Introduction
Fatty acid synthase (FASN) is a key enzyme for
catalyzing endogenous fatty acid synthesis within the cells; the
synthesized fatty acids are involved in the synthesis of cell
constituent structure, such as cell biofilm. In the early 1990s, it
was first discovered that FASN was highly expressed in breast
cancer, and its expression level was closely related to tumor stage
and prognosis. Evidence from subsequent studies has generally
supported this finding and has highlighted the contribution of FASN
in tumor occurrence and development in esophageal, lung and gastric
cancer, malignant melanoma, ovarian, prostate and nasopharyngeal
cancer (1–4). To date, there has been no systematic
study focusing on FASN expression and function in hepatocellular
carcinoma (HCC). The aim of the present study was to verify the
high expression of FASN in HCC cells at the histological and
cellular levels, and to construct FASN shRNA eukaryotic expression
vector for interfering FASN expression in HCC cell line SK-Hep-1,
in an effort to explore the role of FASN in the proliferation,
apoptosis, invasion and migration of HCC cells.
Materials and methods
Cell line and culture
Human normal liver cell line 7702 and HCC cell lines
HepG2, SMMC7721, MHCC97L, MHCC97H and SK-Hep-1 were provided by the
Stem Cell Bank, Chinese Academy of Sciences (Shanghai, China). All
the cells were cultured with DMEM containing 10% fetal bovine serum
in a 5% CO2 incubator at 37°C. FASN mouse anti-human
monoclonal antibody was purchased from Santa Cruz (USA).
HRP-conjugated goat anti-mouse secondary antibody, BCA protein
assay kit and SABC immunohistochemistry kit were purchased from
Wuhan Boster Biological Engineering Co., Ltd. (Wuhan, Hubei,
China). BLOCK-iT™ HiPerform™ Lentiviral Pol II miR RNAi Expression
System with EmGFP and Lipofectamine 2000 (Invitrogen, USA) were
used for the construction of vector. The apoptosis kit was
purchased from Jingmei Biotech Co., Ltd. (Shenzhen, Guangdong,
China).
Harvesting HCC tissue specimens
HCC tissue and tumor-adjacent tissue specimens were
harvested from 20 patients with primary HCC undergoing resection of
HCC in the First Affiliated Hospital of Xi’an Jiaotong University,
China, in 2012. No patients received chemotherapy or radiotherapy
prior to the surgery. HCC diagnosis was performed by imaging and
histopathologic examination. There were 13 males and 7 females. All
participating patients signed the informed consent and the study
was approved by the Ethics Committee of the First Affiliated
Hospital of Xi’an Jiaotong University, College of Medicine. The
obtained tissue specimens were stored at −80°C.
Immunohistochemistry
Paraffin sections were dewaxed in xylene, rehydrated
in gradient alcohol and retrieved with citric acid at high
temperature and high pressure. Then, the sections were immersed in
3% hydrogen peroxide for 10 min to eliminate endogenous peroxidase,
and blocked with serum for 30 min. The sections were incubated with
FASN antibody (1:250) at 4°C overnight, rinsed with
phosphate-buffered saline (PBS) three times, and incubated with
secondary antibody at 28°C for 30 min, and rinsed with PBS again.
The sections were visualized with freshly prepared DAB and
counterstained with hematoxylin for 1 min, followed by tap water
washes, dehydration and mounting. The sections were observed with
the microscope.
Real-time quantitative PCR detection of
FASN expression in different HCC cell lines
FASN and GAPDH primers were synthesized with the
SYBR-Green fluorescent method. The sequences of FASN upstream and
downstream primers were: AAG GAC CTG TCT AGG TTT GAT GC and TGG CTT
CAT AGG TGA CTT CCA. The sequences of GAPDH upstream and downstream
primers were: TGT GGG CAT CAA TGG ATT TGG and ACA CCA TGT ATT CCG
GGT CAA T. PCR conditions were: 95°C (30 sec); 35 cycles of 94°C
(30 sec), 58°C (30 sec), 72°C (50 sec); 58°C (5 min), 55°C (30 sec)
and 95°C (30 sec). Each sample was detected three times. The
results of real-time quantitative PCR were analyzed with the ΔΔCt
method according to the formula: ΔCt = FASN gene Ct − GAPDH gene
Ct. The relative expression level of FASN was calculated using the
2−ΔΔCt method.
Western blot analysis of FASN expression
in different HCC cell lines
After cells at logarithmic phase were cultured in
fresh culture medium 12 h before the detection, total cell protein
was extracted and quantitated with the BCA method. The proteins
were boiled with 5X the sample buffer for 5 min, and added to each
well at 45 μg/hole for electrophoresis. Then, the proteins were
transferred to a PVDF membrane and blocked with 5% skim milk for 1
h at room temperature, incubated with anti-FASN antibody (1:1,000)
at 4°C overnight and secondary antibodies at room temperature for
1.5 h. The images were collected and analyzed with ECL system.
Construction of FASN shRNA eukaryotic
expression vector
First, single-stranded sense and antisense oligo DNA
was synthesized, and the sequences were: TGC TGT CAG GAA GAT AGC
CGA GTT TTG GCC ACT GAC TGA CTC GGC ATG TAT CTT CCT GA and CCT GTC
AGG AAG ATA CAT GCC GAG TCA GTC AGT GGC CAA AAC TCG GCA TGG CTA TCT
TCC TGA C, respectively. After the single-stranded oligo DNA was
annealed, the double-stranded shRNA was inserted into the vector,
which was constructed according to the instructions of BLOCK-iT™
HiPerform™ Lentiviral Pol II miR RNAi Expression System with EmGFP
kit, and then the vector was transformed into competent
bacteria.
