Introduction
Soft tissue sarcomas are rare tumors of mesenchymal
origin with a frequent ability for distant metastasis (1). Fibrosarcomas, tumors belonging to this
group, specifically originate from muscular fibrous tissues, fascia
and tendons (2). Annually,
fibrosarcomas represent 3% of all soft tissue sarcomas and 1% of
new cancer cases diagnosed in the United States and Europe
(2,3). The treatment of fibrosarcomas is
mainly surgical and must be individualized due to the rarity and
pleiotrophy of these tumors (2,3).
Unfractionated heparin (UFH) is a mixture of heavily
sulfated, linear glycosaminoglycan (GAG) chains, which participate
in the regulation of various cell biological functions (4), including the modulation of growth
factor activities (5), the
inhibition of heparanase (5,6) and
changes in extracellular matrix (ECM) composition (7). Its size ranges from 3 to 30 kDa, with
chains in the 12–15 kDa size range most commonly used. On the other
hand, low-molecular-weight heparin (LMWH) consists of LMW fragments
produced by the depolymerization (enzymatic or chemical) of UFH,
which yields chains that are <18 saccharide units long (8). UFH and LMWH have been extensively used
over the years as anticoagulant agents for the prevention of
thromboembolism in cancer patients (9–12). The
long-term utilization of heparin preparations as anti-coagulants
has led to the understanding that UFH and LMWH positively affect
the survival of cancer patients (9,
13–15). Moreover, the effects of
high-molecular-weight haparin (HMWH) and LMWH were found to be
discrete. Thus, according to clinical data, patients who had been
treated with LMWH in order to minize the risk of thrombosis have an
improved survival (3 months) as compared to those treated with UFH
(15), and an increased long-term
survival period (16).
Specifically, the anticancer effect of LMWH has been ascribed to
its inhibition of angiogenesis via the cellular release of tissue
factor pathway inhibitor in endothelial cells (17) or its expression by cancer cells
(18). The anticancer effect of
heparin may be due to its non-anticoagulant derivatives (19), and novel heparin therapeutical
approaches are proposed. Of note, heparin has been found to inhibit
the proliferation of various normal cell types, including vascular
smooth muscle cells, mesanglial cells, fibroblasts and epithelial
cells (20–23). Likewise, the majority of studies
have indicated that heparin inhibits cancer cell growth (24,25),
even though exceptions to the rule have been reported (26,27).
Moreover, using the B6FS fibrosarcoma cell model, we have
previously demonstrated that heparin attenuates the growth ability
of these cells (28).
By contrast, some early studies have suggested that
heparin enhances the spread of cancer cells to other organs and
tissues (29–31). Different mechanisms of action of
heparin have been proposed. Thus, LMWH was found to inhibit the
proliferation, migration, invasion and lung metastatic ability of
HT1080 fibrosarcoma cells through a blockade of the RAGE axis
(32). Chalkiadaki et al
demonstrated that UFH, by activating p53/focal adhesion kinase
(FAK)-dependent signaling, modulated melanoma cell adhesion and
migration (33). The same authors
also demonstrated that LMWH inhibited the ability of melanoma cells
to adhere and to migrate, utilizing a protein kinase C (PKC)α/c-Jun
N-terminal kinase (JNK) signaling axis and resulting in actin
cytoskeletal changes (34).
Fibronectin (FN) is a key ECM component that affects
cell attachment and migration (35). Importantly, FN expression has been
shown to correlate with aggressive cancer progression (35–37).
Fibrosarcoma cells have been demonstrated to specifically adhere to
the FN substrate (38,39). In this study, we investigated the
putative biological roles of UFH and LMWH in the migratory and
adhesive properties of B6FS fibrosarcoma cells.
Materials and methods
Reagents
UFH and LMWH were supplied by Sigma (St. Louis, MO,
USA). Stock solutions of 10 mg/ml were prepared by dissolving
heparin in sterile, RNase- and DNase-free DEPC water (Cayman
Chemical Co., Ann Arbor, MI, USA). Human plasma FN (1 mg/ml) was
obtained by Millipore Corp. (Billerica, MA, USA). RPMI medium and
penicillin-streptomycin were obtained from Biosera (Sussex, UK) and
gentamycin was supplied by Invitrogen Life Technologies (Carlsbad,
CA, USA). Fetal bovine serum (FBS) was purchased by Gibco Life
Technologies (Carlsbad, CA, USA). Fluorescein isothiocyanate
(FITC)-conjugated unfractionated heparin (referred to as
FITC-Heparin) was obtained from Invitrogen Life Technologies.
D-[6-3H(N)]glucosamine hydrochloride was supplied by DuPont de
Nemours (Dreiech, Germany). Heparin lyase II (heparinase II, no EC
number) from Flavobacterium heparinum, chondroitinase AC II
from Arthrobacter aurescens (EC 4.2.2.5), proteinase K and
2X crystallized papain (EC 3.4.22.2) were obtained from Sigma
Chemical Co. (St. Louis, MO, USA). Heparin lyases I and III from
Flavobacterium heparinum (EC 4.2.2.7 and EC 4.2.2.8,
respectively), chondroitinase ABC from Proteus vulgaris (EC
4.2.2.4), keratanase II and the anti-heparitinase stubs antibody
clone (3G10) were from Seikagaku Kogyo Co. (Tokyo, Japan).
Cell culture conditions and transfection
with short intefering RNA (siRNA)
In this study we used the B6FS human fibrosarcoma
cell line (40). The cells were
obtained from the Cell Bank of the Karolinska Institute, Stockholm,
Sweden and were a kind gift from Dr. Anders Hjerpe (Karolinska
Institute). The cells were cultured at 37°C and humidity 5%
CO2 in RPMI supplemented with 10% FBS and antimicrobial
agents (100 IU/ml penicillin, 100 µg/ml streptomycin and
0.5% gentamycin). All experiments were conducted under serum-free
conditions; specifically after 24 h of serum starvation, the cells
were treated with UFH and LMWH (10 µg/ml and 30
µg/ml) for 48 h.
The B6FS cells (450,000 cells per T25 flask) were
incubated with siRNA (100 nM) specific for the FAK gene (siFAK), as
previously described by Hong et al (41) and with siRNA negative control
sequences (siScramble) for 6 h. Specific RNA (Invitrogen Life
Technologies) and Lipofectamine® 2000 (Invitrogen Life
Technologies) (1/50 µl medium) were added to
Opti-MEM© (Invitrogen Life Technologies) for 5 min at
room temperature. In continuation, diluted Lipofectamine 2000 was
mixed with the siRNA for 20 min to induce the formation of
liposome-siRNA complexes. The medium containing siRNA or siScramble
was removed after 6 h of incubation and fresh RPMI 0% FBS medium
supplemented with antibiotics was added. The cells were harvested
for the respective experiments, after 48 h of culture.
Western blot analysis
After 48 h of respective treatments, the harvested
B6FS cells were lysed with RIPA buffer and electrophoresed on an 8%
polyacrylamide gel. Protein bands were transferred onto
nitrocellulose membrane in 10 nM CAPS, pH 11 and 10% methanol. All
membranes were blocked and incubated overnight at 4°C with PBS
containing 0,1% Tween-20 and 5% v/v low fat milk powder. The
respective membranes were incubated with the primary antibodies
diluted in PBS containing 0,1% Tween and 1% v/v low fat milk
powder, for 1 h at room temperature. The following primary
antibodies were used: p-FAK (1:200; MAB1141; Millipore Corp.), FAK
(1:200; sc-557; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA), nuclear factor-κB (NF-κB) p65 subunit (1:200; (sc-109; Santa
Cruz Biotechnology, Inc.) and β-actin (1:2,500; MAB1501; Millipore
Corp.). The immune complexes were detected by peroxidase-conjugated
anti-mouse, anti-goat and anti-rabbit secondary antibodies
(1:10,000; Millipore Corp.), using SuperSignal West Pico
Chemiluminescent substrate (Pierce Biotechnology, Inc., Rockford,
IL, USA).
