Introduction
Lung cancer is one of the leading causes of
cancer-related deaths worldwide. During the past decades, great
advances have been made in the management of lung cancer; however,
the 5-year survival rate is still less than 15% (1). Most lung cancer patients eventually
develop drug resistance following systematic surgery, chemotherapy
and radiotherapy (2). Recently
developed molecularly targeted therapies, such as epidermal growth
factor receptor-tyrosine kinase inhibitors, have improved the
survival time of some patients. Nevertheless, drug resistance is
still a current challenge for molecularly targeted therapies
(3). Recent studies have clearly
demonstrated that natural bioactive compounds can effectively
inhibit growth and proliferation, and induce apoptosis in various
cancer cells, with marginal side effects. This suggests that the
use of natural products is a promising alternative strategy for the
treatment of lung cancer (4).
Fungal-derived chemical compounds with novel
structure and biological activity are potential agents for
anticancer drug development (5,6).
Lentinula edodes is the second most popular edible mushroom
in the world market (7). It has not
only been widely used as a health food for thousands of years in
China, Japan and Korea, but has also become popular in nutritional
and medicinal products throughout Europe and North America
(8). Dried L. edodes
contains carbohydrates, proteins, fiber, lipids and ash (9). As a medicinal material, L.
edodes has many pharmacological activities, including
antibacterial, antiviral, immunomodulatory and antitumor activities
(10), but the molecular
pharmacology of L. edodes remains to be determined.
In our previous studies, we found that Latcripin-13
domain, a novel protein from L. edodes C91-3,
demonstrated tumor-suppressive activity via inducing the apoptosis
of tumor cells without toxicity in normal cells (11). However the underlying molecular
mechanism is largely unknown. Latcripin-13 domain belongs to the
secretion-regulating guanine nucleotide exchange factor (also known
as DelGEF) family, which may be involved in the secretion process.
Latcripin-13 domain contains a regulator of chromosome condensation
(RCC1) domain/β-lactamase-inhibitor protein II (BLIP-II) and a
plant homeodomain (PHD). RCC1 and PHD domains are involved in
several key cellular processes, including nucleocytoplasmic
transport, regulation of spindle formation, nuclear envelope
reassembly at mitosis, gene transcription, cell cycle and apoptosis
(12,13). However, BLIP-II does share
significant sequence identity with the regulator of chromosome
condensation (RCC1) family of proteins. These two families are
clearly related, both having a seven-bladed β-propeller structure,
although they differ in the number of strands per blade; BLIP-II
having three anti-parallel β-strands per blade, while RCC1 has
four-stranded blades (14). BLIP-II
is a secreted protein produced by the soil bacteria Streptomyces
exfoliates SMF19. BLIP-II acts as a potent inhibitor of
β-lactamases such as TEM-1, which is the most widespread resistance
enzyme to penicillin antibiotics (15). Based on the previous study, we
hypothesized that Latcripin-13 domain may induce cancer cell
apoptosis by disrupting pathways important for cell cycle
progression. Whether or not Latcripin-13 domain has the activity of
inhibiting the β-lactamases, will be investigated in the
future.
In this study, we treated human lung carcinoma A549
cells with different concentrations of Latcripin-13 domain for
varying times, analyzed cell apoptosis and cell cycle progression,
and explored the potential molecular mechanisms.
Materials and methods
Preparation and analysis of Latcripin-13
domain
The expression, purification and analysis of the
amino acid sequence and protein structure of Latcripin-13 domain
were performed as previously described (11). In brief, Latcripin-13 domain, cloned
from the transcriptome of L. edodes, was expressed in E.
coli Rosetta-gami (DE3) in the form of inclusion bodies. The
Latcripin-13 domain was purified by Ni-His affinity chromatography
with high purity and refolded by urea gradient dialysis. The amino
acid sequence of Latcripin-13 protein was analyzed using the online
tool ExPASy ProtParam. The protein structure of Latcripin-13 was
analyzed with the circular dichroism (CD) spectra, Swiss-Model,
Pfam and InterPro databases.
Cell line and cell culture
Human lung carcinoma A549 cells were obtained from
the Shanghai Institute of Biochemistry and Cell Biology (Shanghai,
China). The cells were maintained in RPMI-1640 medium supplemented
with 10% fetal bovine serum (FBS), 100 ng/ml streptomycin and 100
U/ml penicillin at 37°C in a humidified atmosphere with 5%
CO2.
Cell viability assay
The effects of Latcripin-13 domain on A549 cell
viability were measured using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT)
assay. Cells were treated with Latcripin-13 domain at various
concentrations. After treatment for 24 or 48 h, medium was replaced
and 20 μl MTT [5 mg/ml in phosphate-buffered saline (PBS)
solution] was added into each well. After incubation for 4 h,
culture supernatants were aspirated, and purple insoluble MTT
product was redissolved in 150 μl of dimethyl sulfoxide
(DMSO). Absorbance at 562 nm was measured via an ELISA reader
(Thermon, USA).
Nuclear staining with Hoechst 33258
The nuclear morphology of cells was evaluated using
the cell-permeable DNA dye, Hoechst 33258. A549 cells
(1×105 cells/ml) were placed in 6-well plates containing
1,500 μl culture medium and permitted to adhere for 24 h.
Different concentrations of Latcripin-13 domain were added. After
48 h, the supernatant was aspirated and 100 μl of Hoechst
33258 was added to each well, followed by incubation at 37°C for 10
min. The stained cells were observed under a fluorescence
microscope at the wavelength of 340 nm.
