Introduction
Esophageal cancer (EC), ranked as the sixth leading
cause of cancer-related mortality worldwide, is one of the most
highly malignant and aggressive cancers (1,2).
Esophageal squamous cell carcinoma (ESCC) is the predominant
histological subtype, accounting for more than 90% of EC cases in
China (3). Although screening
technology and multimodality therapies have remarkably improved
during the past decade, the prognosis of EC remains dismal and the
5-year overall survival rate is still below 15% (4). Accumulative studies have tried to
demonstrate the molecular and biological mechanisms that lead to
EC. A series of risk factors for EC have been established, such as
epidermal growth factor receptor (EGFR), Her-2, p53 and heat shock
proteins (HSPs), which have been found to be associated with the
progression of EC (5–8). However, the mechanisms of oncogenesis
of EC have not been completely clarified. Therefore, the
characterization of molecular markers involved in the
pathophysiological process of ESCC is essential.
The HSPs family, as molecular chaperones, are
biochemical regulators, which function in mediating cell growth,
apoptosis, migration and protein homeostasis (9). HSPs are induced in response not only
to cellular stress, but also to other environmental, physical and
chemical stresses (10). HSPs are
classified into 6 major family members according to molecular
weight: HSP100, HSP90, HSP70, HSP60, HSP40 and small HSPs (11). HSP90 is one of the most abundant
HSPs and more than 200 types of HSP90 client proteins have been
found. A series of previous studies have demonstrated that HSP90 is
activated and upmodulated in a wide variety of human tumors, such
as head and neck squamous cell cancer (HNSCC) (12), colon carcinoma (13) and other adenocarcinomas (14). During the progression of cancers,
HSP90 has a key role in the regulation of cell cycle growth,
signaling, migration and transcription factors, which may lead to
tumorigenesis (15–17). However, there is a paucity of data
on the relationship between activation of HSP90 and the malignancy
of EC.
Hydrogen sulfide (H2S), as a specific
toxic gas, has been qualified as the third gasotransmitter
following nitric oxide (NO) and carbon monoxide (CO) (18–20).
Endogenous H2S is synthesized from L-cysteine by two key
enzymes: cystathionine-β-synthase (CBS) and cystathionine-γ-lyase
(CSE) which are mainly expressed in the enteric neurons and smooth
muscle of the stomach and colon (21,22).
Recently, numerous scientific investigations have proven the
extensive physiological and pathophysiological properties of
H2S on progress of cancer. Previous findings have
demonstrated that H2S promotes cancer cell growth,
proliferation, migration and invasion (23–29),
owing to its vascular relaxant and angiogenesis effects.
H2S enhances the supply of nutrients and blood to the
tumor cells and tissues (29). Our
latest research also showed that exogenous H2S promoted
cancer cell proliferation/anti-apoptosis/angiogenesis/migration
effects via amplifying the activation of NF-κB (30) and p38 MAPK/ERK1/2-COX-2 pathways
(31). However, research focused on
the effect of exogenous H2S on esophageal EC109 cells
and its potential mechanisms is lacking. Hence, we investigated
whether exogenous H2S contributes to cancer progress and
explored these potential effects via activation of HSP90 pathways
in esophageal EC109 cells.
Materials and methods
Materials
NaHS, a donor of H2S, was obtained from
Sigma Chemicals Co. (St. Louis, MO, USA), stored at 2–4°C and
protected from sunlight. GA (a specific inhibitor of HSP90 pathway)
was also purchased from Sigma Chemicals Co. The Cell Counting Kit-8
(CCK-8) was supplied by Dojindo Laboratory (Kumamoto, Japan). Fetal
bovine serum (FBS) and RPMI-1640 medium were obtained from
Gibco-BRL (Grand Island, NY, USA). Anti-MMP2, anti-HSP90,
anti-cleaved caspase-3, anti-bcl-2 antibody and anti-bax antibodies
were supplied by Cell Signaling Technology (Boston, MA, USA).
Horseradish peroxidase (HRP)-conjugated secondary antibody and BCA
protein assay kit were obtained from KangChen Bio-tech, Inc.
(Shanghai, China). Enhanced chemiluminescence (ECL) solution was
purchased from KeyGen Biotech (Nanjing, China). Enzyme-linked
immunosorbent assay (ELISA) was supplied by ExCell Bio Co.
(Shanghai, China).
Cell culture and treatments
The human esophageal carcinoma cells EC109 (EC109
cells) were supplied by Sun Yat-sen University Experimental Animal
Center (Guangzhou, Guangdong, China). The EC109 cells were grown in
RPMI-1640 medium supplemented with 10% FBS under an atmosphere of
5% CO2 and at 37°C with 95% air. The EC109 cells were
treated with 500 µmol/l NaHS for 24 h or co-treated with 500
µmol/l NaHS and 20 µmol/l GA for 24 h.
Western blot analysis
After the indicated treatments, the cells were
harvested and lysed with cell lysis solution at 4°C for 30 min. The
total proteins were quantified using the BCA protein assay kit.
Loading buffer was added to cytosolic extracts, and then after
boiling for 6 min, the same amounts of supernatant from each sample
were fractionated by 10% sodium dodecyl sulphate-polyacrylamide gel
electrophoresis (SDS-PAGE). The total proteins were then
transferred into polyvinylidene difluoride (PVDF) membranes. The
membranes were blocked with 5% fat-free milk for 60 min in fresh
blocking buffer [0.1% Tween-20 in Tris-buffered saline (TBS-T)] at
room temperature, and incubated with either anti-MMP2 (1:1,000
dilution), anti-HSP90 (1:1,000 dilution), anti-bax (1:1,000
dilution), anti-bcl-2 (1:1,000 dilution) and anti-cleaved caspase-3
antibodies (1:1,000 dilution) in freshly prepared TBS-T with 3%
fat-free milk overnight with gentle agitation at 4°C. Membranes
were washed for 5 min with TBS-T for 3 times and incubated with
HRP-conjugated goat anti-rabbit secondary antibody at a
concentration of 1:3,000 dilution (Kangchen Biotech, Shanghai,
China), in TBS-T with 3% fat-free milk for 1.5 h at room
temperature. Then, membranes were washed 3 times with TBS-T for 5
min. The immunoreactive signals were visualized via the ECL. In
order to quantify the protein expression, the X-ray film was
scanned and analyzed with ImageJ 1.47i software. The experiment was
carried out 3 times.
