Introduction
Hepatocellular carcinoma (HCC) is one of the most
common cancer types in both genders. Due to its high mortality, HCC
is the third most common cause of cancer-related death (1). Because of a high incidence of
recurrence after initial treatment and high migratory capacity,
patients with HCC have a poor prognosis with only a 5-year survival
rate of 10% from initial diagnosis (2). The majority of HCC cases occur in
developing countries resulting in a serious economic and social
burden that cannot be ignored.
Viral infections, abnormal metabolic alteration,
epigenetic or genetic changes in liver tissues may finally lead to
HCC. During tumor genesis or progression, the expression levels of
a series of genes are dysregulated such as oncogenes or tumor
suppressors. In addition, the location of specific proteins is also
related to the behavior of cancer cells. Notably, the expression
levels of almost 60% of genes in mammalian cells are regulated by
microRNAs (miRNAs). An increased understanding concerning the
molecular mechanisms involved in HCC will facilitate diagnosis and
effective treatment.
miRNAs are small conserved noncoding RNAs of
approximately 22 nucleotides that function to fine-tune gene
expression by targeting preferential sequences in the 3′
untranslated region (UTR) of mRNAs (3). The roles of miRNAs in cancer
initiation or progression depend on their targets or the specific
tissue origin (4). Several miRNAs
have been shown to be abnormally expressed and to be related to the
prognosis (5), metastasis (6) and therapeutic response of HCC
(7) since the beginning of studies
on the roles of miRNAs in HCC. Moreover, as an important
application that is gradually being explored, various circulating
miRNAs can be prognostic biomarkers for HCC (8,9).
In our previous study, an miRNA expression profile
was carried out in tumor tissues and adjacent non-tumoral liver
tissues from patients with HCC. miR-451 was found to have lower
expression in the tumor tissues. Yet, the function of miR-451
remains unknown. In the present study, the result from the
microassay was further confirmed by qRT-PCR that miR-451 was
downregulated in 24 of the 26 HCC tumor tissues. ATF2, a key
component of activator protein 1 (AP1), was found to be a target of
miR-451 by sequence analysis and luciferase reporter assay. In
contrast to miR-451, ATF2 was increased in tumor tissues where it
was mainly localized in the nucleus. Further study showed that
miR-451 inhibited the migration of HepG2 and SK-Hep-1 cells. These
data revealed the potential function of miR-451 and a new pathway
for the regulation of ATF2 in HCC.
Materials and methods
Clinical tissue specimens
Twenty-six patients with HCCs at the General
Hospital of PLA (Beijing, China) were enrolled. No patient in the
present study received preoperative adjuvant therapy prior to
surgery. The fresh tumoral and peritumoral tissue specimens for RNA
extraction were frozen in liquid nitrogen immediately after
surgery. The clinical features of the patients are characterized in
Table I. Informed consent was
obtained from the patients, and this study was approved by the
Medical Ethics Committee of the General Hospital of PLA.
 | Table IClinical features of the patients with
HCC. |
Table I
Clinical features of the patients with
HCC.
| Patient no. | Age (years) | Gender | Tumor size (cm × cm
× cm) | HbsAg | HCV-Ab | AFP (μg/l) | Cirrhosis |
|---|
| 1 | 66 | M | 4×3×2 | P | N | 38.88 | Yes |
| 2 | 49 | M | 11×7.5×7 | P | N | 1.26 | No |
| 3 | 43 | M | 6.5×6×2.5 | P | N | 3.76 | Yes |
| 4 | 67 | M | 7.5×6.5×4 | N | N | 14,284.00 | Yes |
| 5 | 58 | M | 3×2×2 | N | N | 1,139.00 | Yes |
| 6 | 70 | F | 7×7×6 | N | N | 157.10 | No |
| 7 | 70 | M | 9×8.5×6 | P | N | 3.69 | Yes |
| 8 | 55 | M | 5×4×3 | N | N | 6,044.00 | No |
| 9 | 67 | M | 6×2×2 | N | N | 24.86 | No |
| 10 | 72 | F | 4×3×3 | P | N | 5,830.00 | Yes |
| 11 | 50 | F | 6×6×3.5 | P | N | 5.34 | Yes |
| 12 | 49 | M | 9×6×3 | P | N | 20,000.00 | No |
| 13 | 25 | M | 10×7.5×7 | N | N | 20,000.00 | No |
| 14 | 48 | M | 18×15×8 | P | N | 968.50 | Yes |
| 15 | 52 | M | 6.5×6×3 | P | N | 468.50 | Yes |
| 16 | 40 | M | 7×5×3 | P | N | 210.20 | Yes |
| 17 | 70 | F | 5.5×5×4 | P | N | 862.90 | Yes |
| 18 | 46 | M | 7×6×5 | P | N | 7.25 | Yes |
| 19 | 56 | M | 2.5×2×2 | P | N | 4.09 | No |
| 20 | 60 | M | 2.5×2×2 | P | N | 8.25 | No |
| 21 | 58 | F | 19×15×13 | N | N | 3.81 | No |
| 22 | 42 | M | 4×4×3.5 | P | N | 167.00 | Yes |
| 23 | 47 | F | 3×3×3 | P | N | 8.92 | Yes |
| 24 | 60 | F | 14×11×8 | P | N | 1.41 | No |
| 25 | 62 | F | 4×3×3 | N | N | 2.42 | No |
| 26 | 49 | M | 11×7×4.8 | P | N | 21.06 | No |
Cell lines and cultures
Human liver cancer cell lines HepG2 and SK-Hep-1
were cultured in Dulbecco’s modified Eagle’s medium (DMEM)
(Gibco-BRL, Grand Island, NY, USA) supplemented with 10% fetal
bovine serum (FBS) (Hyclone, Thermo Scientific, Rockford, IL, USA),
100 U/ml penicillin and 100 μg/ml streptomycin in a 5%
CO2 and 95% air incubator at 37°C.
