Introduction
Hepatocellular carcinoma (HCC) is one of the most
common and serious malignancies worldwide (1,2). Due
to its highly aggressiveness and poor prognosis, patients with
advanced HCC are not candidates for local/regional surgical
resection (3–5). Cirrhosis of any etiology is a risk
factor for HCC, which include infection with hepatitis B virus
(HBV) or hepatitis C virus (HCV), alcoholic cirrhosis and exposure
to environmental toxins such as aflatoxin (6–8).
Despite intensive therapeutic intervention, the cure
rate for HCC is extremely limited due to high rates of recurrence
and chemotherapy resistance. Even for those with resected disease,
the recurrence rate can be as high as 50% at 2 years (9–11).
Systematic chemotherapy plays an important role in HCC treatment
especially for patients with advanced HCC, however, systemic
therapy with cytotoxic agents provides marginal benefit, the
response rates are low, and the response duration is typically
short (12,13). Cisplatin is extensively used as a
chemotherapeutic agent for the treatment of HCC. A major problem
with cisplatin treatment of HCC is the development of cisplatin
chemoresistance. Despite the rapid shrinkage in tumor mass
following chemotherapeutic cycles, the acquisition of
chemoresistance becomes a changllenge to oncologists (14). Currently the molecular mechanisms
involved in cancer cell chemoresistance are still largely unclear
(15–17). Therefore, there is an urgent
requirement in revealing the underlying mechanisms responsible for
HCC chemoresistance, which is indispensable for developing
effective chemotherapeutic agents.
Osteopontin (OPN) is a phosphorylated
multifunctional glycoprotein, which is normally secreted by
activated macrophages, leukocytes, T lymphocytes and involved in
tissue remodeling processes (18–20).
OPN binds to either the family of αvβ integrins or the cell-surface
adhesion molecule CD44 to initiate cellular signals exerting its
functions (21,22). Overexpression of OPN has been
reported in a variety of malignancies, including carcinomas of
gastric (23,24), breast (25,26),
prostate (27,28) and lung (29,30).
OPN has recently emerged as a significant protein in the biology of
HCC (31–33). Serum OPN was more sensitive than AFP
in the diagnosis of HCC. OPN overexpression tended to be associated
with the presence of tumor vascular invasion and advanced tumor
grade, it was also found that interference of OPN expression
inhibited the invasion and metastasis of HCC (34,35).
All these data suggest that OPN plays a significant role in the
development and progression of HCC. However, its role in modulating
chemoresistance and molecular mechanism are relatively
unexplored.
In the present study, we tested whether and how OPN
participate in the cisplatin-resistance of HCC. We found that OPN
expression are significantly increased in HCC samples that received
cisplatin-based chemotherapy, and OPN was upregulated when treated
with cisplatin in HCC cell lines. OPN rendered cisplatin
chemoresistance through activation of PI3K/AKT pathway, while
blockage of OPN pathway could regain the sensitivity to
cisplatin.
Materials and methods
Patient samples
Patients were enrolled from Tongji Hospital,
Huazhong University of Science and Technology. All the pathological
sections were obtained with informed consent from the patients, and
the study was approved by the institutional review board of Tongji
Hospital, Huazhong University of Science and Technology. The
diagnosis was based mainly on clinical setting including imaging
(ultrasonography and computerized tomography) and biochemistry (AFP
and liver function enzymes testing). A total of 8 HCC patients were
enrolled, they were diagnosed between July 2009 and July 2013. All
the patients were initially treated with transcatheter arterial
chemoembolization (TACE) after diagnosis using cisplatin. The
tissues were collected before and after TACE.
Cell culture
The human HCC cell lines HepG2, HuH-7 and PLC/PRF/5
were purchased from the Shanghai Institute for Biological Sciences
of the Chinese Academy of Sciences. The cells were grown in
Dulbecco's modified Eagle's medium, supplemented with 10% fetal
bovine serum, 100 U/ml penicillin G, and 100 mg/ml streptomycin.
Cells were grown in a humidified incubator at 37°C in an atmosphere
of 5% CO2 and 95% air.
Immunohistochemistry
The slides were deparafinized, and rehydrated, then
immersed in 3% hydrogen peroxide solution for 10 min, heated in
citrate buffer, pH 6.0, at 95°C for 25 min, cooled at room
temperature for 60 min. The slides were blocked by 10% normal goat
serum at 37°C for 30 min, and then incubated with mouse monoclonal
antibody against OPN (Abcam; 1:500) overnight at 4°C. After washing
with PBS, the slides were incubated with biotionylated second
antibody (diluted 1:100) for 30 min at 37°C, followed by
streptavidin-peroxidase (diluted 1:100) incubation at 37°C for 30
min. Immunolabeling was visualized with a mixture of DAB solution.
Counterstaining was carried out with hematoxylin. Samples were
scored as percentage of positive cells.
Cell cytotoxicity assay
The cells were grown in 96-well culture plates,
treated as indicated and cultured for different time periods. Next,
the cells in each well containing 100 µl medium were
incubated with 10 µl Cell Counting Kit-8 (CCK-8) at 37°C for
2 h. The optical density (OD) of each well was then measured at 450
nm using a microplate reader.
Quantitative real-time PCR
Total RNA was isolated and reverse transcribed.
Real-time PCR was then performed using an ABI 7900 System in the
presence of SYBR-Green. The following gene-specific primers were
used: OPN (forward, 5′-CATCACCTGTGCCATACCAGTT-3′ and reverse,
5′-TTG GAAGGGTCTGTGGGGCTA-3′; and β-actin (forward,
5′-TGGCACCCAGCACAATGAA-3′ and reverse, 5′-CTAAG
TCATAGTCCGCCTAGAAGCA-3′). Target sequences were amplified at 95°C
for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1
min. β-actin was used as endogenous normalization control. All
assays were performed in triplicate. The fold change in mRNAs
expression was determined according to the 2−ΔΔCt
method.
