Introduction
Garlic (Allium sativum) is widely cultivated
and eaten as a food, and for thousands of years has been known to
have efficacy against various diseases. Epidemiological studies
have revealed that an increased intake of garlic reduces the onset
risk of colon, stomach and esophageal cancer (1,2).
Garlic and its organosulfur components have been reported to
suppress colon carcinogenesis in animal experiments (3–5) and to
inhibit the proliferation of cancer cell lines in vitro
(6–13). Its antitumorigenic mechanisms
include inhibition of mutagenic (14,15)
and carcinogenic activity (16),
enhancement of detoxification (3,17) and
protection against DNA mutagenesis (18) from activated carcinogens.
1,2-Dimethylhydrazine (DMH) is a widely used agent
for the chemical inducement of colon carcinogenesis in rodents
(19–21). Subcutaneously injected DMH is
sequentially metabolized to methylazoxymethanol (MAM) in the liver.
This metabolite is transported to the colon via the bile or blood
circulation to cause DNA mutations from G:C to A:T in genes
involved in cell proliferation. Following DMH treatment, the
epithelial cells undergo pathogenesis from minor lesion aberrant
crypt foci (ACF) to adenomas and malignant adenocarcinomas
(22,23).
Aged garlic extract (AGE) is a unique garlic
preparation created through prolonged extraction of fresh garlic.
It is less of an irritant and does not produce the uncomfortable
effects of raw garlic, and is suitable for long-term use. AGE has
various physiological functions, and antitumor (24,25),
immunostimulatory (26),
anti-oxidant (27) and anti-fatigue
effects (28) have been reported.
Although previous studies on the effect of AGE using chemical
carcinogenic agents have been evaluated, indicating its
chemopreventive effect on colon carcinogenesis (29), little research has been performed on
its antitumor effects and mechanisms. AGE activates phase II
enzymes such as glutathione S-transferase (GST), which has been
shown to decrease DNA adduct formation by detoxifying reactive
metabolites, resulting in decreases in DMH-induced colon
carcinogenesis. In the present study, we investigated the antitumor
development effects of AGE in rats that had undergone DMH-induced
colon carcinogenesis, and attempted to elucidate its mechanism of
action by focusing on its anti-proliferative activity and induction
of apoptosis.
Herein, we demonstrated that AGE inhibited
DMH-induced colorectal tumor development by attenuating the
proliferative activity of adenoma and adenocarcinoma cells. This
suppressive effect was associated with cell cycle delay at the G2/M
phase resulting from cyclin B1 and cdk1 downregulation, but not due
to the induction of apoptosis or cell cycle arrest.
Materials and methods
Animals and diet
Four-week-old male F344 rats were purchased from
Charles River Japan (Kanagawa, Japan) and maintained under
controlled temperature (23±2°C), humidity (50±5%) and lighting (12
h light/dark cycle) conditions. All animal studies were approved by
the Animal Care Committee of Prefectural University of Hiroshima.
Animals were allowed to acclimatize for one week before the
experiments were commenced, and were given free access to diet and
tap water ad libitum. AGE provided by Wakunaga
Pharmaceutical Co. Ltd. (Hiroshima, Japan) was produced as follows:
garlic cloves were sliced and soaked in a water-ethanol mixture
solution and naturally extracted for >10 months at room
temperature (26,27). The extract was evaporated until dry
and mixed with basal MF diet (Oriental Yeast Co., Tokyo, Japan) at
a concentration of 3% wt/wt. 1,2-Dimethylhydrazine (DMH) was
purchased from Tokyo Chemical Industry (Tokyo, Japan).
Carcinogenesis protocol
DMH was weighed and dissolved in saline, and the pH
was adjusted to 6.5 with NaHCO3. The experimental
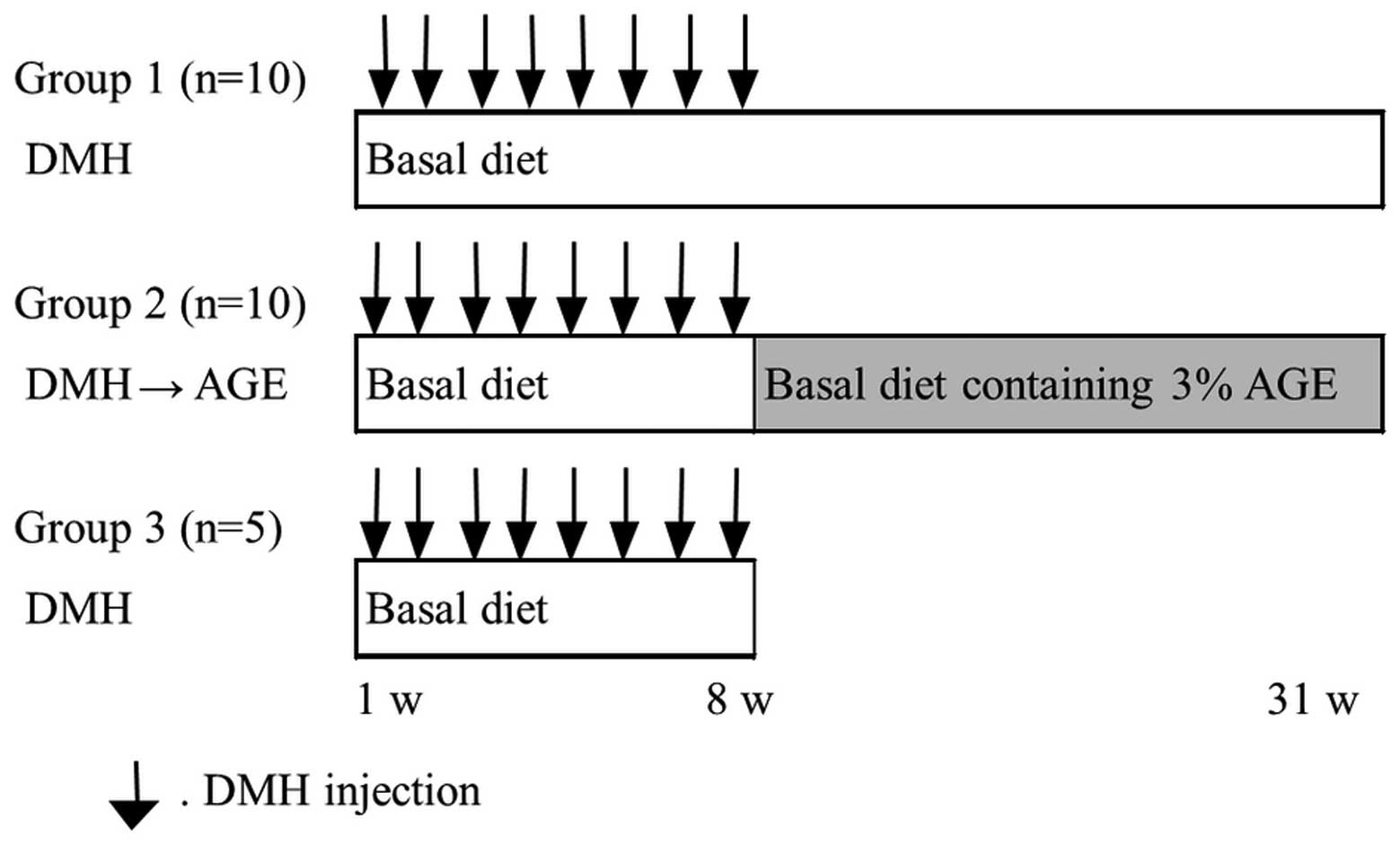
protocol is shown in Fig. 1. A
total of 25 rats were randomly distributed into three groups. The
rats in group 1 (n=10) were fed a basal diet ad libitum
throughout the experimental period and received subcutaneous
injections of DMH at a dose of 20 mg/kg body weight once a week for
the first 8 weeks. Group 2 (n=10) rats received the same course of
DMH injections as group 1 and were fed a basal diet ad
libitum. One week after the last injection of DMH, the rats of
group 2 were fed a diet containing 3% AGE for the remaining
experimental period. Group 3 (n=5) rats received the same course of
DMH injections as group 1. The food intake and body weight of the
rats were recorded weekly. Group 1 and 2 rats were sacrificed at
the end of 31 weeks and group 3 rats at the end of 8 weeks,
whereupon the weights of livers, spleens, kidneys and epididymal
adipose tissue were measured. The entire colon was removed, cut
open along the longitudinal axis from the cecum to the anus, and
placed on a paper towel. Gross tumor number, size and macroscopic
futures were determined, and the colon tissue was fixed in 10%
phosphate-buffered formalin for at least 24 h prior to pathological
examination.
Analysis of aberrant crypts
Fixed colon tissue was stained with 0.5% methylene
blue in distilled water for 5–15 min, and placed luminal side up on
a light microscope slide. The stained whole mount colons were
viewed under a microscope at a magnification of ×10, and ACF were
distinguished from the surrounding normal-appearing crypts by their
large and elliptical luminal openings (30). The number of ACF with more than four
crypts/focus in each colon was counted and divided by the total
number of ACF to evaluate the crypt multiplicity.
