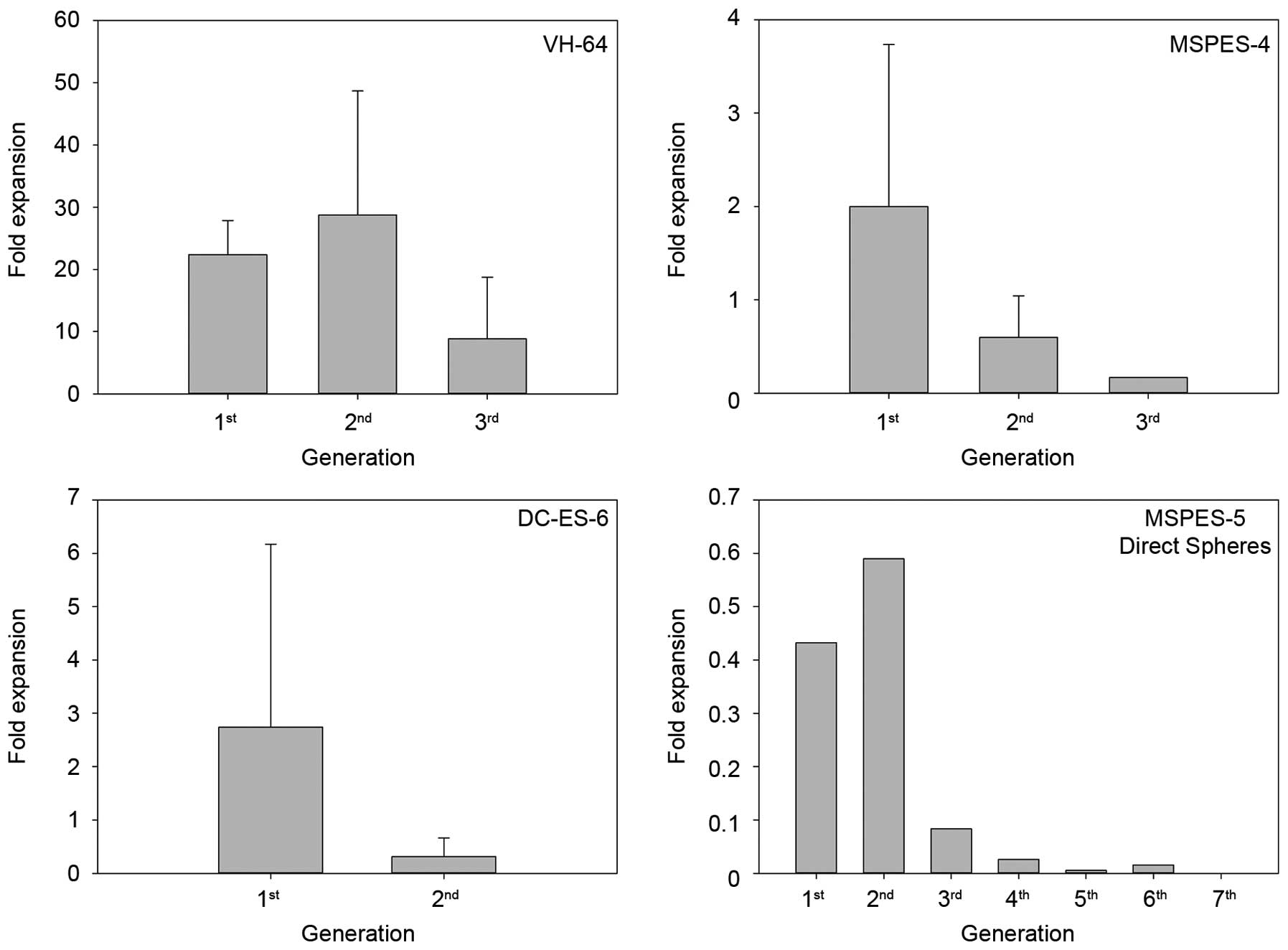
have limited ability to self-renew in vitro
In glioblastoma and other solid tumors, sphere
growth in serum-free medium was associated with enhanced
self-renewal (18,19). Upon disintegration and secondary
replating of VH-64 spheres, single cells continued to form spheres
in serum-free culture (Fig. 3).
