Introduction
Hepatocellular carcinoma (HCC) is a leading cause of
cancer-related mortality worldwide, and is associated with
persistent infection from hepatitis B virus (HBV) or hepatitis C
virus (HCV). These viruses play key roles in hepatocarcinogenesis;
and therefore, HCC is highly prevalent in China due to chronic HBV
and HCV infection (1). HCC patients
show the shortest survival time among cancer patients, with most
patients dying within 12 months of HCC tumor development (2). Furthermore, only 30–40% of HCC
patients are found eligible for potentially curative intervention
(3) upon diagnosis, partially due
to the lack of highly sensitive and specific early-detection
measures. Therefore, the effective identification of new markers
for HCC is urgently needed.
MicroRNAs (miRNAs) are a class of single-stranded
non-coding small RNAs (19–24 nt) that regulate the gene expression
network and are known to contribute to a diverse range of
functions, including development, apoptosis, differentiation and
oncogenesis by binding to specific target mRNAs (4). Circulating miRNAs exist stably in body
fluids and were first reported as a newly identified family of
miRNAs by Valadi et al in 2007 (5). It has become clear that miRNAs
potentially regulate all aspects of cellular activity. Recent
studies have provided clear evidence that miRNAs are abundant in
the liver and modulate a diverse spectrum of liver functions,
including differentiation and development, metabolism, apoptotic
cell death, cell proliferation, viral infection and tumorigenesis
(6,7). Deregulation of miRNA expression may be
a key pathogenic mechanism in many liver diseases, such as HCC,
viral hepatitis and polycystic liver disease (8–11).
Differential miRNA expression in HCC and non-tumor
tissue has been reported in numerous studies (12–17).
Several differentially expressed serum miRNAs, including miR-16,
miR-122, miR-21, miR-223, miR-24, miR-27a, miR-375 and let-7f have
been recently reported in patients with HCC, when compared with
hepatitis B patients and healthy individuals (18,19).
However, the differentially expressed miRNAs found in these studies
varied only between different individuals, and these differences
were either investigated in control vs. HCC patients or cirrhosis
vs. HCC patients. In the present study, we investigated miRNA
expression profiles in cirrhosis patients who went on to develop
hepatitis B virus-related HCC.
Materials and methods
Ethics statement
The present study was approved by the Medical Ethics
Committee of The Third Hospital of Zhenjiang Affiliated to Jiangsu
University, Zhenjiang, China, and was conducted in accordance with
the Declaration of Helsinki. Written informed consent was obtained
from each patient prior to their participation in the present
study.
Study design
A total of 25 patients with cirrhosis that evolved
into HCC, who were treated at The Third Hospital of Zhenjiang
Affiliated to Jiangsu University between January 2005 and December
2012, were enrolled in the present study. In the discovery stage, 2
serum pooled samples from 3 cirrhosis and 3 HCC status samples from
the patients were subjected to deep sequencing using the Illumina
HiSeq 2000 system (Illumina, Inc., San Diego, CA, USA) to identify
statistically significant differential miRNA expression.
Subsequently, differentially expressed miRNAs were validated by
qRT-PCR in serum samples of an independent cohort that included 22
cirrhosis and HCC status samples from patients. All patients were
positive for HBsAg, the surface antigen of HBV, for a period of at
least 6 months and were not co-infected with other types of
hepatitis viruses such as hepatitis A, C, D or E. Patients with any
other liver disease, such as alcoholic, autoimmune or metabolic
liver diseases were excluded. The diagnosis of HCC and cirrhosis
was histopathologically confirmed. As this was a retrospective
study, collection of clinical data from the medical records,
pathology reports, and regular follow-up interviews with the
subjects was utilized. Serum samples used in biochemical tests and
then miRNA detection were from the same specimens.
Demographics and clinical features of the patients
are listed in Table I. Biochemical
characteristics of the patients with cirrhosis that evolved into
HCC are listed in Table II.
 | Table IDemographics and clinical features of
the patients. |
Table I
Demographics and clinical features of
the patients.
| No. | Gender | Age (years) | Smoking status | Alcohol
consumption | Antiviral
treatment | HBeAg | HBV DNA | Genotype |
|---|
| 1 | M | 43 | Yes | No | ETV | N | ND | B |
| 2 | M | 35 | No | Yes | LAM | N | ND | C |
| 3 | M | 46 | No | No | ETV | N | ND | C |
| 4 | F | 44 | Yes | No | ETV | P | ND | C |
| 5 | M | 53 | No | Yes | ETV | N | ND | B |
| 6 | M | 57 | Yes | No | ETV | N | ND | C |
| 7 | M | 63 | Yes | No | LAM | P | ND | C |
| 8 | M | 37 | Yes | No | ETV | P | ND | C |
| 9 | M | 47 | No | Yes | ETV | N | ND | C |
| 10 | M | 53 | No | No | ETV | N | ND | C |
| 11 | F | 67 | Yes | No | LAM | N | ND | B |
| 12 | M | 64 | Yes | No | ETV | N | ND | C |
| 13 | M | 57 | No | No | ETV | N | ND | C |
| 14 | M | 54 | No | No | ETV | P | ND | B |
| 15 | M | 56 | No | No | LAM+ADV | N | ND | B |
| 16 | M | 57 | No | No | ETV | N | ND | C |
| 17 | M | 54 | No | Yes | ETV | N | ND | C |
| 18 | M | 47 | Yes | No | ETV | N | ND | C |
| 19 | M | 45 | Yes | No | ETV | N | ND | B |
| 20 | M | 44 | No | No | LAM | P | ND | C |
| 21 | M | 47 | Yes | No | ETV | N | ND | C |
| 22 | M | 37 | No | No | ETV | N | ND | B |
| 23 | F | 43 | No | No | ETV | P | ND | B |
| 24 | M | 48 | Yes | No | ETV | N | ND | C |
| 25 | M | 51 | No | No | ETV | N | ND | C |
 | Table IIBiochemical characteristics of the
patients with cirrhosis that evolved into HCC. |
Table II
Biochemical characteristics of the
patients with cirrhosis that evolved into HCC.