Plasmid construction and
transfection
For studying the target effect of miR-451 on ATF2,
the 3′UTR segment of ATF2 was subcloned into a modified pGL3
control vector (10) immediately
downstream of the luciferase gene’s stop codon after PCR
amplification of genomic DNA with the primers: pGL3-ATF2-3′UTR (F:
5′-AGTTCTAGATTAAAAACCT GCAGTACAACAGT-3′ and R: 5′-AGTCATATGTTTTCA
GTAACACCCCCATTTAT-3′), and the pGL3-ATF2-3′UTRmut vector was
generated with specific primer substitutions at the miRNA
complementary sites using PCR. Wild-type or mutant inserts were
confirmed by sequencing. DNA transfection was performed using
JetPrime® transfection reagent (Polyplus, France)
according to the manufacturer’s protocol.
miRNA mimics, siRNAs and
transfection
miR-451 mimics (guide, 5′-AAACCGUUACCAUUACUGAGUUU-3′
and passenger, 5′-ACUCAGUAAUGGUAACGGUUUUU-3′), siRNAs against ATF2
(sense, 5′-GUUGGCGAGUCCAUUU GAGTT-3′ and antisense,
5′-CUCAAAUGGACUCGCCAA CTT-3′) and negative control RNA duplex
(sense, 5′-GGCUAC GUCCAGGAGCGCACC-3′ and antisense, 5′-UGCGCUCCU
GGACGUAGCCTT-3′) were synthesized by GenePharma (Shanghai, China).
Cells grown in 6-well plates were transfected with siATF2 (20 nM)
or miR-451 mimics (20 nM) using Interferin® reagent
(Polyplus) according to the manufacturer’s protocol. Total-RNA and
protein were prepared at 48 h and 72 h, respectively, after
transfection.
Real-time RT-PCR for detection of mRNA
and miRNA
Total-RNA from the tissues or the cultured cells was
isolated using TRI® reagent (Sigma-Aldrich, St. Louis,
MO, USA). For mRNA detection, cDNA was synthesized from total-RNA
using Oligo(dT)18 primer and ImProm-II™ reverse
transcriptase system (Promega, Madison, WI, USA) according to the
manufacturer’s protocol. The real-time PCR was performed by using
SYBR Premix Ex Taq (Takara Bio, Dalian, China). Primers used for
mRNA detection were: ATF2 (F: 5′-CTCCAG CTCACACAACTCCA-3′ and R:
5′-TGTTTCAGCTGTGC CAC TTC-3′) and GAPDH (F: 5′-TCAGTGGTGGACCTG
ACCTG3′ and R: 5′-TGCTGTAGCCAAATTCGTTG-3′). Data were normalized to
the GAPDH mRNA level.
For miRNA detection, poly(A)-tailed RNA was used in
real-time RT-PCR (11). In brief,
10 μg total-RNA, 1 mM rATP, 5 μl 10× poly(A) polymerase reaction
buffer and 2 units poly(A) polymerase (NEB, UK) were mixed in a
50-μl reaction system and incubated at 37°C for 60 min according to
the manufacturer. Then the poly(A)-tailed total-RNA was recovered
using water-saturated phenol/chloroform extraction and precipitated
with ethanol. The 1 μg poly(A)-tailed total-RNA was reverse
transcribed using 1 μg miR-RT primer
(5′-GCGAGCACAGAATTAATACGACTCACTATAGG(t)18VN-3′) and 1 μl ImProm
reverse transcriptase (Promega) according to the manufacturer’s
protocol. Real-time PCR was performed using SYBR-Green PCR Mix kit
(Qiagen, Hilden, Germany) according to the manufacturer. Primers
used in qPCR were: miR-451 (F: 5′-AAACCGTTACCATTACTGAGTT-3′ and R:
5′-GCGAGCACAGAATTAATACGAC-3′) and U6 snRNA (F:
5′-CTCGCTTCGGCAGCACA-3′ and R: 5′-AACGCT TCACGAATTTGCGT-3′. The U6
snRNA level was used for normalization.
Western blot analysis
Protein extracts were prepared in a modified RIPA
buffer [50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% (v/v) NP-40, 0.25%
(w/v) Na deoxycholate, 0.5% (w/v) SDS and 1× protease inhibitor
cocktail (Complete Mini, Roche, Switzerland)]. Equal amounts of
proteins were separated by 10% SDS-PAGE and were electrotransferred
onto Immobilon Hybond-C membranes (Amersham Biosciences,
Piscataway, NJ, USA). Primary antibodies against ATF2 (1489;
Epitomics) and β-actin (sc-47778; Santa Cruz) were used and
detected with SuperSignal® West Pico chemiluminescent
substrate (Thermo Scientific).
Luciferase reporter assay
HepG2 cells in 24-well plate were transfected with
mixtures containing 100 ng firefly luciferase reporter plasmid with
the ATF2 3′UTR (named pGL3-ATF2-3′UTR) or ATF2 3′UTR mutant (named
pGL3-ATF2-3′UTRmut) sequence, miR-451 mimics (20 nM), and 1 ng
pRL-TK (a normalization control). Luciferase activities were
measured using a dual-luciferase reporter assay system (Promega) at
48 h after transfection.
Cell migration assay
HepG2 and SK-Hep-1 cells cultured in 6-well plates
were transfected using miR-451 mimics or negative control (NC).
Cells were serum-starved for 18 h in DMEM containing 0.1% FBS from
30 h after transfection. Then the starved cells were trypsinized
and resuspended in DMEM containing 0.1% FBS, and 2×105
cells were added to the upper chamber with a 6.5-mm diameter, 8-μm
pore size membrane (Corning), while the DMEM containing 5% FBS was
placed in the lower compartment of the chamber. After incubation at
37°C for 12 h, cells on the supine surface of the membrane were
removed carefully with a cotton swab, and then the filters were
fixed with 95% ethanol for 30 min, stained with 0.2% crystal violet
solution for 30 min, and finally observed under a microscope.
