Introduction
Breast cancer is the most common type of cancer and
the leading cause of cancer-related death in women (1). Approximately 70% of breast cancer
patients are positive for estrogen receptor (ER) and these patients
are suitable for anti-estrogen therapy. ER-negative breast cancer
is often more malignant and aggressive than ER-positive breast
cancer (2,3). In addition, overexpression of
epidermal growth factor receptor (EGFR) or human EGFR-2 (HER2) is
well correlated to recurrent and metastatic breast cancers
(4,5). These receptors and their down-stream
signaling pathways are widely appreciated as the therapeutic
targets for breast cancer.
Matrix metalloproteinases (MMPs) are a family of
zinc-dependent proteases which are degrading all components of
extracellular matrix as well as cell surface molecules, leading to
regulating a variety of biological responses including cell
migration, invasion, proliferation, apoptosis and angiogenesis
(6-9). A number of MMPs are highly expressed
in cancer tissue from breast cancer patients, and these expression
patterns are closely associated with aggressive phenotypes and poor
survival (10). Although early
detection methods and multimodal approaches for breast cancer
treatment have been made, there has been only modest progress in
improving clinical outcomes for women with metastases. Therefore,
detailed understanding of the biology and its molecular mechanism
underlying the progression of the disease may provide insights into
therapeutic targets and strategies for the treatment of breast
cancer.
Ginger (Zingiber officinale Roscoe,
Zingiberaceae) is a natural dietary rhizome that is widely used as
a traditional medicinal herb as well as a flavoring agent. Ginger
has various bioactive components such as gingerols, shogaols,
paradols and zingerone, indicating the pharmacological roles in
mediating anti-inflammatory and antitumor activities (11-14).
Among the bioactive ingredients from ginger, 6-gingerol and
6-shogaol have been extensively reported to exert antitumor
activities in a variety of cancers by inhibition of cell
proliferation, migration and invasion or induction of apoptosis
(15-20). 10-gingerol, one of the main phenolic
compounds isolated from ginger, has been reported to possess
antitumor activity against ovarian, colon, lung and prostate cancer
cells by inhibition of cell proliferation or induction of apoptosis
(21,22), however, the effects and molecular
mechanisms of 10-gingerol on breast cancer cell growth and
progression are poorly understood. In the present study, we
investigated the regulatory effects and signaling pathways of
10-gingerol on cell proliferation and invasion in MDA-MB-231 breast
cancer cells.
Materials and methods
Cell culture conditions
Human breast cancer cells (MDA-MB-231) from American
Type Culture Collection (Manassas, VA, USA) were grown in 10% fetal
bovine serum-Dulbecco's modified Eagle's medium (FBS-DMEM) (HyClone
Laboratories, Logan, UT, USA).
Preparation of ginger extract and
isolation of 10-gingerol
The dried Zingiber officinale (Z.
officinale) was purchased from Gyeong-dong Oriental Medicine
Market (Seoul, Republic of Korea), identified by Professor Joa Sub
Oh (College of Pharmacy, Dankook University), and deposited at the
herbarium of Gyeonggi Biocenter (Suwon, Republic of Korea). One
thousand two hundred grams of Z. officinale were extracted
three times with 15 liters of ethanol at room temperature for 24 h.
The extract was concentrated, suspended in water, and then
partitioned three times with 1.5 liters of n-hexane. The
n-hexane extract (24 g) was subjected to silica gel column
chromatography (Kieselgel 60, 70-230 mesh, 9×25 cm). Among eight
fractions eluted from column chromatography, the sixth fraction
(0.4 g) was further separated by semi-preparative HPLC (YMC-Pack
ODS A column, 250×20 mm I.D.) eluting with acetonitrile-water
(acetonitrile gradient from 50 to 100%) at a flow speed of 20
ml/min to yield
(S)-5-hydroxy-1-(4-hydroxy-3-methoxyphenyl)-3-tetrade canone
(10-gingerol, 22.5 mg). 1H- and 13C-NMR
spectra were recorded on a Varian 500 MHz NMR spectrometer (Bruker,
Billerica, MA, USA).
