Introduction
Lin28 is a conserved RNA binding protein that was
originally described as an indispensable regulator of developmental
timing in Caenorhabditis elegans (1). Mammals have two homologs, Lin28A and
Lin28B. In mammals, Lin28A, often called Lin28, is ubiquitously
expressed in early embryonic stages and was shown as one of the
four factors that convert fibroblasts into induced pluripotent stem
(iPS) cells (2). Lin28 is readily
expressed in embryos and embryonic stem cells, yet it is either
undetectable or its expression remains at low levels in normal
adult tissues, suggesting that Lin28 may play a critical role in
cell proliferation and/or differentiation during embryonic
development (3,4). Recently, Lin28 was confirmed to
possess the ability to facilitate the reprogramming of human
somatic cells to iPS cells (5).
Furthermore, Lin28A/B promote malignant
transformation, and their expression is associated with advanced
stages of numerous types of tumors, including hepatocarcinoma,
nephroblastoma, ovarian carcinoma and germ cell tumors (6,7). It is
highly expressed in various tumors, such as hepatocellular
carcinoma and colorectal cancer (7–9).
Overexpression of Lin28 has been shown to promote cancer cell
proliferation (10). High
expression of Lin28 is a poor prognostic factor in hepatocellular
cancer and it is significantly associated with lymph node
metastasis in colorectal cancer. These features make Lin28 an
attractive target for antibody-based therapy. Therefore,
understanding the occurrence and expression status of Lin28 has
important implications in the prognosis and treatment of
cancer.
At the molecular level, Lin28 acts as a suppressor
of let-7 microRNA biogenesis (11–14).
Lin28 and its homolog, Lin28B have been known to regulate all let-7
family members through a maturation process and cellular
differentiation (15). Lin28 was
reported to act as a transacting regulator which binds let-7
pre-miRNA to block its maturation in embryonic stem cells (14). This particular function of let-7 was
used in the process of establishing iPS cells from human
fibroblasts in order to enhance the efficiency of cellular
formation (16). A number of
studies have also suggested an association between Lin28 and let-7,
with particular emphasis upon their involvement in cellular
processes such as differentiation and proliferation (13,14).
However, a correlation between Lin28 and let-7 in
gastric cancer cells has not yet been confirmed. In the present
study, we examined the expression of Lin28 and let-7 in gastric
cancer cell lines and investigated the effect of
lentiviral-mediated Lin28 overexpression on proliferation,
migration, cell cycle progression and apoptosis of gastric cancer
cells in vitro.
Materials and methods
Cell culture
The human gastric cancer cell lines BGC-823,
SGC-7901 and HGC-27 and the gastric mucosal cell line GES were
provided by Jiangsu Key Laboratory of Biological Cancer Therapy,
Xuzhou Medical College, China. All cell lines were routinely
maintained in RPMI-1640 medium (Gibco, Garlsbad, CA, USA)
supplemented with 10% fetal bovine serum (FBS) (Invitrogen, USA), 2
mM glutamine, 100 U/ml of penicillin and 100 mg/ml streptomycin
(all from Sigma, St. Louis, MO, USA) at 37°C with 5%
CO2.
Recombinant lentivirus construction
The human Lin28 gene fragments were amplified by
polymerase chain reaction (PCR) technology. The primers for PCR
were synthesized as follows (GeneChem Co., Ltd., Shanghai, China):
Lin28 (6596-2)-P1, GAGGATCCCCGGGTACCGGTCGCCACCATGGGCTCC
GTGTCCAACCAG and Lin28 (6596-2)-P2, TCCTTGTA
GTCCATACCATTCTGTGCCTCCGGGAGCAG. The lentiviral vector Ubi-MCS-GFP
(GeneChem) was digested by the restriction enzyme AgeI. Then
Lin28 gene fragments were ligated into the GV287 lentiviral vector
Ubi-MCS-GFP. The primer, KL6596-P3 (CCCAGTGGATGTCTTTGTGC) located
in the coding sequence of the Lin28 gene was used in PCR to
identify positive transformants. Positive clones, as confirmed by
PCR, were chosen for sequencing. Recombinant lentiviruses, which
coexpress enhanced green fluorescent protein (GFP) and the Lin28
sequence, were produced by 293T cells following the co-transfection
of Ubi-MSC-EGFP-Lin28 and the packaging plasmids pHelper 1.0 and
pHelper 2.0 (GeneChem) using Lipofectamine 2000 (Invitrogen).
Western blot analysis was employed to confirm the overexpression of
Lin28A in the transfected 293T cells as described above.
Lentiviral-expressing green GFP was generated as a control. The
virus titer was detected by quantitative real-time PCR after
concentrating and harvesting the viral supernatant.
Transfection
BGC-823 cells in log phase were cultured in 6-well
plates at a density of 5×104 cells/well and transfected
with Lin28-GFP vectors (Lin28 overexpression group) or GFP vectors
(expressing GFP) at a multiplicity of infection (MOI) of 20 in
serum-free medium. After incubation at 37°C for 16 h, the
transduction medium was replaced with fresh DMEM + 10% FBS.
Lin28A-BGC-823 cells were genetically engineered with a recombinant
lentivirus coexpressing EGFP and Lin28 and used as the
overexpression (OE) group, and GFP-BGC-823 cells were manipulated
with a lentivirus expressing GFP and were used as the negative
control (NC) group. BGC-823 cells without intervention served as
the normal control (CON) group and cultured in the same manner as
described above. After 3 days of transfection, GFP expression was
observed using fluorescence microscopy (MicroPublisher 3.3 RTV;
Olympus, Japan).
RT-PCR assay
RNAs from the different groups were extracted with
TRIzol reagent (Invitrogen) and treated with DNase (Tiangen
Biotech, Beijing, China). cDNA was synthesized from 2 μg of total
RNA according to the manufacturer’s instructions in a total volume
of 10 μl (M-MLV; Promega, Axygen). Negative control reactions were
run without reverse transcriptase. An equal volume of product was
subjected to PCR. The primer sequences were specific to an updated
version of the GenBank sequences. Sequences of primers were used to
detect human Lin28 (NM_024674) and let-7a (MIMAT0000062)
(GeneChem). The amplification conditions were as follows: 40 cycles
of 95°C for 30 sec, 95°C for 5 sec, annealing at 60°C for 30 sec
and 72°C for 15 sec for primer template extension. Gene expression
in each sample was normalized to glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) expression. The fold changes for each mRNA
were calculated using the 2−ΔΔCT method. The different
forward (F) and reverse (R) primer sequences were as follows: the
primers for Lin28 were (forward) 5′-GCCTTCTACTGGAAGATTGG and
(reverse) 5′-GATCTGGAGATGTAAAGCACG; the primers for GAPDH were
(forward) 5′-TGACTTCAACAGCGAC ACCCA and (reverse)
5′-CACCCTGTTGCTGTAGCCAAA.