However, in contrast to the established cell line, primary tumor
cell cultures could not be maintained as spheres for more than 2–3
passages, while parallel monolayer cultures were successfully
established and passaged multiple times. Cells obtained directly
from fresh tumor dissociates (MSPES-5) efficiently formed initial
spheres, but subsequent replating of sphere-derived cells resulted
in a progressive loss of viable cells, and sphere cultures could
not be maintained. Thus, Ewing sarcoma cells are unable to reliably
and continuously form secondary spheres under growth-constraining
conditions, arguing against the presence of self-renewing primitive
cells in these cultures.
Sphere cultures of Ewing sarcoma cells
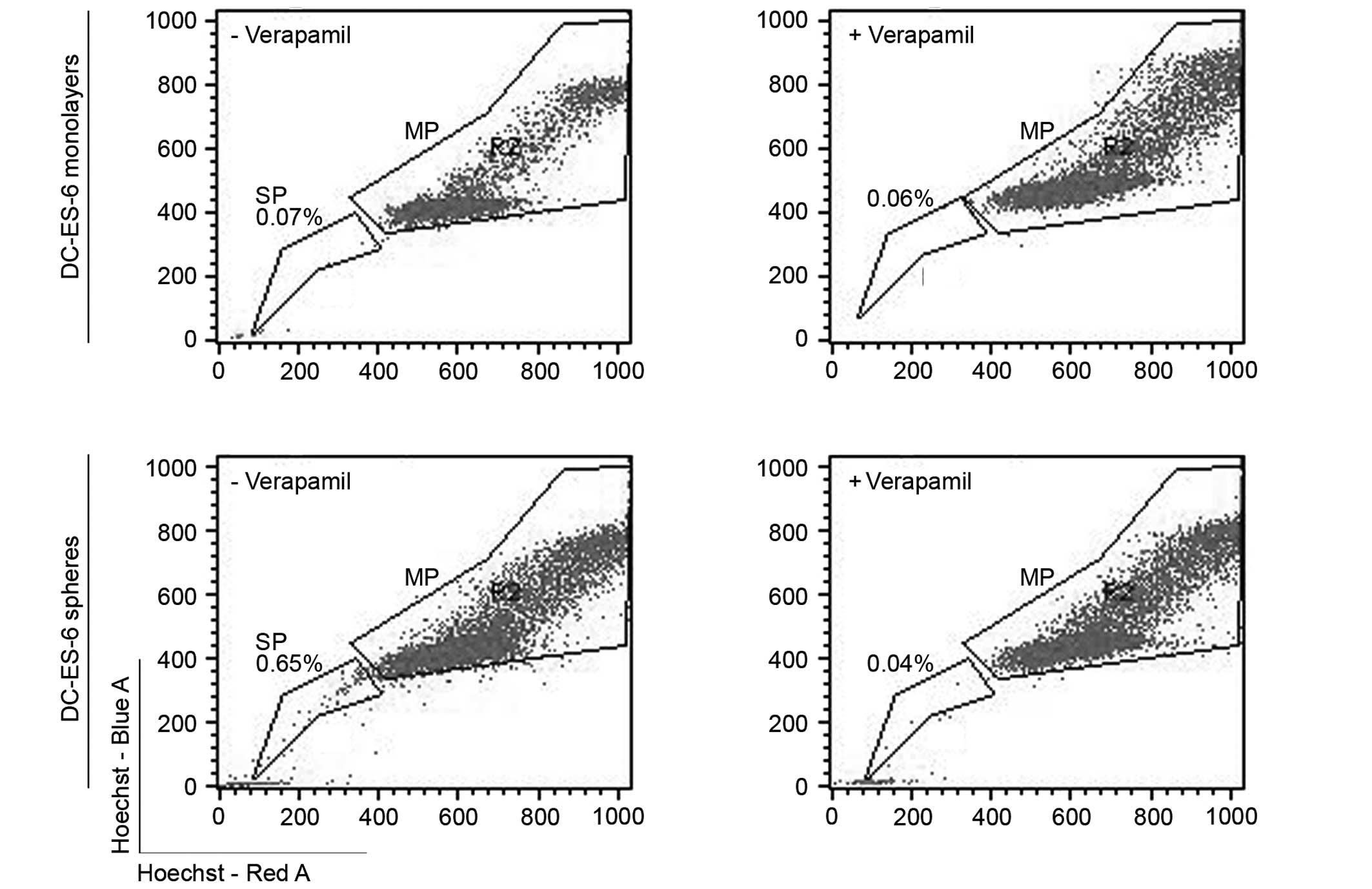
contain variable proportions of SPs
To further address the functional stem cell
characteristics of Ewing sarcoma spheres, we compared the capacity
of sphere- vs. monolayer-cultured cells to enrich for SP cells. The
SP assay relies on the high drug efflux activity of a subset of
cells with supposed tumor-propagating activity and multi-drug
chemoresistance, which is identified by exclusion of the
fluorescent dye Hoechst 33342 (20). To investigate whether sphere culture
enriches for cells with these properties, we quantified the SP in
resuspended monolayer and sphere cultures of VH-64 cells and in two
of the primary Ewing sarcoma cell cultures (Fig. 4, Table
II). Results largely varied between independent experiments
even under identical culture conditions. In VH-64 cells, SPs were
detected in 4/4 sphere cultures and 2/4 monolayer cultures. Whereas
all MSPES-4 cultures lacked SPs, DC-ES-6 sphere cultures but not
monolayers reproducibly had SPs. We conclude that SPs are not a
reliable characteristic of Ewing sarcoma cell cultures irrespective
of in vitro culture conditions.
 | Table IIProportions of SP among VH-64,
