The global burden due to cancer increased to 14.1
million new cases and 8.2 million cancer-associated mortalities in
2012 (1). The outcome of cancer
patients remains poor, despite recent advances in the understanding
of the molecular mechanism of tumorigenesis. Thus, more effective
initial treatments for this intractable disease are required.
Recent therapies under investigation include immunotherapy,
chemotherapy, targeted molecular, antiangiogenic and gene therapy,
radiation enhancement and drugs for overcoming resistance (2). Statins are inhibitors of
3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMGCR), commonly
used as cholesterol-lowering agents (3), that have proven their effectiveness in
the treatment of cardiovascular diseases. Preclinical evidence has
indicated their antiproliferative, pro-apoptotic, anti-invasive and
radio-sensitizing properties (4),
and there are emerging interests in the use of statins as
anticancer agents. In the present study, we reviewed the current
data of statins in cancer.
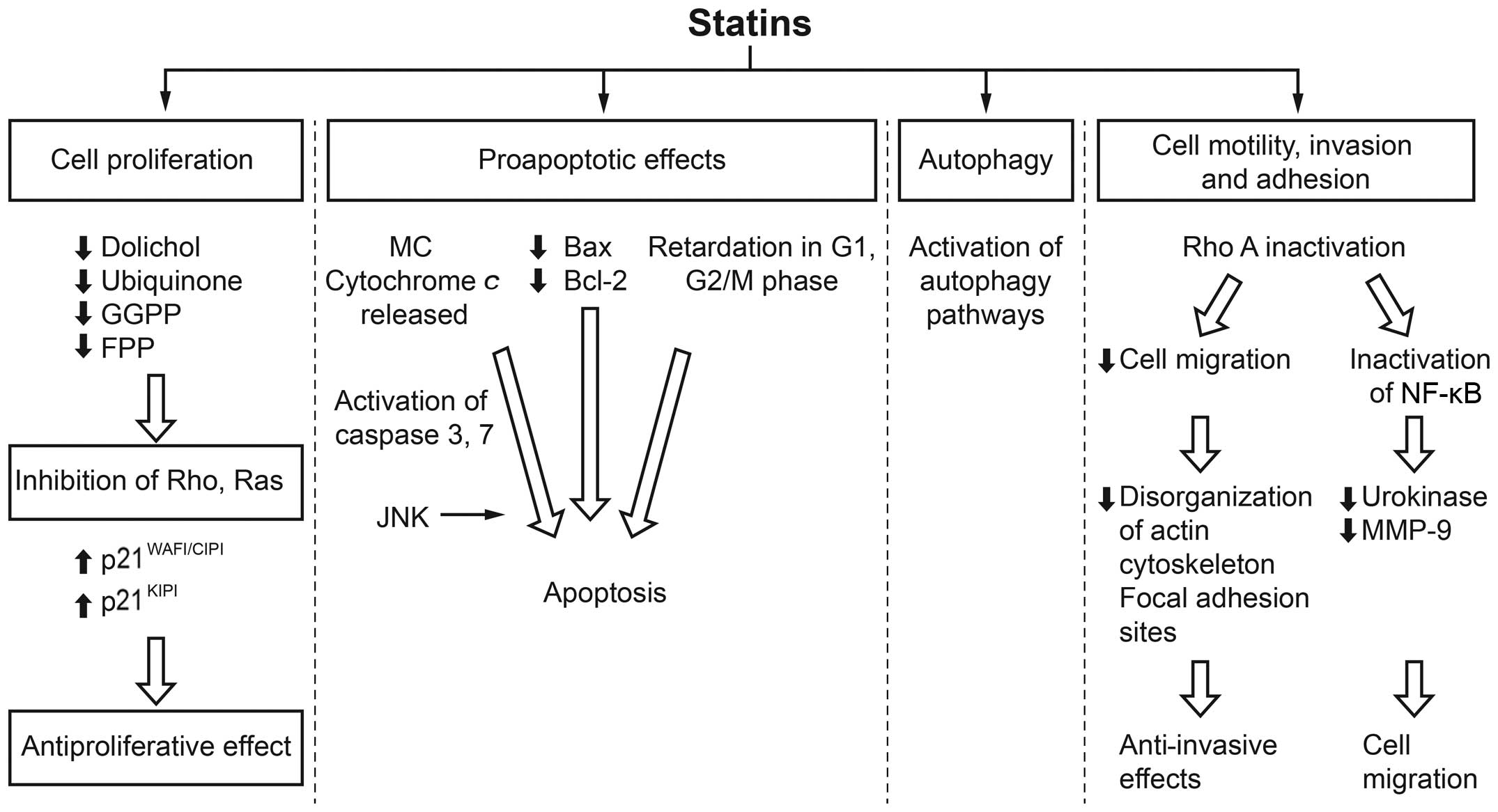
Inhibition of HMGCR by statins is a rate-limiting
step in the mevalonate pathway. The products of the mevalonate
pathway include isoprene units incorporated into sterol and
non-sterol compounds. This inhibition by a statin may result in
decreased levels of mevalonate and its downstream products, which
affects critical cell functions such as membrane integrity, cell
signaling, protein synthesis and cell cycle progression. The effect
of statins on these processes and consequently on tumor cells, may
therefore be able to control tumor initiation, growth and
metastasis (Fig. 1) (5–17).
Accumulating evidence has focused on pre-diagnostic
use of statins in reducing risk of lethal prostate cancer (35). In a prospective cohort study of
34,989 USA male health professionals, the use of statins was
associated with a reduced risk of advanced prostate cancer. The
risk of advanced disease was lower with longer statin use (P
trend=0.003) vs. never use. The relative risk (RR) was 0.60 [95%
confidence interval (CI), 0.35–1.03] for <5 years of use and
0.26 (95% CI, 0.08–0.83) for ≥5 years of use. There was no
association between statin use and risk of total prostate cancer
(RR, 0.96; 95% CI, 0.85–1.09) (36). In a Denmark-based case-control study
(n=42,480), statin use was associated with an overall risk
reduction (6%) that was specifically higher among patients with
advanced prostate cancer (10%) (37). Similarly, a decreased risk in
mortality was noted among 11,772 newly diagnosed non-metastatic
prostate cancer patients in the UK. Furthermore, decreased risks of
prostate cancer mortality and all-cause mortality were reported in
patients who used statins prior to diagnosis [hazard ratio (HR),
0.55, 95% CI, 0.41–0.74; and HR, 0.66, 95% CI, 0.53–0.81,
respectively]. The results were higher compared to those obtained
from patients who initiated the treatment only after diagnosis (HR,
0.82, 95% CI, 0.71–0.96; and HR, 0.91, 95% CI, 0.82–1.01,
respectively) (38). In another
prospective, population-based cohort study (n=1001), statin use
prior to prostate cancer diagnosis was unrelated to prostate cancer
recurrence/progression, but was associated with a decrease in the
risk of prostate cancer-specific mortality (39).
