Introduction
Osteosarcoma (OS) is the most common primary
malignant bone carcinoma in children and adolescents (1). Due to the high propensity for lung
metastasis, survival of OS patients has not improved over the past
30 years (2). A novel therapeutic
strategy is needed for intervention in regards to the unfavorable
prognosis and ineffective treatment for OS patients. Cancer is a
genetic disease and tumors develop through a multi-step process.
Single or multiple mutations in genes related to growth control and
metastasis form the molecular genetic basis of malignant
transformation and tumor progression. Therefore, identification of
molecular targets that can be exploited in the clinic to treat
metastatic disease is desperately needed. Chemokines and chemokine
receptors have been described to act as important targets for the
treatment of cancer (3).
Chemokines, a family of small cytokines that bind to
specific G-protein-coupled seven-span transmembrane receptors, play
an important role in many physiological and pathological processes
(4). In addition to their initially
revealed function in cell trafficking and adhesion, CXC chemokine
receptors (CXCRs) have been reported to participate in the
development, progression and metastasis in a variety of malignant
tumors (5). CXC chemokine receptor
7 (CXCR7), as the receptor for CXCL12 with higher binding affinity,
is overexpressed in glioma and regulates tumor proliferation via
the ERK and AKT pathways (6,7). Based
on the cellular localization, positive expression of CXCR7, mainly
detected in the cytoplasm, mediates anti-apoptotic effects in
glioma cells and indicates a poor prognosis in gallbladder cancer
(8,9) and renal cell carcinoma (10). These data suggest that CXCR7 is a
promising therapeutic target.
Furthermore, the association between CXCR7
expression and clinical and pathological features has been
analyzed, indicating that CXCR7 is associated with tumor grade and
differentiation. CXCR7 exerts a potential function in tumor
progression in pancreatic adenocarcinoma (11) and predicts poor disease-free and
disease-specific survival in cervical cancer (12). Some studies have shown that several
signaling pathways such as CXCR4 and EGFR assist in CXCR7 signaling
transduction promoting tumorigenesis, and that CXCR7 coordinates
β-arrestin-2 and K-Ras-dependent pathways involved in the
development of pancreatic cancer (13). Stromal-derived factor
(SDF)-1-mediated CXCR7 signaling has been found to be expressed in
bladder and thyroid cancers and to promote cell migration and
metastasis (14,15). Thus, targeted inhibition of CXCR7
may provide an effective therapeutic strategy for the treatment of
cancer.
However, few studies show that CXCR7 is weakly
expressed in malignant hematopoietic cells, and has no enhancing
effects on cell proliferation and migration (16). Moreover, it was found that CXCR7 can
activate the PI3K/AKT, ERK and STAT3 pathways facilitating the
development of bladder cancer (17). To elucidate the function and
molecular mechanisms of CXCR7 in human OS, we examined the
expression of CXCR7 by immunohistochemical assay using a tissue
microarray procedure in OS tissues, and constructed lentiviral
vector-mediated Lv-siCXCR7 to observe the effects of CXCR7 on the
biological behaviors of OS cells in vitro and in
vivo. We hypothesized that CXCR7 may stimulate the growth and
invasion of OS cells through regulation of the PI3K/AKT and
β-arrestin pathways.
Materials and methods
Materials
The OS cell lines (MG-63 and U-2 OS) used for the
experiments were obtained from the Institute of Biochemistry and
Cell Biology (Shanghai, China). Lentiviral-mediated CXCR7 siRNA
(Lv-siCXCR7), negative control vector, and virion-packaging
elements were purchased from GeneChem (Shanghai, China). Human OS
tissues and the corresponding adjacent non-cancerous tissues (ANCT)
were collected from patients at the Department of Orthopedic
Surgery, Changzheng Hospital. The tissue microarray of OS was made
by Shanghai Outdo Biotech Co. Ltd. (Shanghai, China). All of the
antibodies were purchased from Cell Signaling Technology (Boston,
MA, USA). CXCR7 primer was synthesized by ABI (Framingham, MA,
USA).
Drugs and reagents
Dulbecco’s modified Eagle’s medium (DMEM) and fetal
bovine serum (FBS) were purchased from Thermo Fisher Scientific
Inc. (Waltham, MA, USA); TRIzol reagent and Lipofectamine 2000 were
obtained from Invitrogen (Carlsbad, CA, USA); M-MLV Reverse
Transcriptase was purchased from Promega (Madison, WI, USA);
SYBR-Green Master Mix was obtained from Takara (Otsu, Japan); and
the ECL Plus kit was obtained from GE Healthcare (Piscataway, NJ,
USA).
Clinical samples and data
The tissue microarray was prepared for
immunohistochemical analysis (IHC). Human OS tissues and the
corresponding ANCT were obtained from biopsies in a total of 45
consecutive OS cases admitted to our hospital from January 2005 to
December 2011. The baseline characteristics of the patients before
neo-adjuvant chemotherapy are summarized in Table I. The study was approved by the
Medical Ethics Committee of the Second Military Medical University
and written informed consent was obtained from the patients or
their parents before sample collection. Two pathologists
respectively reviewed all of the cases.
 | Table ICorrelation of CXCR7 expression with
clinicopathological factors of the OS patients. |
Table I
Correlation of CXCR7 expression with
clinicopathological factors of the OS patients.
| | CXCR7 expression
(n) | |
|---|
| |
| |
|---|
| Variables | Cases (n) | − | + | P-value |
|---|
| Total | 45 | 14 | 31 | |
| Age (years) | | | | 0.850 |
| <20 | 28 | 9 | 19 | |
| ≥20 | 17 | 5 | 12 | |
| Gender | | | | 0.557 |
| Male | 26 | 9 | 17 | |
| Female | 19 | 5 | 14 | |
| Histology | | | | 0.456 |
| Osteoblastic | 18 | 4 | 14 | |
|
Chondroblastic | 15 | 5 | 10 | |
| Fibroblastic | 7 | 2 | 5 | |
| Others | 5 | 3 | 2 | |
| Ennecking
staging | | | | 0.780 |
| I | 13 | 5 | 8 | |
| II | 24 | 7 | 17 | |
| III | 8 | 2 | 6 | |
| Distant
metastasis | | | | 0.004 |
| No | 18 | 10 | 8 | |
| Yes | 27 | 4 | 23 | |
Tissue microarray
The advanced tissue arrayer (ATA-100; Chemicon
International, Temecula, CA, USA) was used to create holes in a
recipient paraffin block and to acquire cylindrical core tissue
biopsies with a diameter of 1 mm from the specific areas of the
‘donor’ block. The tissue core biopsies were transferred to the
recipient paraffin block at defined array positions. The tissue
microarrays contained tissue samples from 45 formalin-fixed
paraffin-embedded cancer specimens with known diagnosis, and the
corresponding ANCT from these patients. The block was incubated in
an oven at 45°C for 20 min to allow complete embedding of the
grafted tissue cylinders in the paraffin of the recipient block,
and then stored at 4°C until microtome sectioning.