Spectrometric analysis of
10-gingerol
1H-NMR (CDCl3, 500 MHz) δ 6.83
(1H, d, J=8.0 Hz, H-5′), 6.69 (1H, d, J=2.0 Hz,
H-2′), 6.67 (1H, dd, J=8.0, 2.0 Hz, H-6′), 4.03 (1H, m,
H-5), 3.88 (3H, s, OCH3), 2.84 (2H, brd, J=7.5
Hz, H-1), 2.75 (2H, brd, J=7.5 Hz, H-2), 2.58 (1H, dd,
J=17.5, 3.0 Hz, H-4b), 2.50 (1H, dd, J=17.0, 9.0 Hz,
H-4a), 1.49 (2H, m, H-6), 1.27-1.51 (14H, m, H-7-H-13), 0.89 (3H,
t, J=7.0 Hz, H-14); 13C-NMR (CDCl3, 125 MHz) δ
211.5 (C-3), 146.4 (C-3′), 144.0 (C-4′), 132.6 (C-1′), 120.7
(C-6′), 114.4 (C-5′), 111.0 (C-2′), 67.7 (C-5), 55.9
(OCH3), 49.4 (C-4), 45.4 (C-2), 36.5 (C-6), 31.9 (C-1),
29.6 (C-9), 29.5 (C-8), 29.3 (C-10), 29.28 (C-11), 25.5 (C-7), 22.7
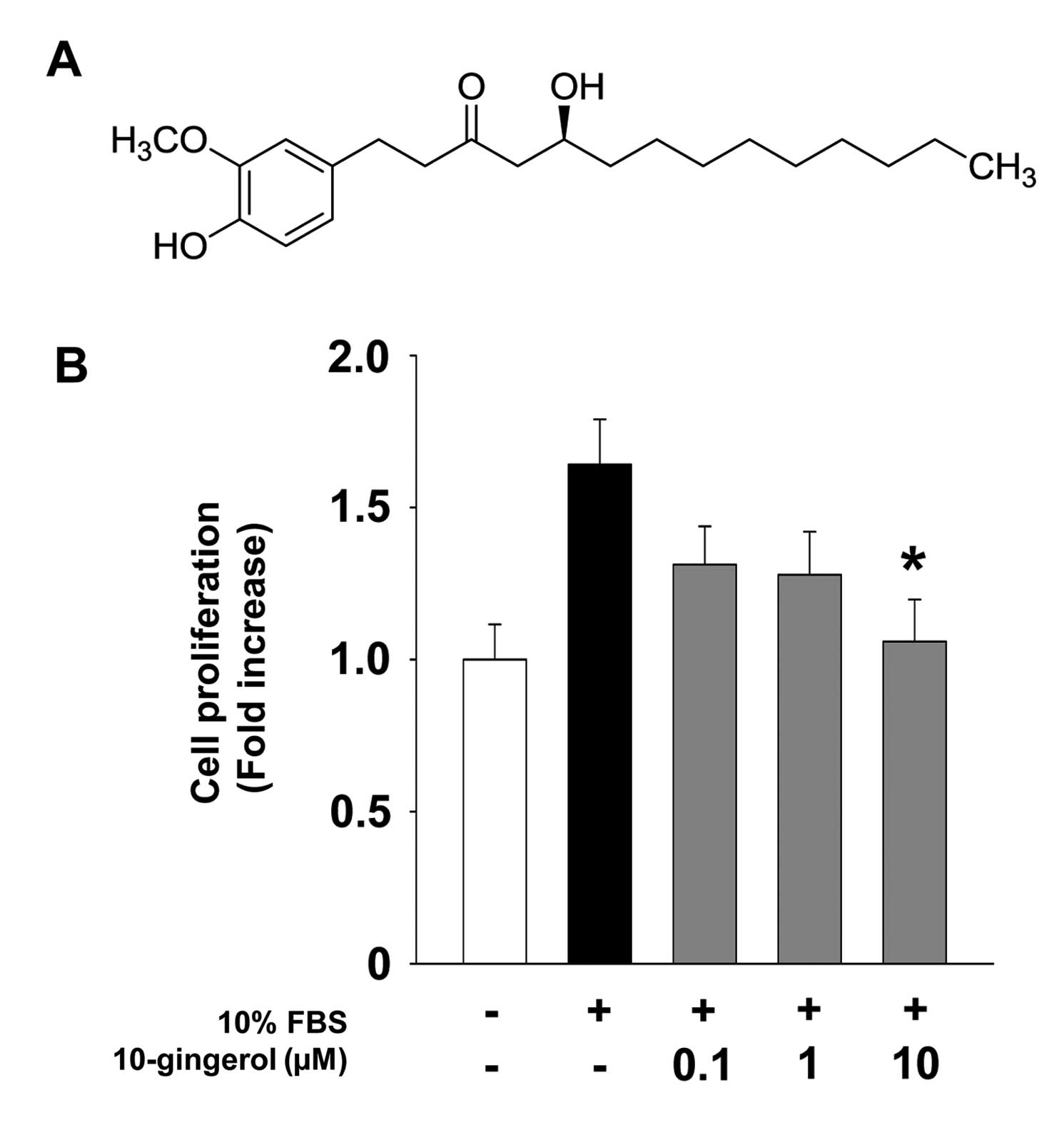
(C-13), 14.1 (C-14). The structure of 10-gingerol is presented in
Fig. 1A.
Reagents
The following pharmacological agents and antibodies
were purchased from commercial sources: LY294002 (Merck Millipore,
Billerica, MA, USA); SB203580 (Cayman Chemical, Ann Arbor, MI,
USA); anti-phospho-extracellular signal-regulated kinase (ERK)
(T202/Y204), anti-phospho-Akt (S473),
anti-phospho-p70S6K (T421/S424) and anti-phospho-p38
mitogen-activated protein kinase (p38MAPK) (T180/Y182)
(Cell Signaling, Beverly, MA, USA); anti-EGFR, anti-ERK, anti-Akt,
anti-p70S6K, anti-p38MAPK, anti-Cdk4,
anti-Cdk2, anti-cyclin D, anti-cyclin E, anti-actin antibodies, and
mouse and rabbit IgG-horseradish peroxidase conjugates (Santa Cruz
Biotechnology, Santa Cruz, CA, USA).
Cell proliferation assay
Subconfluent MDA-MB-231 cells, plated on 6-well
plates (5×104 cells/well, BD Biosciences, Bedford, MA,
USA), were serum-starved for 24 h in basal DMEM to synchronize
cells in G1/G0 phase of cell cycle,
pretreated with 10-gingerol at different concentrations (0.1-10
μM) in the presence or absence of LY294002 (10 μM) or
SB203580 (5 μM) for 30 min, and further incubated with 10%
FBS for 24 h. Following culture for 24 h, the number of cells was
quantified using trypan blue exclusion method as described
previously (23,24). The results from triplicate
determinations (mean ± standard deviation) are presented as the
fold-increase of untreated controls.
Cell cycle analysis
Serum-starved MDA-MB-231 cells, plated on 6-well
plates (5×104 cells/well), were pretreated with
10-gingerol (10 μM) for 30 min, followed by 10% FBS for 24
h. Cells were harvested with trypsin-EDTA, rinsed with phosphate
buffered saline (PBS), and then fixed with ice-cold 70% ethanol for
3 h. After washing with PBS, cells were stained with Muse™ cell
cycle reagent. The profile of cells in the
G1/G0, S and G2/M phases of the
cell cycle was analyzed with a Muse™ cell analyzer (Merck
Millipore) (25).