FASN shRNA transfection of SK-Hep-1 HCC
cells
SK-Hep-1 HCC cells were seeded onto the 6-well
plates at the density of 2×105 cells/hole in the
incubator; when 80–90% confluent at the bottom, SK-Hep-1 cells were
transfected with FASN shRNA. According to the instructions of the
Lipofectamine 2000 kit, the constructed FASN interference vector
and blank vector was diluted with serum-free culture medium in two
centrifugation tubes (1.5 ml; tube 1 and 2), respectively, while
Lipofectamine 2000 was diluted with 100 μl of serum-free medium in
another centrifugation tube (1.5 ml; tube 3). All tubes were placed
at room temperature for 5 min and then 50 μl of the diluted
liposomes was collected from tube 3 and added into tubes 1 and 2,
mixing gently and placing at room temperature for 25 min. The
6-well plates were supplemented with 100 μl of the transfection
solution, covering the cells at the bottom. The culture medium was
replenished 5 h later and the transfection efficiency was detected
24 h later.
Western blot analysis detection of
interference effects of FASN shRNA
At 48 h after transfection, total cell protein was
extracted with the above method.
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2-tetrazolium bromide
(MTT) detection of SK-Hep-1 cell proliferation
The transfected cells were collected and counted at
48 h after transfection. The cells transfected with FASN
interference vector and negative cells at 10,000/ml were seeded in
96-well plates. Each hole was supplemented with the previously
prepared MTT working solution 20 μl (5 mg/ml). The cells were mixed
by pipetting and incubated for 4 h. The culture medium in each hole
was discarded and was supplemented with 150 μl of DMSO, shaking at
room temperature for 10 min. The absorbance of each hole was
measured using a microplate reader and averaged.
Detection of SK-Hep-1 cell apoptosis
At 48 h after transfection, the cells were washed
three times with PBS, then collected and stained according to the
instructions of the apoptosis kit (Jingmei Biotech). The apoptosis
results were analyzed using flow cytometry.
SK-Hep-1 cell migration and invasion
Matrigel was thawed in a refrigerator at 4°C prior
to use. Matrigel diluents were applied to coat the chamber, then
dried. Each hole was supplemented with 50 μl serum-free medium
containing 10 g/l of bovine serum albumin, and cells were incubated
at 37°C for 30 min. At 48 h after transfection, cells were
collected and counted. Subsequently, 150 μl of cell suspension at
1.5×105 cells/ml was pipetted to the chamber, and the
bottom of the 24-well plate was supplemented with 500 μl bovine
fetal serum-containing medium. The cells were cultured for an
additional 48 h. After the culture was completed, the chamber was
taken out and the lower layer of cells was fixed with 95% ethanol
for 5 min, and stained with crystal violet solution at a
concentration of 4 g/l. The cells were counted at five randomly
selected visions.
Statistical analysis
Experimental data were analyzed using SPSS 18.0
software and are expressed as the means ± SD. The difference
between groups was compared using one-way analysis of variance. A
P-value of <0.05 was considered to indicate a statistically
significant result.
Results
FASN is highly expressed in HCC
tissue
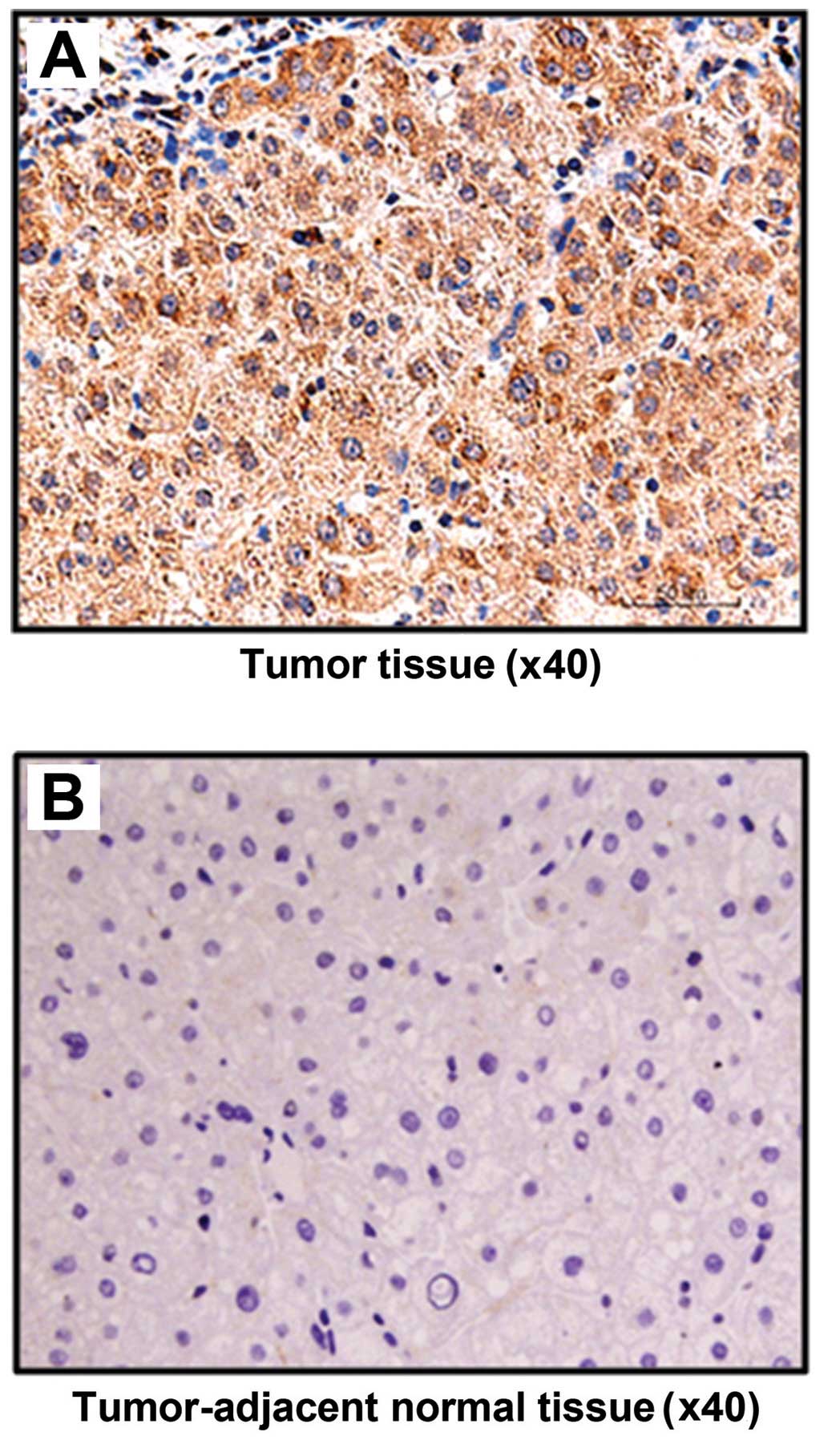
Immunohistochemical staining results showed that
FASN was positively expressed in all 20 HCC patients, accounting
for the positive rate of 100% (20/20). However, the positive
expression rate in tumor-adjacent tissue was only 10% (2/20); there
were significant differences between the two groups (P<0.05).
