Introduction
Neurilemmoma (schwannoglioma) consists of
dermatology neurofibroma and plexiform neurofibroma (PNFs), both of
which are benign tumors. However, PNF has 1/10 malignant
transformation rate and can evolve into malignant peripheral nerve
sheath tumor (MPNST), which always metastasize and has very poor
prognosis (1).
Hypoxia makes great contributions to tumor
progression, oxygen supply is affected by tumor growth and
following neovascularization; hypoxia can result in tumor drug
resistance, metastasis and other serious consequences (2). Intracellular hypoxia can induce the
HIF family to a series of responses, such as glucide metabolic
pathway changes from tricarboxylic acid cycle to anaerobic
glycolysis pathway, neovascularization and apoptosis (3,4).
Internal hypoxic regions exist throughout growth and
evolution of most malignant tumors, and such regions always necrose
and are more prone to tumor metastasis (5). Hypoxia is one of cancer occurrence and
evolution signs (6). The HIF-1α
with high expression level in the hypoxic region of tumor tissues
can upregulate VEGF expression (7),
and can also upregulate E-cadherin suppressor gene expression, and
thus reduce inter-tumor cellular homo-adhesion and facilitate tumor
cell metastasis (8). Since HIF-1α
hydroxylation is oxygen independent, under hypoxic conditions,
HIF-1α can protect itself from being degraded by proteinase von
Hippel-Lindau. Then HIF-1α can combine with the transcriptional
activators p300, HIF-1β and further change its transcriptional
level, and also change transcriptional levels of downstream
microRNAs, including miR-210 (3,9).
MicroRNAs are non-coding RNA with approximately 22
nucleotides (10). They regulate
target protein expression by complete or incomplete matching with
3′ untranslated regions (3′-UTR), which the former can directly
cleave the target mRNA and further reduce target mRNA transcription
and translation, and the later can repress target protein
translation without affecting mRNA stability (11).
It is reported that miR-210 is associated with
various tumors, such as gastroenteric tumor (12), bladder cancer (13), breast carcinoma (14), pancreatic carcinoma (15), and is also one of the major
microRNAs induced by HIF-1α (16).
HIF-1α directly combines with the hypoxia response element (HRE) at
approximately 40 bp upstream of miR-210 promoter transcription
start site, and cis-regulate miR-210 expression (17). miR-210 is also thought to be one of
the tumor hypoxia markers (18–20).
The reported target genes of miR-210 included HOXA1,
HOXA9, HOXA3, E3F3 and ephrin-A3 (EFNA3) (16), but only EFNA3 was still regulated by
miR-210 under hypoxic conditions (21). EFNA3 is a member of Ephrins family,
the biggest subtribe of receptor tyrosine kinases, of which EphA
family receptors are thought to be able to inhibit tumor
vasculogenesis (22,23), and EphB family was thought to be
associated with tumor vascular invasion and generation (24,25).
Our early study results illustrated that miR-210 is
associated with MPNST cell invasion and metastasis, and regulates
EFNA3 protein expression via incomplete complementary paring with
3′-UTR of EFNA3, thus affects focal adhesion kinase (FAK) pathway
in tumor cells and HIF-1α/VEGF-mediated vasculogenesis pathway
(26,27). However, the functions of miR-210
during neurilemmoma hypoxia, and the underlying molecular mechanism
of hypoxia-induced miR-210 upregulation in schwannoma cells is
still vague.
Materials and methods
Cell culture and transfections
The schwannoma RT4-D6P2T cells (ATCC, Manassas, VA,
USA) were cultured in RMPI-1640 (HyClone, Hudson, NH, USA) medium
with 10% fetal bovine serum (Gibco, Carlsbad, CA, USA) at 37°C, 95%
air and 5% CO2 incubator (Thermo Fisher Scientific,
Waltham, MA, USA), or at 37°C, 94% N2, 5% CO2
and 1% O2 tri-gas incubator (Thermo Fisher Scientific)
for hypoxic culture, and were subcultured every 2–3 days.
HIF-1α-shRNA-pRNAT-U6.1/Neo interference plasmids (Auragene,
Changsha, China), or miR-210 complementary Locked Nucleic Acid
(anti-miR-210) (GeneCopopia, Rockville, MD, USA) and lipo2000
(Invitrogen Life Technologies, Carlsbad, CA, USA) mixed liquor was
added into a petri dish with 30–50% cells (Nest, Wuxi, China) for
4–6 h, and then the culture medium was removed and replaced by
fresh culture medium. After the replacement, the resulting solution
was put in a 37°C CO2 incubator for 24–72 h culture and
then the cells were collected for further study.
