Introduction
Lung cancer is reported to be the leading cause of
cancer-related mortality in both men and women worldwide (1,2).
Moreover, the reported overall 5-year survival rate is only 15%
partially due to the low efficacy of current treatment techniques
(1). Thus, novel treatments are
urgently needed for this type of cancer. Pathologically almost 80%
of all lung cancer cases are non-small cell lung cancer (NSCLC).
Cancer-associated fibroblasts (CAFs) have been found to exist in a
large number of NSCLCs (3). With
the development of its research, CAFs have been proven to promote
tumor progression, metastasis and resistance to therapy through
paracrine effects in most solid tumors (4,5).
Soluble factors secreted from CAFs include TGF-β family members,
EGF family members, chemokines and many other molecules. Among all
these factors, stromal cell-derived factor 1 (SDF-1), which is
expressed at elevated level in CAFs, was discovered to perform an
important role in tumor cell survival, metastasis and
chemoresistance through activation of its receptor, CXCR4 (4). However, how SDF-1 functions during the
interaction between CAFs and lung cancer cells is not yet
completely clear.
Chemokine receptors are one type of seven
transmembrane G-protein-coupled receptors. They are classified into
four different groups as CXC, CX3C, CC and XC according to the
chemokines they primarily interact with for signaling (6). CXCR4, one member of the chemokine
receptor family, has been found to be overexpressed in more than 23
different types of cancers including lung, prostate and breast
cancer as well as leukemia (6,7). It
exerts its function in tumor cell survival, metastasis and
therapeutic resistance mainly through the PI3K/AKT/NF-κB axis as
well as the ERK1/2/NF-κB axis after interaction with its ligand
SDF-1 (8). Meanwhile, activation of
NF-κB has been linked to tumor cell survival and cancer therapy
resistance partially through regulating the expression level of
Bcl-xL, one member of the anti-apoptotic genes (9). However, whether NF-κB and Bcl-xL are
involved in the interaction between CAFs and lung cancer cells is
not quite certain.
Although SDF-1 as well as many other soluble factors
are highly expressed in CAFs, the molecular mechanisms controlling
their expression levels are unclear. MicroRNAs, since firstly
discovered in 1993, have been reported to play important roles in
many aspects of cell biology, including cell proliferation,
apoptosis, cell cycle and carcinogenesis (10). Numerous studies have demonstrated
that the expression of microRNAs in tumors are either upregulated
or downregulated compared with their expression level in normal
tissues (11). Downregulation of
microRNAs has been discovered to be related with tumor suppression.
As one of the tumor-suppressing microRNAs, mir-1 was found to be
consistently expressed at a very low level in many tumors including
lung cancer (12), which was
consistent with our data shown in Fig.
2C. Moreover, mir-1 was found to directly regulate the
expression of SDF-1 and CXCR4 in thyroid cancer (13). However, whether mir-1 targets SDF-1
in CAFs and affects its role in the interaction between CAFs and
lung cancer cells has not yet been reported. Therefore, in the
present study, by employing lung cancer cell lines A549 and 95D and
isolation of primary CAFs, we investigated the involvement of SDF-1
in the interaction between CAFs and lung cancer cell lines and
demonstrated that SDF-1 was able to activate NF-κB and Bcl-xL by
interacting with CXCR4 in the A549 and 95D cells. Furthermore, in
our experiments, mir-1 was confirmed to negatively regulate the
expression of SDF-1. In conclusion, we discovered that by secretion
of SDF-1, which was regulated by mir-1, CAFs were able to enhance
cell proliferation and the drug resistance to cisplatin in the A549
and 95D cells. These results also suggest CAFs as a potential
therapeutic target in tumor treatment.
Materials and methods
Reagents and antibodies
Dulbecco's modified Eagle's medium (DMEM):nutrient
mixture F-12 (DMEM/F-12) and fetal bovine serum (FBS) were
purchased from GE Healthcare HyClone (Logan, UT, USA). Rabbit
anti-human fibroblast activation protein (FAP), mouse anti-human
α-smooth muscle actin (α-SMA), mouse anti-human CXCR4, mouse
anti-human NF-κB and rabbit anti-human β-actin antibodies were
provided by Cell Signaling Technology (Danvers, MA, USA). Mouse
anti-human α-SMA and rabbit anti-human Bcl-xL antibodies were
obtained from Santa Cruz Biotechnology (Dallas, TX, USA). Goat
anti-mouse IgG/HRP, goat anti-rabbit IgG/HRP, FITC-labeled goat
anti-mouse IgG and TRITC-labeled goat anti-rabbit IgG were
purchased from KPL, Inc. (Gaithersburg, MD, USA). All primers for
mir-1, SDF-1α, α-SMA, FAP, U6 and GAPDH as well as the sequence of
CXCR4 siRNA and mir-1 mimics were synthesized by GenePharma
(Shanghai, China). Human CXCR4 plasmid was constructed by Yrbio
(Changsha, China).
Primary lung cancer CAF and normal
fibroblast (NF) cultures
Lung cancer and normal lung tissues were obtained
from patients with lung cancer after obtaining informed written
contents. Normal lung tissues were collected from the same patients
at locations at least 5 cm away from the tumor sites. Both types of
tissues were kept in DMEM/F12 supplemented with penicillin (100
U/ml) and streptomycin (50 µg/ml). Primary cell isolation
was performed within 2 h after excision. Briefly, lung cancer and
normal lung tissues were cut into small pieces with a size of ~1
mm3 and planted in cell culture flasks. DMEM/F12
supplemented with 10% FBS, penicillin (100 U/ml) and streptomycin
(50 µg/ml) were added to the flasks. Seventy-two hours
later, the cells outgrew from the explant culture and medium was
changed gently. Then, afterwards, the medium was changed twice a
week. Subculture was performed when cells reached 80% confluency at
the split ratio of 1:3.
Immunofluorescence staining
CAFs and NFs at passages 2–4 were seeded on
coverslips in a 24-well plate on the day before staining. On the
next day, the cells were fixed with 4% paraformaldehyde for 30 min
at room temperature. After permeabilization with 0.3% Triton X-100
for 5 min, the cells were incubated with primary antibodies to FAP
and α-SMA overnight at 4°C. Then, on the second day, FITC and
TRITC-labeled secondary antibodies were added after washing for 3
times with 1X PBS and kept for 2 h at room temperature. Finally,
the coverslips were mounted on microslides and examinated under an
inverted fluorescence microscope. Images were captured under a
magnification of ×200.
MTT assay
Ten microliters of MTT (5 mg/ml) were added into
96-well plates with cells at 5,000 cells/well and incubated for 4 h
in cell incubators at 37°C in the absence of light. Then, culture
medium was gently damped and dimethyl sulfoxide (DMSO) was added to
stop the reaction. Optical densities (ODs) were measured with a
microplate reader at 490 nm.
