Introduction
Multiple myeloma (MM) is a clonal disease of plasma
cells that remains, for the most part, incurable despite recent
advances in treatment strategies and new molecular-targeted
compounds (1–3). Most patients with MM eventually become
resistant to the chemotherapeutic agents and die of disease
progression within 10 years (4). To
improve the prognosis of patients of MM, it is important to
overcome drug resistance (DR). It has been demonstrated that the
marrow microenvironment plays a critical role in myeloma DR
(5–9). The marrow microenvironment consists of
hematopoietic cells, stromal cells and extracellular matrix (ECM).
Interactions between the MM cells and stromal cells/ECM could alter
drug response by blocking apoptosis. This phenomenon was termed
'cell adhesion-mediated drug resistance' (CAM-DR), which is
believed to play a crucial role in MM cells escape the cytotoxic
effects of chemotherapeutic agents (10–13).
However, the underlying molecular mechanisms involved are unclear
to date.
The Homer proteins, which belong to post-synaptic
density (PSD) families, have been shown to be the products of
alternative splicing of an immediately early gene (14,15).
This family of proteins is composed of two major groups: the
short-form proteins (Homer1a and Ania3) and the long-form proteins
(Homer1b/c, Homer2 and Homer3) (16,17).
With a conserved N-terminus of an Enabled/vasodilator-stimulated
phospho-protein (Ena/VASP) homology 1 (EVH-1) domain, they are
capable of binding to a proline-rich consensus sequence (PPXXFR) in
various other scaffolding and signal transduction molecules,
including group 1 metabotropic glutamate receptor (mGluRs) and
1,4,5-trisphosphate receptors (IP3Rs) (18–20).
Additionally, with a coiled-coil (CC) structure in the
carboxyl-terminal regions, Homer1b/c self-multimerize and in turn
couple group 1 mGLuRs and IP3Rs to form multi-protein complexes and
facilitate signal transduction to down-stream pathways (21). The knock-down of Homer1b/c using
specific siRNA protects cortical neurons, as shown against
glutamate-mediated excitotoxicity via anti-apoptotic activity
(14). Treatment of cortical
neurons with glutamate resulted in an increase in Bax with a
decrease in Bcl-2, followed by activation of caspase-9,
representing the classical mitochondrial pathways. Based on the
above research, we speculated that Homer1b/c might be closely
related to apoptosis, however, no report exists on the relationship
between Homer1b/c and cell apoptosis in hematological
malignancies.
In the present study, we first investigated the
expression patterns of Homer1b/c at the protein level in the
doxo-mediated MM cell apoptosis, and then we detected the changing
of Homer1b/c protein expression in cell adhesion model. This study
was conducted to gain greater insight into the pro-apoptotic
effects of Homer1b/c and its functions in CAM-DR of myeloma cells,
possibly providing potential therapies for clinical trials.
Materials and methods
Cell cultures and stimulation
The human MM cell lines RPMI-8226, U266 and bone
marrow stromal cell line HS-5 were obtained from Cell Library,
China Academy of Science. The cell lines RPMI-8226, U266 were
cultured in RPMI-1640 (Gibco-BRL, Grand Island, NY, USA) and the
HS-5 cultured in F12 (Gibco-BRL) supplemented with 10% fetal bovine
serum (FBS), 2 mM L-glutamine and 100 U/ml penicillin-streptomycin
mixture (Gibco-BRL) at 37°C and 5% CO2. To study
apoptosis, cells were seeded onto a 60-mm dish and incubated in a
low concentration of serum (1% FBS) for 24 h prior to treatment
with doxorubicin for different concentrations.
Western blot analysis
Western blot experiments were used to measure
certain proteins. Briefly, the cells were lysed in lysis buffer
[120 Mm Tris (pH 7.4), 135 mM NaCl, 1 mM EDTA, 1% NP40, 0.1% SDS, 1
mM Na3VO4, 1 mM aprotinin and 1 mM PMSF]. An
equivalent amount of protein from each sample was electrophoresed
by 12% sodium dodecylsulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and then transferred to a PVDF membrane. After blocking
with phosphate-buffered saline (PBS) containing 5% non-fat milk and
0.1% Tween-20 overnight, the membrane was incubated with primary
antibody at 4°C overnight. After washing with PBS containing 0.1%
Tween-20 three times, each for 5 min, the membrane was incubated
with HRP-labeled secondary antibody for another 2 h at room
temperature. The membrane was then developed using the ECL
detection systems. The antibodies used in the present study
included: anti-Homer1b/c (anti-rabbit, 1:500; Santa Cruz
Biotechnology), cleaved caspase-3 (anti-rabbit, 1:500; Santa Cruz
Biotechnology), cleaved caspase-3 (anti-rabbit, 1:500; Santa Cruz
Biotechnology), anti-Bax (anti-rabbit, 1:1,000; Cell Signaling
Technology), anti-Bcl-2 (anti-mouse, 1:500; Santa Cruz
Biotechnology) and anti-GAPDH (anti-rabbit, 1:1,000; Sigma).
Preparation of siRNA and transient
transfection
Double-stranded small interfering RNA (siRNA)
transfection was used to knock down Homer1b/c expression. The siRNA
was commercially synthesized (Shanghai GenePharma Co., Ltd.,
Shanghai, China). For controls, scrambled RNA oligonucleotides were
used. For each well, 33.3 nM of each of the three oligos was
transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA,
USA) according to the manufacturer's instructions. After incubation
for 6 h, the medium was replaced with RPMI-1640 containing 10% FBS.
Transfected cells were used for the subsequent experiments 48 h
after transfection.
Cell viability assay
To evaluate the effect of transfection of Homer1b/c
siRNA, cells were seeded on a 96-well cell culture cluster (Corning
Inc., Corning, NY, USA) at a concentration of 5×104/well
in a volume of l00 µl and grown overnight. Cell Counting
kit-8 (CCK-8) reagents (Dojindo Laboratories, Kumamoto, Japan) were
added to the different subset wells, including control, control
siRNA-transfected and Homer1b/c siRNA-transfected cells and then
incubated at 37°C and 5% CO2. The absorbance was
quantified using an automated plate reader at a test wavelength of
450 nm at different times.
Adhesion assays and detection of adhesion
rate
Adhesion of MM cell lines to FN was done as
previously described. In co-culture experiments, HS-5 stromal cells
were seeded first and incubated overnight at 37°C and 5%
CO2. The next morning, stromal cells were washed once
with serum-free medium, and MM cell lines were allowed to adhere
for 2 h in serum-free RPMI-1640. Adhered MM cells were incubated
overnight at 37°C and 5% CO2, non-adhered cells were
removed and RPMI-1640 supplemented with 10% FBS was added for an
additional 24 h. MM cells were incubated with 5 µM of
Calcein-AM (Santa Cruz Biotechnology) for 30 min, washed and
incubated for 45 min to allow unbound dye to diffuse out of the
cells. Labeled cells were allowed to adhere for 2 h and
non-adherent cells were removed with three washes in PBS. The
absorbance was quantified using an automated plate reader at a test
wavelength of 490 nm.