Western blot analysis
Serum-starved cells in 100 mm dishes (BD
Biosciences) were incubated for 15 min or 24 h in 10% FBS in the
presence or absence of 10-gingerol. Cells were rinsed twice with
ice-cold PBS and lysed by incubation in 50 mM Tris-HCl (pH 7.4),
150 mM NaCl, 10% glycerol, 1% Triton X-100, 1 mM EDTA, 100
μg/ml 4-(2-amino-ethyl) benzenesulfonyl fluoride, 10
μg/ml aprotinin, 1 μg/ml pepstatin A, 0.5
μg/ml leupeptin, 80 mM β-glycerophosphate, 25 mM sodium
fluoride and 1 mM sodium orthovanadate for 30 min at 4°C. Cell
lysates were clarified at 13,000 x g for 20 min at 4°C, and the
supernatants were subjected to western blot analysis as described
previously (26,27). Bands of interest were integrated and
quantified by the use of National Institutes of Health (NIH) ImageJ
version 1.34s software.
Invasion assay
The upper side of the Transwell insert (Costar, 6.5
mm diameter insert, 8 μm pore size) (Corning Inc., Corning,
NY, USA) was coated with 50 μl of 1 mg/ml Matrigel (BD
Biosciences) diluted in serum-free DMEM at 37°C. Aliquots (100
μl) of MDA-MB-231 cells (6×105 cells/ml)
resuspended in serum-free DMEM were added to the upper compartment
of the Matrigel-coated Transwell and 600 μl of serum-free
DMEM were added to the lower compartment. After serum starvation
for 2 h, MDA-MB-231 cells were pretreated with 10-gingerol (10
μM), LY294002 (10 μM) or SB203580 (5 μM) for
30 min, followed by serum stimulation for 14 h. The inserts were
fixed with methanol and using a cotton-tipped swab the non-invasive
cells were removed from the top of the membrane. After staining
with 0.04% Giemsa solution (Sigma-Aldrich, St. Louis, MO, USA), the
number of invasive cells was determined from six different fields
using objective magnification, ×200 (28).
Zymogram analysis
Activities of MMPs were measured by zymography
(29,30). Aliquots of conditioned medium were
diluted in sample buffer, and applied to 10% polyacryl-amide gels
containing 1 mg/ml gelatin (Sigma-Aldrich) as a substrate. After
electrophoresis, the gels were incubated in 2.5% Triton X-100 for 1
h to remove SDS and allow re-natu-ralization of MMPs, and further
incubated in developing buffer containing 50 mM Tris-HCl (pH 7.5),
10 mM CaCl2, and 150 mM NaCl for 15 h at 37°C. The gels
were stained with 0.5% Coomassie Brilliant Blue R-250 in 30%
methanol-10% acetic acid for 2 h and followed by destaining with
30% methanol-10% acetic acid. Gelatinolytic activities were
detected as unstained bands against the background of the Coomassie
Blue-stained gelatin.
Statistical analysis
Statistical analysis was performed using Student's
t-test and was based on at least three different experiments. The
results were considered to be statistically significant at
P<0.05.
Results
10-Gingerol inhibits cell proliferation
via downregulation of cell cycle regulatory proteins
We first investigated the effect of 10-gingerol on
cell proliferation in ER-negative MDA-MB-231 breast cancer cells.
10-gingerol treatment inhibited mitogen-stimulated cell
proliferation in a dose-dependent manner (Fig. 1B), and did not alter cell viability
at the highest concentration used in this study (data not shown),
indicating that 10-gingerol-mediated inhibition of cell
proliferation is not mediated by the induction of apoptosis or
cytotoxicity. In addition, our initial experiments indicate that
10-gingerol markedly inhibited ER-positive MCF-7 breast cancer cell
proliferation to levels similar to that observed in MDA-MB-231
cells (data not shown). These findings demonstrate the
anti-proliferative activity of 10-gingerol in breast cancer cells,
independently of ER expression status. We next examined the effect
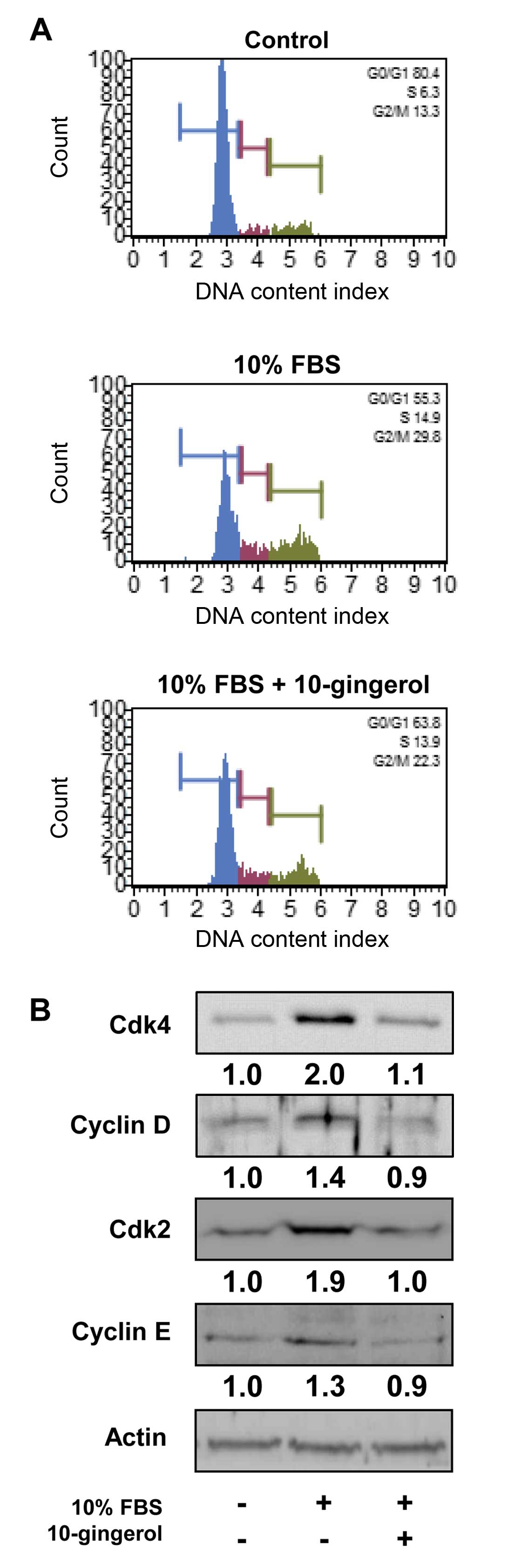
of 10-gingerol on the cell cycle by DNA content analysis (Fig. 2A). Mitogenic stimulation for 24 h
increased the percentage of cells in S phase (6.3 vs. 14.9%) and
G2/M phase (13.3 vs. 29.8%), and simultaneously
decreased the percentage of cells in G1 phase (80.4 vs.
