Introduction
Colorectal carcinoma is a leading cause of
cancer-related mortality worldwide (1). At present, radiotherapy or
chemotherapy using cytotoxic drugs is the major method of cancer
treatment, and many anticancer drugs have been applied clinically
for colon cancer (2,3). However, due to increased concerns in
regards to the side effects and drug resistance associated with
current treatments, an ongoing search for natural antitumor
therapies is underway (4,5). More effective drugs to treat
colorectal carcinoma are necessary, and the development of natural
therapeutics into potential anticancer agents is possible (6). In the past few decades, natural
bioactive substances found in products were considered to be
important antitumor drug sources. Marine seaweeds are widely used
as a source of medicine and have attracted attention due to their
minimal toxicity (7). Recent
studies have demonstrated that the structural variety of
phloroglucinol isolated from Ecklonia cava induces
pharmacological activities by inhibiting apoptosis and protecting
cells against oxidative stress. A number of studies showed that
Ecklonia cava consists of many effective components
(8). In particular, phloroglucinol
is cytotoxic to breast cancer cells and has been studied to
determine its pharmacological and immunological effects (9). However, the biological activities of
phloroglucinol have yet to be fully elucidated. Thus, we selected
phloroglucinol among many types of compounds, and evaluated the
antitumor effects of phloroglucinol in colon cancer cells.
Apoptosis is the key to the normal growth and
differentiation of diverse tissues. Thus, maintaining homeostasis
in normal tissues requires a balance between cell proliferation and
apoptosis. Apoptosis is a regulated and complex process leading to
cell death characterized by nuclear fragmentation, chromatin
condensation, membrane blebbing and cell shrinkage, which
eliminates cancer cells without damaging surrounding tissues
(10,11). Antitumorigenic agents induce
apoptosis-related signaling in cancer cells while differentiation
and development of cell tissues (12). Two major apoptosis signaling
pathways caused by caspase activation fundamentally lead to
apoptosis: an extrinsic pathway (death receptors) and an intrinsic
pathway (mitochondrial) (13,14).
Apoptosis regulated by the mitochondrial pathway, which involves
molecules such as Bcl-2 family members, leads to the release of
cytochrome c into the cytosol (15). The present study was performed to
confirm that phloroglucinol inhibits the growth of HT-29 colon
cancer cells and to determine the molecular mechanism of its
anticancer effect by investigating apoptosis signaling pathways.
Our findings suggest that phloroglucinol affects Fas-induced
apoptosis, a potential factor in colon cancer prevention.
Materials and methods
Cell culture
Human colon cancer cells (ATCC HTB-38) and rat small
intestine epithelial cells (IEC-6, ATCC CRL-1592) were purchased
from the American Type Culture Collection (ATCC; Rockville, MD,
USA). The cells were maintained in a humidified 5% CO2,
95% air, 37°C environment in Roswell Park Memorial Institute
(RPMI)-1640 medium. Dulbecco’s modified Eagle’s medium (DMEM) was
supplemented with penicillin/streptomycin (P/S), and HT-29 and
IEC-6 cell cultures were supplemented with 10% fetal bovine serum
(FBS; HyClone, Inc., South Logan, UT, USA). Cells were cultured to
80% confluency in 100-mm dishes. The medium was replaced every 2
days.
Cell viability
Phloroglucinol was obtained from Sigma- Aldrich (St.
Louis, MO, USA). The effects of diverse phloroglucinol
concentrations on cellular proliferation of HT-29 and IEC-6 cells
were examined colorimetrically after 24 h using the
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxy-
phenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay with Cell
Titer 96® AQueous One solution reagent (Promega,
Madison, WI, USA). To confirm viability, cells were seeded onto
96-well plates at 2×104 cells/well in 100 μl medium and
incubated for 24 h. Attached cells were maintained in serum-free
medium (SFM) for 12 h, after which the medium was replaced with SFM
containing phloroglucinol (0–50 μg/ml) for another 24 h. Cells were
then incubated with MTS solution at 37°C for 30–60 min, and the
absorbance of the solution in each well was measured at 490 nm
using a microplate reader (Benchmark microplate reader; Bio-Rad
Laboratories, Hercules, CA, USA).
Caspase activity
Caspase activities were measured using caspase-3
substrate I (Ac-DEVD-pNA; 235400), caspase-8 substrate I
(Ac-IETD-pNA), caspase-3 inhibitor [Z-D(OMe)- E-(Ome)-V-D(OMe)-FMK;
368057; Calbiochem, San Diego, CA, USA], and caspase-8 inhibitor
(Z-IETD-FMK; R&D Systems, Minneapolis, MN, USA). HT-29 cells
were seeded in culture dishes and grown to 60% confluency. These
cells were treated with 50 μM caspase inhibitor for 1 h and
phloroglucinol for 24 h, followed by addition of caspase lysis
buffer [2.5 mM HEPES (pH 7.5) 5 mM EDTA, 2 mM DTT, 0.1% CHAPS]. A
total of 100 μg/protein/100 μl was collected, and 2 μl of the
substrate was added to the wells. Cells were incubated with caspase
substrate in a shaking incubator at 37°C for 6 h. The absorbance at
405 nm was then determined using an enzyme-linked immunosorbent
assay (ELISA) plate reader (Bio-Rad, Hercules, CA, USA).