Real-time PCR
For real-time PCR, mRNA was isolated using TRIzol
reagent (Invitrogen Life Technologies) according to manufacturer's
instructions. RNA (1 µg) was used for cDNA synthesis, using
the Takara reverse transcription reagent kit (Takara Bio, Dalian,
China). Real-time PCR was performed using the Mx300P cycler
(Stratagene; Agilent Technologies, Inc., Santa Clara, CA, USA). The
KAPA SYBR® FAST Universal qPCR kit (Kapa Biosystems,
Inc., Wilmington, MA, USA) was used for real-time PCR reactions in
a total volume of 20 µl and with suitable specific gene
primers (Table I). The PCR
conditions used for amplification were: 94°C for 15 min followed by
40 cycles at 94°C for 20 sec, 55°C for 30 sec and 72°C for 30 sec,
followed by 72°C for 10 min. Standard curves were run and produced
a linear plot of threshold cycle (Ct) against dilution (log). Gene
levels were quantified according to the concentrations of a
standard curve and are presented as arbitrary units. GAPDH was used
as a housekeeping gene, for comparison between samples.
 | Table IPrimer sequences used for real-time
PCR. |
Table I
Primer sequences used for real-time
PCR.
| Gene | Primer
sequences |
|---|
| FAK | F:
5′-GTGCTCTTGGTTCAAGCTGGAT-3′ |
| R:
5′-ACTTGAGTGAAGTCAGCAAGATGTGT-3′ |
| GAPDH | F:
5′-GGAAGGTGAAGGTCGGAGTCA-3′ |
| R:
5′-GTCATTGATGGCAACAATATCCACT-3′ |
Determination of heparan sulfate (HS)
content
The determination of the HS content was carried out
as previously described by Karamanos et al (42). Briefly, in order to determine the
amount of HS production by the B6FS cells, we performed metabolic
labeling of GAGs by supplementing the cell cultures with
D-[6-3H(N)]glucosamine hydrochloride (10 µCi/ml) during the
period of 16 h prior to the respective harvesting time. Upon the
termination of the incubation period, the cells were harvested and
cell-associated proteoglycans (PGs) were extracted with 50 mM
Tris-HCl, pH 8.0, containing 1% (v/v) Triton X-100 and 0.1% (w/v)
NaCl and the following proteinase inhibitors: phenylmethanesulfonyl
flouride, benzamidine hydrochloride and hexanoic acid at final
concentrations of 2, 5 and 50 mM, respectively. The collected
conditioned medium was concentrated to 1:100 of its original volume
on an YM-10 membrane (Amicon/Millipore). The PGs were then
precipitated by the addition of 4 vol. of 95% (v/v) ethanol
containing 2.5% (w/v) sodium acetate with 40 µl chondroitin
sulfate (CSA; 0.2 mg/l) added as a carrier. Following
centrifugation (11,000 × g for 10 min at 25°C), the precipitates of
Pgs were digested with 2 U/ml proteolytic enzyme papain in 100 mM
phosphate buffer (pH 7.0) at 65°C for 60 min. The GAGs liberated in
this manner were precipitated by the addition of 10 vol. 1% (w/v)
cetylpyridium chloride (CPC) and centrifuged at 10,000 × g for 10
min. The pellets obtained were dissolved in 500 µl of 60
(v/v) propanol-1 containing 0.4% (w/v) CPC. The liberated GAGs were
reprecipitated by the addition of 6 vol. of 95% (v/v) ethanol
containing 2.5% (w/v) sodium acetate. The precipitates were then
washed with ethanol and allowed to dry. For the identification of
galactosaminoglycans (GalAGs)., i.e., chondroitin sulfate (CS)
and/or dermatan sulfate (DS), the GAG preparation was dissolved in
water and digested with an equi-unit mixture (0.2 U/ml) of
chondroitinases ABC and AC II. Aliquots from the supernatant were
analyzed by reversed polarity high-performance capillary
electrophoresis (HPCE), as previously described (42). The determination of HS was carried
out in the GAG preparations which were digested with heparin lyases
I, II and III in combination (0.05 U/ml) in 20 mM acetate buffer,
pH 7.0, containing 1 µmol calcium acetate at 37°C for 90 min
(43). In all cases, the amount of
GAGs was determined from the integrated peak area of the
GAG-derived Δ-disaccharides.
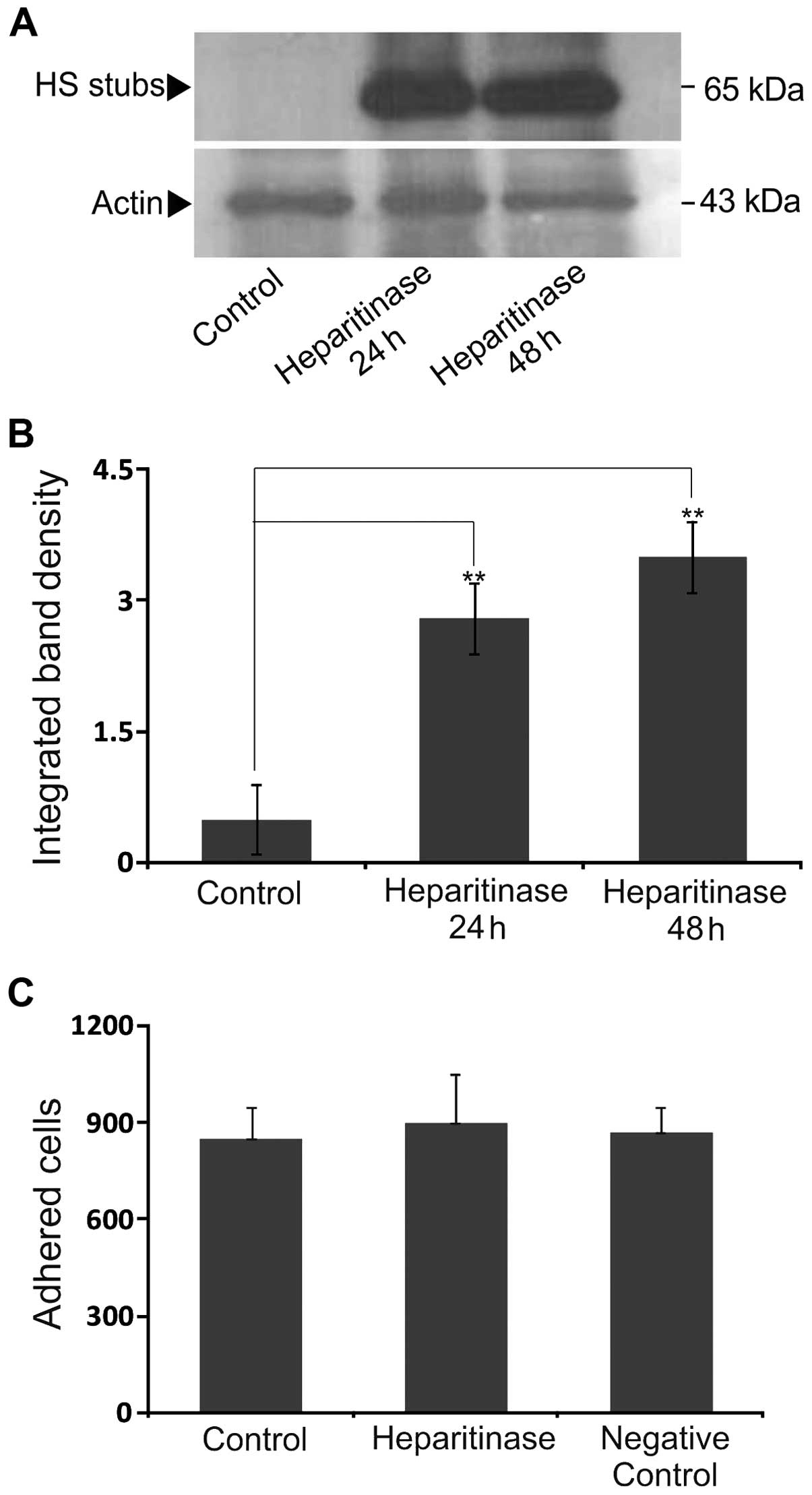
HS digestion
Heparitinase treatments were performed for the
digestion of B6FS cell HS chains, as previously described (24,26).
In brief, cells seeded in 24-well plates were serum-starved for 24
h and then treated with heparitinase (0.001 U/ml) for 24 and 48 h
in 0% FBS medium. The cell extracts treated with heparitinase were
electrophoresed on 8% polyacrylamide Tris/glycine gels and
transferred onto nitrocellulose membranes. After blocking, the
membranes were incubated for 1 h at room temperature with primary
antibodies [mouse anti-heparitinase stubs antibody (clone 3G10;
1:500; CF500913; Seigakagu, Tokyo, Japan); goat anti-actin (1:200;
sc-1616; Santa Cruz Biotechnology, Inc.) and mouse anti-CSA (1:200;
C8035; Sigma)]. The immune complexes were detected following
incubation with peroxidase-conjugated anti-goat or anti-mouse
antibody, 1:4,000 or 1:2,000, respectively, with the SuperSignal
West Pico Chemiluminescent substrate (Pierce Biotechnology,
Inc.)