Data from observational studies have addressed the
risk of glioma among statin users (40,41).
The use of simvastatin and lovastatin for >6 months was
inversely associated with glioma risk (40). A recent large nationwide
case-control study (41) conducted
in Denmark in patients with glioma (2,656 cases and 18,480
controls) also showed a reduction in the risk of glioma among
long-term statin users compared with non-users, and the risk was
inversely related to the intensity of statin treatment among users
[odds ratio (OR), 0.71, 95% CI, 0.44–1.15 for highest intensity
statin users]. This potential chemopreventive effect was limited to
users of lipophilic statins (41).
Population-based studies have shown 19% reductions
in esophageal cancer incidence where statins have been used.
Observational studies have shown that statins reduced the incidence
of adenocarcinoma in patients with Barrett’s esophagus (BE) by 43%;
this effect was further enhanced by a 74% decrease in risk
reduction in patients taking a combination of nonsteroidal
anti-inflammatory drugs and statins (42).
Findings of a meta-analysis showed that statins are
associated with a reduced risk of esophageal cancer, particularly
in patients with BE. In a subset of patients with BE (5 studies,
312 esophageal adenocarcinomas in 2,125 patients), statins were
associated with a significantly decreased risk (41%) of esophageal
adenocarcinomas after adjusting for potential confounders (adjusted
OR, 0.59, 95% CI, 0.45–0.78) (43).
These findings were consistent with those of another meta-analysis
of 11 observational studies. The pooled adjusted data showed statin
use was associated with a lower incidence of the combined
esophageal cancers (OR, 0.81, 95% CI, 0.75–0.88). Furthermore,
their chemopreventive effect was increased in combination with
cyclo-oxygenase inhibitors in reducing the risk of adenocarcinoma
in BE (OR, 0.26, 95% CI, 0.1–0.68) (44).
A meta-analysis of 26 randomized controlled trials
(RCTs) involving 290 gastric cancer and 8 observational studies,
totaling 7,321 gastric cancers indicated a reduced risk of gastric
cancer with statin use (45). In
addition, a meta-analysis of published studies showed a modest
reduction in colorectal cancer risk among statin users (46).
A review of 723 patients diagnosed with primary
inflammatory breast cancer in 1995–2011 showed that hydrophilic
statins were associated with significantly improved
progression-free survival (PFS) rates (47). However, long-term use of statins was
associated with increased risk of invasive ductal carcinoma (IDC;
n=916) and invasive lobular carcinoma (ILC; n=1,068) in a
contemporary population-based case-control study conducted in the
Seattle-Puget Sound region. It was also reported that women
diagnosed with hypercholesterolemia currently using statins for ≥10
years had more than double the risk of IDC (OR, 2.04, 95% CI,
1.17–3.57) and ILC (OR, 2.43, 95% CI, 1.40–4.21) compared with
never users (48).
In the meta-analysis of all the observational
studies published up to January 2012, statin use and long-term
statin use did not significantly affect breast cancer risk.
However, the cumulative meta-analysis showed a change in trend of
reporting risk of breast cancer from positive to negative in statin
users between 1993 and 2011. These findingd do not support the
hypothesis that statins exert a protective effect against breast
cancer (49).
The Cancer in The Ovary and Uterus Study (CITOUS;
case-control study) assessed the use of statins prior to and
following diagnosis in a subset of 424 cases of ovarian and
endometrial cancers and 341 controls using pharmacy records. Use of
statins >1 year prior to diagnosis was associated with risk
reduction, whereas survival improvement was observed among the two
malignancies when statins were ingested only after diagnosis
(50).
The Nurses’ Health and Health Professionals
Follow-Up Study investigated the association between statin use and
renal cell carcinoma (RCC) risk. The reported results were similar
between ever vs. never users of statins. The subgroup analyses of
that study reported that statin use may be associated with a lower
risk of RCC among women with no history of hypertension (51).
Statin use is associated with a reduced risk of
hepatocellular cancer, most strongly in Asian, but also in Western
populations (52). Similarly,
another meta-analysis suggested a favorable effect of statins on
hepatocellular carcinoma in the absence of a duration-risk
relationship (53).
A meta-analysis of all the published articles up to
December 2007 showed no association between statin use on
pancreatic cancer risk among patients using statins daily for
managing hypercholesterolemia (54). These findings were consistent with
those from another meta-analysis, which reported no association
between statin use and pancreatic cancer risk among patients using
statins daily for preventing cardiovascular event (55).
A meta-analysis of observational trials and RCTs did
not support a protective effect of statins on overall lung cancer
risk, and the lung cancer risk among elderly people (56). Nineteen studies (5 RCTs and 14
observational studies) involving 38,013 lung cancer cases suggested
no association between statin use and risk of lung cancer (57). Similarly, a meta-analysis of
published literature did not support the role of statins in
prevention of skin cancer (58).
A retrospective evaluation of 1,502 patients with
urothelial carcinoma of the bladder treated with radical cystectomy
and pelvic lymphadenectomy without neoadjuvant therapy showed
statin users were at a higher risk for disease recurrence and
cancer-specific mortality in a univariate, but not a multivariate
analysis. However, the present study also reported that statin
users were older (P=0.003), had higher body mass index (median 32
vs. 28 kg/m2, P<0.001), and were more likely to have
positive soft tissue surgical margins (9 vs. 4%, P<0.001)
(59). Another meta-analysis with
limited RCTs suggested no association between statin use and risk
of bladder cancer (60).
Numerous population-based case-control studies
conducted in Taiwan did not provide evidence to support an
association between statin use and risk of breast cancer (n=565;
control, n=2,260) (61), esophageal
cancer (n=197; controls, n=788) (62), bladder cancer (n=325; controls,
n=1,300) (63), kidney cancer
(n=177; controls, n=708) (64), and
female lung cancer (n=297; controls, n=1,188) (65).
Another retrospective evaluation of the entire
Danish population diagnosed with cancer between 1995 and 2007 was
performed on 18,721 patients using statins regularly before the
cancer diagnosis vs. 277,204 patients who had never used statins.
The present study concluded that statin use in cancer patients was
associated with reduced cancer-associated mortality as compared to
that in non-users (HR, 0.85, 95% CI, 0.82–0.87) for each of the 13
types of cancer (66). In another
meta-analysis (27 randomized trials), a median of 5 years of statin
therapy was reported to have no effect on the incidence of, or
mortality from, any type of cancer, or the aggregate of all cancers
(67). The effect of statins on the
incidence of different types of cancer reported in various
observational and retrospective studies is presented in Table II.