| Variables | Screening set
| Validation set
|
|---|
| Cirrhosis status
(n=3) | HCC status
(n=3) |
P-valuea | Cirrhosis status
(n=22) | HCC status
(n=22) |
P-valuea |
|---|
| TBIL | 13.67±6.87 | 13.90±6.79 | 0.701 | 16.61±9.48 | 18.62±11.90 | 0.573 |
| ALB | 40.17±3.01 | 41.63±3.78 | 0.745 | 41.66±2.17 | 41.96±3.09 | 0.755 |
| ALT | 35.26±13.25 | 43.62±25.36 | 0.392 | 35.94±21.32 | 32.38±23.57 | 0.363 |
| AST | 43.27±21.76 | 47.25±12.64 | 0.778 | 35.77±14.53 | 45.59±26.45 | 0.277 |
| ALP | 81.31±31.71 | 136.73±93.25 | 0.392 | 95.94±31.39 | 132.88±57.56 | 0.017 |
| GGT | 74.88±38.26 | 151.16±65.74 | 0.249 | 65.67±45.46 | 141.68±67.46 | <0.001 |
| PTA | 98.34±5.13 | 99.53±3.35 | 0.862 | 97.46±4.36 | 98.56±3.21 | 0.764 |
| AFP | 5.23±2.48 | 126.23±73.63 | 0.171 | 6.49±4.44 | 101.46 ±78.4 | 0.021 |
| PLT | 115.37±43.86 | 132.26±48.34 | 0.948 | 132.23±47.98 | 131.28±28.42 | 0.897 |
Illumina sequencing and data
analysis
Procedures and methods of sample collection, RNA
isolation and Illumina sequencing were described in detail in our
previous studies (20,21).
qRT-PCR validation study and data
analysis
qRT-PCR-based relative quantification of miRNAs (300
μl of serum from each participant) was performed with
SYBR® Premix Ex Taq (Takara, Kyoto, Japan) according to
the manufacturer’s instructions using a Rotor-Gene 3000 Real-Time
PCR instrument (Corbett Life Science, Sydney, Australia). miR-24
has been reported to be consistently present in human serum
(22,23). Moreover, our previous experience was
that miR-24 maintained stable expression levels, therefore the
level of miR-24 served as an internal control in the serum miRNA
relative quantitative analysis (20). The specificity of each PCR product
was validated by melt curve analysis at completion of the PCR
amplification cycles. All samples were analyzed in triplicate, and
the cycle threshold (Ct) value was defined as the number of cycles
required for the fluorescent signal to reach the threshold. Using
the comparative Ct method, the relative expression levels of miRNAs
in serum were calculated using the formula for 2−ΔΔCt,
where ΔΔCt = [Ct (target, test) - Ct (ref, test)] - [Ct (target,
calibrator) - Ct (ref, calibrator)]. All primers used were obtained
from Invitrogen (Carlsbad, CA, USA).
Statistical analysis
All Illumina sequencing data were log2
transformed. The differences between samples were calculated using
Chi-square and Fisher’s exact tests. Only the miRNAs with the
fold-difference >2.0 and P<0.01 were considered significant.
Quantitative variables were expressed as mean ± standard deviation
(SD). Comparison of biochemical characteristics was conducted by
paired-samples and the Mann-Whitney test was used to compare the
fold-differences of candidate miRNAs upon qRT-PCR in the validation
data set, between cirrhosis and HCC status. All statistical
analyses were performed using SPSS software, version 21.0 (SPSS,