Immunohistochemical staining
Tissue microarray sections were processed for IHC
analysis of CXCR7 protein as follows. Immunohistochemical
examinations were carried out on 3-mm sections. For anti-CXCR7
immunohistochemistry, unmasking was performed with 10 mM sodium
citrate buffer, pH 6.0, at 90°C for 30 min. For anti-CXCR7
immunohistochemistry, antigen unmasking was not necessary. Sections
were incubated in 0.03% hydrogen peroxide for 10 min at room
temperature to remove endogenous peroxidase activity, and then in
blocking serum [0.04% bovine serum albumin (A2153; Sigma-Aldrich,
Shanghai, China) and 0.5% normal goat serum (X0907; Dako
Corporation, Carpinteria, CA, USA) in phosphate-buffered saline
(PBS)] for 30 min at room temperature. Anti-CXCR7 antibody was used
at a dilution of 1:200. The antibody was incubated overnight at
4°C. Sections were then washed three times for 5 min in PBS.
Non-specific staining was blocked with 0.5% casein and 5% normal
serum for 30 min at room temperature. Finally, staining was
developed using diaminobenzidine substrate, and the sections were
counterstained with hematoxylin. Normal serum or PBS was used to
replace the anti-CXCR7 antibody in the negative controls.
Quantification of protein expression
The expression of CXCR7 was semi-quantitatively
estimated as the total immunostaining scores, which were calculated
as the product of a proportion score and an intensity score. The
proportion and intensity of the staining were evaluated
independently by two observers. The proportion score reflected the
fraction of positive staining cells (0, none; 1, ≤10%; 2, >10 to
≥25%; 3, >25 to 50%; 4, >50%), and the intensity score
represented the staining intensity (0, no staining; 1, weak; 2,
intermediate; and 3, strong). Finally, a total expression score was
given ranging from 0 to 12. Based on the analysis in advance, CXCR7
expression was categorized into two groups: low-level CXCR7
expression (score 0–3) and high-level CXCR7 expression (score
4–12). The scoring was independently assessed by two
pathologists.
Cell culture and transfection
OS cell lines were cultured in DMEM supplemented
with 10% heat-inactivated FBS, 100 U/ml of penicillin, and 100
μg/ml of streptomycin. Cells in this medium were placed in a
humidified atmosphere containing 5% CO2 at 37°C. Cells
were subcultured at a 1:5 dilution in medium containing 300 μg/ml
G418 (an aminoglycoside antibody; commonly used stable transfection
reagent in molecular genetic testing). On the day of transduction,
OS cells were replated at 5×104 cells/well in 24-well
plates containing serum-free growth medium with polybrene (5
mg/ml). When reaching 50% confluency, cells were transfected with
recombinant experimental virus or control virus at the optimal MOI
(multiplicity of infection) of 50, and cultured at 37°C in 5%
CO2 for 4 h. The supernatant was then discarded and
serum containing growth medium was added. At 4 days
post-transduction, the transduction efficiency was measured by the
frequency of green fluorescent protein (GFP)-positive cells.
Positive and stable transfectants were selected and expanded for
further study. The Lv-siCXCR7 vector-infected clone and the
negative control vector-infected cells were designated as the
Lv-siCXCR7 and the NC group.
Quantitative real-time PCR
To quantitatively determine the mRNA expression
level of CXCR7 in OS cells, real-time PCR was performed. Total RNA
was extracted from each clone using TRIzol according to the
manufacturer’s protocol. Reverse transcription was carried out
using M-MLV and cDNA amplification was performed using the
SYBR-Green Master Mix kit according to the manufacturer’s
guidelines. The CXCR7 gene was amplified using a specific
oligonucleotide primer, and the β-actin gene was used as an
endogenous control. The PCR primer sequences were as follows:
CXCR7, 5′-CACCGCATCTCTTCGACTACTCAGATTCAAGAGAT
CTGAGTAGTCGAAGAGATGCTTTTTTG-3′ and 5′-GATC
CAAAAAAGCATCTCTTCGACTACTCAGATCTCTTGA ATCTGAGTAGTCGAAGAGATGC-3′;
β-actin, 5′-CAACG AATTTGGCTACAGCA-3′ and 5′-AGGGGTCTACATGGCA
ACTG-3′. Data were analyzed using the comparative Ct method
(2−ΔΔCt). Three separate experiments were performed for
each clone.
Western blot assay
OS cell lines were harvested and extracted using
lysis buffer (Tris-HCl, SDS, mercaptoethanol and glycerol). Cell
extracts were boiled for 5 min in loading buffer, and then an equal
amount of cell extracts was separated on 15% SDS-PAGE gels.
Separated protein bands were transferred onto polyvinylidene
fluoride (PVDF) membranes, which were subsequently blocked in 5%
skim milk powder. Primary antibodies against CXCR7, p-PI3K, p-AKT,
β-arrestin, PCNA and metalloproteinase-9 (MMP-9) were diluted
according to the manufacturer’s instructions and incubated
overnight at 4°C. Subsequently, horseradish peroxidase-linked
secondary antibodies were added at a dilution of 1:1,000 and
incubated at room temperature for 2 h. The membranes were washed 3
times with PBS, and the immunoreactive bands were visualized using
the ECL Plus kit according to the manufacturer’s instructions. The
relative protein levels in the different cell lines were normalized
to the concentration of β-actin. Three separate experiments were
performed for each clone.
Cell proliferation assay
Cell proliferation was analyzed using the MTT assay.
Briefly, cells infected with the Lv-siCXCR7 virus were incubated in
a 96-well plate at a density of 1×105 cells/well with
DMEM supplemented with 10% FBS. Cells were treated with 20 μl of
MTT dye for 0, 24, 48 and 72 h, and subsequently incubated with 150
μl of DMSO for 5 min. The color reaction was measured at 570 nm
using an enzyme immunoassay analyzer (Bio-Rad, Hercules, CA, USA).