DC-ES-6 and MSPES-4 cells cultured for 8 days as spheres or
monolayers, determined by Hoechst 33342 dye exclusion and gating on
propidium iodide negative cells; addition of verapamil prevents
Hoechst exclusion from SP. |
Table II
Proportions of SP among VH-64,
DC-ES-6 and MSPES-4 cells cultured for 8 days as spheres or
monolayers, determined by Hoechst 33342 dye exclusion and gating on
propidium iodide negative cells; addition of verapamil prevents
Hoechst exclusion from SP.
| Monolayers | Spheres |
|---|
|
|
|
|---|
| Cell line | − Verapamil
(%) | + Verapamil
(%) | − Verapamil
(%) | + Verapamil
(%) |
|---|
| VH-64 | 0.23 | 0.04 | 0.16 | 0.03 |
| 0.39 | 0.70 | 0.17 | 0.00 |
| 0.19 | 0.01 | 0.52 | 0.01 |
| 0.06 | 0.02 | 1.46 | 0.01 |
| DC-ES-6 | 0.01 | 0.01 | 0.31 | 0.15 |
| 0.07 | 0.06 | 0.65 | 0.04 |
| MSPES-4 | 0.03 | 0.08 | 0.27 | 0.30 |
Sphere culture of Ewing sarcoma cells
does not enhance their in vivo tumorigenicity
To directly compare the tumorigenicity of Ewing
sarcoma cells, we transplanted increasing numbers of VH-64, MSPES-4
and DC-ES-6 cells derived from sphere vs. standard monolayer
cultures subcutaneously into the left vs. right flanks of
immunodeficient NSG mice. Transplantation of 5×104
MSPES-4 tumor cells derived from monolayer culture formed a solid
tumor in one of four mice after 10 weeks, whereas analogous numbers
of sphere-cultured MSPES-4 cells failed to induce tumors (Table III). Injection of 5×104
tumor cells from VH-64 and DC-ES-6 did not result in subcutaneous
tumor growth regardless of the culture conditions. Higher numbers
of 2.5×105 tumor cells established solid tumors from all
three cell lines/cultures in a proportion of transplanted animals
(Table III). No consistent
differences in the capacity to initiate tumors were observed
between monolayers and sphere-cultured cells throughout the three
cell lines. These data strongly argue against a higher
tumorigenicity of sphere-cultured cells.