Cell adhesion assay
For the cell attachment assay, we used cells
transfected with siFAK or cells treated with UHF and LMWH (for 48
h). The cells were detached with 5 mM PBS/EDTA. In continuation
5,000 cells/well were seeded onto a flat-bottom 96-well black
plate. The bottom of the wells was coated with FN (5 µg/ml)
for 1 h at 37°C. BSA 1% (30 min at room temperature) was added for
the blocking of non-specific binding sites. The cells were allowed
to adhere for 30 min at 37°C. The number of attached cells was
determined using the CyQUANT fluometric assay (Molecular Probes;
Invitrogen Life Technologies) according to the manufacturer's
instructions.
Cell migration assay
To investigate the motility of cells, 24-well plates
were used. Cells (60,000 cells/well) were seeded for 24 h in RPMI
10% FBS and subsequently treated (as described above) for 48 h. The
cell stroma was wounded by scratching with a sterile 10 µl
pipette tip. The detached cells were washed twice by using fresh
RPMI (serum-free). The wound closure was monitored at 0 and 6 h
using a digital camera (Canon Inc., Tokyo, Japan) connected to a
microscope (Leica, Mannheim, Germany). The quantification of the
wound area was measured using ImageJ software.
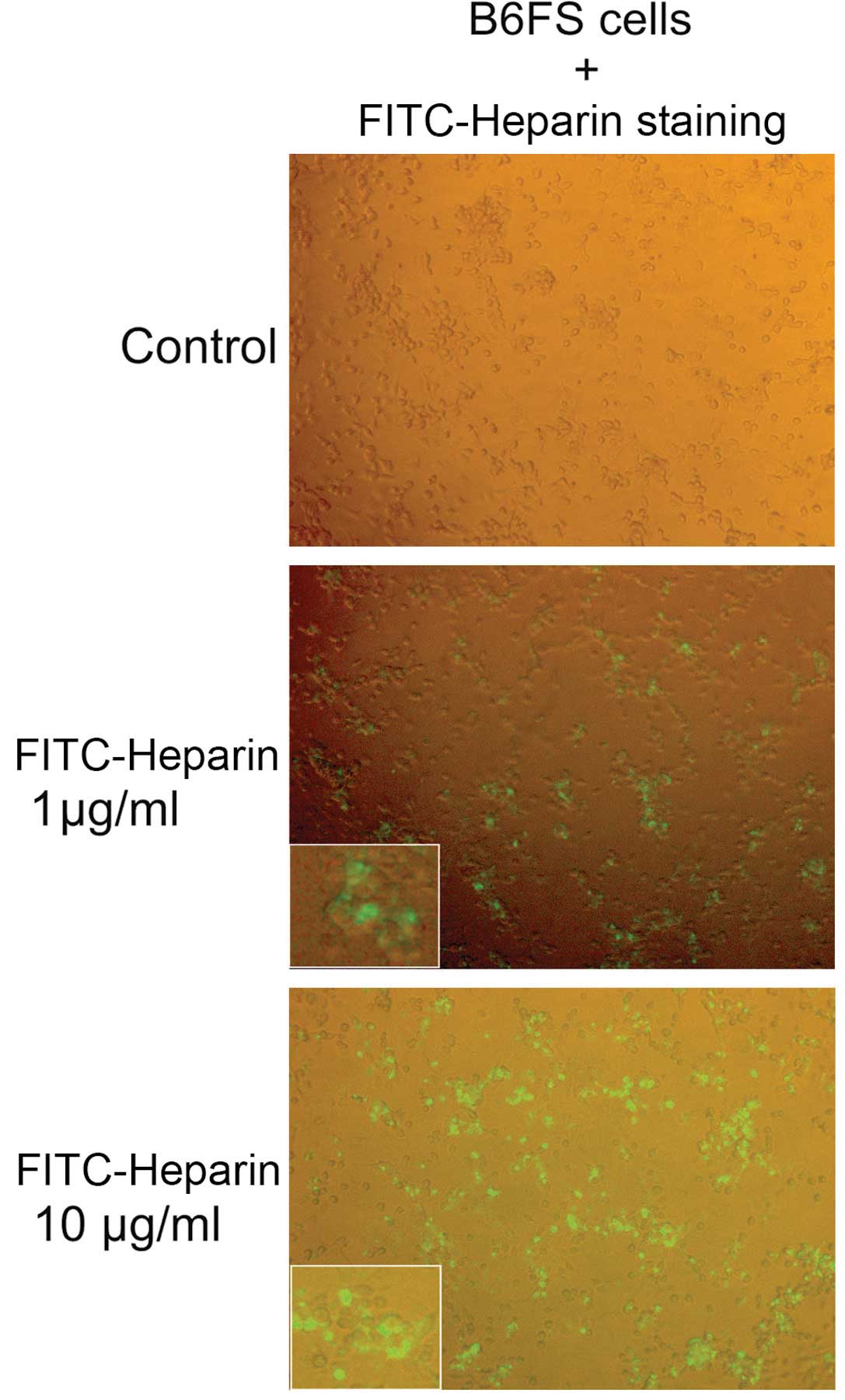
Heparin internalization assay
The B6FS cells were seeded onto a 96-well plate in
RPMI supplemented with 10% FBS for 24 h incubation and
subsequently, FITC-Heparin was added at 1 and 10 µg/ml for
24 h, in serum-free RPMI. Following this incubation, FITC-Heparin
was removed and the cells were gently washed with RPMI (0% FBS).
The concentration and the incubation times were chosen selected
following optimization experiments (data not shown). FITC-Heparin
internalization was visualized under a fluorescence microscope
utilizing Leica DM2500 to acquire images.
Immunofluorescence
The B6FS cells were seeded onto glass coverslips
into 24-well plates (65,000 cells/well) and incubated with 10% FBS
RPMI for 24 h. Following 24 h of serum starvation, the cells were
treated with UFH and LMWH for 48 h. Subsequenlty, the cells were
fixed in 5% formldehyde and 2% sucrose in PBS (incubation for 10
min at room temperature). Following 3 washes with PBS, Triton X
permeabilizing agent was appled for 10 min at room temperature and
washed prior to the addition of fluorescent phalloidin (1:100;
Molecular Probes; Invitrogen Life Technologies) for 20 min in the
dark. Fluorescent phalloidin was used for the detection of actin
filaments. TO-PRO-3 (T3605; Molecular Probes/Thermo Fisher
Scientific, Waltham, MA, USA) was then used for the nuclear
staining (20 min in the dark). The coverslips were placed onto
slides using glycerol and images were collected using a
laser-scanning spectral confocal microscope (TCS SP2; Leica), LCS
Lite software (Leica) and a 63 Apochromat 1.40 numerical aperture
oil objective.