Inc., Chicago, IL, USA). All statistical tests were two-sided and
the results were considered to indicate a statistically significant
result when P<0.05.
Results
Global analysis of miRNAs by deep
sequencing
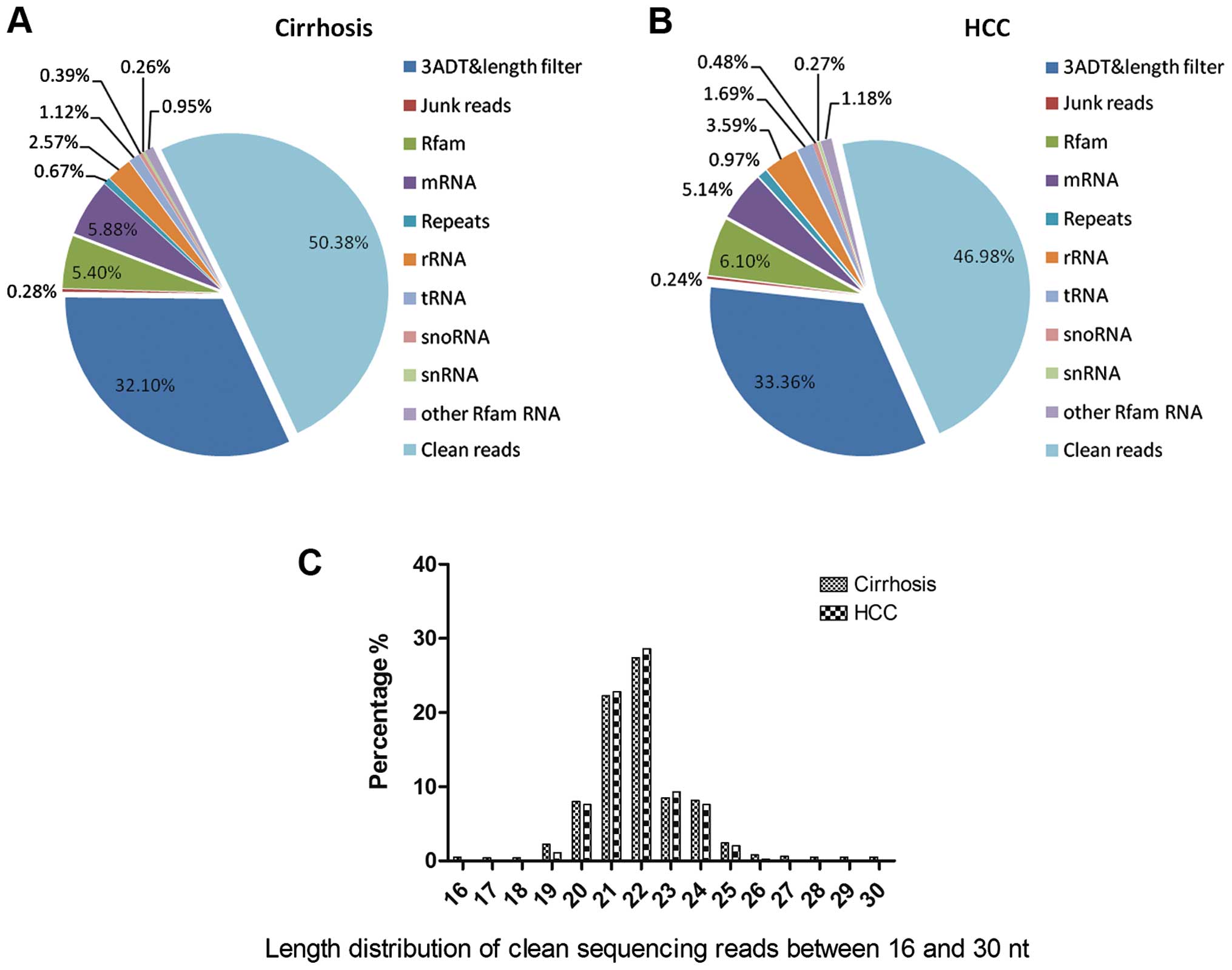
Illumina HiSeq 2000 sequencing of the small RNA
library from the serum of the patients with cirrhosis and HCC
produced 9,846,382 and 9,342,644 raw reads, respectively. After
extensive preprocessing and quality control, the raw reads were
eventually removed, resulting in 425,662 and 426,113 clean reads,
for cirrhosis and HCC status, respectively (Table III, Fig. 1A and B). The distribution of all
reads between 16–30 nt is presented in Fig. 1C. In the present study, we found
that the length of miRNAs was concentrated at 20 and 22 nt. A total
of 1,653 unique reads were mapped to human miRNAs or pre-miRNAs
from the iRbase database, and the pre-miRNAs could be further
mapped to the human genome and expressed sequence tags (ESTs).
 | Table IIIOverview of reads from raw data to
cleaned sequences. |
Table III
Overview of reads from raw data to
cleaned sequences.
| Library | Type | Cirrhosis
| HCC
|
|---|
| Total | (%) of total | Uniq | (%) of uniq | Total | (%) of total | Uniq | (%) of uniq |
|---|
| Raw reads | NA | 9,846,382 | 100 | 958,733 | 100 | 9,342,644 | 100 | 906,960 | 100 |
| 3 ADT and length
filter | Sequence type | 863,846 | 8.77 | 332,485 | 34.68 | 848,736 | 9.08 | 302,543 | 33.36 |
| Junk reads | Sequence type | 6,583 | 0.76 | 4,634 | 0.48 | 5,346 | 0.06 | 2,143 | 0.24 |
| Rfam | RNA class | 447,513 | 4.54 | 59,567 | 6.21 | 512,456 | 5.49 | 55,354 | 6.10 |
| mRNA | RNA class | 342,646 | 3.48 | 47,547 | 4.96 | 235,433 | 2.52 | 46,575 | 5.14 |
| Repeats | RNA class | 76,869 | 0.78 | 11,086 | 1.16 | 98,574 | 1.06 | 8,756 | 0.97 |
| rRNA | RNA class | 432,646 | 4.39 | 32,574 | 3.40 | 242,436 | 2.59 | 32,546 | 3.59 |
| tRNA | RNA class | 123,247 | 1.25 | 18,644 | 1.94 | 115,364 | 1.23 | 15,364 | 1.69 |
| snoRNA | RNA class | 32,536 | 0.33 | 6,537 | 0.68 | 20,123 | 0.22 | 4,365 | 0.48 |
| snRNA | RNA class | 28,328 | 0.29 | 4,234 | 0.44 | 25,346 | 0.27 | 2,456 | 0.27 |
| Other Rfam RNA | RNA class | 175,685 | 1.78 | 15,763 | 1.64 | 17,563 | 0.19 | 10,745 | 1.18 |
| Clean reads | Sequence type | 7,248,031 | 73.61 | 425,662 | 44.40 | 7,221,267 | 77.29 | 426,113 | 46.98 |
Identification of novel miRNAs
In total, 14 novel miRNA genes were identified in
the two disease categories. The length of these candidate miRNAs
ranged from 20 to 24 nt. The localization, sequence, structure and
expression profile of these miRNAs are summarized in Table IV. However, several candidates
among the predicted novel miRNAs were expressed at extremely low
levels.
 | Table IVNovel miRNA candidates represented in
the library. |
Table IV
Novel miRNA candidates represented in
the library.