Western blot analysis
Cell proteins were extracted using lysis buffer [50
mM Tris-HCl (pH 8.8), 150 mM NaCl, 0.1% SDS, 2 mM EDTA, 1 mM PMSF,
1% NP40, 5 μg/ml aprotinin, 1 μg/ml leupeptin] at 4°C on ice. The
lysates were centrifuged at 12,000 rpm for 10 min at 4°C, and the
supernatants were collected. Twenty-five micrograms of protein was
loaded onto a 12% SDS-PAGE gel and subjected to electrophoresis at
20 mA for 62 min under denaturing conditions and then transferred
to nitrocellulose membranes. After blocking in 5% non-fat milk in
Tris-buffered saline Tween-20 (TBST) overnight at 4°C, the
membranes were immunoblotted with anti-Lin28 (1:3,000) or
anti-β-actin (1:5,000) antibodies overnight at 4°C. The following
day, membranes were washed three times with TBST and then incubated
with anti-mouse secondary antibodies in PBS (1:4,000) for 2 h at
room temperature. Bands were detected using an enhanced
chemiluminescence (ECL) system (ECL kit; Santa Cruz, USA). β-actin
was used as an internal control.
Cell proliferation assay
An MTT assay was used to evaluate cell proliferation
according to the manufacturer’s instructions. After recombinant
lentivirus transfection for 4 days, the three groups were seeded in
96-well plates at 2,000 cells/well and cultured for 24, 48, 72, 96
and 120 h. A total of 20 μl of MTT (Sigma) stock solution was added
to 200 μl of medium in each well, and the plates were incubated for
4 h at 37°C. Next, 150 μl of dimethyl sulfoxide was added to each
well, and the plates were incubated for 15 min at room temperature.
The absorbance values were read by an enzyme-linked immunosorbent
assay (490 nm).
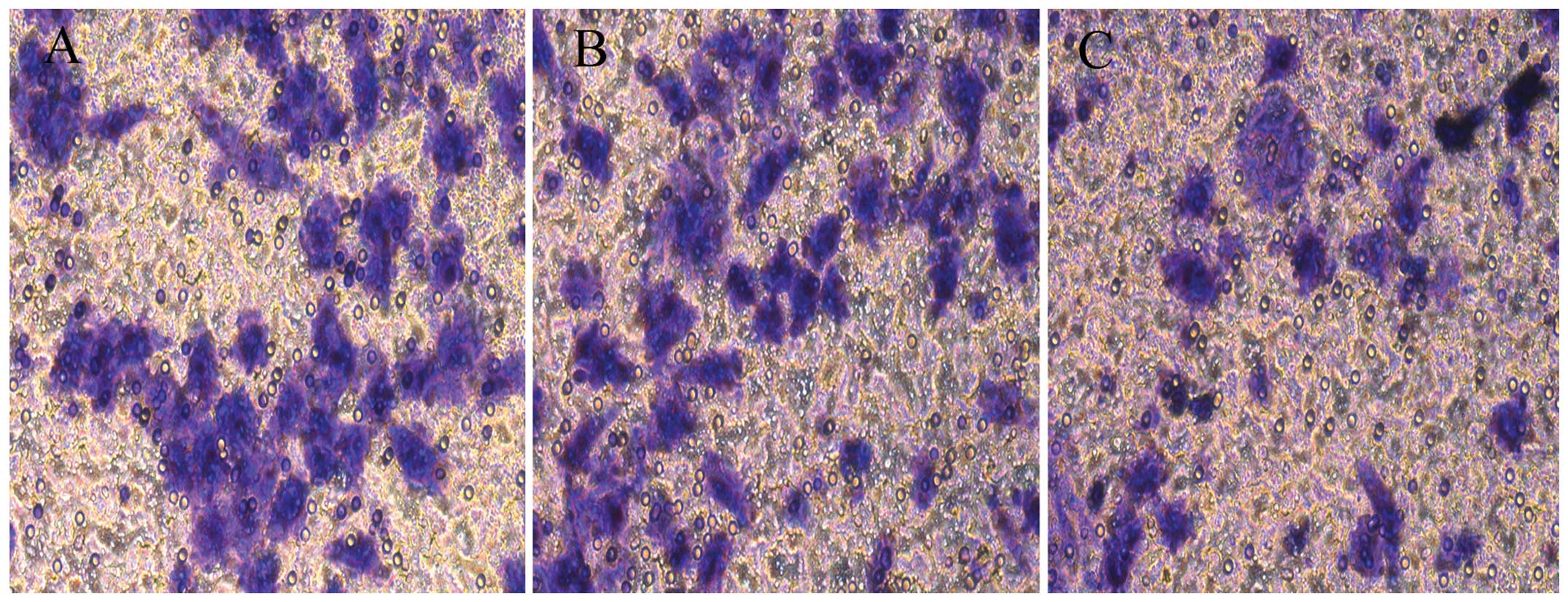
Migration assay
A Transwell assay was used to evaluate cell
migration. After recombinant lentivirus transfection for 4 days,
1×105 cells were plated onto 24-well cell chambers
(3422; Corning Inc., Corning, NY, USA) with an 8-μm pore
polycarbonate membrane. In this assay, the cells were plated in
medium supplemented with 0.1% serum, and the chambers were placed
into 24-well plates with medium containing 10% serum. After 24 h,
invaded cells on the lower membrane surface were fixed and stained
with Giemsa stain while cells that did not migrate through the
pores were removed by cotton swabs. Cells in three random fields
for each insert were counted with fluorescence microscopy
(Olympus). The absorbance values were read by an enzyme-linked
immunosorbent assay (570 nm).