Apoptosis assay
Phloroglucinol treatment-induced apoptosis was
determined using a Muse™ Annexin V and Dead Cell kit (EMD Millipore
Co., Hayward, CA, USA). The cells were seeded onto 6-well plates at
60% confluency, and the medium was replaced with SFM for 4 h,
followed by SFM containing phloroglucinol (0–50 μg/ml). After 24 h,
the cells were collected in 1% FBS-RPMI-1640 medium and mixed using
the Muse cell analyzer (EMD Millipore Co.).
Western blotting
HT-29 cells in 100-mm dishes were cultured to 60%
confluency and then incubated in SFM for 6 h, after which SFM
containing phloroglucinol (0–50 μg/ml) was added to the cells for
24 h. To prepare whole-cell extracts, cells were washed with
phosphate-buffered saline (PBS) and suspended in extraction buffer
[20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% NP-40, 1 mM EGTA, 1 mM
EDTA, 0.25% Na-deoxycholate, 2.5 mM sodium pyrophosphate]
containing protease inhibitors (1 mM sodium orthovanadate, 1 μg/ml
aprotinin, 1 μg/ml pepstatin, 1 μg/ml leupeptin, 1 mM NaF, 1 mM
PMSF) on ice. The extracts were centrifuged at 12,000 rpm for 10
min, and the supernatant was used for western blotting. Boiling
sample buffer was added to the total cell lysate, and the samples
were boiled for 10 min at 100°C. Proteins were separated in 7–15%
SDS-PAGE and transferred onto polyvinylidene fluoride membranes
(Millipore, Billerica, MA, USA). Membranes were blocked for 1 h 30
min at room temperature in blocking buffer [1% bovine serum albumin
(BSA) in Tris-buffered saline-Tween-20 (TBS-T)], followed by
incubation with primary antibodies (1:1,000 in 1% BSA/TBS-T)
overnight at 4°C or for 2 h 30 min at room temperature. The
membranes were then washed three times for 10 min in TBS-T, and
horse-radish peroxidase (HRP)-conjugated goat, mouse or rabbit
secondary antibody was added (1:10,000 in 1% BSA/TBS-T). Reactive
bands were detected using Super Signal West Pico chemiluminescent
substrate (Thermo Fisher Scientific Inc., Rockford, IL, USA) and
expressed on Kodak X-ray film.
Statistical analysis
Statistical analyses were performed using SPSS
software (v. 18.0; SPSS Inc., Chicago, IL, USA). The results are
represented as means ± standard deviation (SD). Differences between
groups were determined using Duncan’s multiple range test.
Statistical significance was set at P<0.05.
Results
Phloroglucinol inhibits proliferation of
the HT-29 cells
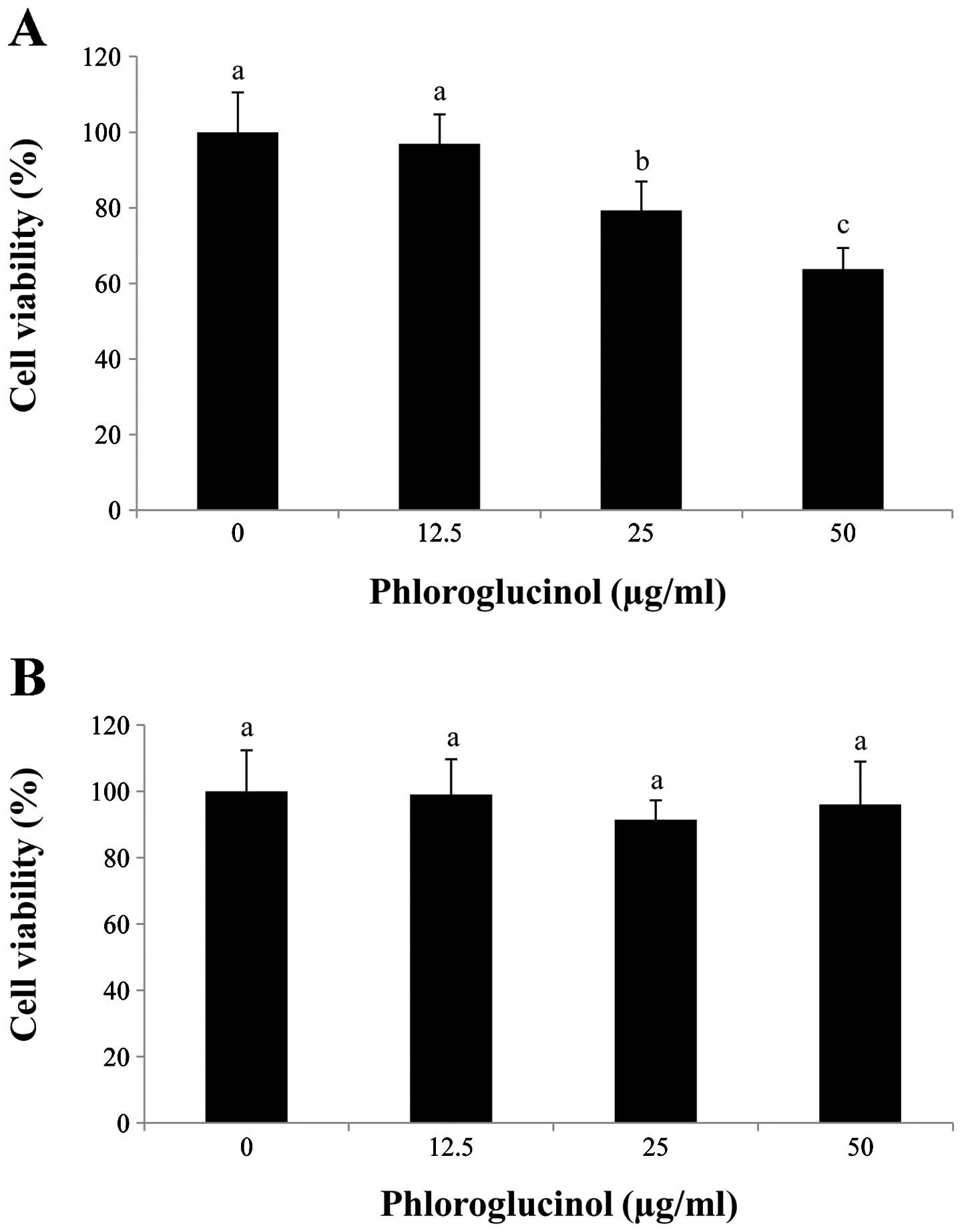
In preliminary studies, we determined the effects of
phloroglucinol treatment (0, 12.5, 25 and 50 μg/ml) on HT-29 colon
cancer cells using the MTS assay (Fig.