Western blot analysis
Cell lysates were extracted using RIPA lysis buffer
containing protease inhibitor cocktail. Protein concentrations were
determined using BCA method. Cell lysates containing 40 µg
of protein were loaded and separated on 10% SDS-PAGE gels and
subsequently transferred to polyvinylidene difluoride membranes
(PVDF). Membranes were blocked in 5% milk solution, incubated at
4°C overnight with following primary antibodies: mouse monoclonal
to OPN (Abcam; 1:500), mouse monoclonal to PI3K (Abcam; 1:1,000),
rabbit polyclonal to phospho-PI3K (Abcam; 1:800), rabbit polyclonal
to AKT (Cell Signaling Technology; 1:800), rabbit polyclonal to
phospho-AKT (Cell Signaling Technology; 1:1,000), rabbit monoclonal
to caspase-3 (Cell Signaling Technology; 1:1,000), rabbit
monoclonal to actin (Santa Cruz Biotechnology; 1:2,000). They were
then washed, and incubated with horseradish peroxidase conjugated
secondary antibody at dilution 1:5,000 for 1 h at room temperature.
Membranes were then washed and developed using ECL substrate.
Densitometry was performed using ImageJ software.
FACScan analysis
The HCC cells were harvested with fresh 0.25%
trypsin solution and washed using PBS. The cells were resuspended
in incubation buffer with Annexin V (1 mg/ml) and propidium iodide
(PI) (1 mg/ml). The cells were analyzed using a Becton-Dickinson
FACSort and data were analyzed using WinMDI software. Cells that
were PI negative and Annexin V negative are considered healthy
cells, PI negative and Annexin V positive cells are considered
apoptotic, and cells that are positive to both PI and Annexin V are
considered necrotic.
Statistical analysis
Each experiment was performed independently at least
three times. Values were expressed as mean ± SD. A two-tailed
Student's t-test was used to estimate intergroup differences if not
otherwise stated. P<0.05 was considered to be statistically
significant.
Results
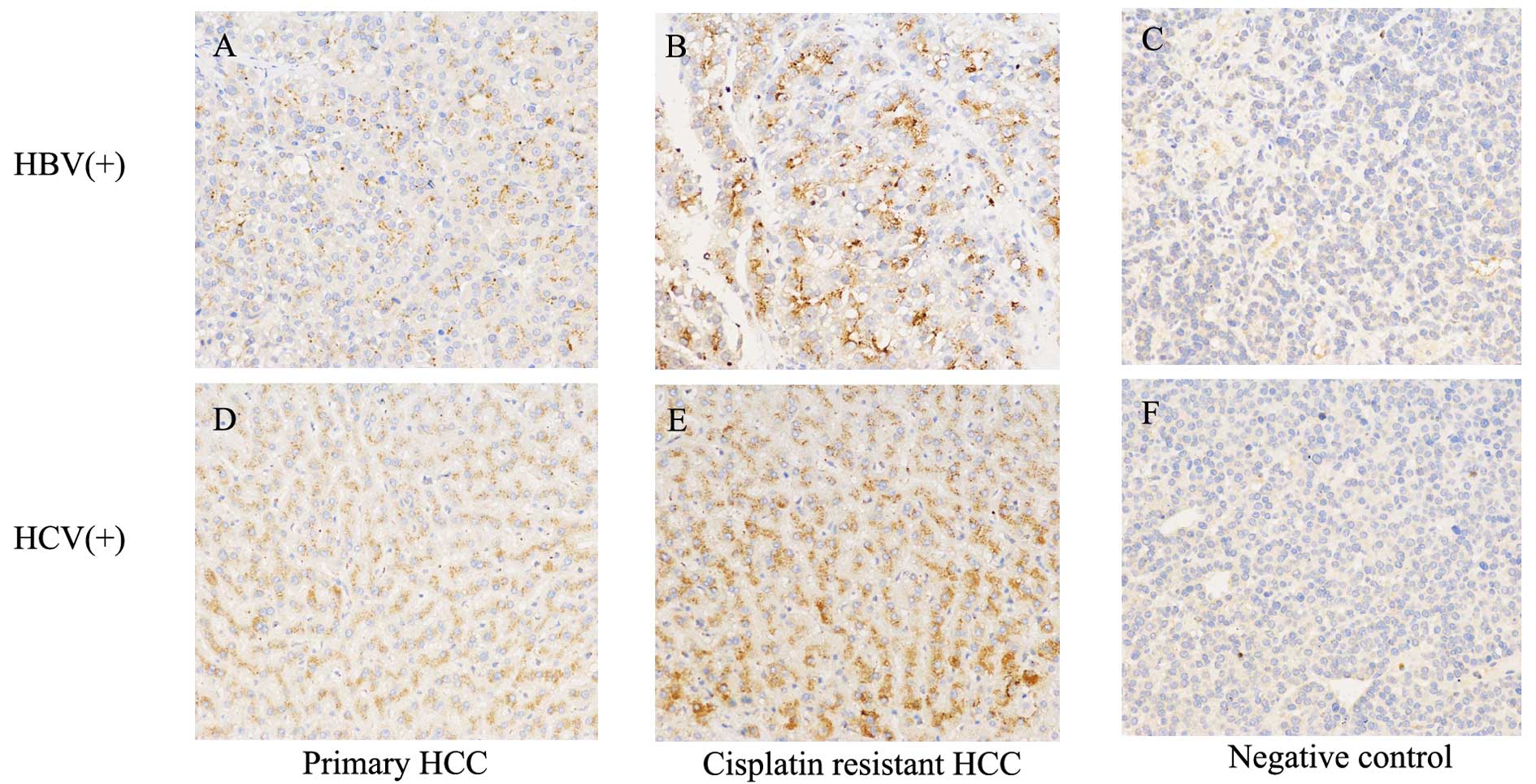
OPN is highly expressed in cisplatin
resistant HCC samples
To study the underlying mechanism for the resistance
of HCC to cisplatin treatment, we collected 8 patients, of whom the
specimens before and after received cisplatin-based chemotherapy
were available. We first focused on identifying the changes in gene
expression after cisplatin-based chemotherapy. We found that OPN
expression levels were significantly increased in HCC patients
after chemotherapy (Fig. 1). The
HCC is frequently associated with hepatitis virus infection in
China, in the present study, 5 of the patients were HBV-associated
HCC, and 3 of them were HCV-related HCC. We found that OPN
expression levels were significantly increased after chemotherapy
regardless of their serum virology status (Fig. 1A and B).