Immunohistochemical analysis
For detection of ATF2 expression in tissues, the
sections (4-μm) were prepared and subjected to deparaffinized in
xylol and rehydrated in a graded alcohol series. Antigen retrieval
was performed by boiling tissue sections in EDTA buffer (pH 8.0)
for 5 min at 100°C in a pressure cooker. Endogenous peroxidase
activity was blocked with hydrogen peroxidase (0.3%), and
nonspecific proteins were blocked with 5% BSA. Incubation with the
primary antibody against ATF2 (1:200) was carried out overnight at
4°C, followed by the biotinylated secondary antibody and detection
with diaminobenzidine (Dako) and counterstained with Mayer’s
hematoxylin. For each tissue specimen, ATF2 expression was scored
on a scale of 0–3 according to the extent (0–100%) and intensity
(strong, 3; moderate, 2; and weak, 1) which was evaluated by an
observer in a blinded manner, and the average was calculated.
Statistical analysis
All values are reported as means ± SD. Differences
were estimated by the two-tailed Student’s t-test using Excel
software. Spearman’s rank correlation was carried out to evaluate
the relationship between miR-451 and ATF2 expression. P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-451 is underexpressed in primary HCC
lesions
The clinical features of the HCC patients in this
study are described in Table I.
Twenty-six pairs of fresh tumoral and peritumoral tissues were
employed for detecting the expression of miR-451 by real-time
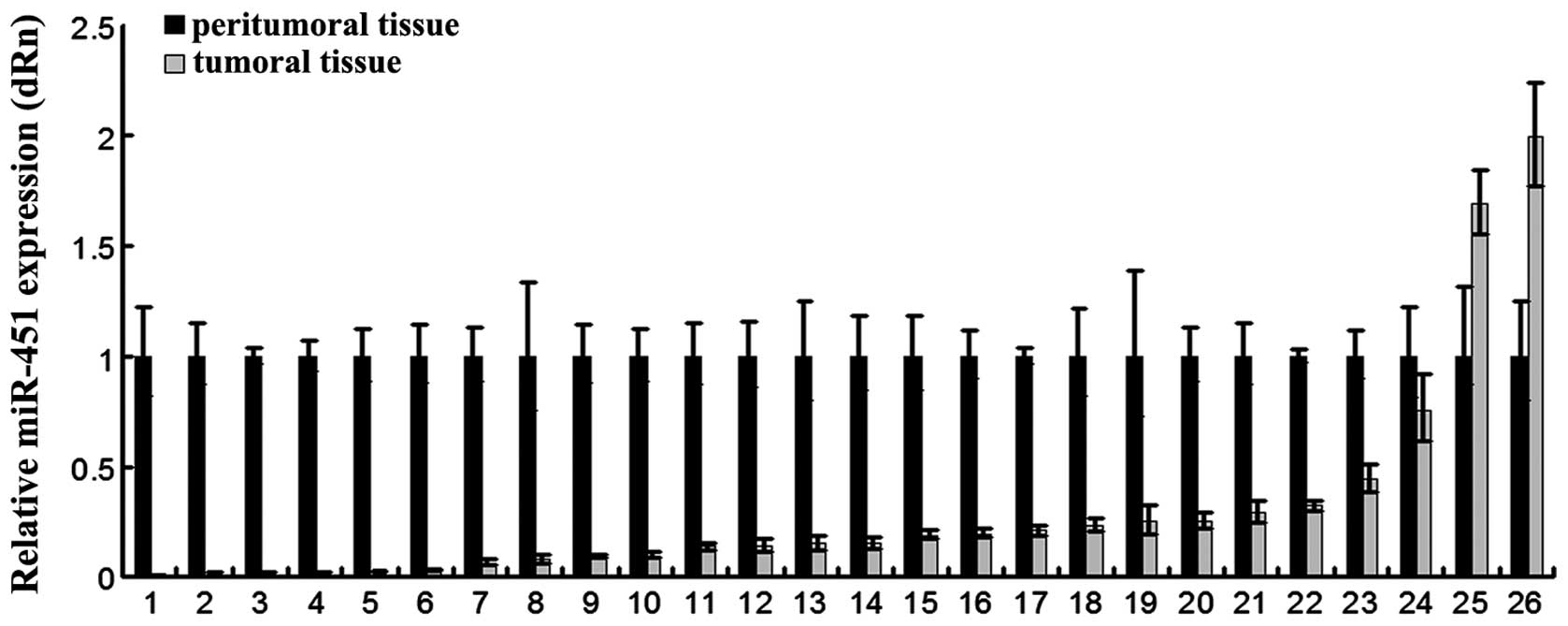
RT-PCR. As shown in Fig. 1, miR-451
was underexpressed (1.3- to 315-fold) in 92% of the tumors (24 of
26 patients) compared to the matching peritumoral tissues. The
results suggest that miR-451 could be involved in most processes of
HCC.