Measurement of cell viability
The EC109 cells were seeded in 96-well plates at
some concentration of 1×104/ml and incubated at 37°C.
The CCK-8 assay was employed to assess the cell viability of EC109
cells. After the indicated treatments, 10 µl CCK-8 solution
at a 1/10 dilution was added to each well and then the plate was
incubated for 1.5 h in the incubator. Absorbance at 450 nm was
assayed using a microplate reader (Molecular Devices, Sunnyvale,
CA, USA). The means of the optical density (OD) of 3-wells in the
indicated groups were used to calculate the percentage of cell
viability according to the formula below: Cell viability (%) = (OD
treatment group/OD control group) × 100%. The experiment was
carried out 5 times.
ELISA for detection of VEGF in culture
supernatant
EC109 cells were cultured in 96-well plates. After
the different indicated treatments, the level of VEGF in the
culture media was tested by ELISA according to the manufacturer's
instructions. The experiment was performed at least 5 times.
Transwell migration assay
The EC109 cells were harvested and washed twice with
phosphate-buffered saline (PBS). After washing, 1×105
cells were resuspended in 200 µl Dulbecco's modified Eagle's
medium (DMEM), and added to the upper chamber of the Transwell
membrane (Transwell permeable support with a 5.0-µm
polycarbonate membrane, 6.5-mm insert and 24-well plate; Corning
Costar, Tewksbury, MA, USA), and 600 µl of 10% FBS-DMEM was
added to each bottom chamber. Four groups in the upper chamber were
included in the assay: i) control; ii) NaHS, NaHS (500
µmol/l); iii) NaHS + GA (a specific inhibitor of HSP90
pathway), NaHS (500 µmol/l) + GA (20 µmol/l); iv) GA,
GA (20 µmol/l). After 24 h at 37°C, cells that migrated to
the lower chambers were counted. Triplicate experiments were
performed with each group, and the means and standard deviations
were calculated.
Statistical analysis
All data are presented as the mean ± SEM.
Differences between groups were analyzed by one-way analysis of
variance (ANOVA) using SPSS 13.0 (SPSS, Inc., Chicago, IL, USA)
software, and followed by LSD post hoc comparison test. Statistical
significance was considered at P<0.05.
Results
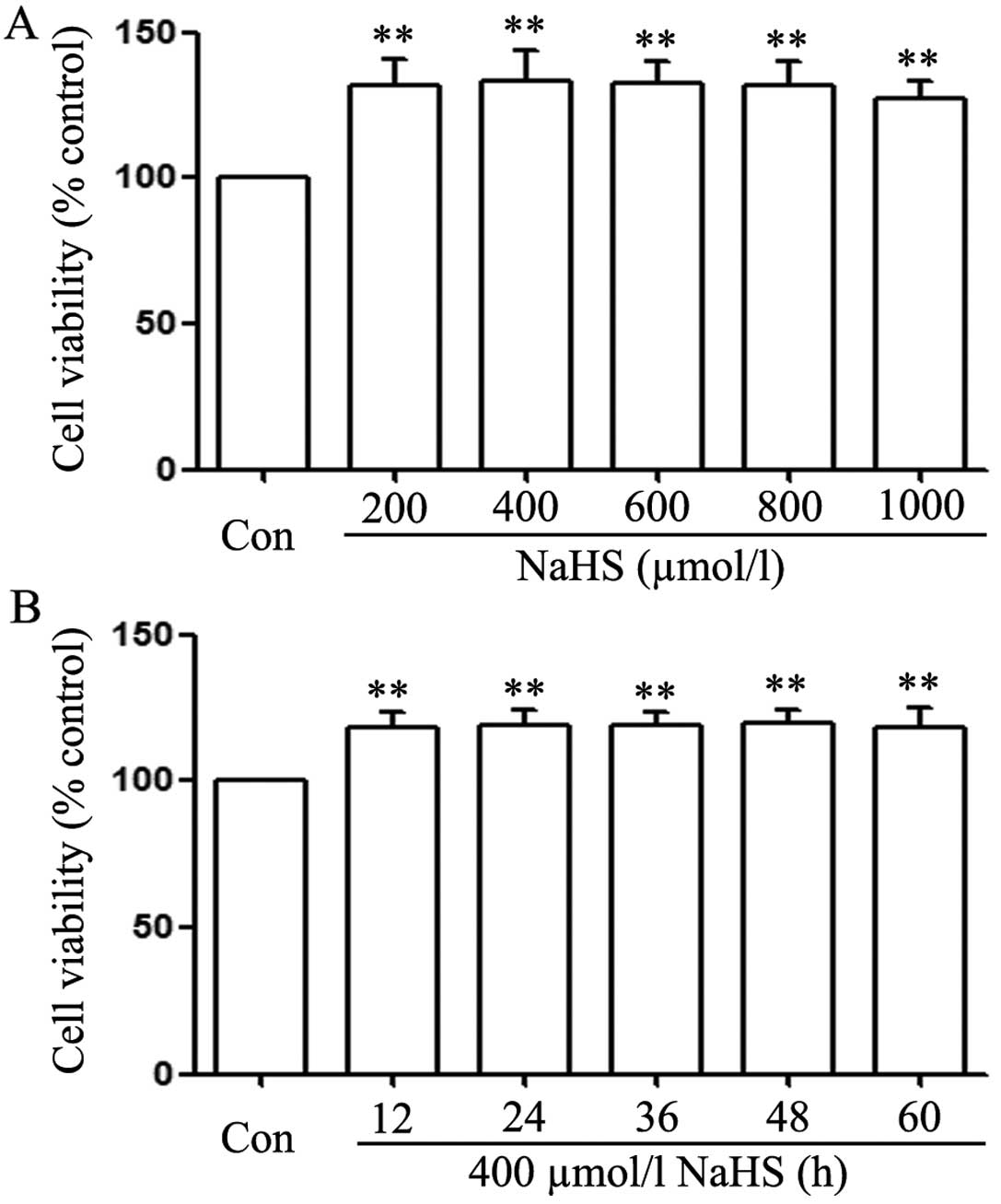
NaHS promotes cell proliferation in EC109
esophageal cells
In order to test the effect of exogenous
H2S on human EC cell proliferation, the dose-response
study with varying doses (200, 400, 600, 800 and 1,000
µmol/l) of NaHS (a donor of H2S) for 24 h was
performed to calculate the effective doses of NaHS. As shown in
Fig. 1A, the doses of NaHS from 200
to 1,000 µmol/l markedly promoted cell proliferation,
leading to an increase in cell viability and reaching a peak at 400
µmol/l. Therefore, 400 µmol/l NaHS was used in the
subsequent time-response study with different treatment times (12,
24, 36, 48 and 60 h). As shown in Fig.