Histological analysis
After ACF counting, the colons were sectioned into
eight parts, which were then divided into three in the longitudinal
axis direction, and dehydrated and embedded in paraffin wax. For
pathological analysis, 4-μm sections of formalin-fixed
paraffin-embedded tissues were prepared. Deparaffinized and
rehydrated slides were stained by hematoxylin and eosin (H&E),
and sections were examined under a light microscope at ×200
magnification (Olympus, Japan). Tumor histology was classified as
adenomas and adenocarcinomas by one investigator and one
pathologist under blinded conditions according to WHO criteria
(31). Tumor grade and depth of
invasion were analyzed based on UICC TNM classification (6th
edition).
PCNA immunohistochemical staining
Tissue sections (4-μm) were deparaffinized and
rehydrated with PBS, and then retrieved in a microwave oven in
target retrieval solution (Dako, Glostrup, Denmark) for 10 min.
Endogenous peroxidase activity was blocked with 3% hydrogen
peroxidase for 10 min at room temperature. The sections were
incubated overnight at 4°C with anti-PCNA antibody (clone PC10,
Dako) at a 1:200 dilution. The sections were rinsed with PBS,
incubated with biotinylated secondary antibody (LSAB2 System-HRP,
Dako) for 60 min at room temperature, incubated with HRP-linked
tertiary antibody and then stained with 3,3′ diaminobenzidine
(DAB). Finally, all sections were counterstained with hematoxylin.
Cells with brown nuclei were considered positive. Scoring of
PCNA-positive indices was evaluated using ImageJ software (NIH,
Bethesda, MD, USA).
Cell culture and AGE treatment
DLD-1 human colon cancer cells (ATCC CCL-221) and
MRC-5 human lung fibroblast cells (ATCC CCL-171) were purchased
from the American Type Culture Collection (ATCC, Manassas, VA,
USA). DLD-1 and MRC-5 cells were cultured in RPMI-1640 (Nakarai,
Japan) supplemented with 10% heat-inactivated fetal bovine serum
(FBS) (HyClone, Logan, UT, USA), penicillin (100 U/ml), and
streptomycin (100 μg/ml) in a humidified atmosphere with 5%
CO2 at 37°C. Culture media containing different
concentrations of AGE were freshly prepared at the time of each
experiment. When cell confluency reached 50–60%, the cells were
treated with 0, 1, 5 or 10 mg/ml AGE.
Cell proliferation assay
Cells (4×103) were seeded in 96-well
plates and incubated with different doses of AGE for varying
durations, each in triplicate. After each period of incubation,
cells were fixed by 50% trichloroacetic acid (TCA) at 4°C for 1 h
and stained with 0.4% sulforhodamine B (Sigma-Aldrich, St. Louis,
MO, USA) solution for 20 min. The unincorporated stain was removed
with 1% acetic acid. The dye was solubilized in 10 mM Tris for 5
min. The color reaction was quantified using a plate reader at 565
nm.
Cell synchronization
DLD-1 cells were synchronized at the G1/S boundary
by double thymidine block. The cells were pre-synchronized at S
phase by incubation with 2 mM thymidine (Wako, Japan) for 15 h, and
released by changing the medium to fresh medium without thymidine
for 8 h. Cells were then resynchronized at the G1/S phase boundary
by incubation with 2 mM thymidine for 15 h. Cells were washed and
incubated in fresh medium or AGE-containing medium to promote
re-entry into the cell cycle.
Cell cycle analysis
The cell cycle distribution of the DLD-1 cells was
measured by flow cytometry. Cells were harvested and fixed with
ice-cold 70% ethanol for 1 h. Cells were treated with 100 μg/ml of
RNaseA (Wako) and 25 μg/ml of propidium iodide (PI) (Sigma) for 30
min at 37°C, and then analyzed using a FACSCalibur flow cytometer
(Becton Dickinson Bioscience, Franklin Lakes, NJ, USA).
Western blotting
Cells treated with or without AGE at the indicated
concentrations and times were harvested, and then resuspended in
Cell-LyEX cell lysis buffer (Toyo B-Net, Japan) supplemented with
Protease Phosphatase Inhibitor Cocktail (Thermo Fisher Scientific,
Inc., Rockford, IL, USA) on ice for 1 h. Whole cell lysates were
centrifuged at 20,600 × g and the supernatants were used for
western blotting. For the preparation of nuclear extracts, the
cells were resuspended in Buffer A (10 mM HEPES (pH 7.5), 10 mM
KCl, 0.1 mM EGTA, 0.1 mM EDTA, 1 mM DTT, 0.5 mM PMSF, proteinase
inhibitors and 0.5% Nonidet P-40) on ice for 15 min. The
centrifuged pellet was resuspended in Buffer B [20 mM HEPES (pH
7.5), 400 mM NaCl, 1 mM EGTA, 1 mM EDTA, 1 mM DTT, 1 mM PMSF and
proteinase inhibitors], and vortexed on ice for 15 min. The
centrifuged supernatant at 20,600 × g was obtained as nuclear
extract protein. Equal amounts of proteins (10 or 20 μg) were
boiled and separated by sodium dodecylsulfate polyacrylamide gel
electrophoresis (SDS-PAGE), transferred to polyvinylidene
difluoride (PVDF) membranes (Millipore, Billerica, MA, USA) and
blocked with 2% BSA (w/v) in TBST (10 mM Tris, 150 mM NaCl and
0.05% Tween-20) for 1 h at room temperature. Membranes were
incubated with primary antibodies against cyclin B1 (clone 12231),
cdk1 (clone 9112), cyclin D1 (clone 2926), NF-κB p65 (clone 8242),
caspase-3 (clone 9662) (Cell Signaling Technology, Beverly, MA,
USA), β-actin (MBL, Japan) or lamin B1 (clone 133741, Abcam, USA)
at a 1:1000 dilution at 4°C overnight and then incubated with
horseradish peroxidase-conjugated secondary antibody (Invitrogen,
Carlsbad, CA, USA) at a 1:2000 dilution at room temperature for 1
h. Blots were visualized using a chemiluminescence western blotting
kit (Luminate Forte Western HRP Substrate, Millipore). Analysis was
performed with the ImageQuant LAS 4000 analyzer, and results were
analyzed with ImageQuant TL software (GE Healthcare, Milwaukee, WI,
USA).
Measurement of caspase-3 activity
DLD-1 cells (4.5×103) were seeded in
96-well half well plates and incubated with 0, 1, 5 or 10 mg/ml AGE
in triplicate for 2 days; 0.05 μM staurosporine (Sigma) was used as
a positive control. Caspase-3 activity was measured using a
commercially available Caspase-Glo 3/7 assay (Promega, Madison, WI,
USA) according to the manufacturer’s instructions.
Statistical analysis
The data obtained in this study are presented as
means ± standard error (SE). The significance of the differences
obtained in the in vivo experiments was evaluated by
unpaired two-tailed Student’s t-tests. For the in vitro
experiments, the statistical significance was compared between each
treatment group and the control using one-way analysis of variance
(ANOVA) and Tukey-Kramer’s tests. A P-value of <0.05 was
considered significant.
Results
General observations
Throughout the study, no macroscopic abnormalities
were observed in any of the groups. Body, liver, spleen, kidney,
epididymal adipose tissue weights, and daily consumption of food
are shown in Table I. Body and
organ weight did not differ significantly in each group, nor did
average food intake, indicating the long-term safety of AGE
administration.
 | Table IFinal body weight, mean food intake
and organ weight of the rats. |
Table I
Final body weight, mean food intake
and organ weight of the rats.