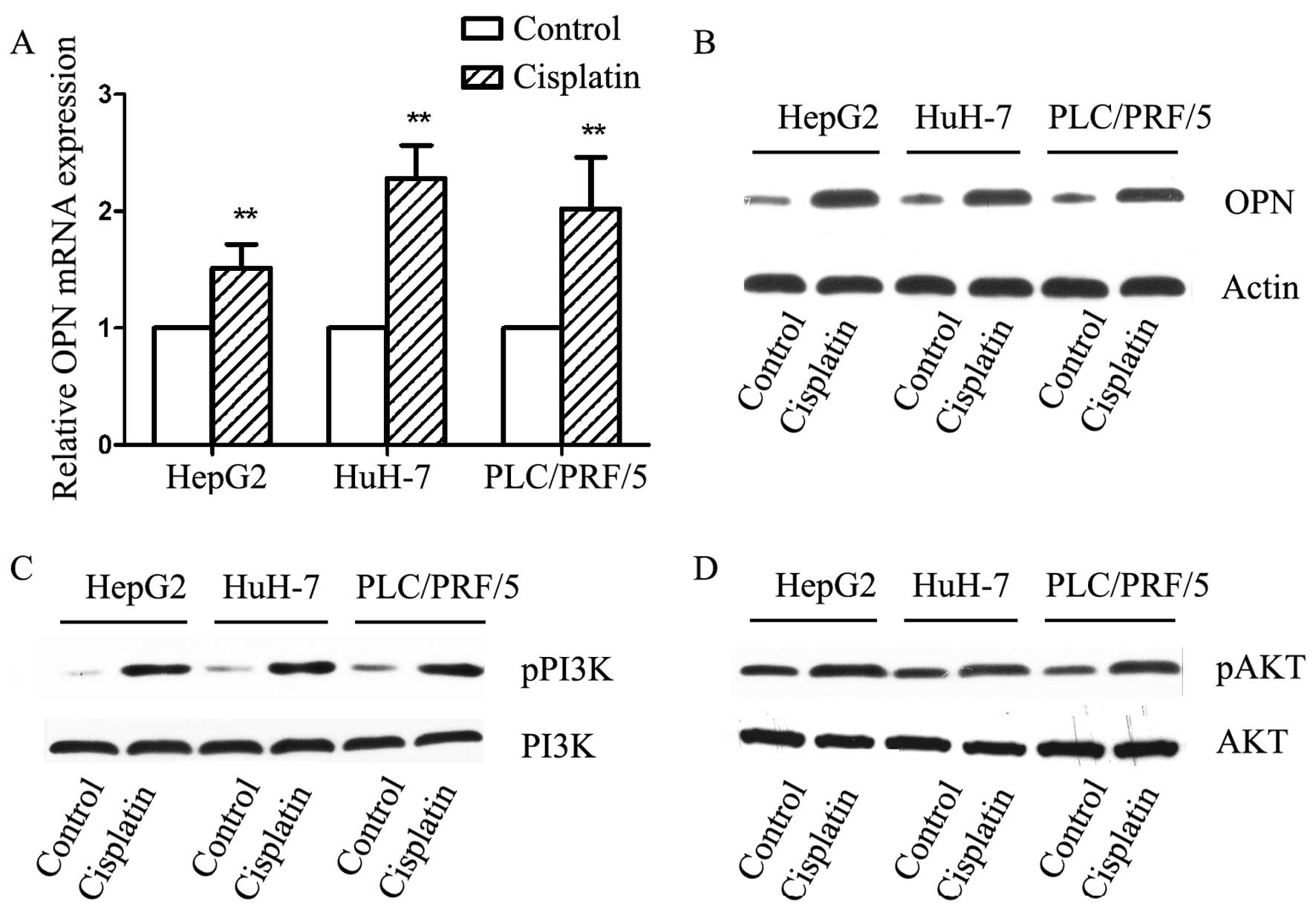
Cisplatin increases osteopotin and
activates PI3K/AKT in HCC cells
We then explored the effect of cisplatin in
vitro, we treated three HCC cell lines with 10 µM
cisplatin for 24 h, which was less toxic in our preliminary study.
mRNA levels were detected by qPCR, we found that in all three cell
lines, OPN expression was significantly elevated after cisplatin
treatment with a average fold-change of 1.94±0.44 (Fig. 2A). We further examined the protein
level of OPN by immunoblotting. In agreement with qPCR data, OPN
expression was statistically increased after treated by cisplatin
(Fig. 2B). The involved signaling
pathway in cisplatin treated HCC cells was investigated examining
the phosphorylation of PI3K/AKT. PI3K activation was observed after
exposure to cisplatin, which was consistent in all three cell lines
(Fig. 2C). The phosphorylation of
AKT was also found after exposure to cisplatin (Fig. 2D).