In a phase I–II trial of lovastatin in anaplastic
astrocytoma and glioblastoma multiforme, 18 patients received
lovastatin between 20 and 30 mg/kg/day for 7 days followed by a
3-week rest. Lovastatin was considered well tolerated, as no
patient reported myalgia and only 2 patients reported mild joint
pain. Nine of 18 patients received concurrent radiation with no
neurological toxicity, indicating that the combination was
potentially safe. Of those who received concurrent radiation, 2
minor and 2 partial responses (duration range, 160–236 days) were
observed. One patient each on lovastatin monotherapy showed partial
and minor response, and stable disease. Notably, the patient who
had partial response accomplished a response duration of >405
days, at which time lovastatin was discontinued due to cost-related
issues (68).
Similarly, another phase I study evaluated the
safety and tolerability of lovastatin using escalating doses in 88
cancer patients with advanced solid tumors. A majority of patients
had prostate cancer or central nervous system tumors. Myopathy was
found to be a dose-limiting toxicity and ubiquinone administration
was associated with reversal of lovastatin-induced myopathy.
Myopathy was prevented by its prophylactic administration in a
56-patient cohort. In the absence of supplementation, lovastatin
was well tolerated up to 25 mg/kg/day for 7 days followed by a
3-week rest. One anaplastic astrocytoma patient treated with
lovastatin at 30 and 35 mg/kg/day who progressed after surgical
resection of the tumor, irradiation and 2 cycles of carmustine had
a minor response (45% tumor size reduction) maintained for 8 months
(69).
Prolongation of overall survival and PFS was
documented in MM patients with lovastatin plus thalidomide and
dexamethasone (TDL) vs. thalidomide and dexamethasone alone. The
TDL regimen was safe and well tolerated (70).
Simvastatin in combination with conventional FOLFIRI
[irinotecan, 5-fluorouracil (5-FU), and leucovorin] in metastatic
colorectal cancer patients showed promising antitumor activities
(71). An exploratory subgroup
analysis in non-small cell lung carcinoma patients with wild-type
epidermal growth factor receptor (EGFR) non-adenocarcinomas showed
higher RR, 40 vs. 0%, P=0.043) and longer PFS (3.6 vs. 1.7 months,
P=0.027) with simvastatin plus gefitinib vs. gefitinib alone
(72). Moreover, low-dose
simvastatin to gemcitabine in advanced pancreatic cancer does not
provide clinical benefit or results in increased toxicity (73).
The 6-month interventions with atorvastatin did not
provide convincing evidence of colorectal cancer risk reduction in
a multicenter phase II trial, although the relatively small sample
size limited statistical power (74).
In patients with RCC and metastasis, zoledronate
with fluvastatin or atorvastatin as bone-targeting therapy affected
certain bone biomarkers and provided bone response in several
patients. However, no statistically significant improvement in time
to skeletal events was observed (75). The survival of pediatric brain stem
tumor patients was significantly increased with metronomic
treatment with carboplatin and vincristine associated with
fluvastatin and thalidomide (76).
In patients with acute myeloid leukemia (AML),
pravastatin with idarubicin plus high-dose cytarabine (Ida-HDAC)
decreased the total and low-density lipoprotein (LDL) cholesterol
in almost all patients. The encouraging response rates suggest
further trials evaluating the effect of cholesterol modulation on
response in AML should be conducted (77). Chemoembolization and pravastatin
combination significantly improved (P=0.003) survival of patients
with advanced hepatocellular carcinoma vs. those receiving
chemoembolization alone (78).
Pravastatin in combination with epirubicin,
cisplatin, and capecitabine did not improve outcome in advanced
gastric cancer patients in a randomized phase II trial (79). Summary of clinical trials that used
statins as monotherapy or as a combination in patients with
different cancer types are presented in Table III.
Statins act by arresting cells in the late G1 phase
of the cell cycle and can affect cell synchronization in the
radiosensitive phase (5). The late
G1 and G2-M phases are most sensitive to radiation therapy;
therefore, statins potentially sensitized cells to radiation in the
late G1 phase (80,81). The antitumor effect of lovastatin as
a radiosensitizer on B-cell rat lymphoma (L-TACB) was higher than
that of individual therapy (82).
The underlying molecular mechanism involved Ras, which confers
intrinsic resistance to radiation since in vitro studies
using osteosarcoma cells demonstrated that lovastatin decreases
this radiation resistance (80,81).
Furthermore, HMGCR may serve as a predictive marker of response to
postoperative radiotherapy in ductal carcinoma in situ
(DCIS) (83). A retrospective
cohort study suggested an association between statin therapy and
improvement in response of rectal cancer to neoadjuvant
chemoradiation (84).
Statins have shown anticancer potential with
numerous chemotherapeutic agents. Simvastatin showed additive
activity and mutual sensitization with doxorubicin by triggering
caspase activation in human rhabdomyosarcoma cells (85). When combined with 5-FU or cisplatin
as chemotherapy, lovastatin acts by inhibiting geranylgeranylation
but not farnesylation of target protein(s) in colon cancer cells
(10). Lovastatin or simvastatin
with cytosine arabinoside significantly enhances the
antiproliferative effect of each drug in leukemia cell lines and
this may be beneficial in the leukemia treatment (86,87). A
similar synergy of simvastatin with
N,N′-bis(2-chloroethyl)-N-nitrosourea or β-interferon
produces antiproliferative activity in human glioma cells (88). Cerivastatin also increases the
cytotoxicity of 5-FU in chemoresistant colorectal cancer cell lines
by inhibiting nuclear factor-κB DNA-binding activity (89).
A phase I study in AML patients showed synergistic
effects of the addition of pravastatin to a conventional
chemotherapy regimen (idarubicin and high-dose cytarabine)
(77). Statin use in combination
with concurrent chemoradiotherapy in preoperative rectal carcinoma
patients was associated with improved pathologic complete response
at the time of surgery (90). HMGCR
expression was reported as an independent predictor of prolonged
recurrence-free survival in primary ovarian cancer. Future studies
are required to evaluate HMGCR expression as a surrogate marker of
response to statin treatment, particularly in conjunction with
current chemotherapeutic regimens (91). Synergistic effects are observed in
patients with relapsed or refractory myeloma by the addition of
lovastatin to thalidomide and dexamethasone (70).