 | Table IIIEwing sarcoma cells derived from
sphere culture do not exhibit higher in vivo tumorigenicity
than monolayer cells.a |
Table III
Ewing sarcoma cells derived from
sphere culture do not exhibit higher in vivo tumorigenicity
than monolayer cells.a
| Incidence of tumor
generation |
|---|
|
|
|---|
| VH-64 | MSPES-4 | DC-ES-6 |
|---|
|
|
|
|
|---|
| Cell dose | Monolayer | Spheres | Monolayer | Spheres | Monolayer | Spheres |
|---|
|
5×104 | 0/2 | 0/2 | 1/4 | 0/4 | 0/4 | 0/4 |
|
2.5×105 | 1/2 | 1/2 | 1/4 | 3/4 | 1/4 | 1/4 |
Discussion
Sphere growth of glioblastoma and other solid cancer
cell lines under growth-constraining conditions was found to
represent and select for cells with self-renewing and clonogenic
potential that were tumorigenic in vivo (12,19,21–29).
In the present study, we showed that anchorage-independent growth
of Ewing sarcoma cells in serum-free medium is not associated with
stem-cell like phenotype and behavior. Throughout various cell
lines and biopsy-derived cell cultures, sphere-cultured Ewing
sarcoma cells had a limited capacity to self-renew by continued
sphere formation. Moreover, sphere-derived cells were not superior
to monolayer-cultured cells to initiate tumors in immunodeficient
mice. These results appear to be inconsistent with previous reports
of an enhanced tumorigenicity of cells from solid tumor spheres,
including one study in Ewing sarcoma (14). A potential reason for discrepant
functional results in individual investigations is that spheres
from extracranial tumors were often generated from established cell
lines (14,25,29).
Although cellular hierarchies of tumorigenicity can be preserved in
some cancer cell lines (30),
freshly isolated primary tumor samples and early cultures of tumor
cells more closely reflect the biology of the disease. Here, we
were able to establish parallel sphere and monolayer cultures of
CD99high Ewing sarcoma cells from four relapse biopsies.
Among individual Ewing sarcoma cell lines and cultures, we observed
substantial heterogeneity of both phenotype and function. However,
of four biopsies, all obtained from patients with aggressive,
relapsed disease, none was able to reinitiate spheres in
vitro for more than 2–3 passages. Thus, failure to self-renew
in vitro was a common characteristic of various Ewing
sarcomas.
Further arguments against the enrichment of Ewing
sarcoma spheres for cells with cancer stem cell properties were
obtained by phenotyping and side population (SP) analysis.
Expression of CD133 has been associated with higher tumorigenicity
of individual subpopulations in various solid tumors (19,31,32),
although the role of this marker in Ewing sarcoma is controversial.
Following one report that CD133 expression indeed marks a
subpopulation of Ewing sarcoma cells with tumor-initiating capacity
(17), others found that this
association was restricted to a limited proportion of Ewing
sarcomas (33). An alternative
surface marker proposed to reflect enhanced tumorigenicity in Ewing
sarcoma is the neural crest stem cell marker CD57 (14). In the present study, expression of
both CD133 and CD57 was variable among individual cell lines and
cultures. While individual cell cultures had substantially higher
surface CD57 expression than the respective monolayers, no
consistent association with the two culture conditions was
identified. The heterogeneity of our findings argues against a
significant role of CD133 and CD57 as markers of cells with
tumor-initiating capacity in Ewing sarcomas. The potential of SPs
identified by exclusion of the fluorescent dye Hoechst 33342 to
reflect the cancer stem cell pool in solid malignancies remains a
matter of debate. Although SPs were shown to define a cell
population with cancer stem cell characteristics in some tumors
(34,35), failure of SP to represent the cancer
stem cell compartment was reported in glioblastoma (36). Among the Ewing sarcoma cell lines
and cultures investigated here, the presence of SPs was
inconsistent, even between individual experiments using the same
cell line. Thus, Hoechst dye 33342 exclusion does not appear to be
a reliable marker of subpopulations with distinct characteristics
in Ewing sarcoma. Ultimately, we obtained in vivo evidence
in a highly immunodeficient mouse model that the tumorigenicity of
sphere-derived Ewing sarcoma cells is low and not superior to tumor
cells cultured as monolayers.