Enzyme-linked immunosorbent assay
(ELISA)
The secretion of SDF-1 from CAFs and NFs into
culture medium was measure by SDF-1α (human) ELISA kit (Abnova,
Taipei, Taiwan). CAFs and NFs were maintained in T-25 culture
flasks. When cells reached confluency, the medium was replenished
with 5 ml fresh medium. Twenty-four hours later, the culture
supernatant was collected and kept at −80°C before measurement. All
steps were carried out according to the manufacturer's
instructions.
CXCR4 overexpression and silencing
CXCR4 upregulation or downregulation in the A549 and
95D cells were carried out with CXCR4 plasmids or CXCR4 siRNA
transfection with Lipofectamine 2000 in a 6-well plate,
respectively. The sequences of CXCR4 siRNA were:
5′-GGGACUAUGACUCCAUGAATTUUCAUGGAGUCAUAGUCCCTT-3′. All steps were
carried out according to the manufacturer's instructions. Western
blotting was used to evaluate the transfection efficacy 24 h after
transfection.
Assessment of cell proliferation
The cell proliferation rate of the A549 and 95D
cells after CXCR4 overexpression and silencing was measured with
MTT assay. Cells were divided into 3 groups: cells only, cells +
CXCR4 (CXCR4-overexpressing cells) and cells + CXCR4 siRNA
(CXCR4-silenced cells). Twenty-four hours after transfection, the
cells were seeded into a 96-well plate, and MTT assay was carried
out 24, 48 and 72 h after cells were seeded.
The cell proliferation rate of the A549 and 95D
cells after incubation with CAFs or addition of the CAF supernatant
was assessed with the MTT assay. In the present study, AMD3100, one
type of CXCR4 inhibitor and recombinant human chemokine SDF-1 were
used to verify their effect on the proliferation rate of the A549
and 95D cells. The cells in the present study were divided into
groups as follows: cells only, cells + CAF supernatant, cells + 10
ng/ml SDF-1, cells + CAFs co-culture, and cells + CAFs co-culture +
10 µg/ml AMD3100. A549 or 95D cells were seeded into a
96-well plate 24 h before co-culturing with CAFs or addition of
SDF-1, AMD3100 or CAF supernatant. Afterwards, MTT assay was
carried out 24, 48 and 72 h, later.
Assessment of the drug resistance to
cisplatin
Drug resistance of the A549 and 95D cells after
CXCR4 overexpression and silencing was measured using the MTT
assay. The cells were divided into 3 groups: cells only, cells +
CXCR4 (CXCR4-overexpressing cells) and cells + CXCR4 siRNA
(CXCR4-silenced cells). Twenty-four hours after transfection, the
cells were seeded into a 96-well plate, and cisplatin was added on
the next day. Concentrations of cisplatin applied in the present
study were 0, 0.6, 1.2, 2.4, 3.6 and 7.2 µg/ml. MTT assay
was carried out 24 h after cisplatin was added. Cell viability at
the concentration of 0 µg/ml was set as 100%; cell viability
at all other concentrations was calculated accordingly.
The drug resistance of the A549 and 95D cells after
incubation with the CAFs or addition of the CAF supernatant was
tested using the MTT assay. In the present study, AMD3100, one type
of CXCR4 inhibitor and recombinant human chemokine SDF-1 were used
to verify their effect on the drug resistance of the A549 and 95D
cells. The cells in the present study were divided into groups as
follows: cells only, cells + CAF supernatant, cells + 10 ng/ml
SDF-1, cells + CAFs co-culture, and cells + CAFs co-culture +10
µg/ml AMD3100. A549 or 95D cells were seeded into a 96-well
plate 24 h before co-culturing with CAFs or the addition of SDF-1,
AMD3100 or CAF supernatant. Cisplatin was added also on the next
day after cells were seeded. Concentrations of cisplatin applied in
the present study were 0, 0.6, 1.2, 2.4, 3.6 and 7.2 µg/ml.
MTT assay was carried out 24 h after cisplatin was added. Cell
viability at the concentration of 0 µg/ml was set as 100%;
cell viability at all other concentrations was calculated,
accordingly.
MicroRNA mir-1 overexpression
The expression level of mir-1 in CAFs was elevated
by transient mir-1 mimic transfection with Lipofectamine 2000 in a
6-well plate. The duplex sequences of mir-1 mimics were: sense,
5′-ACAUACUUCUUUACAUUCCATT-3′ and antisense,
5′-UGGAAUGUAAAGAAGUAUGUAU-3′. All steps were carried out according
to the manufacturer′s instructions. Quantitative PCR was utilized
to evaluate the transfection efficacy 24 and 48 h after
transfection.
Quantative PCR
Total RNA was extracted from the cells using TRIzol
reagent and used for cDNA synthesis. mir-1 reverse transcription
was conducted with a microRNA-specific primer using the miScript
Reverse Transcription kit (Qiagen, Hilden, Germany). For mRNA
reverse transcription, total RNA was reverse transcribed with the
SuperScript Reverse Transcription kit (Thermo Fisher, Waltham, MA,
USA). Quantitative real-time PCR was performed using SYBR-Green
Master Mix (Bio-Rad, Hercules, CA, USA). The following primers were
used: mir-1-forward, 5′-CTG TCACTCGAGCTGCTGGAATG-3′ and
mir-1-reverse, 5′-ACCGTGTCGTGGAGTCGGCAATT-3′; SDF-1α-forward,
5′-CCAAACTGTGCCCTTCAGAT-3′ and SDF-1α-reverse,
5′-CTTTAGCTTCGGGTCAATGC-3′; α-SMA-forward,
5′-AGCTACCCGCCCAGAAACTA-3′ and α-SMA-reverse,
5′-ATGATGCCGTGCTCGATAGG-3′; FAP-forward, 5′-TGTGCATTGTCTTACGCCCT-3′
and FAP-reverse, 5′-GAGTATCTCCAAAGCATGGTTCTA-3′; GAPDH-forward,
5′-CCAGGTGGTCTCCTCTGA-3 and GAPDH-reverse,
5′-GCTGTAGCCAAATCGTTGT-3′; U6-forward, 5′-CTCGCTTCGGCAGCACA-3′ and
U6-reverse, 5′-AACGCTTCACGAATTTGCGT-3′. The mRNA expression values
were normalized to GAPDH. The microRNA expression values were
normalized to U6. Relative expression levels of microRNA or mRNA
were analyzed using the Bio-Rad C1000 Thermal Cycler.
Western blotting
Whole-cell protein was extracted with protein lysis
solution [50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% SDS, 0.5%
sodiumdeoxycholate and 0.5% Triton X-100] on ice. Protease
inhibitors were added into the cellular lysate. The protein
concentration was measured using a bicinchoninic acid assay.
Western blot analysis was performed as previously described
(14) using the following
antibodies: mouse anti-human CXCR4, mouse anti-human NF-κB, rabbit
anti-human Bcl-xL and rabbit anti-human β-actin antibodies.