Cell cycle analysis
Cell cycle distribution was assayed using the Cell
Apoptosis PI detection kit (KeyGen Biotech, Nanjing, China)
according to the manufacturer's protocol. Briefly, 1×106
cells were harvested and washed with 1X buffer A after Latcripin-13
domain treatment. The cells were fixed with cold 70% ethanol for 24
h at −20°C, and then resuspended in 500 μl 1X buffer A,
followed by incubation with 5 μl propidium iodide (PI) for
30 min in the dark. After 30 min, fluorescence-activated cells were
sorted in the FACSCalibur™ flow cytometer (BD Biosciences, San
Jose, CA, USA) with excitation at 488 nm and detection at 620 nm.
Data were gated to exclude cellular debris. The proportions of G1,
S and G2-M phase cells were calculated from the DNA content
histograms.
Assay of mitochondrial membrane
potential
Mitochondrial membrane potential (Δψm) was measured
using the mitochondrial membrane sensor kit containing the dye JC-1
(KeyGen Biotech) according to the manufacturer's instruction. Cells
were treated with Latcripin-13 domain for 48 h, and then harvested
for flow cytometric analysis.
Western blot analysis
Protein extracts of A549 cells treated with or
without Latcripin-13 domain were prepared by lysing cells in RIPA
buffer on ice for 30 min. Samples were centrifuged at 15,000 × g
for 10 min. Protein concentration was determined with the BCA
protein assay (KeyGen Biotech). For each sample, 30 μg/lane
of protein was loaded onto 12% poly-acrylamide gels and transferred
to Total Blot NC membranes (Pall, USA), followed by blocking with
5% fat-free milk and incubation with the appropriate specific
primary and secondary antibodies. The antibodies used were as
follows: NF-κB (p65; ZSGB-BIO, Beijing, China);
phosphorylated-GSK3β (S9; Bioworld Technology, Nanjing, China);
GSK3β, Bcl-2, Bax, cyclin D1, CDK4, β-actin (Proteintech, Wuhan,
China); GAPDH (TransGen Biotech, Beijing, China); secondary
antibody (ZSGB-BIO). Signals were detected using a chemiluminescent
gel imaging system according to the manufacturer's instructions
(Bio-Rad).
Caspase activation assay
Caspase-3, caspase-8 and caspase-9 activation was
determined using Caspase Colorimetric assay kits (KeyGen Biotech)