Real-time polymerase chain reaction
(qPCR)
Total RNAs were extracted from the cells using
TRIzol (Invitrogen Life Technologies) reagent, and prepared with a
RevertAid First Strand cDNA Synthesis kit (Fermentas Inc., Ontario,
Canada). SYBR green qPCR assay was carried out to find the relative
expression levels of miR-210, compared with the internal control
gene U6, and calculated using the 2−∆∆Ct method. The
rat-miR-210 primers (RmiRQP3171), U6 primers (RmiRQP9003) and qPCR
Mix were purchased from GeneCopoeia.
Western blot analysis
Cells (107) were collected and put into
200 µl RIPA (Invitrogen Life Technologies) lysate for extracting
total proteins, of which 50 µg were used for western blot analysis.
The EFNA3 and β-actin antibodies were from Sigma (St. Louis, MO,
USA), HIF-1α, P62 and elf4E antibodies were from Abcam (Cambridge,
MA, USA), VEGF antibodies were from Immunoway (Suzhou, Jiangsu,
China).
Cell cycle, apoptosis, migration
assay, and ultrastructural observation
Cell cycle: the cells were treated for 24 h, and
then Trypsins (Auragene) were used to dissociate the cells, and PBS
was used to wash and resuspend the cells. Ethyl alcohol (70%) was
added into the resuspension, then the resuspension was cultured at
4°C for 18 h. Cells were again washed with PBS and the washed cells
were resuspended in Staining Solution (50 µg/ml of PI, 1 mg/ml of
RNase A, 0.1% Triton X-100 in PBS). The stained cells
(1×106) were then analyzed with a flow cytometer
(Beckman Coulter, Brea, CA, USA). Cell apoptosis: cells
(5×105) were resuspended in Annexin V-FITC and propidium
iodide (PI) labeling solution, and then Annexin V Apoptosis
Detection kit FITC (eBioscience, Affymetrix, San Diego, CA, USA)
was used for immediate testing. Cell migration: Cellular invasion
ability was tested by Transwell assay. The cells were cultured
under normoxic/hypoxic for 24 h, and then were dissociated and
inoculated in serum-free medium in the upper chamber with well
placed Matrigel for another 24 h in normoxic/hypoxic conditions.
The remaining cells in the upper chamber were carefully removed,
and the cells that entered into the Matrigel were stained by the
staining solution with 75% ethyl alcohol and crystal violet
(Beyotime Biotechnology, Jiangsu, China) for 25 min. Dried at room
temperature, and then were photographed with an inverted microscope
(Motic, Xiamen, China). Ultrastructural observation: 24 h after the
treatment, the cells were placed, respectively, in normoxic and
hypoxic conditions for another 24 h culture, dissociated with
Trypsin-EDTA (Auragene), washed twice with PBS, immobilized with
2.5% glutaraldehyde precooled at 4°C overnight, post-immobilized
with 2% osmic acid, dehydrated with ethanol-acetone, embedded with
epoxy resin, sliced, and then observed with a transmission electron
microscope (Hitachi, Tokyo, Japan) under 80 kV.
H&E, IHC, ISH and IF analysis
The schwannoma tissue microarray (SO808) was
purchased from Alenabio (Shangxi, China), and hematoxylin and eosin
(H&E) staining procedures were as previously described
(28). Immunology and Histology
Chemistry (IHC) test: after being dewaxed, hydrated, antigen
retrieved and H2O2 confined, the tissue chip
was exposed to rabbit anti-VEGF polyclonal antibodies (1:100,
Immunoway), incubated overnight at 4°C, then exposed to goat
anti-rabbit secondary antibodies (1:800, Jackson Immuno Research
Inc., West Grove, PA, USA), incubated at room temperature for 30
min, and the VEGF protein expression was observed with an optical
microscope (Upototech, Changchun, China). In Situ
Hybridization (ISH) test: Rat-miR-210 probe sequence:
5′-ACAGATCAGCCGCTGTCACACGCAC-3′, synthesized at BGI Tech (Shenzhen,
China). Hybridization probe mixed solution (1:500) was instilled
into the chips, using the IsHyb In Situ Hybridization kit
(Biochain, Newark, CA, USA) according to the instructions, then
observed and photographed the results with an optical microscope
(Upototech). Immunofluorescent assay (IF) test: the cells were
inoculated in a 6-well dish (the density <80%), after being
immobilized, the anti-LC3B II/I antibodies (ab48394) by Abcam were
used for IF test, with the primary antibodies diluted at a ratio of
1:1000, and the green fluorescent protein expression was observed
with an inverted microscope (Motic).