miR-451 interacts with the 3′UTR of ATF2
mRNA
miRNAs usually carry out their function by binding
to the 3′UTR and inhibiting the expression of target mRNAs. By
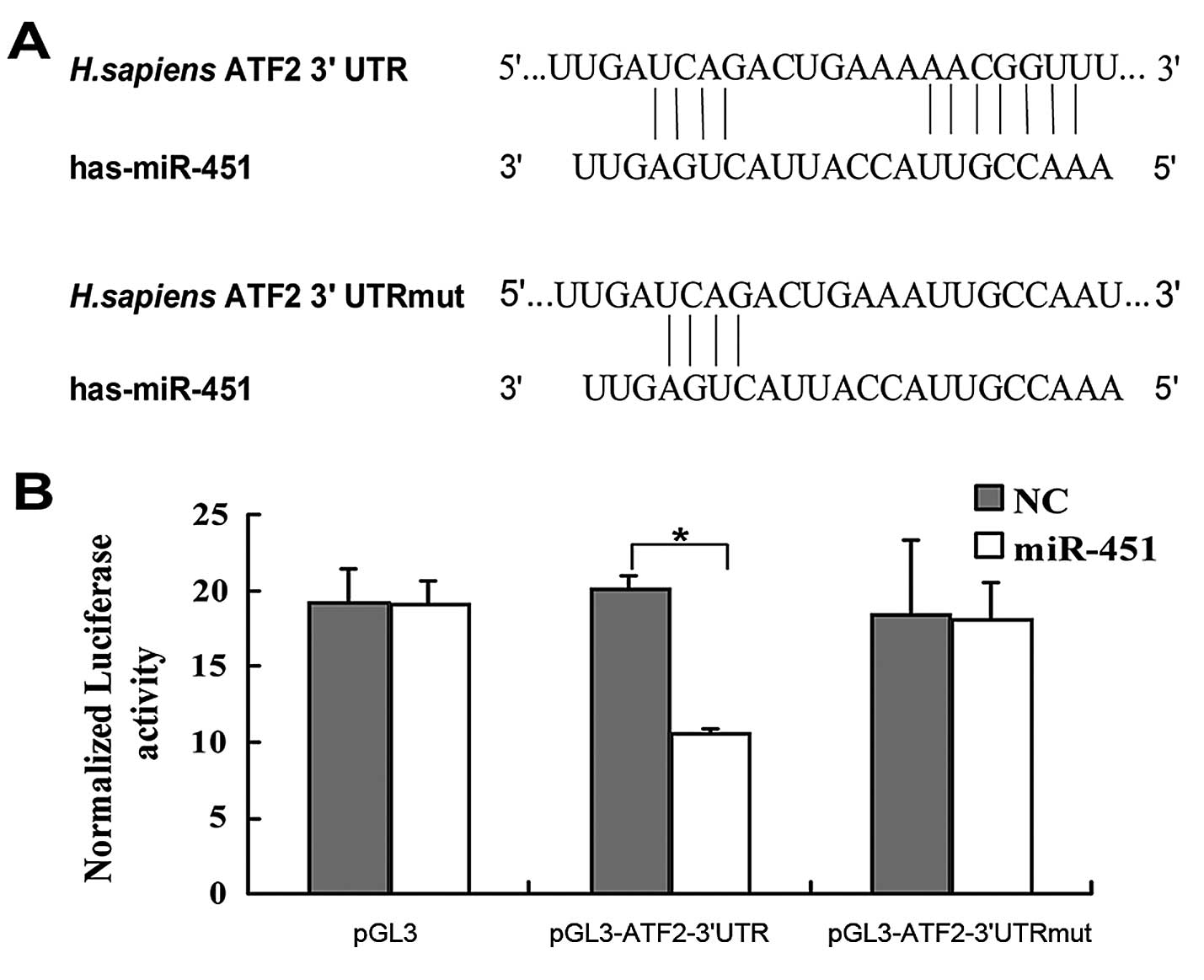
bioinformatic analysis using TargetScan (www.targetscan.org), we found that the 3′UTR of ATF2
contained a putative binding site for miR-451 (Fig. 2A). To investigate whether ATF2 is a
direct target of miR-451, the human ATF2 wild-type 3′UTR and
mutant-type 3′UTR containing the mutant sequence at the miR-451
binding site were subcloned into the pGL3 vector separately after
the firefly luciferase opening reading frame (ORF). These vectors
were cotransfected with miR-451 mimics or the negative control (NC)
RNA duplex into the HepG2 cells, respectively. The dual-luciferase
reporter assay showed that miR-451 significantly decreased the
relative luciferase activity (~37%) of the reporter containing the
wild-type 3′UTR as compared to the negative control RNA but no
decrease in luciferase activity of the reporter was detected when
the putative miR-451 binding site was mutated (Fig. 2B). This result suggests that ATF2 is
a direct target of miR-451.
miR-451 suppresses the expression of
ATF2
Inhibition of expression by miRNAs may be mediated
by degrading mRNAs (12,13) or impeding translation without
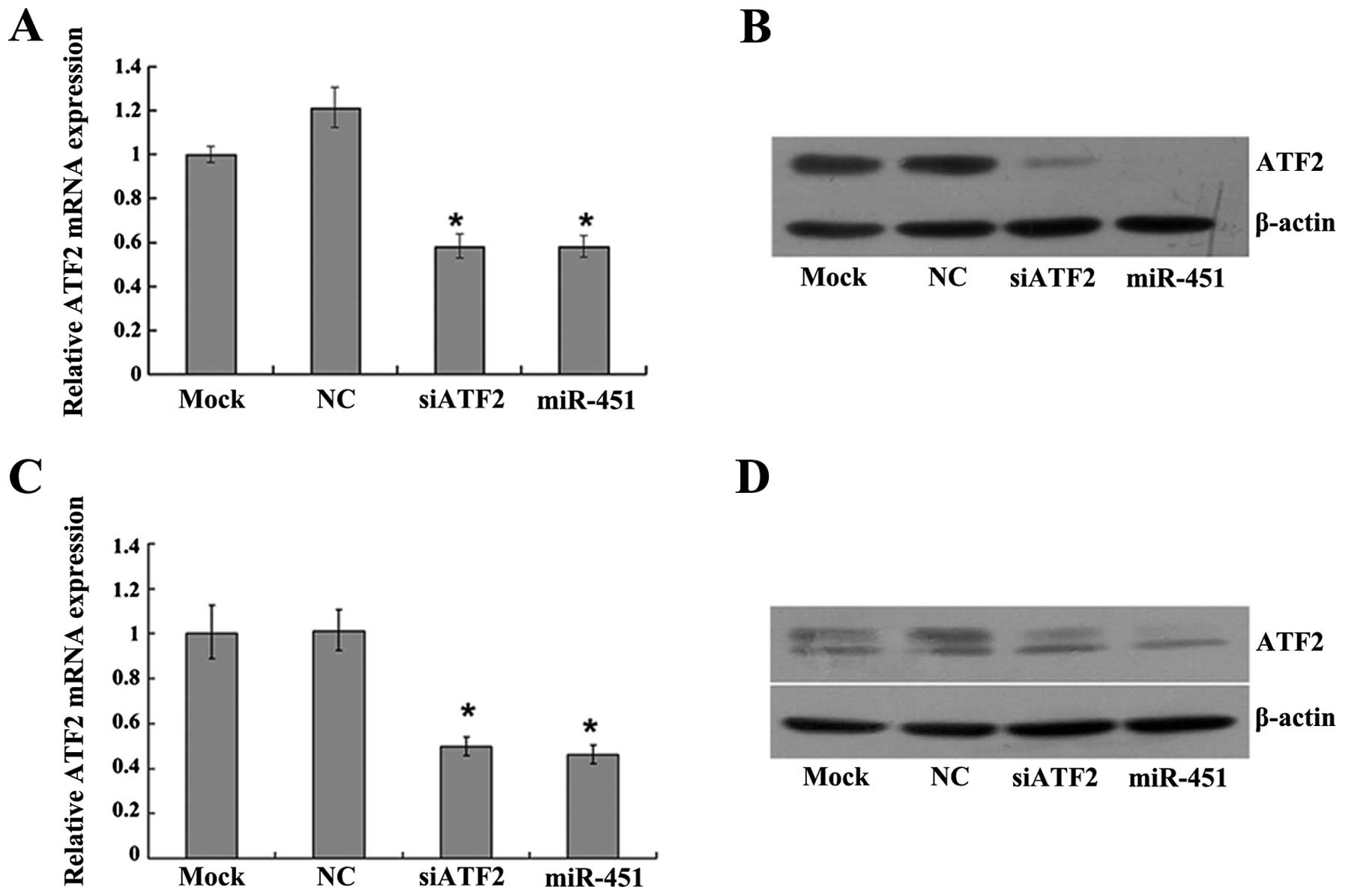
affecting the stability of mRNAs (14). To ascertain whether miR-451 inhibits