Drug cytotoxicity assay
For drug cytotoxicity assays, MM cells were washed
once after transfected with siRNA and adhered to FN or stromal
cells as previously described. After 24 h, drugs or vehicle control
was added to each well and incubated for 48 h, then medium
containing drugs was removed, suspended and the attached MM cells
were collected. The survival cells were assessed by CCK-8
assay.
Flow cytometry-based Annexin V/PI
staining
The flow cytometry assay was performed to measure
the degree of apoptosis and necrosis using an ApoScreen Annexin V
kit (SouthernBiotech, Birmingham, AL, USA) according to the
manufacturer's protocol. Briefly, RPMI-8226 and U266 cells were
digested by 0.1% trypsin and resuspended in cold binding buffer (10
mM HEPES, pH 7.4, 140 mM NaCl, 2.5 mM CaCl2, 0.1% BSA)
at concentrations between 1×106 and 1×107
cells/ml. Labeled Annexin V (10 µl) was added to 100
µl of the cell suspension. After 15-min incubation on ice,
380 µl binding buffer and 10 µl propidium iodide (PI)
solution were added to the cell suspension. Subsequently, the
number of stained cells was assessed by a flow cytometer (BD
FACSAriaII).
Statistical analysis
All experiments were repeated at least three times
per condition. The data are described as mean ± SEM. Data were
analyzed using the two-tailed t-test for statistical analysis.
P-value <0.05 was considered to indicate a statistically
significant result.
Results
Increased Homer1b/c expression in
doxo-mediated MM cell apoptosis
The knockdown of Homer1b/c using specific siRNA has
been reported to protect cortical neurons against glutamate-induced
apoptosis via classical mitochondrial apoptotic pathway (14). First, we examined the cell viability
after incubation for 48 h with doxorubicin for different
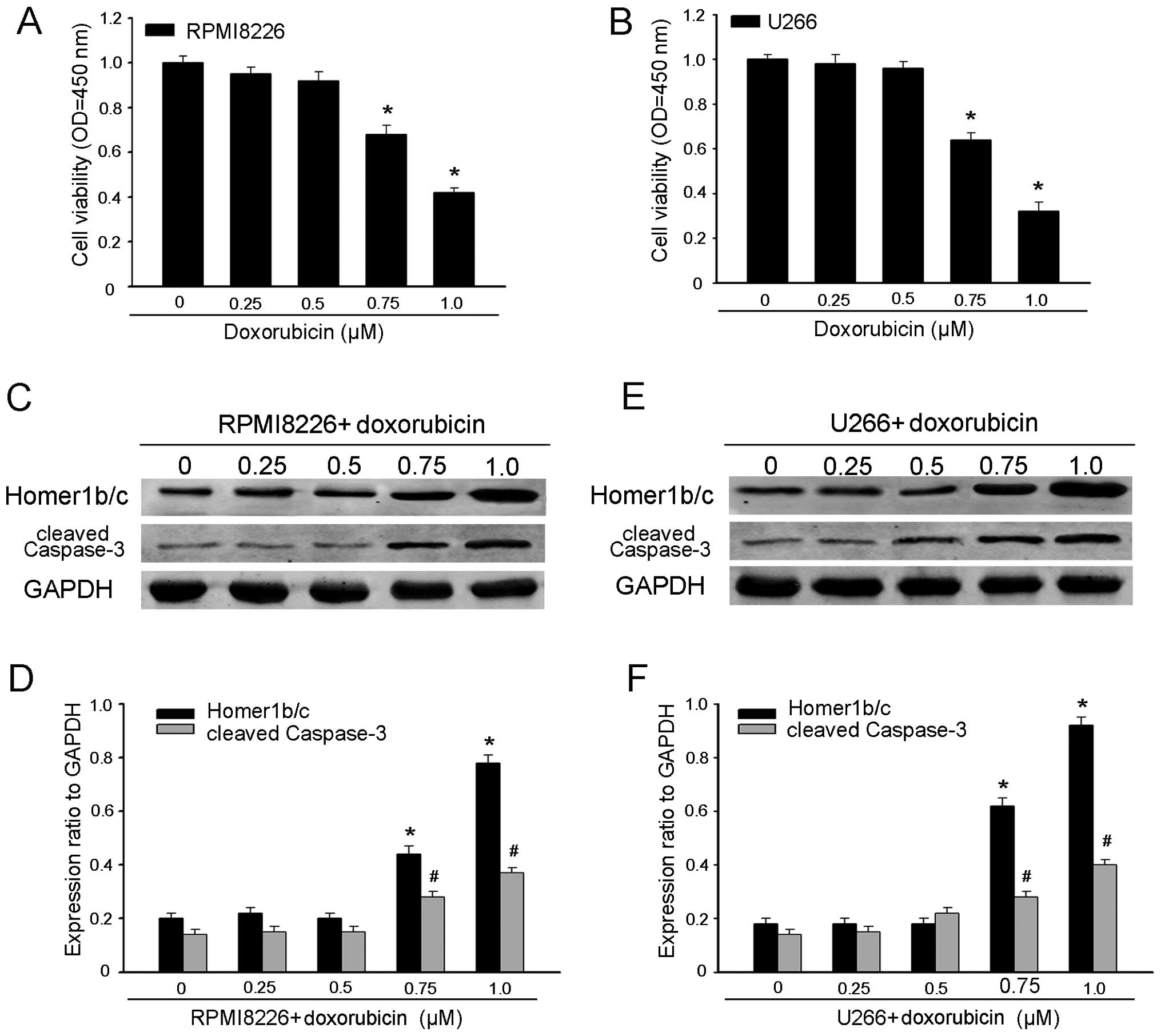
concentrations. As shown in Fig. 1A and
B, both RPMI-8226 and U266 cell viability decreased along with
the increased concentration of doxorubicin. Herein, we examined
whether Homer1b/c also participated in the regulation of
doxorubicin-induced MM cells apoptosis. Exposing RPMI-8226 and U266
cells to gradually increasing concentration of doxorubicin for 48 h
induced an apparent increase in the protein level of cleaved
caspase-3, one of the key executioners of apoptotic cell death,
measured by western blot analysis. Concomitantly with an increase
in cleaved caspase-3 expression, doxorubicin also resulted in an
increase in Homer1b/c expression (Fig.