1B, treatment of EC109 cells with 400 µmol/l NaHS for
the indicated times all markedly promoted cell proliferation,
reaching the maximal proliferative effect at 24 h. Based on the
aforementioned results, EC109 esophageal cells were treated with
400 µmol/l NaHS for 24 h in all subsequent experiments.
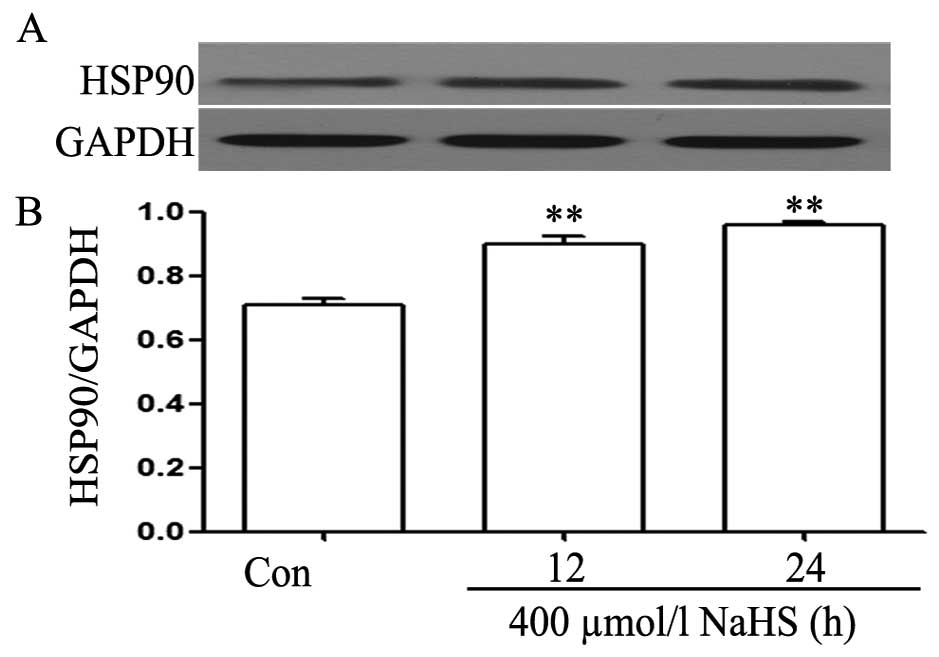
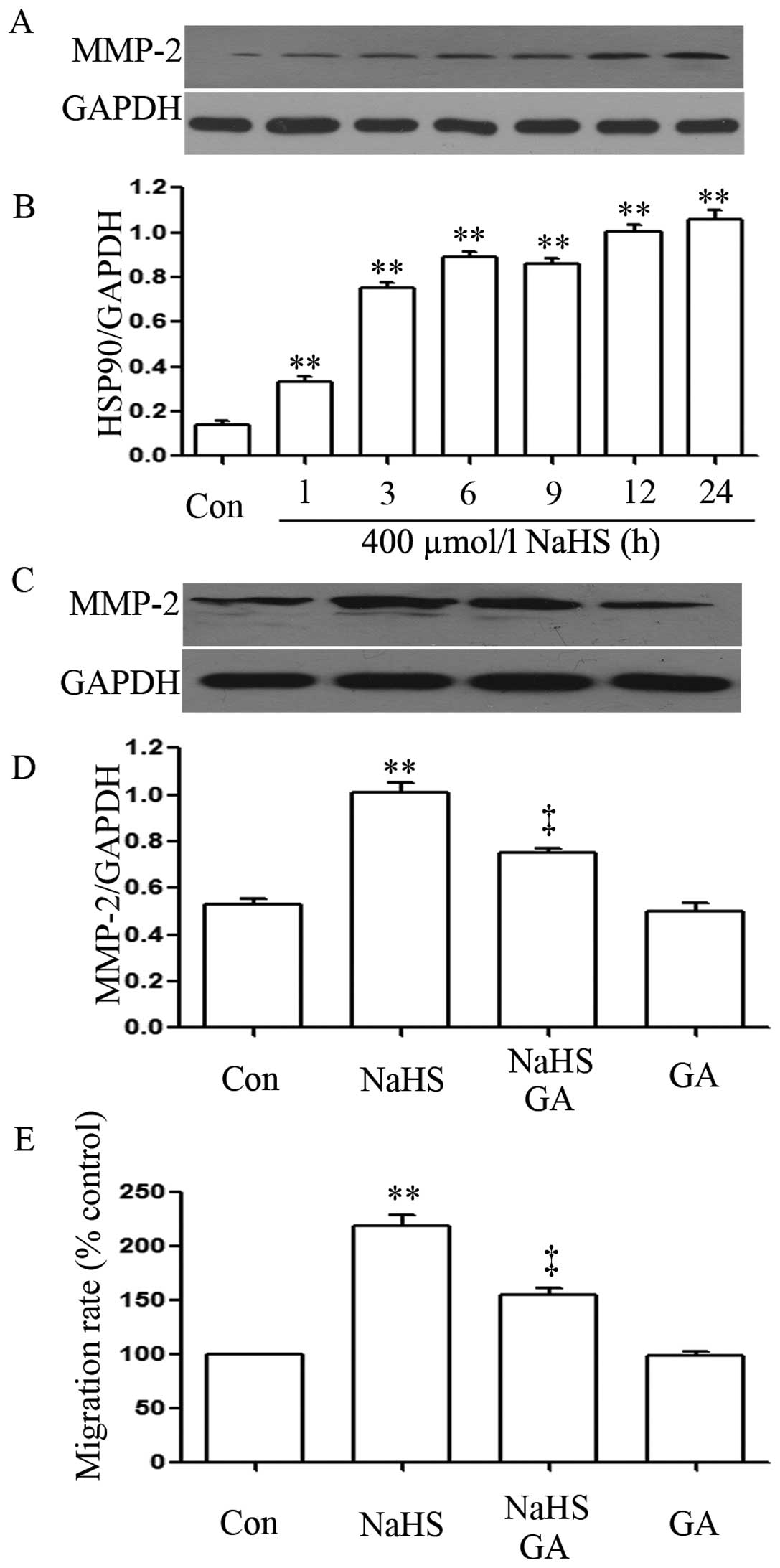
NaHS upregulates the expression levels of
HSP90 in EC109 esophageal cells
We observed the effects of NaHS on the expression
levels of HSP90. As shown in Fig. 2A
and B, exposure of EC109 cells for the indicated time (12 and
24 h) to 400 µmol/l NaHS markedly enhanced the expression of
HSP90, reaching a peak at 24 h.