following the manufacturer's instructions. In brief,
2×106 cells were collected following treatment with
various concentrations of Latcripin-13 domain (50, 100
μg/ml) for 48 h. After washing with PBS, cells were lysed in
50 ml of cold lysis buffer and incubated on ice for 20 min. The
cell lysate was centrifuged at 15,000 rpm for 10 min at 4°C.
Reaction buffer/dithiothreitol (DTT) was then added to the
supernatant. After incubation at 37°C for 2 h with substrate
DEVD-pNA, the absorbance was measured at the wavelength of 405 nm
using an ELISA microreader. Caspase-3, −8 and −9 activity was
denoted as U and calculated from the standard curve generated with
pNA solutions of different concentrations.
Statistical analysis
All the data are presented as mean ± standard
deviations of three independent experiments. Statistical analyses
were performed by one-way ANOVA using SPSS 11.0 software.
Significant difference was set at P<0.05.
Results
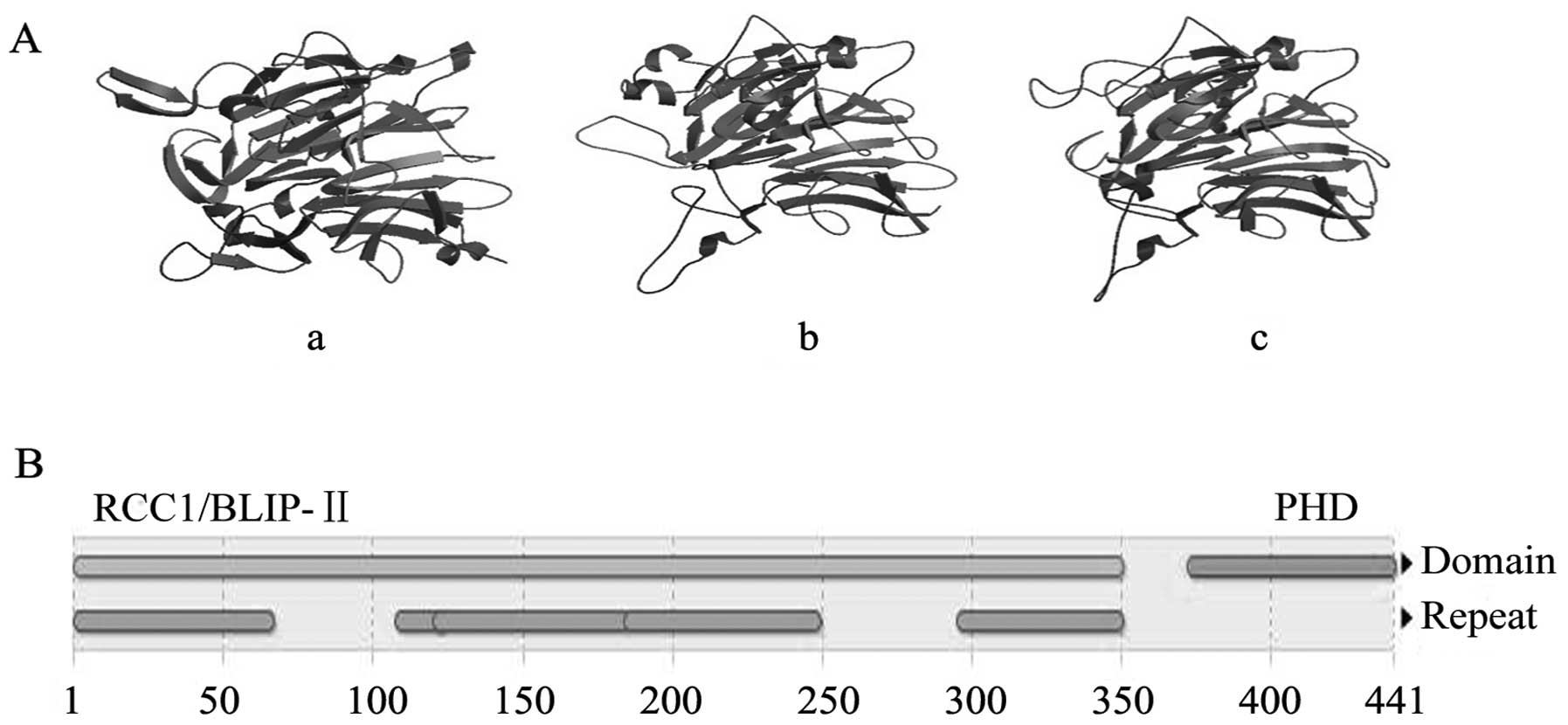
Preparation and properties of
Latcripin-13 domain
The amino acid sequence of the Latcripin-13 domain
was obtained according to the gene sequence (GenBank accession
number: KF682439, the sites from 235 to 1557). The number of amino
acids of Latcripin-13 domain was 441 with molecular weight of
46705.2 Da and theoretical isoelectric point of 5.07. The CD
spectra of the Latcripin-13 domain exhibited a high content of
α-helix (4.7%), β-sheets (38.7%), turns (21.6%) and unordered
(35%), as predicted using the Swiss-Model database. The InterPro
database showed that Latcripin-13 domain belongs to the
secretion-regulating guanine nucleotide exchange factor (also known
as DelGEF) family containing the RCC1/BLIP-II and PHD domain. The
model building results of Latcripin-13 domain by Swiss-Model
database are showed in Fig. 1.
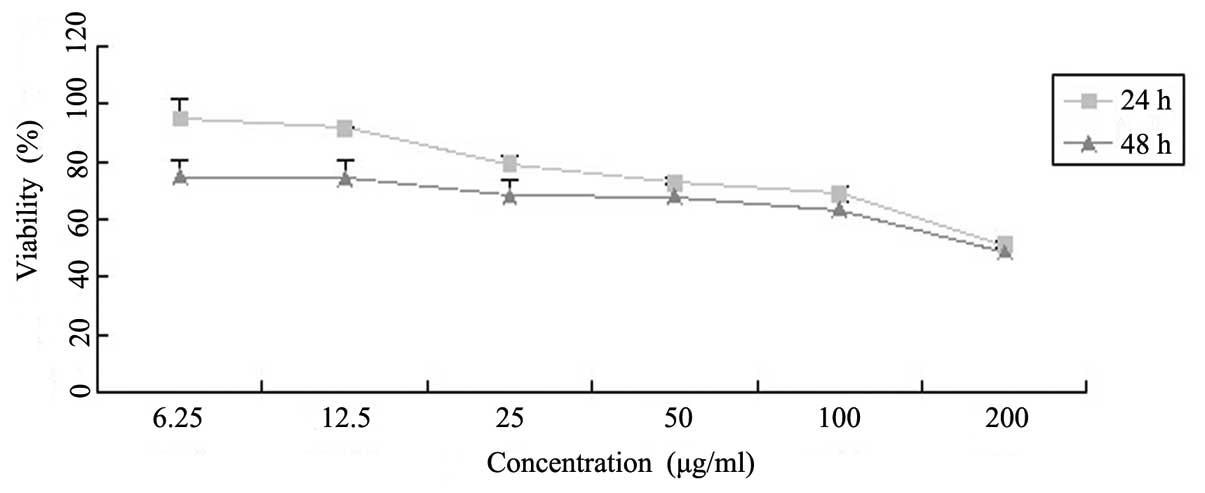
Latcripin-13 domain inhibits the
proliferation of A549 cells
To assay the effects of Latcripin-13 domain on the
proliferation of A549 cells, we treated A549 cells with varying
concentrations of Latcripin-13 domain for 24 and 48 h, and
determined cell viability with the MTT assay. As shown in Fig. 2, Latcripin-13 domain inhibited the
viability of the A549 cells in dose- and time-dependent
manners.