1). Our data showed that phloroglucinol treatment decreased
HT-29 cell proliferation in a concentration-dependent manner. In
comparison with the control, 50 μg/ml phloroglucinol for 24 h
inhibited cell viability by 60%. In contrast, IEC-6 cells were
unaffected by the phloroglucinol treatment.
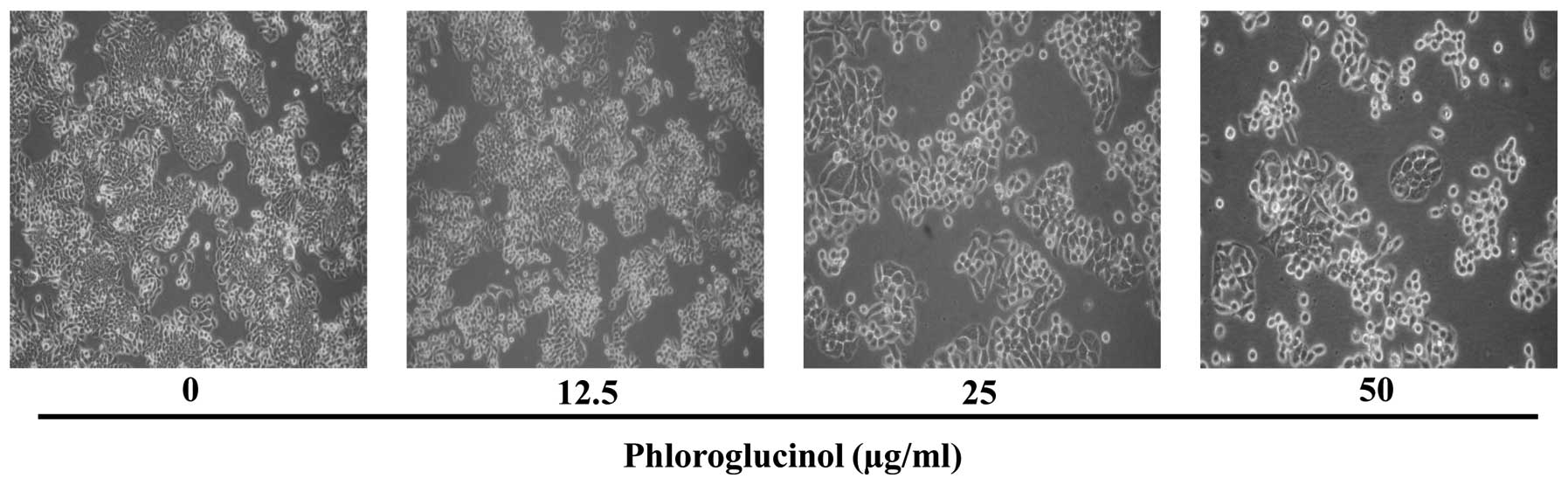
Phloroglucinol induces morphological
changes in the HT-29 cells
Since phloroglucinol significantly reduced HT-29
cell viability, we used these concentrations to determine
morphological changes using light microscopy (Fig. 2). We found that phloroglucinol
decreased cell growth as well as cell size in a
concentration-dependent manner.