Briefly, equal amounts of protein (50 ng) were separated by
SDS-PAGE and transferred to polyvinylidene difluoride (PVDF)
membranes. HRP-conjugated goat anti-rabbit or mouse IgG was used as
a secondary antibody. Bound fragments were detected with ECL
chemiluminescence kit (Pierce) and exposed on X-film.
Statistical analysis
The Student's t-test was applied to analyze
statistical differences between two groups. p<0.05 was
considered to be statistically significant. Each test data from
independent experiments were repeated at least 3 times.
Results
Isolation and culture of CAFs and
NFs
To investigate the effect of CAFs on the drug
resistance of lung cancer, we isolated CAFs and NFs from lung
cancer and normal lung tissues of the same patient following
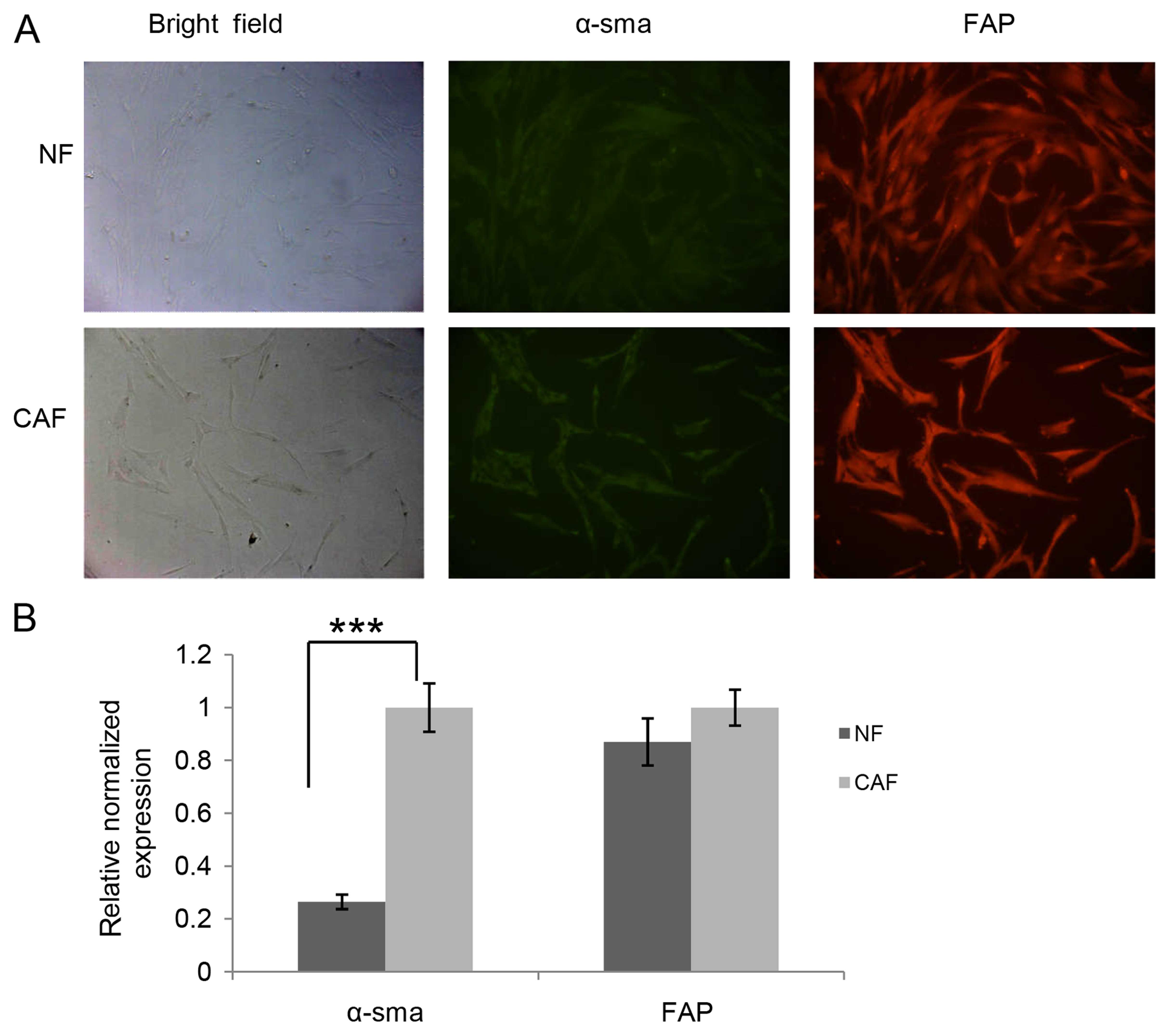
written content. Since CAFs and NFs display similar spindle-like
morphology in culture, we carried out immunofluorescence staining
and quantitative PCR to compare the expression level of α-SMA and
FAP. CAFs exhibited a higher amount of α-SMA at the mRNA (Fig. 1B) and protein level (Fig. 1A) than NFs, whereas there were no