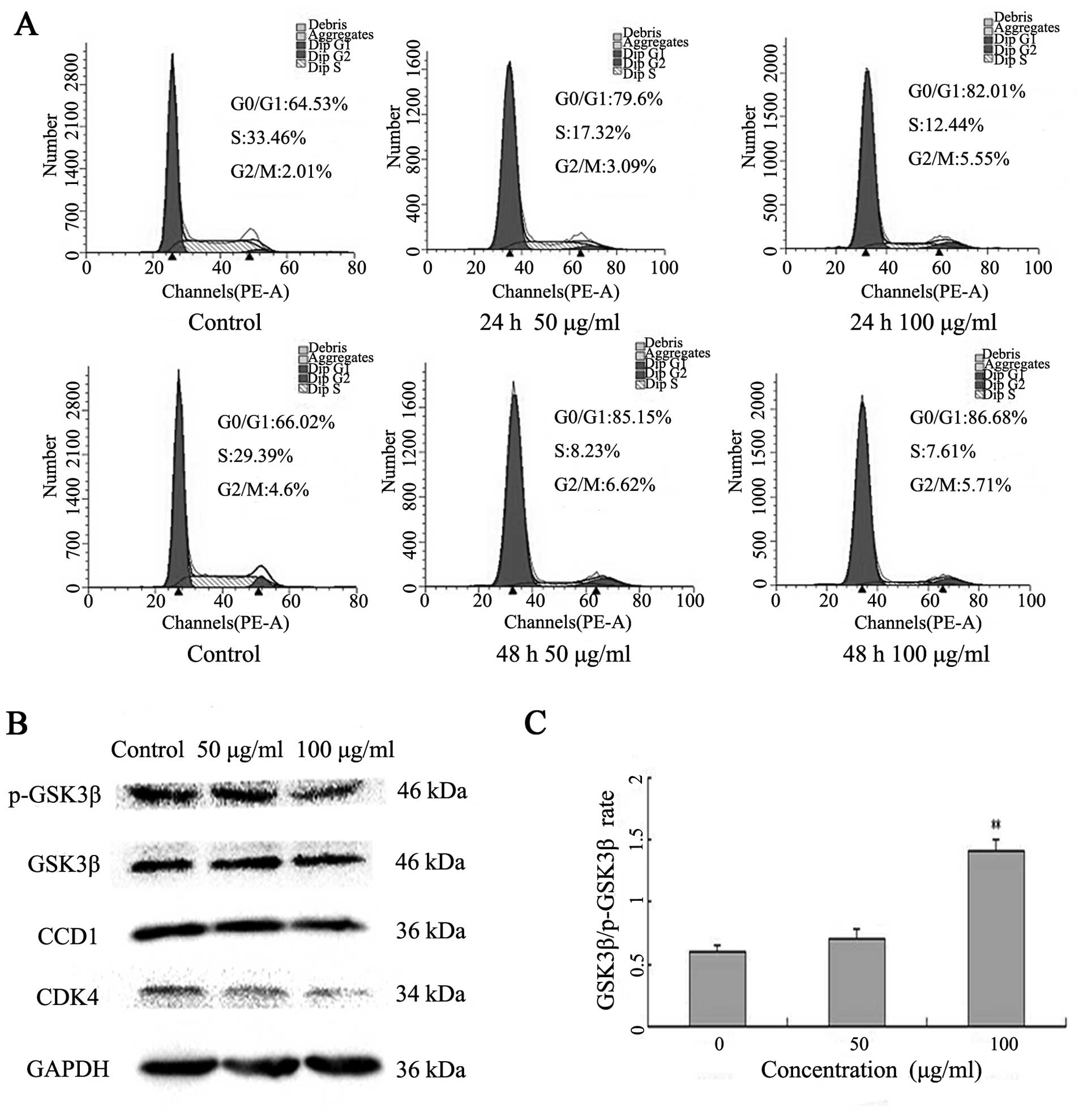
Latcripin-13 domain induces cell cycle
arrest at G1 phase in A549 cells
To investigate the underlying mechanism by which
Latcripin-13 domain inhibits cell proliferation of A549 cells, we
analyzed the cell cycle progression following Latcripin-13 domain
treatment. A549 cells were incubated with 50 or 100 μg/ml of
Latcripin-13 domain for 24 and 48 h. The distribution of the cell
cycle was analyzed using PI staining. Treatment with 50 or 100
μg/ml of Latcripin-13 domain for 24 h led to 79.6 and
82.01%, respectively, of cells in the G0/G1 phase compared with
64.53% in the control group. Moreover, 85.15 and 86.68% of cells
were in G0/G1 phase following treatment for 48 h with 50 and 100
μg/ml, respectively (Fig.
3A). Thus, Latcripin-13 domain arrested A549 cells in the cell
cycle at the G0/G1 phase.
To determine the molecular mechanism underlying cell
cycle arrest in the G1 phase by Latcripin-13 domain, the expression
of G1 phase regulatory proteins, CDK4, cyclin D1, GSK3β and
phosphorylated GSK3β were examined after treatment with
Latcripin-13 domain for 48 h by western blotting. We found that 100
μg/ml of Latcripin-13 domain significantly decreased cyclin
D1 and CDK4, and increased the ratio of GSK3β/phosphorylated GSK3β
in the A549 cells (Fig. 3B and C).
These results indicated that Latcripin-13 domain arrested cell
cycle at the G1 phase, at least in part by decreasing cyclin
D1/CDK4 and phosphorylation of GSK3β.