55.3%), compared with untreated controls. However, 10-gingerol
prevented the increase in S phase (14.9 vs. 13.9%) and
G2/M phase (29.8 vs. 22.3%), and the decrease in
G1 phase (55.3 vs. 63.8%) associated with mitogenic
stimulation. These observations suggest that 10-gingerol inhibits
the transition from G1 phase of the cell cycle to S
phase, resulting in G1 arrest, which is well correlated
with inhibition of cell proliferation (Fig. 1B). Based on these findings, we
analyzed the changes of cell cycle regulatory proteins in
10-gingerol-treated MDA-MB-231 cells. 10-Gingerol treatment
markedly suppressed mitogen-induced expression of cyclin-dependent
kinases (Cdks) and cyclins to levels observed in untreated controls
(Fig. 2B). Collectively, these
findings indicate that 10-gingerol downregulates the expression of
cell cycle regulatory proteins, resulting in inhibition of cell
cycle progression and proliferation.
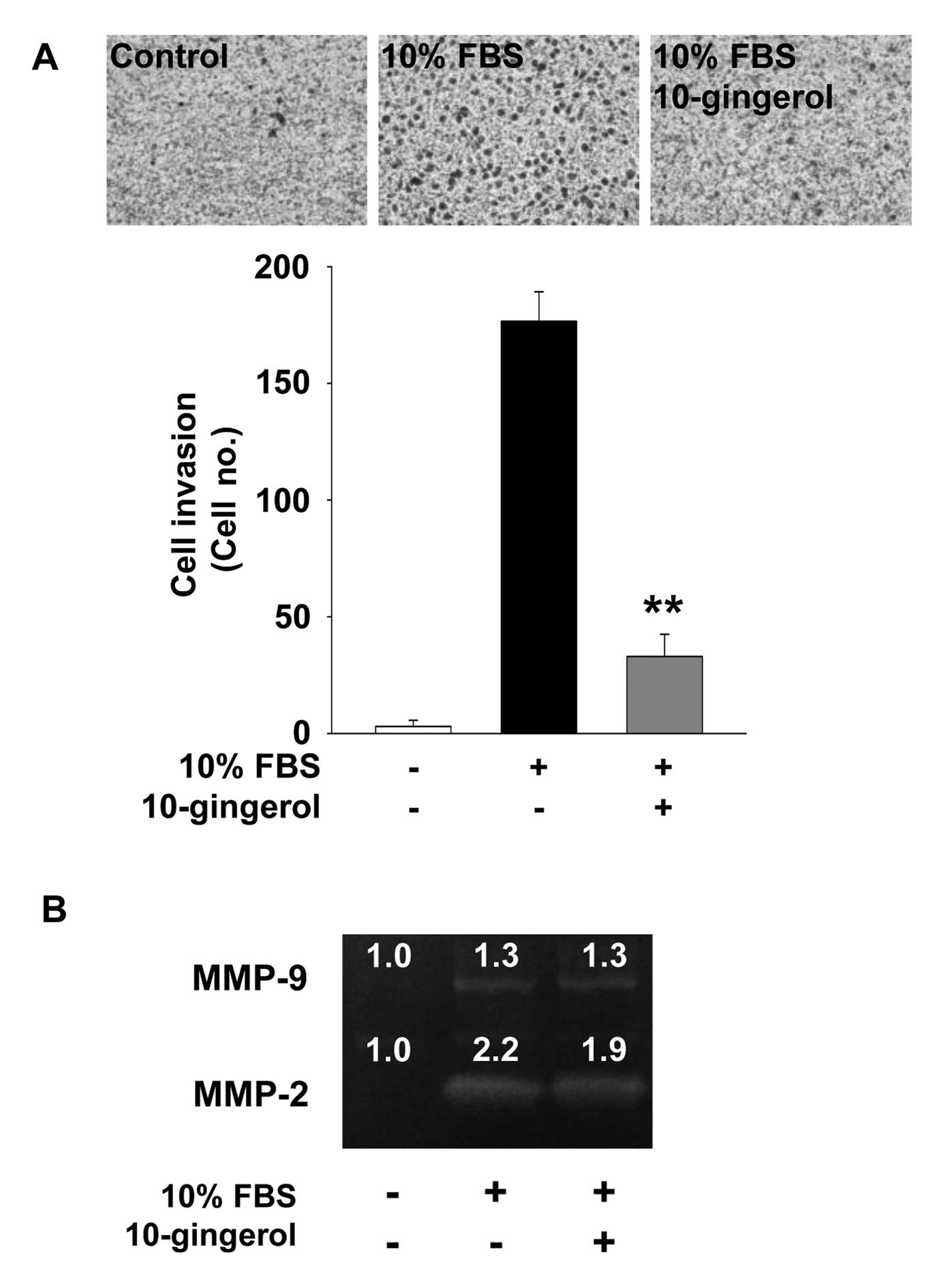
10-Gingerol inhibits cell invasion
We next examined the effect of 10-gingerol on cell
invasion which plays pivotal roles in cancer progression.