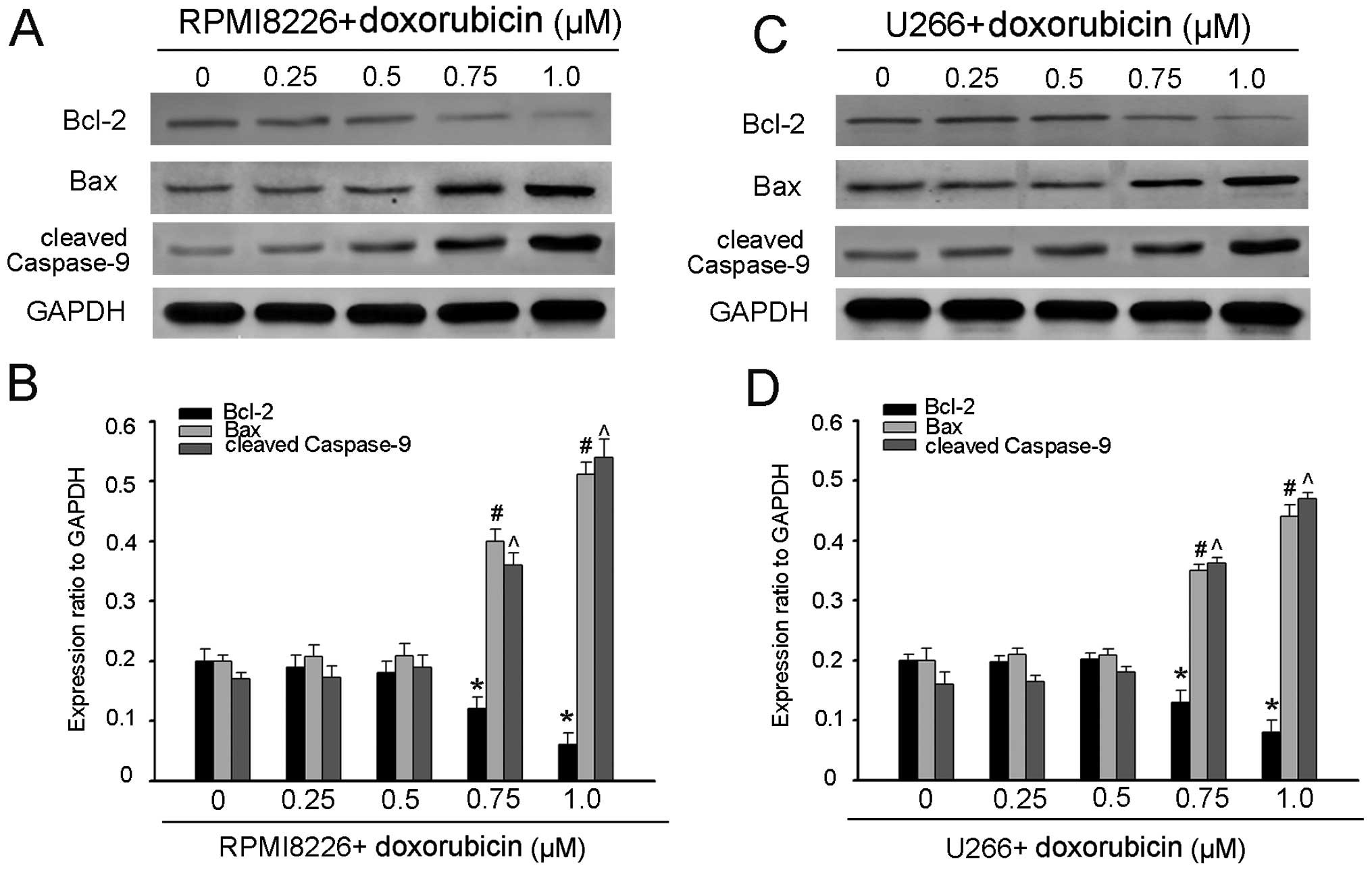
1C–F). To further confirm the involvement of the mitochondria
pathway, we investigated the expression levels of Bcl-2 and Bax,
and the caspase-9 activity. As expected, treatment of MM cells with
doxorubicin resulted in an increase in Bax with a decrease in Bcl-2
and activation of caspase-9 (Fig.
2). These results suggested that Homer1b/c participated in the
process of the doxorubicin-mediated MM cell apoptosis. Possibly it
promotes doxorubicin-induced apoptosis via classical mitochondrial
apoptotic pathway, which should be further proved.
Homer1b/c acts as pro-apoptotic factor in
MM cells
To further determine the function of Homer1b/c in MM
cell apoptosis, Homer1b/c siRNA was transfected into RPMI-8226
cells, which significantly downregulated the expression of
Homer1b/c in cells, confirmed by western blot analysis (Fig. 3A and B). We subsequently evaluated
the expression of cleaved caspase-3 by western blot analysis, and
found that the expression of cleaved caspase-3 was downregulated in
the cells at the protein level (Fig. 3C
and D). To investigate whether the pro-apoptotic functions of
Homer1b/c is mediated by mitochondrial pathways, we detected the
expression levels of Bcl-2 and Bax and the caspase-9 activity after
RPMI-8226 cell transfection with Homer1b/c siRNA by western blot
analysis and found that transfection with Homer1b/c siRNA resulted
in an increase in Bcl-2 with a decrease in Bax and cleaved
caspase-9 (Fig. 3C and D). These
results confirmed the pro-apoptotic effects of Homer1b/c. In order
to further detect whether Homer1b/c is the direct cause of cell
apoptosis, Homer1b/c siRNA and control siRNA cells were incubated
for 48 h with 1 µM doxorubicin. In the presence of the
chemotherapy drugs, the expression of cleaved caspase-3, Bax and
cleaved caspase-9 decreased more significantly in the Homer-1b/c
siRNA cells than in those from the control group. At the same time,
the Bcl-2 increased more in Homer1b/c siRNA cells (Fig. 3C and D). Cell viability assay also
suggested that the cell activity was significantly increased in
Homer1b/c siRNA cells and knocking Homer1b/c down protected MM
cells against drug-induced apoptosis (Fig. 3E). These results supported previous
research that promotion of MM cell apoptosis by Homer1b/c was
through the mitochondrial pathways (14), but whether there are other possible
mechanisms included still needs further research.