endogenous ATF2 expression, miR-451 mimics were transfected into
HepG2 and SK-Hep-1 cells, respectively. Total-RNA and protein were
extracted at 48 h and 72 h after transfection. Results from RT-PCR
and western blot analysis showed that ATF2 mRNA (Fig. 3A and C) and protein (Fig. 3B and D) were significantly reduced
after transfection of miR-451 mimics as compared to the negative
control. The results revealed that miR-451 suppresses ATF2
expression at the post-transcriptional level by degrading its
mRNA.
A significant negative correlation
between miR-451 and ATF2 expression is observed in human HCC
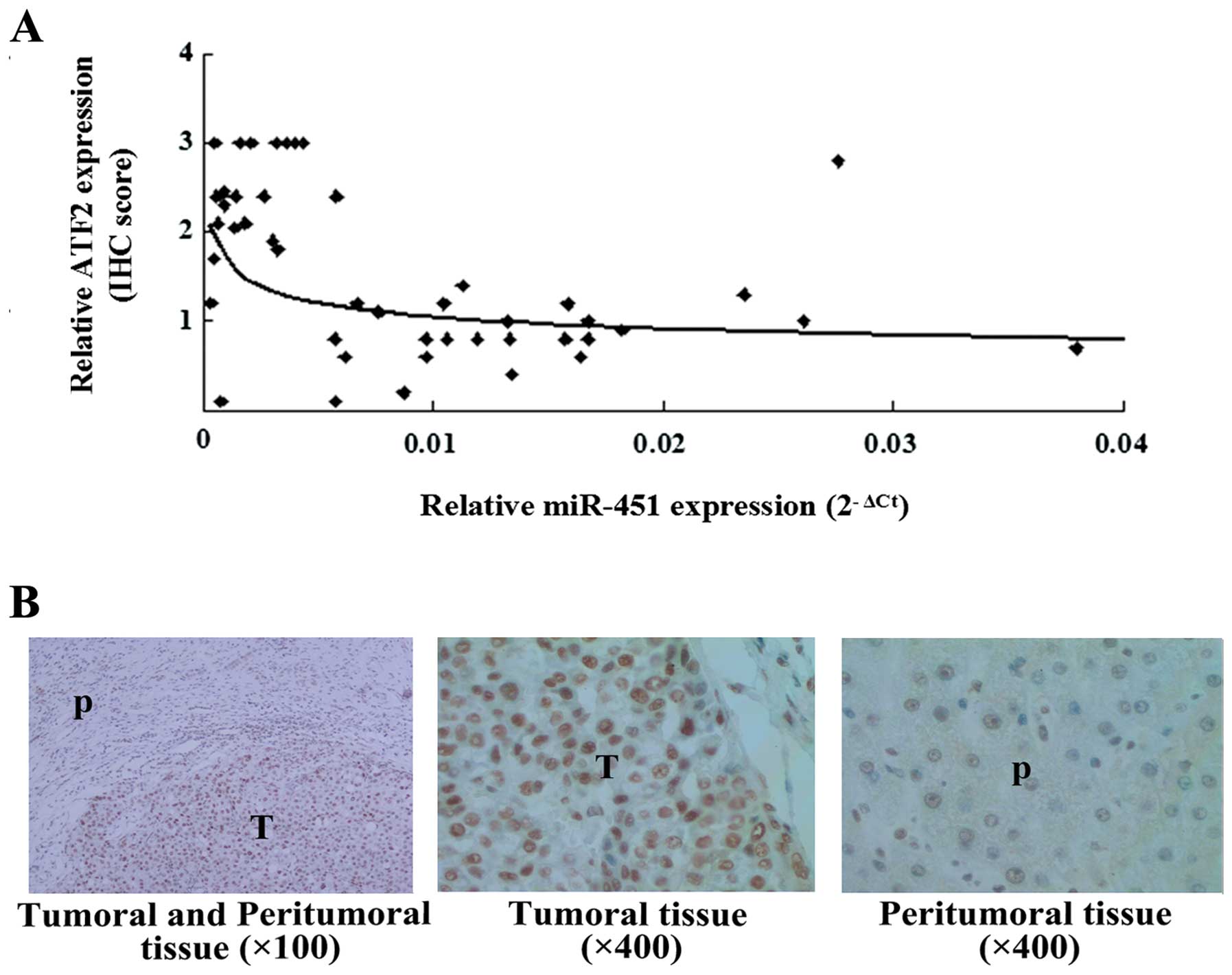
To determine the relationship between the expression
of miR-451 and ATF2, miR-451 and ATF2 expression in the 26 pairs of
tissues (including tumoral and peritumoral tissues from the 26
samples) was detected, which was represented by 2−ΔCt
and ATF2 IHC score. Based on Spearman’s rank analysis, an inverse
correlation between miR-451 and ATF2 (r=−0.5028, P=0.0002) was
shown (Fig. 4A). Research has
revealed that the location of ATF2 is related to its role in cancer
(15). Further observation in this
study showed that the upregulated ATF2 was mainly located in the
nucleus of cells in the HCC tissues (Fig. 4B), which provides evidence that ATF2
has oncogenic activity in liver cancer. These results suggest that
downregulation of miR-451 could contribute to the upregulation of
ATF2 and the tumorigenesis of HCC.