We conclude that tumor spheres propagated in the
absence of serum can not be generally assumed to be enriched for
cells with tumor-initiating capacity in Ewing sarcoma. Tumorigenic
subsets may be heterogeneous, dynamic and sensitive to undergo
phenotypic and functional alterations during both in vitro
and in vivo growth. Reliable phenotypes and functional
characteristics that enrich for subpopulations with stem-cell like
properties have not yet been identified in this disease. Such
markers could be useful for the development of novel therapeutics,
since tumor-initiating subpopulations of solid cancers are
considered the prime targets of therapy (37).
From the translational point of view, the sphere
model, despite its inadequacy to reflect tumor-initiating
properties, may be a useful additional tool for preclinical in
vitro testing of novel therapies. Three-dimensional in
vitro culture models are now increasingly acknowledged for
preclinical evaluation of immunotherapeutics and mechanisms of
immune evasion (38–41). In Ewing sarcoma, susceptibility of
tumor cells to lysis by NK cells was indeed found to critically
depend on in vitro culture conditions, with multicellular
clusters being substantially more resistant than monolayers or
single cell suspensions (4). This
discrepancy provides a potential explanation why the potent
antitumor activity of NK cells in preclinical experiments (42,43)
fails to translate into clinical responses to adoptive transfer of
these cells (43,44). Thus, even in the absence of the
capacity to enrich for tumor-initiating cells, sphere cultures of
tumor cells could add information to the preclinical evaluation of
novel drugs and immune targeted therapies.
Acknowledgements
Statistical support was provided by René Schmidt,
Institute of Biostatistics and Clinical Research. The present study
was supported by an institutional grant by IMF Muenster (AL 121005,
to C.R. and B.A.), by funding provided by the local charity
‘Freundeskreis KMT’ (to K.L), and by a grant from the Dr
Mildred-Scheel-Stiftung der Deutschen Krebshilfe (109566, to
C.R.).
References
|
1
|
Paulussen M, Craft AW, Lewis I, et al:
Results of the EICESS-92 study: two randomized trials of Ewing’s
sarcoma treatment - cyclophosphamide compared with ifosfamide in
standard-risk patients and assessment of benefit of etoposide added
to standard treatment in high-risk patients. J Clin Oncol.
26:4385–4393. 2008.PubMed/NCBI
|
|
2
|
Paulussen M, Ahrens S, Burdach S, et al:
Primary metastatic (stage IV) Ewing tumor: survival analysis of 171
patients from the EICESS studies. European Intergroup Cooperative
Ewing Sarcoma Studies. Ann Oncol. 9:275–281. 1998. View Article : Google Scholar
|
|
3
|
Stahl M, Ranft A, Paulussen M, et al: Risk
of recurrence and survival after relapse in patients with Ewing
sarcoma. Pediatr Blood Cancer. 57:549–553. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cho D, Shook DR, Shimasaki N, Chang YH,
Fujisaki H and Campana D: Cytotoxicity of activated natural killer
cells against pediatric solid tumors. Clin Cancer Res.
16:3901–3909. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mackall CL, Rhee EH, Read EJ, et al: A
pilot study of consolidative immunotherapy in patients with
high-risk pediatric sarcomas. Clin Cancer Res. 14:4850–4858. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thiel U, Pirson S, Müller-Spahn C, et al:
Specific recognition and inhibition of Ewing tumour growth by
antigen-specific allo-restricted cytotoxic T cells. Br J Cancer.