significant differences in FAP expression at the mRNA (Fig. 1B) and protein level (Fig. 1A) between CAFs and NFs. Meanwhile,
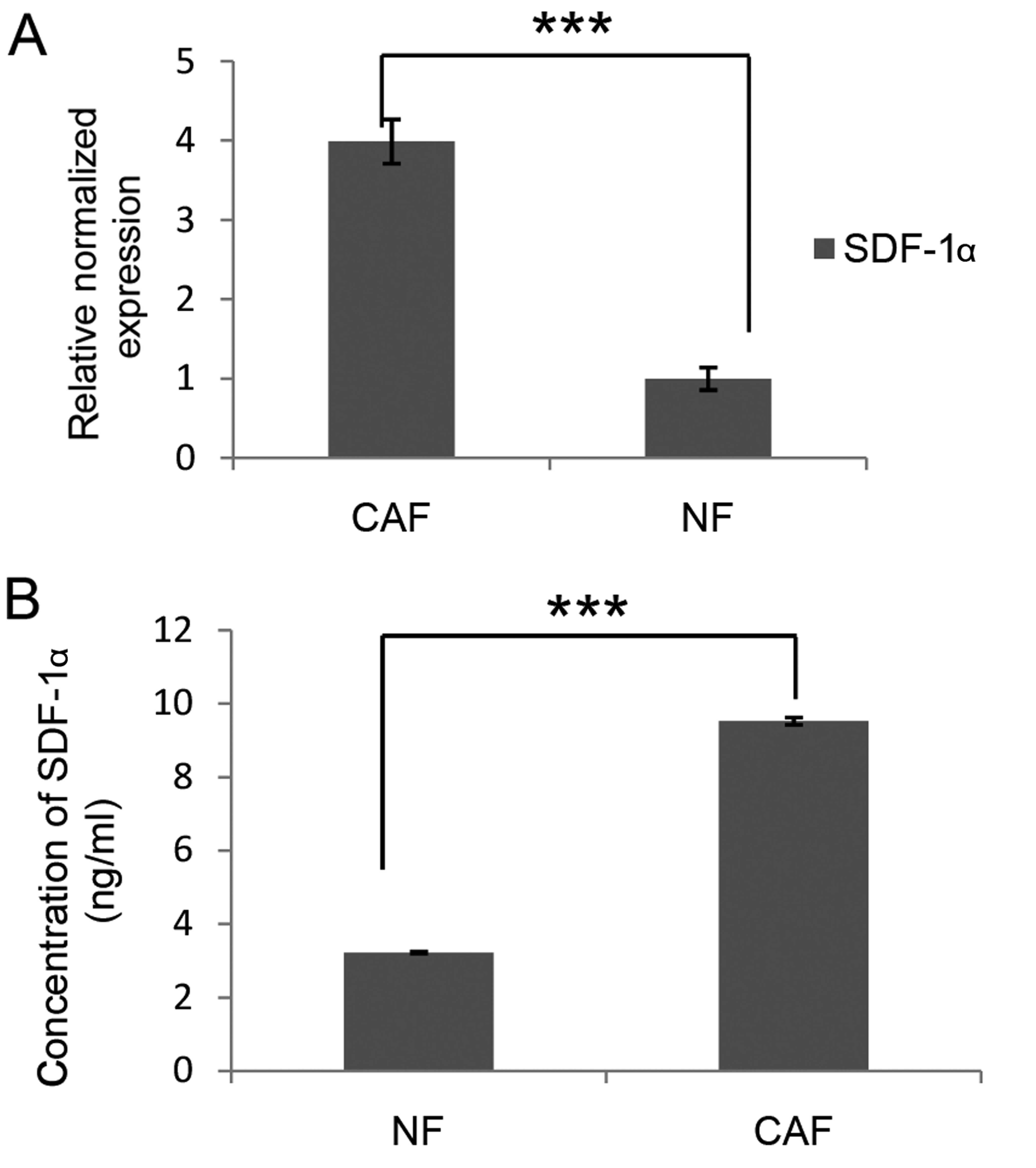
we also compared the expression and secretion of SDF-1α from CAF
and NFs. Data in Fig. 2A showed
that CAFs transcribed much more SDF-1 mRNA than NFs. Furthermore,
ELISA data indicated that the concentration of SDF-1α in the CAF
culture supernatant was 9.53 ng/ml, while that in the NF culture
supernatant was only 3.23 ng/ml (Fig.
2B). Local NFs are considered to be one origin of CAFs. The
expression of α-SMA and SDF-1α is significantly upregulated during
transformation from NFs to activated CAFs (15). Taken together, these results imply
that CAFs derived from patients with lung cancer may be
reprogrammed from local NFs and exert their functions via secretion
of SDF-1α.
Effects of CXCR4 on cell proliferation
and drug resistance of A549 and 95D cells
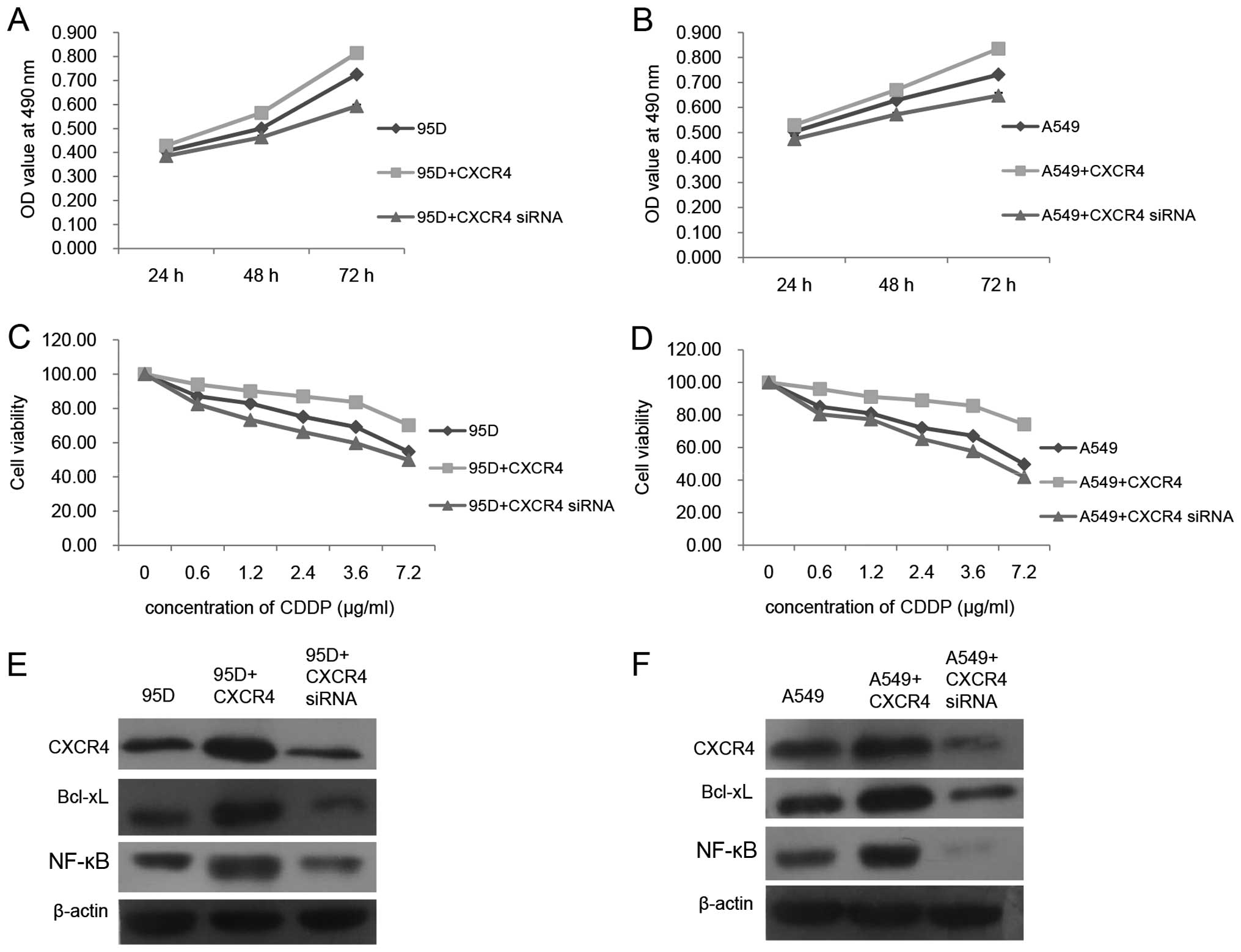
To illuminate the function of CXCR4 in the cell
proliferation and drug resistance of A549 and 95D cells, we
performed CXCR4 plasmid transfection and CXCR4 siRNA transfection
to upregulate and downregulate its expression level in A549 and 95D
cells, respectively. As shown in Fig.