10-Gingerol treatment markedly inhibited mitogen-induced invasion
of MDA-MB-231 cells (Fig. 3A).
Expression and activation of MMPs have been reported to enhance
cell migration and invasion by degrading the components of
extracellular matrix and cell surfaces (6-9). Based
on 10-gingerol-mediated inhibition of cell invasion, we examined
the activity of MMPs in MDA-MB-231 cells. 10-gingerol treatment
marginally inhibited the activity of MMP-2, but not MMP-9,
suggesting that inhibition of cell invasion by 10-gingerol is
mediated, at least in part, through the suppression of MMP-2
activity (Fig. 3B).
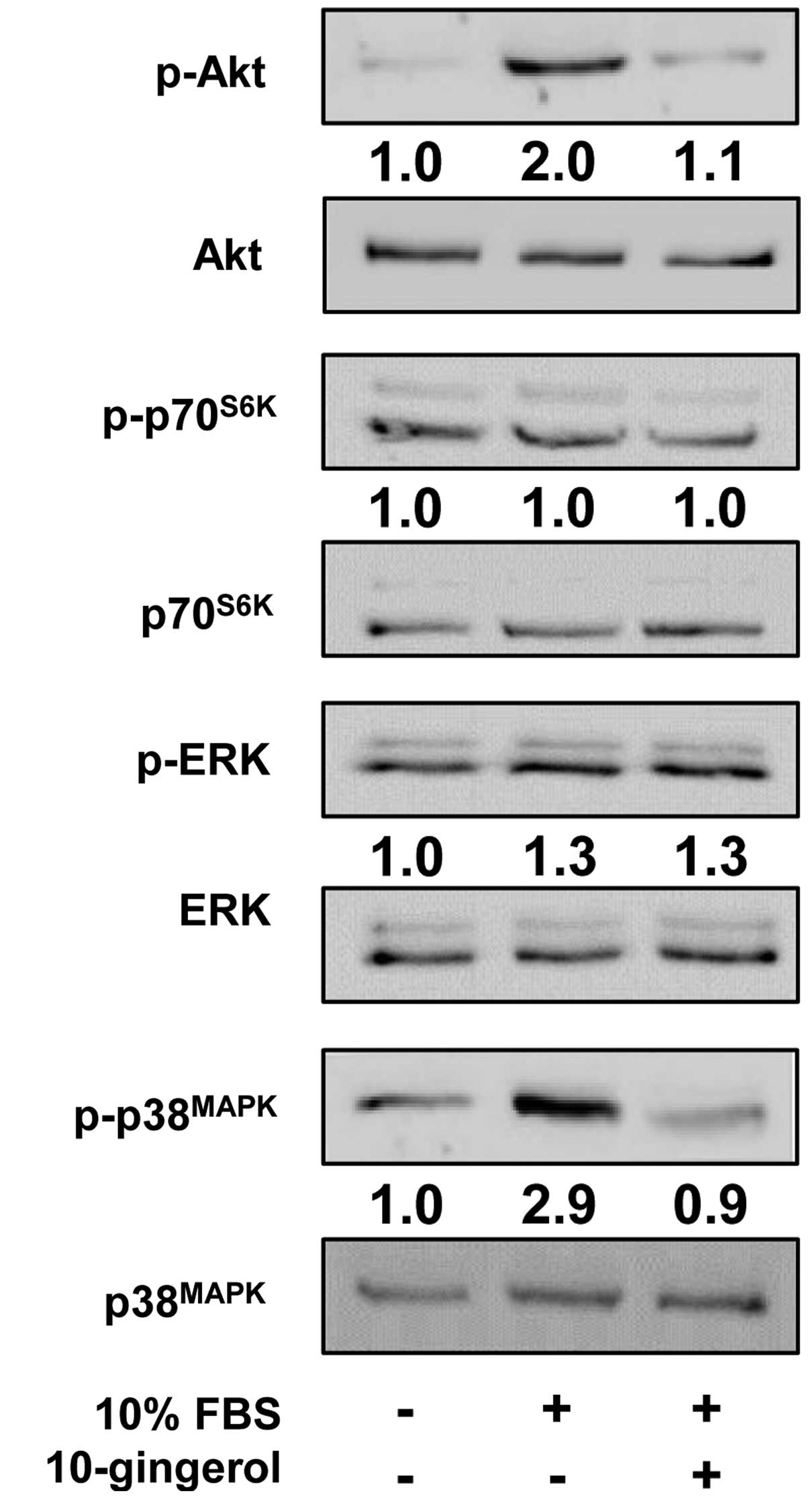
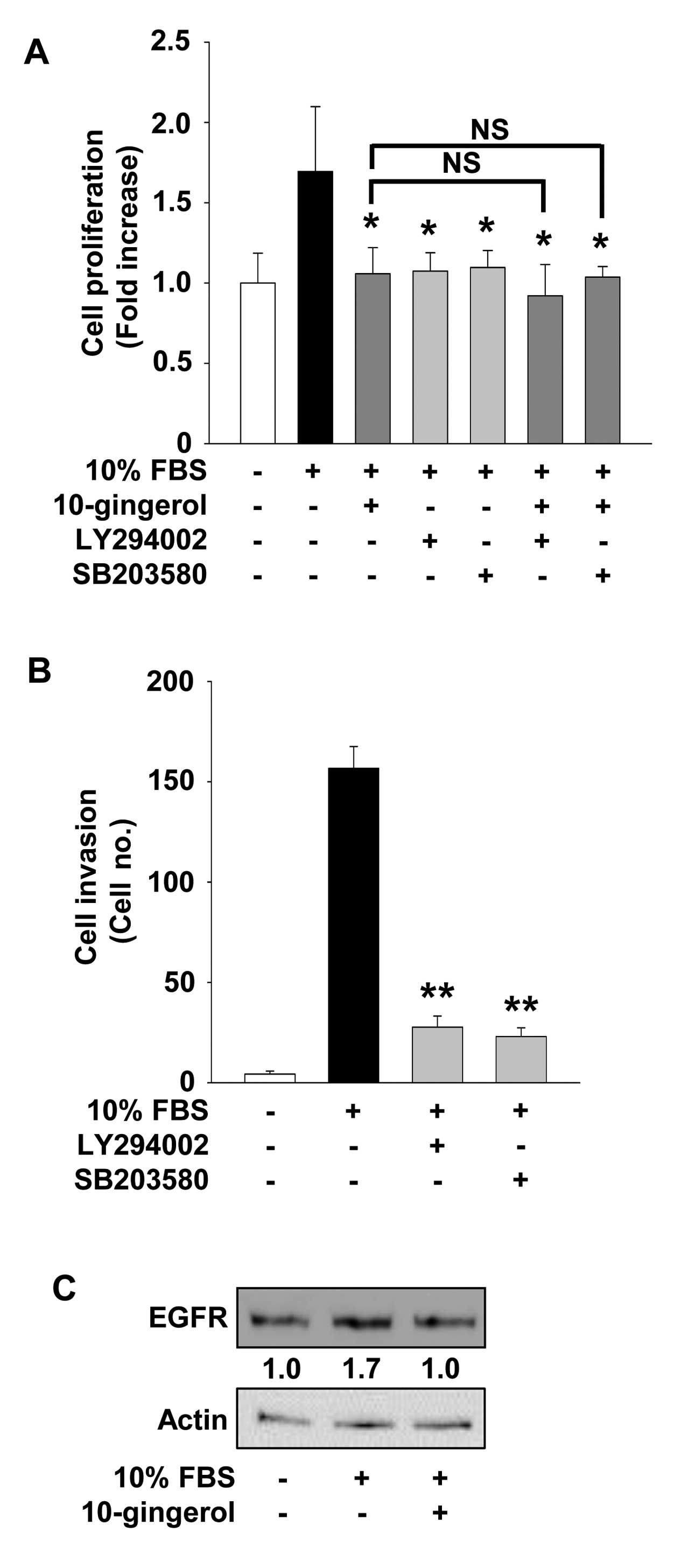
Regulatory effects of 10-gingerol on cell
proliferation and invasion are mediated through inactivation of
Akt- and p38MAPK-dependent signaling pathways
To investigate the molecular mechanism by which
10-gingerol modulates cell proliferation and invasion, we examined
the changes in activation of intracellular signaling pathways such
as Akt, p70S6K, ERK and p38MAPK in
10-gingerol-treated MDA-MB-231 cells. Mitogenic stimulation
increased the phosphorylation/activation of Akt, ERK and
p38MAPK, but not that of p70S6K, as compared
with unstimulated controls. 10-Gingerol treatment markedly
inhibited the phosphorylation of Akt and p38MAPK in
MDA-MB-231 cells (Fig. 4). Finally,
pretreatment of cells with LY294002, an inhibitor of
phosphoinositide 3-kinase (PI3K)/Akt pathway, or SB203580, an
inhibitor of p38MAPK pathway, mimicked the suppressive
effects of 10-gingerol on cell proliferation and invasion in