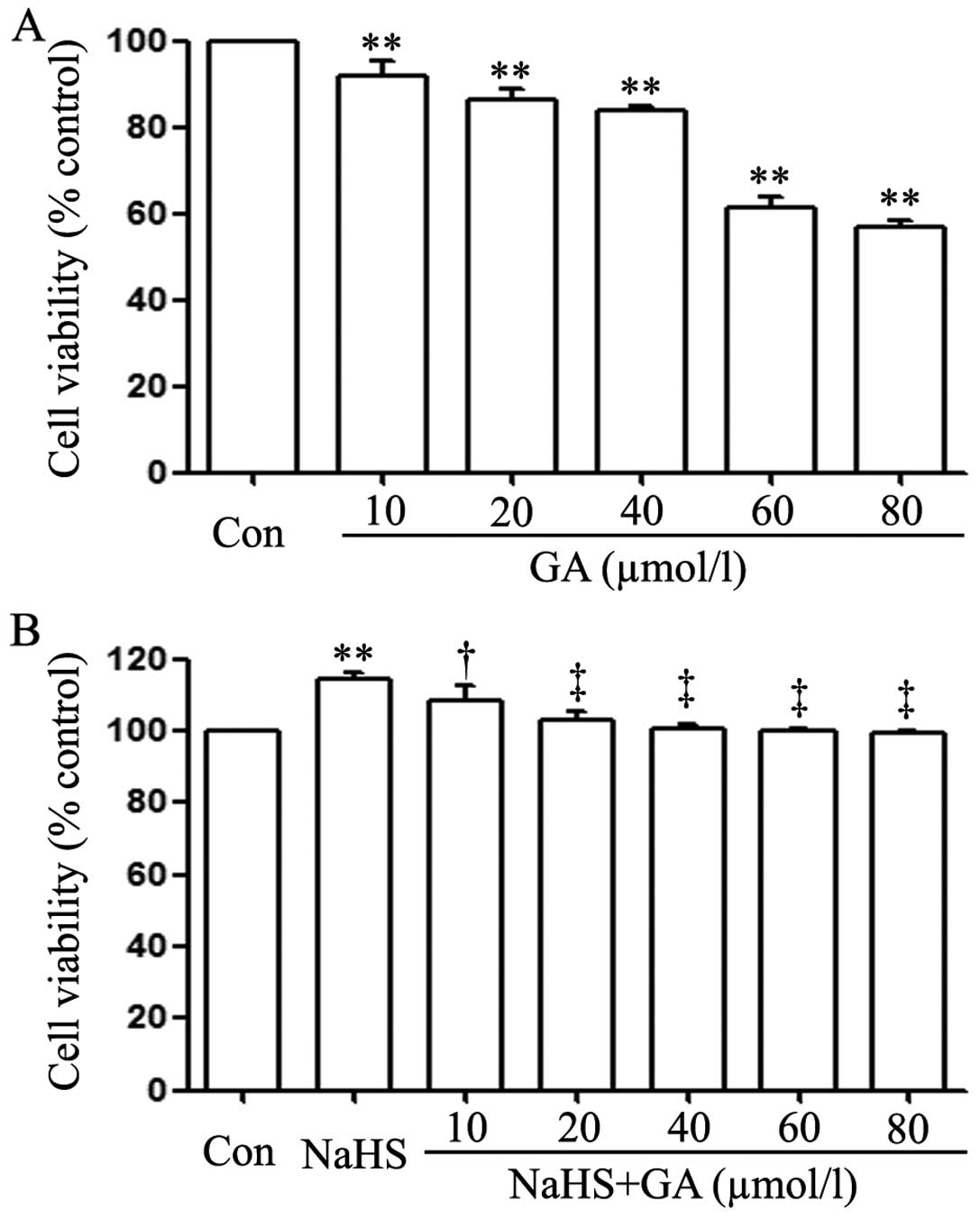
GA (a specific inhibitor of HSP90
pathway) reduces the cell proliferation in EC109 esophageal
cells
EC109 cells were treated with different doses of GA
(10, 20, 40, 60 and 80 µmol/l) for 24 h. As shown in
Fig. 3A, the doses of GA from 10 to
80 µmol/l markedly reduced cell proliferation, dropping to a
bottom at 80 µmol/l.
GA alleviates NaHS-induced cell
proliferation in EC109 cells
As shown in Fig. 3B,
exposure of EC109 cells to 400 µmol/l NaHS for 24 h induced
cell proliferation, leading to an increase in cell viability.
However, the increased cell viability was repressed by co-treatment
with different doses of GA (a specific inhibitor of HSP90 pathway)
for 24 h. As shown in Fig. 3A, at
the dose of 10 µmol/l, the cell viability did not change. On
the contrary, the dose of GA from 20 to 80 µmol/l
significantly suppressed the cell proliferation, leading to a
decrease in cell viability and reaching the minimum at 20
µmol/l. According to the aforementioned results, EC109 cells
were co-treated with 400 µmol/l NaHS and 20 µmol/l GA
for 24 h in all following experiments.