3A and B, CXCR4 upregulation elevated the cell proliferation
rate of the A549 and 95D cells, whereas CXCR4 silencing had exactly
the opposite effects. Simultaneously, chemoresistance of A549 and
95D to cisplatin (CDDP) was enhanced or suppressed as the CXCR4
expression level was increased or decreased, respectively (Fig. 3C and D). As reported, CXCR4 plays a
role in tumor cell growth, survival and drug resistance mainly
through the PI3K/AKT/NF-κB axis as well as the ERK1/2/NF-κB axis
(8). Meanwhile, NF-κB activation or
upregulation usually leads to transcriptional activation of genes
such as Bcl-xL to suppress apoptosis (9). Therefore, western blotting was
utilized to reveal the involvement of Bcl-xL and NF-κB during CXCR4
overexpression and silencing. As demonstrated in Fig. 3E and F, we obtained similar data
from the A549 and 95D cells. The protein levels of Bcl-xL and NF-κB
were both escalated as CXCR4 was overexpressed, while their protein
levels were both declined as CXCR4 was silenced. These data
indicated that, in the A549 and 95D cells, activation of NF-κB and
Bcl-xL could be possible signaling transduction factors for CXCR4
to promote its role in cell proliferation and drug resistance.
Effects of CAFs on cell proliferation and
drug resistance of A549 and 95D cells
To reveal the effects of CAFs on cell proliferation
and drug resistance of A549 and 95D, a Transwell-base co-culture
system and CAF culture supernatant were applied to the A549 and 95D
cells, respectively. As in the Transwell-base co-culture system,
A549 and 95D cells were seeded in 96-well plates at the
concentration of 5,000/well, and CAFs were seeded in the inserts at
the concentration of 5,000/well, as well. As shown in Fig. 4A and B, the OD values of the 95D and
A549 cells were both increased after co-culture with the CAFs.
Moreover, cell viability of the 95D and A549 cells in the cisplatin
suspension was also increased after co-culture with the CAFs. CAFs
are routinely cultivated in T25 flasks. When they reached
confluency, which was ~1×106 in each flask according to
our counting, the culture medium was replaced with 5 ml fresh
medium. Twenty-four hours later, the culture supernatant was
collected. With the addition of the CAF culture supernatant, the OD
values of the 95D and A549 cells were also increased compared with
that in the control group (Fig. 4A and
B). Meanwhile, the cell viability of the 95D and A549 cells in
cisplatin suspension was increased with the addition of the CAF
culture supernatant as well (Fig. 4C
and D). The data we obtained using co-culture techniques and
supernatant were consistent with each other. As reported, CAFs are
capable of affecting tumor growth, survival and chemoresistance by
secretion of TGF-β1, SDF-1 and other small molecules (5). To verify the involvement of SDF-1 in
the present study, we simultaneously assessed the effects of SDF-1α
on the cell proliferation and drug resistance of the A549 and 95D
cells. As indicated by the data in Fig.
4, SDF-1α exhibited capability similar to the CAF culture
supernatant as well as the CAF co-culture system. Furthermore, we
added CXCR4 inhibitor-AMD3100 to test whether or not CXCR4 was
involved. As hypothesized, 10 µg/ml AMD3100 attenuated the
cell viability of the A549 and 95D cells in the cisplatin
suspension and slowed down the cell proliferation rate of the A549
and 95D cells compared with all other groups. These data indicate
that the SDF-1/CXCR4 axis played an important role in the effects
of CAFs on the cell proliferation and drug resistance of the A549
and 95D cells.
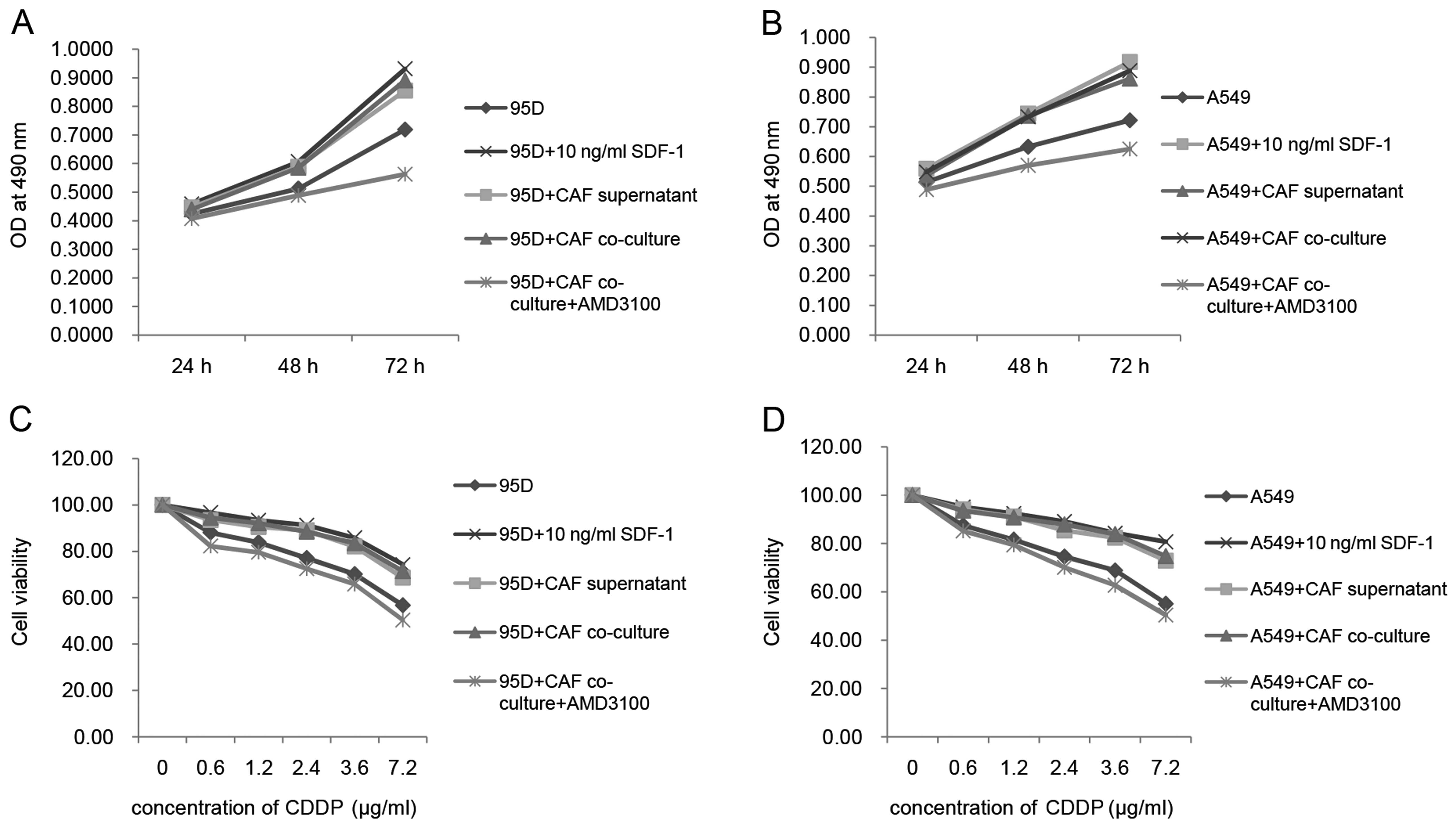 | Figure 4CAFs enhance the cell proliferation
and drug resistance of A549 and 95D cells through SDF-1 secretion.