104:948–956. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Erkizan HV, Kong Y, Merchant M, et al: A
small molecule blocking oncogenic protein EWS-FLI1 interaction with
RNA helicase A inhibits growth of Ewing’s sarcoma. Nat Med.
15:750–756. 2009.PubMed/NCBI
|
|
8
|
Braun S, Pantel K, Müller P, et al:
Cytokeratin-positive cells in the bone marrow and survival of
patients with stage I, II, or III breast cancer. N Engl J Med.
342:525–533. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Woelfle U, Breit E, Zafrakas K, et al:
Bi-specific immunomagnetic enrichment of micrometastatic tumour
cell clusters from bone marrow of cancer patients. J Immunol
Methods. 300:136–145. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Freedman VH and Shin SI: Cellular
tumorigenicity in nude mice: correlation with cell growth in
semi-solid medium. Cell. 3:355–359. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shin SI, Freedman VH, Risser R and Pollack
R: Tumorigenicity of virus-transformed cells in nude mice is
correlated specifically with anchorage independent growth in vitro.
Proc Natl Acad Sci USA. 72:4435–4439. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee J, Kotliarova S, Kotliarov Y, et al:
Tumor stem cells derived from glioblastomas cultured in bFGF and
EGF more closely mirror the phenotype and genotype of primary
tumors than do serum-cultured cell lines. Cancer Cell. 9:391–403.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lawlor ER, Scheel C, Irving J and Sorensen
PH: Anchorage-independent multi-cellular spheroids as an in vitro
model of growth signaling in Ewing tumors. Oncogene. 21:307–318.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wahl J, Bogatyreva L, Boukamp P, et al:
Ewing’s sarcoma cells with CD57-associated increase of
tumorigenicity and with neural crest-like differentiation capacity.
Int J Cancer. 127:1295–1307. 2010.
|
|
15
|
Ottaviano L, Schaefer KL, Gajewski M, et
al: Molecular characterization of commonly used cell lines for bone
tumor research: a trans-European EuroBoNet effort. Genes
Chromosomes Cancer. 49:40–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Landuzzi L, De Giovanni C, Nicoletti G, et
al: The metastatic ability of Ewing’s sarcoma cells is modulated by
stem cell factor and by its receptor c-kit. Am J Pathol.
157:2123–2131. 2000.
|
|
17
|
Suvà ML, Riggi N, Stehle JC, et al:
Identification of cancer stem cells in Ewing’s sarcoma. Cancer Res.
69:1776–1781. 2009.
|
|
18
|
Al Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003.
|
|
19
|
Singh SK, Hawkins C, Clarke ID, et al:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goodell MA, Rosenzweig M, Kim H, et al:
Dye efflux studies suggest that hematopoietic stem cells expressing
low or undetectable levels of CD34 antigen exist in multiple
species. Nat Med. 3:1337–1345. 1997. View Article : Google Scholar
|
|
21
|
Coulon A, Flahaut M, Mühlethaler-Mottet A,
et al: Functional sphere profiling reveals the complexity of
neuroblastoma tumor-initiating cell model. Neoplasia. 13:991–1004.
2011.PubMed/NCBI
|
|
22
|
Hansford L, Smith K, Datti A, et al: Tumor
initiating cells from neuroblastoma, a neural crest-derived tumor.
Int J Dev Neurosci. 24:4892006. View Article : Google Scholar
|
|
23
|
Mahller YY, Williams JP, Baird WH, et al:
Neuroblastoma cell lines contain pluripotent tumor initiating cells
that are susceptible to a targeted oncolytic virus. PLoS One.