The staining in HCC tissue was significantly more visible than that
in tumor-adjacent tissue, and positive staining was placed in the
cytoplasm (Fig. 1).
FASN is highly expressed in HCC cell
lines
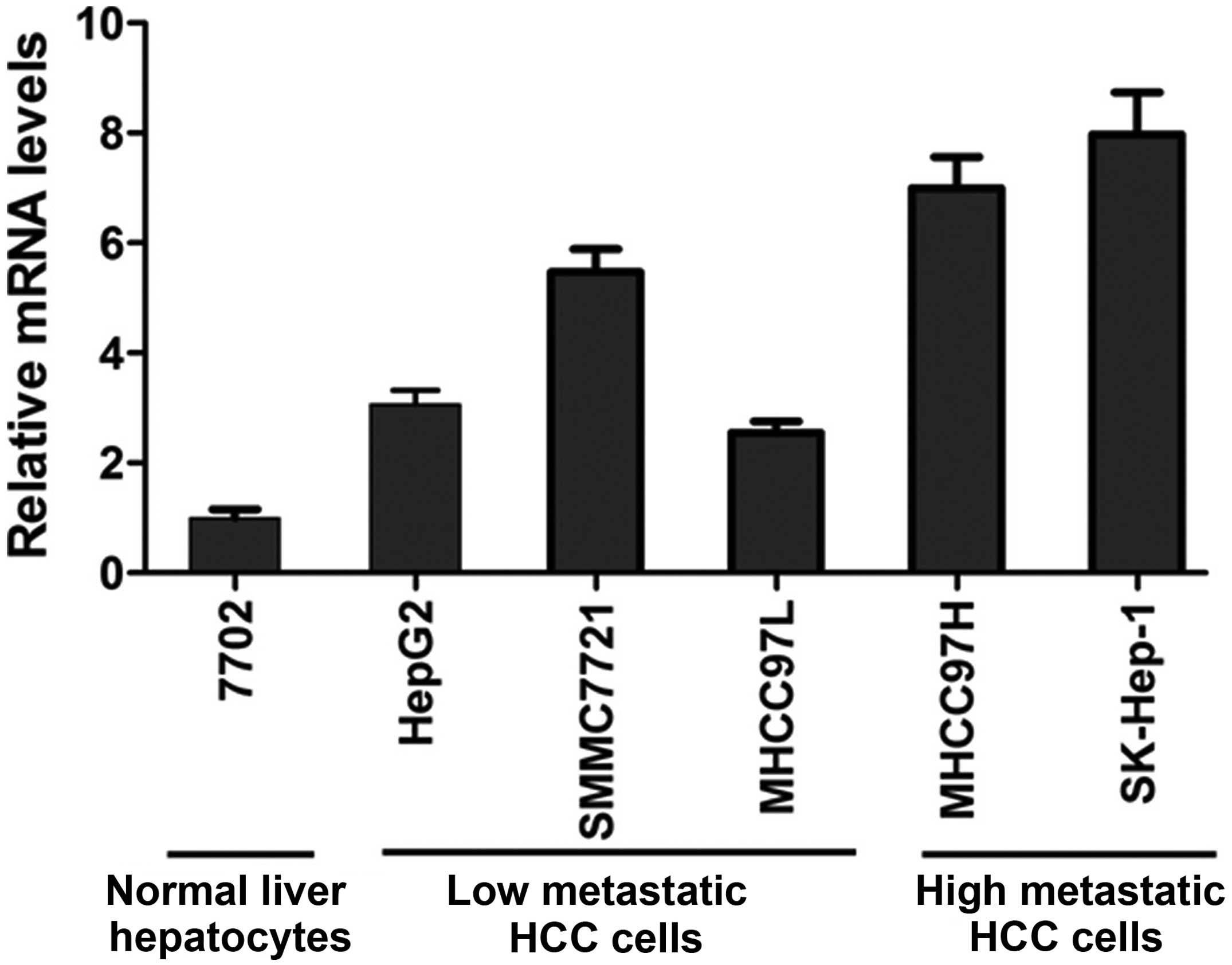
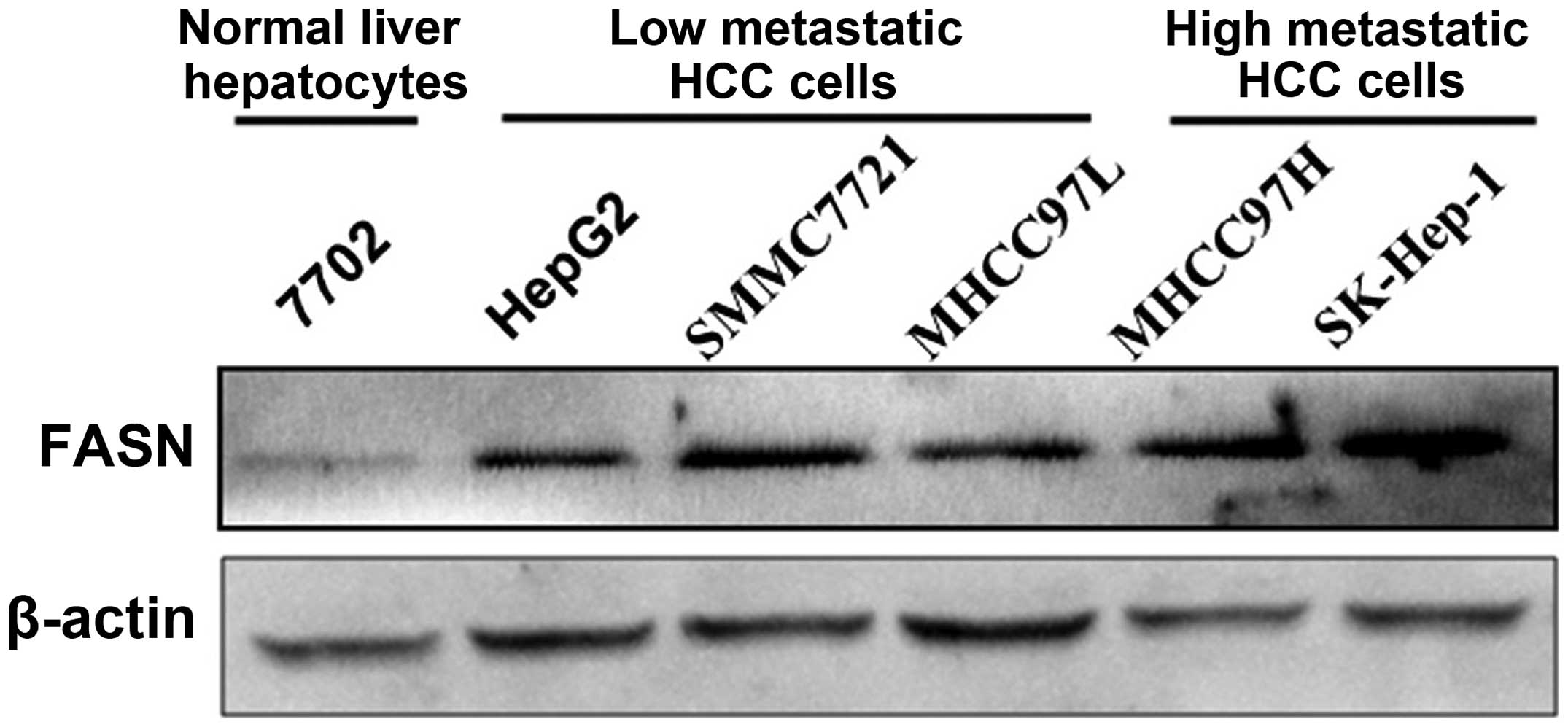
FASN mRNA and protein expression levels in normal
liver cell line 7702 and HCC cell lines HepG2, SMMC7721, MHCC97L,
MHCC97H and SK-Hep-1 were detected with real-time quantitative PCR
and western blot analysis, respectively. The results showed that
FASN mRNA and protein expression levels in HCC cell lines HepG2,