The proliferation activity was calculated for each clone.
Transwell invasion assay
Transwell filters were coated with Matrigel (3.9
μg/μl; 60–80 μl) on the upper surface of a polycarbonate membrane
(diameter, 6.5 mm; pore size, 8 μm). After incubation at 37°C for
30 min, the Matrigel solidified and served as the extracellular
matrix for analysis of tumor cell invasion. Harvested cells
(1×105) in 100 μl of serum-free DMEM were added into the
upper compartment of the chamber. A total of 200 μl of conditioned
medium derived from NIH3T3 cells was used as a source of
chemoattractant, which was placed in the bottom compartment of the
chamber. After 24 h of incubation at 37°C with 5% CO2,
the medium was removed from the upper chamber. The non-invaded
cells on the upper side of the chamber were scraped off with a
cotton swab. Cells that had migrated from the Matrigel into the
pores of the inserted filter were fixed with 100% methanol, stained
with hematoxylin, then mounted and dried at 80°C for 30 min. The
number of cells invading through the Matrigel was counted in 3
randomly selected visual fields from the central and peripheral
portion of the filter by using an inverted microscope (×200
magnification). Each assay was repeated 3 times.
Subcutaneous tumor model and gene
therapy
Six-week-old female immunodeficient mice (BALB/c-nu)
were bred at the laboratory animal facility (Institute of Chinese
Academy of Sciences, Shanghai), and were housed individually in
microisolator ventilated cages with free access to water and food.
All experimental procedures were performed according to the
regulations and internal biosafety and bioethics guidelines of the
Second Military Medical University and the Shanghai Municipal
Science and Technology Commission. Three mice were injected
subcutaneously with 1×107 OS cells (U-2 OS) in 50 μl of
PBS pre-mixed with an equal volume of Matrigel matrix
(Becton-Dickinson). Mice were monitored daily and developed
subcutaneous tumors. When the tumor size reached ~5 mm in length,
the tumors were surgically removed, cut into 1–2 mm3
pieces, and re-seeded individually into other mice. When the tumor
size reached ~5 mm in length, the mice were randomly assigned to
the NC group and Lv-siCXCR7 group. In the treatment group, 15 μl of
Lv-siCXCR7 was injected into the subcutaneous tumors using a
multi-site injection format. Injections were repeated every other
day after initial treatment. The tumor volume every 3 days was
measured with a caliper, using the formula: Volume = (length ×
width)2/2.
Statistical analysis
SPSS 20.0 was used for the statistical analysis.
Kruskal-Wallis H and Chi-square tests were used to analyze the
expression levels in all groups. One-way analysis of variance
(ANOVA) was used to analyze the differences between groups. The LSD
method of multiple comparisons was used when the probability for
ANOVA was statistically significant. Statistical significance was
set at P<0.05.
Results
Expression of CXCR7 in OS tissues
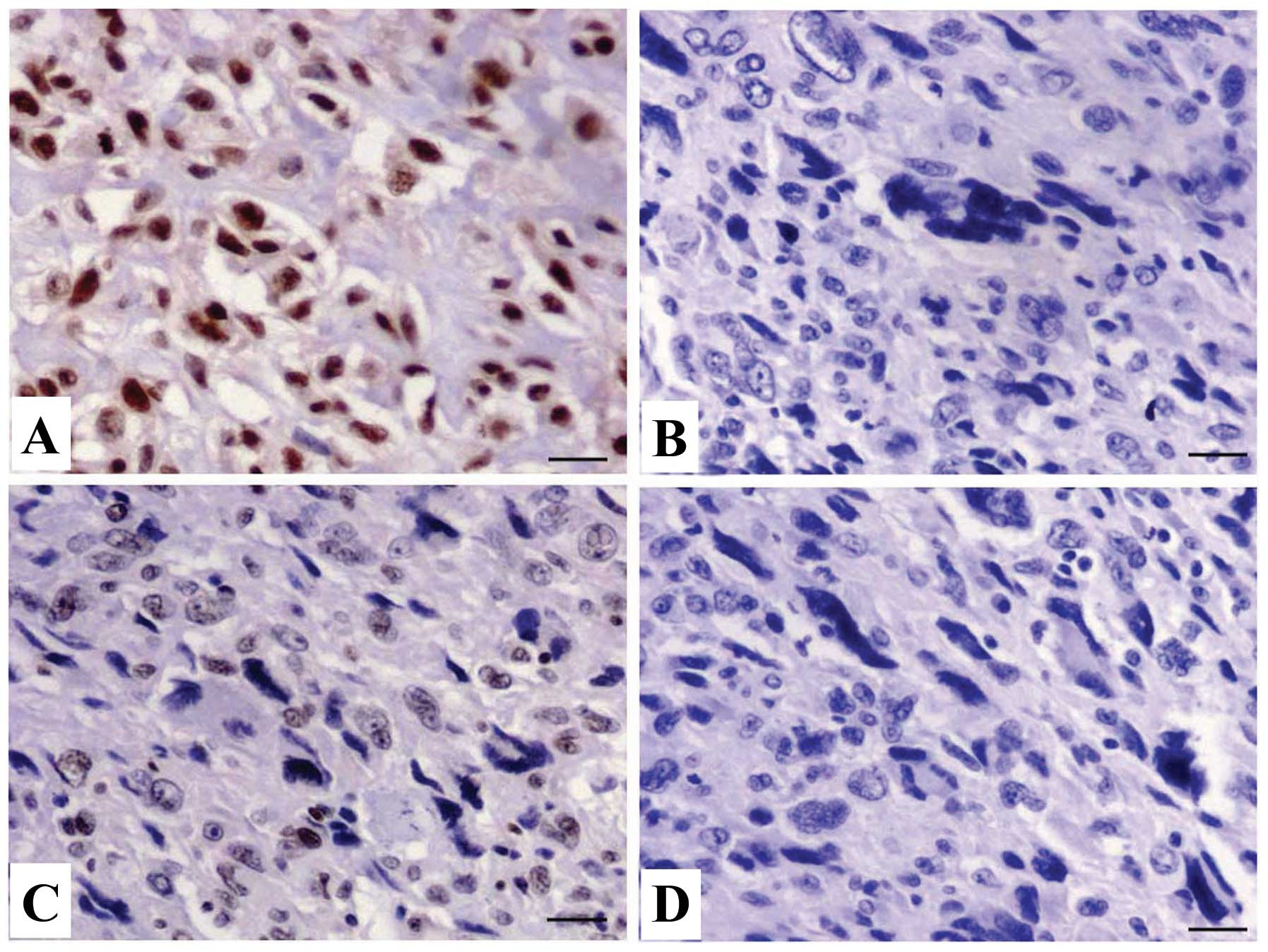
The expression of CXCR7 protein was evaluated using
IHC staining in OS tissues. The different levels of positive
expression of CXCR7 protein were detected in OS (Fig. 1A and B) and ANCT tissues (Fig. 1C and D). Positive CXCR7
immunostaining was mainly localized in the cytoplasm of OS tissue
cells. According to the CXCR7 immunoreactive intensity, the
positive expression of CXCR7 in OS tissues was significantly
increased compared with that in ANCT (P=0.033) (Table II).