| Novel miR_name | miR_seq | Genome ID | Strand | Start | End | MFE | Cirrhosis
count | HCC count | P-value |
|---|
|
PC-5p-1700_1443 |
TGTAGGCAAGGGAAGTCGGC |
gi|224514641|ref|NT_167214.1| | + | 116070 | 116319 | 0.6 | 1,613 | 4,551 | 5.61E-42 |
| PC-3p-8816_210 |
AGGACGGTGGTCATGGAAGTC |
gi|224589820|ref|NC_000008.10| | − | 109304597 | 109304710 | 0.6 | 859 | 3,508 | 6.89E-30 |
| PC-3p-6084_321 |
ACGGGCTTGGCAGAATCAGC |
gi|224589807|ref|NC_000016.9| | − | 81902013 | 81902146 | 0.5 | 228 | 835 | 3.41E-13 |
| PC-5p-7578_250 |
AATTTCATCGTGATGGGGA |
gi|224589811|ref|NC_000002.11| | − | 210764476 | 210764537 | 0.6 | 168 | 605 | 2.02E-11 |
|
hsa-mir-7641-2-p3 |
TCGGGCCTGGTTAGTACTTGGA | 224589805 | + | 76070552 | 76070604 | 0.5 | 456 | 559 | 3.07E-11 |
|
hsa-mir-6240-p5_1ss17GT |
TGCCCAGTGCTCTGAATGTC |
gi|224589802|ref|NC_000011.9| | + | 77597474 | 77597582 | 0.6 | 300 | 534 | 6.26E-11 |
| PC-5p-250006_9 |
ATGTGATGCATCGCTTCTGT |
gi|224589811|ref|NC_000002.11| | − | 170685375 | 170685445 | 0.9 | 28 | 340 | 3.91E-08 |
|
hsa-mir-4459-p5_1ss4TC |
AGGCGGAGGTTGCAGTGAGC | 224589817 | − | 53371348 | 53371413 | 0.6 | 25 | 177 | 1.38E-06 |
|
PC-5p-156137_14 |
GCCGACTACAACATCCAGAA |
gi|224589820|ref|NC_000008.10| | + | 106730487 | 106730585 | 0.8 | 21 | 155 | 2.81E-06 |
|
PC-3p-1704_1400 |
AGGGCTGGGTCGGTCGGGCTGG |
gi|224514641|ref|NT_167214.1| | + | 116070 | 116319 | 0.6 | 2,580 | 140 | 1.14E-06 |
|
hsa-mir-566-p3_1ss6AG |
AGGCGGAGGTTGCAGTGAGC | 224589815 | + | 50210759 | 50210852 | 0.5 | 31 | 60 | 4.93E-04 |
| PC-5p-32366_69 |
GTGGAAATCATGTGGGCTTT |
gi|224589804|ref|NC_000013.10| | + | 61836053 | 61836109 | 0.6 | 34 | 49 | 8.14E-04 |
| PC-5p-42534_51 |
AGAATGAAACTCAAAGGAAT |
gi|224589804|ref|NC_000013.10| | − | 32584468 | 32584537 | 0.6 | 31 | 47 | 6.86E-04 |
|
hsa-mir-451a-p3 |
TTTAGTAATGGTAATGGTTCT | 224589808 | − | 27188387 | 27188458 | 1.5 | 80 | 25 | 1.15E-02 |
Analysis of differentially expressed
miRNAs
When the cirrhosis and HCC status samples were
compared, the differential expression levels of 127 miRNAs showed
significant variability (Fig. 2).
Among these, 22 miRNAs showed a >2-fold upregulation
(P<0.01), and 2 miRNAs showed a >2-fold downregulation
(P<0.01) in the cirrhosis and HCC patients (Table V).
 | Table VDifferential expression of miRNAs
between HCC and cirrhosis. |
Table V
Differential expression of miRNAs
between HCC and cirrhosis.
| No. | miR_name | Fold-change | Fold-change
(log2) | Up/down | miR_seq |
|---|
| 1 | hsa-miR-455-5p | 28.37 | 4.83 | Up |
UAUGUGCCUUUGGACUACAUCG |
| 2 | hsa-let-7e-5p | 26.25 | 4.71 | Up |
UGAGGUAGGAGGUUGUAUAGUU |
| 3 |
hsa-miR-483-5p_R−1 | 22.60 | 4.50 | Up |
AAGACGGGAGGAAAGAAGGGAG |
| 4 |
hsa-miR-190b_R+1 | 22.36 | 4.48 | Up |
UGAUAUGUUUGAUAUUGGGUUU |
| 5 | hsa-miR-30e-3p | 20.01 | 4.32 | Up |
CUUUCAGUCGGAUGUUUACAGC |
| 6 | hsa-miR-486-5p | 17.41 | 4.12 | Up |
TCCTGTACTGAGCTGCCCCGAG |
| 7 |
hsa-miR-584-5p_R−1 | 11.08 | 3.47 | Up |
UUAUGGUUUGCCUGGGACUGG |
| 8 | hsa-miR-10a-5p | 10.45 | 3.38 | Up |
UACCCUGUAGAUCCGAAUUUGUG |
| 9 | hsa-miR-574-3p | 9.38 | 3.23 | Up |
UGAGUGUGUGUGUGUGAGUGUGU |
| 10 |
hsa-miR-193b-5p | 9.13 | 3.19 | Up |
CGGGGTTTTGAGGGCGAGATGA |
| 11 |
hsa-miR-4532_R+2 | 8.33 | 3.06 | Up |
CCCCGGGGAGCCCGGCGCG |
| 12 | hsa-miR-206 | 7.69 | 2.94 | Up |
UGGAAUGUAAGGAAGUGUGUGG |
| 13 |
hsa-miR-28-5p_R−2 | 7.43 | 2.89 | Up |
AAGGAGCUCACAGUCUUGAG |
| 14 | hsa-miR-433-3p | 6.13 | 2.62 | Up |
AUCAUGAUGGGCUCCUCGGUGU |
| 15 |
hsa-miR-3187-3p | 6.04 | 2.59 | Up |
TTGGCCATGGGGCTGCGCGG |
| 16 | hsa-miR-98-5p | 5.71 | 2.51 | Up |
UGAGGUAGUAAGUUGUAUUGUU |
| 17 |
hsa-miR-4433b-5p | 5.29 | 2.40 | Up |
AUGUCCCACCCCCACUCCUGU |
| 18 | hsa-miR-497-5p | 4.94 | 2.30 | Up |
CAGCAGCACACTGTGGTTTGT |
| 19 |
hsa-mir-1285-1-p5 | 4.60 | 2.20 | Up |
GAUCUCACUUUGUUGCCCAGG |
| 20 | hsa-miR-141-3p | 3.29 | 1.72 | Up |
UAACACUGUCUGGUAAAGAUGG |
| 21 |
hsa-miR-100-5p_R−1 | 2.99 | 1.58 | Up |
AACCCGUAGAUCCGAACUUGUG |
| 22 |
hsa-miR-99b-3p_R−2 | 2.36 | 1.24 | Up |
CACCCGUAGAACCGACCUUG |
| 32 |
hsa-miR-199a-5p | 0.50 | −0.99 | Down |