| | | | | Organ weight |
|---|
| | | | |
|
|---|
| Group | Treatment | n | Body weight at 31
weeks (g) | Food intake
(g/day/rat) | Liver (g) | Spleen (g) | Kidney (g) | Epididymal adipose
tissue (g) |
|---|
| 1 | DMH | 10 | 401.0±8.6 | 14.6±0.1 | 7.87±0.21 | 0.72±0.01 | 1.01±0.03 | 11.65±0.51 |
| 2 | DMH→AGEa | 10 | 381.0±7.4 | 14.5±0.1 | 7.62±0.22 | 0.71±0.03 | 1.00±0.02 | 10.09±0.56 |
Total number and multiplicity of ACF
All rats treated with DMH for 8 weeks showed
development of 114.2±20 ACF at the end of the injection course
(Table II; group 3). In contrast,
our previous study demonstrated that ACF were absent in
DMH-untreated rats. DMH induced a total of 245.6±29 ACF per rat
(group 1), while AGE intake after DMH treatment (group 2) reduced
the total number of ACF (178.4±25). Furthermore, the number of foci
containing more than four aberrant crypts (AC)/focus was
significantly decreased in group 2 (24.8±4) compared with group 1
(50.6±10) (P=0.038) (Table II,
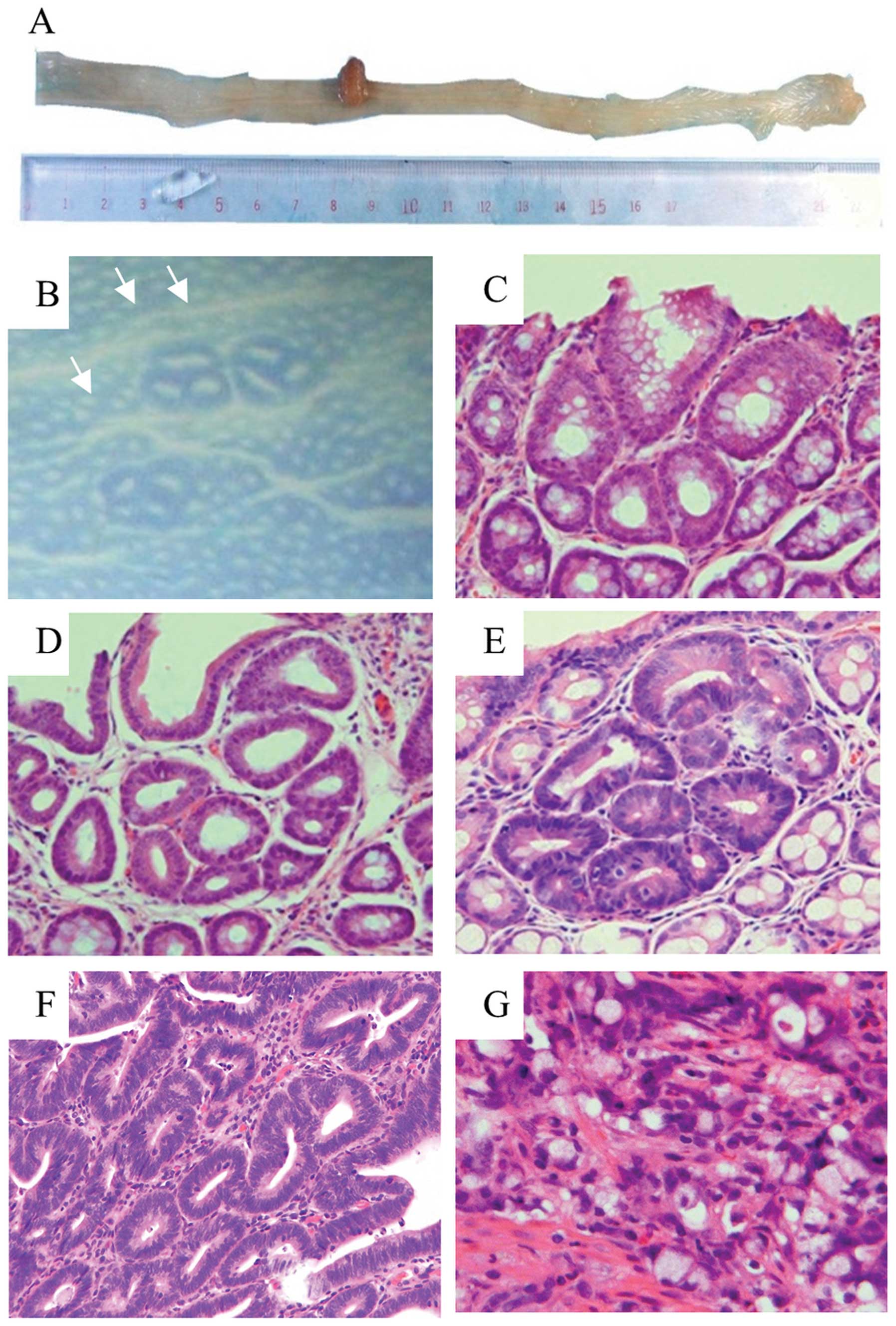
Fig. 2B; original magnification,
×10).
 | Table IITotal number and multiplicity of ACF
in the colon tissue. |
Table II
Total number and multiplicity of ACF
in the colon tissue.
| Group | Treatment | n | Weeks | Incidence (%) | Total ACFa | ACF with >4
AC |
|---|
| 1 | DMH | 10 | 31 | 100 | 245.6±29.3 | 50.6±10.5 |
| 2 | DMH→AGE | 10 | 31 | 100 | 178.4±25.7 | 24.8±4.4b |
| 3 | DMH | 5 | 8 | 100 | 114.2±20.8 | 2.0±1.0 |
Effect of AGE on DMH-induced colorectal
tumor development
All grossly visible tumors were semi-pedunculated,
located in the middle and distal regions of the colon, and
histologically diagnosed as well-differentiated adenocarcinomas
(Fig. 2A and F). There were no
differences in gross tumor incidence (number of rats bearing tumors
per group) and multiplicity (number of tumors per rat) between
group 2 and group 1. However, the mean tumor diameter of group 2
(5.0±1.5 mm) was smaller than group 1 (7.3±1.4 mm), but with no
significant difference (Table
III). On the other hand, the multiplicity of microscopic tumors
histologically diagnosed as adenomas in group 2 was decreased
compared with group 1 (P=0.003) (Table
IV) (Fig. 2C–E). Moreover, the
percentage of adenomas with severe dysplasia was significantly
reduced in group 2 (P=0.037). The pathological multiplicity of
colon adenocarcinomas in group 2 and group 1 were as follows:
well-differentiated adenocarcinoma, 0.2±0.1 and 0.8±0.2; moderately
differentiated adenocarcinoma, 0.2±0.1 and 0.3±0.1; poorly
differentiated adenocarcinoma, 0.1±0.1 and 0.0±0.0; mucinous
adenocarcinoma was not observed; and signet-ring cell carcinoma,
0.0±0.0 and 0.2±0.1, respectively (Fig.
2G). The total multiplicity of adenocarcinomas in group 2 was
decreased compared with group 1 (0.5±0.2 and 1.3±0.3, P=0.078). The
depth of invasion of colon adenocarcinomas in group 2 and group 1
was as follows: early colorectal cancer (mucosa and submucosa),
0.5±0.2 and 1.2±0.3; and advanced colorectal carcinoma (muscularis,
subserosa and serosa), 0.0±0.0 and 0.1±0.1, respectively (Table V).
 | Table IIIIncidence, multiplicity and size of
the gross colon tumors. |
Table III
Incidence, multiplicity and size of
the gross colon tumors.
| | | Gross lesions of
adenocarcinoma |
|---|
| | |
|
|---|
| Group | Treatment | n | Incidence (%) |
Multiplicitya | Tumor diameter
(mm) |
|---|
| 1 | DMH | 10 | 30 | 0.3±0.2 | 7.3±1.4 |
| 2 | DMH→AGE | 10 | 30 | 0.3±0.2 | 5.0±1.5 |
 | Table IVIncidence, multiplicity and
distribution of the microscopic colon adenomas. |
Table IV
Incidence, multiplicity and
distribution of the microscopic colon adenomas.
| | | | Distribution
(%) |
|---|
| | | |
|
|---|
| Group | n | Incidence (%) | Multiplicity | Mild | Moderate | Severe |
|---|
| 1 | 10 | 100 | 32.4±5.0 | 62.0±4.1 | 32.4±3.5 | 5.6±1.4 |
| 2 | 10 | 90 | 12.5±2.7b | 76.6±6.4 | 30.8±5.7 | 1.6±1.1a |
 | Table VPathological characteristics of the
microscopic colon adenocarcinomas. |
Table V
Pathological characteristics of the
microscopic colon adenocarcinomas.