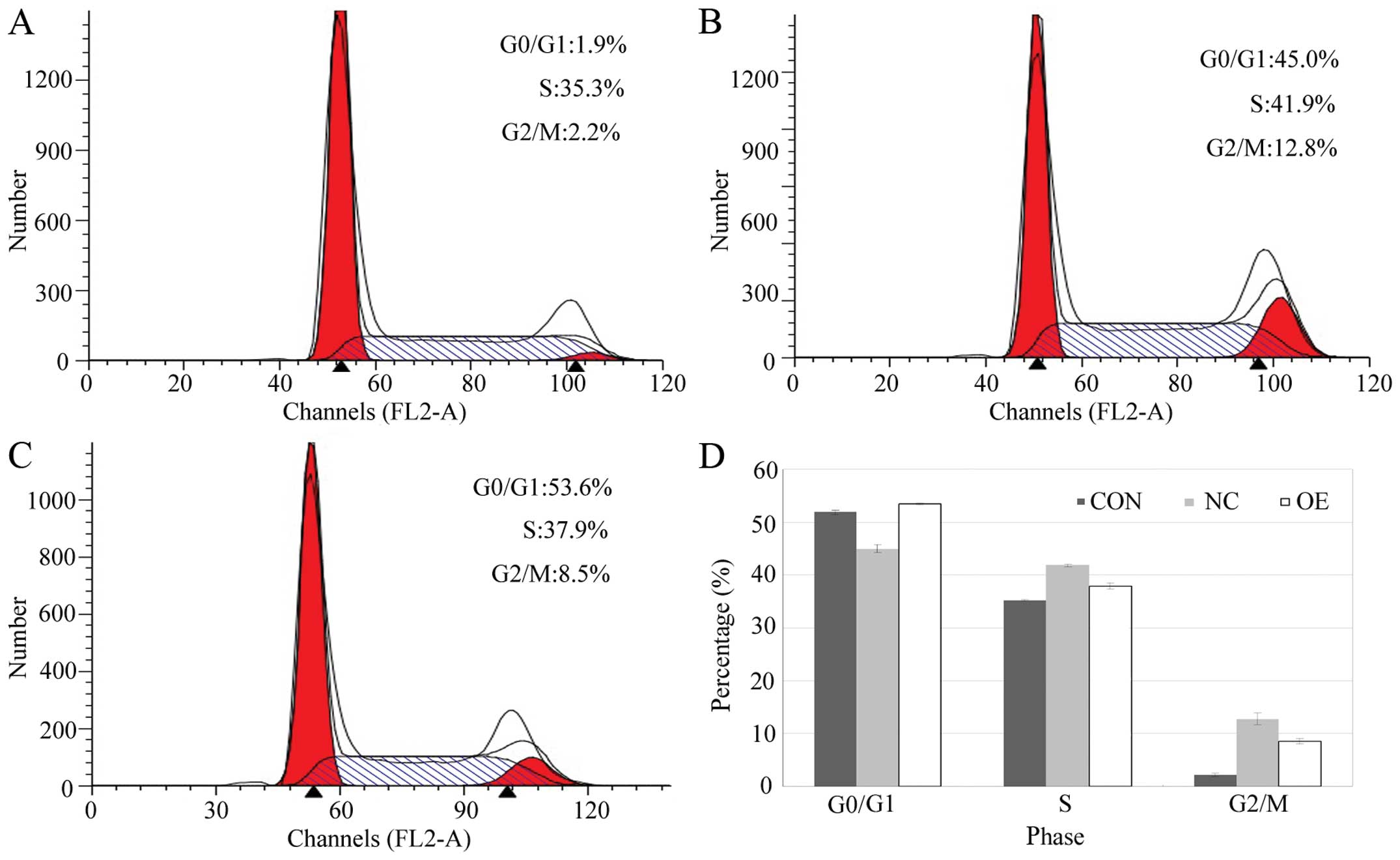
Cell cycle analysis
To analyze cell cycle distribution, DNA content per
duplicate was analyzed using flow cytometry. After recombinant
lentivirus transfection for 6 days, the cells in the three groups
were harvested by trypsinization, centrifuged and washed three
times with 4°C PBS. The cells were then fixed in 70% ethanol at 4°C
for 1 h. The fixed cells were stained with 50 μg/ml propidium
iodide (PI) containing 50 μg/ml RNase A (DNase free) for 15 min at
room temperature in the dark and analyzed by fluorescence-activated
cell sorting (FACSCalibur; BD Biosciences, San Jose, CA, USA). The
results were analyzed using CellQuest software (Becton-Dickinson
and Co., Franklin Lakes, NJ, USA). The cell cycle distribution was
evaluated by calculating the proportion of cells in the G0/G1, S
and G2/M stages. In each independent experiment, three parallel
wells were contructed, and the procedures were carried out in
triplicate.