Bisulfite sequencing PCR (BSP)
BSP primers were designed according to the predicted
CpG island in Rat-miR-210 promoter region: forward
5′-GGAAGGATATGTTTTGGATTGTATTAA-3′, reverse
5′-TAAAACCCACCCTACAAAACTACAAC-3′, the PCR products were cloned into
pGEM-T Easy vector, 15–20 clones were randomly selected for
sequencing, and 10 correct clones were selected for methylation
analysis.
Statistical analysis
The data are shown as the mean ± SE. Statistical
analysis were performed using SPSS 11.0 software, Student's t-test
was used to analyze the differences between two groups, with 95%
confidence interval. Pearson's Chi-square test was used to analyze
the relationships of microvascular density (MVD) vs. VEGF and MVD
vs. miR-210, with 99% confidence interval. P<0.05 was considered
to indicate a statistically significant difference; and P<0.01,
highly significant.
Results
Expression of miR-210 and
relationships with MVD and VEGF in schwannoma tissues
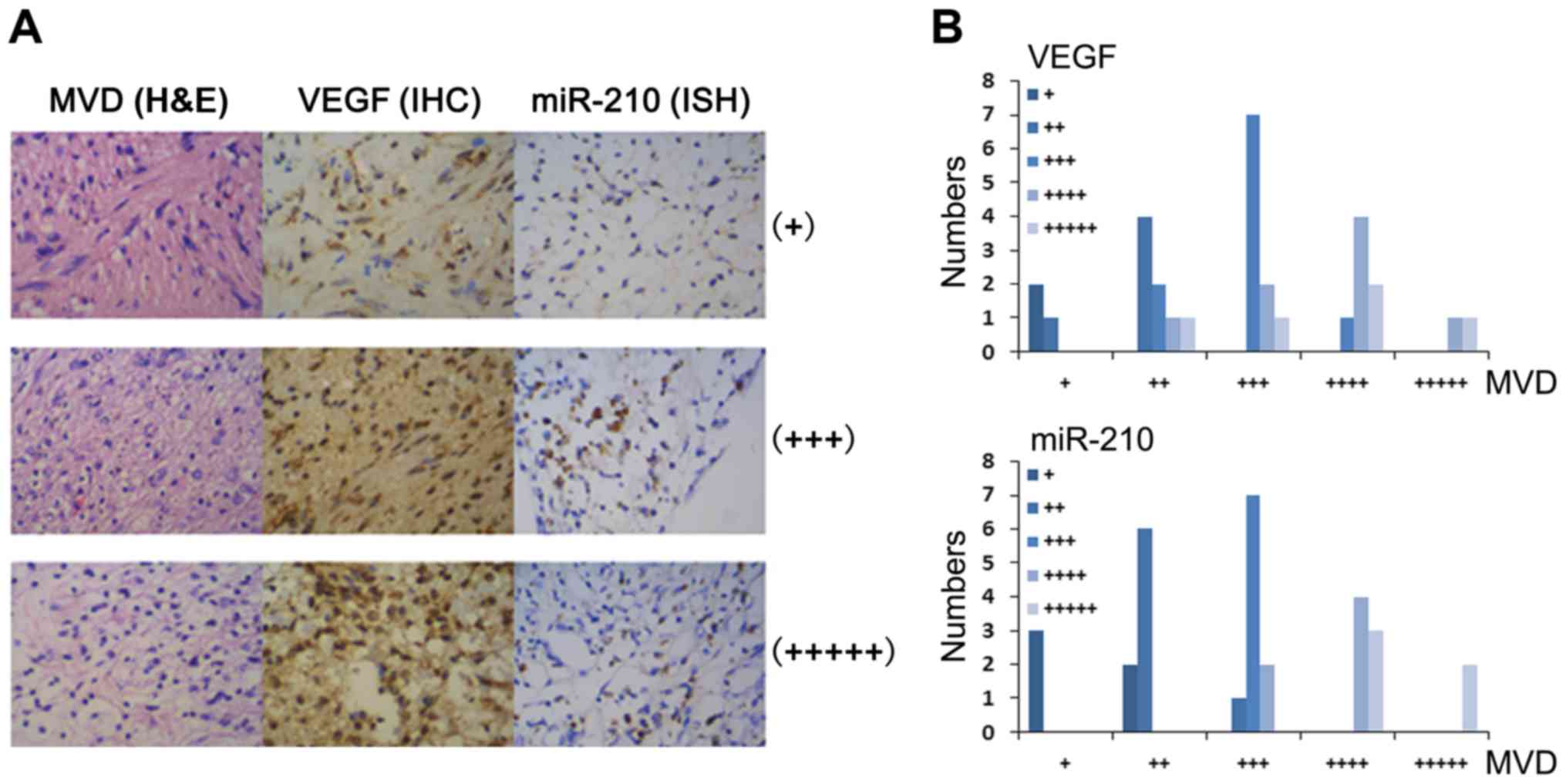
As hypoxia is one of the key factors stimulating
tumor vasculogenesis, we stained central neurilemmoma tissue
microarray (with 30 cases of schwannoma tissues from different
sources, 2 spots for each case) by H&E, scored microvascular
density (MVD), and then tested the expression of VEGF and miR-210
using the same chips by IHC and ISH, respectively (Fig. 1A). The bar chart of Pearson's