SMMC7721, MHCC97L, MHCC97H and SK-Hep-1 were higher than those in
normal liver cell line 7702 (P<0.05; Figs. 2 and 3). In addition, highly metastatic liver
cancer cell lines MHCC97H and SK-Hep-1 had a higher expression
level than low metastatic liver cancer cell lines HepG2, SMMC7721
and MHCC97L (P<0.05; Figs. 2 and
3).
FASN shRNA interference of FASN
expression
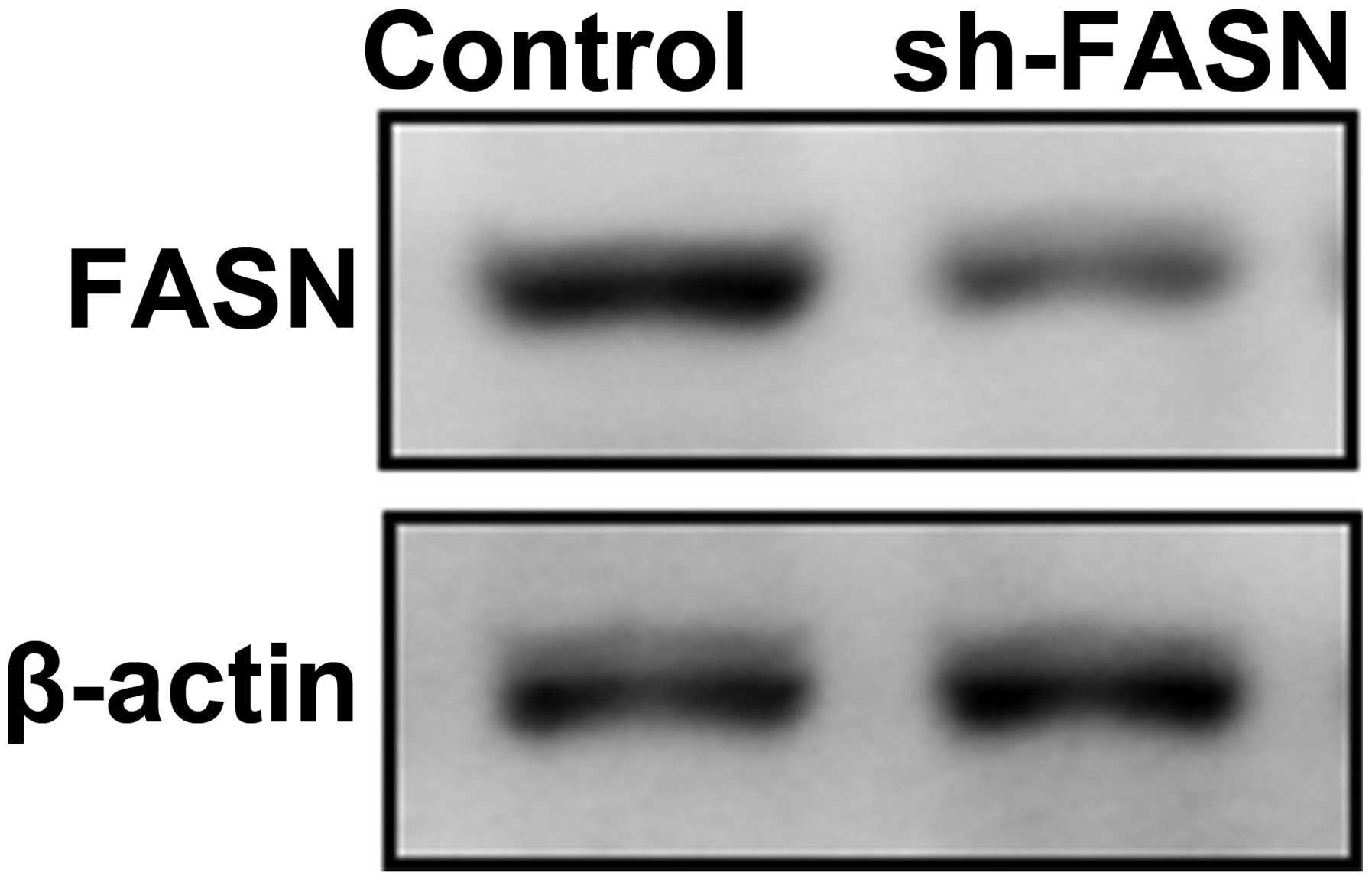
To explore the function of FASN, we first
constructed an interference vector of FASN expression, and
identified its interference efficiency (Fig. 4).