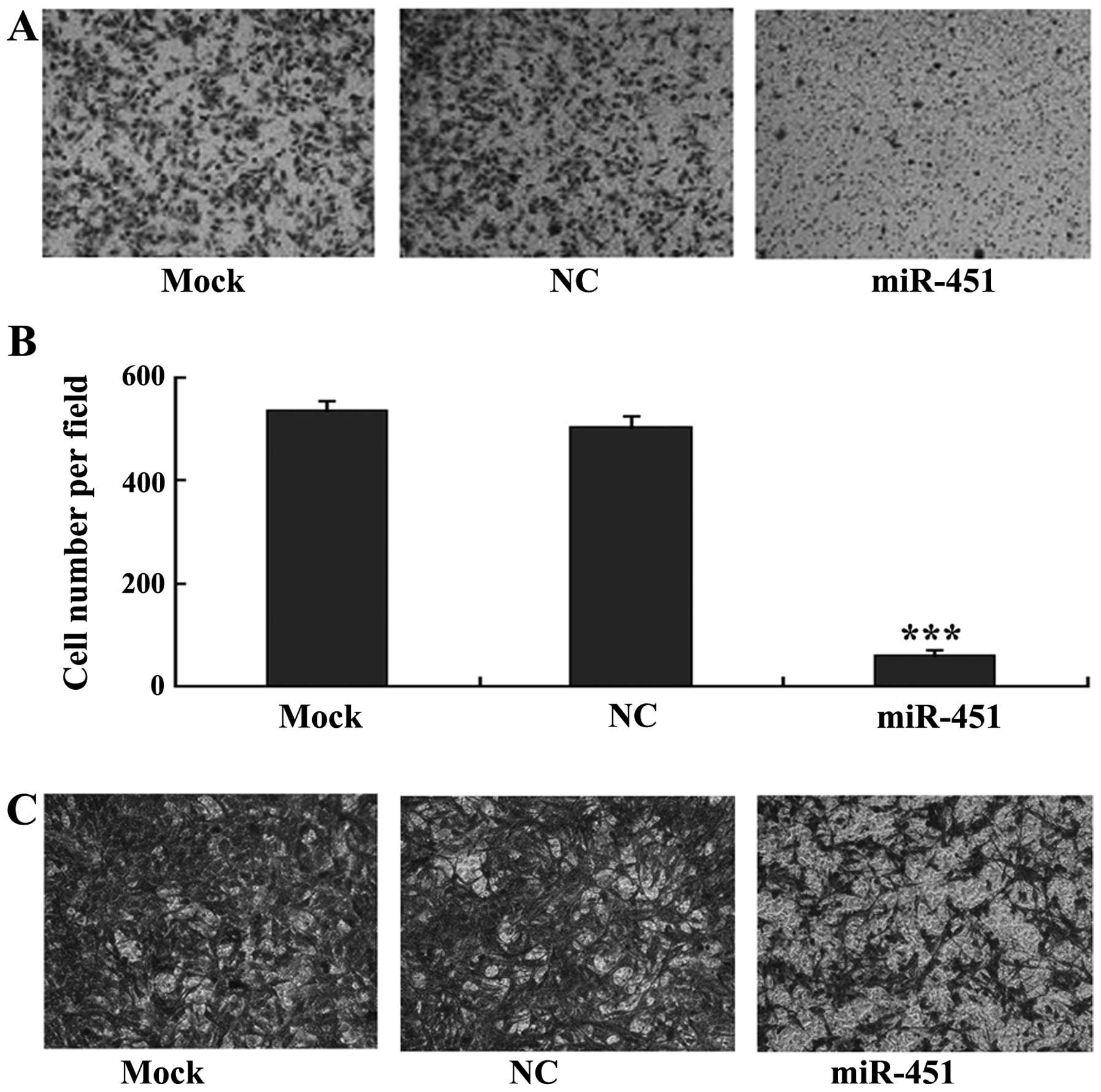
miR-451 suppresses the migration of HepG2
and SK-Hep-1 cells
miR-451 has been reported to inhibit the migration
of lung cancer cells (16). To
evaluate its potential function in liver cancer, an in vitro
transwell migration assay was performed using liver carcinoma cell
lines, HepG2 and SK-Hep-1. The cells were cultured in DMEM
containing 0.1% FBS in the upper Transwell chamber and DMEM
containing only 5% FBS without cultured cells in the lower chamber.
The data revealed that the numbers of HepG2 (Fig. 5A and B) and SK-Hep-1 (Fig. 5C) cells that migrated across the
membrane were significantly reduced following transfection with
miR-451 as compared to the number in the negative control cells.
The results suggest that overexpression of miR-451 suppresses the
migratory ability of liver carcinoma cells.