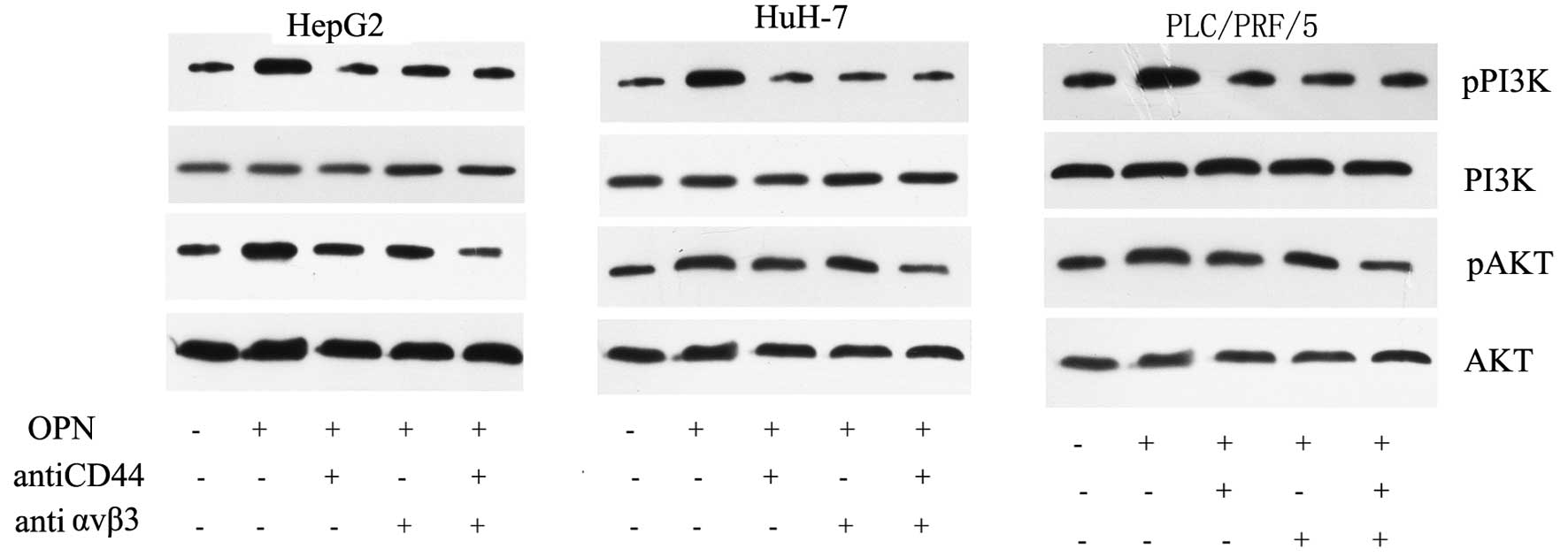
Osteopontin activates PI3K/AKT through
CD44 and αvβ3
We further examined whether the activation of
PI3K/Akt pathways was induced by OPN. We treated HCC cells with 0.5
µM OPN for 4 h. To inhibit the combination of OPN with its
receptors, we incubated the cells with either anti-CD44 (20
µg/ml) or anti-αvβ3 antibody (20 µg/ml). As shown in
Fig. 4, OPN strongly activated the
phosphorylation of PI3K and Akt in all three HCC cell lines. In
contrast, anti-CD44 and anti-αvβ3 antibody reversed the activation
of the PI3K/AKT signaling pathway. While single antibody could
partially reduce the phosphorylation of PI3K/AKT, the combination
of the two antibodies showed a synergistic effect and could largely
reverse the effect (Fig. 3). These
data indicate that both the CD44 and αvβ3 participate in the
OPN-induced PI3K/AKT pathway activation in HCC cells.
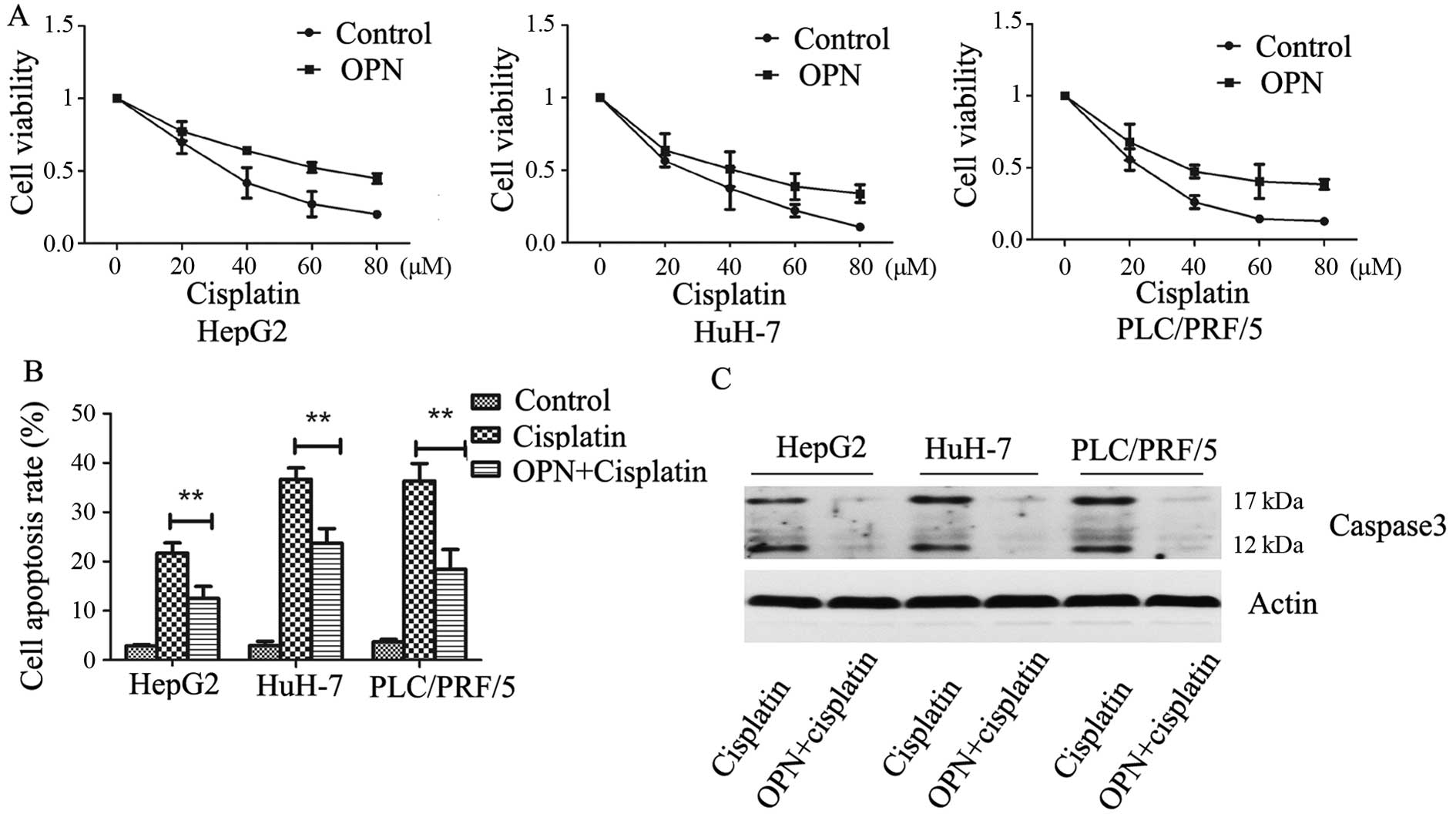
Osteopontin enhances resistance to
cisplatin in HCC
To study the role of OPN in the resistance of HCC
cells to cisplatin, the human hepatoma cell lines were treated with
increasing concentrations of cisplatin plus 0.5 µM OPN and