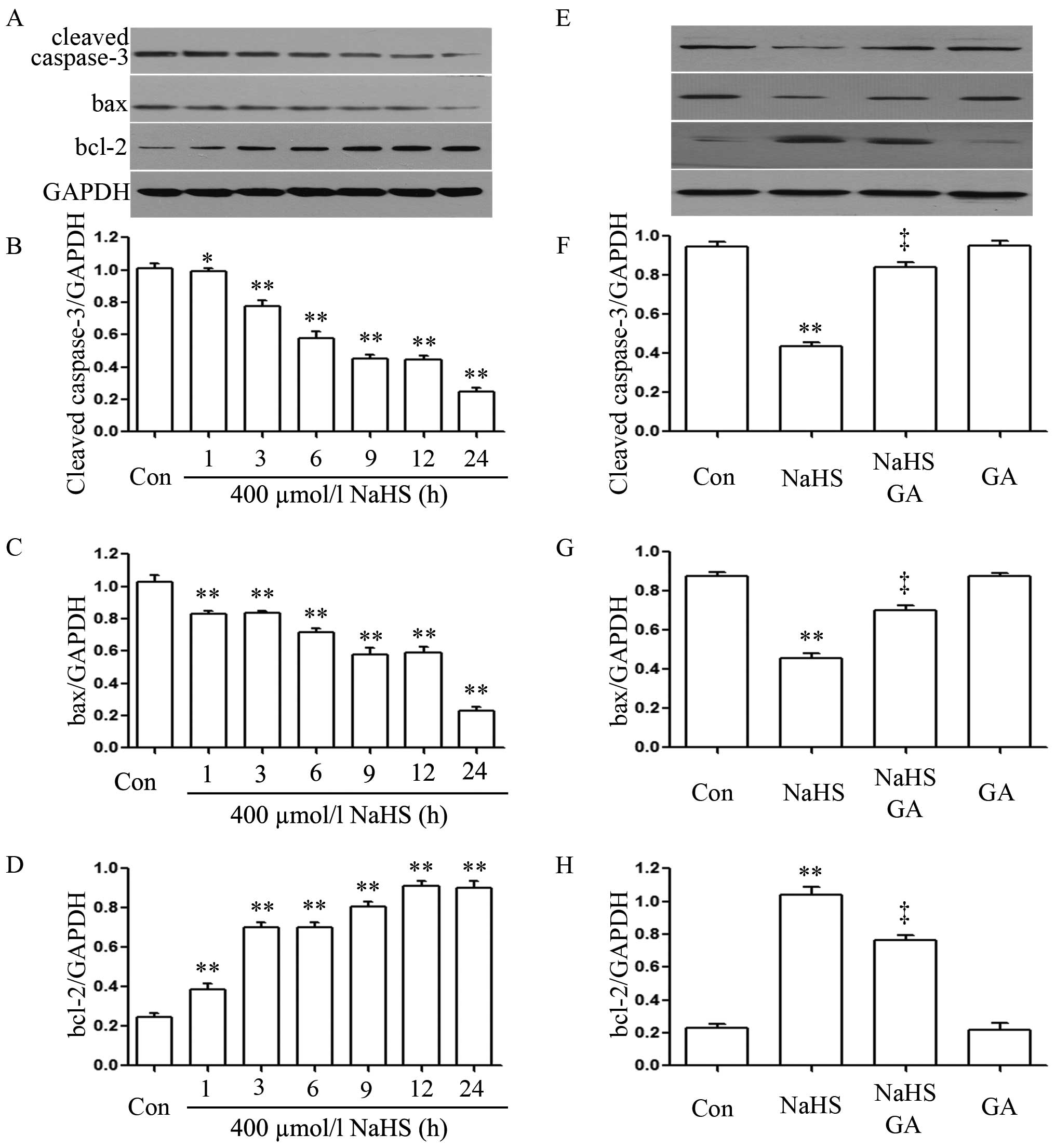
NaHS alleviates the expression level of
cleaved caspase-3 and bax, and upregulates the expression levels of
bcl-2 in EC109 esophageal cells
In order to observe the effects of NaHS on the
expression levels of cleaved caspase-3, bax and bcl-2 in EC109
cells, EC109 cells were exposed to 400 µmol/l NaHS for
different times (1, 3, 6, 9, 12 and 24 h). As shown in Fig. 4A, NaHS significantly enhanced the
expression levels of bcl-2 reaching a peak at 12 h, whereas the
expression level of caspase-3 and bax was markedly decreased.
GA inhibits NaHS-induced increased
expression levels of bcl-2 and upregulates NaHS-induced decreased
caspase-3 expression in EC109 esophageal cells
As shown in Fig. 4E,
EC109 cells were exposed to 400 µmol/l NaHS for 24 h. The
expression levels of bcl-2 were significantly increased; on the
contrary, the expression level of caspase-3 and bax were markedly
decreased. Notably, co-treatment of EC109 cells with 400
µmol/l NaHS and 20 µmol/l GA for 24 h considerably
depressed NaHS-induced increased expression levels of bcl-2;
however, expression of caspase-3 and bax was considerably
downregulated. Treatment of cells with 20 µmol/l GA for 24 h
did not alter the basal expression levels of caspase-3, bax or
bcl-2.
NaHS upregulates the expression level of
MMP-2
In order to observe the effects of NaHS on the
expression levels of MMP-2 in EC109 cells, EC109 cells were exposed
to 400 µmol/l NaHS for different times (1, 3, 6, 9, 12 and
24 h). As shown in Fig. 5A, NaHS
significantly enhanced the expression levels of MMP-2, which peaked
at 24 h. Notably, co-treatment of EC109 cells with 400
µmol/l NaHS and 20 µmol/l GA for 24 h considerably
depressed NaHS-induced increased expression levels of MMP-2.
Treatment of cells with 20 µmol/l GA for 24 h did not alter
the basal expression levels of MMP-2.
The migration rate and Transwell
migration assay
As shown in Fig. 5E,
NaHS strengthened the migration rate in EC109 esophageal cells
while GA depressed NaHS-induced increased migration rate. Treatment
of cells with 20 µmol/l GA for 24 h did not alter the
migration rate compared with control group.
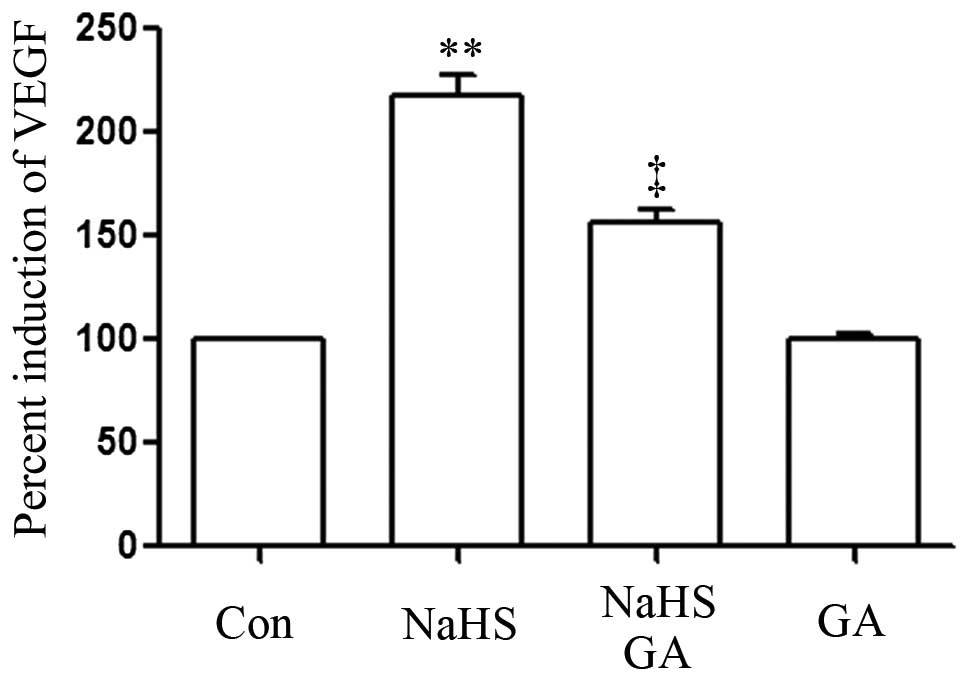
GA suppresses NaHS-induced upregulated
production of VEGF in EC109 esophageal cells
As shown in Fig. 6,
the level of VEGF was markedly increased in NaHS-induced EC109
cells compared with the control group (P<0.01). However, the
increased level of VEGF was significantly suppressed by
co-treatment with GA and NaHS.