| Histology |
|---|
| | Differentiated
type | Undifferentiated
type | |
|---|
| |
|
| |
|---|
| Group | n | Well differentiated
adenocarcinoma | Moderately
differentiated adenocarcinoma | Total | Poorly
differentiated adenocarcinoma | Mucinous
adenocarcinoma | Signet-ring cell
carcinoma | Total | Total/rat |
|---|
| 1 | 10 | 0.8±0.2 | 0.3±0.1 | 1.1±0.3 | 0.0±0.0 | 0.0±0.0 | 0.2±0.1 | 0.2±0.1 | 1.3±0.3 |
| 2 | 10 | 0.2±0.1 | 0.2±0.1 | 0.4±0.2a | 0.1±0.1 | 0.0±0.0 | 0.0±0.0 | 0.1±0.1b | 0.5±0.2c |
|
| Depth of
invasion |
| | Early colorectal
cancer | Advanced colorectal
carcinomas | |
| |
|
| |
| Group | n | m | sm | Total | mp | ss | se | Total | Total/rat |
|
| 1 | 10 | 0.9±0.3 | 0.3±0.1 | 1.2±0.3 | 0.1±0.1 | 0.0±0.0 | 0.0±0.0 | 0.1±0.1 | 1.3±0.3 |
| 2 | 10 | 0.2±0.1 | 0.3±0.1 | 0.5±0.2a | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.5±0.2c |
PCNA-labeling index in the colon tumor
lesions
Proliferating cell nuclear antigen (PCNA) is
considered to be a marker of cell proliferation, and is
overexpressed in various cancers. We found that AGE decreased
DMH-induced colorectal adenoma and adenocarcinoma formation. To
investigate the effect of AGE on the proliferative activity of
these dysplastic cells, PCNA activity in adenoma and adenocarcinoma
lesions was examined by immunohistochemical (IHC) staining. Prior
to IHC analysis, serial paraffin sections of colon tissues were
classified as normal mucosa, adenomas or adenocarcinomas using
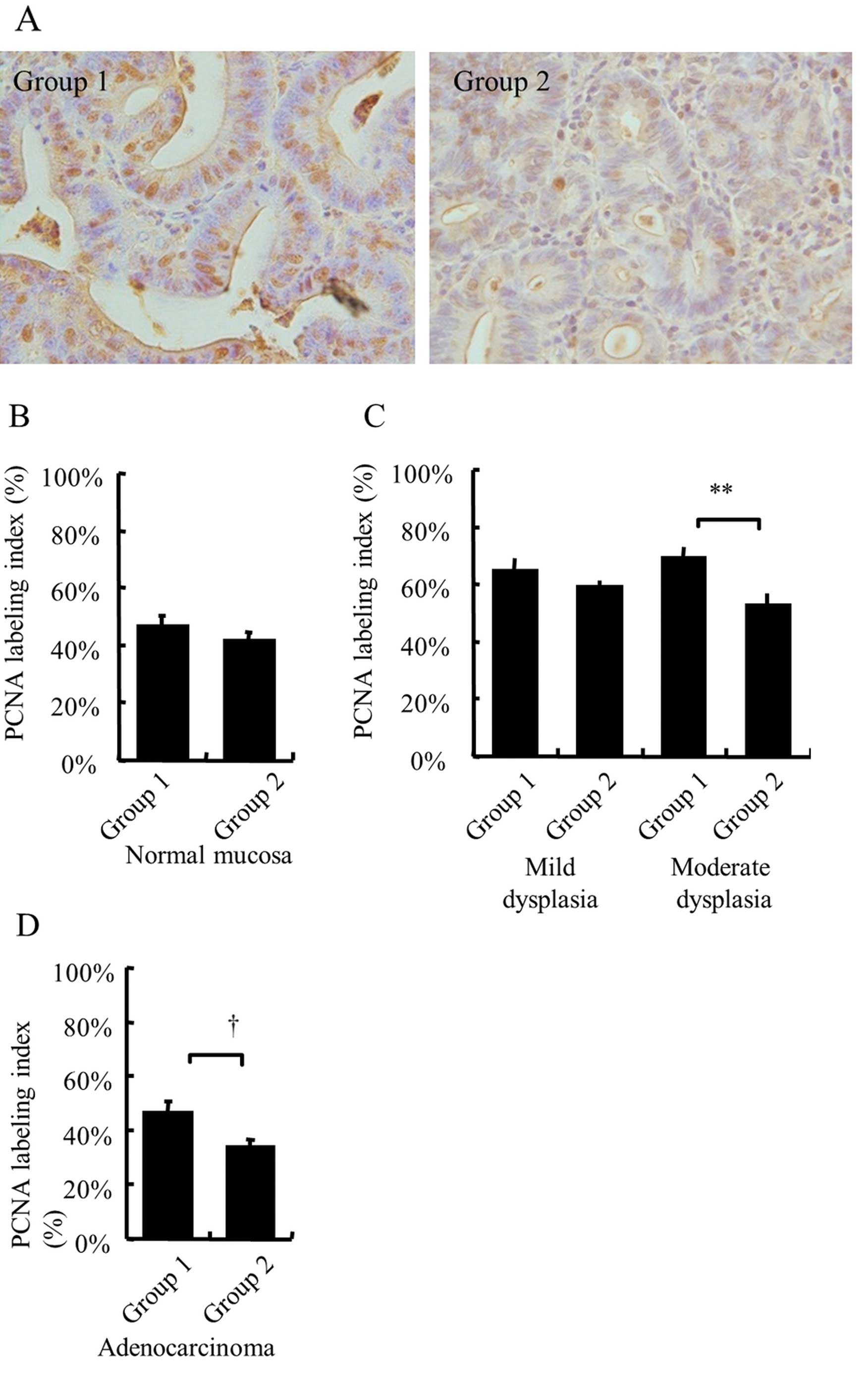
H&E staining. Fig. 3A shows the
proliferative activity of the grossly visible tumors diagnosed as
well-differentiated adenocarcinoma lesions as measured by PCNA
expression. Qualitative microscopic evaluation of the PCNA labeling
index showed that it was substantially increased in group 2
(P=0.054) (Fig. 3A and D).
Similarly, the PCNA labeling index in the adenoma lesions was
significantly decreased in group 2 (55±3%) compared with group 1
(67±3%) (P=0.005). Moreover, in the classification by histological
type of adenomas, the AGE group showed a significantly decreased
PCNA labeling index compared with the DMH group for moderate
dysplasia (53±4 and 69±4%, respectively) (P=0.01), but did not
differ with respect to mild dysplasia (59±2 and 65±4%,
respectively) (P=0.3; Fig. 3C). In
contrast, there was no difference in the PCNA labeling index for
normal colon mucosa between the groups (Fig. 3B). These results indicate that AGE
suppressed the proliferation of middle- and late-stage DMH-induced
colon tumors.
Effect of AGE on the proliferation of
colon cancer
To gain insight into the mechanism of tumor cell
inhibition by AGE, sulforhodamine B assays were performed. DLD-1 or
MRC-5 cells were treated with AGE at different concentrations for
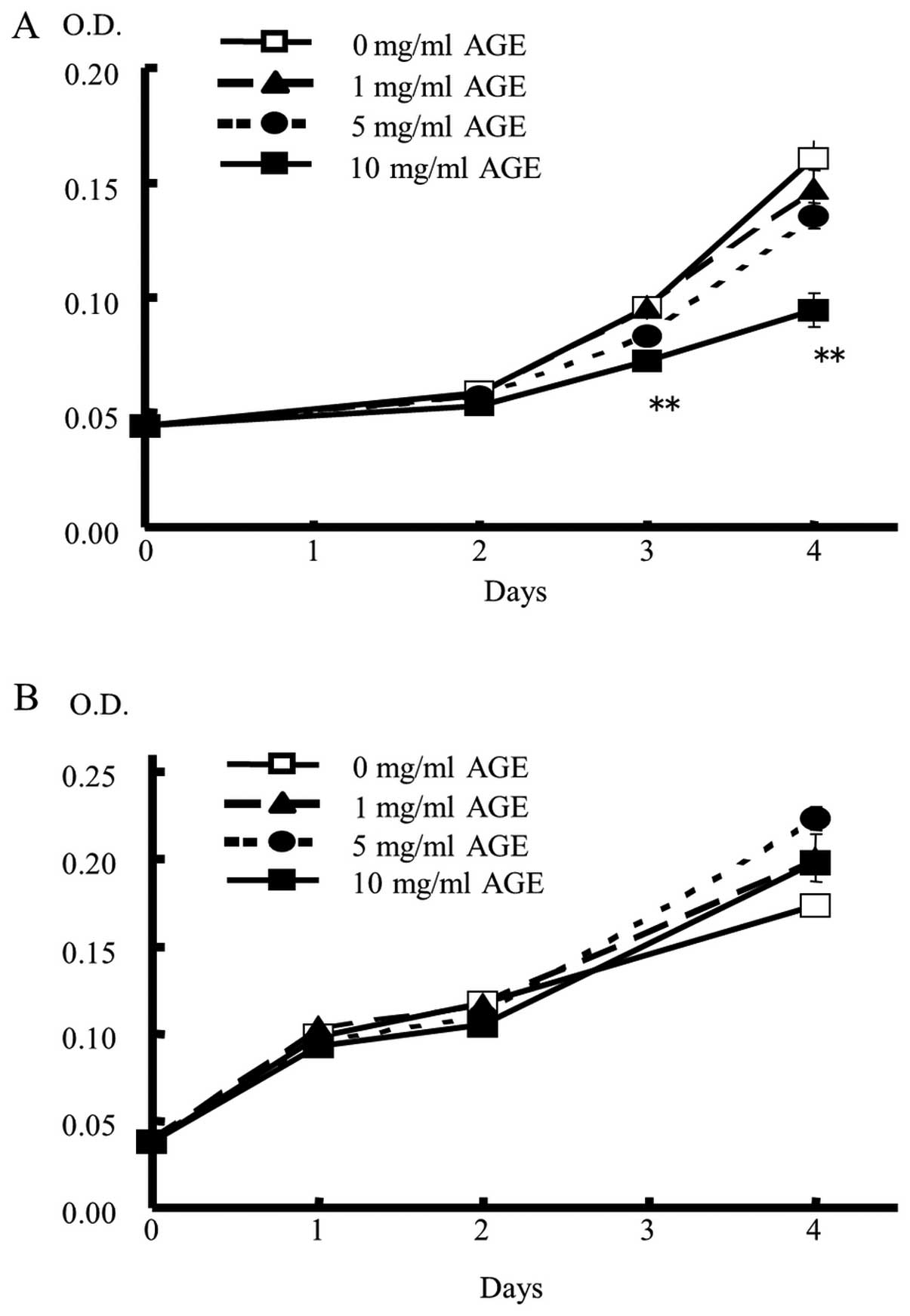
varying durations in triplicate experiments (Fig. 4). Over a culture period of >2
days, DLD-1 cell proliferation was significantly suppressed by AGE
in a dose- and time-dependent manner, as evidenced by statistically
significant suppression at 10 mg/ml concentration compared with no
AGE treatment (Fig. 4A). In
contrast, AGE showed no suppressive effect on normal fibroblast
MRC-5 cells under any condition (Fig.