Chi-squared test for MVD/VEGF and MVD/miR-210 relevance is shown in
Fig. 1B, and the scores and
P-values as shown in Table I. VEGF
positive stain became darker and miR-210 expression level increased
with the tumor tissue MVD elevation, the MVD vs VEGF and MVD vs.
miR-210 relevant levels had statistical significance at the level
of confidence for interval of 99% (P=0.005 <0.01, P=0.025
<0.05).
 | Table I.Bar chart of MVD and VEGF (Pearson's
correlation=0.710)/miR-210 (Pearson's correlation=0.931). |
Table I.
Bar chart of MVD and VEGF (Pearson's
correlation=0.710)/miR-210 (Pearson's correlation=0.931).
|
| VEGF |
|---|
|
|
|
|---|
| MVD | (+) | (++) | (+++) | (++++) | (+++++) | SUM |
|---|
| (+) | 2 | 1 | 0 | 0 | 0 | 3 |
| (++) | 0 | 4 | 2 | 1 | 1 | 8 |
| (+++) | 0 | 0 | 7 | 2 | 1 | 10 |
| (++++) | 0 | 0 | 1 | 4 | 2 | 7 |
| (+++++) | 0 | 0 | 0 | 1 | 1 | 2 |
| Total | 2 | 5 | 10 | 8 | 5 | 30 |
|
|
| miR-210 |
|
|
|
| MVD | (+) | (++) | (+++) | (++++) | (+++++) | SUM |
|
| (+) | 3 | 0 | 0 | 0 | 0 | 3 |
| (++) | 2 | 6 | 0 | 0 | 0 | 8 |
| (+++) | 0 | 1 | 7 | 2 | 0 | 10 |
| (++++) | 0 | 0 | 0 | 4 | 3 | 7 |
| (+++++) | 0 | 0 | 0 | 0 | 2 | 2 |
| Total | 5 | 7 | 7 | 6 | 5 | 30 |
Effects on miR-210/EFNA3 expression in
rat schwann cells RT4-D6P2T under hypoxia
In order to explore the functions of miR-210 in
schwannoma cells, we used RT4-D6P2T cells as a model for related