ACAGUAGUCUGCACAUUGGUUA |
| 23 |
hsa-miR-1228-5p | 0.49 | −1.03 | Down |
GUGGGCGGGGGCAGGUGUGUG |
| 24 | hsa-miR-202-3p | 0.48 | −1.05 | Down |
AGAGGTATAGGGCATGGGAA |
| 29 | hsa-miR-92a-3p | 0.46 | −1.13 | Down |
TATTGCACTTGTCCCGGCCTGT |
| 25 |
hsa-miR-6852-5p | 0.45 | −1.14 | Down |
CCCUGGGGUUCUGAGGACAUG |
| 31 | hsa-miR-30b-5p | 0.45 | −1.14 | Down |
UGUAAACAUCCUACACUCAGCU |
| 33 | hsa-miR-511-5p | 0.44 | −1.18 | Down |
GUGUCUUUUGCUCUGCAGUCA |
| 27 | hsa-miR-320a | 0.37 | −1.44 | Down |
AAAAGCTGGGTTGAGAGGGCGA |
| 28 |
hsa-mir-6127-p3 | 0.36 | −1.48 | Down |
UGAGGGAGUGGGUGGGAGG |
| 26 | hsa-miR-26a-5p | 0.36 | −1.48 | Down |
UUCAAGUAAUCCAGGAUAGGCU |
| 30 | hsa-miR-885-5p | 0.35 | −1.50 | Down |
TCCATTACACTACCCTGCCTCT |
| 34 | hsa-miR-30a-3p | 0.32 | −1.63 | Down |
UGUAAACAUCCUCGACUGGAAG |
| 35 |
hsa-miR-4454_L−2 | 0.19 | −2.39 | Down |
GGAUCCGAGUCACGGCACCA |
| 36 | hsa-let-7c-5p | 0.08 | −3.61 | Down |
UGAGGUAGUAGGUUGUAUGGUU |
| 37 | hsa-miR-30c-5p | 0.06 | −4.17 | Down |
UGUAAACAUCCUACACUCUCAGC |
| 38 | hsa-let-7f-5p | 0.05 | −4.23 | Down |
UGAGGUAGUAGAUUGUAUAGUU |
| 39 | hsa-miR-122-5p | 0.03 | −5.00 | Down |
UGGAGUGUGACAAUGGUGUUUG |
| 40 | hsa-miR-192-5p | 0.03 | −5.00 | Down |
CUGACCUAUGAAUUGACAGCC |
Validation of the differentially
expressed miRNAs
We used qRT-PCR to confirm the expression of 40
candidate miRNAs that were selected from the previous step in an
independent cohort consisting of 22 serum samples. The threshold
value for the miRNAs was determined as Ct <35 and the detection
rate >75%. We then calculated the 2−ΔΔCt of 40
candidate miRNAs in the 2 status types. Eight of the 40 miRNAs had
significantly differential expression levels between the 2 statuses
(Table VI). These miRNAs were
hsa-miR-122-5p, hsa-miR-192-5p, hsa-miR-486-5p, hsa-miR-193b-5p,
hsa-miR-206, hsa-miR-141-3p, hsa-miR-199a-5p and
hsa-miR-26a-5p.
 | Table VIExpression profiles of candidate
miRNAs upon qRT-PCR in the validation set. |
Table VI
Expression profiles of candidate
miRNAs upon qRT-PCR in the validation set.
| No. | miR_name | HCC vs. cirrhosis
|
|---|
| P-value | Fold-change |
|---|
| 1 | hsa-miR-455-5p | 0.108 | 1.58 |
| 2 | hsa-let-7e-5p | 0.063 | 4.50 |
| 3 |
hsa-miR-483-5p_R−1 | 0.108 | 3.19 |
| 4 |
hsa-miR-190b_R+1 | 0.246 | 3.47 |
| 5 | hsa-miR-30e-3p | ND | ND |
| 6 |
hsa-miR-486-5p | 0.006 | 2.42 |
| 7 |
hsa-miR-584-5p_R−1 | 0.108 | 3.25 |
| 8 | hsa-miR-10a-5p | 0.144 | 1.43 |
| 9 | hsa-miR-574-3p | ND | ND |
| 10 |
hsa-miR-193b-5p | 0.008 | 2.64 |
| 11 |
hsa-miR-4532_R+2 | 0.023 | 1.23 |
| 12 |
hsa-miR-206 | 0.003 | 3.64 |
| 13 |
hsa-miR-28-5p_R−2 | 0.069 | 2.45 |
| 14 | hsa-miR-433-3p | 0.108 | 1.64 |
| 15 |
hsa-miR-3187-3p | ND | ND |
| 16 | hsa-miR-98-5p | 0.723 | 1.63 |
| 17 |
hsa-miR-4433b-5p | ND | ND |
| 18 | hsa-miR-497-5p | <0.001 | 1.33 |
| 19 |
hsa-mir-1285-1-p5 | 0.012 | 1.64 |
| 20 |
hsa-miR-141-3p | 0.001 | 2.44 |
| 21 |
hsa-miR-100-5p_R−1 | 0.289 | 1.54 |
| 22 |
hsa-miR-99b-3p_R−2 | 0.125 | 2.14 |
| 23 |
hsa-miR-1228-5p | 0.268 | 2.54 |
| 24 | hsa-miR-202-3p | ND | ND |
| 25 |
hsa-miR-6852-5p | ND | ND |
| 26 |
hsa-miR-26a-5p | 0.002 | 0.64 |
| 27 | hsa-miR-320a | 0.056 | 0.53 |
| 28 |
hsa-mir-6127-p3 | 0.108 | 0.77 |
| 29 | hsa-miR-92a-3p | 0.256 | 0.87 |
| 30 | hsa-miR-885-5p | 0.442 | 0.65 |
| 31 | hsa-miR-30b-5p | 0.256 | 0.45 |
| 32 |
hsa-miR-199a-5p | <0.001 | 0.53 |
| 33 | hsa-miR-511-5p | 0.284 | 0.45 |
| 34 | hsa-miR-30a-3p | 0.084 | 0.54 |
| 35 |
hsa-miR-4454_L−2 | ND | ND |
| 36 | hsa-let-7c-5p | 0.073 | 0.65 |
| 37 | hsa-miR-30c-5p | 0.077 | 0.76 |
| 38 | hsa-let-7f-5p | 0.112 | 0.78 |
| 39 |
hsa-miR-122-5p | <0.001 | 0.12 |
| 40 |
hsa-miR-192-5p | <0.001 | 0.24 |
Discussion
Since the discovery of circulating miRNAs, several
studies have been conducted to investigate their potential as novel
biomarkers in body fluid. Circulating miRNAs have already been
shown to be relevant biomarkers for cancer detection, applicable in
non-invasive diagnostic testing and have demonstrated several other
successful applications (24–27).