Preclinical data based on cancer cell lines and
animal models demonstrate encouraging anticancer activity of
statins. Similarly, several population-based and retrospective
studies demonstrate chemopreventive and survival benefit of statins
in various types of cancer. However, this benefit has not been
confirmed/proven or validated in clinical trials, and is attributed
to the absence of well-conducted large-scale phase III RCTs that
have addressed the antitumor effects of statins in cancer. In fact,
a majority of the trials thus far are phase I and/or small or
poorly conducted phase II clinical trials with a small sample size
and inadequate power. Moreover, genetic and non-genetic factors
also may contribute to the inter-individual variation in statin
response. In ovarian cancer, HMGCR expression was reported as an
independent predictor of prolonged recurrence-free survival
(91). Lipkin et al,
identified a single-nucleotide polymorphism in the HMGCR
gene that significantly modified the chemopreventive activity of
statins for colorectal cancer risk (92). Heterogeneous nuclear
ribonucleoprotein A1 (HNRNPA1) overexpression was recently reported
to reduce HMGCR enzyme activity, enhance LDL-C uptake, and increase
cellular apolipoprotein B (93).
This may explain the inter-individual variation of drug response to
statins. Advances in molecular biology may be useful to identify
markers responsive to statin treatment and tailor base statin
treatment based on genotypic profile, in the direction of
personalized medicine.
Studies suggest statins can modulate the outcome of
various cancer types and notably can target cancer vs. normal
cells. The microenvironments seem to regulate the statin effect in
different types of cancer. The side-effects appear to be limited,
manageable and may be associated with genetic and non-genetic
factors. Future studies should concentrate on evaluating statins in
large-scale phase III RCTs in cancer patients to establish the
precise effect of stains in cancer prevention and treatment.
This study was supported in part by King Fahad
Medical City, Riyadh, Saudi Arabia.
|
1
|
Ferlay J, Soerjomataram I, Ervik M, et al:
GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC
CancerBase No. 11 (Internet). Lyon, France: International Agency
for Research on Cancer; 2013, Available from: http://globocan.iarc.fr.
Accessed.
|
|
2
|
Kanu OO, Mehta A, Di C, Lin N, et al:
Glioblastoma multiforme: a review of therapeutic targets. Expert
Opin Ther Targets. 13:701–718. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goldstein JL and Brown MS: Regulation of
the mevalonate pathway. Nature. 343:425–430. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gazzerro P, Proto MC, Gangemi G, et al:
Pharmacological actions of statins: a critical appraisal in the
management of cancer. Pharmacol Rev. 64:102–146. 2012. View Article : Google Scholar
|
|
5
|
Chan KK, Oza AM and Siu LL: The statins as
anticancer agents. Clin Cancer Res. 9:10–19. 2003.PubMed/NCBI
|
|
6
|
Baserga R: Molecular biology of the cell
cycle. Int J Radiat Biol Relat Stud Phys Chem Med. 49:219–226.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tapia-Pérez JH, Kirches E, Mawrin C,
Firsching R and Schneider T: Cytotoxic effect of different statins
and thiazolidinediones on malignant glioma cells. Cancer Chemother
Pharmacol. 67:1193–1201. 2011. View Article : Google Scholar
|
|
8
|
Rao S, Lowe M, Herliczek TW and Keyomarsi
K: Lovastatin mediated G1 arrest in normal and tumor breast cells
is through inhibition of CDK2 activity and redistribution of p21
and p27, independent of p53. Oncogene. 17:2393–2402. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Collisson EA, Kleer C, Wu M, et al:
Atorvastatin prevents RhoC isoprenylation, invasion, and metastasis
in human melanoma cells. Mol Cancer Ther. 2:941–948.
2003.PubMed/NCBI
|
|
10
|
Agarwal B, Bhendwal S, Halmos B, Moss SF,
Ramey WG and Holt PR: Lovastatin augments apoptosis induced by
chemotherapeutic agents in colon cancer cells. Clin Cancer Res.
5:2223–2229. 1999.PubMed/NCBI
|
|
11
|
Marcelli M, Cunningham GR, Haidacher SJ,
et al: Caspase-7 is activated during lovastatin-induced apoptosis
of the prostate cancer cell line LNCaP. Cancer Res. 58:76–83.
1998.PubMed/NCBI
|
|
12
|
Koyuturk M, Ersoz M and Altiok N:
Simvastatin induces apoptosis in human breast cancer cells: p53 and
estrogen receptor independent pathway requiring signalling through
JNK. Cancer Lett. 250:220–228. 2007. View Article : Google Scholar
|
|
13
|
Baetta R, Donetti E, Comparato C, et al:
Pro-apoptotic effect of atorvastatin on stimulated rabbit smooth
muscle cells. Pharmacol Res. 36:115–121. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang IK, Lin-Shiau SY and Lin JK:
Induction of apoptosis by lovastatin through activation of
caspase-3 and DNase II in leukaemia HL-60 cells. Pharmacol Toxicol.
86:83–91. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He Z, Mangala LS, Theriot CA, Rohde LH, Wu
H and Zhang Y: Cell killing and radiosensitizing effects of
atorvastatin in PC3 prostate cancer cells. J Radiat Res.
53:225–233. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Denoyelle C, Vasse M, Körner M, et al:
Cerivastatin, an inhibitor of HMG-CoA reductase, inhibits the
signaling pathways involved in the invasiveness and metastatic
properties of highly invasive breast cancer cell lines: an in vitro
study. Carcinogenesis. 22:1139–1148. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bouterfa HL, Sattelmeyer V, Czub S,
Vordermark D, Roosen K and Tonn JC: Inhibition of Ras farnesylation
by lovastatin leads to downregulation of proliferation and
migration in primary cultured human glioblastoma cells. Anticancer
Res. 20:2761–2771. 2000.PubMed/NCBI
|
|
18
|
Schmidmaier R, Baumann P, Bumeder I,
Meinhardt G, Straka C and Emmerich B: First clinical experience
with simvastatin to overcome drug resistance in refractory multiple
myeloma. Eur J Haematol. 79:240–243. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sondergaard TE, Pedersen PT, Andersen TL,
et al: A phase II clinical trial does not show that high dose
simvastatin has beneficial effect on markers of bone turnover in
multiple myeloma. Hematol Oncol. 27:17–22. 2009. View Article : Google Scholar
|
|
20
|
Bjarnadottir O, Romero Q, Bendahl PO, et
al: Targeting HMG-CoA reductase with statins in a
window-of-opportunity breast cancer trial. Breast Cancer Res Treat.
138:499–508. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Garwood ER, Kumar AS, Baehner FL, et al:
Fluvastatin reduces proliferation and increases apoptosis in women
with high grade breast cancer. Breast Cancer Res Treat.
119:137–144. 2010. View Article : Google Scholar
|
|
22
|
Inano H, Suzuki K, Onoda M and Wakabayashi
K: Anti-carcinogenic activity of simvastatin during the promotion
phase of radiation-induced mammary tumorigenesis of rats.