4:e42352009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rainusso N, Brawley VS, Ghazi A, et al:
Immunotherapy targeting HER2 with genetically modified T cells
eliminates tumor-initiating cells in osteosarcoma. Cancer Gene
Ther. 19:212–217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Walter D, Satheesha S, Albrecht P, et al:
CD133 positive embryonal rhabdomyosarcoma stem-like cell population
is enriched in rhabdospheres. PLoS One. 6:e195062011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang S, Balch C, Chan MW, et al:
Identification and characterization of ovarian cancer-initiating
cells from primary human tumors. Cancer Res. 68:4311–4320. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brown CE, Starr R, Martinez C, et al:
Recognition and killing of brain tumor stem-like initiating cells
by CD8+ cytolytic T cells. Cancer Res. 69:8886–8893.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gibbs CP, Kukekov VG, Reith JD, et al:
Stem-like cells in bone sarcomas: implications for tumorigenesis.
Neoplasia. 7:967–976. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhong Y, Guan K, Guo S, et al: Spheres
derived from the human SK-RC-42 renal cell carcinoma cell line are
enriched in cancer stem cells. Cancer Lett. 299:150–160. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Charafe-Jauffret E, Ginestier C, Iovino F,
et al: Breast cancer cell lines contain functional cancer stem
cells with metastatic capacity and a distinct molecular signature.
Cancer Res. 69:1302–1313. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
O’Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110.
2007.PubMed/NCBI
|
|
32
|
Yin S, Li J, Hu C, et al: CD133 positive
hepatocellular carcinoma cells possess high capacity for
tumorigenicity. Int J Cancer. 120:1444–1450. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jiang X, Gwye Y, Russell D, et al: CD133
expression in chemo-resistant Ewing sarcoma cells. BMC Cancer.
10:1162010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hirschmann-Jax C, Foster AE, Wulf GG, et
al: A distinct ‘side population’ of cells with high drug efflux
capacity in human tumor cells. Proc Natl Acad Sci USA.
101:14228–14233. 2004.
|
|
35
|
Murase M, Kano M, Tsukahara T, et al: Side
population cells have the characteristics of cancer stem-like
cells/cancer-initiating cells in bone sarcomas. Br J Cancer.
101:1425–1432. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Broadley KW, Hunn MK, Farrand KJ, et al:
Side population is not necessary or sufficient for a cancer stem
cell phenotype in glioblastoma multiforme. Stem Cells. 29:452–461.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Barrett LE, Granot Z, Coker C, et al:
Self-renewal does not predict tumor growth potential in mouse
models of high-grade glioma. Cancer Cell. 21:11–24. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Feder-Mengus C, Ghosh S, Weber WP, et al:
Multiple mechanisms underlie defective recognition of melanoma
cells cultured in three-dimensional architectures by
antigen-specific cytotoxic T lymphocytes. Br J Cancer.
96:1072–1082. 2007. View Article : Google Scholar
|
|
39
|
Fischer K, Hoffmann P, Voelkl S, et al:
Inhibitory effect of tumor cell-derived lactic acid on human T
cells. Blood. 109:3812–3819. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hirschhaeuser F, Leidig T, Rodday B,
Lindemann C and Mueller-Klieser W: Test system for trifunctional
antibodies in 3D MCTS culture. J Biomol Screen. 14:980–990. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jääskeläinen J, Kalliomäki P, Paetau A and
Timonen T: Effect of LAK cells against three-dimensional tumor
tissue. In vitro study using multi-cellular human glioma spheroids
as targets. J Immunol. 142:1036–1045. 1989.PubMed/NCBI
|
|
42
|
Lakshmikanth T, Burke S, Ali TH, et al:
NCRs and DNAM-1 mediate NK cell recognition and lysis of human and
mouse melanoma cell lines in vitro and in vivo. J Clin Invest.
119:1251–1263. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Parkhurst MR, Riley JP, Dudley ME and
Rosenberg SA: Adoptive transfer of autologous natural killer cells
leads to high levels of circulating natural killer cells but does
not mediate tumor regression. Clin Cancer Res. 17:6287–6297. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Geller MA, Cooley S, Judson PL, et al: A
phase II study of allogeneic natural killer cell therapy to treat
patients with recurrent ovarian and breast cancer. Cytotherapy.
13:98–107. 2011. View Article : Google Scholar : PubMed/NCBI
|