To date, three methods have mainly been used for the analysis of
the expression profiles of circulating miRNAs in serum: qRT-PCR,
microarray and next-generation sequencing technology (28). Although qRT-PCR has been widely
employed for miRNA quantification, it is only capable of detecting
a limited number of miRNAs at any one time. Microarray analysis, a
high-throughput method, is capable of detecting only known
fragments and is not suitable for detection of low-abundant miRNAs
or for distinguishing between miRNAs with single nucleic acid
polymorphisms. Compared to these techniques, next-generation
sequencing technology appears to be more suitable for miRNA
profiling. Thus, the Roche 454 genome Sequencer, the Illumina
genome Analyzer and the ABI SOLiD System sequencing platforms have
become widely available and used over the past few years.
Several studies have shown that many miRNAs are
dysregulated in HCC (17,29,30)
and have also considered the potential of circulating miRNA levels
to affect HCC progression. The high stability of miRNAs in
circulation suggests them for use as potentially ideal biomarkers,
particularly for early-stage detection (4). Various studies have observed and
explored the upregulation of circulating miR-21 (18,31),
miR-222 (31) and miR-223 (32) in the serum/plasma of HBV- or
HCV-associated HCC patients.
Downregulation of miRNAs is also a common finding in
HBV-related HCC; in this case, these miRNAs act as tumor-suppressor
genes. The pathological mechanisms of tumor-suppressive miRNAs is
involved in cell cycle arrest, increased apoptosis and eventual
reductions in tumor angiogenesis and metastasis by inhibiting
migration and invasion. Among these downregulated miRNAs, miR-122
and miR-199 appear to be particularly important in HCC (33–35).
In the present study, we also found that the miRNA downregulated in
cirrhosis status that evolved into HCC was miR-122, a
liver-specific miRNA that is abundant in the liver and plays an
important role in regulating hepatocyte development and
differentiation (36,37). The overexpression of miR-122 has
been found to induce apoptosis and suppress proliferation in the
human liver carcinoma cell lines Hepg2 and Hep3B in vitro
(38), and has been demonstrated
in vivo directly by the generation of miR-122-knockout mice
in liver cancer (39,40).
The present study revealed that serum
hsa-miR-486-5p, hsa-miR-193b-5p, hsa-miR-206, hsa-miR-141-3p,
hsa-miR-199a-5p, hsa-miR-122-5p, hsa-miR-192-5p and hsa-miR-26a-5p
were potential circulating markers for HCC diagnosis, and 4 of
these 8 miRNAs (miR-122, miR-199, miR-192 and miR-26a) in the
present study have been previously reported to show differential
expression (19,41,42).
At the circulating blood level, Xu et al
(18) reported that miR-21, miR-122
and miR-223 could be utilized in discriminating HCC patients from a
healthy group. Qu et al (43) found that miR-16 has moderate
diagnostic accuracy in HCC. Li et al (14) reported an extraordinarily high
diagnostic accuracy for serum miRNA profiles in the diagnosis of
HCC [area under the curve (AUC) = 0.97-1.00] with miR-10a,
miR-125b, miR-223, miR-23a, miR-23b, miR-342-3p, miR-375, miR-423,
miR-92a and miR-99a. However, the need for different markers for
different group comparisons with different critical values in their
study (HCC vs. healthy, HCC vs. HBV, healthy vs. HBV, healthy vs.
HCV and HBV vs. HCV) raised concerns about the robustness of these
markers.
In our previous study (20), we established a logistic model of
miRNAs for the diagnosis of HCC in a larger sample size and
independent validation set. However, in the previous study, the
cirrhosis and HCC patients were different individuals. While the
present study was limited by the sample size, its innovation was
the successful investigation of two phases of disease status in the
same individuals.
Acknowledgments
The authors thank LC Bio-Tech Inc. for the expert
technical assistance. This study was supported by the Natural
Science Foundation of Jiangsu Province, China (BK2011151)
(http://www.jstd.gov.cn/), the Medical Project of
the Health Department, Jiangsu Province (H201248) (http://www.jswst.gov.cn/), the Preventive Medicine
Research Projects of Jiangsu Province (Y2012016) (http://www.jswst.gov.cn/), and the Social Development
Project of Zhenjiang City (SH201346) (http://kjj.zhenjiang.gov.cn/).