H2S demonstrates
proliferation, anti-apoptosis, angiogenesis and migration effects
on EC109 esophageal cells via amplifying the activation of HSP90
pathway
We found that NaHS upregulated HSP90 activity
resulting in an elevated rate of H2S production level,
which in turn modulated protein expressions of caspase-3, bax,
bcl-2, MMP-2 and VEGF. The downregulated caspase-3 and bax directly
induced anti-apoptosis, led to decreased apoptosis and increased
cell viability of EC109 cells. MMP-2 contributes to cancer cell
invasion and migration. The increased production of VEGF stimulates
angiogenesis, promoting the supply of nutrients and blood to the
tumor. Conversely, the above properties of H2S were
significantly inhibited by the co-condition of 400 µmol/l
NaHS and 20 µmol/l GA for 24 h.
Discussion
In the present study, we demonstrated a novel
finding of H2S on esophageal EC109 cells and provided
evidence to reveal its potential mechanisms. These findings support
our hypothesis that preconditioning with exogenous H2S
mediates proliferation, anti-apoptotic, angiopoiesis and migration
effects in esophageal cancer. The molecular mechanisms of
H2S are not yet fully understood. It is known that
H2S is produced in the body mainly by two crucial
enzymes, CBS and CSE, which are mainly found in the central nervous
system (CNS) (32). A recent study
emphasized that H2S played an important role in various
physiological and pathological processes of the nervous system as a
neuromodulator and neuroprotectant (33). Furthermore, H2S could
exert protection to nerve cancer cells, such as PC12 cells
(34). However, the performance of
H2S on the cancer cells are comparatively complicated
and extremely controversial. On the one hand, H2S has
shown its anticancer ability based on anti-inflammatory effect,
anti-apoptosis and activation of some signal pathways (35,36).
On the other hand, H2S can exert totally opposite
properties via amplifying the activation of NF-κB pathway (30) and p38 MAPK/ERK1/2-COX-2 pathways
(31) in other cancer cells. In
order to confirm our hypothesis, EC109 cells were treated with NaHS
(a donor of H2S) and some typical pathway-related
biomarkers were detected. Unexpectedly, we found two interesting
results. Firstly, the optimal concentration of NaHS that induced
maximal effect of proliferation was 400 µmol/l, which was in
the range of physiological doses of H2S (0.2–1 mmol/l).
This indicated that H2S may participate in the
esophageal cancer growth. Secondly, previous studies have suggested
that HSPs can prevent pro-apoptotic signaling and apoptosis
(10). Therefore, it is necessary
to assess apoptotic factors and apoptosis in EC109 cells. Treatment
of cells with 400 µmol/l NaHS for 24 h markedly diminished
cell apoptosis by upregulating the expression of bcl-2 and
decreasing the expression of caspase-3 and bax, which are
pro-apoptotic Bcl-2 family proteins. Moreover, the aforementioned
NaHS-induced effects were inhibited by GA. HSPs can inhibit the
activity of pro-apoptotic Bcl-2 proteins to prevent
permeabilization of the outer mitochondrial membrane and release of
apoptogenic factors (10). The
disruption of apoptosome formation represents another mechanism by
which HSPs can prevent caspase activation and induction of
apoptosis. The aforementioned results were consistent with previous
studies (15–17). These data demonstrated that
H2S exerted its cell proliferation and anti-apoptosis
effects in EC109 cells via activating the HSP90 pathway, and
H2S may be involved in esophageal cancer growth under
physiological conditions. Given that the previous study showed that
H2S-protected PC12 cells from formaldehyde induced
apoptosis (34), our findings imply
that H2S exerts a cytoprotective effect for EC109
cells.
A large number of experiments have shown that
H2S can contribute to VEGF production (37–41).
The present study also found that H2S significantly
increased the production of VEGF in EC109 cells, and the effect was
similarly suppressed by the specific HSP90 pathway inhibitor. VEGF
is one of the most potent and pivotal angiogenic factors, and is
crucial for the persistent proliferation and metastasis of tumor
cells (42). Therefore, we
hypothesized that H2S promotes the supply of blood and
nutrients to the tumor via angiogenesis effect. Further studies are
needed to explore our hypothesis in vivo. It is well known
that tumor invasion and metastasis require increased expressions of
MMPs. Among the MMPs, MMP-2 and MMP-9 have been thought to be key
enzymes in this process since they degrade type IV collagen, which
is one of the important components of extracellular matrix
(43). Growing evidence reveals
that the upregulated expression of MMPs, particularly the
gelatinase (MMP-2 and MMP-9), is closely associated with metastasis
potential in several types of carcinomas (44–47).
The present study demonstrated that HSP90 activation strongly
increases the expression of MMP-2 protein in EC109 cells, which
indicated that H2S was involved in EC109 cell invasion
and migration.
To investigate the complicated mechanism for NaHS
induced pro-proliferative effect, anti-apoptosis, angiogenesis and
migration in EC109 cells, we studied the HSP90 pathway, which has
been previously demonstrated, linked to cancer progression by
regulation of cell proliferation, signaling and apoptosis (15–17).