4B).
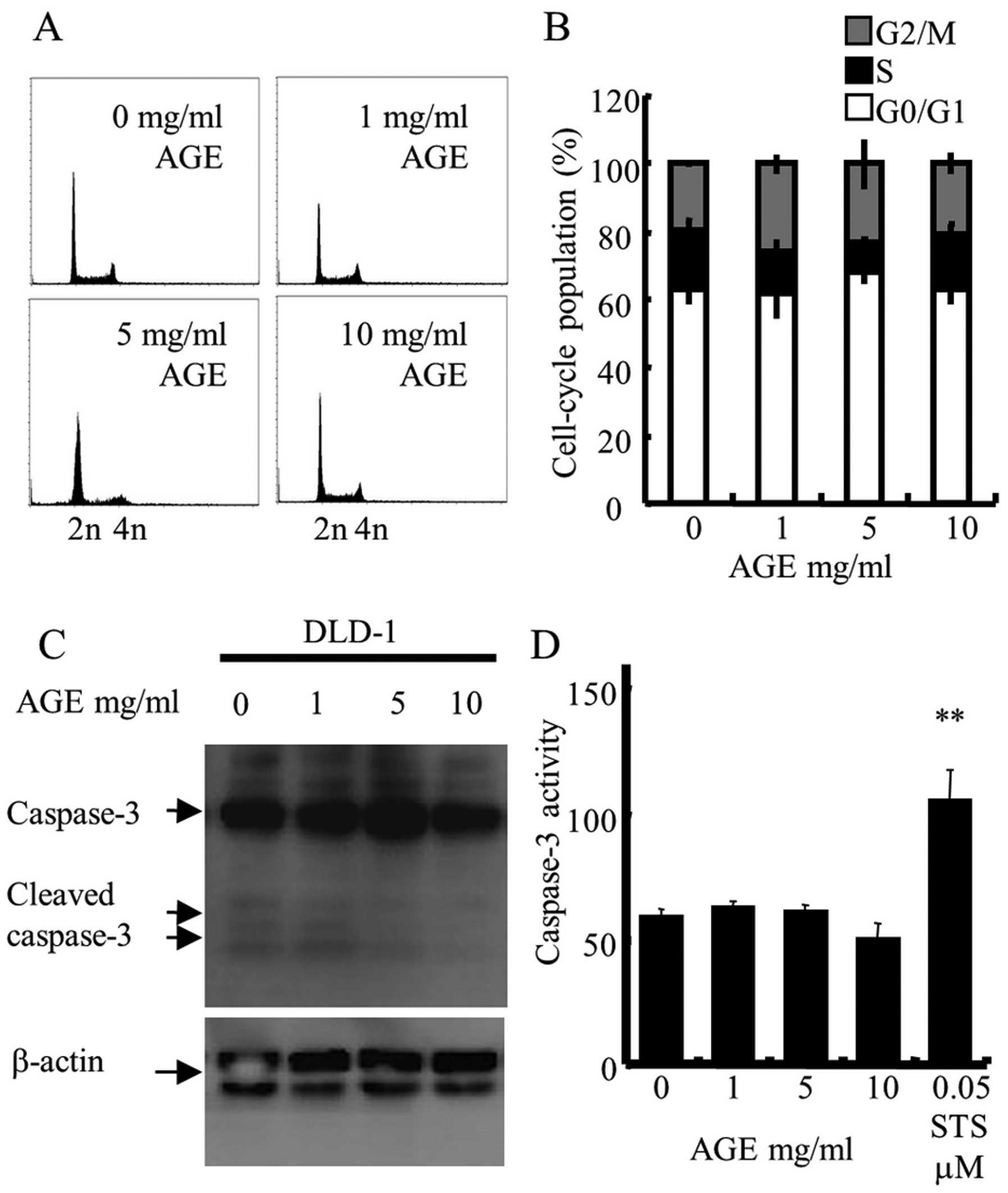
Next, to investigate whether the growth suppressive
effects of AGE were correlated with cell cycle arrest or apoptosis
induction, which is a generally accepted mechanism for
anti-proliferative agents (32),
the cell cycle distribution of DLD-1 cells was examined by flow
cytometric analysis. DLD-1 cells were treated with 0, 1, 5 or 10
mg/ml AGE for 2 days, and harvested cells were stained with PI.
Fig. 5A and B show that AGE did not
promote cell cycle arrest at any phase, and did not increase the
sub-G1 population, an indicator of apoptosis. Results of the
immunoblotting of whole cell lysates treated with AGE for 2 days
showed that AGE did not induce the expression of cleaved caspase-3,
a protein that plays a central role in the execution-phase of cell
apoptosis (33) (Fig. 5C). In addition, AGE did not increase
the activity of caspase-3 in the DLD-1 cells (Fig. 5D). These results indicate that the
anti-proliferative activity of AGE against DLD-1 cells was
independent of cell cycle arrest or apoptotic cell death.
Effect of AGE on cell cycle
progression
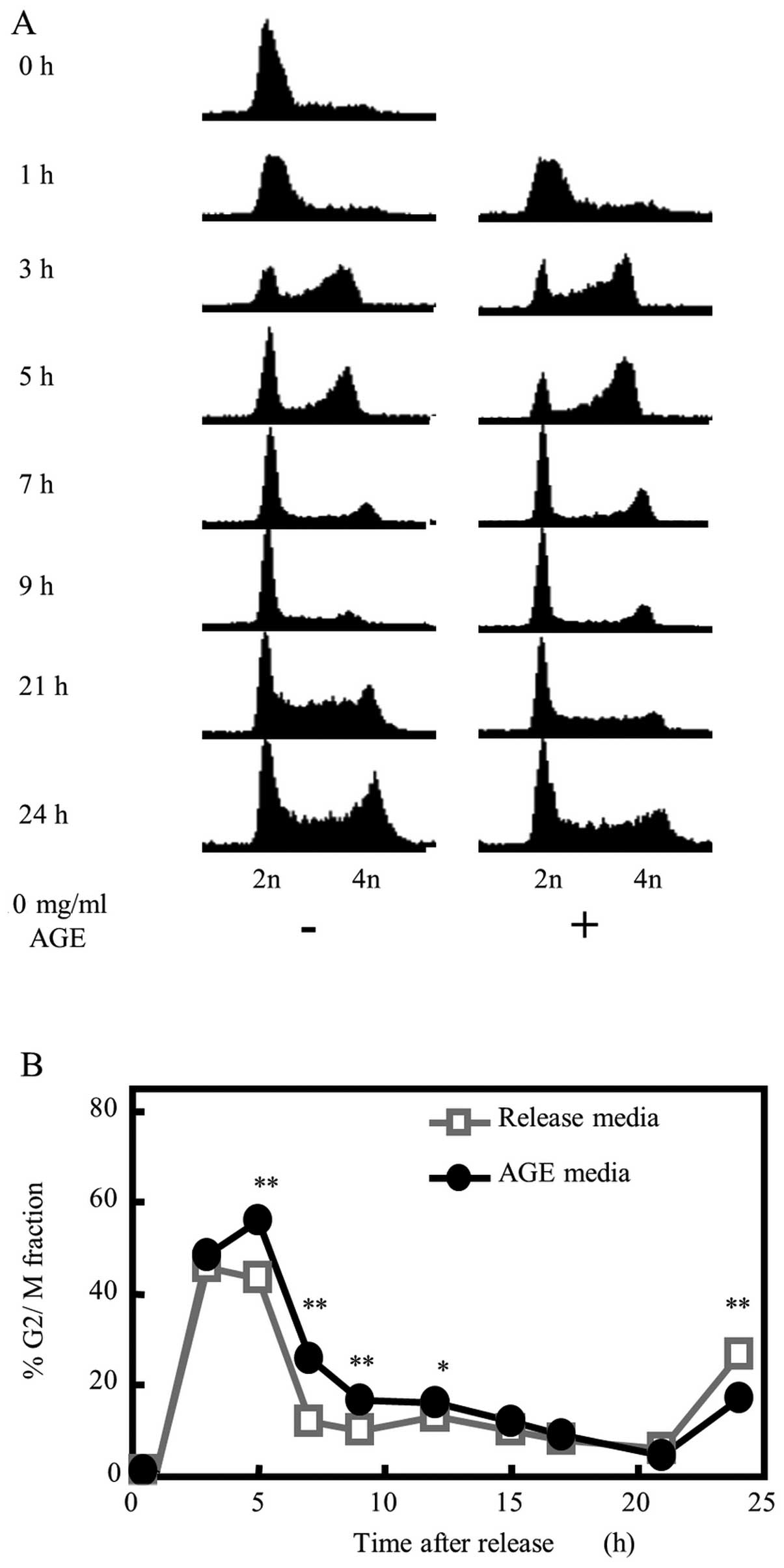
To determine whether the AGE-mediated growth
suppression of colon cancer cells was related to the speed of cell
cycle progression, we synchronized DLD-1 cells at the G1/S phase
boundary by double thymidine block. The medium of synchronized
cells was replaced with fresh culture medium with or without 10
mg/ml AGE (Fig. 6A and B). In the
release medium condition, DLD-1 cells progressed into the G2/M
phase at 3 h post-release, and began to shift to the next G1 phase
from the G2/M phase at 5 h. Most cells were located in the G1 phase
of the next cycle by 7 h. The cells began to shift to the next S
phase at 21 h, and to shift to the next G2/M phase at 24 h. In
contrast, cells released with 10 mg/ml AGE-supplemented medium
showed a cell cycle progression delay of 2–3 h compared with those
that received release medium alone. Many cell populations were
still in the G2/M phase at 5 h post-release. Furthermore, there
were significantly more cells remaining in the G2/M phase as
compared with the release medium-treated cells at 9 h. Moreover, a
transition delay to the next S phase was confirmed in
AGE-supplemented condition at 21 to 24 h post-release.