(A and B) MTT assay was applied to measure the proliferation rate
of A549 and 95D cells after co-cultured with CAFs or addition of
CAF supernatant. In the present study, cells + 10 ng/ml SDF-1 was
set as a positive control. CAF supernatant, CAF co-culture and 10
ng/ml SDF-1 exerted similar positive effects on the proliferation
of A549 and 95D cells. AMD3100, one type of CXCR4 inhibitor, was
added to the cells co-cultured with CAFs to block the interaction
between SDF-1 and CXCR4. In this group, proliferation of the A549
and 95D cells was decreased compared with that in all other groups,
even in the control group. (C and D) MTT assay data indicated that
drug-resistance of the A549 and 95D cells was increased after
co-cultured with CAFs or addition of CAFs supernatant.
Concentrations of cisplatin applied in the present study were 0,
0.6, 1.2, 2.4, 3.6 and 7.2 µg/ml. This assay was performed
24 h after drug addition into cell culture. Cell viability at the
concentration of 0 µg/ml was set as 100%; cell viability at
all other concentrations was accordingly calculated. In the present
study, cells + 10 ng/ml SDF-1 was set as a positive control. CAF
supernatant, CAF co-culture and 10 ng/ml SDF-1 exerted similar
positive effects on the drug-resistance of A549 and 95D cells.
AMD3100 (10 µg/ml), one type of CXCR4 inhibitor, was added
to the cells co-cultured with CAFs to block the interaction between
SDF-1 and CXCR4. In this group, the drug resistance of the A549 and
95D cells was inhibited compared with that in all other groups,
even in the control group. |
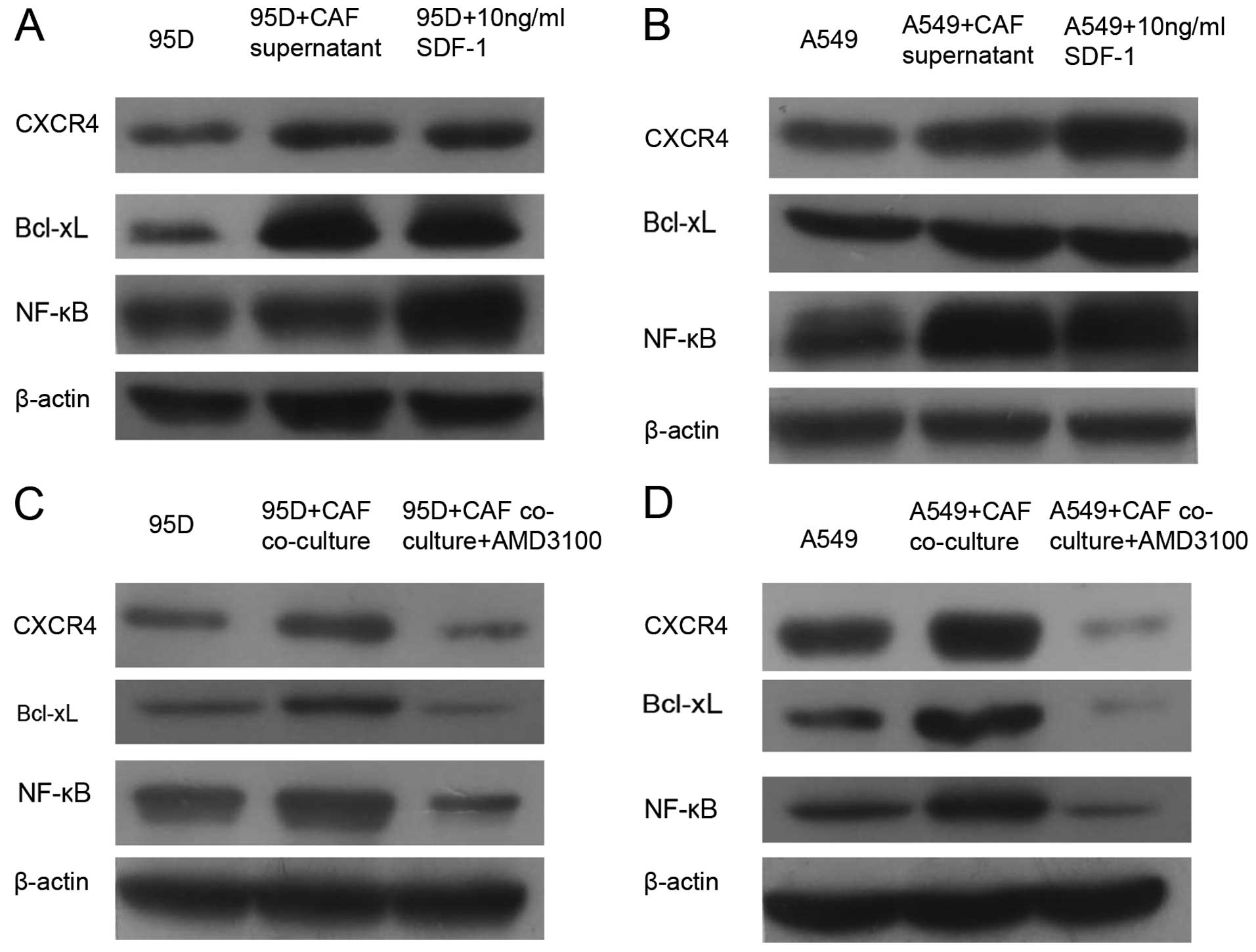
Effects of CAFs on signaling
transductions in the A549 and 95D cells
To elucidate the signaling passways in A549 and 95D
cells that are influenced by the paracrine effect of CAFs, we
compared the protein levels of NF-κB and Bcl-xL among the groups by
western blotting. As shown in Fig.
5, the CAF supernatant and CAF co-culture system had similar
effects on the expression of CXCR4, NF-κB and Bcl-xL. The protein
level of CXCR4 was increased by addition of the CAF supernatant as
well as co-culture with CAFs. In addition, subsequently, the
expression levels of NF-κB and Bcl-xL were both upregulated. To
confirm the participation of SDF-1, we also measured the expression
level of CXCR4, NF-κB and Bcl-xL after addition of recombinant
human SDF-1. As indicated in Fig. 5A
and B, the protein levels of CXCR4, NF-κB and Bcl-xL were all
elevated by direct addition of 10 ng/ml SDF-1 into the cell culture
of A549 and 95D. Furthermore, we also assessed the expression of
Bcl-xL and NF-κB after blocking the activation of CXCR4 with
AMD3100. As shown in Fig. 5C and D,
both Bcl-xL and NF-κB were downregulated following the addition of
10 µg/ml AMD3100 into the cell culture of A549 and 95D.
Taken together, these above data suggested that upregulation of
NF-κB and Bcl-xL through the SDF-1/CXCR4 axis could be the
molecular mechanisms behind the paracrine effects of CAFs on the
A549 and 95D cells.