Statistical analysis
Statistical significance was evaluated by one-way
ANOVA analysis with Turkey's post-test, using GraphPad Prism
(version 4.0) software. A value of p<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of heparin in B6FS fibrosarcoma
cell functions
Initially, we examined the effects of UFH and LMWH
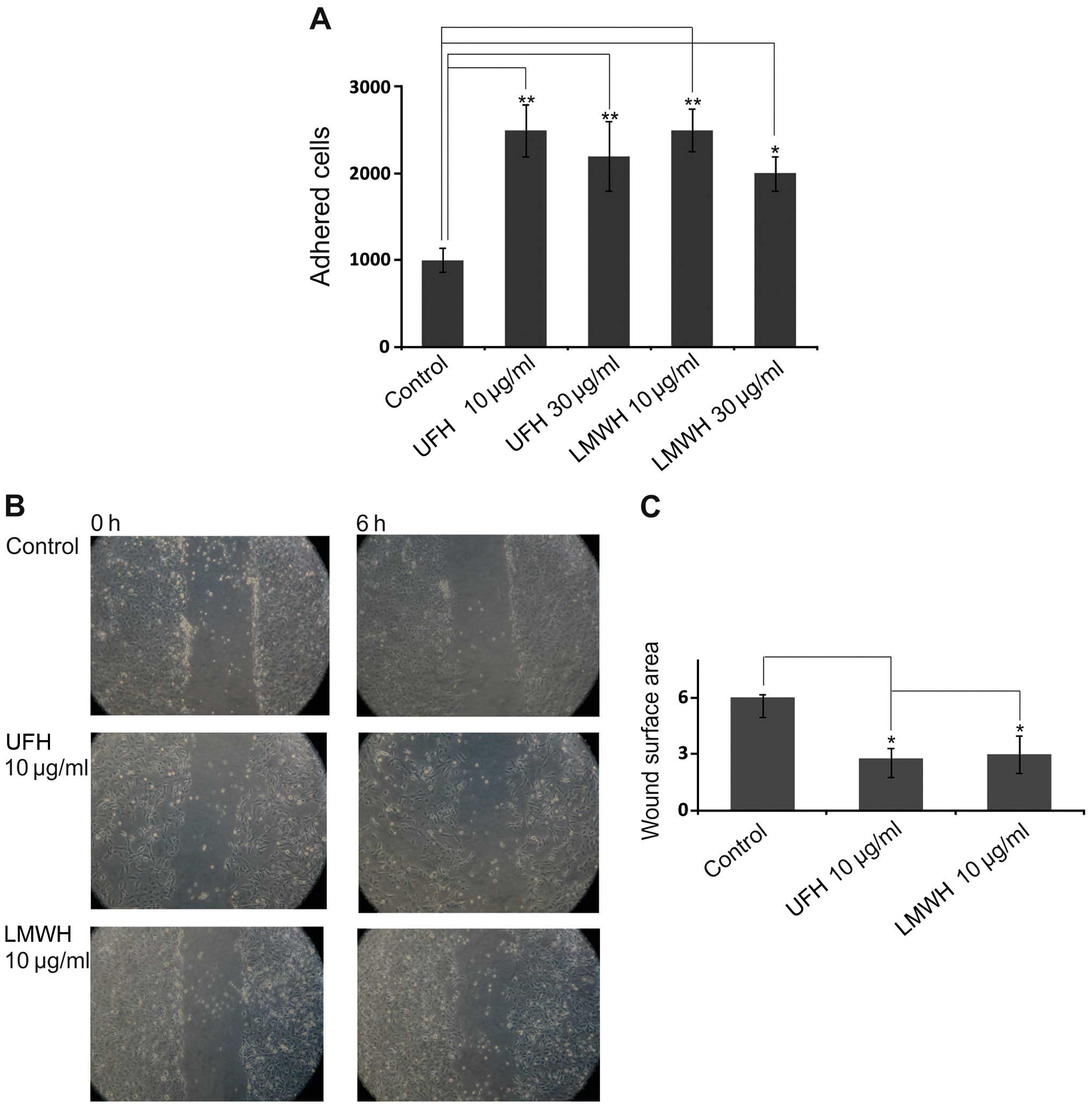
on FN-dependent B6FS cell adhesion. As shown in Fig. 1A, both UFH and LMWH enhanced B6FS
cell adhesion (p<0.01, p<0.05); the maximal effect being
evident at the concentration of 10 µg/ml. Of note, the
molecular weight of the heparin preparations did not modify their
effects on cellular function. Thus, UFH and LMWH had the same
promoting effect on B6FS cell adhesion. This suggests that the
effects of heparin are attributed to its oligosaccharide
structure.
In continuation, we analyzed the effects of heparin
on B6FS fibrosarcoma cell migration. Subsequent to pre-treatment
with UFH and LMWH (10 µg/ml) an enhancement of fibrosarcoma
cell migration was observed (p<0.05; Fig. 1B and C). To exclude false-positive
results due to traces of heparin-binding growth factors, heat
treatment was applied for the utilized heparin preparation. The
effects of heparin on cell adhesion/migration were not affected by
heat treatment (data not shown).
The participation of FAK is necessary for
heparin-induced cell adhesion
FAK is a 125-kDa cytoplasmic tyrosine kinase protein
which is primarily positioned at adhesion sites and plays a key
role in the processes of cell adhesion and migration (44). Importantly, FAK expression has been
shown to be closely associated with (even in early reports)
aggressive cancer behavior (45).
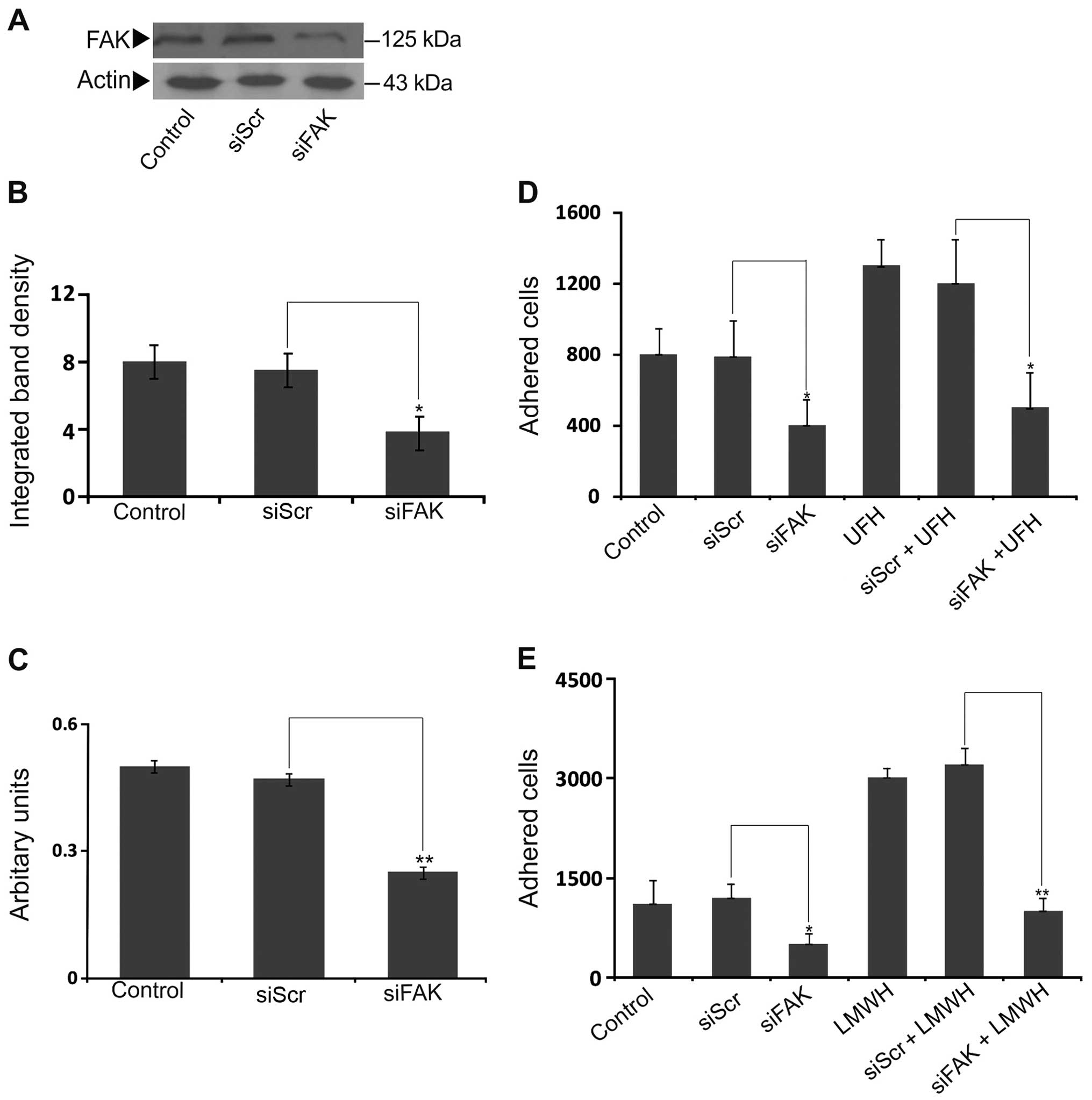
To examine the putative participation of FAK in heparin-dependent
adhesion, the B6FS cells were transfected with siRNA specific for
the FAK gene as previously described (41). This approach resulted in an
efficient downregulation of FAK both at the mRNA (p<0.01) and
protein level (p<0.05) (Fig.
2A–C). Our results demonstrated that the FAK-deficient cells
exhibited a marked decrease in their basal level ability to adhere
to the FN substrate (p<0.05; Fig.
2D). Importantly, both the UFH and LMWH stimulatory effects on
B6FS cell adhesion were abolished in the FAK-deficient cells
(p<0.05 and p<0.01, respectively) (Fig. 2D and E). Therefore, the effect of
heparin on fibrosarcoma cell adhesion was FAK-dependent.