Effect of AGE on the expression levels of
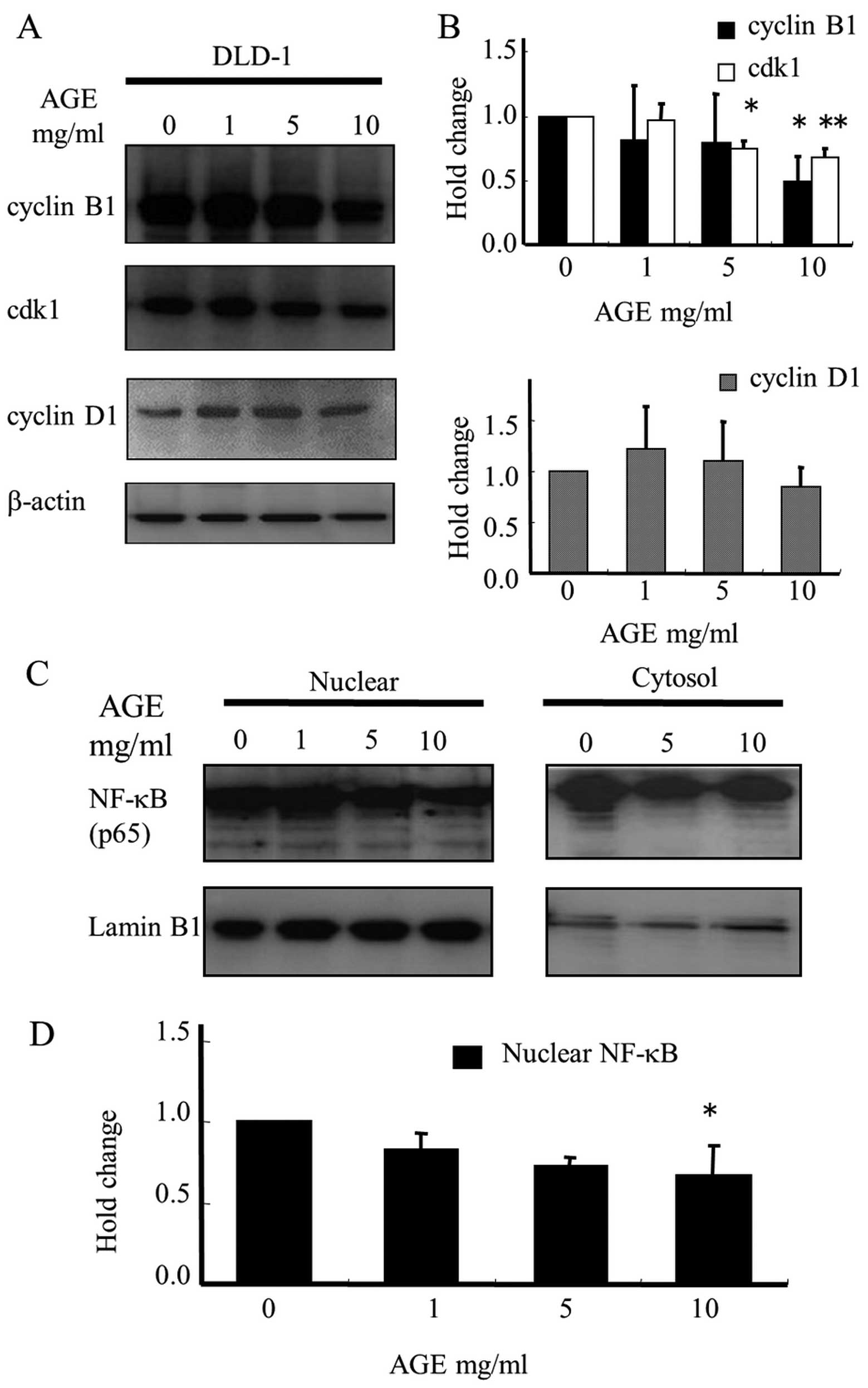
cell cycle-related proteins in DLD-1 cells
To investigate the mechanism of AGE-mediated
proliferative inhibition based on cell cycle delay, we determined
its effect on the levels of proteins related to cell cycle
regulation by immunoblotting. Cyclin B1 has been reported to be an
essential cell cycle component required for the transition from G2
to M phase (34–37). Cyclin-dependent kinase 1 (cdk1)
forms a complex with cyclin B1 to regulate cell cycle progression.
As shown in Fig. 7A and B, AGE
treatment for 2 days caused a decrease in cyclin B1 and cdk1
protein levels in the DLD-1 cells, but did not affect the
expression of cyclin D1, which is required for G1/S transition. The
quantitative results demonstrated that 10 mg/ml AGE suppressed the
expression of cyclin B1 and cdk1 by up to 50 and 30%, respectively.
In brief, AGE induced a cell cycle delay in DLD-1 cells by
modulating the expression of regulatory proteins involved in the
G2/M phase cell cycle checkpoints.
Nuclear factor-κB (NF-κB) is associated with cell
proliferation, cell cycle progression and promotion of tumor growth
via control of cyclin B1 expression levels (38,39).
To determine whether the activation of NF-κB could be inhibited by
AGE, the major component of the NF-κB complex, p65, located in the
nucleus, was assessed by immunoblotting. Western blot analysis data
showed that AGE treatment significantly attenuated the
translocation of p65 from the cytosol to the nucleus in a
dose-dependent manner (Fig. 7C and
D).
Discussion
Many studies suggest that garlic has anticancer
properties. Recent studies have demonstrated several molecular
mechanisms for its anti-carcinogenic effects in animal models,
including its direct suppression of cancer cell proliferation and
also its indirect action of inhibiting carcinogen activation by
enhancing detoxification though the induction of anti-oxidant
enzymes or modulation of cytochrome P450-dependent mono-oxygenases
(40). In this study, to
investigate the effect of AGE against tumor development independent
of its detoxification effects on carcinogenic agents, rats were
administered an AGE-containing basal diet from one week after a
final DMH injection to the end of experiment, and colonic tumors
were evaluated pathologically. The ultimate carcinogenic metabolite
of DMH is responsible for the methylation of DNA bases in tissue
including colorectal epithelial, resulting in the development of
dysplastic aberrant crypts and progression to adenomas, followed by
adenocarcinoma development (19,21).
In this experiment, ACF development was observed in all rats at the
end of the DMH treatment (Table
II, group 3), thus confirming that the carcinogenic initiation
by this DMH treatment protocol was certainly occurring in the rat
colon at the time that AGE intake was commenced; the effect of AGE
on tumors was evaluated as the effect on dysplastic cells that
exhibited elevated growth activity in the tumor development phase.
Pretlow et al showed that aberrant crypts with high
multiplicity (>4 crypts/focus) correlate with tumor incidence
(41). Furthermore, it is well
known that the proliferative activity of dysplastic cells is
elevated with the grade of tumor. AGE feeding, however, did not
inhibit gross tumorigenesis, but the mean tumor diameter was
decreased by AGE. It is considered that these gross tumors could
have been formed by initial DMH treatment and had already developed
at the time of AGE feeding. On the other hand, we demonstrated that
AGE substantially reduced DMH-induced colon adenocarcinomas
(P=0.07) and significantly reduced adenomas (P<0.01) and ACF
with higher crypt multiplicities (P<0.05) (Tables II–IV). Further analysis of the histological
type of adenomas revealed that AGE significantly reduced the
percentage of adenomas with severe dysplasia rather than those with
mild or moderate dysplasia. Our results suggest that the antitumor
effect of AGE is due to its inhibitory effect on the development of
these dysplastic lesions (Tables
II–V).