 | Table IIExpression of CXCR7 protein in OS
tissues. |
Table II
Expression of CXCR7 protein in OS
tissues.
| | CXCR7 expression
(n) | | | | |
|---|
| |
| | | | |
|---|
| Target | Sample | − | + | ++ | +++ | Total | Positive rate
(%) | χ2 | P-value |
|---|
| CXCR7 | OS | 14 | 15 | 9 | 7 | 45 | 68.9 | | |
| ANCT | 21 | 17 | 5 | 2 | 45 | 53.3 | 4.547 | 0.033 |
Correlation of CXCR7 expression with
clinicopathological parameters
The association of CXCR7 expression with various
clinical and pathological factors was analyzed. As shown in
Table I, increased expression of
CXCR7 was closely correlated with the distant metastasis of OS
(P=0.004). However, no significant association was found between
CXCR7 expression and other factors including age, gender, histology
and Ennecking staging of the tumor (all P>0.05).
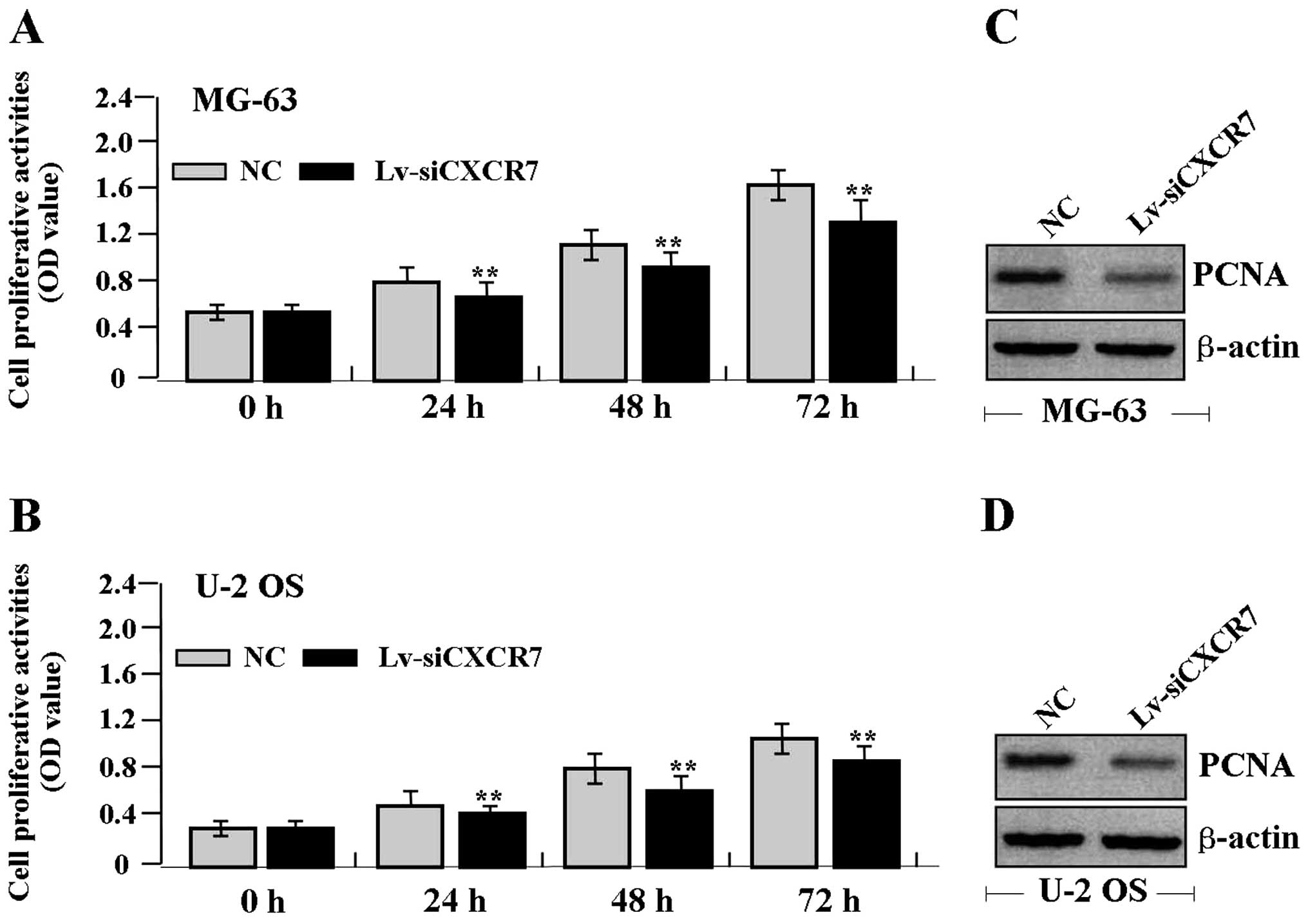
Effect of CXCR7 knockdown on the
expression of PI3K, AKT and β-arrestin
First, lentiviruses of different multiplicity of
infections (MOIs) were used to transfect OS cell lines (MG-63 and
U-2 OS), and the transfection efficiency of Lv-siCXCR7 (MOI=50) was
>65%. After Lv-siCXCR7 was transfected into the OS cell lines
for 24 h, the expression levels of CXCR7 mRNA (Fig. 2A and B) and protein (Fig. 2C and D), p-PI3K, and p-AKT and
β-arrestin proteins (Fig. 2E and F)
were detected by real-time PCR and western blot assays, indicating
a reduced expression of CXCR7, p-PI3K, p-AKT and β-arrestin in the
Lv-siCXCR7 group when compared with the levels in the NC group.