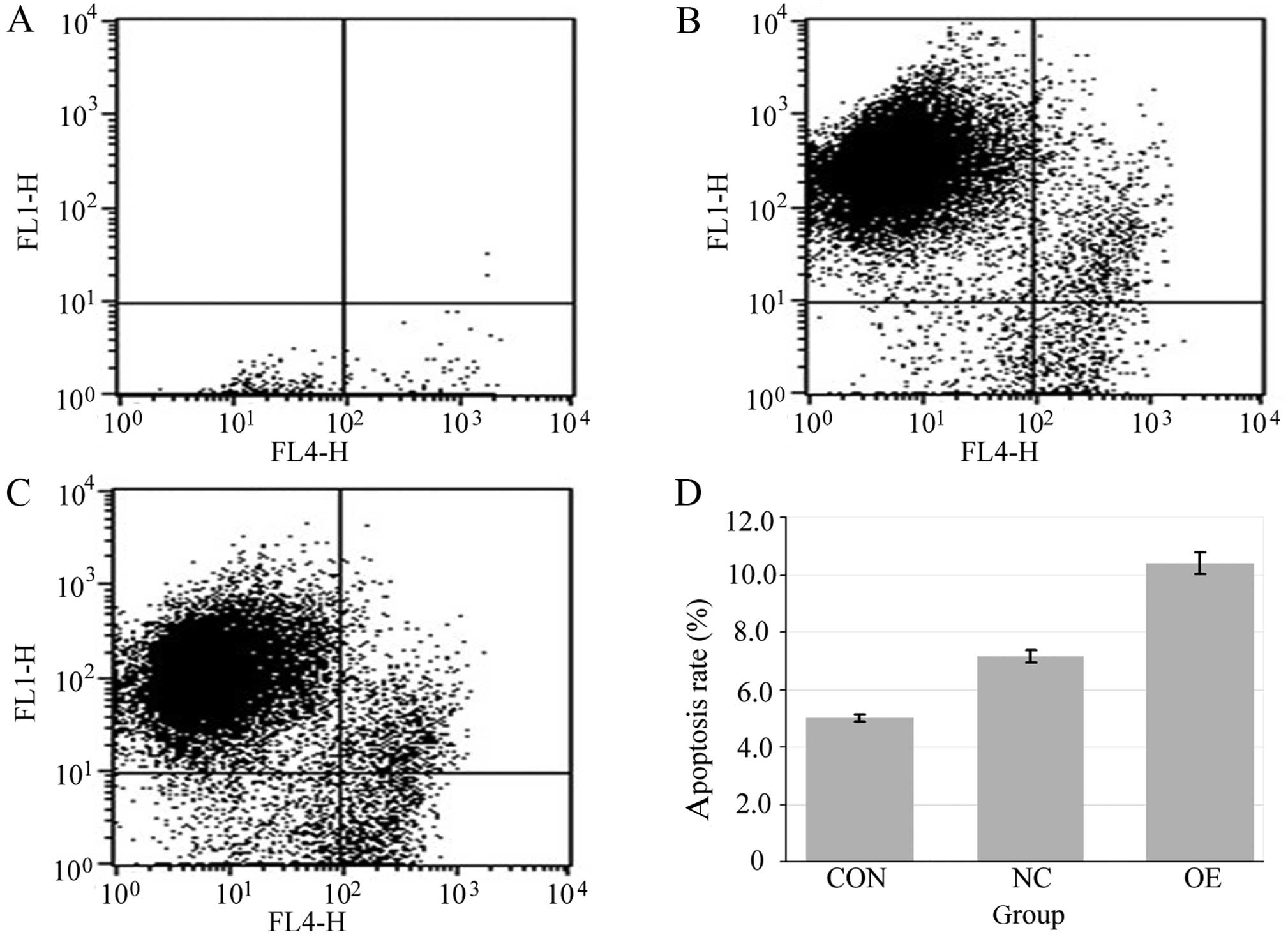
Apoptosis analysis
The Annexin V-APC apoptosis detection kit
(eBioscience Inc., San Diego, CA, USA) was used to evaluate
apoptosis. After recombinant lentivirus transfection for 9 days,
cell in the three groups were harvested and centrifuged for 5 min
at 1,500 rmp, and then suspended at a density of 1×106
cells/ml. Subsequently, the cells were diluted by buffer, and 5 μl
Annexin V was added to each sample. Incubation was carried out for
another 15 min, and samples were analyzed using flow cytometry (BD
Biosciences) and observed by fluorescence microscopy (Olympus).
Data obtained were analyzed using CellQuest software.
Statistical analysis
Statistical analysis was performed with SPSS 13.0
software (version 13.0). The Student’s t-test, one-way ANOVA and
Pearson’s Chi-square test were used according to the data
characteristics. P<0.05 was considered to indicate a
statistically significant result. The quantitative data are
presented as means ± standard deviation.
Results
Lin28 and let-7 expression in gastric
cancer cell lines
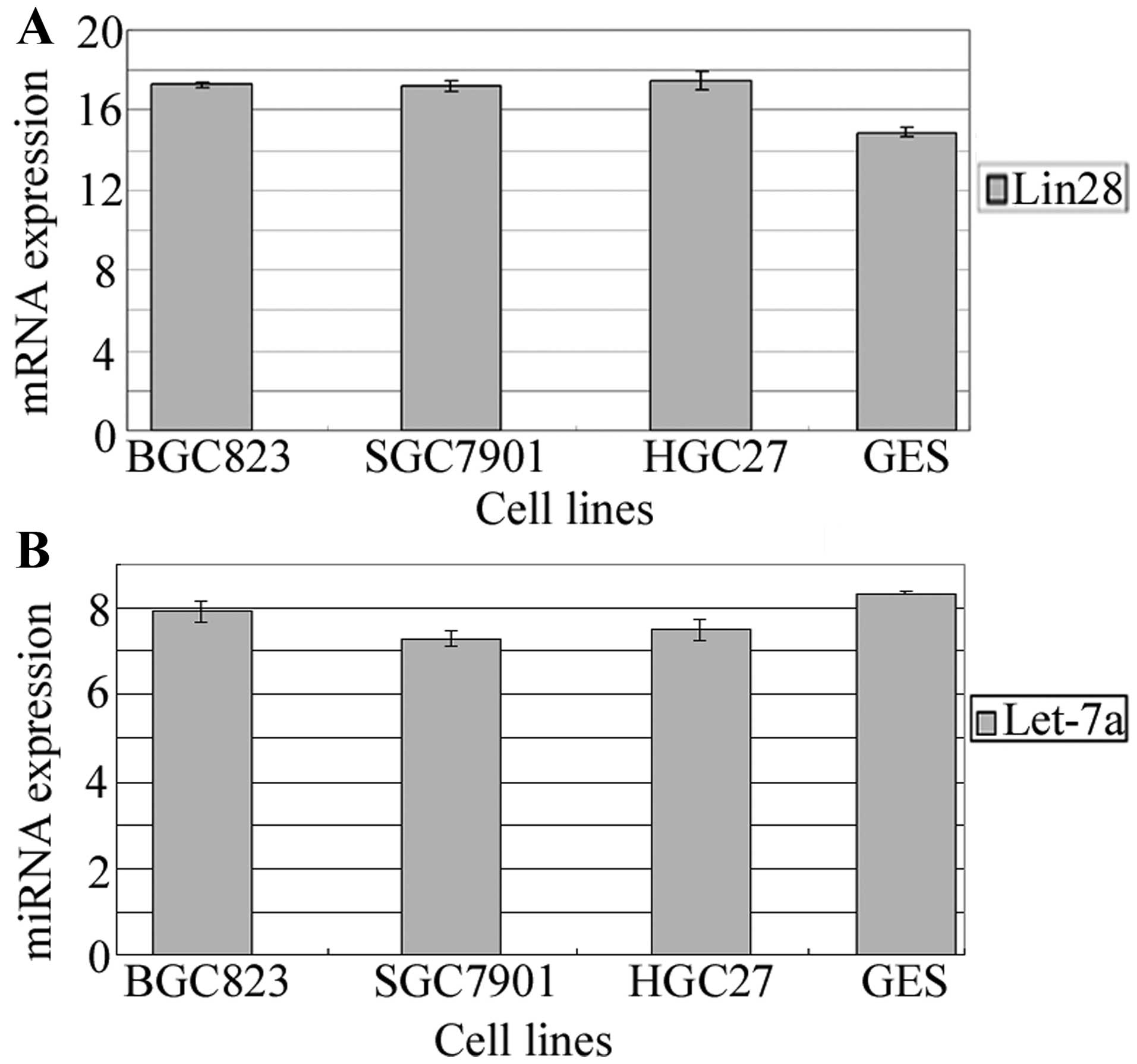
Using real-time PCR to quantify Lin28 mRNA and
hsa-let-7a-5p in four human gastric cancer cell lines and a normal
gastric epithelium cell line, we found that Lin28 mRNA was
moderately expressed in the GES cells and expressed at a low level
in the BGC-823, SGC-7901 and HGC-27 cells. let-7a miRNA was highly
expressed in both GES cells and the gastric cancer cell lines
(Fig. 1). The expression of Lin28
mRNA was inversely correlated with let-7a (r=−0.727, P=0.007).