MDA-MB-231 cells (Fig. 5A and B).
Co-treatment with 10-gingerol did not significantly enhance the
anti-proliferative activity of these pharmacological inhibitors,
indicating that 10-gingerol and these inhibitors may share similar
roles and mechanisms of action in regulating cellular processes of
MDA-MB-231 cells.
10-Gingerol suppresses the expression of
EGFR
The EGFR is a receptor tyrosine kinase which is
highly expressed or activated in various types of human cancers
including breast cancer (4,31). Therefore, EGFR and its down-stream
signaling pathways have been known as key therapeutic targets for
cancer treatment. 10-Gingerol treatment markedly suppressed
mitogen-induced expression of EGFR in MDA-MB-231 cells to levels
observed in unstimulated controls (Fig.
5C). Taken together, these observations suggest that
anti-proliferative and anti-invasive activities of 10-gingerol in
MDA-MB-231 breast cancer cells might be correlated with suppression
of EGFR expression.
Discussion
Ginger has popularly been consumed as a flavoring
agent and traditional medicinal herb for the treatment of a variety
of disorders such as pain, inflammation, asthma, hypertension and
diabetes through antioxidative, anti-inflammatory and
anti-hyperglycemic activities (32). In addition, antitumor effects of
ginger and its components including gingerols and shogaols have
been reported in various types of human cancers (11-13,15-22).
However, no pharmacological effects and detailed molecular
mechanisms of 10-gingerol on breast cancer cell proliferation and
invasion have been clearly investigated to date.