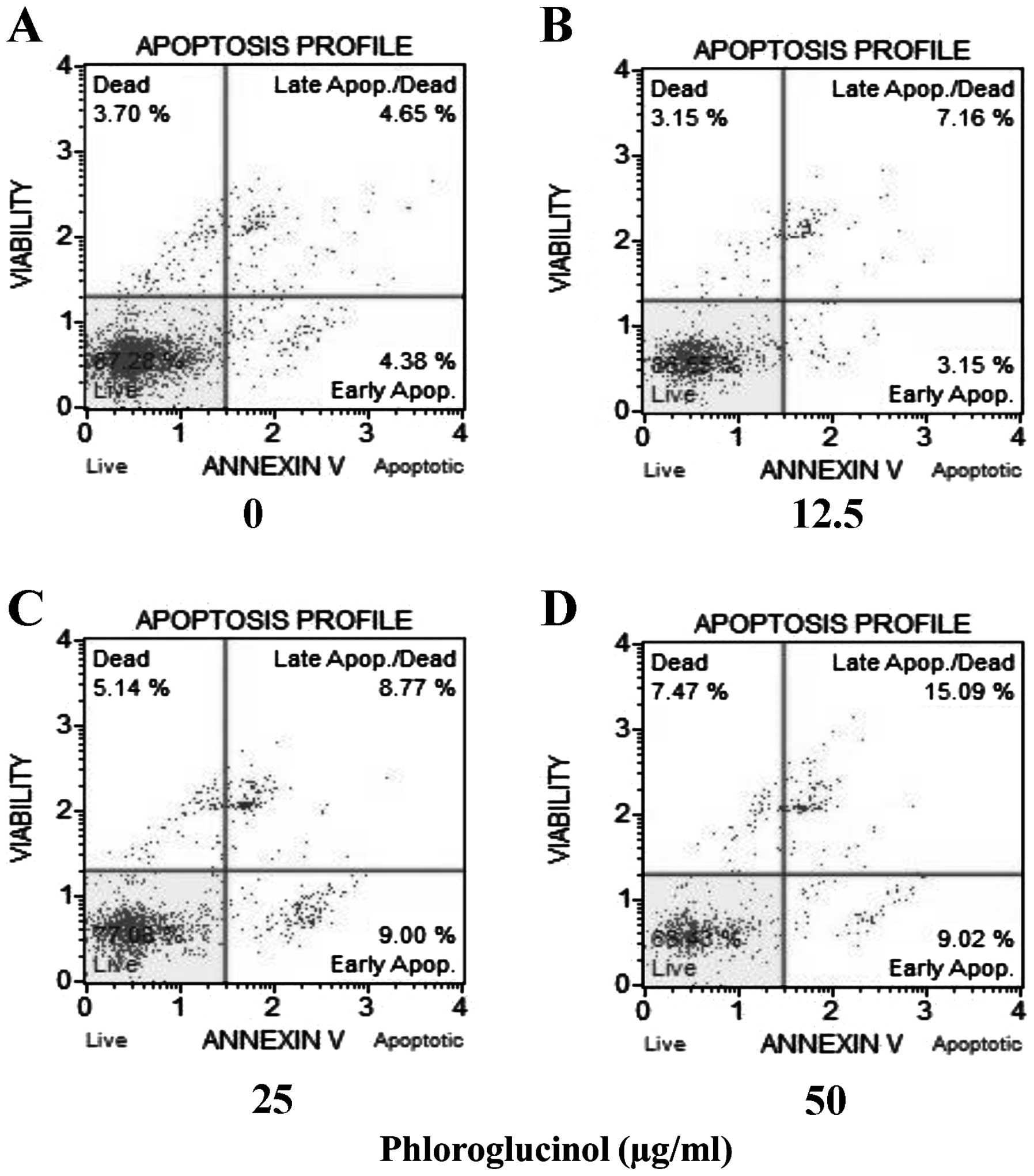
Phloroglucinol increases the rate of
cellular apoptosis in a concentration-dependent manner as revealed
by the Annexin V assay
Colon cancer HT-29 cells were treated with
phloroglucinol (0, 12.5, 25 and 50 μg/ml), and then the percentages
of necrotic and apoptotic cells were evaluated by staining with
7-aminoactinomycin D (7-ADD) and Annexin V. In each analysis,
non-apoptotic viable cells showed negative staining with Annexin V
and 7-AAD. During early apoptosis, cells were Annexin V-positive
and 7-AAD-negative; during late apoptosis, cells were Annexin
V-positive and 7-AAD-positive. Mechanically injured cells were
Annexin V-negative and 7-AAD-positive. In the present study,
phloroglucinol treatment of HT-29 cells increased the percentage of
apoptotic cells in a concentration-dependent manner. Untreated cell
populations contained 87.28% living cells and 3.7% necrotic cells.
Treatment with phloroglucinol (12.5, 25 or 50 μg/ml) for 24 h
resulted in 10.31, 17.77 and 24.11% apoptotic cells, respectively
(Fig. 3).