Effect of heparin on FAK activation
Heparin has been shown to antagonize the function of
integrin adhesion receptors (46),
which trigger FAK clustering (47),
and to have an effect on FAK expression and activation (33,34).
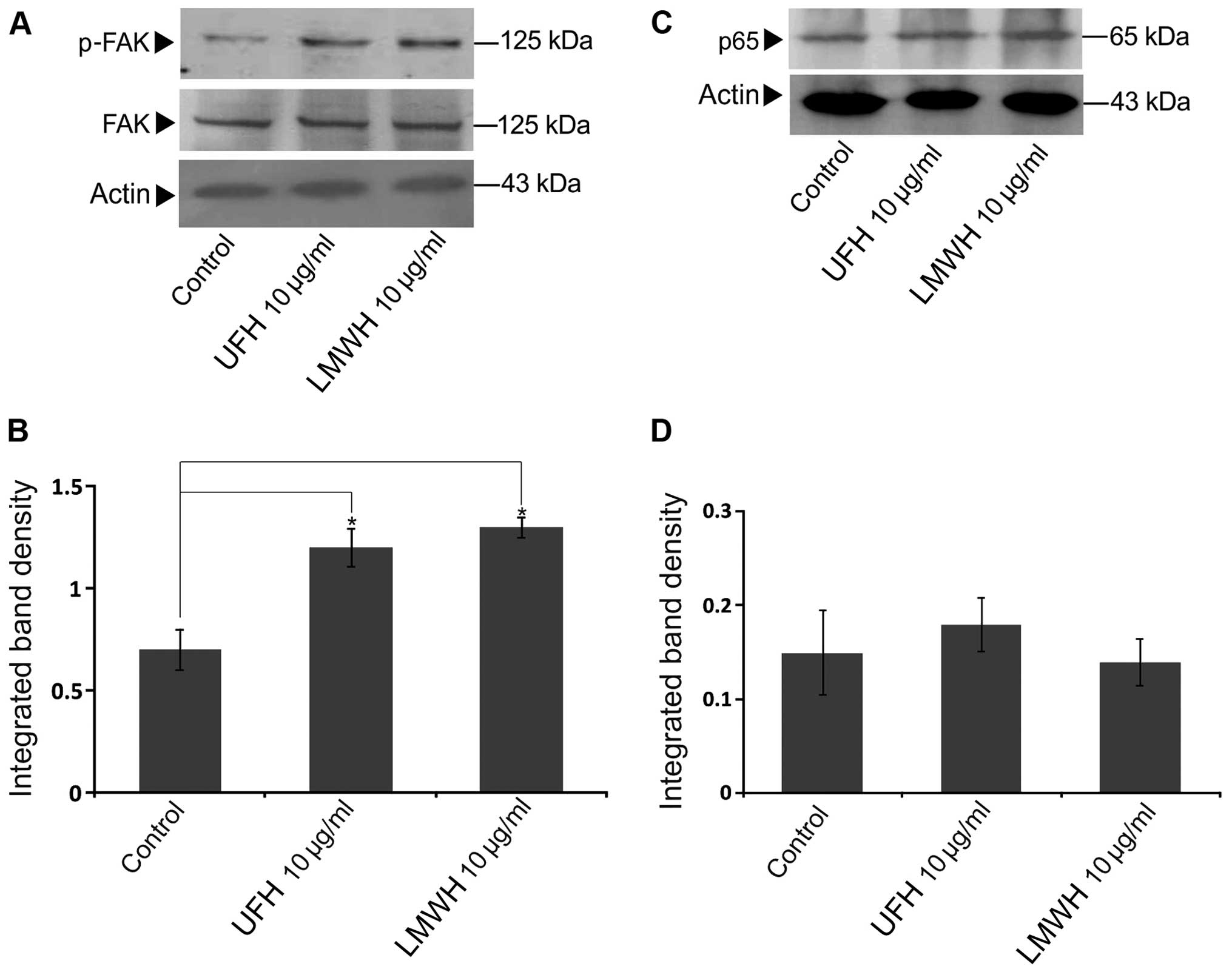
Thus, we investigated the possible role of heparin in FAK
expression and activation. To this end, the heparin-treated B6FS
cell extracts were probed with antibodies against FAK and p-FAK. As
shown in Fig. 3A and B, both UFH
and LMWH significantly activated FAK (Y397) at 10 µg/ml,
whereas no effect of heparin on total FAK protein expression was
evident (Fig. 3A and B).
NF-κB has been previoulsy demonstrated to stimulate
FAK promoter activity and upregulation (41). Heparin has been suggested to
modulate NF-κB translocation to the nucleus and its transcriptional
activity (48). In this study, we
examined the possible role of NF-κB in heparin-induced FAK
activation by using a specific antibody for the p65 subunit of
NF-κB. As shown in Fig. 3C and D,
UFH and LMWH did not affect the protein expression of the p65
subunit of NF-κB. Thus, p65 does not participate in the
heparin-induced upregulation of FAK (Fig. 3C and D).
Heparin affects cytoskeleton organization
in B6FS cells
The phenotype of cancer cells is directly associated
with their adhesive and migratory properties, which are of utmost
importance for the process of cancer metastasis (49). FAK, has been postulated as the key
conduit point which regulates the flow of signals from the ECM to
the actin cytoskeleton (50). In
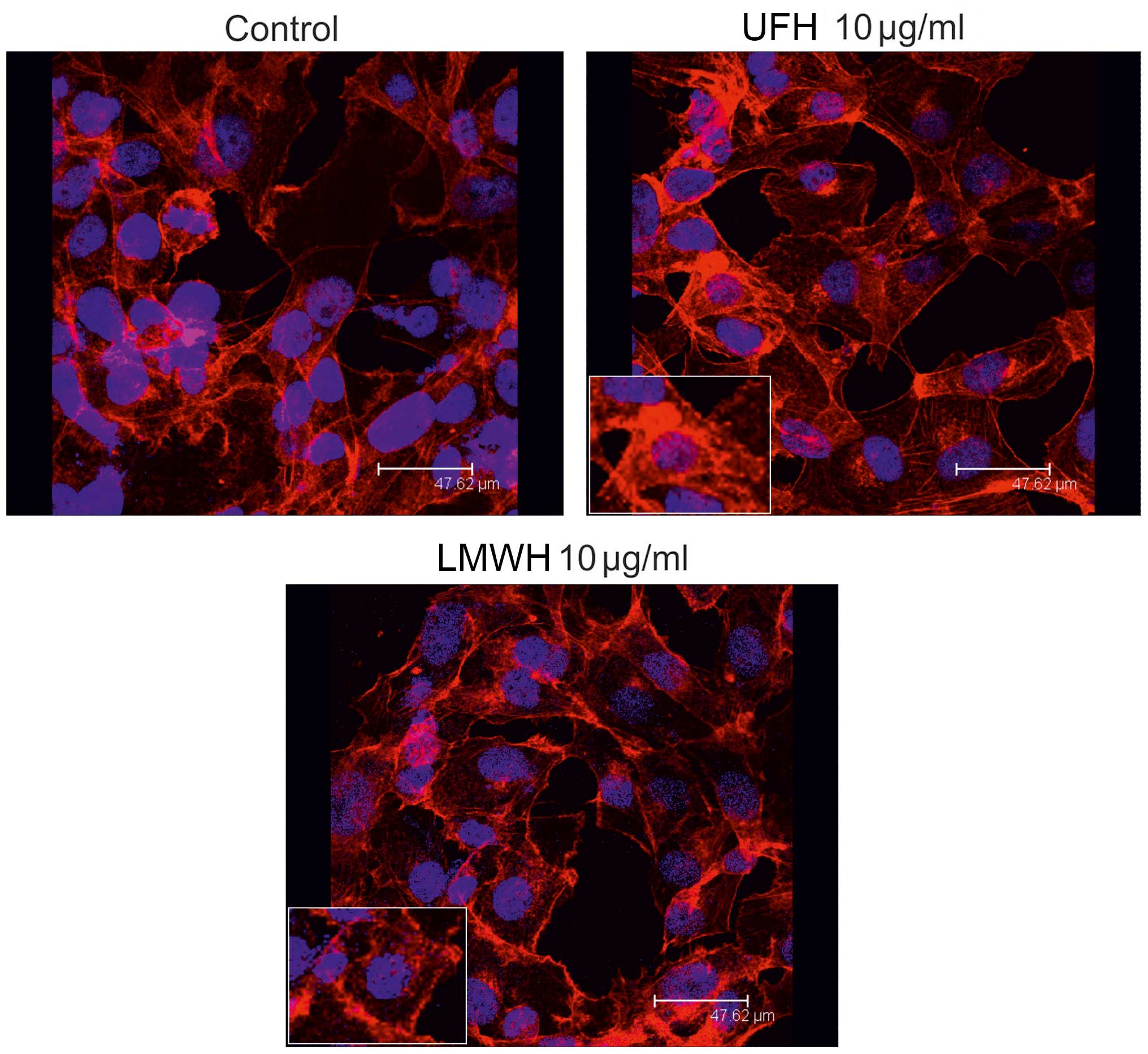
this study, we investigated the possible effect of heparin on actin
cytoskeleton organization in B6FS fibrosarcoma cells. To this end,
rhodamine-conjugated phalloidin staining was used. The results of
confocal miscroscopy indicated that B6FS cell actin cytoskeleton
organization was enhanced. Thus, both the HMW- and LMWH-treated
cells were found to have the fully spread, adhesive phenotype with
the cells being stretched by the tensile forces of actin stress
fibers (Fig. 4).