References
|
1
|
Zemel R, Issachar A and Tur-Kaspa R: The
role of oncogenic viruses in the pathogenesis of hepatocellular
carcinoma. Clin Liver Dis. 15:261–279. vii–x. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cui XW, Chang JM and Dietrich CF: Should
hepatocellular carcinoma screening with ultrasound be recommended
in hepatocellular carcinoma high-incidence areas? Hepatology.
61:1091–1092. 2015. View Article : Google Scholar
|
|
3
|
Lo SS, Dawson LA, Kim EY, Mayr NA, Wang
JZ, Huang Z and Cardenes HR: Stereotactic body radiation therapy
for hepatocellular carcinoma. Discov Med. 9:404–410.
2010.PubMed/NCBI
|
|
4
|
Petrelli A, Perra A, Cora D, Sulas P,
Menegon S, Manca C, Migliore C, Kowalik MA, Ledda-Columbano GM,
Giordano S, et al: MicroRNA/gene profiling unveils early molecular
changes and nuclear factor erythroid related factor 2 (NRF2)
activation in a rat model recapitulating human hepatocellular
carcinoma (HCC). Hepatology. 59:228–241. 2014. View Article : Google Scholar
|
|
5
|
Valadi H, Ekström K, Bossios A, Sjöstrand
M, Lee JJ and Lötvall JO: Exosome-mediated transfer of mRNAs and
microRNAs is a novel mechanism of genetic exchange between cells.
Nat Cell Biol. 9:654–659. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li Y and Kowdley KV: MicroRNAs in common
human diseases. Genomics Proteomics Bioinformatics. 10:246–253.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bala S, Petrasek J, Mundkur S, Catalano D,
Levin I, Ward J, Alao H, Kodys K and Szabo G: Circulating microRNAs
in exosomes indicate hepatocyte injury and inflammation in
alcoholic, drug-induced, and inflammatory liver diseases.
Hepatology. 56:1946–1957. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Waidmann O, Bihrer V, Pleli T, Farnik H,
Berger A, Zeuzem S, Kronenberger B and Piiper A: Serum microRNA-122
levels in different groups of patients with chronic hepatitis B
virus infection. J Viral Hepat. 19:e58–e65. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Waidmann O, Köberle V, Brunner F, Zeuzem
S, Piiper A and Kronenberger B: Serum microRNA-122 predicts
survival in patients with liver cirrhosis. PLoS One. 7:e456522012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang J, Li J, Shen J, Wang C, Yang L and
Zhang X: MicroRNA-182 downregulates metastasis suppressor 1 and
contributes to metastasis of hepatocellular carcinoma. BMC Cancer.
12:2272012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lamba V, Ghodke-Puranik Y, Guan W and
Lamba JK: Identification of suitable reference genes for hepatic
microRNA quantitation. BMC Res Notes. 7:1292014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kutay H, Bai S, Datta J, Motiwala T,
Pogribny I, Frankel W, Jacob ST and Ghoshal K: Downregulation of
miR-122 in the rodent and human hepatocellular carcinomas. J Cell
Biochem. 99:671–678. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ladeiro Y, Couchy G, Balabaud C,
Bioulac-Sage P, Pelletier L, Rebouissou S and Zucman-Rossi J:
MicroRNA profiling in hepatocellular tumors is associated with
clinical features and oncogene/tumor suppressor gene mutations.
Hepatology. 47:1955–1963. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li LM, Hu ZB, Zhou ZX, Chen X, Liu FY,
Zhang JF, Shen HB, Zhang CY and Zen K: Serum microRNA profiles
serve as novel biomarkers for HBV infection and diagnosis of
HBV-positive hepatocarcinoma. Cancer Res. 70:9798–9807. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meng F, Henson R, Wehbe-Janek H, Ghoshal
K, Jacob ST and Patel T: MicroRNA-21 regulates expression of the
PTEN tumor suppressor gene in human hepatocellular cancer.
Gastroenterology. 133:647–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pineau P, Volinia S, McJunkin K, Marchio
A, Battiston C, Terris B, Mazzaferro V, Lowe SW, Croce CM and
Dejean A: miR-221 overexpression contributes to liver
tumorigenesis. Proc Natl Acad Sci USA. 107:264–269. 2010.
View Article : Google Scholar :
|
|
17
|
Huang X and Jia Z: Construction of
HCC-targeting artificial miRNAs using natural miRNA precursors. Exp
Ther Med. 6:209–215. 2013.PubMed/NCBI
|
|
18
|
Xu J, Wu C, Che X, Wang L, Yu D, Zhang T,
Huang L, Li H, Tan W, Wang C, et al: Circulating microRNAs, miR-21,
miR-122, and miR-223, in patients with hepatocellular carcinoma or
chronic hepatitis. Mol Carcinog. 50:136–142. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou J, Yu L, Gao X, Hu J, Wang J, Dai Z,
Wang JF, Zhang Z, Lu S, Huang X, et al: Plasma microRNA panel to
diagnose hepatitis B virus-related hepatocellular carcinoma. J Clin
Oncol. 29:4781–4788. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tan Y, Ge G, Pan T, Wen D, Chen L, Yu X,
Zhou X and Gan J: A serum microRNA panel as potential biomarkers
for hepatocellular carcinoma related with hepatitis B virus. PLoS
One. 9:e1079862014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tan Y, Ge G, Pan T, Wen D and Gan J: A
pilot study of serum microRNAs panel as potential biomarkers for
diagnosis of nonalcoholic fatty liver disease. PLoS One.