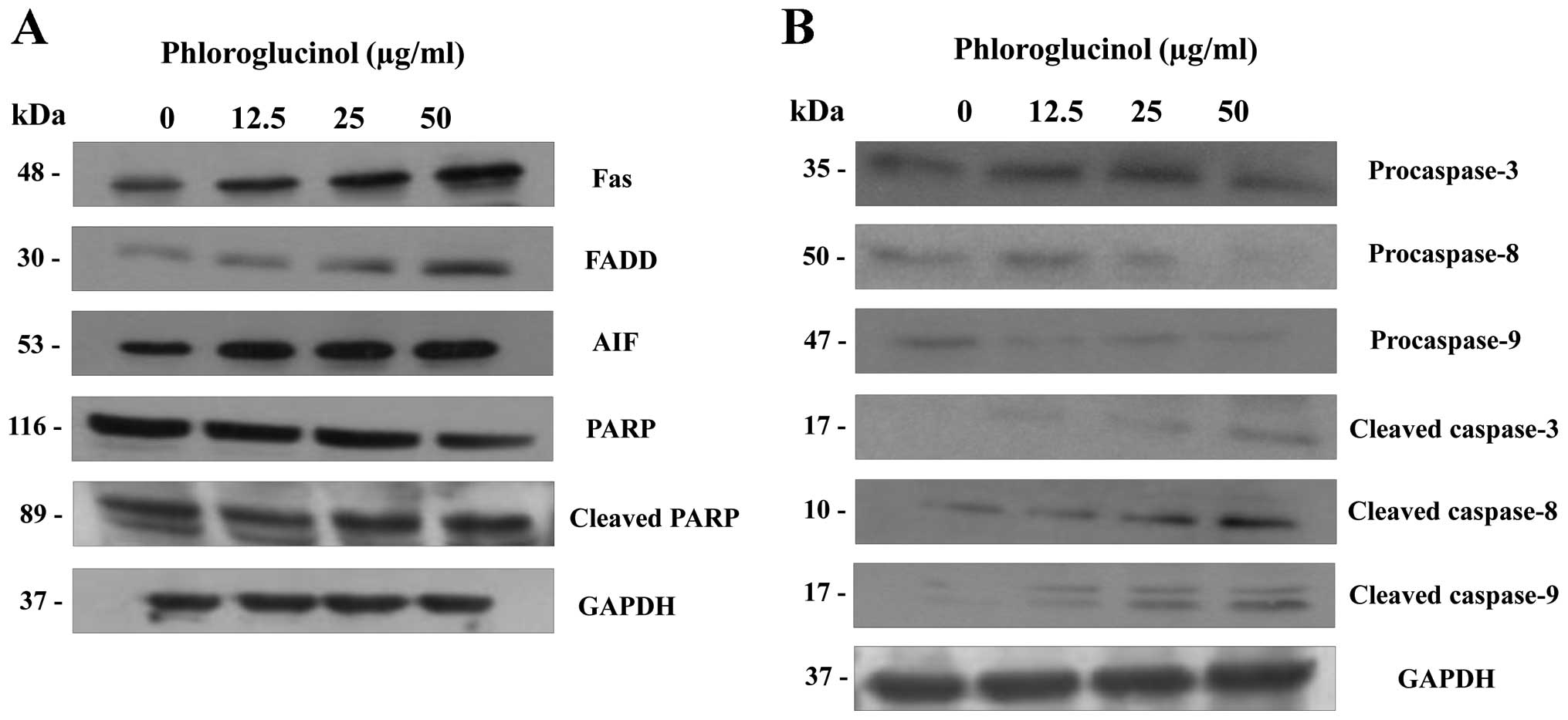
Phloroglucinol induces the expression of
apoptosis regulatory proteins
The multifactorial process of apoptosis is triggered
by two main pathways (16). As
shown in Fig. 4, we focused on the
extrinsic pathway mediated by Fas. This pathway is regulated by
activation of tumor necrosis factor (TNF) receptors and cell
surface death receptors, such as Fas (CD95, APO-1) (17). The Fas signaling pathway upregulates
downstream signal transduction, including members of the caspase
family. Subsequently, apoptotic substrates are cleaved, including
poly(ADP-ribose) polymerase (PARP) (18). In the present study, phloroglucinol
treatment increased the expression of FAS and FADD and cleaved
PARP, caspase-3, -8 and -9 (Fig.
4). These findings indicate that phloroglucinol induces
apoptosis through the Fas signaling pathway.
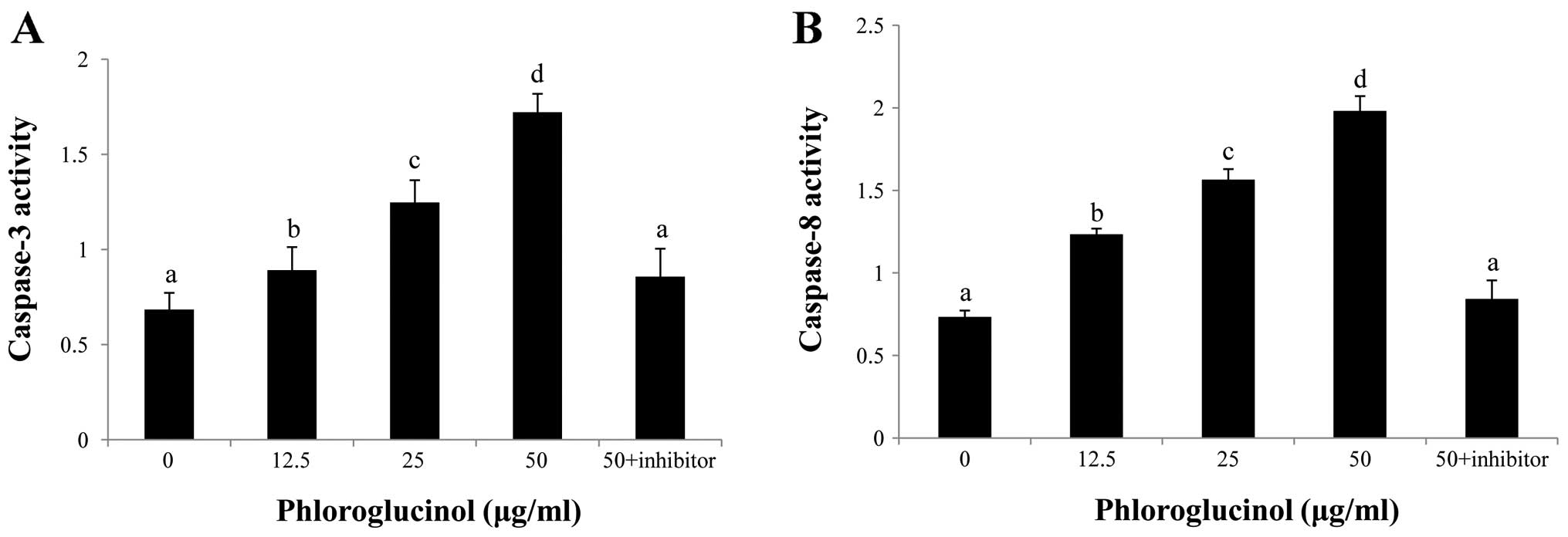
Phloroglucinol induces activation of
caspase-3 and caspase-8
To confirm which caspases are involved in
phloroglucinol-induced apoptosis, caspase inhibitors were
evaluated. Our results demonstrated that caspase-3 and -8
inhibitors potently repressed phloroglucinol-induced apoptosis
(Fig. 5). These results indicate
that caspase-3 and caspase-8 activation are involved in
phloroglucinol-induced apoptosis.