One of the common events in tumor development is
cell cycle deregulation. PCNA is known as a cell proliferation
marker; the degree of PCNA expression generally correlates well
with the mitotic activity of neoplastic cells and tumor grade
(42,43). Garlic derivative diallyl disulfide
(DADS)-mediated inhibition of breast cancer cell proliferation was
correlated with reduced PCNA activity (44). Another garlic derivative,
s-allylcysteine (SAC), inhibited PCNA protein levels in a mouse
tumor xenograft model of oral cancer (45). Our experiment showed that AGE
inhibited the expression of PCNA in high-grade adenoma and
adenocarcinoma cells with no effect on normal colon ductal cells
(Fig. 3). That is, AGE suppressed
the proliferation of tumor cells in which the proliferation was
abnormally enhanced. This could be due to tumor-specific
suppression of proliferation by AGE; it is well known that tumor
cells that proliferate much faster contain very high levels of PCNA
compared with normal mucosa. These results indicate that the
antitumor effect of AGE on the development of colonic tumors is
correlated with suppression of the proliferative activity of
dysplastic lesions.
We investigated the growth inhibitory mechanism of
tumor cells by AGE using DLD-1 human colon cancer cells. Matsuura
et al demonstrated the growth inhibitory effect of AGE
against HT29, SW480 and SW620 colorectal cancer cell lines in
vitro (25). We demonstrated
that AGE suppressed DLD-1 proliferation but did not inhibit that of
MRC-5 normal fibroblasts (Fig. 4).
However, the growth inhibitory effect of AGE was gradual and the
difference was observed following treatment of more than 2 days.
The doubling time of cancer cells is approximately 24 h; if agents
with strong effects on apoptosis or cell cycle arrest are used, the
growth inhibitory effect could be observed even after one day of
incubation. This indicates that AGE does not have a strong growth
inhibitory effect on cells.
The anti-carcinogenic effect of several organosulfur
compounds (OSCs) derived from garlic have been reported in animal
models induced by a variety of chemical carcinogens and in many
cultured cancer cell lines including breast, colon, prostate,
gastric and lung cancer (40).
Recent studies have revealed that certain OSCs can suppress the
proliferation of cancer cells by regulating cell cycle progression
such as the induction of G2/M phase arrest, and have also been
shown to induce apoptosis via the intrinsic pathway by altering the
ratio of the BCL-2 family of proteins both in cell culture and
in vivo models. Caspase-3 is a critical executioner of
apoptosis, and cleavage to its active form induces apoptosis
(40). However, in the present
study, AGE did not induce the expression of cleaved caspase-3 in
DLD-1 cells and did not promote cell cycle arrest in any specific
phase (Fig. 5). Thus, the effect of
AGE on growth inhibition was not due to the induction of apoptosis
or cell cycle arrest. Interestingly, however, as a result of the
synchronized cell cycle analysis, which was conducted to study the
correlation between cell growth suppression and cell cycle
progression, AGE promoted delayed cell cycle progression of 2–3 h
at the G2/M phase compared with normal conditions (Fig. 6), which was followed by the
downregulation of cyclin B1 and cdk1 protein expression (Fig. 7).
Eukaryotic cell cycle progression involves
sequential activation of cdk1 associated with cyclin B1. A complex
of cyclin B1 and cdk1 plays a major role in regulation of the G2 to
M transition (46). Ryu et
al showed that the decrease in cyclin B1 and cdk1 expression
was correlated with G2/M arrest (47). These results suggest that
AGE-mediated cell cycle delay at the G2/M checkpoint but not
apoptosis induction is associated with a slight decrease in cyclin
B1 and cdk1 protein levels. The cell proliferation suppressive
effect of AGE in DLD-1 cells was observed at more than 2 days of
treatment in a dose dependent-manner and did not cause cell death
even at 4 days of treatment (Fig.
4). We showed that AGE inhibited cell proliferation slowly,
without apoptosis or cell cycle arrest, and was correlated with the
results obtained following a delay of 2–3 h compared with the
normal cell doubling time. Delay of the cell cycle at the G2/M
phase caused a decrease in the population of cells in the S phase,
and was consistent with the suppressed PCNA labeling index of colon
tumor tissue from the AGE-fed rats.
NF-κB transcription factor is constitutively
activated in human pancreatic, breast, prostate and lung cancer
cells as well as colorectal carcinomas, which provides favorable
conditions for cancer cell proliferation (10,48–51).
Furthermore, NF-κB is activated in human colon tumor lesions and
adenomatous polyps (52). NF-κB
activation involves its translocation from the cytoplasm to the
nucleus, where it binds to target sequences to regulate gene
expression (48). NF-κB is blocked
by over-expression of the mutant form of its inhibitor, IκB, which
reduces NF-κB translocation to the nucleus and decreases cell
survival via downregulation of cyclin B1 expression (38,39).
In this manner, NF-κB regulates cell proliferation by controlling
cyclin B1 expression levels. Pai et al showed that SAC
included in AGE was able to inhibit the activation of NF-κB in a
mouse xenograft tumor model (45).
In this study, we demonstrated that AGE reduced the amount of NF-κB
in the nuclei of DLD-1 cells (Fig.
7). That is, it can be considered that the lower expression
levels of cyclin B1 and cdk1 by the action of AGE on tumor cells is
associated with its reduction in NF-κB activity.
In conclusion, the results of the present study
indicate that dietary administration of AGE effectively attenuated
colon tumor cell progression in a rat tumor model. The possible
mechanisms proposed here revealed that the antitumor effect of AGE
is to delay the rate of cellular proliferation by lowering cyclin
B1 and cdk1 expression levels through attenuation of NF-κB
activity. To the best of our knowledge, this is the first report
that AGE-mediated suppression of tumor progression is related to
the downregulation of cell cycle-associated proteins. AGE is
appropriate for long-term administration due to its low toxicity,
and thus is suitable as a chemopreventive agent against colon
tumor.
Acknowledgements
This study was supported in part by the important
Research Grant from the Prefectural University of Hiroshima. The
authors thank Drs Kodera and Imamura of Wakunaga Pharmaceutical Co.
for the preparation of AGE, and Hiroshima Tsuchiya General Hospital
for technical support.
References
|
1
|
Fleischauer AT, Poole C and Arab L: Garlic
consumption and cancer prevention: meta-analyses of colorectal and
stomach cancers. Am J Clin Nutr. 72:1047–1052. 2000.PubMed/NCBI
|
|
2
|
Gao CM, Takezaki T, Ding JH, Li MS and
Tajima K: Protective effect of allium vegetables against both
esophageal and stomach cancer: a simultaneous case-referent study
of a high-epidemic area in Jiangsu Province, China. Jpn J Cancer
Res. 90:614–621. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sumiyoshi H and Wargovich MJ:
Chemoprevention of 1,2-dimethylhydrazine-induced colon cancer in
mice by naturally occurring organosulfur compounds. Cancer Res.
15:5084–5087. 1990.
|
|
4
|
Hatono S, Jimenez A and Wargovich MJ:
Chemopreventive effect of S-allylcysteine and its relationship to
the detoxification enzyme glutathione S-transferase.
Carcinogenesis. 17:1041–1044. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang X, Jiao F, Wang QW, et al: Aged black
garlic extract induces inhibition of gastric cancer cell growth in
vitro and in vivo. Mol Med Rep. 5:66–72. 2012.
|
|
6
|
Xiao D, Pinto JT, Soh JW, Deguchi A, et
al: Induction of apoptosis by the garlic-derived compound
S-allylmercaptocysteine (SAMC) is associated with microtubule
depolymerization and c-Jun NH(2)-terminal kinase 1 activation.
Cancer Res. 63:6825–6837. 2003.PubMed/NCBI
|
|
7
|
Knowles LM and Milner JA: Depressed p34
cdc2 kinase activity and G2/M phase arrest induced by diallyl
disulfide in HCT-15 cells. Nutr Cancer. 30:169–174. 1998.
View Article : Google Scholar
|
|
8
|
Shirin H, Pinto JT, Kawabata Y, et al:
Antiproliferative effects of S-allylmercaptocysteine on colon
cancer cells when tested alone or in combination with sulindac
sulfide. Cancer Res. 61:725–731. 2001.PubMed/NCBI
|
|
9
|
Hosono T, Fukao T, Ogihara J, Ito Y, Shiba
H, Seki T and Ariga T: Diallyl trisulfide suppresses the
proliferation and induces apoptosis of human colon cancer cells
through oxidative modification of beta-tubulin. J Biol Chem.
280:41487–41493. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ban JO, Yuk DY, Woo KS, et al: Inhibition
of cell growth and induction of apoptosis via inactivation of
NF-kappaB by a sulfur-compound isolated from garlic in human colon
cancer cells. J Pharmacol Sci. 104:374–383. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sriram N, Kalayarasan S, Ashokkumar P,
Sureshkumar A and Sudhandiran G: Diallyl sulfide induces apoptosis
in Colo 320 DM human colon cancer cells: involvement of caspase-3,
NF-kappaB, and ERK-2. Mol Cell Biochem. 311:157–165. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liang D, Qin Y, Zhao W, et al:
S-allylmercaptocysteine effectively inhibits the proliferation of
colorectal cancer cells under in vitro and in vivo conditions.
Cancer Lett. 310:69–76. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dong M, Yang G, Liu H, Liu X, Lin S, Sun D
and Wang Y: Aged black garlic extract inhibits HT29 colon cancer
cell growth via the PI3K/Akt signaling pathway. Biomed Rep.