9:e1051922014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peltier HJ and Latham GJ: Normalization of
microRNA expression levels in quantitative RT-PCR assays:
Identification of suitable reference RNA targets in normal and
cancerous human solid tissues. RNA. 14:844–852. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang H, Li QY, Guo ZZ, Guan Y, Du J, Lu
YY, Hu YY, Liu P, Huang S and Su SB: Serum levels of microRNAs can
specifically predict liver injury of chronic hepatitis B. World J
Gastroenterol. 18:5188–5196. 2012.PubMed/NCBI
|
|
24
|
Li G, Cai G, Li D and Yin W: MicroRNAs and
liver disease: Viral hepatitis, liver fibrosis and hepatocellular
carcinoma. Postgrad Med J. 90:106–112. 2014. View Article : Google Scholar
|
|
25
|
Li ZJ, Ou-Yang PH and Han XP: Profibrotic
effect of miR-33a with Akt activation in hepatic stellate cells.
Cell Signal. 26:141–148. 2014. View Article : Google Scholar
|
|
26
|
Marin JJ, Bujanda L and Banales JM:
MicroRNAs and cholestatic liver diseases. Curr Opin Gastroenterol.
30:303–309. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Papaconstantinou I, Karakatsanis A,
Gazouli M, Polymeneas G and Voros D: The role of microRNAs in liver
cancer. Eur J Gastroenterol Hepatol. 24:223–228. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mei Q, Li X, Meng Y, Wu Z, Guo M, Zhao Y,
Fu X and Han W: A facile and specific assay for quantifying
microRNA by an optimized RT-qPCR approach. PLoS One. 7:e468902012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Salvi A, Abeni E, Portolani N, Barlati S
and De Petro G: Human hepatocellular carcinoma cell-specific miRNAs
reveal the differential expression of miR-24 and miR-27a in
cirrhotic/non-cirrhotic HCC. Int J Oncol. 42:391–402. 2013.
|
|
30
|
Xu RH, Zheng LY, He DL, et al: Profiling
of differentially expressed microRNAs (miRNAs) during
differentiation of rat hepatic oval cells (HOCs) into
hepatocellular carcinoma (HCC) cells. Clin Transl Oncol.
17:230–237. 2015. View Article : Google Scholar
|
|
31
|
Li J, Wang Y, Yu W, Chen J and Luo J:
Expression of serum miR-221 in human hepatocellular carcinoma and
its prognostic significance. Biochem Biophys Res Commun. 406:70–73.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tomimaru Y, Eguchi H, Nagano H, Wada H,
Kobayashi S, Marubashi S, Tanemura M, Tomokuni A, Takemasa I,
Umeshita K, et al: Circulating microRNA-21 as a novel biomarker for
hepatocellular carcinoma. J Hepatol. 56:167–175. 2012. View Article : Google Scholar
|
|
33
|
Murakami Y, Yasuda T, Saigo K, Urashima T,
Toyoda H, Okanoue T and Shimotohno K: Comprehensive analysis of
microRNA expression patterns in hepatocellular carcinoma and
non-tumorous tissues. Oncogene. 25:2537–2545. 2006. View Article : Google Scholar
|
|
34
|
Jiang J, Gusev Y, Aderca I, Mettler TA,
Nagorney DM, Brackett DJ, Roberts LR and Schmittgen TD: Association
of microRNA expression in hepatocellular carcinomas with hepatitis
infection, cirrhosis, and patient survival. Clin Cancer Res.
14:419–427. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hou J, Lin L, Zhou W, Wang Z, Ding G, Dong
Q, Qin L, Wu X, Zheng Y, Yang Y, et al: Identification of miRNomes
in human liver and hepatocellular carcinoma reveals miR-199a/b-3p
as therapeutic target for hepatocellular carcinoma. Cancer Cell.
19:232–243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Morita K, Taketomi A, Shirabe K, Umeda K,
Kayashima H, Ninomiya M, Uchiyama H, Soejima Y and Maehara Y:
Clinical significance and potential of hepatic microRNA-122
expression in hepatitis C. Liver Int. 31:474–484. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chang J, Nicolas E, Marks D, Sander C,
Lerro A, Buendia MA, Xu C, Mason WS, Moloshok T, Bort R, et al:
miR-122, a mammalian liver-specific microRNA, is processed from hcr
mRNA and may downregulate the high affinity cationic amino acid
transporter CAT-1. RNA Biol. 1:106–113. 2004. View Article : Google Scholar
|
|
38
|
Datta J, Kutay H, Nasser MW, Nuovo GJ,
Wang B, Majumder S, Liu CG, Volinia S, Croce CM, Schmittgen TD, et
al: Methylation mediated silencing of microRNA-1 gene and its role
in hepatocellular carcinogenesis. Cancer Res. 68:5049–5058. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hsu SH, Wang B, Kota J, Yu J, Costinean S,
Kutay H, Yu L, Bai S, La Perle K, Chivukula RR, et al: Essential
metabolic, anti-inflammatory, and anti-tumorigenic functions of
miR-122 in liver. J Clin Invest. 122:2871–2883. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tsai WC, Hsu SD, Hsu CS, Lai TC, Chen SJ,
Shen R, Huang Y, Chen HC, Lee CH, Tsai TF, et al: MicroRNA-122
plays a critical role in liver homeostasis and
hepatocarcinogenesis. J Clin Invest. 122:2884–2897. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kota J, Chivukula RR, O’Donnell KA,
Wentzel EA, Montgomery CL, Hwang HW, Chang TC, Vivekanandan P,
Torbenson M, Clark KR, et al: Therapeutic microRNA delivery
suppresses tumorigenesis in a murine liver cancer model. Cell.
137:1005–1017. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ji J, Shi J, Budhu A, Yu Z, Forgues M,
Roessler S, Ambs S, Chen Y, Meltzer PS, Croce CM, et al: MicroRNA
expression, survival, and response to interferon in liver cancer. N
Engl J Med. 361:1437–1447. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Qu KZ, Zhang K, Li H, Afdhal NH and
Albitar M: Circulating microRNAs as biomarkers for hepatocellular
carcinoma. J Clin Gastroenterol. 45:355–360. 2011. View Article : Google Scholar : PubMed/NCBI
|