Regulation of mir-1 on SDF-1 synthesis in
CAFs and its subsequent effects on downstream signaling
transduction in A549 and 95D cells
mir-1, as a tumor-suppressor microRNA, was reported
to be expressed at a very low level in lung cancer and other cancer
types (12). SDF-1 was identified
as one of its targets in head and neck tumors (13). In the present study, we upregulated
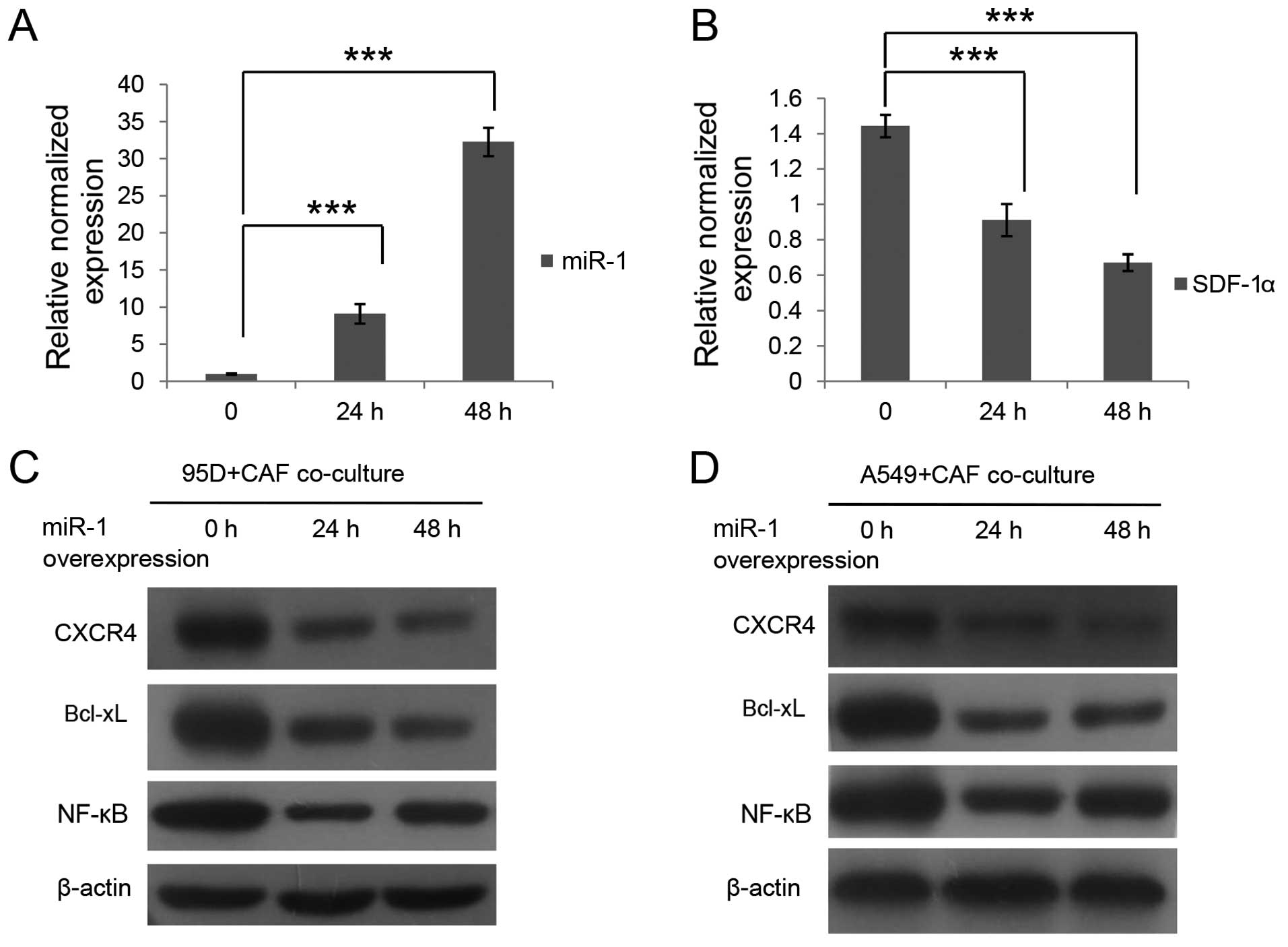
mir-1 expression in the CAFs by mir-1 mimic transfection (Fig. 6A). In addition, consequently, the
expression level of SDF-1 was decreased as indicated in Fig. 6B. We also detected the expression
levels of CXCR4, NF-κB and Bcl-xL in the A549 and 95D cells after
co-culture with mir-1-overexpressing CAFs. As demonstrated by the
data in Fig. 6C and D, the protein
levels of CXCR4, NF-κB and Bcl-xL in the A549 and 95D cells
declined after co-culture with the mir-1-overexpressing CAFs,
although changes were not apparently time-dependent. These results
revealed that mir-1 negatively regulated the expression of SDF-1
and that mir-1 overexpression consequently downregulated the
expression of CXCR4, NF-κB and Bcl-xL in the A549 and 95D cells by
co-culturing with mir-1-upregulated CAFs.
Discussion
The tumor microenvironment plays an important role
in cancer development and progression. CAFs, the dominant component
of the tumor microenvironment, have been shown to be crucial for
tumor cell proliferation, survival and therapeutic resistance
(4). In this present study, we
first isolated CAFs from patient tissues and demonstrated that they
promoted cell proliferation and chemoresistance to cisplatin in
lung cancer cell lines A549 and 95D in a paracrine manner.
Secondly, using ELISA and quantitative PCR, we found that a higher
amount of SDF-1 existed in the CAFs when compared with that in the
NFs. Thirdly, we found that SDF-1 facilitated lung cancer cell
proliferation and drug resistance via the CXCR4-mediated signaling
passway which involved NF-κB and Bcl-xL. Moreover, we also
confirmed that the expression level of SDF-1 in CAFs was negatively
regulated by microRNA mir-1.
CAFs are heterogeneous and poorly defined to date.
α-SMA, FAP, vimentin and many other markers have been reported to
characterize them (16). In the
present study, we employed α-SMA and FAP. As shown in our results,
α-SMA and FAP were both highly expressed in the CAFs, which
indicated that lung cancer CAFs may resemble myofibroblasts since
co-expression of α-SMA and FAP is characteristic of myofibroblasts
(4). However, we also found that
FAP was expressed in NFs and no significant difference at the mRNA
level was detected between NFs and CAFs. Meanwhile, a higher
expression level of SDF-1 was observed in the CAFs when compared
with that in the NFs. Therefore, α-SMA and SDF-1 may be superior
markers of lung cancer CAFs than FAP.