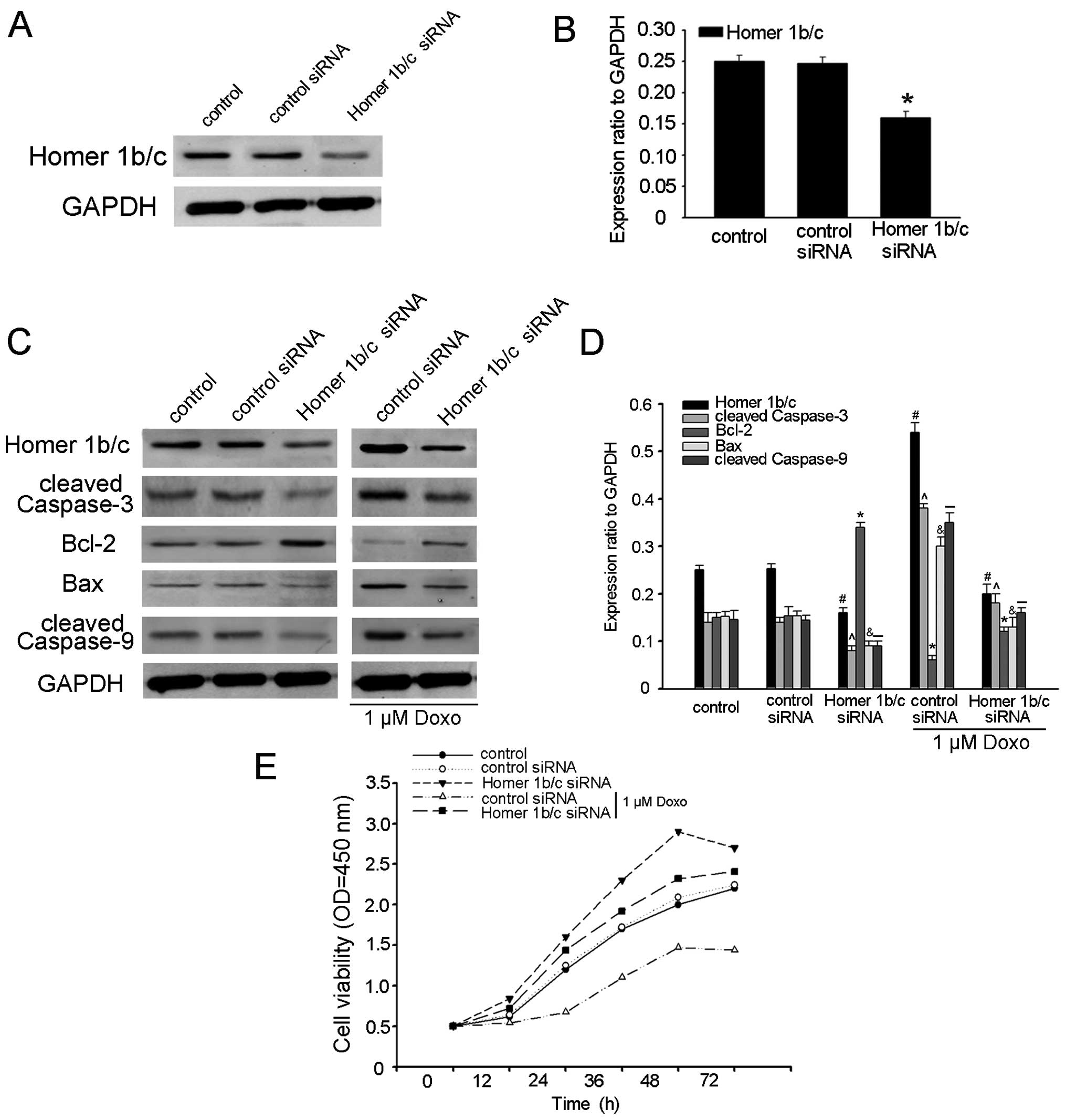 | Figure 3Homer1b/c acts as a pro-apoptotic
factor in MM cells. (A) RPMI-8226 cells were transiently
transfected with siRNA targeting either Homer1b/c or a scrambled
sequence (control siRNA) as described above for 48 h and immunoblot
analysis of Homer1b/c and GAPDH was performed. (B) The bar chart
demonstrated the ratio of Homer1b/c protein to GAPDH by
densitometry after control-siRNA or Homer1b/c-siRNA determined by
western blot analysis. (C) After incubated with or without 1
µM doxorubicin, normal cells and cells transfected with
Homer1b/c siRNA or control siRNA were lysed, then analyzed by
western blot analysis using antibodies against cleaved-caspase-3,
Bcl-2, Bax, cleaved caspase-9 and GAPDH. (D) The bar chart
demonstrates the ratio of cleaved-caspase-3, Bcl-2, Bax, cleaved
caspase-9 to GAPDH for the above by densitometry. The data are mean
± SD of three independent experiments
(#,^,*,&,-P<0.05 compared with the control
group). (E) In vitro cell viability was examined by CCK-8
assay at the indicated time. Data show mean ± SD of triplicates
from one experiment representative of three experiments performed
(*P<0.05 compared with control or cells transfected
with control siRNA). |
The expression of Homer1b/c associated
with cell adhesion in MM cells
Cell adhesion-mediated drug resistance (CAM-DR) is
thought to be a major obstacle in the treatment of myeloma
(13,22–25).
To investigate the role of Homer1b/c in CAM-DR, we built the MM
cell adhesion model, in which RPMI-8226 and U266 cells were
adherent to the FN and HS-5 cells (3). After 48-h incubation, Homer1b/c
expression was detected by western blot analysis. As shown in
Fig. 4A and B, the quantity of
Homer1b/c expression was decreased in adherent cells, as compared
to that in suspended cells, which suggested that Homer1b/c might
participate in the process of cell adhesion. The expression of
cleaved caspase-3, Bax, Bcl-2 and cleaved caspase-9 was detected by
western blot analysis and we found that the protein level of Bax,
cleaved caspase-9 and cleaved caspase-3 increased while Bcl-2
decreased (Fig. 4C and D). All
these results indicated that Homer1b/c might play a part in the
process of CAM-DR. To further verify the role of Homer1b/c in
CAM-DR, cell adhesion assay was performed following Homer1b/c siRNA
transfection. RPMI-8226 and U266 cells were transiently transfected
with Homer1b/c-siRNA or control siRNA for 48 h and stained with
calcein for 30 min. Then they were cultured in suspension, or
plated on FN coated surface or pre-established stromal cells and
cultured for another 2 h. Cell adhesion assay revealed cell
adhesion rate was significantly increased after knockdown of
Homer1b/c (Fig. 4E and F). Taken
together these data suggested that Homer1b/c expression might
negatively correlate with cell adhesion. The relationship between
the pro-apoptotic role of Homer1b/c and the MM CAM-DR has not been
fully shown, and more studies should be carried out.