These results suggest that Lin28 may play important roles in
gastric cancer progression by acting as a tumor-suppressor gene and
let-7a may also play an important role.
Lenti-Lin28 upregulates the expression of
Lin28 in the BGC-823 cells
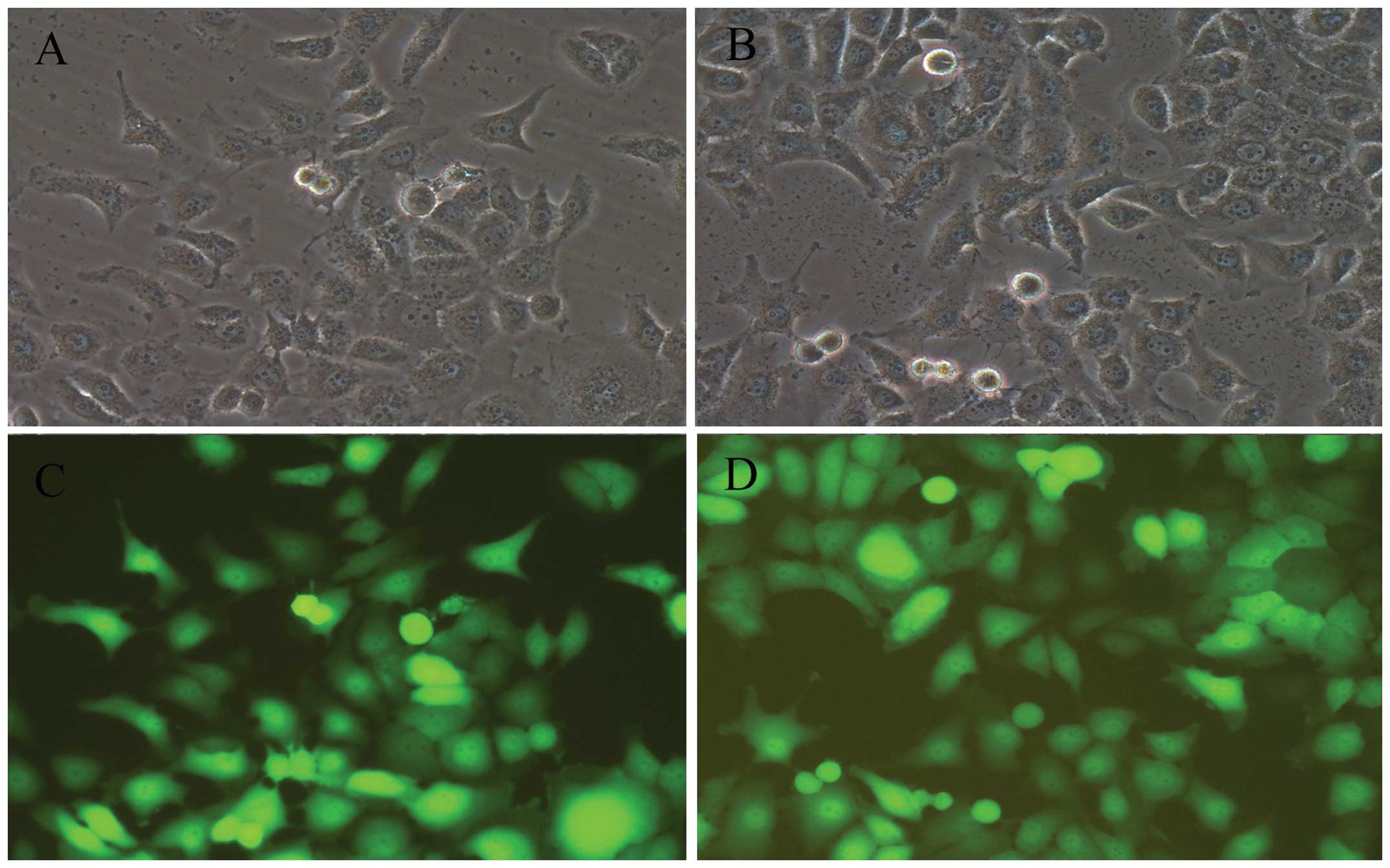
After lentivirus transduction at 20 MOI for 72h, the
cell viability was ~90, and 80% of the BGC-823 cells steadily
delivered the GFP gene (Fig. 2).