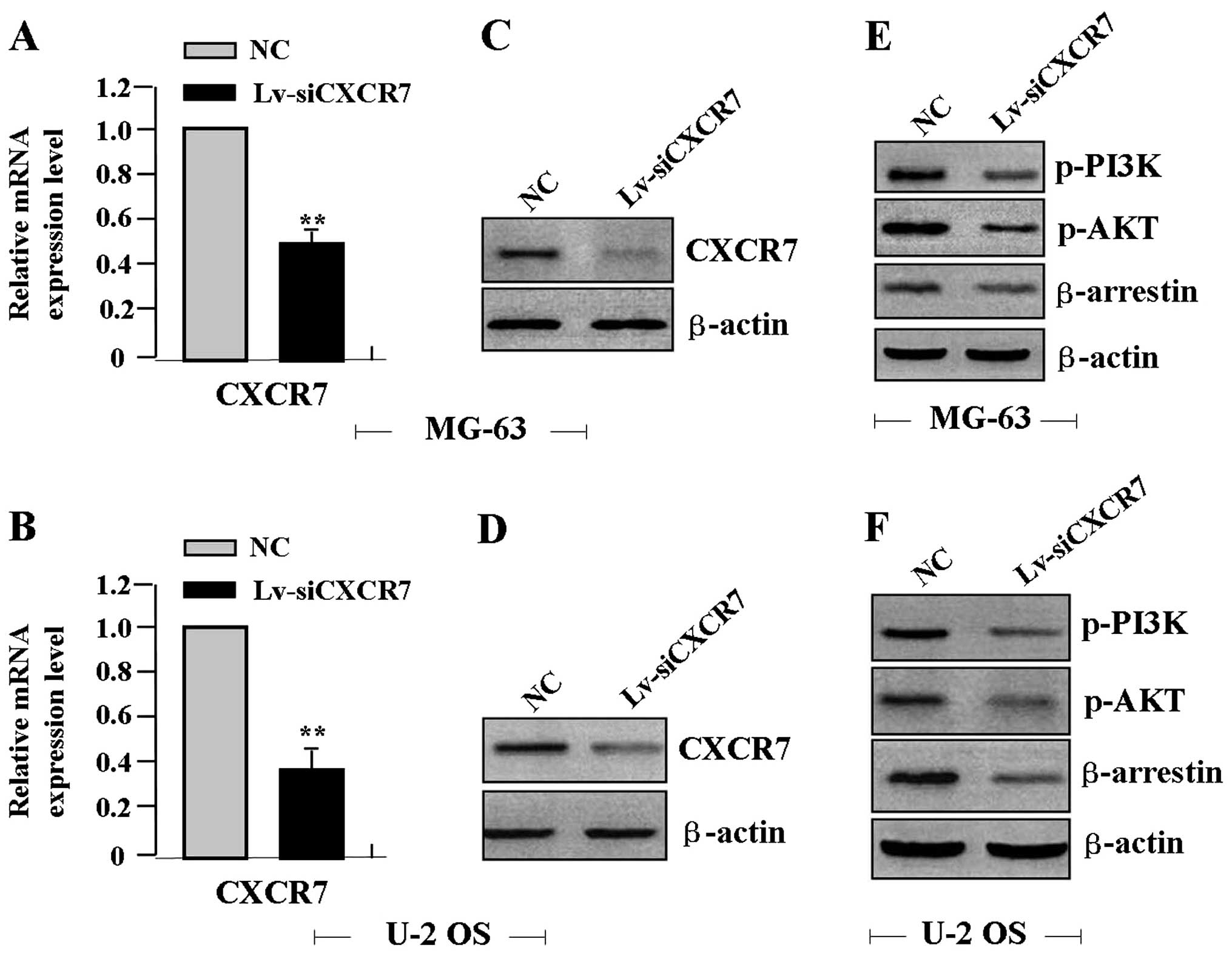 | Figure 2Effect of CXCR7 on the expression of
PI3K, AKT and β-arrestin. After OS cells were transfected with
Lv-siCXCR7 for 24 h, the expression levels of CXCR7, PI3K, AKT and
β-arrestin were detected by (A and B) real-time PCR and (C-F)
western blot assays, revealing decreased expression of CXCR7, PI3K,
AKT and β-arrestin in the Lv-siCXCR7 group when compared with
levels in the NC group (each **P<0.01). CXCR7, CXC
chemokine receptor 7; Lv-siCXCR7, lentiviral vector-mediated CXCR7
siRNA; OS, osteosarcoma; NC, negative control. |
Effect of CXCR7 knockdown on cell
proliferation
Deregulated cell proliferation is a hallmark of
cancer. To verify the effect of CXCR7 on tumor growth in OS cells,
we examined cell proliferative activities by MTT assay. The results
showed that knockdown of CXCR7 diminished the proliferative
activities of OS cells in a time-dependent manner compared to the
NC group (Fig. 3A and B). In
addition, the expression level of PCNA protein, examined by western
blot assay (Fig. 3C and D), was
found to be significantly downregulated in the Lv-siCXCR7 group
compared with the NC group.