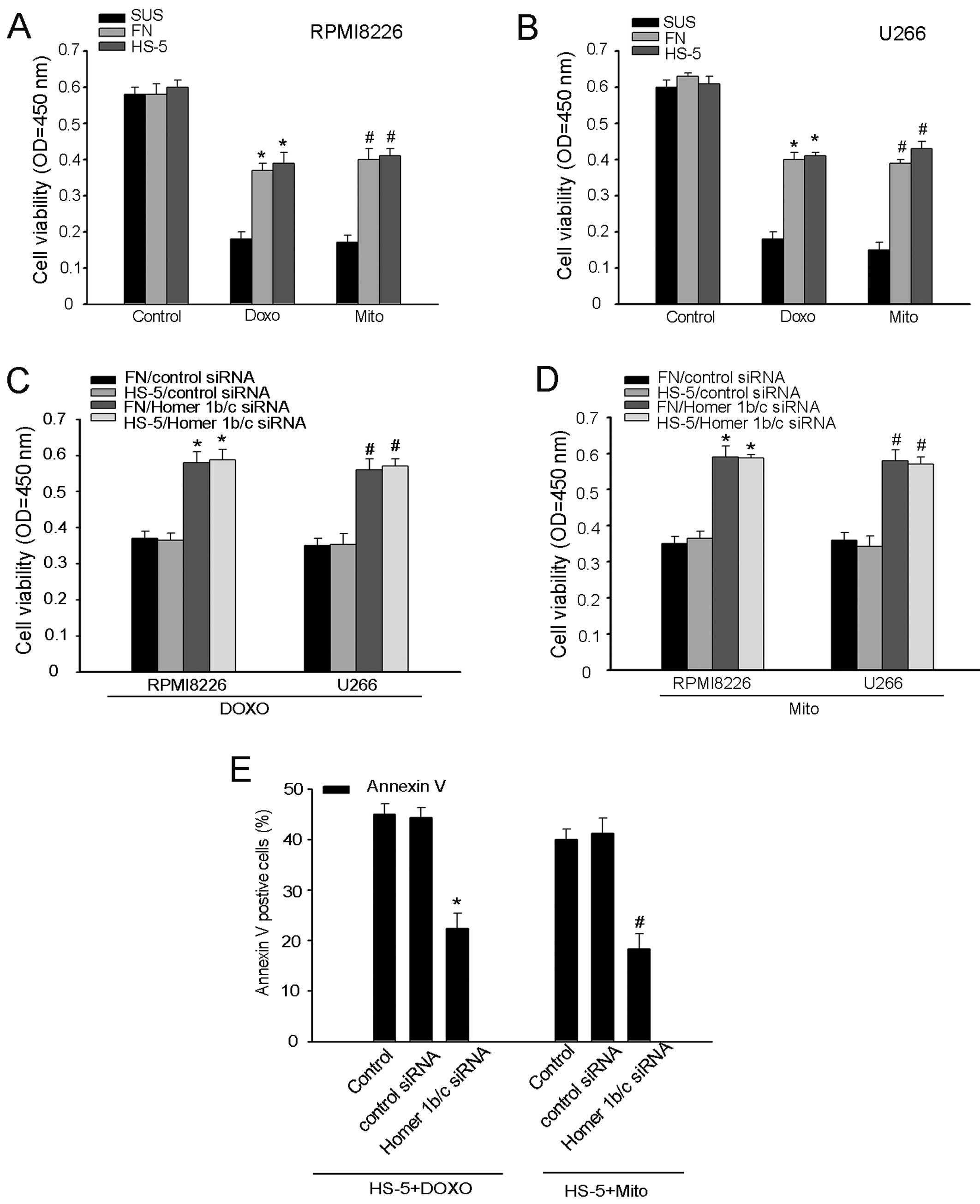
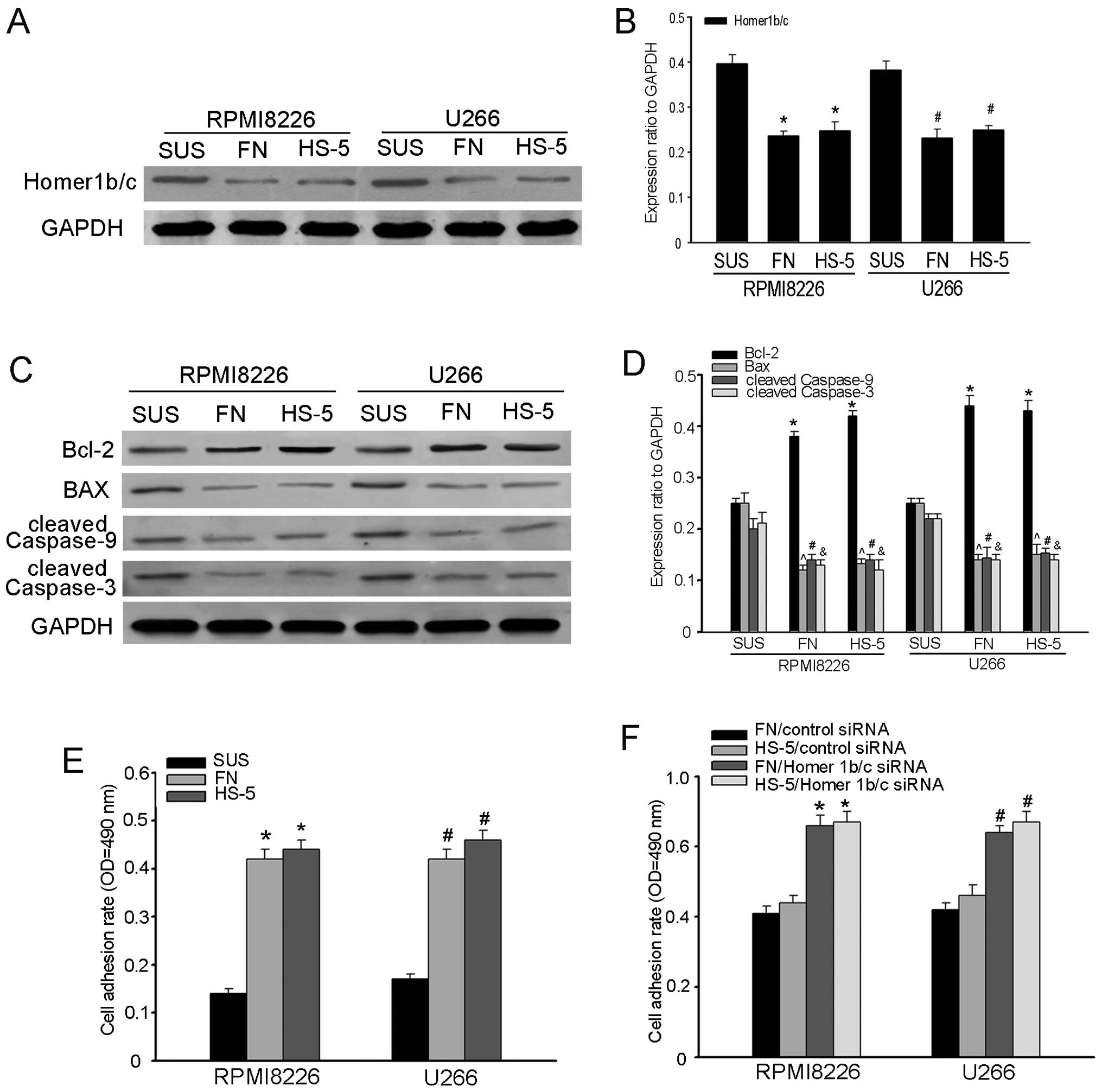 | Figure 4The expression of Homer1b/c and cell
adhesion in MM cells. (A and C) Western blot analysis of expression
of Homer1b/c, Bcl-2, Bax, cleaved caspase-9 and cleaved caspase-3
in a cell adhesion model of RPMI-8226 and U266 cells adhered to FN
or HS-5 cells. Cells in suspension were used as negative control.