As shown in Fig. 2,
the FASN expression reached the peak in SK-Hep-1 cells, thus we
transfected SK-Hep-1 cells with RNA interference vector to knock
down the expression of FASN. The cell proliferation, apoptosis,
migration and invasion were observed.
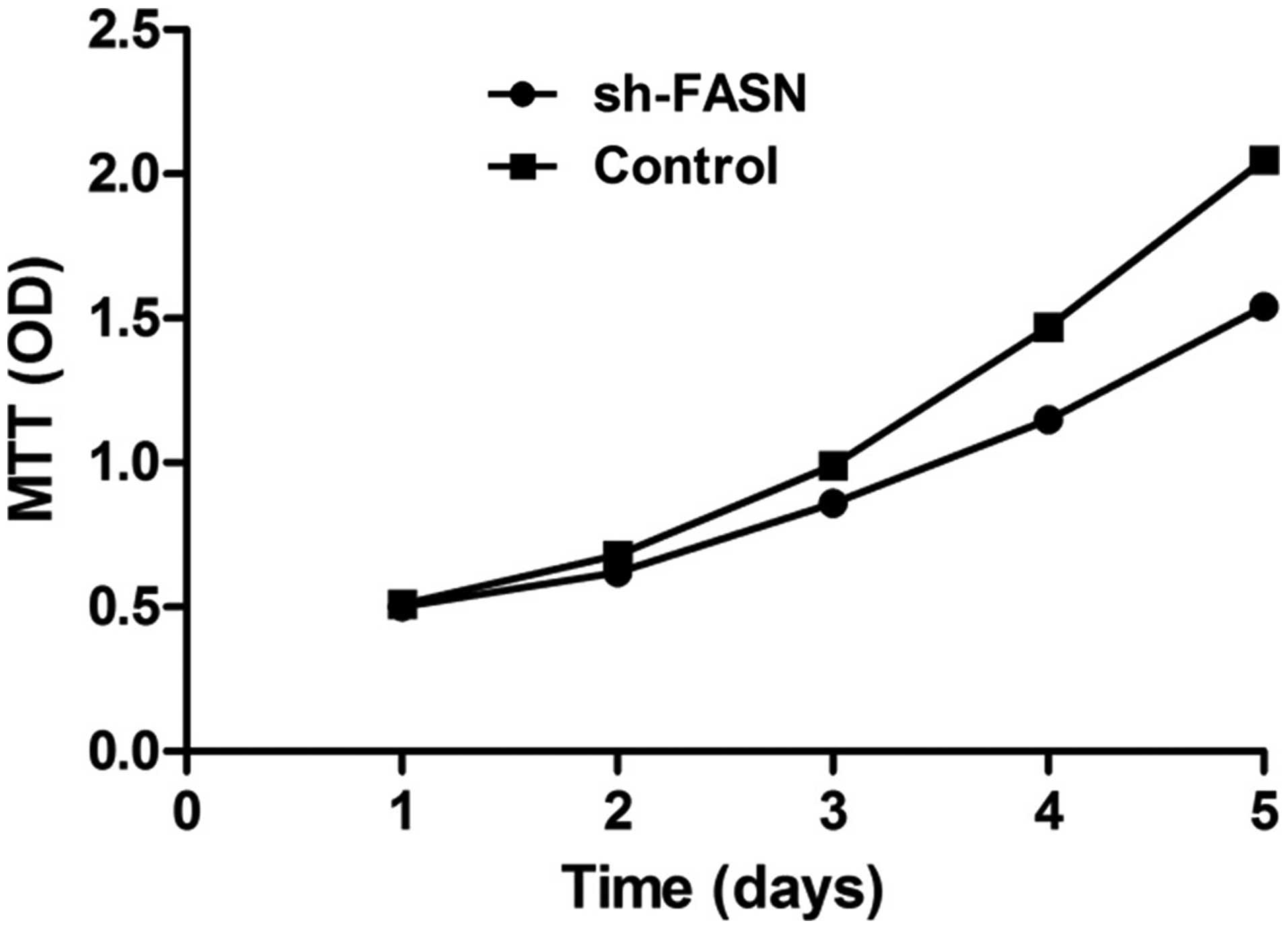
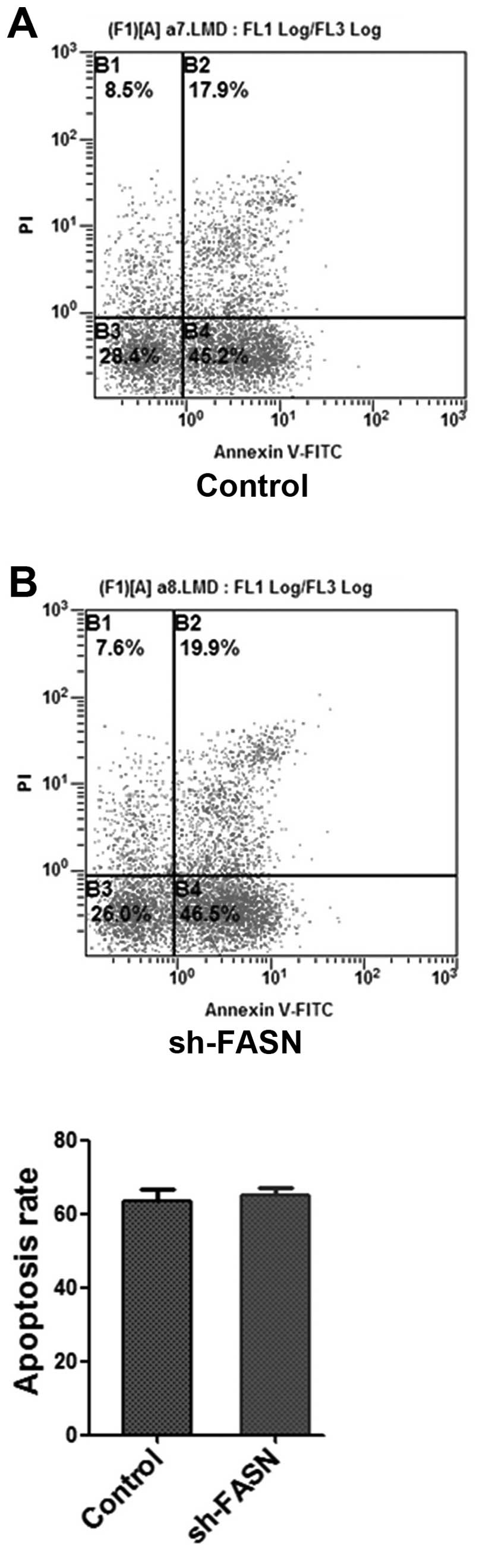
Effects of sh-FASN on SK-Hep-1 cell
proliferation and apoptosis
After the FASN expression in SK-Hep-1 cells was
knocked down, the cell proliferation and apoptosis were determined
with MTT assay and flow cytometry. The results showed that the cell
proliferation was significantly inhibited by the knockdown
(Fig. 5), while the apoptosis did
not change significantly (Fig.
6).
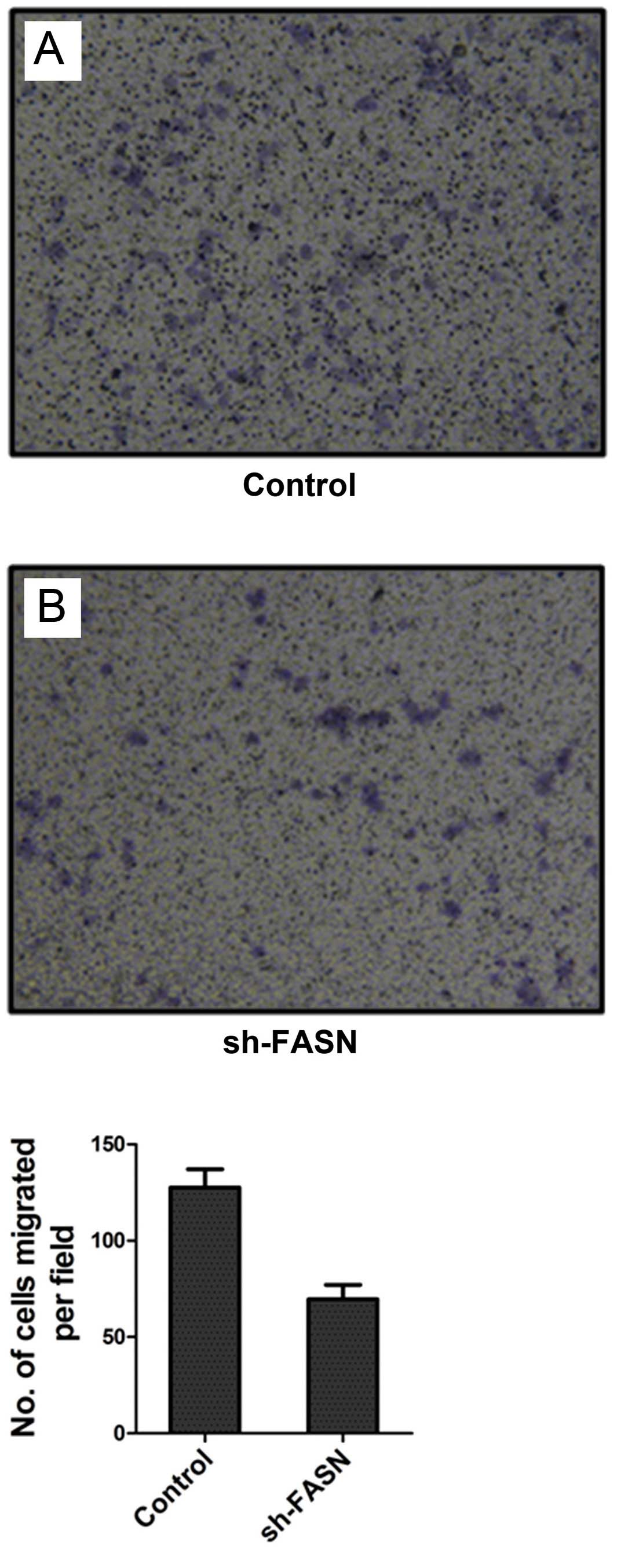
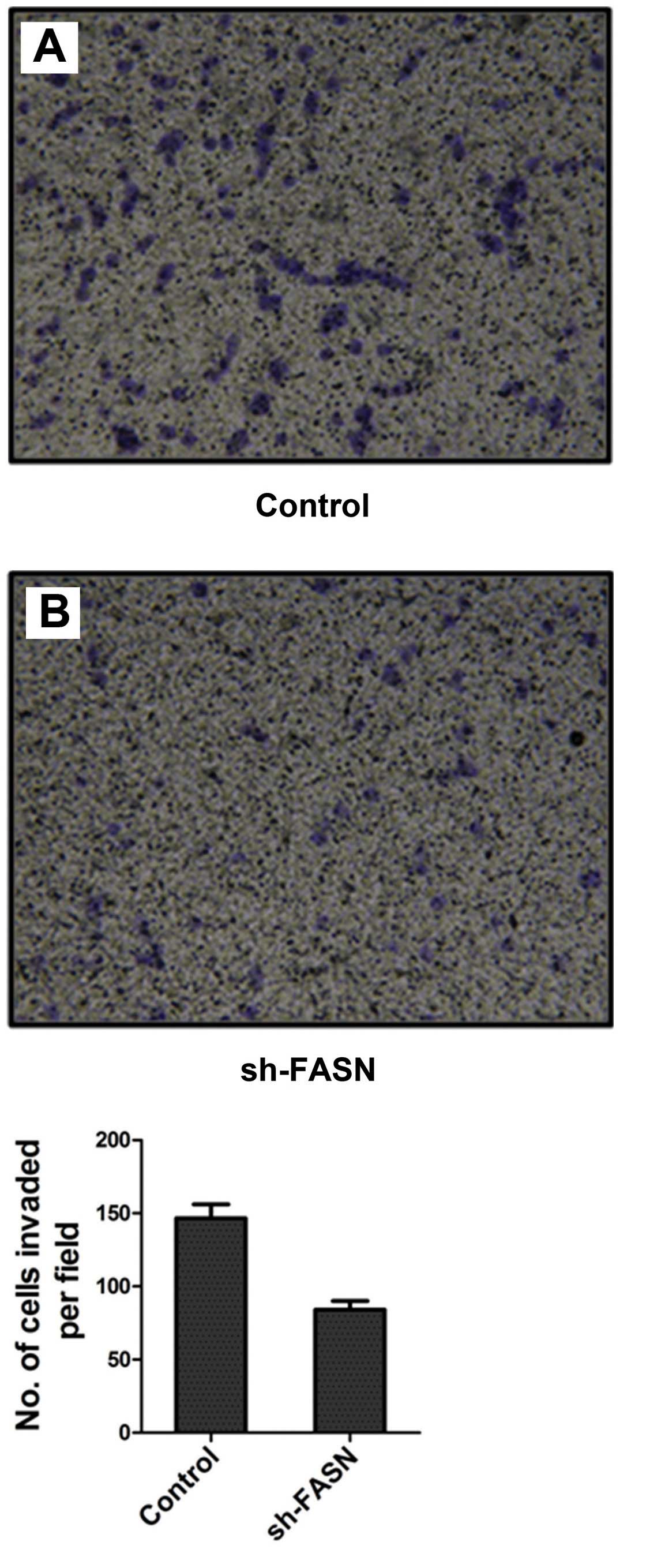
Effects of sh-FASN on SK-Hep-1 cell
migration and invasion
After FASN expression in SK-Hep-1 cells was knocked
down, the cell migration and invasion were detected by Transwell
chamber test. The results showed that the number of cells that
migrated and invaded into the bottom of the membrane was
significantly reduced after the knockdown (Figs. 7 and 8).