Therapeutic resistance of lung cancer, particularly
NSCLC, is mainly due to improved survival ability of cells and
metastasis (1). Numerous studies
have established the correlation between CXCR4 expression and poor
prognosis in NSCLC. CXCR4 overexpression was reported to be
associated with poor survival in stage IV NSCLC patients (17). In our results, elevated CXCR4
expression in lung cancer cell lines A549 and 95D promoted cell
proliferation and drug resistance to cisplatin. The protein levels
of NF-κB and Bcl-xL were found to be increased in the
CXCR4-overexpressing A549 and 95D cells as well as in tumor cells
co-cultured with CAFs. Furthermore, co-culture with the CAFs also
induced upregulation of CXCR4 expression in the A549 and 95D cells,
which was attenuated by addition of AMD3100. In previous studies by
other research groups, NF-κB was found to suppress apoptosis by
activating TRAF1 and TRAF2, as well as bcl-2 homologues A1/Bfl-1
and Bcl-xL (9). Moreover, NF-κB was
also found to be involved in cell growth and angiogenesis by
regulating the expression of ICAM-1 and Cox-2 (9). Taken together, our data may provide
explanations for the correlation between CXCR4 expression and the
poor outcome of lung cancer and offer evidence for the potential
therapeutic application of targeting CAFs or the SDF-1/CXCR4
axis.
MicroRNAs exert their regulatory role in a variety
of biological process. Recently, they have been demonstrated to
function as oncogenes or tumor-suppressor genes in many types of
cancers. mir-1 as well as mir-148 have been identified as tumor
suppressors (10,11,18).
Both were discovered to be expressed at a very low level in many
types of tumors including lung cancer. mir-148 was reported to
suppress metastasis and mir-1 was found to be involved in tumor
cell proliferation, metastasis and apoptosis (10,18).
However, not much is known concerning their functions in CAFs. In
the present study, mir-1 expression in lung cancer CAFs was
measured, which was lower than that in the NFs. Our data also
indicated that mir-1 negatively regulates the expression of SDF-1,
which plays an important role during the interaction between CAFs
and tumor cells. Thus, microRNA expression was altered in CAF
formation and affected CAF functions. Meanwhile, to the best of our
knowledge, this was the first study to report the function of mir-1
in lung cancer CAFs. In the future, more research is warranted to
obtain a better understanding of the functions of mirRNAs in CAFs,
in particular in the whole tumor microenvironment.
In summary, we discovered that CAFs were capable of
influencing the cell proliferation and drug resistance of A549 and
95D cells by SDF-1 secretion. In addition, SDF-1 upregulated the
expression of CXCR4 which subsequently elevated the expression of
NF-κB and Bcl-xL. Meanwhile, microRNA mir-1 mediated the expression
of SDF-1 in the CAFs. Conclusively, we revealed a
mir-1/SDF-1/CXCR4/NF-κB/Bcl-xL signaling pathway behind the
interaction between CAFs and the lung cancer cell lines, which
explains the reason why CAFs increased the proliferation rate and
drug resistance capacities of the A549 and 95D cells. These results
also identify CAFs as a potential therapeutic target in tumor
treatment.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (no. 81370128).
References
|
1
|
Wald O, Shapira OM and Izhar U:
CXCR4/CXCL12 axis in non small cell lung cancer (NSCLC) pathologic
roles and therapeutic potential. Theranostics. 3:26–33. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim SH, Choe C, Shin YS, Jeon MJ, Choi SJ,
Lee J, Bae GY, Cha HJ and Kim J: Human lung cancer-associated
fibroblasts enhance motility of non-small cell lung cancer cells in
co-culture. Anticancer Res. 33:2001–2009. 2013.PubMed/NCBI
|
|
4
|
Franco OE, Shaw AK, Strand DW and Hayward
SW: Cancer associated fibroblasts in cancer pathogenesis. Semin
Cell Dev Biol. 21:33–39. 2010. View Article : Google Scholar :
|
|
5
|
Karagiannis GS, Poutahidis T, Erdman SE,
Kirsch R, Riddell RH and Diamandis EP: Cancer-associated
fibroblasts drive the progression of metastasis through both
paracrine and mechanical pressure on cancer tissue. Mol Cancer Res.
10:1403–1418. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chatterjee S, Behnam Azad B and Nimmagadda
S: The intricate role of CXCR4 in cancer. Adv Cancer Res.
124:31–82. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen Y, Jacamo R, Konopleva M, Garzon R,
Croce C and Andreeff M: CXCR4 downregulation of let-7a drives
chemoresistance in acute myeloid leukemia. J Clin Invest.
123:2395–2407. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Teicher BA and Fricker SP: CXCL12
(SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 16:2927–2931.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Perona R and Sánchez-Pérez I: Control of
oncogenesis and cancer therapy resistance. Br J Cancer. 90:573–577.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Han C, Yu Z, Duan Z and Kan Q: Role of
microRNA-1 in human cancer and its therapeutic potentials. Biomed
Res Int. 2014:4283712014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aprelikova O and Green JE: MicroRNA
regulation in cancer-associated fibroblasts. Cancer Immunol
Immunother. 61:231–237. 2012. View Article : Google Scholar
|
|
12
|
Li J, Dong X, Wang Z and Wu J: MicroRNA-1
in cardiac diseases and cancers. Korean J Physiol Pharmacol.
18:359–363. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leone V, D'Angelo D, Rubio I, de Freitas
PM, Federico A, Colamaio M, Pallante P, Medeiros-Neto G and Fusco
A: MiR-1 is a tumor suppressor in thyroid carcinogenesis targeting
CCND2, CXCR4, and SDF-1alpha. J Clin Endocrinol Metab.
96:E1388–E1398. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van Solingen C, de Boer HC, Bijkerk R,
Monge M, van Oeveren-Rietdijk AM, Seghers L, de Vries MR, van der
Veer EP, Quax PH, Rabelink TJ, et al: MicroRNA-126 modulates
endothelial SDF-1 expression and mobilization of
Sca-1+/Lin− progenitor cells in ischaemia.
Cardiovasc Res. 92:449–455. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang L, Xu AM, Liu S, Liu W and Li TJ:
Cancer-associated fibroblasts in digestive tumors. World J
Gastroenterol. 20:17804–17818. 2014.PubMed/NCBI
|
|
16
|
Du H, Chen D, Zhou Y, Han Z and Che G:
Fibroblast phenotypes in different lung diseases. J Cardiothorac
Surg. 9:1472014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Otsuka S, Klimowicz AC, Kopciuk K,
Petrillo SK, Konno M, Hao D, Muzik H, Stolte E, Boland W, Morris D,
et al: CXCR4 overexpression is associated with poor outcome in
females diagnosed with stage IV non-small cell lung cancer. J
Thorac Oncol. 6:1169–1178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hanoun N, Delpu Y, Suriawinata AA, Bournet
B, Bureau C, Selves J, Tsongalis GJ, Dufresne M, Buscail L,
Cordelier P, et al: The silencing of microRNA 148a production by
DNA hypermethylation is an early event in pancreatic
carcinogenesis. Clin Chem. 56:1107–1118. 2010. View Article : Google Scholar : PubMed/NCBI
|