It has been reported that HSP90 can be activated by various stimuli
both in normal and in cancer cells (10). Herein, we found that NaHS activated
HSP90 pathway in EC109 cells. Notably, GA, an inhibitor of HSP90,
blocked NaHS-induced HSP90 pathway activation by decreasing
expression levels of bcl-2, MMP-2 and VEGF, and increasing
caspase-3 and bax expression. These results suggest that HSP90
activation is necessary in NaHS-induced EC109 cell progression.
In conclusion, H2S-induced cell
proliferation, anti-apoptosis, angiogenesis and migration in EC109
esophageal cells. These effects may be mediated by the activation
of HSP90 pathway, leading to overexpression levels of MMP-2, bcl-2
and VEGF, downregulation of caspase-3 and bax, increased cell
viability, and decreased number of apoptotic cells. In esophageal
cancer, the findings provide novel insight into a unified concept
and identify H2S as an endogenous tumor-promoting factor
and anticancer drug target. The deeper mechanism of H2S
in EC109 esophageal cells is still unclear and needs to be further
investigated.
References
|
1
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wheeler JB and Reed CE: Epidemiology of
esophageal cancer. Surg Clin North Am. 92:1077–1087. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hongo M, Nagasaki Y and Shoji T:
Epidemiology of esophageal cancer: Orient to Occident. Effects of
chronology, geography and ethnicity. J Gastroenterol Hepatol.
24:729–735. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
5
|
Kaneko K, Kumekawa Y, Makino R, Nozawa H,
Hirayama Y, Kogo M, Konishi K, Katagiri A, Kubota Y, Muramoto T, et
al: EGFR gene alterations as a prognostic biomarker in advanced
esophageal squamous cell carcinoma. Front Biosci. 15:65–72. 2010.
View Article : Google Scholar
|
|
6
|
Delektorskaya VV, Chemeris GY, Zavalishina
LE, Ryazantseva AA, Grigorchuk AY, Kononets PV and Davydov MI:
Squamous cell carcinoma of the esophagus: Evaluation of the status
of epidermal growth factor receptors (EGFR and HER-2) by
immunohistochemistry and in situ hybridization. Bull Exp Biol Med.
149:615–620. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Berg D, Wolff C, Langer R, Schuster T,
Feith M, Slotta-Huspenina J, Malinowsky K and Becker KF: Discovery
of new molecular subtypes in oesophageal adenocarcinoma. PLoS One.
6:e239852011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Langer R, Ott K, Specht K, Becker K,
Lordick F, Burian M, Herrmann K, Schrattenholz A, Cahill MA,
Schwaiger M, et al: Protein expression profiling in esophageal
adenocarcinoma patients indicates association of heat-shock protein
27 expression and chemotherapy response. Clin Cancer Res.
14:8279–8287. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Morimoto RI: Cells in stress:
Transcriptional activation of heat shock genes. Science.
259:1409–1410. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Beere HM: 'The stress of dying': The role
of heat shock proteins in the regulation of apoptosis. J Cell Sci.
117:2641–2651. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mehta TA, Greenman J, Ettelaie C,
Venkatasubramaniam A, Chetter IC and McCollum PT: Heat shock
proteins in vascular disease - a review. Eur J Vasc Endovasc Surg.
29:395–402. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cohen SM, Mukerji R, Samadi AK, Zhang X,
Zhao H, Blagg BS and Cohen MS: Novel C-terminal Hsp90 inhibitor for
head and neck squamous cell cancer (HNSCC) with in vivo efficacy
and improved toxicity profiles compared with standard agents. Ann
Surg Oncol. 19(Suppl 3): S483–S490. 2012. View Article : Google Scholar
|
|
13
|
Drecoll E, Nitsche U, Bauer K, Berezowska
S, Slotta-Huspenina J, Rosenberg R and Langer R: Expression
analysis of heat shock protein 90 (HSP90) and Her2 in colon
carcinoma. Int J Colorectal Dis. 29:663–671. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jego G, Hazoumé A, Seigneuric R and
Garrido C: Targeting heat shock proteins in cancer. Cancer Lett.
332:275–285. 2013. View Article : Google Scholar
|
|
15
|
Lu X, Xiao L, Wang L and Ruden DM: Hsp90
inhibitors and drug resistance in cancer: The potential benefits of
combination therapies of Hsp90 inhibitors and other anti-cancer
drugs. Biochem Pharmacol. 83:995–1004. 2012. View Article : Google Scholar :
|
|
16
|
Den RB and Lu B: Heat shock protein 90
inhibition: Rationale and clinical potential. Ther Adv Med Oncol.
4:211–218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chehab M, Caza T, Skotnicki K, Landas S,
Bratslavsky G, Mollapour M and Bourboulia D: Targeting Hsp90 in
urothelial carcinoma. Oncotarget. 6:8454–8473. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang R: Two's company, three's a crowd:
Can H2S be the third endogenous gaseous transmitter?
FASEB J. 16:1792–1798. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kilburn KH, Thrasher JD and Gray MR:
Low-level hydrogen sulfide and central nervous system dysfunction.
Toxicol Ind Health. 26:387–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guidotti TL: Hydrogen sulfide: Advances in
understanding human toxicity. Int J Toxicol. 29:569–581. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Han YF, Huang X, Guo X, Wu YS, Liu DH, Lu
HL, Kim YC and Xu WX: Evidence that endogenous hydrogen sulfide
exerts an excitatory effect on gastric motility in mice. Eur J
Pharmacol. 673:85–95. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schicho R, Krueger D, Zeller F, Von
Weyhern CW, Frieling T, Kimura H, Ishii I, De Giorgio R, Campi B
and Schemann M: Hydrogen sulfide is a novel prosecretory
neuromodulator in the Guinea-pig and human colon. Gastroenterology.