Carcinogenesis. 18:1723–1727. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Narisawa T, Fukaura Y, Tanida N, Hasebe M,
Ito M and Aizawa R: Chemopreventive efficacy of low dose of
pravastatin, an HMG-CoA reductase inhibitor, on
1,2-dimethylhydrazine-induced colon carcinogenesis in ICR mice.
Tohoku J Exp Med. 180:131–138. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Narisawa T, Morotomi M, Fukaura Y, Hasebe
M, Ito M and Aizawa R: Chemoprevention by pravastatin, a
3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor, of
N-methyl-N-nitrosourea-induced colon carcinogenesis in F344 rats.
Jpn J Cancer Res. 87:798–804. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Clutterbuck RD, Millar BC, Powles RL, et
al: Inhibitory effect of simvastatin on the proliferation of human
myeloid leukaemia cells in severe combined immunodeficient (SCID)
mice. Br J Haematol. 102:522–527. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kikuchi T, Nagata Y and Abe T: In vitro
and in vivo antiproliferative effects of simvastatin, an HMG-CoA
reductase inhibitor, on human glioma cells. J Neurooncol.
34:233–239. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hawk MA, Cesen KT, Siglin JC, Stoner GD
and Ruch RJ: Inhibition of lung tumor cell growth in vitro and
mouse lung tumor formation by lovastatin. Cancer Lett. 109:217–222.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Matar P, Rozados VR, Binda MM, Roggero EA,
Bonfil RD and Scharovsky OG: Inhibitory effect of lovastatin on
spontaneous metastases derived from a rat lymphoma. Clin Exp
Metastasis. 17:19–25. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Matar P, Rozados VR, Roggero EA and
Scharovsky OG: Lovastatin inhibits tumor growth and metastasis
development of a rat fibrosarcoma. Cancer Biother Radiopharm.
13:387–393. 1998. View Article : Google Scholar
|
|
30
|
Alonso DF, Farina HG, Skilton G, Gabri MR,
De Lorenzo MS and Gomez DE: Reduction of mouse mammary tumor
formation and metastasis by lovastatin, an inhibitor of the
mevalonate pathway of cholesterol synthesis. Breast Cancer Res
Treat. 50:83–93. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Broitman SA, Wilkinson JT IV, Cerda S and
Branch SK: Effects of monoterpenes and mevinolin on murine colon
tumor CT-26 in vitro and its hepatic ‘metastases’ in vivo. Adv Exp
Med Biol. 401:111–130. 1996. View Article : Google Scholar
|
|
32
|
Jani JP, Specht S, Stemmler N, et al:
Metastasis of B16F10 mouse melanoma inhibited by lovastatin, an
inhibitor of cholesterol biosynthesis. Invasion Metastasis.
13:314–324. 1993.PubMed/NCBI
|
|
33
|
Feleszko W, Mlynarczuk I, Balkowiec-Iskra
EZ, et al: Lovastatin potentiates antitumor activity and attenuates
cardiotoxicity of doxorubicin in three tumor models in mice. Clin
Cancer Res. 6:2044–2052. 2000.PubMed/NCBI
|
|
34
|
Feleszko W, Bałkowiec EZ, Sieberth E, et
al: Lovastatin and tumor necrosis factor-α exhibit potentiated
antitumor effects against Ha-ras-transformed murine tumor via
inhibition of tumor-induced angiogenesis. Int J Cancer. 81:560–567.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mucci LA and Stampfer MJ: Mounting
evidence for prediagnostic use of statins in reducing risk of
lethal prostate cancer. J Clin Oncol. 32:1–2. 2014. View Article : Google Scholar
|
|
36
|
Platz EA, Leitzmann MF, Visvanathan K, et
al: Statin drugs and risk of advanced prostate cancer. J Natl
Cancer Inst. 98:1819–1825. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jespersen CG, Norgaard M, Friis S, Skriver
C and Borre M: Statin use and risk of prostate cancer: a Danish
population-based case-control study, 1997–2010. Cancer Epidemiol.
38:42–47. 2014. View Article : Google Scholar
|
|
38
|
Yu O, Eberg M, Benayoun S, et al: Use of
statins and the risk of death in patients with prostate cancer. J
Clin Oncol. 32:5–11. 2014. View Article : Google Scholar
|
|
39
|
Geybels MS, Wright JL, Holt SK, Kolb S,
Feng Z and Stanford JL: Statin use in relation to prostate cancer
outcomes in a population-based patient cohort study. Prostate.
73:1214–1222. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ferris JS, McCoy L, Neugut AI, Wrensch M
and Lai R: HMG CoA reductase inhibitors, NSAIDs and risk of glioma.
Int J Cancer. 131:E1031–E1037. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gaist D, Andersen L, Hallas J, Sørensen
HT, Schroder HD and Friis S: Use of statins and risk of glioma: a
nationwide case-control study in Denmark. Br J Cancer. 108:715–720.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tsibouris P, Vlachou E and Isaacs PE: Role
of chemoprophylaxis with either NSAIDs or statins in patients with
Barrett’s esophagus. World J Gastrointest Pharmacol Ther. 5:27–39.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Singh S, Singh AG, Singh PP, Murad MH and
Iyer PG: Statins are associated with reduced risk of esophageal
cancer, particularly in patients with Barrett’s esophagus: a
systematic review and meta-analysis. Clin Gastroenterol Hepatol.
11:620–629. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Beales IL, Hensley A and Loke Y: Reduced
esophageal cancer incidence in statin users, particularly with
cyclo-oxygenase inhibition. World J Gastrointest Pharmacol Ther.
4:69–79. 2013.PubMed/NCBI
|
|
45
|
Wu XD, Zeng K, Xue FQ, Chen JH and Chen
YQ: Statins are associated with reduced risk of gastric cancer: a
meta-analysis. Eur J Clin Pharmacol. 69:1855–1860. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lytras T, Nikolopoulos G and Bonovas S:
Statins and the risk of colorectal cancer: an updated systematic
review and meta-analysis of 40 studies. World J Gastroenterol.
20:1858–1870. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Brewer TM, Masuda H, Liu DD, et al: Statin
use in primary inflammatory breast cancer: a cohort study. Br J
Cancer. 109:318–324. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
McDougall JA, Malone KE, Daling JR,
Cushing-Haugen KL, Porter PL and Li CI: Long-term statin use and
risk of ductal and lobular breast cancer among women 55 to 74 years
of age. Cancer Epidemiol Biomarkers Prev. 22:1529–1537. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Undela K, Srikanth V and Bansal D: Statin
use and risk of breast cancer: a meta-analysis of observational
studies. Breast Cancer Res Treat. 135:261–269. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lavie O, Pinchev M, Rennert HS, Segev Y
and Rennert G: The effect of statins on risk and survival of
gynecological malignancies. Gynecol Oncol. 130:615–619. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu W, Choueiri TK and Cho E: Statin use
and the risk of renal cell carcinoma in 2 prospective US cohorts.