Discussion
Increasing evidence indicates that cell metabolism
abnormalities are closely related to tumor occurrence and
development (5). Abnormal
metabolism of tumor cells mainly refers to the abnormalities in the
metabolism of glucose and lipid, the glycolytic activity and fatty
acid synthesis activity of tumor cells are enhanced (6,7). FASN
is the key molecule for catalyzing fatty acid synthesis, and human
FASN gene is located at chromosome 17. FASN is highly expressed in
a number of malignant tumors; it can promote the synthesis of
endogenous fatty acids in tumor cells, then the synthesized fatty
acids provide energy for the proliferation of tumor cells. Previous
studies addressing prostate cancer revealed that FASN mRNA and
protein expression levels in cancer tissue were significantly
increased compared with normal prostate tissue around the lesions,
and the expression levels gradually increased in normal epithelium,
epithelial hyperplasia tissue, highly proliferative and prostate
cancer tissues. These findings indicated the contribution of FASN
in prostate cancer (8,9). FASN expression in renal carcinoma
tissue was significantly higher than that in the tumor-adjacent
normal tissue, indicating that FASN expression is involved in the
lymph node metastasis, tumor classification and prognosis (10). A high FASN expression was also found
in bladder cancer; the expression levels were significantly
correlated with the tumor classification of bladder cancer, and
high FASN expression can be regarded as an indicator of bladder
cancer prognosis (11).
Interference on FASN expression could inhibit the proliferation of
endometrial cancer cells, while promoting apoptosis (12). Furthermore, FASN can promote the
invasion and migration of osteosarcoma cells (13,14).
As FASN occurs in the progression of various tumors,
investigations into FASN as a potential target for cancer therapy
has been widely studied and has achieved considerable progress
(15,16). Cerulenin is the first discovered
FASN inhibitor, but its application in antitumor treatment has been
limited due to unstable chemical property. C75 is a stable FASN
inhibitor, which can dose-dependently inhibit the expression of
FASN in tumor cells, and was shown to exhibit antitumor activity in
the in vivo experiments (17).