Heparin internalization in B6FS
fibrosarcoma cells
In continuation, we hypothesized that heparin
affects fibrosarcoma cell motility and cell attachment through its
internalization. Indeed, the cellular internalization of heparin
has been demonstrated in previous studies (51,52).
In this study, FITC-Heparin was utilized to investigate the
internalization of heparin in B6FS fibrosarcoma cells. As shown in
Fig. 5, FITC-Heparin was taken up
by the B6FS cells in a dose-dependent manner, with the majority of
cells exhibiting strong staining. The results of immunoflourescence
demonstrated that FITC-Heparin was located, not only in the
cytoplasmic region, but also in the nucleus of fibrosarcoma
cells.
Effect of cell-associated HS chains on
B6FS cell adhesion
HS exhibits structural similarities with heparin,
but does not have as many 'highly sulfated' sequences consisting of
tri-sulfated disaccharide units, which are sequences with the
highest binding affinity to heparin/HS-binding proteins (53). Biochemical analyses of the
metabolically [3H]-labeled CS/DS/HS/PGs produced by the B6FS cells
demonstrated that the HS amount was 63.1% of the total B6FS
cell-associated GAGs (data not shown). Therefore, we examined the
possible involvement of endogenous HS chains on B6FS cell adhesion.
Initially, treatment of the cells with heparitinase and concomitant
blotting with the monoclonal antibody 3G10, which recognizes a
neoepitope generated by heparitinase digestion, demonstrated an
efficient removal of HS chains at the 24 h time point (Fig. 6A and B). The specific digestion of
cell-associated HS with heparitinase did not affect their adhesion
to FN (Fig. 6C). These results
suggest that B6FS cell adhesion is independent of their
cell-associated HS content.
Discussion
In the present study, the effects of UFH and LMWH on
fibrosarcoma cell motility functions were examined. We demonstrated
that both heparin preparations affected fibrosarcoma cell motility
and adhesion. The stimulatory effects of both UFH and LMWH on B6FS
cell adhesion were FAK-dependent and resulted in actin cytoskeleton
reorganization.
Fibrosarcoma is a tumor of mesenchymal origin that
is mostly composed of transformed fibroblasts and abundant is in
ECM microenvironment (2,54). The B6FS cell line originates from a
poorly differentiated fibrosarcoma (40). In poorly differentiated tumors,
cancer cells are characterized by scant phenotypic similarity with
cells of origin, mild pleomorphism, as well as intense mitotic
activity (55,56). It is noteworthy that fibrosarcoma is
a particularly heterogeneous tumor type, taking into account
morphology, differentiation and behavior (57).
In this study, we demonstrated a promoting effect of
UFH and LMWH on the motility of B6FS cells. Specifically, both UFH
and LMWH were found to enhance cell adhesion onto FN, as well as
the migration of B6FS fibrosarcoma cells. Heparin and the closely
resembling HS chains, via the activation of key cell signaling
pathways (for fibrosarcoma cells), act as extracellular regulators
for many cell functions (5,58,59).
Previously, we demonstrated a negative regulatory effect of LMWH on
melanoma cell adhesion and migration via the PKCα/JNK signaling
pathway (34). Furthermore, LMWH
was found to inhibit the proliferation, migration, invasion and the
induction of lung metastases of HT1080 fibrosarcoma cells, through
a blockade of the RAGE axis (32).
Likewise, in the present study, we examined the mechanisms of
action of heparin in B6FS cells by focusing on FAK (60). FAK, as its name implies, is a key
constituent of the focal adhesion complex, which is an actin-based
anchoring junction limited to the cell-ECM interface in mammalian
tissues. It is used by motile cells, such as fibroblasts and
metastatic cancer cells for their attachment and movement under
physiological or pathological conditions (61). By generating FAK-deficient cells, as
previously described (33,41) we demonstrated that the effects of
UFH and LMWH on B6FS cell adhesion were FAK-dependent. As regards
the effect of heparin on cell adhesion, in other cell models, it
has been shown that the interaction of heparin activates integrin
and results in the tyrosine-phosphorylation of focal
adhesion-associated proteins, such as FAK, Src and paxillin in
endothelial cells (62). On the
other hand, heparin has been shown to block osteoblast adhesion
onto osteoactivin in an FAK/ERK-dependent manner (63). In the present study, however, both
UFH and LMWH were was found to enhance FAK (Y397) phosphorylation
in B6FS fibrosarcoma cells. FAK activation is closely associated
with actin cytoskeleton organization. Importantly, both heparin
preparations were demonstrated to induce morphological changes in
the B6FS cell actin cytoskeleton. Indeed, actin polymerization
results in the formation of the actin stress fibers, which are
necessary for efficient cell motility functions. Treatment of
fibrosarcoma cells with both UFH and LMWH enhanced the formation of
actin filaments and induced the well 'spread' migratory active
phenotype. This effect of heparin on the organization of the
cytoskeleton is well correlated with the heparin-dependent
enhancement of B6FS cell adhesion and migration.
The internalization of heparin has been demonstrated
in various cell models. Thus, heparin stabilizes lens
epithelium-derived growth factor (LEDGF) and facilitates its
translocation from the ECM to the nucleus (64). In a murine macrophage cell line,
heparin was found to initiate the phogocytosis of gelatin-latex
particles (65), whereas we
previously demonstrated an internalization of UFH in melanoma cells
(33). In continuation, in the
present study, we investigated the putative internalization of
heparin in the B6FS cell model utilizing FITC-Heparin. This
approach showed a dose-dependent internalization of FITC-Heparin
and its subsequent localization into the B6FS cell cytoplasm and
nucleus. Importantly, other authors have provided data which showed
the heparin nuclear localization and regulation of gene expression
of several cell types (66,67), even though the mechanism of heparin
uptake remains unspecified (48).
Thus, heparin is suggested to regulate the transactivation of
transcription factors, such as Jun/c-Fos/AP-1 (68) and to inhibit NF-κB transcriptional
activity (48). Moreover, NF-κB was
suggested to stimulate FAK activation (41). In the B6FS cells, no effect of
heparin on NF-κB, subunit p63, transactivation was evident and the
intracellular mechanism of heparin action resulting in FAK/actin
cytoskeleton-dependent enhanced adhesion and migration requires
further study.
In the present study, the enzymatic cleavage of
endogenous HS chains did not affect the adhesion of B6FS cell line.
These data suggests that the exogenous addition of heparin
specifically activates signaling pathways responsible for the
promotion of cell adhesion and migration and highlights the
putative role of heparin/HS content of the cancer microenvironment.
The effects of heparin seem to be tissue and cell line-specific.
Thus, studies have suggested that heparin exerts an inhibitory
effect on both cancer and normal cell proliferation (21–23,28).
On the other hand, a dose-dependent stimulatory role of heparin on
the proliferation of HT29, SW1116 and HCT116 human colon cancer
cells, with no involvement of endogenous HS chains was proposed
(27).
In this study, we report that both UFH and LMWH
through a FAK/actin cytoskeleton axis, enhance the adhesion and
migration of B6FS fibrosarcoma cells. The responsiveness of
fibrosarcoma cell motility to the exogenous heparin/HS content of
the cancer microenvironment may play a role in their ability to
metastasize. The exact mechanisms of action of heparin require
further investigation, which is currently under way in our
laboratories.