cell toxicity was assayed using cell counting reagent. We found
that OPN treatment markedly increased cell resistance to cisplatin
compared with PBS control in HepG2, HuH7 and PLC/PRF/5 cells
(Fig. 4A). To induce HCC cell
apoptosis, the cells were treated with 40 µM cisplatin and
0.5 µM OPN. The apoptosis rate was then analyzed by flow
cytometry, the data showed that OPN strongly enhanced the
resistance of HCC cells to cisplatin (Fig. 4B). We also detected the expression
of activated caspase-3, the data showed that OPN significantly
inhibited cisplatin-induced caspase-3 cleavage (Fig. 4C). These data suggest that OPN
inhibited cisplatin-induced cell apoptosis.
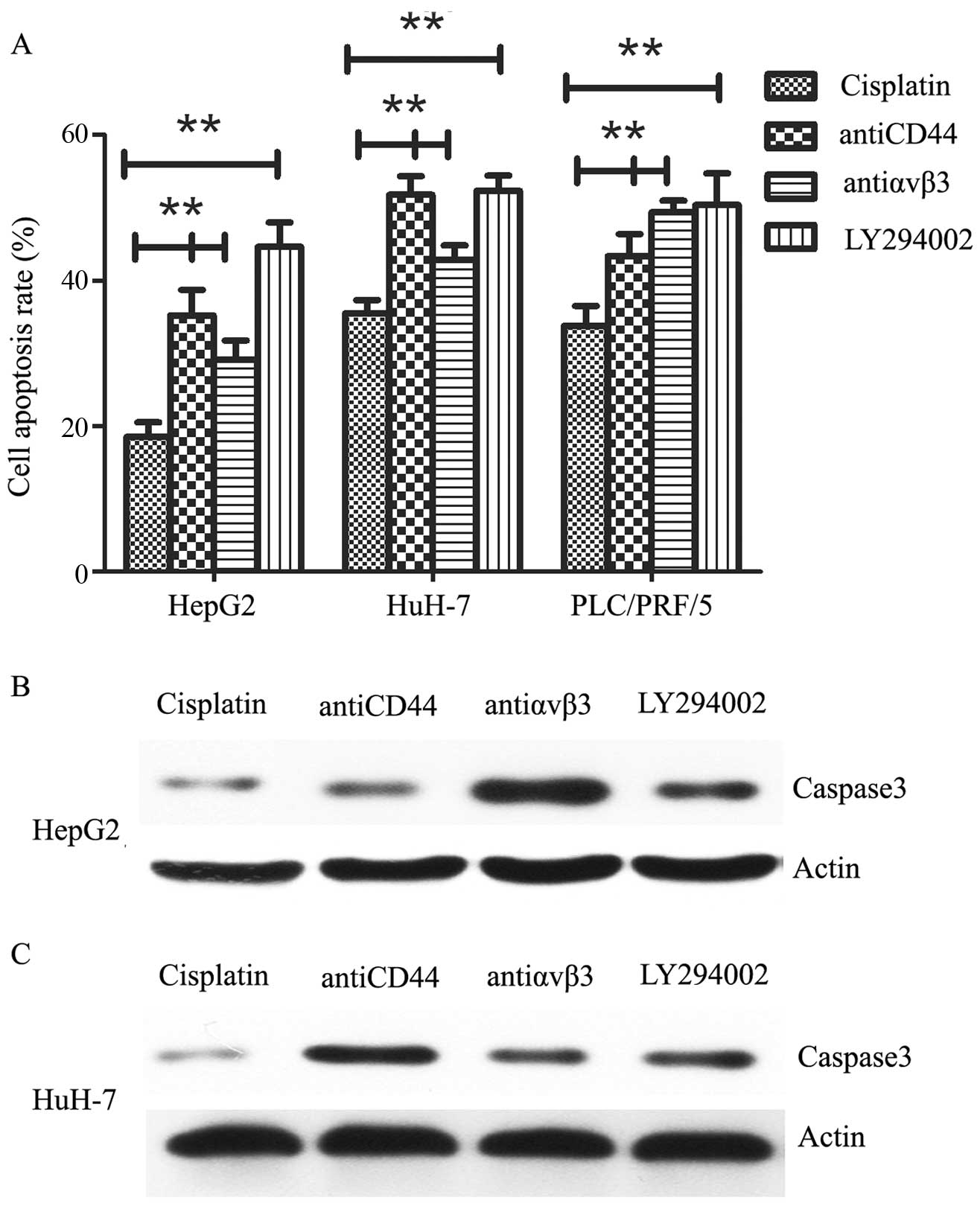
Blockage of OPN pathway sensitizes HCC
cells to cisplatin treatment
To determine whether OPN-induced cisplatin
resistance could be reversed, we pre-treated the cells with
anti-CD44 (20 µg/ml), anti-αvβ3 (20 µg/ml) or
LY294002 (10 µM) for 24 h, followed by 40 µM
cisplatin for 24 h. We found that cisplatin-induced cell apoptosis
was significantly activated by targeting of OPN receptors. In
consistent, cell apoptosis was also activated by blockage of
PI3K/AKT signaling pathway (Fig.