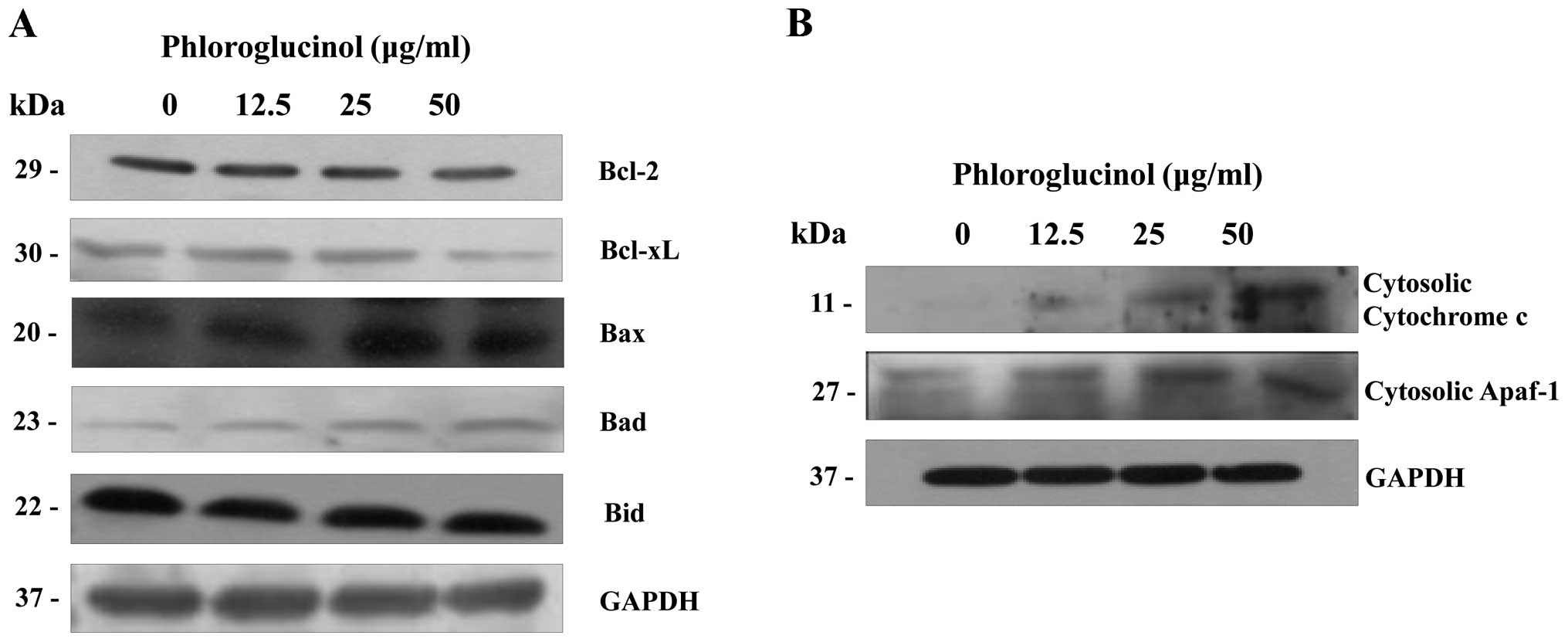
Phloroglucinol induces mitochondrial
membrane potential dissipation and cytochrome c release
The mitochondria- related pathway is a significant
apoptotic pathway characterized by cytochrome c release from
the mitochondria into the cytosol and by disorganized mitochondrial
transmembrane potential (19). In
addition, the mitochondrial apoptosis pathway commonly involves
Bcl-2 family proteins. Variations in the proportion of these
members affect the life or death of cells (20). In order to determine whether
cytochrome c release is enhanced in phloroglucinol-treated
HT-29 cells, we analyzed the cytosolic expression levels of
cytochrome c and Bcl-2 family proteins. Levels of Bcl-xL and
Bcl-2 expression were simultaneously suppressed (Fig. 6A); conversely, levels of Bax, Bad,
and Bid expression were increased. Subsequently, the expression
levels of cytochrome c and apoptotic protease activating factor-1
(Apaf-1) in the cytosol were also increased (Fig. 6B). These results indicate that
collaboration among the Bcl-2 family proteins was involved in
mitochondrial change during the advancement of apoptosis by
phloroglucinol.