Data presented in this study show that 10-gingerol
treatment markedly inhibits the proliferation of breast cancer
cells through downregulation of cell cycle regulatory proteins such
as Cdks and cyclins, and this anti-proliferative activity of
10-gingerol seems to be independent of ER expression status.
Moreover, 10-gingerol strongly abrogates breast cancer cell
invasion, which might be mediated partially through inhibition of
MMP-2 activity. Previous studies demonstrate that 6-gingerol or
6-shogaol inhibits cell invasion in different cell lines including
breast cancer and liver cancer by differential modulation of MMP-2
and MMP-9 activity (15,18,33).
These findings indicate that the regulatory effect of bioactive
phenolic components including 10-gingerol, 6-gingerol or 6-shogaol
on MMP activity might be dependent on the cell/tissue types or the
changes in expression of endogenous inhibitors, tissue inhibitors
of metalloproteinases (34).
EGFR is frequently overexpressed in ER-negative
breast cancer patients with aggressive phenotype and poor clinical
outcome, indicating the potential role of EGFR and its down-stream
signaling components as therapeutic targets for the treatment of
ER-negative breast cancers (35).
EGFR-dependent down-stream signaling pathways include the
activation of PI3K/Akt, Ras/Raf/ERK, p38MAPK, c-Jun
N-terminal kinase, phospholipase Cγ, and focal adhesion kinase,
which are implicated in cell proliferation, survival, migration and
invasion (4). In the present study
we demonstrated that 10-gingerol-mediated inhibition of breast
cancer cell proliferation and invasion is mediated through
inactivation of Akt and p38MAPK activity as evidenced by
treatment with LY294002 and SB203580, respectively. Furthermore,
10-gingerol treatment markedly suppressed the expression of EGFR in
ER-negative MDA-MB-231 cells as well as ER-positive MCF-7 cells
(data not shown). This finding is similar to the patterns of
10-gingerol inhibition of cell proliferation in both ER-negative
MDA-MB-231 and ER-positive MCF-7 cells. In conclusion, we
demonstrate for the first time that 10-gingerol inhibits
mitogen-induced Akt and p38MAPK
phosphorylation/activation and EGFR expression, leading to
inhibition of breast cancer cell proliferation and invasion. These
findings warrant further evaluation and preclinical development of
10-gingerol as a potent antitumor agent in combination with
conventional or molecular targeted therapies for the treatment of
breast cancer.
Acknowledgments
The present study was supported by the research fund
of Dankook University in 2014.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guo M, Wang M, Deng H, Zhang X and Wang
Z-Y: A novel anticancer agent Broussoflavonol B downregulates
estrogen receptor (ER)-α36 expression and inhibits growth of
ER-negative breast cancer MDA-MB-231 cells. Eur J Pharmacol.
714:56–64. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Munoz J, Wheler J and Kurzrock R:
Expression of estrogen and progesterone receptors across human
malignancies: New therapeutic opportunities. Cancer Metastasis Rev.
Dec 28–2014.Epub ahead of print. PubMed/NCBI
|
|
4
|
Masuda H, Zhang D, Bartholomeusz C,
Doihara H, Hortobagyi GN and Ueno NT: Role of epidermal growth
factor receptor in breast cancer. Breast Cancer Res Treat.
136:331–345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dawood S, Broglio K, Buzdar AU, Hortobagyi
GN and Giordano SH: Prognosis of women with metastatic breast
cancer by HER2 status and trastuzumab treatment: An
institutional-based review. J Clin Oncol. 28:92–98. 2010.
View Article : Google Scholar :
|
|
6
|
Bourboulia D and Stetler-Stevenson WG:
Matrix metalloproteinases (MMPs) and tissue inhibitors of
metalloproteinases (TIMPs): Positive and negative regulators in
tumor cell adhesion. Semin Cancer Biol. 20:161–168. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stetler-Stevenson WG: Tissue inhibitors of
metalloproteinases in cell signaling: Metalloproteinase-independent
biological activities. Sci Signal. 1:re62008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vandenbroucke RE and Libert C: Is there
new hope for therapeutic matrix metalloproteinase inhibition? Nat
Rev Drug Discov. 13:904–927. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hadler-Olsen E, Winberg J-O and
Uhlin-Hansen L: Matrix metalloproteinases in cancer: Their value as
diagnostic and prognostic markers and therapeutic targets. Tumour
Biol. 34:2041–2051. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oyagbemi AA, Saba AB and Azeez OI:
Molecular targets of [6]-gingerol: Its potential roles in cancer
chemoprevention. Biofactors. 36:169–178. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shukla Y and Singh M: Cancer preventive
properties of ginger: A brief review. Food Chem Toxicol.
45:683–690. 2007. View Article : Google Scholar
|
|
13
|
Baliga MS, Haniadka R, Pereira MM, D'Souza
JJ, Pallaty PL, Bhat HP and Popuri S: Update on the chemopreventive
effects of ginger and its phytochemicals. Crit Rev Food Sci Nutr.
51:499–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee T-Y, Lee K-C, Chen S-Y and Chang H-H:
6-Gingerol inhibits ROS and iNOS through the suppression of PKC-α
and NF-kappaB pathways in lipopolysaccharide-stimulated mouse
macrophages. Biochem Biophys Res Commun. 382:134–139. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee HS, Seo EY, Kang NE and Kim WK:
[6]-Gingerol inhibits metastasis of MDA-MB-231 human breast cancer
cells. J Nutr Biochem. 19:313–319. 2008. View Article : Google Scholar
|
|
16
|
Lee S-H, Cekanova M and Baek SJ: Multiple
mechanisms are involved in 6-gingerol-induced cell growth arrest
and apoptosis in human colorectal cancer cells. Mol Carcinog.