(B and D) The bar chart demonstrates the ratio of Homer1b/c, Bcl-2,
Bax, cleaved caspase-9 and cleaved caspase-3 to GAPDH for the above
by densitometry. (E) RPMI-8226 and U266 cells pre-incubated with 5
µM of Calcein-AM were adhered to FN or HS-5 cells for 2 h.
After 2 h, non-adherent cells were removed and the cell adhesion
rate was measured on an automated plate reader. (F) RPMI-8226 and
U266 cells transfected with siRNA targeting either Homer1b/c or a
scrambled sequence (control siRNA) as described above for 48 h and
the cell adhesion rate was measured as described above. In (A-F), A
representative of three independent experiments is shown
(*,#P<0.05 compared with the control group). FN,
fibronectin; SUS, suspension. |
The role of Homer1b/c in MM CAM-DR
In the process of CAM-DR, cell adhesion ability is
directly relevant to cell tolerance effects of chemotherapy agents.
To further confirm the role of Homer1b/c in CAM-DR, we tested the
sensitivity of MM cells to chemotherapeutic agents. Consistent with
the exiting studies, the sensitivity of adherent RPMI-8226 and U266
cells to doxorubicin and mitoxantrone is lower that of cells in
suspension (Fig. 5A and B)
(2,12,26).
The drug resistance was further increased in both RPMI-8226 and
U266 cells after interference with Homer1b/c siRNA (Fig. 5C and D). We also used a flow
cytometry assay to detect the apoptotic cells adherent to stromal
cells by assessing the levels of Annexin V-positive cells. We found
that meddling with Homer1b/c promoted the chemotherapy
drug-mediated cell apoptosis (Fig.
5E). In conclusion, Homer1b/c was a pro-apoptotic factor in
MM.
Discussion
The present study analyzed the role of Homer1b/c in
MM and described that the expression of Homer1b/c was associated
with the CAM-DR. Based on existing research, the cell apoptosis
model was established to study the potential functions of Homer1b/c
at cellular and molecular levels. Western blot analysis documented
that the expression of Homer1b/c in doxorubicin-medicated MM cells
increased apoptosis. We also evaluated the activity of caspase-3,
an apoptosis marker, using western blot analysis (Fig. 1A and B). The results suggested that
Homer1b/c was involved in cell apoptosis after being incubated with
drugs, but the exact roles of Homer1b/c remained unclear. We also
documented the expression of Bcl-2 and Bax, and the caspase-9
activity with the increased concentration of doxorubicin. Treatment
of MM cells with doxorubicin resulted in an increase in Bax with a
decrease in Bcl-2 and activation of caspase-9 (Fig. 2). All these changes following
doxorubicin treatment were attenuated by knocking down Homer1b/c
(Fig. 3C and D), indicating that
the protective effects of Homer1b/c knockdown might be mediated by
the mitochondrial pathways (27–30).
We hypothesized, based on the present study, that Homer1b/c might
play a negative role in MM CAM-DR. Next, MM cell adhesion model was
constructed. As expected, the protein expression level of Homer1b/c
decreased after MM cells had adhered to FN or HS-5 cells. Also, the
expression of Bcl-2 increased and Bax, cleaved caspase-9, cleaved
caspase-3 decreased in the cell adhesion model. Cell adhesion assay
revealed that cell adhesion rate was significantly increased after
knockdown of Homer1b/c. In cell adhesion model, we confirmed that
the MM cell adhesion-mediated drug resistance did exist and found
that CAM-DR could be increased after knocking Homer1b/c down. All
these results suggested that Homer1b/c might play an essential
pro-apoptotic role and be at least partially relevant to CAM-DR
through mitochondrial pathways.
Multiple myeloma is a clonal B-cell malignancy
characterized by the infiltration and growth of malignant plasma
cells in the bone marrow microenvironment (4,31). The
extracellular matrix and stromal cells in bone marrow
microenvironment protecting MM cells from drug-induced apoptosis
are one of the most important reasons for drug resistance and
relapse, which is the so-called CAM-DR. Interactions of MM cells
with BM microenvironment play an important role in the pathogenesis
of myeloma and in the development of drug resistance. In the
myeloma cells after chemotherapy, the expression of both
anti-apoptosis and pro-apoptosis proteins was induced. Bcl-2, a key
apoptosis-suppressor protein, has gained more attention (28,32).
On the contrary, Bax as a pro-apoptotic member of Bcl-2 family can
induce destructive changes in mitochondria (33,34).
It has been confirmed that mitochondria could mediate intrinsic
apoptotic pathways through release of cytochrome c,
regulation of Bcl-2 family proteins, and cleavage of caspase-9 and
-3. So the Bcl-2/Bax ratio is important to MM cell fate after
chemotherapy. However, it has been reported that downregulation of
Homer1b/c using specific siRNA protects neurons against
neurotoxicity-induced apoptosis through an increase in Bcl-2 with a
decrease in Bax (14,35,36).
Thus, we suspected that, after chemotherapy, increased expression
of Homer1b/c might promote cell apoptosis through the classical
mitochondrial apoptotic pathway. Our follow-up research confirmed
our suspicions. Doxorubicin is a broad-spectrum chemotherapy drug.
It plays a role in embedding the DNA and inhibiting the synthesis
of nucleic acid. Clinically, it is used for the treatment of
various tumors, such as MM, acute myeloid leukemia (AML), acute
lymphoblastic leukemia (ALL), lung cancer, ovarian cancer, and
breast cancer (37–40). So in the model of the present study,
we used doxorubicin to induced cell apoptosis. We found that the
alteration of Homer1b/c was related to Bcl-2, Bax, cleavage of
caspase-9 and -3, which was consistent with our hypothesis that
Homer1b/c might play an essential pro-apoptotic role.
Some studies have shown that Homer1b/c could
regulate diverse cell functions being able to assemble signaling
complexes (41). However, the role
of Homer1b/c in development of MM has remained unclear. In the
present study, we have demonstrated that the expression of
Homer1b/c was significantly decreased in MM cells adhered to FN or
HS-5 cells, which suggested that Homer1b/c might be involved in the
processes of CAM-DR. All the data were compatible with the
hypothesis that decreased levels of Homer1b/c in the adhered cells
might have an impact on cell survival through the mitochondrial
pathway.