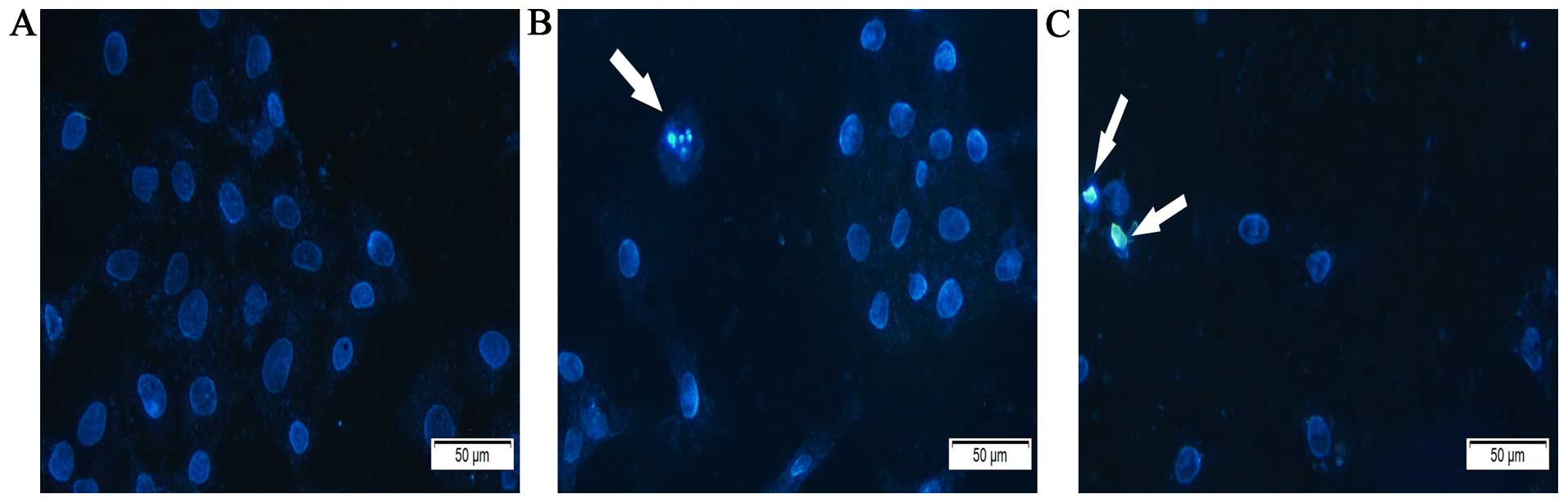
Latcripin-13 domain induces apoptosis in
A549 cells
To assess whether induction of apoptosis contributed
to the inhibition of cell proliferation of A549 cells by
Latcripin-13 domain, we treated A549 cells with 0, 5 or 100
μg/ml of Latcripin-13 domain for 48 h and assessed the cell
morphology under a microscope following Hoechst 33258 staining. In
comparison to the control, Latcripin-13 domain induced apparent
nuclear fragmentation and chromatin condensation, the typical
morphological characteristics of apoptotic cells (Fig. 4). These results showed that
Latcripin-13 domain induced apoptosis of the A549 cells.
Latcripin-13 domain induces apoptosis by
mitochondria-mediated pathway in A549 cells
To support the above observation, we evaluated the
mitochondrial function of Latcripin-13 domain-treated A549 cells by
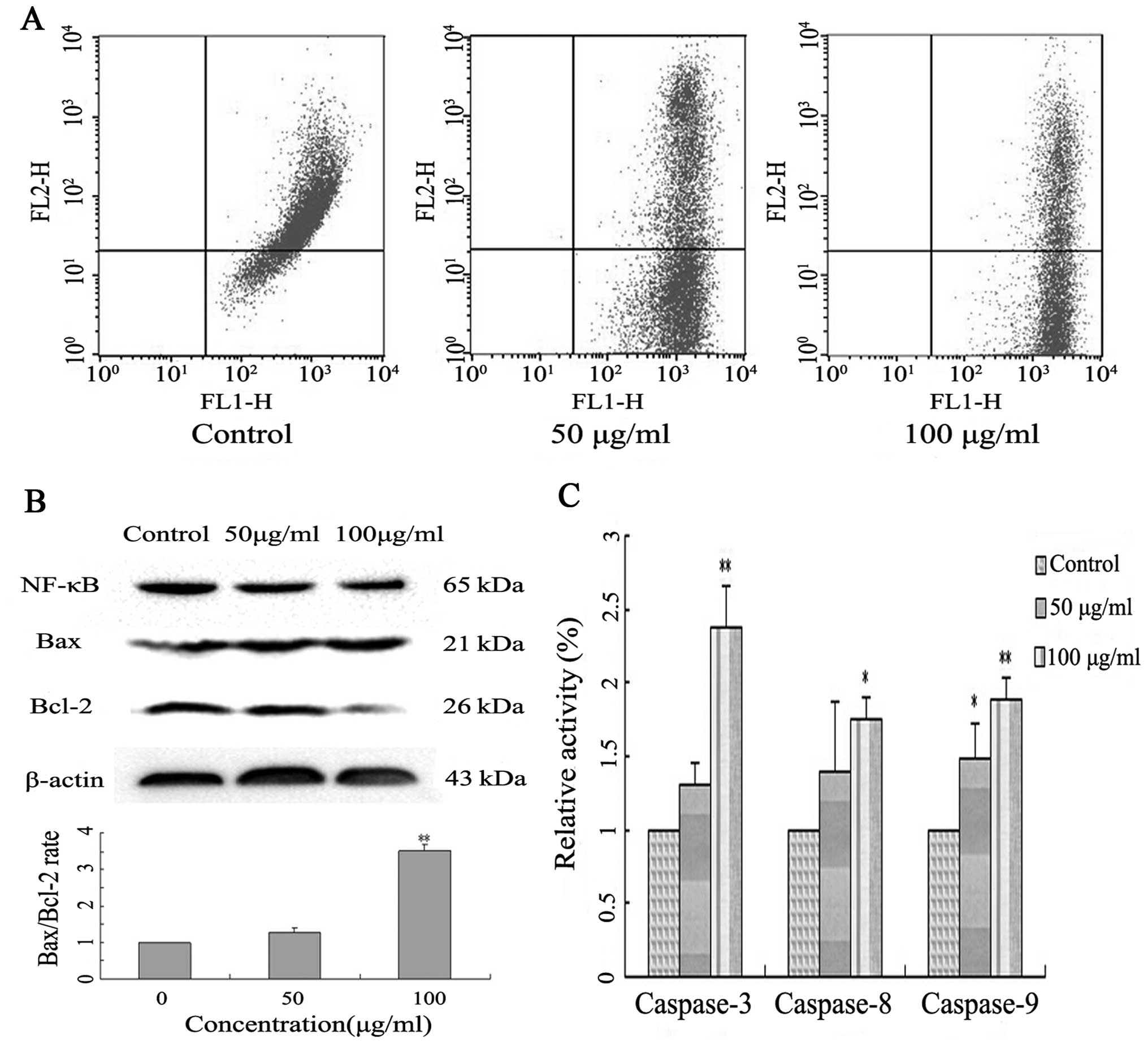
measuring mitochondrial membrane potential (MMP, Δψm) using the
fluorochrome JC-1 and flow cytometry. A549 cells treated with
Latcripin-13 domain demonstrated significant dissipation of the MMP
in a concentration-dependent manner (Fig. 5A), indicating that treatment with
Latcripin-13 domain led to loss of mitochondrial membrane potential
in the A549 cells.