Discussion
In the present study, we have confirmed that miR-451
expression is downregulated in human HCCs and it is a direct
negative regulator of ATF2 by targeting its 3′UTR, which
contributes to an increase in ATF2 in the nucleus of neoplastic
tumor tissues inversely related to that of miR-451. Functional
analysis revealed that miR-451 suppressed the migratory ability of
hepatoma cell lines, HepG2 and SK-Hep-1. Thus, miR-451, as a
suppressor, has an important role in the progression of hepatic
carcinoma.
Dysregulated expression of miRNAs, that function as
oncogenes or tumor suppressors, occurs in various types of cancers
in a tissue-specific manner. However, widespread deregulation of
miRNAs usually occurs in the same type of cancer. For example, in
human HCC, miR-22 (17), let-7g
(18), miR-29 (19), miR-124 and miR-199b-5p were found to
be expressed at a lower level, indicating a poor survival or
prognosis for patients with HCC. miR-451 has been reported to be
highly conserved across vertebrates, and its biogenesis is
independent of Dicer (20), mainly
functioning as a tumor suppressor (16,21),
or acting to change the response to stress and start conditional
control of cell proliferation and migration (22,23).
Suppression of miR-451 expression by tamoxifen was found to promote
breast cancer cell survival and endocrine resistance (24), while transfection with the mature
miR-451 was found to disperse neurospheres, and inhibit
glioblastoma cell growth (25). In
gastric cancer, miR-451 was also found to be decreased when
compared to normal gastric mucosa (26). In the present study, we confirmed
that miR-451 was markedly decreased in HCC and suppressed the
migration of hepatoma cells; thus, it is considered as a potential
tumor suppressor in HCC similar to its function in other
tumors.
ATF2 may form homodimeric or heterodimeric activator
protein 1 (AP1) which is involved in a series of eukaryotic
cellular functions from cell proliferation and development to
stress response and apoptosis (26,27).
ATF2 can elicit oncogene activities or tumor-suppressor activities
depending on the cell or tissue type (28). For example, in melanoma, interfering
with ATF2 transcriptional activity can inhibit the proliferation of
melanoma cells in culture and the formation of tumors and
metastasis (29,30). Contrarily, expression of
transcriptionally inactive ATF2 in the presence of oncogene
activation (such as Ras mutations) in non-melanoma skin cancers
increases papilloma formation owing to the deregulated expression
of genes that promote proliferation, such as CTNNB1 (31). Consistent with its cell cycle
regulatory role, enhanced expression of ATF2 increased cell
proliferation in mouse cancer models (32,33).
However, how ATF2 elicits oncogenes or suppressors
is not fully known. Increasing research suggests that subcellular
localization of ATF2 is a crucial factor for its contrary
activities. In melanomas, nuclear ATF2 is associated with
metastasis and poor prognosis while cytoplasmic ATF2 is associated
with nonmalignant skin cancers and more favorable prognosis
(34). Its subcellular localization
could be regulated by PKCɛ that promotes oncogenic functions of
ATF2 in the nucleus while blocking its apoptotic function in the
mitochondria (15). In this
research, we found that ATF2 was significantly increased in tumor
tissue where it was localized in the nucleus in HCC compared to
adjacent normal tissues. The detailed role of ATF2 in HCC requires
further investigation. Choi et al noted that ATF2 can bind
directly to HIF-1α thereby competing with p53 leading to inhibition
of the degradation of HIF-1α, and is essential for the full
induction and maintaining the stability of HIF-1α (35). A previous study confirmed that ATF2
is essential for hepatocyte survival in embryo development
(27). Collectively, we speculate
that ATF2 plays an oncogenic role in HCC.
Notably, ATF2 usually plays an important role as a
transcriptional factor during response to stress. For example,
transient elevation of ATF2 was observed in the MAPK pathway by
hypoxia or low glucose. In contrast, to maintain survival and
migration of tumor cells such as glioma, miR-451 downregulation is
necessary at low energy (23).
Thereby whether regulation by miR-451 of ATF2 is involved in
cellular adaptation in response to cellular stress is a new topic
which warrants further study.
Tumor invasion and metastasis are often associated
with enhanced synthesis of matrix metalloproteinases (MMPs), and
among these, MMP2 and MMP9 are of central importance. As an
oncogene, ATF2 mediates MMP2 transcriptional activation induced by
p38 in breast epithelial cells (36). If miR-451 could deregulate ATF2
expression, the migration of hepatoma cells could be inhibited. In
the present study, miR-451 was found to inhibit the migration of
hepatoma cell lines, HepG2 and SK-Hep-1.
In conclusion, miR-451 is a critical regulator of
ATF2 in HCC and suppresses the migration of liver cancer cells.
These findings suggest that miR-451 is a potential target for liver
cancer therapy, and could be developed as a diagnostic or
prognostic factor.
Acknowledgements
The present study was partially supported by the
Chinese State Key Projects for Basic Research (nos. 2010CB912801
and 2009CB521804), and the Chinese National Natural Science
Foundation Projects (nos. 81072021, 31170713 and 31270836).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun H, Wei Y, Tu H, et al: Expressions of
COX-2, PKC-alpha and miR-101 in gastric cancer and their
correlations. Nan Fang Yi Ke Da Xue Xue Bao. 33:559–562. 2013.(In
Chinese).
|
|
3
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lujambio A and Lowe SW: The microcosmos of
cancer. Nature. 482:347–355. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Augello C, Vaira V, Caruso L, et al:
MicroRNA profiling of hepatocarcinogenesis identifies C19MC cluster
as a novel prognostic biomarker in hepatocellular carcinoma. Liver
Int. 32:772–782. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ying Q, Liang L, Guo W, et al:
Hypoxia-inducible microRNA-210 augments the metastatic potential of
tumor cells by targeting vacuole membrane protein 1 in
hepatocellular carcinoma. Hepatology. 54:2064–2075. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fornari F, Gramantieri L, Giovannini C, et
al: MiR-122/cyclin G1 interaction modulates p53 activity and
affects doxorubicin sensitivity of human hepatocarcinoma cells.