Abbreviations:
|
GAG
|
glycosaminoglycan
|
|
UFH
|
unfractionated heparin
|
|
LMWH
|
low-molecular-weight heparin
|
|
FN
|
fibronectin
|
|
FAK
|
focal adhesion kinase
|
References
|
1
|
Meric F, Hess KR, Varma DG, Hunt KK,
Pisters PW, Milas KM, Patel SR, Benjamin RS, Plager C, Papadopoulos
NE, et al: Radiographic response to neoadjuvant chemotherapy is a
predictor of local control and survival in soft tissue sarcomas.
Cancer. 95:1120–1126. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nedea EA and DeLaney TF: Sarcoma and skin
radiation oncology. Hematol Oncol Clin North Am. 20:401–429. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Linhardt RJ: 2003 Claude S. Hudson Award
address in carbohydrate chemistry. Heparin: Structure and activity.
J Med Chem. 46:2551–2564. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Engelberg H: Actions of heparin that may
affect the malignant process. Cancer. 85:257–272. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vlodavsky I and Friedmann Y: Molecular
properties and involvement of heparanase in cancer metastasis and
angiogenesis. J Clin Invest. 108:341–347. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tyagi SC, Kumar S and Katwa L:
Differential regulation of extracellular matrix metalloproteinase
and tissue inhibitor by heparin and cholesterol in fibroblast
cells. J Mol Cell Cardiol. 29:391–404. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Smorenburg SM and Van Noorden CJ: The
complex effects of heparins on cancer progression and metastasis in
experimental studies. Pharmacol Rev. 53:93–105. 2001.PubMed/NCBI
|
|
9
|
Elias EG, Shukla SK and Mink IB: Heparin
and chemotherapy in the management of inoperable lung carcinoma.
Cancer. 36:129–136. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kakkar AK and Macbeth F: Antithrombotic
therapy and survival in patients with malignant disease. Br J
Cancer. 102(Suppl 1): S24–S29. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Malavaki CJ, Theocharis AD, Lamari FN,
Kanakis I, Tsegenidis T, Tzanakakis GN and Karamanos NK: Heparan
sulfate: Biological significance, tools for biochemical analysis
and structural characterization. Biomed Chromatogr. 25:11–20. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Karamanos NK and Tzanakakis GN:
Glycosaminoglycans: From 'cellular glue' to novel therapeutical
agents. Curr Opin Pharmacol. 12:220–222. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Halkin H, Goldberg J, Modan M and Modan B:
Reduction of mortality in general medical in-patients by low-dose
heparin prophylaxis. Ann Intern Med. 96:561–565. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chahinian AP, Propert KJ, Ware JH, Zimmer
B, Perry MC, Hirsh V, Skarin A, Kopel S, Holland JF, Comis RL, et
al: A randomized trial of anticoagulation with warfarin and of
alternating chemotherapy in extensive small-cell lung cancer by the
Cancer and Leukemia group B. J Clin oncol. 7:993–1002.
1989.PubMed/NCBI
|
|
15
|
Hettiarachchi RJ, Smorenburg SM, Ginsberg
J, Levine M, Prins MH and Büller HR: Do heparins do more than just
treat thrombosis? The influence of heparins on cancer spread.
Thromb Haemost. 82:947–952. 1999.PubMed/NCBI
|
|
16
|
von Tempelhoff GF, Harenberg J, Niemann F,
Hommel G, Kirkpatrick CJ and Heilmann L: Effect of low molecular
weight heparin (Certoparin) versus unfractionated heparin on cancer
survival following breast and pelvic cancer surgery: A prospective
randomized double-blind trial. Int J Oncol. 16:815–824.
2000.PubMed/NCBI
|
|
17
|
Mousa SA and Mohamed S: Anti-angiogenic
mechanisms and efficacy of the low molecular weight heparin,
tinzaparin: Anti-cancer efficacy. Oncol Rep. 12:683–688.
2004.PubMed/NCBI
|
|
18
|
Ettelaie C, Fountain D, Collier ME, Beeby
E, Xiao YP and Maraveyas A: Low molecular weight heparin suppresses
tissue factor-mediated cancer cell invasion and migration in vitro.
Exp Ther Med. 2:363–367. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alyahya R, Sudha T, Racz M, Stain SC and
Mousa SA: Anti-metastasis efficacy and safety of non-anticoagulant
heparin derivative versus low molecular weight heparin in surgical
pancreatic cancer models. Int J Oncol. 46:1225–1231. 2015.
|
|
20
|
Tiozzo R, Cingi MR, Pietrangelo A,
Albertazzi L, Calandra S and Milani MR: Effect of heparin-like
compounds on the in vitro proliferation and protein synthesis of
various cell types. Arzneimittelforschung. 39:15–20.
1989.PubMed/NCBI
|
|
21
|
Au YP, Kenagy RD, Clowes MM and Clowes AW:
Mechanisms of inhibition by heparin of vascular smooth muscle cell
proliferation and migration. Haemostasis. 23(Suppl 1): 177–182.
1993.PubMed/NCBI
|
|
22
|
Bennett MR, Evan GI and Newby AC:
Deregulated expression of the c-myc oncogene abolishes inhibition
of proliferation of rat vascular smooth muscle cells by serum
reduction, interferon-gamma, heparin, and cyclic nucleotide
analogues and induces apoptosis. Circ Res. 74:525–536. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miralem T, Wang A, Whiteside CI and
Templeton DM: Heparin inhibits mitogen-activated protein
kinase-dependent and -independent c-fos induction in mesangial
cells. J Biol Chem. 271:17100–17106. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nikitovic D, Assouti M, Sifaki M, Katonis
P, Krasagakis K, Karamanos NK and Tzanakakis GN: Chondroitin
sulfate and heparan sulfate-containing proteoglycans are both
partners and targets of basic fibroblast growth factor-mediated
proliferation in human metastatic melanoma cell lines. Int J
Biochem Cell Biol. 40:72–83. 2008. View Article : Google Scholar
|
|
25
|
Nikitovic D, Zafiropoulos A, Tzanakakis
GN, Karamanos NK and Tsatsakis AM: Effects of glycosaminoglycans on
cell proliferation of normal osteoblasts and human osteosarcoma
cells depend on their type and fine chemical compositions.
Anticancer Res. 25:2851–2856. 2005.PubMed/NCBI
|
|
26
|
Chatzinikolaou G, Nikitovic D,
Asimakopoulou A, Tsatsakis A, Karamanos NK and Tzanakakis GN:
Heparin - a unique stimulator of human colon cancer cells' growth.
IUBMB Life. 60:333–340. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chatzinikolaou G, Nikitovic D, Berdiaki A,
Zafiropoulos A, Katonis P, Karamanos NK and Tzanakakis GN: Heparin
regulates colon cancer cell growth through p38 mitogen-activated
protein kinase signalling. Cell Prolif. 43:9–18. 2010. View Article : Google Scholar
|
|
28
|
Fthenou E, Zafiropoulos A, Tsatsakis A,
Stathopoulos A, Karamanos NK and Tzanakakis GN: Chondroitin sulfate
A chains enhance platelet derived growth factor-mediated signalling
in fibrosarcoma cells. Int J Biochem Cell Biol. 38:2141–2150. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Boeryd B: Action of heparin and
plasminogen inhibitor (EACA) on metastatic tumour spread in an
isologous system. Acta Pathol Microbiol Scand. 65:395–404.
1965.PubMed/NCBI
|
|
30
|
Hagmar B and Norrby K: Evidence for
effects of heparin on cell surfaces influencing experimental
metastases. International journal of cancer Int J Cancer. 5:72–84.
1970. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maat B: Extrapulmonary colony formation
after intravenous injection of tumour cells into heparin-treated
animals. Br J Cancer. 37:369–376. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takeuchi A, Yamamoto Y, Munesue S,
Harashima A, Watanabe T, Yonekura H, Yamamoto H and Tsuchiya H: Low
molecular weight heparin suppresses receptor for advanced glycation
end products-mediated expression of malignant phenotype in human
fibrosarcoma cells. Cancer Sci. 104:740–749. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chalkiadaki G, Nikitovic D, Berdiaki A,
Katonis P, Karamanos NK and Tzanakakis GN: Heparin plays a key
regulatory role via a p53/FAK-dependent signaling in melanoma cell
adhesion and migration. IUBMB Life. 63:109–119. 2011.PubMed/NCBI
|
|
34
|
Chalkiadaki G, Nikitovic D, Katonis P,
Berdiaki A, Tsatsakis A, Kotsikogianni I, Karamanos NK and
Tzanakakis GN: Low molecular weight heparin inhibits melanoma cell
adhesion and migration through a PKCa/JNK signaling pathway
inducing actin cytoskeleton changes. Cancer Lett. 312:235–244.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pankov R and Yamada KM: Fibronectin at a
glance. J Cell Sci. 115:3861–3863. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Clark EA, Golub TR, Lander ES and Hynes
RO: Genomic analysis of metastasis reveals an essential role for
RhoC. Nature. 406:532–535. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Inoue T, Nabeshima K, Shimao Y, Kataoka H
and Koono M: Modulation of fibronectin synthesis by cancer
cell-fibroblast interaction. Int J Oncol. 9:721–730.