We next evaluated the anti-apoptotic proteins NF-κB,
Bcl-2 and the pro-apoptotic protein Bax in the Latcripin-13
domain-treated A549 cells by western blotting. Latcripin-13 domain
downregulated the expression of NF-κB, Bcl-2, and moderately
increased the expression of Bax (Fig.
5B). Quantification analysis showed that Latcripin-13 domain
induced a concentration-dependent increase in the Bax/Bcl-2 ratio
in the A549 cells, an indication of cell apoptosis (Fig. 5B).
To assess whether the Latcripin-13-induced apoptosis
in A549 cells is caspase-dependent, caspase-3, −8 and −9 enzyme
activity was analyzed using a Caspase Colorimetric assay. In
comparison to the control, 100 μg/ml of Latcripin-13 domain
significantly induced the activation of caspase-3 (P<0.01),
caspase-8 and caspase-9 (P<0.05) (Fig. 5C).
Discussion
In the present study, we demonstrated that
Latcripin-13 domain inhibited proliferation and induced G1 phase
cell cycle arrest of the A549 cells. The cell cycle arrest was
accompanied by decreased cyclin D1 and CDK4, and an increased ratio
of GSK3β/phosphorylated GSK3β. Importantly, Latcripin-13 domain
induced the apoptosis of the A549 cells, as demonstrated by loss of
mitochondrial membrane potential, increase in the Bax/Bcl-2 ratio
and activation of the caspases. Our results indicated that
Latcripin-13 domain arrested cell cycle progression and induced
apoptosis in lung carcinoma A549 cells, suggesting that
Latcripin-13 domain is a potential agent for the treatment of lung
cancer.
Uncontrolled regulation of the cell cycle, leading
to unrestrained cell proliferation, is a hallmark of cancer. Normal
cell cycle progression requires the sequential expression of
cyclins, resulting in the activation of the cyclin-dependent
kinases (CDKs) to phosphorylate target proteins (16). Cyclin D1 is a positive cell-cycle
regulator necessary for the transition of cells from G1 to S phase.
Cyclin D1 is expressed relatively early in the G1 phase as a
crucial control of the G1 checkpoint of the cell cycle, after which
cyclin D1/CDK4/6 forms a complex that is important in cell cycle
regulation by retinoblastoma (Rb) phosphorylation (17–19).
Overexpression of cyclin D1 is among the most commonly observed
alterations in human malignant disorders. In this study, we found
that Latcripin-13 domain inhibited proliferation and arrested A549
cells in G1 phase, accompanied by a decrease in cyclin D1
expression. These results suggest that Latcripin-13 domain may
inhibit the growth of cancer cells by decreasing cyclin D1.
Cyclin D1 is overexpressed in most cancers (20). Genetic mutations or aberrant control
of cyclin D1 expression contribute to cyclin D1 upregulation and
accelerated G1 to S phase transition (21). GSK-3 is a serine-threonine kinase
involved in a variety of physiologic functions such as glycogen
metabolism, gene expression and apoptosis (22–24).
Moreover, GSK-3β plays a key role in the regulation of cyclin D1 by
both regulating cyclin D1 mRNA transcription and
ubiquitin-dependent proteolysis of cyclin D1 protein (25). Accordingly, GSK-3β is implicated in
the pathogenesis of a number of diseases, including diabetes,
bipolar disorder, Alzheimer's disease, heart failure and cancer
(26–29). Proteasome-dependent degradation of
cyclin D1 is triggered by GSK-3β-mediated phosphorylation at
threonine 286 (Thr286), which targets cyclin D1 for ubiquitination
and proteolytic destruction (30,31).
Our study showed that with an increasing concentration of
Latcripin-13 domain, the GSK3β/phosphorylated GSK3β ratio
increased. Our results suggest that Latcripin-13 domain may inhibit
the G1 to S phases transition of A549 cells via a GSK3β-cyclin D1
signaling pathway.
Cell apoptosis is characterized by cytoplasmic
shrinkage, chromatin condensation and DNA fragmentation (32,33).
As shown in Fig. 2, the growth of
A549 cells was inhibited by Latcripin-13 domain in a dose- and
time-dependent manner. Fluorescence microscopy of cells stained
with the DNA-binding dye Hoechst 33258 revealed that Latcripin-13
peptide treatment resulted in significant cell shrinkage and
obvious chromatin condensation (Fig.
4).
Cell apoptosis is controlled by the mitochondrial
Fas signaling pathways (34).
Dysfunction of mitochondria results in a permeabilization of the
outer membrane and concomitant release of mitochondrial apoptotic
components into the cytosol, leading to caspase activation
(35). Mitochondrial membrane
permeability is controlled by a balance of pro- and anti-apoptotic
proteins, including Bax and Bcl-2. In addition, apoptosis in cancer
cells is mediated by cell surface death receptors, activation of
which leads to activated caspase-8 and downstream caspases, such as
caspase-3 (36). Activated
caspase-8 can also cleave and stimulate Bid protein, which further
promotes caspase-9 activation (37,38).