131:1542–1552. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cai WJ, Wang MJ, Ju LH, Wang C and Zhu YC:
Hydrogen sulfide induces human colon cancer cell proliferation:
Role of Akt, ERK and p21. Cell Biol Int. 34:565–572. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cao Q, Zhang L, Yang G, Xu C and Wang R:
Butyrate-stimulated H2S production in colon cancer
cells. Antioxid Redox Signal. 12:1101–1109. 2010. View Article : Google Scholar
|
|
25
|
Du SX, Xiao J, Guan F, Sun LM, Wu WS, Tang
H, Du JB, Tang CS and Jin HF: Predictive role of cerebrospinal
fluid hydrogen sulfide in central nervous system leukemia. Chin Med
J. 124:3450–3454. 2011.
|
|
26
|
Levine J, Ellis CJ, Furne JK, Springfield
J and Levitt MD: Fecal hydrogen sulfide production in ulcerative
colitis. Am J Gastroenterol. 93:83–87. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pupo E, Pla AF, Avanzato D, Moccia F, Cruz
JE, Tanzi F, Merlino A, Mancardi D and Munaron L: Hydrogen sulfide
promotes calcium signals and migration in tumor-derived endothelial
cells. Free Radic Biol Med. 51:1765–1773. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rose P, Moore PK, Ming SH, Nam OC,
Armstrong JS and Whiteman M: Hydrogen sulfide protects colon cancer
cells from chemopreventative agent beta-phenylethyl isothiocyanate
induced apoptosis. World J Gastroenterol. 11:3990–3997. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Szabo C, Coletta C, Chao C, Módis K,
Szczesny B, Papapetropoulos A and Hellmich MR: Tumor-derived
hydrogen sulfide, produced by cystathionine-β-synthase, stimulates
bioenergetics, cell proliferation, and angiogenesis in colon
cancer. Proc Natl Acad Sci USA. 110:12474–12479. 2013. View Article : Google Scholar
|
|
30
|
Zhen Y, Pan W, Hu F, Wu H, Feng J, Zhang Y
and Chen J: Exogenous hydrogen sulfide exerts
proliferation/anti-apoptosis/angiogenesis/migration effects via
amplifying the activation of NF-κB pathway in PLC/PRF/5 hepatoma
cells. Int J Oncol. 46:2194–2204. 2015.PubMed/NCBI
|
|
31
|
Zhen Y, Zhang W, Liu C, He J, Lu Y, Guo R,
Feng J, Zhang Y and Chen J: Exogenous hydrogen sulfide promotes C6
glioma cell growth through activation of the p38 MAPK/ERK1/2-COX-2
pathways. Oncol Rep. 34:2413–2422. 2015.PubMed/NCBI
|
|
32
|
Tan BH, Wong PT and Bian JS: Hydrogen
sulfide: A novel signaling molecule in the central nervous system.
Neurochem Int. 56:3–10. 2010. View Article : Google Scholar
|
|
33
|
Zhang X and Bian JS: Hydrogen sulfide: A
neuromodulator and neuroprotectant in the central nervous system.
ACS Chem Neurosci. 5:876–883. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang JM, Zhou CF, Gao SL, Tian Y, Wang
CY, Wang L, Gu HF and Tang XQ: BDNF-TrkB pathway mediates
neuroprotection of hydrogen sulfide against formaldehyde-induced
toxicity to PC12 cells. PLoS One. 10:e01194782015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kashfi K: Anti-cancer activity of new
designer hydrogen sulfide-donating hybrids. Antioxid Redox Signal.
20:831–846. 2014. View Article : Google Scholar :
|
|
36
|
Ma K, Liu Y, Zhu Q, Liu CH, Duan JL, Tan
BK and Zhu YZ: H2S donor, S-propargyl-cysteine,
increases CSE in SGC-7901 and cancer-induced mice: Evidence for a
novel anti-cancer effect of endogenous H2S? PLoS One.
6:e205252011. View Article : Google Scholar
|
|
37
|
Bir SC, Kolluru GK, McCarthy P, Shen X,
Pardue S, Pattillo CB and Kevil CG: Hydrogen sulfide stimulates
ischemic vascular remodeling through nitric oxide synthase and
nitrite reduction activity regulating hypoxia-inducible factor-1α
and vascular endothelial growth factor-dependent angiogenesis. J Am
Heart Assoc. 1:e0040932012. View Article : Google Scholar
|
|
38
|
Holwerda KM, Burke SD, Faas MM, Zsengeller
Z, Stillman IE, Kang PM, van Goor H, McCurley A, Jaffe IZ,
Karumanchi SA, et al: Hydrogen sulfide attenuates sFlt1-induced
hypertension and renal damage by upregulating vascular endothelial
growth factor. J Am Soc Nephrol. 25:717–725. 2014. View Article : Google Scholar :
|
|
39
|
Köhn C, Dubrovska G, Huang Y and Gollasch
M: Hydrogen sulfide: Potent regulator of vascular tone and
stimulator of angiogenesis. Int J Biomed Sci. 8:81–86. 2012.
|
|
40
|
Polhemus DJ, Kondo K, Bhushan S, Bir SC,
Kevil CG, Murohara T, Lefer DJ and Calvert JW: Hydrogen sulfide
attenuates cardiac dysfunction after heart failure via induction of
angiogenesis. Circ Heart Fail. 6:1077–1086. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tao BB, Liu SY, Zhang CC, Fu W, Cai WJ,
Wang Y, Shen Q, Wang MJ, Chen Y, Zhang LJ, et al: VEGFR2 functions
as an H2S-targeting receptor protein kinase with its
novel Cys1045-Cys1024 disulfide bond serving as a specific
molecular switch for hydrogen sulfide actions in vascular
endothelial cells. Antioxid Redox Signal. 19:448–464. 2013.
View Article : Google Scholar :
|
|
42
|
Leung WK, To KF, Go MY, Chan KK, Chan FK,
Ng EK, Chung SC and Sung JJ: Cyclooxygenase-2 upregulates vascular
endothelial growth factor expression and angiogenesis in human
gastric carcinoma. Int J Oncol. 23:1317–1322. 2003.PubMed/NCBI
|
|
43
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jones JL, Shaw JA, Pringle JH and Walker
RA: Primary breast myoepithelial cells exert an invasion-suppressor
effect on breast cancer cells via paracrine down-regulation of MMP
expression in fibroblasts and tumour cells. J Pathol. 201:562–572.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li C, Li F, Zhao K, Yao J, Cheng Y, Zhao
L, Li Z, Lu N and Guo Q: LFG-500 inhibits the invasion of cancer
cells via downregulation of PI3K/AKT/NF-κB signaling pathway. PLoS
One. 9:e913322014. View Article : Google Scholar
|
|
46
|
Puzovic V, Brcic I, Ranogajec I and
Jakic-Razumovic J: Prognostic values of ETS-1, MMP-2 and MMP-9
expression and co-expression in breast cancer patients. Neoplasma.
61:439–446. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ruan M, Zhang Z, Li S, Yan M, Liu S, Yang
W, Wang L and Zhang C: Activation of Toll-like receptor-9 promotes
cellular migration via up-regulating MMP-2 expression in oral
squamous cell carcinoma. PLoS One. 9:e927482014. View Article : Google Scholar : PubMed/NCBI
|