1996.PubMed/NCBI
|
|
38
|
Mytilinaiou M, Bano A, Nikitovic D,
Berdiaki A, Voudouri K, Kalogeraki A, Karamanos NK and Tzanakakis
GN: Syndecan-2 is a key regulator of transforming growth factor
beta 2/Smad2-mediated adhesion in fibrosarcoma cells. IUBMB Life.
65:134–143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kouvidi K, Berdiaki A, Nikitovic D,
Katonis P, Afratis N, Hascall VC, Karamanos NK and Tzanakakis GN:
Role of receptor for hyaluronic acid-mediated motility (RHAMM) in
low molecular weight hyaluronan (LMWHA)-mediated fibrosarcoma cell
adhesion. J Biol Chem. 286:38509–38520. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Thurzo V, Popovic M, Matoska J, Blasko M,
Grófová M, Lizonová A and Steno M: Human neoplastic cells in tissue
culture: Two established cell lines derived from giant cell tumor
and fibrosarcoma. Neoplasma. 23:577–587. 1976.PubMed/NCBI
|
|
41
|
Hong IK, Jin YJ, Byun HJ, Jeoung DI, Kim
YM and Lee H: Homophilic interactions of Tetraspanin CD151
up-regulate motility and matrix metalloproteinase-9 expression of
human melanoma cells through adhesion-dependent c-Jun activation
signaling pathways. J Biol Chem. 281:24279–24292. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Karamanos NK, Axelsson S, Vanky P,
Tzanakakis GN and Hjerpe A: Determination of hyaluronan and
galactosaminoglycan disaccharides by high-performance capillary
electrophoresis at the attomole level. Applications to analyses of
tissue and cell culture proteoglycans. J Chromatogr A. 696:295–305.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Karamanos NK, Vanky P, Tzanakakis GN,
Tsegenidis T and Hjerpe A: Ion-pair high-performance liquid
chromatography for determining disaccharide composition in heparin
and heparan sulphate. J Chromatogr A. 765:169–179. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Schaller MD, Borgman CA, Cobb BS, Vines
RR, Reynolds AB and Parsons JT: pp125FAK a structurally distinctive
protein-tyrosine kinase associated with focal adhesions. Proc Natl
Acad Sci USA. 89:5192–5196. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Weiner TM, Liu ET, Craven RJ and Cance WG:
Expression of focal adhesion kinase gene and invasive cancer.
Lancet. 342:1024–1025. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fritzsche J, Simonis D and Bendas G:
Melanoma cell adhesion can be blocked by heparin in vitro:
Suggestion of VLA-4 as a novel target for antimetastatic
approaches. Thromb Haemost. 100:1166–1175. 2008.
|
|
47
|
Moyano JV, Maqueda A, Albar JP and
Garcia-Pardo A: A synthetic peptide from the heparin-binding domain
III (repeats III4-5) of fibronectin promotes stress-fibre and
focal-adhesion formation in melanoma cells. Biochem J. 371:565–571.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dudás J, Ramadori G, Knittel T, Neubauer
K, Raddatz D, Egedy K and Kovalszky I: Effect of heparin and liver
heparan sulphate on interaction of HepG2-derived transcription
factors and their cis-acting elements: Altered potential of
hepatocellular carcinoma heparan sulphate. Biochem J. 350:245–251.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Geiger B and Yamada KM: Molecular
architecture and function of matrix adhesions. Cold Spring Harb
Perspect Biol. 3:32011. View Article : Google Scholar
|
|
50
|
Parsons JT, Martin KH, Slack JK, Taylor JM
and Weed SA: Focal adhesion kinase: A regulator of focal adhesion
dynamics and cell movement. Oncogene. 19:5606–5613. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Castellot JJ Jr, Wong K, Herman B, Hoover
RL, Albertini DF, Wright TC, Caleb BL and Karnovsky MJ: Binding and
internalization of heparin by vascular smooth muscle cells. J Cell
Physiol. 124:13–20. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bârzu T, Pascal M, Maman M, Roque C,
Lafont F and Rousselet A: Entry and distribution of fluorescent
antiproliferative heparin derivatives into rat vascular smooth
muscle cells: Comparison between heparin-sensitive and
heparin-resistant cultures. J Cell Physiol. 167:8–21. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Casu B and Lindahl U: Structure and
biological interactions of heparin and heparan sulfate. Adv
Carbohydr Chem Biochem. 57:159–206. 2001. View Article : Google Scholar
|
|
54
|
Shrivastava S, Nayak SK, Nayak P and Sahu
S: Fibrosarcoma of maxilla: A rare case report. J Oral Maxillofac
Pathol. 20:1622016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Thway K, Robertson D, Jones RL, Selfe J,
Shipley J, Fisher C and Isacke CM: Endosialin expression in soft
tissue sarcoma as a potential marker of undifferentiated
mesenchymal cells. Br J Cancer. Jul 19–2016.Epub ahead of print.
View Article : Google Scholar :
|
|
56
|
Noujaim J, Thway K, Sheri A, Keller C and
Jones RL: Histology-Driven Therapy: The Importance of Diagnostic
Accuracy in Guiding Systemic Therapy of Soft Tissue Tumors. Int J
Surg Pathol. 24:5–15. 2016. View Article : Google Scholar
|
|
57
|
Blizniukov OP and Zamogil'naia IaA:
Pleomorphic fibrosarcoma. Vopr Onkol. 58:54–60. 2012.In
Russian.
|
|
58
|
Rabenstein DL: Heparin and heparan
sulfate: Structure and function. Nat Prod Rep. 19:312–331. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chen Q, Zhou Z, Shan L, Zeng H, Hua Y and
Cai Z: The importance of Src signaling in sarcoma. Oncol Lett.
10:17–22. 2015.PubMed/NCBI
|
|
60
|
Hauck CR, Hsia DA and Schlaepfer DD: The
focal adhesion kinase - a regulator of cell migration and invasion.
IUBMB Life. 53:115–119. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hall JE, Fu W and Schaller MD: Focal
adhesion kinase: Exploring Fak structure to gain insight into
function. Int rev Cell Mol Biol. 288:185–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Medeiros VP, Paredes-Gamero EJ, Monteiro
HP, Rocha HA, Trindade ES and Nader HB: Heparin-integrin
interaction in endothelial cells: Downstream signaling and heparan
sulfate expression. J Cell Physiol. 227:2740–2749. 2012. View Article : Google Scholar
|
|
63
|
Moussa FM, Hisijara IA, Sondag GR, Scott
EM, Frara N, Abdelmagid SM and Safadi FF: Osteoactivin promotes
osteoblast adhesion through HSPG and αvβ1 integrin. J Cell Biochem.
115:1243–1253. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Fatma N, Singh DP, Shinohara T and Chylack
LT Jr: Heparin's roles in stabilizing, potentiating, and
transporting LEDGF into the nucleus. Invest Ophthalmol Vis Sci.
41:2648–2657. 2000.PubMed/NCBI
|
|
65
|
van de Water L III, Schroeder S, Crenshaw
EB III and Hynes RO: Phagocytosis of gelatin-latex particles by a
murine macrophage line is dependent on fibronectin and heparin. J
Cell Biol. 90:32–39. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kovalszky I, Dudás J, Oláh-Nagy J, Pogány
G, Töváry J, Timár J, Kopper L, Jeney A and Iozzo RV: Inhibition of
DNA topoisomerase I activity by heparan sulfate and modulation by
basic fibroblast growth factor. Mol Cell Biochem. 183:11–23. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Timar J and Paterson H: Localization and
production of proteoglycans by HT1080 cell lines with altered N-ras
expression. Cancer Lett. 53:145–150. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Busch SJ, Martin GA, Barnhart RL, Mano M,
Cardin AD and Jackson RL: Trans-repressor activity of nuclear
glycosaminoglycans on Fos and Jun/AP-1 oncoprotein-mediated
transcription. J Cell Biol. 116:31–42. 1992. View Article : Google Scholar : PubMed/NCBI
|