Caspase-3, a downstream effector of both apoptosis pathways, is
activated to regulate the caspase signaling cascade, eventually
inducing apoptosis (39,40). Thus, these three caspases play
important roles in the induction, transduction and amplification of
intracellular apoptotic signals (41). Our results showed that Latcripin-13
domain decreased MMP, accompanied by an increase in Bax, but a
decrease in Bcl-2. In addition, Latcripin-13 domain at 100
μg/ml significantly activated caspase-3, −8 and −9 in A549
cells. Importantly, NF-κB was also downregulated by Latcripin-13
domain (Fig. 5B). RCC1 is a
eukaryotic nuclear protein that acts as a guanine nucleotide
exchange factor for Ran, a member of the Ras GTPase family. RCC1
mediates a Ran-GTP gradient necessary for the regulation of spindle
formation and nuclear assembly during mitosis, as well as for the
transport of macromolecules across the nuclear membrane during
interphase (42). The nuclear
RanGTP level is diminished during the early stages of apoptosis,
which correlates with immobilization of RCC1 on the chromosomes.
Nuclear localization signal (NLS)-containing proteins that
transport to the nucleus have been limited, including NF-κB-p65,
which has important roles in rescuing cells from apoptosis. Wong
et al has reported that RCC1 reads the histone code created
by caspase-activated Mst1 to initiate apoptosis by reducing the
level of RanGTP in the nucleus (43). Therefore, we propose that
Latcripin-13 domain may reduce the concentration of nuclears RanGTP
and recruit the Fas-associated via death domain (FADD) to bind to
death receptors, which in turn activates caspase-8 to inhibit NF-κB
signaling.
In conclusion, our data indicate that Latcripin-13
domain inhibits the proliferation of A549 cells via G1 cell cycle
arrest and also inhibits the mitochondrial-mediated pathway. In
addition, Latcripin-13 domain induces apoptosis through caspase-8
and NF-κB signaling. These findings provide further mechanistic
evidence of the anticancer activity of Latcripin-13 domain.
Acknowledgments
This study was financially supported by the National
Natural Science Foundation of China (no. 81472836).
Abbreviations:
|
RCC1
|
regulator of chromosome
condensation
|
|
PHD
|
plant homeodomain
|
|
L. edodes
|
Lentinula edodes
|
|
CD
|
circular dichroism
|
|
BLIP-II
|
β-lactamase-inhibitor protein II
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide
|
References
|
1
|
Jahangeer S, Forde P, Soden D and Hinchion
J: Review of current thermal ablation treatment for lung cancer and
the potential of electrochemotherapy as a means for treatment of
lung tumors. Cancer Treat Rev. 39:862–871. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu CY, Hu HY, Pu CY, Huang N, Shen HC, Li
CP and Chou YJ: Pulmonary tuberculosis increases the risk of lung
cancer: A population-based cohort study. Cancer. 117:618–624. 2011.
View Article : Google Scholar
|
|
3
|
Kerkentzes K, Lagani V, Tsamardinos I,
Vyberg M and Røe OD: Hidden treasures in 'ancient' microarrays:
gene-expression portrays biology and potential resistance pathways
of major lung cancer subtypes and normal tissue. Front Oncol.
4:2512014. View Article : Google Scholar
|
|
4
|
Surh YJ: Cancer chemoprevention with
dietary phytochemicals. Nat Rev Cancer. 3:768–780. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Long BH and Fairchild CR: Paclitaxel
inhibits progression of mitotic cells to G1 phase by interference
with spindle formation without affecting other microtubule
functions during anaphase and telephase. Cancer Res. 54:4355–4361.
1994.PubMed/NCBI
|
|
6
|
Patel S and Goyal A: Recent developments
in mushrooms as anticancer therapeutics: A review. Biotech. 2:1–15.
2012.
|
|
7
|
Hadeler H: Medicinal Mushrooms You Can
Grow. Cariaga Publishing House. 1995.
|
|
8
|
Lee KH, Morris-Natschke SL, Yang X, Huang
R, Zhou T, Wu SF, Shi Q and Itokawa H: Recent progress of research
on medicinal mushrooms, foods, and other herbal products used in
traditional Chinese medicine. J Tradit Complement Med. 2:84–95.
2012.PubMed/NCBI
|
|
9
|
Xu X, Yan H, Tang J, Chen J and Zhang X:
Polysaccharides in Lentinus edodes: Isolation, structure,
immunomodulating activity and future prospective. Crit Rev Food Sci
Nutr. 54:474–487. 2014. View Article : Google Scholar
|
|
10
|
Bisen PS, Baghel RK, Sanodiya BS, Thakur
GS and Prasad GB: Lentinus edodes: A macrofungus with
pharmacological activities. Curr Med Chem. 17:2419–2430. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang J, Zhong M, Liu B, Sha L, Lun Y,
Zhang W, Li X, Wang X, Cao J, Ning A, et al: Expression and
functional analysis of novel molecule - Latcripin-13 domain from
Lentinula edodes C91-3 produced in prokaryotic
expression system. Gene. 555:469–475. 2015. View Article : Google Scholar
|
|
12
|
Bienz M: The PHD finger, a nuclear
protein-interaction domain. Trends Biochem Sci. 31:35–40. 2006.