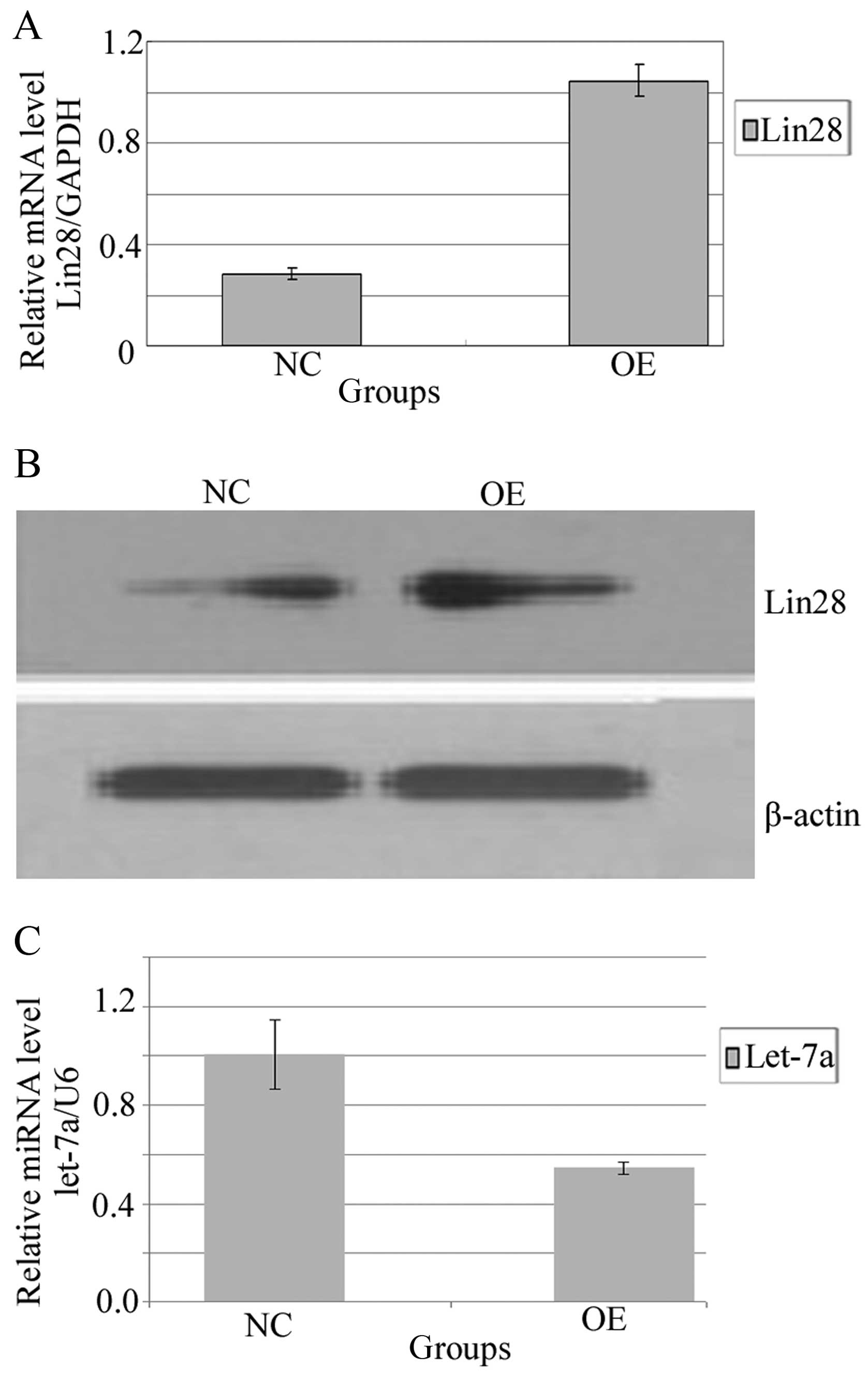
RT-PCR analysis was performed to detect Lin28 expression in the
BGC-823 cells. The mRNA level of Lin28 was low or undetectable in
the NC group, yet exposure of the BGC-823 cells to lenti-Lin28 for
72 h significantly elevated the level of Lin28. The Lin28A mRNA
levels in the OE and NC groups were 28.5±2.3 and 104.5±6.4%,
respectively (P=0.0027) (Fig. 3A).
Moreover, to verify whether the increase in Lin28 mRNA was
translated into upregulation of the protein, we assayed the protein
expression of Lin28 in BGC-823 cells. In accordance with the mRNA
level, western blotting showed that the OE group expressed a higher
protein level following lentivirus Lin28 administration (Fig. 3B).
Overexpression of Lin28 downregulates
let-7a expression in the BGC-823 cells
We assessed let-7a expression in the NC and OE
groups with RT-PCR. Let-7a miRNA levels were 100.7±14.1 and
54.4±2.5%, respectively (Fig. 3C).
The expression level of let-7a miRNA in the OE group was
significantly lower than that in the NC group (P=0.02647). The
overexpression of Lin28 mRNA was inversely correlated with let-7a
(r=−0.863, P=0.003). Systematic overexpression of Lin28 markedly
downregulated the miRNA expression of let-7a.