5A). In addition, we tested caspase-3 expression by western
blot analysis, the expression of activated caspase-3 was also shown
to be upregulated after OPN pathway inhibiton (Fig. 5B). These data indicate that OPN
pathway might have an important function in cisplatin resistance in
HCC cells.
Discussion
Systematic chemotherapy plays important roles in HCC
treatment especially for patients with advanced HCC (5). Although cisplatin is a common
therapeutic agent used for chemotherapy in HCC patients, its
curative effect is significantly limited due to chemoresistance of
HCC (36). Much effort has been
exerted in analyzing the role of OPN in the development and
progression in a variety of malignancies (23,25,27,29).
In the present study, we performed a retrospective analysis on the
OPN gene expression before and after cisplatin-based chemotherapy
in HCC patients. We found that OPN was upregulated in the HCC
patients who received cisplatin treatment. The present study
included both HBV and HCV-related HCC, and data showed the
upregulation of OPN after cisplatin treatment was independent of
virus infection status. In a study that enrolled 131 patients with
HCC, plasma OPN was found to be significantly elevated compared to
healthy subjects (32). It is also
reported that OPN level in plasma was directly correlated with the
tumor number. Elevated plasma level of OPN is thus regarded as a
potential prognostic biomarker as well as a marker of early HCC
detection (33). It has also been
shown that oncogenic activation of the OPN is common in HCC, and
overexpression of OPN is closely correlated with intrahepatic
metastasis, early recurrence and a worse prognosis (37–40).
Our data support the idea that OPN might participate in the
chemoresistance of HCC.
To confirm the above hypothesis, we explored the
effect of cisplatin on OPN expression in vitro. In agreement
with patient-derived samples, cisplatin treatment for 24 h resulted
in increased OPN expression in human HCC cells both at mRNA and
protein levels. In parallel, we found that PI3K/AKT signaling
pathway was activated after exposure to cisplatin. PI3K/Akt pathway
is the most extensively studied and has been demonstrated to be a
critical mechanism of drug resistance in HCC cells (41–43).
We then ask whether OPN could activate PI3K/AKT pathway in
vitro, we showed here that phosphorylation of PI3K/AKT occurred
as early as 4 h after OPN incubation. OPN binds to the family of
αvβ integrins, and the cell-surface adhesion molecule CD44, to
initiate inhibition of cellular signal (18). We found that single antibody
inhibitor partially reduced the phosphorylation of PI3K/AKT, the
combination of the two inhibitors completely reversed the effect. A
previous study showed that OPN regulates HCC cell behaviour in a
CD44-dependent manner (34). Our
data indicated that OPN induced PI3K/AKT signaling pathway was
dependent on CD44 and αvβ3.
Elevated expression of OPN has been associated with
tumor invasion, progression or metastasis in multiple cancers. Pang
et al (25) demonstrated
that OPN expression is critical for breast cancer growth and
knockdown of OPN enhanced MDA-MB-231 breast cancer cells apoptosis.
Research has also shown the abnormal expressions of OPN in
chemo-resistant cancer cells. It is reported that acquired
cisplatin resistance in the small cell lung cancer line is also
associated with OPN expression, which involved the maintaining of
the anti-apoptotic bcl-2 protein (44). Consistent with these reports, we
found that incubation with OPN inhibited cisplatin-induced cell
apoptosis. Blockage of OPN by antibody targeted inhibition promoted
cisplatin-induced apoptosis. Although high levels of the OPN
pathway were responsible for mediating the acquired resistance to
cisplatin, the downstream molecules used by OPN is still unclear.
PI3K/Akt signaling is an aberrant pathway in HCC, and this pathway
may be the critical target for therapeutic design (41,43),
we therefore tested whether abnormal activation of PI3K/AKT
signaling mediated by OPN represents a novel pathway regulating
chemoresistance. Consistently, cell apoptosis was also activated by
blockage of PI3K/AKT signaling pathway.
In conclusion, our results demonstrated that OPN
functions as a chemoresistant gene in HCC, which involves
activation of PI3K/AKT pathway. Our findings suggest that the
combination of cisplatin treatment and OPN pathway blockage could
be a therapeutic strategy for HCC. However, further work is needed
to determine the extrapolation of in vitro results to an
in vivo situation.
References
|
1
|
Venook AP, Papandreou C, Furuse J and de
Guevara LL: The incidence and epidemiology of hepatocellular
carcinoma: A global and regional perspective. Oncologist. 15(Suppl
4): 5–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eggert T, McGlynn KA, Duffy A, Manns MP,
Greten TF and Altekruse SF: Fibrolamellar hepatocellular carcinoma
in the USA, 2000-2010: A detailed report on frequency, treatment
and outcome based on the Surveillance, Epidemiology, and End
Results database. United European Gastroenterol J. 1:351–357. 2013.