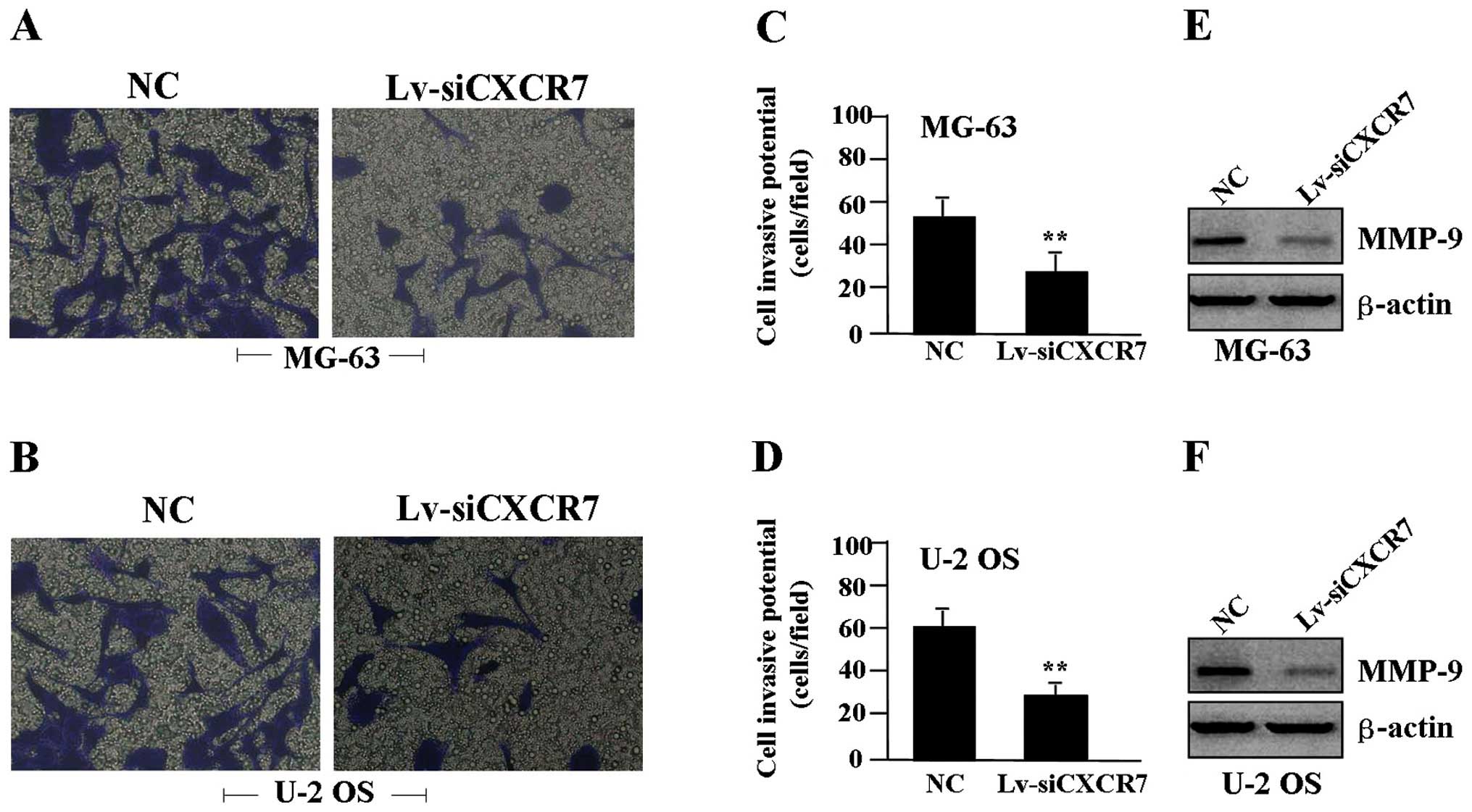
Effect of CXCR7 knockdown on cell
invasion
To determine the effect of CXCR7 on cell invasion, a
Transwell assay was performed. The invasive potential of tumor
cells in the Transwell assay was determined by the ability of cells
to invade a matrix barrier containing laminin and type IV collagen,
the major components of the basement membrane. Representative
micrographs of the Transwell filters are shown in Fig. 4A and B. The invasive potential of
the OS cells was apparently weakened in the Lv-siCXCR7 group
compared to the NC group (P<0.01) (Fig. 4C and D). In addition, the expression
level of MMP-9 protein, examined by western blot assay (Fig. 4E and F), was found to be
significantly downregulated in the Lv-siCXCR7 group compared with
the level in the NC group.
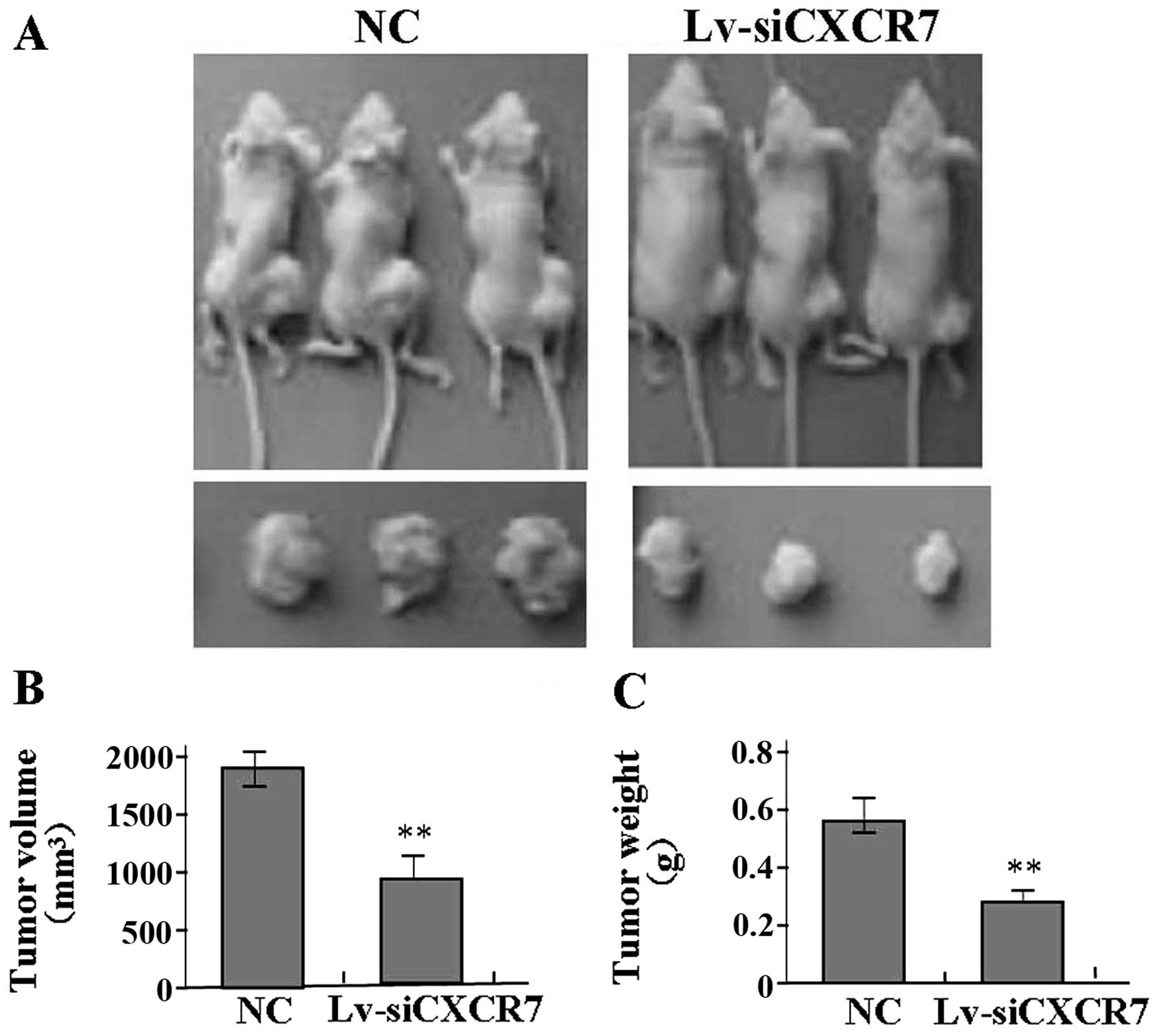
Effect of CXCR7 knockdown on xenograft
tumor growth
Our in vitro experiments demonstrated an
inhibitory effect of CXCR7 knockdown on OS growth. Therefore, we
further investigated the effect of CXCR7 on U-2 OS xenograft tumor
growth in vivo. The mean volume of the tumors in the
experimental mice before treatment was 68.32±14.25 mm3.