Cancer Res. 69:5761–5767. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qu KZ, Zhang K, Li H, Afdhal NH and
Albitar M: Circulating microRNAs as biomarkers for hepatocellular
carcinoma. J Clin Gastroenterol. 45:355–360. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tomimaru Y, Eguchi H, Nagano H, et al:
Circulating microRNA-21 as a novel biomarker for hepatocellular
carcinoma. J Hepatol. 56:167–175. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Q, Fu H, Sun F, et al: miR-16 family
induces cell cycle arrest by regulating multiple cell cycle genes.
Nucleic Acids Res. 36:5391–5404. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fu HJ, Zhu J, Yang M, et al: A novel
method to monitor the expression of microRNAs. Mol Biotechnol.
32:197–204. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bagga S, Bracht J, Hunter S, et al:
Regulation by let-7 and lin-4 miRNAs results in target mRNA
degradation. Cell. 122:553–563. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lim LP, Lau NC, Garrett-Engele P, et al:
Microarray analysis shows that some microRNAs downregulate large
numbers of target mRNAs. Nature. 433:769–773. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mathonnet G, Fabian MR, Svitkin YV, et al:
MicroRNA inhibition of translation initiation in vitro by targeting
the cap-binding complex eIF4F. Science. 317:1764–1767. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lau E, Kluger H, Varsano T, et al:
PKCepsilon promotes oncogenic functions of ATF2 in the nucleus
while blocking its apoptotic function at mitochondria. Cell.
148:543–555. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang XC, Tian LL, Jiang XY, et al: The
expression and function of miRNA-451 in non-small cell lung cancer.
Cancer Lett. 311:203–209. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang J, Yang Y, Yang T, et al:
microRNA-22, downregulated in hepatocellular carcinoma and
correlated with prognosis, suppresses cell proliferation and
tumourigenicity. Br J Cancer. 103:1215–1220. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ji J, Zhao L, Budhu A, et al: Let-7g
targets collagen type I alpha2 and inhibits cell migration in
hepatocellular carcinoma. J Hepatol. 52:690–697. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xiong Y, Fang JH, Yun JP, et al: Effects
of microRNA-29 on apoptosis, tumorigenicity, and prognosis of
hepatocellular carcinoma. Hepatology. 51:836–845. 2010.PubMed/NCBI
|
|
20
|
Yang JS, Maurin T, Robine N, et al:
Conserved vertebrate mir-451 provides a platform for
Dicer-independent, Ago2-mediated microRNA biogenesis. Proc Natl
Acad Sci USA. 107:15163–15168. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang R, Wang ZX, Yang JS, Pan X, De W and
Chen LB: MicroRNA-451 functions as a tumor suppressor in human
non-small cell lung cancer by targeting ras-related protein 14
(RAB14). Oncogene. 30:2644–2658. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Godlewski J, Nowicki MO, Bronisz A, et al:
MicroRNA-451 regulates LKB1/AMPK signaling and allows adaptation to
metabolic stress in glioma cells. Mol Cell. 37:620–632. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Godlewski J, Bronisz A, Nowicki MO,
Chiocca EA and Lawler S: microRNA-451: A conditional switch
controlling glioma cell proliferation and migration. Cell Cycle.
9:2742–2748. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bergamaschi A and Katzenellenbogen BS:
Tamoxifen downregulation of miR-451 increases 14-3-3ζ and promotes
breast cancer cell survival and endocrine resistance. Oncogene.
31:39–47. 2012.PubMed/NCBI
|
|
25
|
Gal H, Pandi G, Kanner AA, et al: miR-451
and imatinib mesylate inhibit tumor growth of glioblastoma stem
cells. Biochem Biophys Res Commun. 376:86–90. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hai T and Hartman MG: The molecular
biology and nomenclature of the activating transcription
factor/cAMP responsive element binding family of transcription
factors: activating transcription factor proteins and homeostasis.
Gene. 273:1–11. 2001. View Article : Google Scholar
|
|
27
|
Breitwieser W, Lyons S, Flenniken AM, et
al: Feedback regulation of p38 activity via ATF2 is essential for
survival of embryonic liver cells. Genes Dev. 21:2069–2082. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Eferl R and Wagner EF: AP-1: a
double-edged sword in tumorigenesis. Nat Rev Cancer. 3:859–868.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bhoumik A, Gangi L and Ronai Z: Inhibition
of melanoma growth and metastasis by ATF2-derived peptides. Cancer
Res. 64:8222–8230. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bhoumik A, Huang TG, Ivanov V, et al: An
ATF2-derived peptide sensitizes melanomas to apoptosis and inhibits
their growth and metastasis. J Clin Invest. 110:643–650. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bhoumik A, Fichtman B, Derossi C, et al:
Suppressor role of activating transcription factor 2 (ATF2) in skin
cancer. Proc Natl Acad Sci USA. 105:1674–1679. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vale-Cruz DS, Ma Q, Syme J and LuValle PA:
Activating transcription factor-2 affects skeletal growth by
modulating pRb gene expression. Mech Dev. 125:843–856. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nakamura T, Okuyama S, Okamoto S, Nakajima
T, Sekiya S and Oda K: Down-regulation of the cyclin A promoter in
differentiating human embryonal carcinoma cells is mediated by
depletion of ATF-1 and ATF-2 in the complex at the ATF/CRE site.
Exp Cell Res. 216:422–430. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Berger AJ, Kluger HM, Li N, et al:
Subcellular localization of activating transcription factor 2 in
melanoma specimens predicts patient survival. Cancer Res.
63:8103–8107. 2003.PubMed/NCBI
|
|
35
|
Choi JH, Cho HK, Choi YH and Cheong J:
Activating transcription factor 2 increases transactivation and
protein stability of hypoxia-inducible factor 1alpha in
hepatocytes. Biochem J. 424:285–296. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Song H, Ki SH, Kim SG and Moon A:
Activating transcription factor 2 mediates matrix
metalloproteinase-2 transcriptional activation induced by p38 in
breast epithelial cells. Cancer Res. 66:10487–10496. 2006.
View Article : Google Scholar
|