In conclusion, in the present study, we delineated
the role of Homer1b/c in multiple myeloma (MM). We found that
Homer1b/c plays an important pro-apoptotic role through classical
mitochondrial apoptotic pathway. Since cell adhesion-medicated drug
resistance remains a major obstacle for treatment of MM, we built a
cell adhesion model in MM and detected the change of Homer1b/c
protein expression. Homer1b/c siRNA reversed the high rate of MM
cell adhesion to either FN or HS-5 cells. Consistent with the
decreased adhesion rate, the cell also exhibited decreased drug
resistance. Further study should be performed to determine whether
there might be other signaling pathways for Homer1b/c during MM
cell apoptosis and CAM-DR. Aside from the pro-apoptosis effect, it
still calls for further study to confirm the clinical relevance of
Homer1b/c in the disease progression.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (nos. 81201858, 81172879 and
81372537) and the Natural Science Foundation of Jiangsu province
Grant (BK2012231); A Project Funded by the Priority Academic
Program Development of Jiangsu Higher Education Institutions
(PAPD).
References
|
1
|
Azab AK, Runnels JM, Pitsillides C, Moreau
AS, Azab F, Leleu X, Jia X, Wright R, Ospina B, Carlson AL, et al:
CXCR4 inhibitor AMD3100 disrupts the interaction of multiple
myeloma cells with the bone marrow microenvironment and enhances
their sensitivity to therapy. Blood. 113:4341–4351. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bjorklund CC, Baladandayuthapani V, Lin
HY, Jones RJ, Kuiatse I, Wang H, Yang J, Shah JJ, Thomas SK, Wang
M, et al: Evidence of a role for CD44 and cell adhesion in
mediating resistance to lenalidomide in multiple myeloma:
Therapeutic implications. Leukemia. 28:373–383. 2014. View Article : Google Scholar
|
|
3
|
Shao S, Huang X, Wang Y, He S, Xu X, Zhu
X, Yang X, Ding Z, Yao L, Huang Y, et al: A role for activator of
G-protein signaling 3 (AGS3) in multiple myeloma. Int J Hematol.
99:57–68. 2014. View Article : Google Scholar
|
|
4
|
Krönke J, Udeshi ND, Narla A, Grauman P,
Hurst SN, McConkey M, Svinkina T, Heckl D, Comer E, Li X, et al:
Lenalidomide causes selective degradation of IKZF1 and IKZF3 in
multiple myeloma cells. Science. 343:301–305. 2014. View Article : Google Scholar :
|
|
5
|
Abdi J, Garssen J and Redegeld F:
Toll-like receptors in human multiple myeloma: New insight into
inflammation-related pathogenesis. Curr Mol Med. 14:423–431. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abdi J, Mutis T, Garssen J and Redegeld
FA: Toll-like receptor (TLR)-1/2 triggering of multiple myeloma
cells modulates their adhesion to bone marrow stromal cells and
enhances bortezomib-induced apoptosis. PLoS One. 9:e966082014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cho HY and Lee SW: TLR5 activation by
flagellin induces doxorubicin resistance via interleukin-6 (IL-6)
expression in two multiple myeloma cells. Cell Immunol. 289:27–35.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Murray ME, Gavile CM, Nair JR, Koorella C,
Carlson LM, Buac D, Utley A, Chesi M, Bergsagel PL, Boise LH, et
al: CD28-mediated pro-survival signaling induces chemotherapeutic
resistance in multiple myeloma. Blood. 123:3770–3779. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pei XY, Dai Y, Felthousen J, Chen S,
Takabatake Y, Zhou L, Youssefian LE, Sanderson MW, Bodie WW, Kramer
LB, et al: Circumvention of Mcl-1-dependent drug resistance by
simultaneous Chk1 and MEK1/2 inhibition in human multiple myeloma
cells. PLoS One. 9:e890642014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fei M, Hang Q, Hou S and Ruan C: Cell
adhesion to fibronectin down-regulates the expression of Spy1 and
contributes to drug resistance in multiple myeloma cells. Int J
Hematol. 98:446–455. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kiziltepe T, Ashley JD, Stefanick JF, Qi
YM, Alves NJ, Handlogten MW, Suckow MA, Navari RM and Bilgicer B:
Rationally engineered nanoparticles target multiple myeloma cells,
overcome cell-adhesion-mediated drug resistance, and show enhanced
efficacy in vivo. Blood Cancer J. 2:e642012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Neri P and Bahlis NJ: Targeting of
adhesion molecules as a therapeutic strategy in multiple myeloma.
Curr Cancer Drug Targets. 12:776–796. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Neri P, Ren L, Azab AK, Brentnall M,
Gratton K, Klimowicz AC, Lin C, Duggan P, Tassone P, Mansoor A, et
al: Integrin β7-mediated regulation of multiple myeloma cell
adhesion, migration, and invasion. Blood. 117:6202–6213. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen T, Fei F, Jiang XF, Zhang L, Qu Y,
Huo K and Fei Z: Down-regulation of Homer1b/c attenuates
glutamate-mediated excitotoxicity through endoplasmic reticulum and
mitochondria pathways in rat cortical neurons. Free Radic Biol Med.
52:208–217. 2012. View Article : Google Scholar
|
|
15
|
Yao YX, Jiang Z and Zhao ZQ: Knockdown of
synaptic scaffolding protein Homer 1b/c attenuates secondary
hyperalgesia induced by complete Freund's adjuvant in rats. Anesth
Analg. 113:1501–1508. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lv MM, Cheng YC, Xiao ZB, Sun MY, Ren PC
and Sun XD: Down-regulation of Homer1b/c attenuates group I
metabotropic glutamate receptors dependent Ca2+
signaling through regulating endoplasmic reticulum Ca2+
release in PC12 cells. Biochem Biophys Res Commun. 450:1568–1574.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakano-Kobayashi A, Tai Y, Nadif Kasri N
and Van Aelst L: The X-linked mental retardation protein OPHN1
interacts with Homer1b/c to control spine endocytic zone
positioning and expression of synaptic potentiation. J Neurosci.
34:8665–8671. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
de Bartolomeis A, Tomasetti C, Cicale M,
Yuan PX and Manji HK: Chronic treatment with lithium or valproate
modulates the expression of Homer1b/c and its related genes Shank
and Inositol 1,4,5-trisphosphate receptor. Eur
Neuropsychopharmacol. 22:527–535. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fei F, Rao W, Zhang L, Chen BG, Li J, Fei
Z and Chen Z: Downregulation of Homer1b/c improves neuronal
survival after traumatic neuronal injury. Neuroscience.