Previous studies demonstrated that fatty acid
synthesis activity was significantly enhanced in HCC tissue. These
metabolic changes can be explained by the high expression of
several fatty acid synthetase in HCC cells. The fatty acid
synthetase is mainly SREBP1, ACLY, ACC, FASN and SCD1, wherein FASN
is the dominant one (18). However,
these previous studies have some limitations, such as small size of
HCC tissue samples, lack of detection of FASN expression levels in
HCC cell lines, and lack of investigations into the biological
significance of FASN high expression in HCC cells, that is the
influence on malignant tumors. The present study demonstrated that
FASN was highly expressed in HCC cell lines HepG2, SMMC7721,
MHCC97L, MHCC97H and SK-Hep-1, compared with normal hepatocyte
7702. FASN expression also showed significant differences between
highly metastatic HCC cells (MHCC97H and SK-Hep-1) and low
metastatic HCC cells (HepG2, SMMC7721 and MHCC97L). Our findings
indicate that FASN expression may be involved in HCC metastasis.
The high expression of FASN within cells may be regulated at the
transcriptional level, rather than the post-transcriptional level,
as detected by real-time quantitative PCR. In addition,
immunohistochemical staining results also found a high expression
of FASN in HCC tissue at the tissue level. Our findings
demonstrated that FASN is highly expressed in both HCC cells and
tissues. In the in vitro experiments, the eukaryotic
expression vector targeting FASN interference was constructed and
transfected into HCC cell line SK-Hep-1, to knock down FASN
expression. Furthermore, cell proliferation, apoptosis, migration
and invasion were observed. The results showed that the knockdown
of FASN could inhibit cell proliferation, invasion and migration,
leaving cell apoptosis unaffected. It was previously reported that
FASN promoted cell proliferation and inhibited apoptosis in
endometrial cancer (12).
Similarly, we supported their outcomes on the proliferation of HCC
cells, although the apoptosis was not affected. The main reason for
this discrepancy is that FASN may be involved in the development
and progression of various types of tumor cells through different
mechanisms. In addition, we confirmed that knockdown of FASN
expression inhibited the invasion and migration of HCC cells,
indicating the contribution of FASN to malignant tumor metastasis.
Inhibiting FASN expression in HCC cells is a challenging issue and
requires further investigation.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (no. 30971340).
References
|
1
|
Daker M, Bhuvanendran S, Ahmad M, Takada K
and Khoo AS: Deregulation of lipid metabolism pathway genes in
nasopharyngeal carcinoma cells. Mol Med Rep. 7:731–741.
2013.PubMed/NCBI
|
|
2
|
Grunt TW, Wagner R, Grusch M, et al:
Interaction between fatty acid synthase- and ErbB-systems in
ovarian cancer cells. Biochem Biophys Res Commun. 385:454–459.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou Y, Niu C, Li Y, et al: Fatty acid
synthase expression and esophageal cancer. Mol Biol Rep.
39:9733–9739. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cerne D, Zitnik IP and Sok M: Increased
fatty acid synthase activity in non-small cell lung cancer tissue
is a weaker predictor of shorter patient survival than increased
lipoprotein lipase activity. Arch Med Res. 41:405–409. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cairns RA, Harris I, McCracken S and Mak
TW: Cancer cell metabolism. Cold Spring Harb Symp Quant Biol.
76:299–311. 2011. View Article : Google Scholar
|
|
6
|
Glaysher J: Lipid metabolism and cancer.
Curr Opin Lipidol. 24:530–531. 2013. View Article : Google Scholar
|
|
7
|
Kuhajda FP: Fatty-acid synthase and human
cancer: new perspectives on its role in tumor biology. Nutrition.
16:202–208. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pflug BR, Pecher SM, Brink AW, Nelson JB
and Foster BA: Increased fatty acid synthase expression and
activity during progression of prostate cancer in the TRAMP model.
Prostate. 57:245–254. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Swinnen JV, Roskams T, Joniau S, et al:
Overexpression of fatty acid synthase is an early and common event
in the development of prostate cancer. Int J Cancer. 98:19–22.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Horiguchi A, Asano T, Asano T, Ito K,
Sumitomo M and Hayakawa M: Fatty acid synthase over expression is
an indicator of tumor aggressiveness and poor prognosis in renal
cell carcinoma. J Urol. 180:1137–1140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sugino T, Baba K, Hoshi N, Aikawa K,
Yamaquchi O and Suzuki T: Overexpression of fatty acid synthase in
human urinary bladder cancer and combined expression of the
synthase and Ki-67 as a predictor of prognosis of cancer patients.
Med Mol Morphol. 44:146–150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pizer ES, Kurman RJ, Pasternack GR and
Kuhajda FP: Expression of fatty acid synthase is closely linked to
proliferation and stromal decidualization in cycling endometrium.
Int J Gynecol Pathol. 16:45–51. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu ZL, Zhou Y, Luo QF, et al: Inhibition
of fatty acid synthase supresses osteosarcoma cell invasion and
migration. Indian J Pathol Microbiol. 55:163–169. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Long XH, Mao JH, Peng AF, Zhou Y, Huang SH
and Liu ZL: Tumor suppressive microRNA-424 inhibits osteosarcoma
cell migration and invasion via targeting fatty acid synthase. Exp
Ther Med. 5:1048–1052. 2013.PubMed/NCBI
|
|
15
|
Alli PM, Pinn ML, Jaffee EM, McFadden JM
and Kuhajda FP: Fatty acid synthase inhibitors are chemopreventive
for mammary cancer in neu-N transgenic mice. Oncogene.
24:39–46. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Menendez JA, Mehmi I, Verma VA, Teng PK
and Lupu R: Pharmacological inhibition of fatty acid synthase
(FAS): a novel therapeutic approach for breast cancer
chemoprevention through its ability to suppress Her-2/neu (erbB-2)
oncogene-induced malignant transformation. Mol Carcinog.
41:164–178. 2004. View
Article : Google Scholar
|
|
17
|
Pandey PR, Liu W, Xing F, Fukuda K and
Watabe K: Anti-cancer drugs targeting fatty acid synthase (FAS).
Recent Pat Anticancer Drug Discov. 7:185–197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Calvisi DF, Wang C, Ho C, et al: Increased
lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes
development of human hepatocellular carcinoma. Gastroenterology.
140:1071–1083. 2011. View Article : Google Scholar : PubMed/NCBI
|