47:197–208. 2008. View
Article : Google Scholar :
|
|
17
|
Brown AC, Shah C, Liu J, Pham JTH, Zhang
JG and Jadus MR: Ginger's (Zingiber officinale Roscoe) inhibition
of rat colonic adenocarcinoma cells proliferation and angiogenesis
in vitro. Phytother Res. 23:640–645. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Weng C-J, Wu C-F, Huang H-W, Ho C-T and
Yen G-C: Anti-invasion effects of 6-shogaol and 6-gingerol, two
active components in ginger, on human hepatocarcinoma cells. Mol
Nutr Food Res. 54:1618–1627. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim MO, Lee M-H, Oi N, Kim SH, Bae KB,
Huang Z, Kim DJ, Reddy K, Lee SY, Park SJ, et al: [6]-shogaol
inhibits growth and induces apoptosis of non-small cell lung cancer
cells by directly regulating Akt1/2. Carcinogenesis. 35:683–691.
2014. View Article : Google Scholar :
|
|
20
|
Fan J, Yang X and Bi Z: 6-Gingerol
inhibits osteosarcoma cell proliferation through apoptosis and AMPK
activation. Tumour Biol. 36:1135–1141. 2015. View Article : Google Scholar
|
|
21
|
Kim JS, Lee SI, Park HW, Yang JH, Shin TY,
Kim YC, Baek NI, Kim SH, Choi SU, Kwon BM, et al: Cytotoxic
components from the dried rhizomes of Zingiber officinale Roscoe.
Arch Pharm Res. 31:415–418. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sang S, Hong J, Wu H, Liu J, Yang CS, Pan
MH, Badmaev V and Ho CT: Increased growth inhibitory effects on
human cancer cells and anti-inflammatory potency of shogaols from
Zingiber officinale relative to gingerols. J Agric Food Chem.
57:10645–10650. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cho Y-R, Choi SW and Seo D-W: The in vitro
antitumor activity of Siegesbeckia glabrescens against ovarian
cancer through suppression of receptor tyrosine kinase expression
and the signaling pathways. Oncol Rep. 30:221–226. 2013.PubMed/NCBI
|
|
24
|
Kim H-J, Ko H-Y, Choi S-W and Seo D-W:
Anti-angiogenic effects of Siegesbeckia glabrescens are mediated by
suppression of the Akt and p70S6K-dependent signaling pathways.
Oncol Rep. 33:699–704. 2015.
|
|
25
|
Lee HN, Kim J-K, Kim JH, Lee SJ, Ahn EK,
Oh JS and Seo DW: A mechanistic study on the anti-cancer activity
of ethyl caffeate in human ovarian cancer SKOV-3 cells. Chem Biol
Interact. 219:151–158. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim SH, Cho Y-R, Kim H-J, Oh JS, Ahn EK,
Ko HJ, Hwang BJ, Lee SJ, Cho Y, Kim YK, et al: Antagonism of
VEGF-A-induced increase in vascular permeability by an integrin
α3β1-Shp-1-cAMP/PKA pathway. Blood. 120:4892–4902. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim H-J, Cho Y-R, Kim SH and Seo D-W:
TIMP-2-derived 18-mer peptide inhibits endothelial cell
proliferation and migration through cAMP/PKA-dependent mechanism.
Cancer Lett. 343:210–216. 2014. View Article : Google Scholar
|
|
28
|
Lee HN, Joo J-H, Oh JS, Choi SW and Seo
D-W: Regulatory effects of Siegesbeckia glabrescens on non-small
cell lung cancer cell proliferation and invasion. Am J Chin Med.
42:453–463. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yoon HJ, Cho Y-R, Joo J-H and Seo D-W:
Knockdown of integrin α3β1 expression induces proliferation and
migration of non-small cell lung cancer cells. Oncol Rep.
29:662–668. 2013.
|
|
30
|
Cho Y-R, Kim JH, Kim J-K, Ahn EK, Ko HJ,
In JK, Lee SJ, Bae U, Kim YK, Oh JS, et al: Broussonetia kazinoki
modulates the expression of VEGFR-2 and MMP-2 through the
inhibition of ERK, Akt and p70S6K dependent signaling pathways: Its
implication in endothelial cell proliferation, migration and
tubular formation. Oncol Rep. 32:1531–1536. 2014.PubMed/NCBI
|
|
31
|
Jamdade VS, Sethi N, Mundhe NA, Kumar P,
Lahkar M and Sinha N: Therapeutic targets of triple-negative breast
cancer: A review. Br J Pharmacol. 172:4228–4237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ali BH, Blunden G, Tanira MO and Nemmar A:
Some phyto-chemical, pharmacological and toxicological properties
of ginger (Zingiber officinale Roscoe): A review of recent
research. Food Chem Toxicol. 46:409–420. 2008. View Article : Google Scholar
|
|
33
|
Weng C-J, Chou C-P, Ho C-T and Yen G-C:
Molecular mechanism inhibiting human hepatocarcinoma cell invasion
by 6-shogaol and 6-gingerol. Mol Nutr Food Res. 56:1304–1314. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Seo D-W, Li H, Guedez L, Wingfield PT,
Diaz T, Salloum R, Wei BY and Stetler-Stevenson WG: TIMP-2 mediated
inhibition of angiogenesis: An MMP-independent mechanism. Cell.
114:171–180. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Burness ML, Grushko TA and Olopade OI:
Epidermal growth factor receptor in triple-negative and basal-like
breast cancer: Promising clinical target or only a marker? Cancer
J. 16:23–32. 2010. View Article : Google Scholar : PubMed/NCBI
|