267:187–194. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zeng X, Pan ZG, Shao Y, Wu XN, Liu SX, Li
NL and Wang WM: SKF-96365 attenuates toxin-induced neuronal injury
through opposite regulatory effects on Homer1a and Homer1b/c in
cultured rat mesencephalic cells. Neurosci Lett. 543:183–188. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miletic G, Miyabe T, Gebhardt KJ and
Miletic V: Increased levels of Homer1b/c and Shank1a in the
post-synaptic density of spinal dorsal horn neurons are associated
with neuropathic pain in rats. Neurosci Lett. 386:189–193. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Azab F, Vali S, Abraham J, Potter N, Muz
B, de la Puente P, Fiala M, Paasch J, Sultana Z, Tyagi A, et al:
PI3KCA plays a major role in multiple myeloma and its inhibition
with BYL719 decreases proliferation, synergizes with other
therapies and overcomes stroma-induced resistance. Br J Haematol.
165:89–101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Emmons MF, Gebhard AW, Nair RR, Baz R,
McLaughlin ML, Cress AE and Hazlehurst LA: Acquisition of
resistance toward HYD1 correlates with a reduction in cleaved α4
integrin expression and a compromised CAM-DR phenotype. Mol Cancer
Ther. 10:2257–2266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fei M, Hang Q, Hou S, He S and Ruan C:
Adhesion to fibronectin induces p27(Kip1) nuclear accumulation
through down-regulation of Jab1 and contributes to cell
adhesion-mediated drug resistance (CAM-DR) in RPMI 8,226 cells. Mol
Cell Biochem. 386:177–187. 2014. View Article : Google Scholar
|
|
25
|
Noborio-Hatano K, Kikuchi J, Takatoku M,
Shimizu R, Wada T, Ueda M, Nobuyoshi M, Oh I, Sato K, Suzuki T, et
al: Bortezomib overcomes cell-adhesion-mediated drug resistance
through downregulation of VLA-4 expression in multiple myeloma.
Oncogene. 28:231–242. 2009. View Article : Google Scholar
|
|
26
|
Huang X, Wang Y, Nan X, He S, Xu X, Zhu X,
Tang J, Yang X, Yao L, Wang X, et al: The role of the orphan G
protein-coupled receptor 37 (GPR37) in multiple myeloma cells. Leuk
Res. 38:225–235. 2014. View Article : Google Scholar
|
|
27
|
Morelli MB, Offidani M, Alesiani F,
Discepoli G, Liberati S, Olivieri A, Santoni M, Santoni G, Leoni P
and Nabissi M: The effects of cannabidiol and its synergism with
bortezomib in multiple myeloma cell lines. A role for transient
receptor potential vanilloid type-2. Int J Cancer. 134:2534–2546.
2014. View Article : Google Scholar
|
|
28
|
Paulus A, Chitta K, Akhtar S, Personett D,
Miller KC, Thompson KJ, Carr J, Kumar S, Roy V, Ansell SM, et al:
AT-101 downregulates BCL2 and MCL1 and potentiates the cytotoxic
effects of lenalidomide and dexamethasone in preclinical models of
multiple myeloma and Waldenström macroglobulinaemia. Br J Haematol.
164:352–365. 2014. View Article : Google Scholar
|
|
29
|
Ramírez-Labrada A, López-Royuela N,
Jarauta V, Galán-Malo P, Azaceta G, Palomera L, Pardo J, Anel A,
Marzo I and Naval J: Two death pathways induced by sorafenib in
myeloma cells: Puma-mediated apoptosis and necroptosis. Clin Transl
Oncol. 17:121–132. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sook SH, Lee HJ, Kim JH, Sohn EJ, Jung JH,
Kim B, Kim JH, Jeong SJ and Kim SH: Reactive oxygen
species-mediated activation of AMP-activated protein kinase and
c-Jun N-terminal kinase plays a critical role in
beta-sitosterol-induced apoptosis in multiple myeloma U266 cells.
Phytother Res. 28:387–394. 2014. View
Article : Google Scholar
|
|
31
|
Stephens M, McKenzie H and Jordens CF: The
work of living with a rare cancer: Multiple myeloma. J Adv Nurs.
70:2800–2809. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guo S, Zhi Y, Yang H, Yu Y, Wang Y, Zhang
J, Wang G, Zhang L, Sun B and Zhang Y: Bcl-2 expression is
associated with poor prognosis of solitary plasmacytoma of bone.
Ann Hematol. 93:471–477. 2014. View Article : Google Scholar
|
|
33
|
Ding JH, Yuan LY, Huang RB and Chen GA:
Aspirin inhibits proliferation and induces apoptosis of multiple
myeloma cells through regulation of Bcl-2 and Bax and suppression
of VEGF. Eur J Haematol. 93:329–339. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Korpis K, Weber F, Brune S, Wünsch B and
Bednarski PJ: Involvement of apoptosis and autophagy in the death
of RPMI 8226 multiple myeloma cells by two enantiomeric sigma
receptor ligands. Bioorg Med Chem. 22:221–233. 2014. View Article : Google Scholar
|
|
35
|
Fresquet V, Rieger M, Carolis C,
García-Barchino MJ and Martinez-Climent JA: Acquired mutations in
BCL2 family proteins conferring resistance to the BH3 mimetic
ABT-199 in lymphoma. Blood. 123:4111–4119. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yadav P, Singh DD, Mukesh M, Kataria RS,
Yadav A, Mohanty AK and Mishra BP: Expression profiling of glucose
transporter 1 (GLUT1) and apoptotic genes (BAX and BCL2) in milk
enriched mammary epithelial cells (MEC) in riverine buffalo during
lactation. Anim Biotechnol. 25:151–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mantawy EM, El-Bakly WM, Esmat A, Badr AM
and El-Demerdash E: Chrysin alleviates acute doxorubicin
cardiotoxicity in rats via suppression of oxidative stress,
inflammation and apoptosis. Eur J Pharmacol. 728:107–118. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pei XM, Yung BY, Yip SP, Ying M, Benzie IF
and Siu PM: Desacyl ghrelin prevents doxorubicin-induced myocardial
fibrosis and apoptosis via the GHSR-independent pathway. Am J
Physiol Endocrinol Metab. 306:E311–E323. 2014. View Article : Google Scholar
|
|
39
|
Poornima P, Kumar VB, Weng CF and Padma
VV: Doxorubicin induced apoptosis was potentiated by neferine in
human lung adenocarcima, A549 cells. Food Chem Toxicol. 68:87–98.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shokoohinia Y, Hosseinzadeh L, Moieni-Arya
M, Mostafaie A and Mohammadi-Motlagh HR: Osthole attenuates
doxorubicin-induced apoptosis in PC12 cells through inhibition of
mitochondrial dysfunction and ROS production. Biomed Res Int.
2014:1568482014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chiarello C, Bortoloso E, Carpi A, Furlan
S and Volpe P: Negative feedback regulation of Homer 1a on
norepinephrine-dependent cardiac hypertrophy. Exp Cell Res.
319:1804–1814. 2013. View Article : Google Scholar : PubMed/NCBI
|