Cancer. 118:797–803. 2012. View Article : Google Scholar
|
|
52
|
Singh S, Singh PP, Singh AG, Murad MH and
Sanchez W: Statins are associated with a reduced risk of
hepatocellular cancer: a systematic review and meta-analysis.
Gastroenterology. 144:323–332. 2013. View Article : Google Scholar
|
|
53
|
Pradelli D, Soranna D, Scotti L, et al:
Statins and primary liver cancer: a meta-analysis of observational
studies. Eur J Cancer Prev. 22:229–234. 2013. View Article : Google Scholar
|
|
54
|
Bonovas S, Filioussi K and Sitaras NM:
Statins are not associated with a reduced risk of pancreatic cancer
at the population level, when taken at low doses for managing
hyper-cholesterolemia: evidence from a meta-analysis of 12 studies.
Am J Gastroenterol. 103:2646–2651. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Cui X, Xie Y, Chen M, et al: Statin use
and risk of pancreatic cancer: a meta-analysis. Cancer Causes
Control. 23:1099–1111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Deng Z, Zhang S, Yi L and Chen S: Can
statins reduce risk of lung cancer, especially among elderly
people? A meta-analysis. Chin J Cancer Res. 25:679–688. 2013.
|
|
57
|
Tan M, Song X, Zhang G, et al: Statins and
the risk of lung cancer: a meta-analysis. PLoS One. 8:e573492013.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Li X, Wu XB and Chen Q: Statin use is not
associated with reduced risk of skin cancer: a meta-analysis. Br J
Cancer. 110:802–807. 2014. View Article : Google Scholar :
|
|
59
|
da Silva RD, Xylinas E, Kluth L, et al:
Impact of statin use on oncologic outcomes in patients with
urothelial carcinoma of the bladder treated with radical
cystectomy. J Urol. 190:487–492. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhang XL, Geng J, Zhang XP, et al: Statin
use and risk of bladder cancer: a meta-analysis. Cancer Causes
Control. 24:769–776. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chan TF, Wu CH, Lin CL and Yang CY: Statin
use and the risk of breast cancer: a population-based case-control
study. Expert Opin Drug Saf. 13:287–293. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chan TF, Chiu HF, Wu CH, Lin CL and Yang
CY: Statin use and the risk of esophageal cancer: a
population-based case-control study. Expert Opin Drug Saf.
12:293–298. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kuo CC, Chiu HF, Lee IM, Kuo HW, Lee CT
and Yang CY: Statin use and the risk of bladder cancer: a
population-based case-control study. Expert Opin Drug Saf.
11:733–738. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Chiu HF, Kuo CC, Kuo HW, Lee IM, Lee CT
and Yang CY: Statin use and the risk of kidney cancer: a
population-based case-control study. Expert Opin Drug Saf.
11:543–549. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Cheng MH, Chiu HF, Ho SC and Yang CY:
Statin use and the risk of female lung cancer: a population-based
case-control study. Lung Cancer. 75:275–279. 2012. View Article : Google Scholar
|
|
66
|
Nielsen SF, Nordestgaard BG and Bojesen
SE: Statin use and reduced cancer-related mortality. N Engl J Med.
367:1792–1802. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Cholesterol Treatment Trialists’ (CTT)
Collaboration. Emberson JR, Kearney PM, Blackwell L, et al: Lack of
effect of lowering LDL cholesterol on cancer: meta-analysis of
individual data from 175,000 people in 27 randomised trials of
statin therapy. PLoS One. 7:e298492012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Larner J, Jane J, Laws E, Packer R, Myers
C and Shaffrey M: A phase I–II trial of lovastatin for anaplastic
astrocytoma and glioblastoma multiforme. Am J Clin Oncol.
21:579–583. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Thibault A, Samid D, Tompkins AC, et al:
Phase I study of lovastatin, an inhibitor of the mevalonate
pathway, in patients with cancer. Clin Cancer Res. 2:483–491.
1996.PubMed/NCBI
|
|
70
|
Hus M, Grzasko N, Szostek M, et al:
Thalidomide, dexamethasone and lovastatin with autologous stem cell
transplantation as a salvage immunomodulatory therapy in patients
with relapsed and refractory multiple myeloma. Ann Hematol.
90:1161–1166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Lee J, Jung KH, Park YS, et al:
Simvastatin plus irinotecan, 5-fluorouracil, and leucovorin
(FOLFIRI) as first-line chemotherapy in metastatic colorectal
patients: a multicenter phase II study. Cancer Chemother Pharmacol.
64:657–663. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Han JY, Lee SH, Yoo NJ, et al: A
randomized phase II study of gefitinib plus simvastatin versus
gefitinib alone in previously treated patients with advanced
non-small cell lung cancer. Clin Cancer Res. 17:1553–1560. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Hong JY, Nam EM, Lee J, et al: Randomized
double-blinded, placebo-controlled phase II trial of simvastatin
and gemcitabine in advanced pancreatic cancer patients. Cancer
Chemother Pharmacol. 73:125–130. 2014. View Article : Google Scholar
|
|
74
|
Limburg PJ, Mahoney MR, Ziegler KL, et al:
Randomized phase II trial of sulindac, atorvastatin, and prebiotic
dietary fiber for colorectal cancer chemoprevention. Cancer Prev
Res. 4:259–269. 2011. View Article : Google Scholar
|
|
75
|
Manoukian GE, Tannir NM, Jonasch E, Qiao
W, Haygood TM and Tu SM: Pilot trial of bone-targeted therapy
combining zoledronate with fluvastatin or atorvastatin for patients
with metastatic renal cell carcinoma. Clin Genitourin Cancer.
9:81–88. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
López-Aguilar E, Sepúlveda-Vildósola AC,
Betanzos-Cabrera Y, et al: Phase II study of metronomic
chemotherapy with thalidomide, carboplatin-vincristine-fluvastatin
in the treatment of brain stem tumors in children. Arch Med Res.
39:655–662. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Kornblau SM, Banker DE, Stirewalt D, et
al: Blockade of adaptive defensive changes in cholesterol uptake
and synthesis in AML by the addition of pravastatin to idarubicin +
high-dose Ara-C: a phase 1 study. Blood. 109:2999–3006. 2007.
|
|
78
|
Graf H, Jüngst C, Straub G, et al:
Chemoembolization combined with pravastatin improves survival in
patients with hepatocellular carcinoma. Digestion. 78:34–38. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Konings IR, van der Gaast A, van der Wijk
LJ, de Jongh FE, Eskens FA and Sleijfer S: The addition of
pravastatin to chemotherapy in advanced gastric carcinoma: a
randomised phase II trial. Eur J Cancer. 46:3200–3204. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
McKenna WG, Weiss MC, Bakanauskas VJ, et
al: The role of the H-ras oncogene in radiation resistance and
metastasis. Int J Radiat Oncol Biol Phys. 18:849–859. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Miller AC, Kariko K, Myers CE, Clark EP
and Samid D: Increased radioresistance of EJras-transformed human
osteo-sarcoma cells and its modulation by lovastatin, an inhibitor
of p21ras isoprenylation. Int J Cancer. 53:302–307. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Rozados VR, Hinrichsen LI, McDonnell J and
Scharovsky OG: Lovastatin enhances in vitro radiation-induced
apoptosis of rat B-cell lymphoma cells. J Exp Clin Cancer Res.