View Article : Google Scholar
|
|
13
|
Yamaguchi R and Newpor J: A role for
Ran-GTP and Crm1 in blocking re-replication. Cell. 113:115–125.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lim D, Park HU, De Castro L, Kang SG, Lee
HS, Jensen S, Lee KJ and Strynadka NCJ: Crystal structure and
kinetic analysis of beta-lactamase inhibitor protein-II in complex
with TEM-1 beta-lactamase. Nat Struct Biol. 8:848–852. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fryszczyn BG, Adamski CJ, Brown NG, Rice
K, Huang W and Palzkill T: Role of β-lactamase residues in a common
interface for binding the structurally unrelated inhibitory
proteins BLIP and BLIP-II. Protein Sci. 23:1235–1246. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bernardi A, Frozza RL, Hoppe JB, Salbego
C, Pohlmann AR, Battastini AM and Guterres SS: The
antiproliferative effect of indomethacin-loaded lipid-core
nanocapsules in glioma cells is mediated by cell cycle regulation,
differentiation, and the inhibition of survival pathways. Int J
Nanomedicine. 8:711–728. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Blagosklonny MV and Pardee AB: The
restriction point of the cell cycle. Cell Cycle. 1:103–110. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Giacinti C and Giordano A: RB and cell
cycle progression. Oncogene. 25:5220–5227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Caldon CE, Sutherland RL and Musgrove E:
Cell cycle proteins in epithelial cell differentiation:
Implications for breast cancer. Cell Cycle. 9:1918–1928. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pines J: Four-dimensional control of the
cell cycle. Nat Cell Biol. 1:E73–E79. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hsieh TC, Yang CJ, Lin CY, Lee YS and Wu
JM: Control of stability of cyclin D1 by quinone reductase 2 in
CWR22Rv1 prostate cancer cells. Carcinogenesis. 33:670–677. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Embi N, Rylatt DB and Cohen P: Glycogen
synthase kinase-3 from rabbit skeletal muscle. Separation from
cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J
Biochem. 107:519–527. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Troussard AA, Tan C, Yoganathan TN and
Dedhar S: Cell-extracellular matrix interactions stimulate the AP-1
transcription factor in an integrin-linked kinase- and glycogen
synthase kinase 3-dependent manner. Mol Cell Biol. 19:7420–7427.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Turenne GA and Price BD: Glycogen synthase
kinase3 beta phosphorylates serine 33 of p53 and activates p53's
transcriptional activity. BMC Cell Biol. 2:122001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takahashi-Yanaga F and Sasaguri T:
GSK-3beta regulates cyclin D1 expression: A new target for
chemotherapy. Cell Signal. 20:581–589. 2008. View Article : Google Scholar
|
|
26
|
Haq S, Choukroun G, Kang ZB, Ranu H,
Matsui T, Rosenzweig A, Molkentin JD, Alessandrini A, Woodgett J,
Hajjar R, et al: Glycogen synthase kinase-3beta is a negative
regulator of cardiomyocyte hypertrophy. J Cell Biol. 151:117–130.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Antos CL, McKinsey TA, Frey N, Kutschke W,
McAnally J, Shelton JM, Richardson JA, Hill JA and Olson EN:
Activated glycogen synthase-3 beta suppresses cardiac hypertrophy
in vivo. Proc Natl Acad Sci USA. 99:907–912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
van de Schans VA, van den Borne SW,
Strzelecka AE, Janssen BJ, van der Velden JL, Langen RC,
Wynshaw-Boris A, Smits JF and Blankesteijn WM: Interruption of Wnt
signaling attenuates the onset of pressure overload-induced cardiac
hypertrophy. Hypertension. 49:473–480. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Martinez A, Castro A, Dorronsoro I and
Alonso M: Glycogen synthase kinase 3 (GSK-3) inhibitors as new
promising drugs for diabetes, neurodegeneration, cancer, and
inflammation. Med Res Rev. 22:373–384. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Diehl JA, Zindy F and Sherr CJ: Inhibition
of cyclin D1 phosphorylation on threonine-286 prevents its rapid
degradation via the ubiquitin-proteasome pathway. Genes Dev.
11:957–972. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Diehl JA, Cheng M, Roussel MF and Sherr
CJ: Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis
and subcellular localization. Genes Dev. 12:3499–3511. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lawen A: Apoptosis - an introduction.
Bioessays. 25:888–896. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Robertson JD and Orrenius S: Molecular
mechanisms of apoptosis induced by cytotoxic chemicals. Crit Rev
Toxicol. 30:609–627. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zimmermann KC, Bonzon C and Green DR: The
machinery of programmed cell death. Pharmacol Ther. 92:57–70. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jourdan M, Reme T, Goldschmidt H, Fiol G,
Pantesco V, De Vos J, Rossi JF, Hose D and Klein B: Gene expression
of anti- and pro-apoptotic proteins in malignant and normal plasma
cells. Br J Haematol. 145:45–58. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hu W and Kavanagh JJ: Anticancer therapy
targeting the apoptotic pathway. Lancet Oncol. 4:721–729. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gupta S: Molecular steps of death receptor
and mitochondrial pathways of apoptosis. Life Sci. 69:2957–2964.
2001. View Article : Google Scholar
|
|
38
|
Jin Z and El-Deiry WS: Overview of cell
death signaling pathways. Cancer Biol Ther. 4:139–163. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lei JC, Yu JQ, Yin Y, Liu YW and Zou G:
Alantolactone induces activation of apoptosis in human hepatoma
cells. Food Chem Toxicol. 50:3313–3319. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang Q, Wu J, Hu Z and Li D: Induction of
HL-60 apoptosis by ethyl acetate extract of Cordyceps sinensis
fungal mycelium. Life Sci. 75:2911–2919. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ola MS, Nawaz M and Ahsan H: Role of Bcl-2
family proteins and caspases in the regulation of apoptosis. Mol
Cell Biochem. 351:41–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Makde RD, England JR, Yennawar HP and Tan
S: Structure of RCC1 chromatin factor bound to the nucleosome core
particle. Nature. 467:562–566. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wong CH, Chan H, Ho CY, Lai SK, Chan KS,
Koh CG and Li HY: Apoptotic histone modification inhibits nuclear
transport by regulating RCC1. Nat Cell Biol. 11:36–45. 2009.
View Article : Google Scholar
|