View Article : Google Scholar
|
|
3
|
Jaka H, Mshana SE, Rambau PF, Masalu N,
Chalya PL and Kalluvya SE: Hepatocellular carcinoma:
Clinicopathological profile and challenges of management in a
resource-limited setting. World J Surg Oncol. 12:2462014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Naugler WE, Alsina AE, Frenette CT,
Rossaro L and Sellers MT: Building the multidisciplinary team for
management of patients with hepatocellular carcinoma. Clin
Gastroenterol Hepatol. 13:827–835. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Crissien AM and Frenette C: Current
management of hepatocellular carcinoma. Gastroenterol Hepatol (NY).
10:153–161. 2014.
|
|
6
|
Knudsen ES, Gopal P and Singal AG: The
changing landscape of hepatocellular carcinoma: Etiology, genetics,
and therapy. Am J Pathol. 184:574–583. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kew MC: The role of cirrhosis in the
etiology of hepatocellular carcinoma. J Gastrointest Cancer.
45:12–21. 2014. View Article : Google Scholar
|
|
8
|
Tornai I: Role of environmental factors in
the etiology of hepatocellular carcinoma. Orv Hetil. 151:1132–1136.
2010.In Hungarian. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Senkerikova R, Frankova S, Sperl J,
Oliverius M, Kieslichova E, Filipova H, Kautznerova D, Honsova E,
Trunecka P and Spicak J: Incidental hepatocellular carcinoma: Risk
factors and long-term outcome after liver transplantation.
Transplant Proc. 46:1426–1429. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kamiyama T, Tahara M, Nakanishi K, Yokoo
H, Kamachi H, Kakisaka T, Tsuruga Y, Matsushita M and Todo S:
Long-term outcome of laparoscopic hepatectomy in patients with
hepatocellular carcinoma. Hepatogastroenterology. 61:405–409.
2014.PubMed/NCBI
|
|
11
|
Takaki S, Sakaguchi H, Anai H, Tanaka T,
Yamamoto K, Morimoto K, Nishiofuku H, Inoue M, Sueyoshi S, Nagata
T, et al: Long-term outcome of transcatheter subsegmental and
segmental arterial chemoemobolization using lipiodol for
hepatocellular carcinoma. Cardiovasc Intervent Radiol. 35:544–554.
2012. View Article : Google Scholar
|
|
12
|
Otsuji K, Takai K, Nishigaki Y, Shimizu S,
Hayashi H, Imai K, Suzuki Y, Hanai T, Ideta T, Miyazaki T, et al:
Efficacy and safety of cisplatin versus miriplatin in transcatheter
arterial chemoembolization and transarterial infusion chemotherapy
for hepatocellular carcinoma: A randomized controlled trial.
Hepatol Res. 45:514–522. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ishikawa T, Kubota T, Abe S, Watanabe Y,
Sugano T, Inoue R, Iwanaga A, Seki K, Honma T and Yoshida T:
Hepatic arterial infusion chemotherapy with cisplatin before
radical local treatment of early hepatocellular carcinoma (JIS
score 0/1) improves survival. Ann Oncol. 25:1379–1384. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Terazawa T, Kondo S, Hosoi H, Morizane C,
Shimizu S, Mitsunaga S, Ikeda M, Ueno H and Okusaka T:
Transarterial infusion chemotherapy with cisplatin plus S-1 for
hepatocellular carcinoma treatment: A phase I trial. BMC Cancer.
14:3012014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
An J, Wang X, Guo P, Zhong Y, Zhang X and
Yu Z: Hexabromocyclododecane and polychlorinated biphenyls increase
resistance of hepatocellular carcinoma cells to cisplatin through
the phosphatidylinositol 3-kinase/protein kinase B pathway. Toxicol
Lett. 229:265–272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fang D, Guo Y, Zhu Z and Chen W: Silence
of p15 expression by RNAi enhances cisplatin resistance in
hepatocellular carcinoma cells. Bosn J Basic Med Sci. 12:4–9.
2012.PubMed/NCBI
|
|
17
|
Kim Y, Jang M, Lim S, Won H, Yoon KS, Park
JH, Kim HJ, Kim BH, Park WS, Ha J, et al: Role of cyclophilin B in
tumorigenesis and cisplatin resistance in hepatocellular carcinoma
in humans. Hepatology. 54:1661–1678. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Butler WT: Structural and functional
domains of osteopontin. Ann N Y Acad Sci. 760:6–11. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dai J, Cao Z, Kang Y, Fan K, Ji G, Yang H,
Wang H, Gao J, Wang H and Guo Y: A functional motif QLYxxYP is
essential for osteopontin induced T lymphocyte activation and
migration. Biochem Biophys Res Commun. 380:715–720. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cao Z, Dai J, Fan K, Wang H, Ji G, Li B,
Zhang D, Hou S, Qian W, Zhao J, et al: A novel functional motif of
osteopontin for human lymphocyte migration and survival. Mol
Immunol. 45:3683–3692. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kumar S, Sharma P, Kumar D, Chakraborty G,
Gorain M and Kundu GC: Functional characterization of stromal
osteopontin in melanoma progression and metastasis. PLoS One.