Discussion
Apoptosis is an essential process involved in
homeostasis and maintenance of multicellular organisms by
extirpating superfluous cells (21). Furthermore, induction of apoptosis
is critical to remedy cancer (22).
In this study, we investigated the interaction between the
extrinsic and intrinsic pathways of apoptosis induced by
phloroglucinol in human colon cancer HT-29 cells. We determined
that phloroglucinol inhibits cell proliferation by inducing
apoptosis: we observed altered cellular morphology (whole cell
numbers and cell size were reduced in a density-dependent manner)
following changes in the levels of pro-apoptotic and anti-apoptotic
proteins and caspase activation. Moreover, we also showed evidence
that the cell apoptosis ratio increased following treatment with
high phloroglucinol concentrations.
The death receptor-mediated pathway is triggered by
FasL and its receptor FADD (23,24).
FADD then induces caspase-8 via interaction with death effector
regions, leading to activation of caspase-8 and the apoptotic
protease cascade via effector caspase activation (25,26).
In this study, phloroglucinol increased the extrinsic signaling
protein expression levels of Fas, FADD and caspase-8.
Caspases, which are cysteine proteases, play an
important role in regulating the apoptotic response (27). Activation of apoptotic signaling
pathways results in cytochrome c release from the
mitochondria in concert with cleavage and activation of caspase-9,
which in turn also induces caspase-3 activation (28,29).
In the present study, we found that phloroglucinol- induced HT-29
cell apoptosis was associated with activation of caspase-3, -8 and
-9.
Moreover, cell apoptosis is modulated by Bcl-2
family members, which negatively or positively regulate apoptosis.
Anti-apoptotic Bcl-2 and Bcl-xL proteins are able to intercept
diverse apoptotic signals, whereas pro-apoptotic proteins, such as
Bax and Bad, can cause the release of apoptosis palpating factors
into the cytoplasm (30,31). Our data showed that phloroglucinol
induced a decrease in Bcl-2 and Bcl-xL expression and an increase
in Bax and Bad expression. This alteration may trigger the release
of cytochrome c and Apaf-1 into the cytosol and the cleavage
of poly(ADP-ribose) polymerase (32). In this study, the release of
apoptosis-promoting factors such as cytosolic cytochrome c
and Apaf-1 was increased following phloroglucinol treatment.
Therefore, these findings suggest that phloroglucinol induces
apoptosis through mitochondrial dysfunction by modulating the
protein levels of Bcl-2 family members. These findings may aid in
the understanding of the mechanism underlying induction of
apoptosis by phloroglucinol in carcinoma cells.
In conclusion, our study demonstrated that
phloroglucinol significantly inhibited growth and induced apoptosis
of HT-29 colon cancer cells via both the extrinsic and intrinsic
cell death signaling pathways. This research provides a mechanism
for the antitumorigenic activity of phloroglucinol. In the
apoptotic process, phloroglucinol upregulated pro-apoptotic and
downregulated anti-apoptotic proteins, followed by caspase
activation. Our results partially explain the effect of
phloroglucinol on HT-29 colon cancer cell apoptosis. Although a
fully detailed mechanism is not clear, phloroglucinol could be used
as a potential therapeutic candidate in the prevention or treatment
of colorectal cancer in the future.
Acknowledgements
This research was supported by Fishery
Commercialization Technology Development Program through iPET
(Korea Institute of Planning and Evaluation for Technology in Food,
Agriculture, Forestry and Fisheries) funded by Ministry of Oceans
and Fisheries (MOF) (111090-03-3-HD110).
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu Y,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar
|
|
2
|
Matsui T, Omura K, Kawakami K, Morita S
and Sakamoto J: Genotype of thymidylate synthase likely to affect
efficacy of adjuvant 5-FU based chemotherapy in colon cancer. Oncol
Rep. 16:1111–1115. 2006.PubMed/NCBI
|
|
3
|
Oehler C and Ciernik IF: Radiation therapy
and combined modality treatment of gastrointestinal carcinomas.
Cancer Treat Rev. 32:119–138. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park EJ and Pezzuto JM: Botanicals in
cancer chemoprevention. Cancer Metast Rev. 21:231–255. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stan SD, Kar S, Stoner GD and Singh SV:
Bioactive food components and cancer risk reduction. J Cell
Biochem. 104:339–356. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wei A, Zhou D, Xiong C, Cai Y and Ruan J:
A novel non-aromatic B-ring flavonoid: isolation, structure
elucidation and its induction of apoptosis in human colon HT-29
tumor cell via the reactive oxygen species-mitochondrial
dysfunction and MAPK activation. Food Chem Toxicol. 49:2445–2452.
2011. View Article : Google Scholar
|
|
7
|
Mhadhebi L, Mhadhebi A, Robert J and
Bouraoui A: Antioxidant, anti-inflammatory and antiproliferative
effects of aqueous extracts of three mediterranean brown seaweeds
of the genus cystoseira. Iran J Pharm Res. 13:207–220.