During the entire tumor growth period, the tumor growth activity
was determined. The tumors treated with Lv-siCXCR7 grew
substantially slower compared to the NC group (Fig. 5A). When the tumors were harvested,
the average weight and volume of the tumors in Lv-siCXCR7 group
were significantly reduced when compared with these parameters in
the NC group (Fig. 5B and C),
suggesting that CXCR7 knockdown blocked the growth of OS cells.
Discussion
CXCR7, a chemokine receptor, has been previously
demonstrated to have an impact on growth, apoptosis,
invasion/metastasis and prognosis in many types of cancers. CXCR7
was found to be differentially expressed in pancreatic cancer,
while knockdown of CXCR7 resulted in decreased migration and
invasion (18). The expression of
CXCR7 was found to be upregulated in renal cell (10) and human hepatocellular carcinoma
(19), and human brain tumors
(20). Overexpression of CXCR7 was
found to be associated with metastasis and poor survival of
patients with hepatocellular carcinoma, indicating its potential
function as a therapeutic target (21). CXCR4 and CXCR7 are frequently
co-expressed in human cancer tissues and cell lines (12,13).
Given the demonstrated importance of CXCR7 in tumor pathogenesis,
we further examined the expression of CXCR7 in human OS tissues.
The present study showed that CXCR7 was markedly upregulated in the
cytoplasm of cells in the OS tissues when compared to the cells in
the adjacent non-tumor tissues, and was positively correlated with
distant metastasis of OS, suggesting that CXCR7 may represent a new
biomarker involved in the development of OS.
Furthermore, the function of CXCR-7 in cancer
warrants further research. Some studies have shown that CXCR-7 and
IL-8 can contribute to tumor proliferation and metastasis in renal
cell carcinoma (22). Activation of
CXCR7 on tumor blood vessels facilitates the progression of colon
cancer with lung metastasis (23).
High expression of CXCR7 also leads to progression, metastasis and
angiogenesis in brain tumors, and regulates the transendothelial
migration in lymphoma (24,25). To confirm the function of CXCR7 in
cancer, our present study showed that knockdown of CXCR7 suppressed
the proliferation and invasion of OS cells. Likewise, the findings
of Zheng et al support our study, indicating that targeted
inhibition of the CXCR7 axis suppresses proliferation, invasion and
angiogenesis in hepatocellular carcinoma (26), suggesting that CXCR7 may be a
critical therapeutic target for the treatment of cancer.
In response to the regulatory mechanisms of CXCR7 in
cancer, a few studies have shown that MAPK, ERK and
β-arrestin-linked signaling pathways are recruited and activated by
the CXCR4·CXCR7 complex implicated in cell migration (27). The PI3K/AKT pathway mediates the
CXCR7 signaling regulating cell survival and prognosis in head and
neck cell carcinoma (28). CXCR7
promotes proliferation and motility in bladder cancer through
activation of the AKT and ERK pathways (17,29).
Therefore, further study needs to be carried out to investigate the
regulatory mechanisms of CXCR7 in OS cells. Our present findings
indicated that knockdown of CXCR7 downregulated the expression of
p-PI3K, p-AKT and β-arrestin in OS cells, suggesting that CXCR7 may
be involved in the progression of OS via regulation of the PI3K/AKT
and β-arrestin pathways.
PCNA is a nuclear protein that is expressed in
proliferating cells and may be required for maintaining cell
proliferation, and may be used as a marker for cell proliferation
of colon cancer (30). MMP-9 is
thought to be a key enzyme involved in the degradation of type IV
collagen, and a high level of MMP-9 in tissues is associated with
tumor invasion and metastasis (31). It has been reported that the AKT and
β-arrestin pathways promote growth and invasion of tumor cells via
regulation of PCNA and MMP-9 expression (32). In the present study, it was found
that knockdown of CXCR7 also downregulated the expression of PCNA
and MMP-9 in OS cells, suggesting that CXCR7 may promote the
progression of OS through AKT and β-arrestin pathway-mediated
regulation of PCNA and MMP-2 expression.
In conclusion, our findings indicate that
upregulation of CXCR7 expression is correlated with distant
metastasis of OS, and knockdown of CXCR7 inhibits the growth and
invasion of OS cells through inhibition of the PI3K/AKT and
β-arrestin pathways, suggesting that CXCR7 may serve as a potential
therapeutic target for the treatment of cancer.
References
|
1
|
Chen X, Luther G, Zhang W, et al: The E-F
hand calcium-binding protein S100A4 regulates the proliferation,
survival and differentiation potential of human osteosarcoma cells.