Overexpression of Lin28 inhibits gastric
cancer cell proliferation and migration
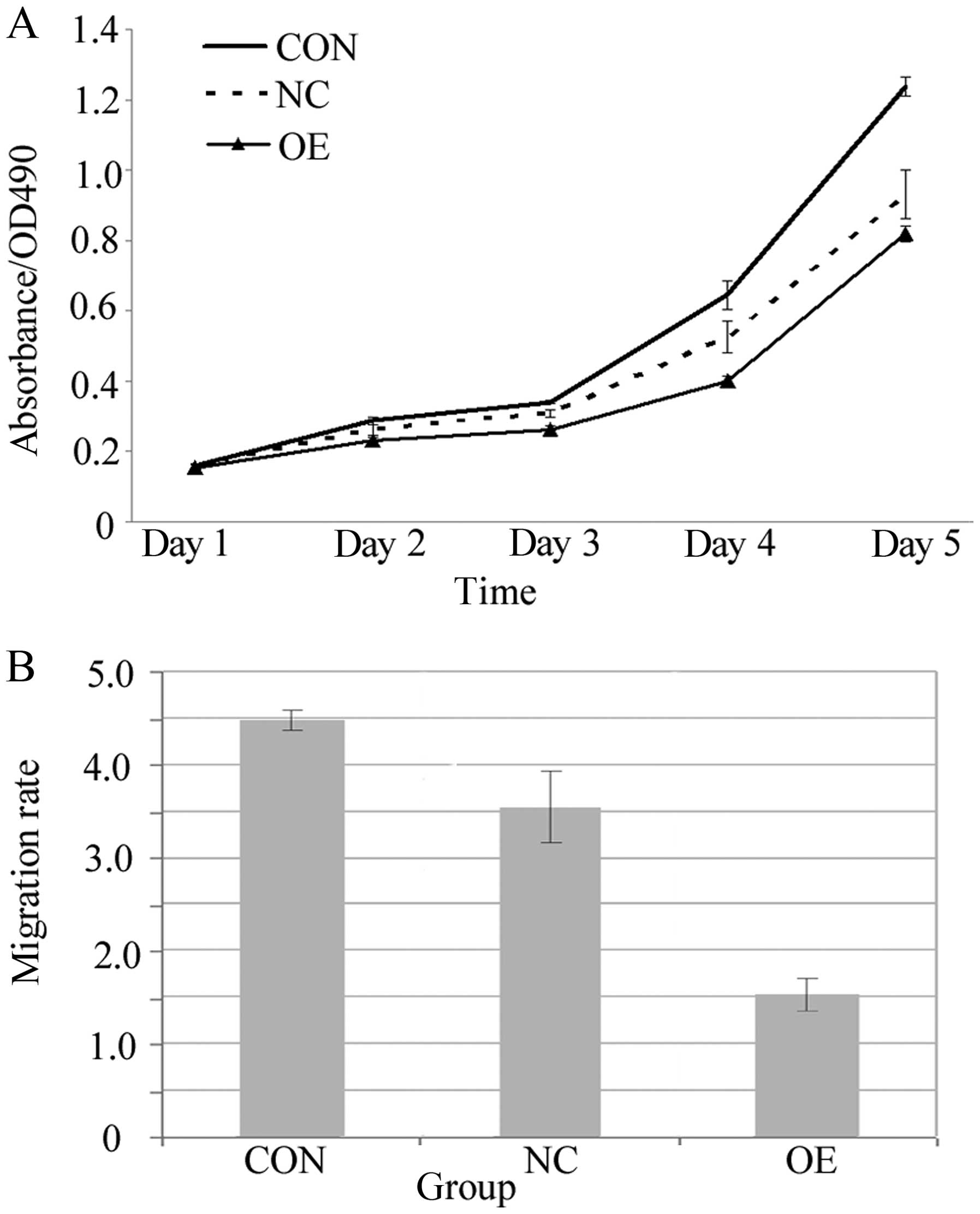
An MTT assay was employed to study the impact of
overexpression of Lin28 on cell proliferation. The number of
proliferating cells was determined by measuring the optical density
(OD) value at 490 nm. The cell lines in all of the groups were
cultured for 144 h, and the OD values were then determined and
compared among the groups. The results indicated that the rate of
cell proliferation was slower in the OE group than the rates in the
CON and NC groups (P<0.05, Fig.
4A). A Transwell assay was used to determine the influence of
the overexpression of Lin28 on cell migration capability.
Differences in migration were distinctly observed among the three
groups after Giemsa staining (Fig.
5). The observations revealed that the migration rate was
significantly decreased in the OE group when compared with the
rates in the CON and NC groups (P<0.05, Fig. 4B).
Overexpression of Lin28 affects cell
cycle progression and the rate of apoptosis
Cell cycle profiles were monitored by flow
cytometric analysis. Compared with that of the CON and NC groups,
the percentage of cells in the G0/G1 phase in the OE group was
significantly increased, while the percentage of these cells in the
G2/M phase was significantly decreased (Fig. 6). These findings indicate that the
inhibitory effect on cell proliferation by Lin28 may be due to
G0/G1 cell cycle arrest and increased apoptosis in BGC-823 cells.
The rate of apoptosis in the OE group was significantly increased
when compared with the rates in the CON and NC groups (Fig. 7).
Discussion
In the present study, we investigated the
association of Lin28 expression with let-7 in gastric cancer cells.
We found that Lin28 expression was downregulated while let-7a was
highly expressed in the gastric cancer cell lines, and that Lin28
was inversely correlated with let-7a. Lin28 transfection induced
downregulation of let-7a miRNA and suppression of proliferation,
migration and cell cycle progression in the gastric cancer cells.
These results suggest that Lin28 may suppress tumor malignancy of
gastric cancer cells through inhibition of let-7 microRNA
biogenesis and could be a potential target for gastric cancer
therapy.
A possible interference of Lin28 towards let-7
maturation process has been reported both in developmental stages
and in various human malignancies. This inverse relationship
between Lin28 and let-7 miRNA is present in mammalian cells, where
Lin28A/B are mainly expressed in undifferentiated cells, and mature
let-7 is only detectable upon differentiation or tissue development
(13,14,17).
In undifferentiated cells, Lin28 is highly expressed
and blocks the biogenesis of let-7 miRNA. Upon differentiation,
Lin28 expression is reduced and leads to increased levels of mature
let-7. Let-7 can silence gene expression of proto-oncogenes (Ras,
c-Myc, Hmga2), cell cycle progression factors (cyclin D1 and D3,
Cdk4), components of the PI3K-mTOR pathway and Lin28 itself,
thereby establishing a positive feedback loop. Besides its role in
differentiation, a Lin28/let-7 regulatory network is apparently
involved in several cellular processes such as development and
physiology, proliferation, oncogenesis, as well as metabolism
(18,19). In the present study, our results
revealed that an inverse relationship between Lin28 and let-7a
miRNA was also present in gastric cancer cells.
The roles of Lin28A and Lin28B are considered to be
homogeneous in the oncogenesis of various types of cancer (14), yet the details of the mechanism
remain unknown. Several studies have shown that Lin28B is
associated with an aggressive biological behavior in several human
malignancies (8,20). For instance, Viswanathan et
al reported that activation of Lin28B may be associated with
rare amplification or translocation of various oncogenes (7). When Lin28B was overexpressed in colon
cancer, cellular proliferation in tumor cells was elevated, which
then often resulted in metastasis and aggressive tumorigenesis
(21). In ovarian cancer, higher
expression of Lin28B appears to be linked with poor prognosis of
patients possibly via mediation of insulin-like growth factor-II
(13).