2014.PubMed/NCBI
|
|
8
|
Kang HS, Chung HY, Kim JY, Son BW, Jung HA
and Choi JS: Inhibitory phlorotannins from the edible brown alga
Ecklonia stolonifera on total reactive oxygen species (ROS)
generation. Arch Pharm Res. 27:194–198. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim MM, Ta QV, Mendis E, Rajapakse N, Jung
WK, Byun HG, Jeon YJ and Kim SK: Phlorotannins in Ecklonia
cava extract inhibit matrix metalloproteinase activity. Life
Sci. 79:1436–1443. 2006.PubMed/NCBI
|
|
10
|
Lin ML, Chen SS, Lu YC, Liang RY, Ho YT,
Yang CY and Chung JG: Rhein induces apoptosis through induction of
endoplasmic reticulum stress and Ca2+-dependent
mitochondrial death pathway in human nasopharyngeal carcinoma
cells. Anticancer Res. 27:3313–3322. 2007.PubMed/NCBI
|
|
11
|
Debatin KM and Krammer PH: Death receptors
in chemotherapy and cancer. Oncogene. 23:2950–2966. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu W, Lee SK, Jung MJ, Heo SI, Hur JH and
Wang MH: Induction of cell cycle arrest and apoptosis by the ethyl
acetate fraction of Kalopanax pictus leaves in human colon
cancer cells. Bioresour Technol. 101:9366–9372. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Degterev A and Yuan J: Expansion and
evolution of cell death programmes. Nat Rev Mol Cell Biol.
9:378–390. 2008. View
Article : Google Scholar
|
|
14
|
Tan ML, Ooi JP, Ismail N, Moad Al and
Muhammad TS: Programmed cell death pathways and current antitumor
targets. Pharm Res. 26:1547–1560. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sakinah SA, Handayani ST and Hawariah LP:
Zerumbone induced apoptosis in liver cancer cells via modulation of
Bax/Bcl-2 ratio. Cancer Cell Int. 7:1–11. 2007.PubMed/NCBI
|
|
16
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grimm S and Brdiczka D: The permeability
transition pore in cell death. Apoptosis. 12:841–855. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cryns V and Yuan J: Proteases to die for.
Genes Dev. 12:1551–1570. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gogvadze V and Orrenius S: Mitochondrial
regulation of apoptotic cell death. Chem Biol Interact. 163:4–14.
2006. View Article : Google Scholar
|
|
20
|
Kim TH, Zhao Y, Ding WX, Shin JN, He X,
Seo YW, Chen J, Rabinowich H, Amoscato AA and Win XM:
Bid-cardiolipin interaction at mitochondrial contact site
contributes to mitochondrial cristae reorganization and cytochrome
c release. Mol Biol Cell. 15:3061–3072. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Danial NN and Korsmeyer SJ: Cell death:
critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fesik SW: Promoting apoptosis as a
strategy for cancer drug discovery. Nat Rev Cancer. 5:876–885.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nagata S and Golstein P: The Fas death
factor. Science. 267:1449–1456. 1995. View Article : Google Scholar
|
|
24
|
Chinnaiyan AM, O’Rourke K, Tewari M and
Dixit VM: FADD, a novel death domain-containing protein, interacts
with the death domain of Fas and initiates apoptosis. Cell.
81:505–512. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gupta SC, Reuter S, Phromnoi K, Park B,
Hema PS, Nair M and Aggarwal BB: Nimbolide sensitizes human colon
cancer cells to TRAIL through reactive oxygen species-and
ERK-dependent up-regulation of death receptors, p53, and Bax. J
Biol Chem. 286:1134–1146. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Khosravi-Far R and Esposti MD: Death
receptor signals to mitochondria. Cancer Biol Ther. 3:1051–1057.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song G, Mao YB, Cai QF, Yao LM, Ouyang GL
and Bao SD: Curcumin induces human HT-29 colon adenocarcinoma cell
apoptosis by activating p53 and regulating apoptosis-related
protein expression. Braz J Med Biol Res. 38:1791–1798. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Juo P, Kuo CJ, Yuan J and Blenis J:
Essential requirement for caspase-8/FLICE in the initiation of the
Fas-induced apoptotic cascade. Curr Biol. 8:1001–1008. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Varfolomeev EE, Schuchmann M, Luria V,
Chiannilkulchai N, Beckmann JS, Mett IL, Rebrikov D, Brodianski VM,
Kemper OC, Kollet O, et al: Targeted disruption of the mouse
caspase 8 gene ablates cell death induction by the TNF receptors,
Fas/Apol, and DR3 and is lethal prenatally. Immunity. 9:267–276.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vaskivuo TE, Stenback F and Tapanainen JS:
Apoptosis and apoptosis related factors Bcl-2, Bax, tumor necrosis
factor-alpha, and NF-kappaB in human endometrial hyperplasia and
carcinoma. Cancer. 95:1463–1471. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim R: Unknotting the roles of Bcl-2 and
Bcl-xL in cell death. Biochem Biophys Res Commun. 333:336–343.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Park HK, Kim IH, Kim JK and Nam TJ:
Induction of apoptosis and the regulation of ErbB signaling by
laminarin in HT-29 human colon cancer cells. Int J Mol Med.
32:291–295. 2013.PubMed/NCBI
|