24:55–61. 2005.PubMed/NCBI
|
|
83
|
Butt S, Butt T, Jirström K, et al: The
target for statins, HMG-CoA reductase, is expressed in ductal
carcinoma-in situ and may predict patient response to radiotherapy.
Ann Surg Oncol. 21:2911–2919. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Mace AG, Gantt GA, Skacel M, Pai R, Hammel
JP and Kalady MF: Statin therapy is associated with improved
pathologic response to neoadjuvant chemoradiation in rectal cancer.
Dis Colon Rectum. 56:1217–1227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Werner M, Sacher J and Hohenegger M:
Mutual amplification of apoptosis by statin-induced mitochondrial
stress and doxorubicin toxicity in human rhabdomyosarcoma cells. Br
J Pharmacol. 143:715–724. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Holstein SA and Hohl RJ: Interaction of
cytosine arabinoside and lovastatin in human leukemia cells. Leuk
Res. 25:651–660. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Lishner M, Bar-Sef A, Elis A and Fabian I:
Effect of simvastatin alone and in combination with cytosine
arabinoside on the proliferation of myeloid leukemia cell lines. J
Investig Med. 49:319–324. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Soma MR, Pagliarini P, Butti G, Paoletti
R, Paoletti P and Fumagalli R: Simvastatin, an inhibitor of
cholesterol biosynthesis, shows a synergistic effect with
N,N′-bis(2-chloroethyl)-N-nitrosourea and β-interferon on human
glioma cells. Cancer Res. 52:4348–4355. 1992.PubMed/NCBI
|
|
89
|
Wang W, Collie-Duguid E and Cassidy J:
Cerivastatin enhances the cytotoxicity of 5-fluorouracil on
chemosensitive and resistant colorectal cancer cell lines. FEBS
Lett. 531:415–420. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Katz MS, Minsky BD, Saltz LB, Riedel E,
Chessin DB and Guillem JG: Association of statin use with a
pathologic complete response to neoadjuvant chemoradiation for
rectal cancer. Int J Radiat Oncol Biol Phys. 62:1363–1370. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Brennan DJ, Brandstedt J, Rexhepaj E, et
al: Tumour-specific HMG-CoAR is an independent predictor of
recurrence free survival in epithelial ovarian cancer. BMC Cancer.
10:1252010. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Lipkin SM, Chao EC, Moreno V, et al:
Genetic variation in 3-hydroxy-3-methylglutaryl CoA reductase
modifies the chemo-preventive activity of statins for colorectal
cancer. Cancer Prev Res. 3:597–603. 2010. View Article : Google Scholar
|
|
93
|
Yu CY, Theusch E, Lo K, et al: HNRNPA1
regulates HMGCR alternative splicing and modulates cellular
cholesterol metabolism. Hum Mol Genet. 23:319–332. 2014. View Article : Google Scholar
|
|
94
|
Sławińska-Brych A, Zdzisińska B and
Kandefer-Szerszeń M: Fluvastatin inhibits growth and alters the
malignant phenotype of the C6 glioma cell line. Pharmacol Rep.
66:121–129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Yongjun Y, Shuyun H, Lei C, Xiangrong C,
Zhilin Y and Yiquan K: Atorvastatin suppresses glioma invasion and
migration by reducing microglial MT1-MMP expression. J
Neuroimmunol. 260:1–8. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Crosbie J, Magnussen M, Dornbier R,
Iannone A and Steele TA: Statins inhibit proliferation and
cytotoxicity of a human leukemic natural killer cell line. Biomark
Res. 1:332013. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Al-Haidari AA, Syk I and Thorlacius H:
HMG-CoA reductase regulates CCL17-induced colon cancer cell
migration via geranylgeranylation and RhoA activation. Biochem
Biophys Res Commun. 446:68–72. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Ishikawa S, Hayashi H, Kinoshita K, et al:
Statins inhibit tumor progression via an enhancer of zeste homolog
2-mediated epigenetic alteration in colorectal cancer. Int J
Cancer. 135:2528–2536. 2014. View Article : Google Scholar :
|
|
99
|
Chang HL, Chen CY, Hsu YF, et al:
Simvastatin induced HCT116 colorectal cancer cell apoptosis through
p38MAPK-p53-survivin signaling cascade. Biochim Biophys Acta.
1830:4053–4064. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Rentala S, Chintala R, Guda M, Chintala M,
Komarraju AL and Mangamoori LN: Atorvastatin inhibited
Rho-associated kinase 1 (ROCK1) and focal adhesion kinase (FAK)
mediated adhesion and differentiation of
CD133+CD44+ prostate cancer stem cells.
Biochem Biophys Res Commun. 441:586–592. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Peng X, Li W, Yuan L, Mehta RG, Kopelovich
L and McCormick DL: Inhibition of proliferation and induction of
autophagy by atorvastatin in PC3 prostate cancer cells correlate
with downregulation of Bcl2 and upregulation of miR-182 and p21.
PLoS One. 8:e704422013. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Al-Husein B, Goc A and Somanath PR:
Suppression of interactions between prostate tumor cell-surface
integrin and endothelial ICAM-1 by simvastatin inhibits
micrometastasis. J Cell Physiol. 228:2139–2148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Fang Z, Tang Y, Fang J, et al: Simvastatin
inhibits renal cancer cell growth and metastasis via AKT/mTOR, ERK
and JAK2/STAT3 pathway. PLoS One. 8:e628232013. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Islam M, Sharma S, Kumar B and Teknos TN:
Atorvastatin inhibits RhoC function and limits head and neck cancer
metastasis. Oral Oncol. 49:778–786. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Yu X, Pan Y, Ma H and Li W: Simvastatin
inhibits proliferation and induces apoptosis in human lung cancer
cells. Oncol Res. 20:351–357. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Pelaia G, Gallelli L, Renda T, et al:
Effects of statins and farnesyl transferase inhibitors on ERK
phosphorylation, apoptosis and cell viability in non-small lung
cancer cells. Cell Prolif. 45:557–565. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Chen J, Liu B, Yuan J, et al: Atorvastatin
reduces vascular endothelial growth factor (VEGF) expression in
human non-small cell lung carcinomas (NSCLCs) via inhibition of
reactive oxygen species (ROS) production. Mol Oncol. 6:62–72. 2012.