2:250–254. 2014.PubMed/NCBI
|
|
14
|
Soni KB, Lahiri M, Chackradeo P, Bhide SV
and Kuttan R: Protective effect of food additives on
aflatoxin-induced mutagenicity and hepatocarcinogenicity. Cancer
Lett. 115:129–133. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Knasmüller S, de Martin R, Domjan G and
Szakmary A: Studies on the antimutagenic activities of garlic
extract. Environ Mol Mutagen. 13:357–365. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Amagase H and Milner JA: Impact of various
sources of garlic and their constituents on
7,12-dimethylbenz[a]anthracene binding to mammary cell DNA.
Carcinogenesis. 14:1627–1631. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sparnins VL, Barany G and Wattenberg LW:
Effects of organosulfur compounds from garlic and onions on
benzo[a] pyrene-induced neoplasia and glutathione S-transferase
activity in the mouse. Carcinogenesis. 9:131–134. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tadi PP, Tee RW and Lau BH: Organosulfur
compounds of garlic modulate mutagenesis, metabolism, and DNA
binding of aflatoxin B1. Nutr Cancer. 15:87–95. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Perše M and Cerar A: Morphological and
molecular alterations in 1,2 dimethylhydrazine and azoxymethane
induced colon carcinogenesis in rats. J Biomed Biotechnol.
2011:1–14. 2011. View Article : Google Scholar
|
|
20
|
Tammariello AE and Milner JA: Mouse models
for unraveling the importance of diet in colon cancer prevention. J
Nutr Biochem. 21:77–88. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen J and Huang XF: The signal pathways
in azoxymethane-induced colon cancer and preventive implications.
Cancer Biol Ther. 8:1313–1317. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takahashi M and Wakabayashi K: Gene
mutations and altered gene expression in azoxymethane-induced colon
carcinogenesis in rodents. Cancer Sci. 95:475–480. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lijinsky W, Saavedra JE and Reuber MD:
Organ-specific carcinogenesis in rats by methyl- and
ethylazoxyalkanes. Cancer Res. 45:76–79. 1985.PubMed/NCBI
|
|
24
|
Tanaka S, Haruma K, Kunihiro M, et al:
Effects of aged garlic extract (AGE) on colorectal adenomas: a
double-blinded study. Hiroshima J Med Sci. 53:39–45. 2004.
|
|
25
|
Matsuura N, Miyamae Y, Yamane K, et al:
Aged garlic extract inhibits angiogenesis and proliferation of
colorectal carcinoma cells. J Nutr. 136(Suppl 3): 842–846.
2006.
|
|
26
|
Kyo E, Uda N, Kasuga S and Itakura Y:
Immunomodulatory effects of aged garlic extract. J Nutr.
131:S1075–S1079. 2006.
|
|
27
|
Morihara N, Sumioka I, Moriguchi T, Uda N
and Kyo E: Aged garlic extract enhances production of nitric oxide.
Life Sci. 71:509–517. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Morihara N, Ushijima M, Kashimoto N, et
al: Aged garlic extract ameliorates physical fatigue. Biol Pharm
Bull. 29:962–966. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Katsuki T, Hirata K, Ishikawa H, Matsuura
N, Sumi S and Itoh H: Aged garlic extract has chemopreventative
effects on 1,2-dimethylhydrazine-induced colon tumors in rats. J
Nutr. 136(Suppl 3): 847–851. 2006.
|
|
30
|
McLellan EA, Medline A and Bird RP:
Sequential analyses of the growth and morphological characteristics
of aberrant crypt foci: putative preneoplastic lesions. Cancer Res.
51:5270–5274. 1991.PubMed/NCBI
|
|
31
|
Pindborg JJ, Reichart PA, Smith CJ and
Waal I: Histological typing of cancer and precancer of the oral
mucosa. 2nd edition. WHO; Geneva: pp. 21–31. 1997
|
|
32
|
Ferreira CG, Epping M, Kruyt FA and
Giaccone G: Apoptosis: target of cancer therapy. Clin Cancer Res
2002. 8:2024–2034. 2002.
|
|
33
|
Alnemri ES, Livingston DJ, Nicholson DW,
Salvesen G, Thornberry NA, Wong WW and Yuan J: Human ICE/CED-3
protease nomenclature. Cell. 87:1711996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Muschel RJ, Zhang HB, Iliakis G and
McKenna WG: Cyclin B expression in HeLa cells during the G2 block
induced by ionizing radiation. Cancer Res. 51:5113–5117.
1991.PubMed/NCBI
|
|
35
|
Metting NF and Little JB: Transient
failure to dephosphorylate the cdc2-cyclin B1 complex accompanies
radiation-induced G2-phase arrest in HeLa cells. Radiat Res.
143:286–292. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kao GD, McKenna WG, Maity A, Blank K and
Muschel RJ: Cyclin B1 availability is a rate-limiting component of
the radiation-induced G2 delay in HeLa cells. Cancer Res.
57:753–758. 1997.PubMed/NCBI
|
|
37
|
Azzam EI, de Toledo SM, Gooding T and
Little JB: Intercellular communication is involved in the bystander
regulation of gene expression in human cells exposed to very low
fluences of alpha particles. Radiat Res. 150:497–504. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guo G, Yan-Sanders Y, Lyn-Cook BD, et al:
Manganese superoxide dismutase-mediated gene expression in
radiation-induced adaptive responses. Mol Cell Biol. 23:2362–2378.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ozeki M, Tamae D, Hou DX, Wang T, Lebon T,
Spitz DR and Li JJ: Response of cyclin B1 to ionizing radiation:
regulation by NF-kappaB and mitochondrial antioxidant enzyme MnSOD.
Anticancer Res. 24:2657–2663. 2004.PubMed/NCBI
|
|
40
|
Herman-Antosiewicz A, Powolny AA and Singh
SV: Molecular targets of cancer chemoprevention by garlic-derived
organo-sulfides. Acta Pharmacol Sin. 28:1355–1364. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pretlow TP1, Cheyer C and O’Riordan MA:
Aberrant crypt foci and colon tumors in F344 rats have similar
increases in proliferative activity. Int J Cancer. 56:599–602.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hur K, Kim JR, Yoon BI, Lee JK, Choi JH,
Oh GT and Kim DY: Overexpression of cyclin D1 and cyclin E in
1,2-dimethylhydrazine dihydrochloride-induced rat colon
carcinogenesis. J Vet Sci. 1:121–126. 2000.
|
|
43
|
Janakiram NB, Mohammed A, Qian L, Choi CI,
Steele VE and Rao CV: Chemopreventive effects of RXR-selective
rexinoid bexarotene on intestinal neoplasia of Apc(Min/+) mice.
Neoplasia. 14:159–168. 2012.PubMed/NCBI
|
|
44
|
Powolny AA and Singh SV: Multitargeted
prevention and therapy of cancer by diallyl trisulfide and related
Allium vegetable-derived organosulfur compounds. Cancer Lett.
269:305–314. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pai MH, Kuo YH, Chiang EP and Tang FY:
S-Allylcysteine inhibits tumour progression and the
epithelial-mesenchymal transition in a mouse xenograft model of
oral cancer. Br J Nutr. 14:28–38. 2012. View Article : Google Scholar
|
|
46
|
Xiao D, Vogel V and Singh SV: Benzyl
isothiocyanate-induced apoptosis in human breast cancer cells is
initiated by reactive oxygen species and regulated by Bax and Bak.
Mol Cancer Ther. 5:2931–2945. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ryu DS, Kim SH and Lee DS:
Anti-proliferative effect of polysaccharides from Salicornia
herbacea on induction of G2/M arrest and apoptosis in human colon
cancer cells. J Microbiol Biotechnol. 19:1482–1489. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Huang S, Pettaway CA, Uehara H, Bucana CD
and Fidler IJ: Blockade of NF-kappaB activity in human prostate
cancer cells is associated with suppression of angiogenesis,
invasion, and metastasis. Oncogene. 20:4188–4197. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lee SH, Lee CW, Lee JW, et al: Induction
of apoptotic cell death by 2′-hydroxycinnamaldehyde is involved
with ERK-dependent inactivation of NF-kappaB in TNF-alpha-treated
SW620 colon cancer cells. Biochem Pharmacol. 70:1147–1157. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Saccani A, Schioppa T, Porta C, et al: p50
nuclear factor-kappaB overexpression in tumor-associated
macrophages inhibits M1 inflammatory responses and antitumor
resistance. Cancer Res. 66:11432–11440. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tang X, Liu D, Shishodia S, et al: Nuclear
factor-kappaB (NF-kappaB) is frequently expressed in lung cancer
and preneoplastic lesions. Cancer. 107:2637–2646. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hardwick JC, van den Brink GR, Offerhaus
GJ, van Deventer SJ and Peppelenbosch MP: NF-kappaB, p38 MAPK and
JNK are highly expressed and active in the stroma of human colonic
adenomatous polyps. Oncogene. 20:819–827. 2001. View Article : Google Scholar : PubMed/NCBI
|