Research has also highlighted the roles of Lin28 in
the biological behavior of gastric cancer. In previous studies, it
has been reported that Lin28 is expressed at a low level in gastric
carcinoma tissues when compared with the expression in the
corresponding normal tissues, and positive expression of Lin28
protein was found to be correlated with poor outcome. Thus,
positive expression of Lin28 protein serves as an independent
prognostic factor (22). Lin28
expression was also associated with pathologic tumor response in
locally advanced gastric cancer patients undergoing neoadjuvant
chemotherapy and Lin28 could serve as a predictive biomarker for
neoadjuvant chemotherapy in patients with gastric cancer (23). However, in the present study, the
results revealed that overexpression of Lin28 inhibited the
proliferation, migration, cell cycle progression and induced the
apoptosis of gastric cancer cells, and Lin28 is associated with
suppressive biological behavior in gastric cancer cells. To clarify
the precise roles of Lin28, further investigation is required
including further analysis of the molecular mechanisms.
Collectively, the present study demonstrated that
Lin28 inhibited the proliferation, migration and cell cycle
progression of gastric cancer cells, while let-7 microRNA may play
an important role in this process. We suggest that Lin28 may be a
candidate predictor or an anticancer therapeutic target for gastric
cancer patients.
Acknowledgements
This study was supported by Grants from the Open
Project of Jiangsu Province Tumor Biological Therapy Institute (no.
ZL1203), and the Natural Science Foundation Project of Jiangsu
Provincial Education Department (no. 13KJB320029).
References
|
1
|
Moss EG, Lee RC and Ambros V: The cold
shock domain protein LIN-28 controls developmental timing in C.
elegans and is regulated by the lin-4 RNA. Cell. 88:637–646. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu J, Vodyanik MA, Smuga-Otto K, et al:
Induced pluripotent stem cell lines derived from human somatic
cells. Science. 318:1917–1920. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
West JA, Viswanathan SR, Yabuuchi A, et
al: A role for Lin28 in primordial germ-cell development and
germ-cell malignancy. Nature. 460:909–913. 2009.PubMed/NCBI
|
|
4
|
Wong SS, Ritner C, Ramachandran S, et al:
miR-125b promotes early germ layer specification through
Lin28/let-7d and preferential differentiation of mesoderm in human
embryonic stem cells. PLoS One. 7:e361212012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gonzalez F, Barragan Monasterio M,
Tiscornia G, et al: Generation of mouse-induced pluripotent stem
cells by transient expression of a single nonviral polycistronic
vector. Proc Natl Acad Sci USA. 106:8918–8922. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thornton JE and Gregory RI: How does Lin28
let-7 control development and disease? Trends Cell Biol.
22:474–482. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Viswanathan SR, Powers JT, Einhorn W, et
al: Lin28 promotes transformation and is associated with advanced
human malignancies. Nat Genet. 41:843–848. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo Y, Chen Y, Ito H, Watanabe A, Ge X,
Kodama T and Aburatani H: Identification and characterization of
lin-28 homolog B (LIN28B) in human hepatocellular carcinoma. Gene.
384:51–61. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saiki Y, Ishimaru S, Mimori K, et al:
Comprehensive analysis of the clinical significance of inducing
pluripotent stemness-related gene expression in colorectal cancer
cells. Ann Surg Oncol. 16:2638–2644. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pan L, Gong Z, Zhong Z, Dong Z, Liu Q, Le
Y and Guo J: Lin-28 reactivation is required for let-7 repression
and proliferation in human small cell lung cancer cells. Mol Cell
Biochem. 355:257–263. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Heo I, Joo C, Cho J, Ha M, Han J and Kim
VN: Lin28 mediates the terminal uridylation of let-7 precursor
microRNA. Mol Cell. 32:276–284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Newman MA, Thomson JM and Hammond SM:
Lin-28 interaction with the Let-7 precursor loop mediates regulated
microRNA processing. RNA. 14:1539–1549. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rybak A, Fuchs H, Smirnova L, Brandt C,
Pohl EE, Nitsch R and Wulczyn FG: A feedback loop comprising lin-28
and let-7 controls pre-let-7 maturation during neural stem-cell
commitment. Nat Cell Biol. 10:987–993. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Viswanathan SR, Daley GQ and Gregory RI:
Selective blockade of microRNA processing by Lin28. Science.
320:97–100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Viswanathan SR and Daley GQ: Lin28: a
microRNA regulator with a macro role. Cell. 140:445–449. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thomson JA, Itskovitz-Eldor J, Shapiro SS,
Waknitz MA, Swiergiel JJ, Marshall VS and Jones JM: Embryonic stem
cell lines derived from human blastocysts. Science. 282:1145–1147.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Darr H and Benvenisty N: Genetic analysis
of the role of the reprogramming gene LIN-28 in human embryonic
stem cells. Stem Cells. 27:352–362. 2009. View Article : Google Scholar
|
|
18
|
Thornton JE, Chang HM, Piskounova E and
Gregory RI: Lin28-mediated control of let-7 microRNA expression by
alternative TUTases Zcchc11 (TUT4) and Zcchc6 (TUT7). RNA.
18:1875–1885. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mayr F and Heinemann U: Mechanisms of
Lin28-mediated miRNA and mRNA regulation - a structural and
functional perspective. Int J Mol Sci. 14:16532–16553. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang TC, Zeitels LR, Hwang HW, et al:
Lin-28B transactivation is necessary for Myc-mediated let-7
repression and proliferation. Proc Natl Acad Sci USA.
106:3384–3389. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
King CE, Cuatrecasas M, Castells A,
Sepulveda AR, Lee JS and Rustgi AK: LIN28B promotes colon cancer
progression and metastasis. Cancer Res. 71:4260–4268. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu C, Shen J, Xie S, Jiang Z, Huang L and
Wang L: Positive expression of Lin28 is correlated with poor
survival in gastric carcinoma. Med Oncol. 30:3822013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Teng RY, Zhou JC, Jiang ZN, et al: The
relationship between Lin28 and the chemotherapy response of gastric
cancer. Onco Targets Ther. 6:1341–1345. 2013. View Article : Google Scholar : PubMed/NCBI
|