Cell Physiol Biochem. 32:1083–1096. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roth M, Linkowski M, Tarim J, et al:
Ganglioside GD2 as a therapeutic target for antibody-mediated
therapy in patients with osteosarcoma. Cancer. 120:548–554. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goguet-Surmenian E, Richard-Fiardo P,
Guillemot E, et al: CXCR7-mediated progression of osteosarcoma in
the lungs. Br J Cancer. 109:1579–1585. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun X, Cheng G, Hao M, et al:
CXCL12/CXCR4/CXCR7 chemokine axis and cancer progression. Cancer
Metastasis Rev. 29:709–722. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hattermann K and Mentlein R: An infernal
trio: the chemokine CXCL12 and its receptors CXCR4 and CXCR7 in
tumor biology. Ann Anat. 195:103–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Singh AK, Arya RK, Trivedi AK, et al:
Chemokine receptor trio: CXCR3, CXCR4 and CXCR7 crosstalk via
CXCL11 and CXCL12. Cytokine Growth Factor Rev. 24:41–49. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Odemis V, Lipfert J, Kraft R, et al: The
presumed atypical chemokine receptor CXCR7 signals through
Gi/o proteins in primary rodent astrocytes and human
glioma cells. Glia. 60:372–381. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yao X, Zhou L, Han S, et al: High
expression of CXCR4 and CXCR7 predicts poor survival in gallbladder
cancer. J Int Med Res. 39:1253–1264. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hattermann K, Held-Feindt J, Lucius R, et
al: The chemokine receptor CXCR7 is highly expressed in human
glioma cells and mediates antiapoptotic effects. Cancer Res.
70:3299–3308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang L, Chen W, Gao L, et al: High
expression of CXCR4, CXCR7 and SDF-1 predicts poor survival in
renal cell carcinoma. World J Surg Oncol. 10:2122012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gebauer F, Tachezy M, Effenberger K, et
al: Prognostic impact of CXCR4 and CXCR7 expression in pancreatic
adenocarcinoma. J Surg Oncol. 104:140–145. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schrevel M, Karim R, ter Haar NT, et al:
CXCR7 expression is associated with disease-free and
disease-specific survival in cervical cancer patients. Br J Cancer.
106:1520–1525. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Heinrich EL, Lee W, Lu J, et al: Chemokine
CXCL12 activates dual CXCR4 and CXCR7-mediated signaling pathways
in pancreatic cancer cells. J Transl Med. 10:682012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gosalbez M, Hupe MC, Lokeshwar SD, et al:
Differential expression of stroma derived factor-1 isoforms in
bladder cancer. J Urol. Nov 26–2013.(Epub ahead of print).
View Article : Google Scholar
|
|
15
|
Liu Z, Sun DX, Teng XY, et al: Expression
of stromal cell-derived factor 1 and CXCR7 in papillary thyroid
carcinoma. Endocr Pathol. 23:247–253. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tarnowski M, Liu R, Wysoczynski M, et al:
CXCR7: a new SDF-1-binding receptor in contrast to normal
CD34+ progenitors is functional and is expressed at
higher level in human malignant hematopoietic cells. Eur J
Haematol. 85:472–483. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hao M, Zheng J, Hou K, et al: Role of
chemokine receptor CXCR7 in bladder cancer progression. Biochem
Pharmacol. 84:204–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo JC, Li J, Yang YC, et al:
Oligonucleotide microarray identifies genes differentially
expressed during tumorigenesis of DMBA-induced pancreatic cancer in
rats. PLoS One. 8:e829102013. View Article : Google Scholar
|
|
19
|
Monnier J, Boissan M, L’Helgoualc’h A, et
al: CXCR7 is up-regulated in human and murine hepatocellular
carcinoma and is specifically expressed by endothelial cells. Eur J
Cancer. 48:138–148. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hattermann K, Mentlein R and Held-Feindt
J: CXCL12 mediates apoptosis resistance in rat C6 glioma cells.
Oncol Rep. 27:1348–1352. 2012.PubMed/NCBI
|
|
21
|
Xue TC, Han D, Chen RX, et al: High
expression of CXCR7 combined with alpha fetoprotein in
hepatocellular carcinoma correlates with extra-hepatic metastasis
to lung after hepatectomy. Asian Pac J Cancer Prev. 12:657–663.
2011.PubMed/NCBI
|
|
22
|
Gahan JC, Gosalbez M, Yates T, et al:
Chemokine and chemokine receptor expression in kidney tumors:
molecular profiling of histological subtypes and association with
metastasis. J Urol. 187:827–833. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guillemot E, Karimdjee-Soilihi B, Pradelli
E, et al: CXCR7 receptors facilitate the progression of colon
carcinoma within lung not within liver. Br J Cancer. 107:1944–1949.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang T, Xia QJ, Chen JB, et al: Expression
of the CXCL12/SDF-1 chemokine receptor CXCR7 in human brain
tumours. Asian Pac J Cancer Prev. 13:5281–5286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zabel BA, Lewén S, Berahovich RD, et al:
The novel chemokine receptor CXCR7 regulates trans-endothelial
migration of cancer cells. Mol Cancer. 10:732011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zheng K, Li HY, Su XL, et al: Chemokine
receptor CXCR7 regulates the invasion, angiogenesis and tumor
growth of human hepatocellular carcinoma cells. J Exp Clin Cancer
Res. 29:312010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Décaillot FM, Kazmi MA, Lin Y, et al:
CXCR7/CXCR4 heterodimer constitutively recruits β-arrestin to
enhance cell migration. J Biol Chem. 286:32188–32197.
2011.PubMed/NCBI
|
|
28
|
Liu FY, Zhao ZJ, Li P, et al: NF-κB
participates in chemokine receptor 7-mediated cell survival in
metastatic squamous cell carcinoma of the head and neck. Oncol Rep.
25:383–391. 2011.
|
|
29
|
Yates TJ, Knapp J, Gosalbez M, et al:
C-X-C chemokine receptor 7: a functionally associated molecular
marker for bladder cancer. Cancer. 119:61–71. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Risio M: Cell proliferation in colorectal
tumor progression: an immunohistochemical approach to intermediate
biomarkers. J Cell Biochem (Suppl). 16G:79–87. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hornebeck W, Lambert E, Petitfrère E and
Bernard P: Beneficial and detrimental influences of tissue
inhibitor of metalloproteinase-1 (TIMP-1) in tumor progression.
Biochimie. 87:377–383. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dong S, Kong J, Kong F, et al:
Insufficient radiofrequency ablation promotes
epithelial-mesenchymal transition of hepatocellular carcinoma cells
through Akt and ERK signaling pathways. J Transl Med. 11:2732013.
View Article : Google Scholar
|