View Article : Google Scholar
|
|
108
|
Guterres FA, Martinez GR, Rocha ME and
Winnischofer SM: Simvastatin rises reactive oxygen species levels
and induces senescence in human melanoma cells by activation of
p53/p21 pathway. Exp Cell Res. 319:2977–2988. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Pich C, Teiti I, Rochaix P, et al: Statins
reduce melanoma development and metastasis through MICA
overexpression. Front Immunol. 4:622013. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Gopalan A, Yu W, Sanders BG and Kline K:
Simvastatin inhibition of mevalonate pathway induces apoptosis in
human breast cancer cells via activation of JNK/CHOP/DR5 signaling
pathway. Cancer Lett. 329:9–16. 2013. View Article : Google Scholar
|
|
111
|
Park YH, Jung HH, Ahn JS and Im YH: Statin
induces inhibition of triple negative breast cancer (TNBC) cells
via PI3K pathway. Biochem Biophys Res Commun. 439:275–279. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Zhao Z, Cao X, Pan Y, Sha S, Zhao T and
Zhang T: Simvastatin downregulates HER2 via upregulation of PEA3 to
induce cell death in HER2-positive breast cancer cells. Oncol Res.
20:187–195. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Qi XF, Zheng L, Lee KJ, et al: HMG-CoA
reductase inhibitors induce apoptosis of lymphoma cells by
promoting ROS generation and regulating Akt, Erk and p38 signals
via suppression of mevalonate pathway. Cell Death Dis. 4:e5182013.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Zeng M, Gu WY, Jiang TX, et al: Effects of
simvastatin on PI3K/AKT signaling pathway in human acute monocytic
leukemia cell line SHI-1. Zhongguo Shi Yan Xue Ye Xue Za Zhi.
20:268–272. 2012.(In Chinese). PubMed/NCBI
|
|
115
|
Yang SS, Li R, Qu X, Fang W and Quan Z:
Atorvastatin decreases Toll-like receptor 4 expression and
downstream signaling in human monocytic leukemia cells. Cell
Immunol. 279:96–102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Shi J, Zhu J, Zhao H, Zhong C, Xu Z and
Yao F: Mevalonate pathway is a therapeutic target in esophageal
squamous cell carcinoma. Tumour Biol. 34:429–435. 2013. View Article : Google Scholar
|
|
117
|
Ma L, Niknejad N, Gorn-Hondermann I,
Dayekh K and Dimitroulakos J: Lovastatin induces multiple stress
pathways including LKB1/AMPK activation that regulate its cytotoxic
effects in squamous cell carcinoma cells. PLoS One. 7:e460552012.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Tu YS, Kang XL, Zhou JG, Lv XF, Tang YB
and Guan YY: Involvement of Chk1-Cdc25A-cyclin A/CDK2 pathway in
simvastatin induced S-phase cell cycle arrest and apoptosis in
multiple myeloma cells. Eur J Pharmacol. 670:356–364. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Araki M, Maeda M and Motojima K:
Hydrophobic statins induce autophagy and cell death in human
rhabdomyosarcoma cells by depleting geranylgeranyl diphosphate. Eur
J Pharmacol. 674:95–103. 2012. View Article : Google Scholar
|
|
120
|
Liu H, Wang Z, Li Y, Li W and Chen Y:
Simvastatin prevents proliferation and bone metastases of lung
adenocarcinoma in vitro and in vivo. Neoplasma. 60:240–246. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Liao J, Chung YT, Yang AL, et al:
Atorvastatin inhibits pancreatic carcinogenesis and increases
survival in
LSL-KrasG12D-LSL-Trp53R172H-Pdx1-Cre mice.
Mol Carcinog. 52:739–750. 2013. View Article : Google Scholar
|
|
122
|
Vitols S, Angelin B and Juliusson G:
Simvastatin impairs mitogen-induced proliferation of malignant
B-lymphocytes from humans - in vitro and in vivo studies. Lipids.
32:255–262. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Higgins MJ, Prowell TM, Blackford AL, et
al: A short-term biomarker modulation study of simvastatin in women
at increased risk of a new breast cancer. Breast Cancer Res Treat.
131:915–924. 2012. View Article : Google Scholar
|
|
124
|
Bansal D, Undela K, D’Cruz S and Schifano
F: Statin use and risk of prostate cancer: a meta-analysis of
observational studies. PLoS One. 7:e466912012. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Park HS, Schoenfeld JD, Mailhot RB, et al:
Statins and prostate cancer recurrence following radical
prostatectomy or radiotherapy: a systematic review and
meta-analysis. Ann Oncol. 24:1427–1434. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Singh PP and Singh S: Statins are
associated with reduced risk of gastric cancer: a systematic review
and meta-analysis. Ann Oncol. 24:1721–1730. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
van der Spek E, Bloem AC, Sinnige HA and
Lokhorst HM: High dose simvastatin does not reverse resistance to
vincris-tine, adriamycin, and dexamethasone (VAD) in myeloma.
Haematologica. 92:e130–e131. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Han JY, Lim KY, Yu SY, Yun T, Kim HT and
Lee JS: A phase 2 study of irinotecan, cisplatin, and simvastatin
for untreated extensive-disease small cell lung cancer. Cancer.
117:2178–2185. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Kim WS, Kim MM, Choi HJ, et al: Phase II
study of high-dose lovastatin in patients with advanced gastric
adenocarcinoma. Invest New Drugs. 19:81–83. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Lazzeroni M, Guerrieri-Gonzaga A, Serrano
D, et al: Breast ductal lavage for biomarker assessment in high
risk women: rationale, design and methodology of a randomized phase
II clinical trial with nimesulide, simvastatin and placebo. BMC
Cancer. 12:5752012. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Kawata S, Yamasaki E, Nagase T, et al:
Effect of pravastatin on survival in patients with advanced
hepatocellular carcinoma. A randomized controlled trial. Br J
Cancer. 84:886–891. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Lersch C, Schmelz R, Erdmann J, et al:
Treatment of HCC with pravastatin, octreotide, or gemcitabine - a
critical evaluation. Hepatogastroenterology. 51:1099–1103.
2004.PubMed/NCBI
|
|
133
|
Knox JJ, Siu LL, Chen E, et al: A Phase I
trial of prolonged administration of lovastatin in patients with
recurrent or metastatic squamous cell carcinoma of the head and
neck or of the cervix. Eur J Cancer. 41:523–530. 2005. View Article : Google Scholar : PubMed/NCBI
|