8:e691162013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu M, Liu Q, Yi K, Wu L and Tan X: Effects
of osteopontin on functional activity of late endothelial
progenitor cells. J Cell Biochem. 112:1730–1736. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qiu Y, Hu Y, Zhang ZY, Ye L, Xu FH,
Schneider ME, Ma XL, Du YX, Zuo XB, Zhou FS, et al: Genetic
association of osteopontin (OPN) and its receptor CD44 genes with
susceptibility to Chinese gastric cancer patients. J Cancer Res
Clin Oncol. 140:2143–2156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu J, Liu Q, Wan Y, Zhao Z, Yu H, Luo H
and Tang Z: Osteopontin promotes the progression of gastric cancer
through the NF-κB pathway regulated by the MAPK and PI3K. Int J
Oncol. 45:282–290. 2014.PubMed/NCBI
|
|
25
|
Pang H, Lu H, Song H, Meng Q, Zhao Y, Liu
N, Lan F, Liu Y, Yan S, Dong X, et al: Prognostic values of
osteopontin-c, E-cadherin and β-catenin in breast cancer. Cancer
Epidemiol. 37:985–992. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang H, Guo M, Chen JH, Wang Z, Du XF,
Liu PX and Li WH: Osteopontin knockdown inhibits αv,β
integrin-induced cell migration and invasion and promotes apoptosis
of breast cancer cells by inducing autophagy and inactivating the
PI3K/Akt/mTOR pathway. Cell Physiol Biochem. 33:991–1002. 2014.
View Article : Google Scholar
|
|
27
|
Hsieh IS, Huang WH, Liou HC, Chuang WJ,
Yang RS and Fu WM: Upregulation of drug transporter expression by
osteopontin in prostate cancer cells. Mol Pharmacol. 83:968–977.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thoms JW, Dal Pra A, Anborgh PH,
Christensen E, Fleshner N, Menard C, Chadwick K, Milosevic M,
Catton C, Pintilie M, et al: Plasma osteopontin as a biomarker of
prostate cancer aggression: Relationship to risk category and
treatment response. Br J Cancer. 107:840–846. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang T, Zhang DM, Zhao D, Hou XM, Liu XJ,
Ling XL and Ma SC: The prognostic value of osteopontin expression
in non-small cell lung cancer: A meta-analysis. J Mol Histol.
45:533–540. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu TT, Han ZG, Shan L, Tao J, Zhang T,
Yuan SF and Shen HL: Expression of osteopontin in non-small cell
lung cancer and correlative relation with microvascular density.
Asian Pac J Cancer Prev. 15:29–32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
El-Din Bessa SS, Elwan NM, Suliman GA and
El-Shourbagy SH: Clinical significance of plasma osteopontin level
in Egyptian patients with hepatitis C virus-related hepatocellular
carcinoma. Arch Med Res. 41:541–547. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shang S, Plymoth A, Ge S, Feng Z, Rosen
HR, Sangrajrang S, Hainaut P, Marrero JA and Beretta L:
Identification of osteopontin as a novel marker for early
hepatocellular carcinoma. Hepatology. 55:483–490. 2012. View Article : Google Scholar
|
|
33
|
Kim SH, Chung YH, Yang SH, Kim JA, Jang
MK, Kim SE, Lee D, Lee SH, Lee D, Kim KM, et al: Prognostic value
of serum osteopontin in hepatocellular carcinoma patients treated
with transarterial chemoembolization. Korean J Hepatol. 15:320–330.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Phillips RJ, Helbig KJ, Van der Hoek KH,
Seth D and Beard MR: Osteopontin increases hepatocellular carcinoma
cell growth in a CD44 dependant manner. World J Gastroenterol.
18:3389–3399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu MC, Lee YS, Lin SE, Wu HY, Chen TC, Lee
WC, Chen MF and Tsai CN: Recurrence and poor prognosis following
resection of small hepatitis B-related hepatocellular carcinoma
lesions are associated with aberrant tumor expression profiles of
glypican 3 and osteopontin. Ann Surg Oncol. 19(Suppl 3): S455–S463.
2012. View Article : Google Scholar
|
|
36
|
Daniele B: Current issues in the
management of hepatocellular carcinoma. Ann Oncol. 24(Suppl 2):
ii52013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Qin L: Osteopontin is a promoter for
hepatocellular carcinoma metastasis: A summary of 10 years of
studies. Front Med. 8:24–32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang R, Pan X, Huang Z, Weber GF and
Zhang G: Osteopontin enhances the expression and activity of MMP-2
via the SDF-1/CXCR4 axis in hepatocellular carcinoma cell lines.
PLoS One. 6:e238312011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lin F, Li Y, Cao J, Fan S, Wen J, Zhu G,
Du H and Liang Y: Overexpression of osteopontin in hepatocellular
carcinoma and its relationships with metastasis, invasion of tumor
cells. Mol Biol Rep. 38:5205–5210. 2011. View Article : Google Scholar
|
|
40
|
Chen RX, Xia YH, Xue TC, Zhang H and Ye
SL: Down-regulation of osteopontin inhibits metastasis of
hepatocellular carcinoma cells via a mechanism involving MMP-2 and
uPA. Oncol Rep. 25:803–808. 2011.
|
|
41
|
Cheng L, Luo S, Jin C, Ma H, Zhou H and
Jia L: FUT family mediates the multidrug resistance of human
hepatocellular carcinoma via the PI3K/Akt signaling pathway. Cell
Death Dis. 4:e9232013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang XJ, Feng CW and Li M: ADAM17 mediates
hypoxia-induced drug resistance in hepatocellular carcinoma cells
through activation of EGFR/PI3K/Akt pathway. Mol Cell Biochem.
380:57–66. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li QL, Gu FM, Wang Z, Jiang JH, Yao LQ,
Tan CJ, Huang XY, Ke AW, Dai Z, Fan J, et al: Activation of
PI3K/AKT and MAPK pathway through a PDGFRβ-dependent feedback loop
is involved in rapamycin resistance in hepatocellular carcinoma.
PLoS One. 7:e333792012. View Article : Google Scholar
|
|
44
|
Gu T, Ohashi R, Cui R, Tajima K, Yoshioka
M, Iwakami S, Sasaki S, Shinohara A, Matsukawa T, Kobayashi J, et
al: Osteopontin is involved in the development of acquired
chemo-resistance of cisplatin in small cell lung cancer. Lung
Cancer. 66:176–183. 2009. View Article : Google Scholar : PubMed/NCBI
|