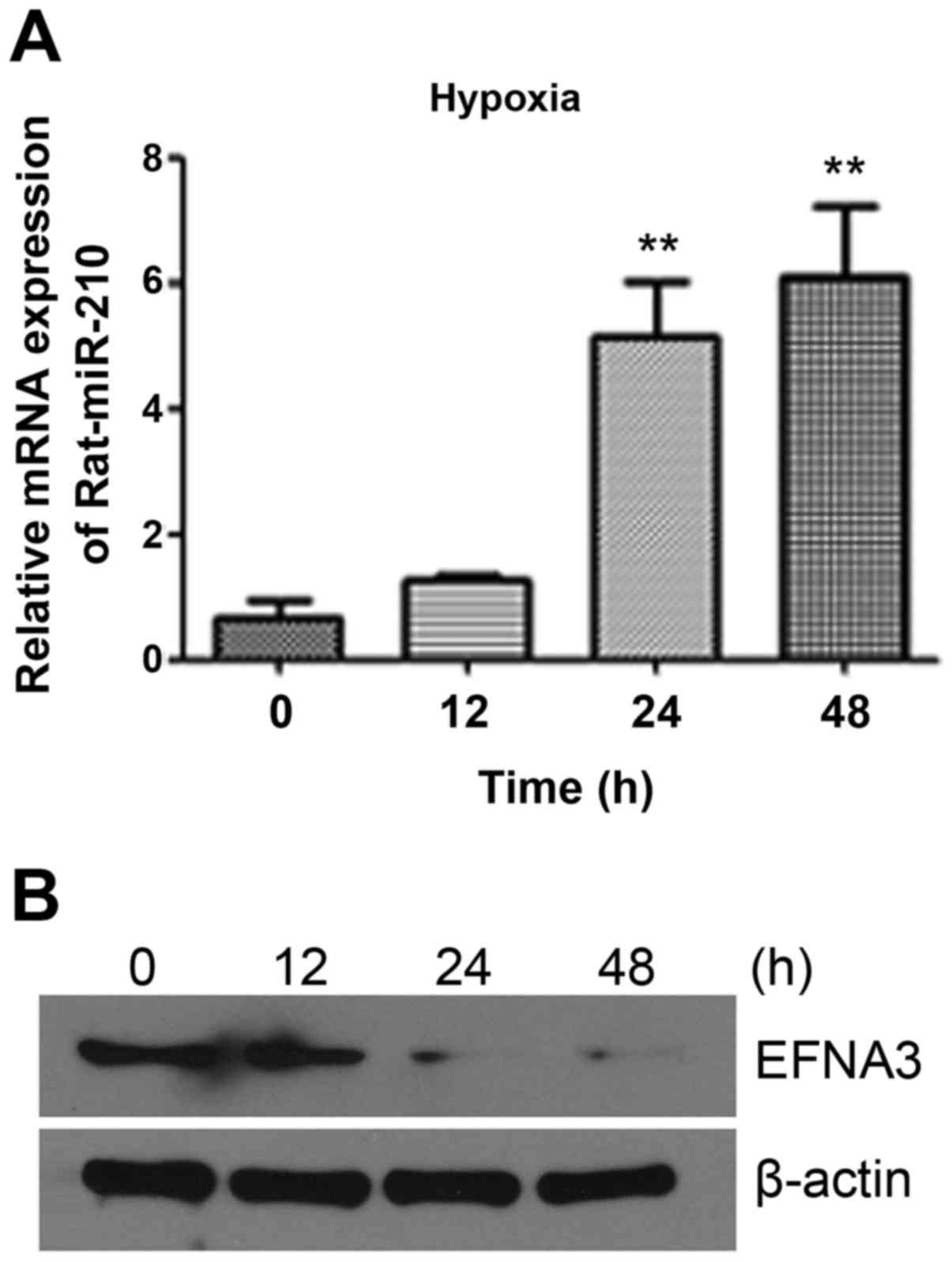
research. First, to find whether hypoxia could affect miR-210/EFNA3
expression, the expression levels of miR-210 and EFNA3 were
detected under hypoxia for 0, 12, 24 and 48 h in RT4-D6P2T cells.
As shown in Fig. 2, miR-210
expression increased slightly after 12 h, increased by 4–6 times
after 24 h, and further increased after 48 h, but not as much as 24
h. While the expression trend of EFNA3 was opposite to that of
miR-210. The expression of EFNA3 significantly decreased after 12 h
hypoxic culture, and could hardly be detected after 24 h or 48 h.
This indicated that hypoxia induced the expression of miR-210 in
RT4-D6P2T. EFNA3, one of the target genes of miR-210, is still
negatively regulated by miR-210 during hypoxic culture and shows a
reverse expression trend to miR-210. This result was consistent
with previous results (21). Thus,
miR-210/EFNA 3 may be involved in schwannoma cell hypoxia. As
expression of both miR-210 and EFNA3 changed significantly after 24
h in hypoxia, so we chose the hypoxic of 5% O2 for 24 h
as the following experimental conditions.
Effects on RT4-D6P2T cellular
functions under hypoxia
As hypoxia changes miR-210/EFNA3 expression in
schwannoma RT4-D6P2T cell line, we wanted to explore the effects of
hypoxia on the functions of such cells, to find which function
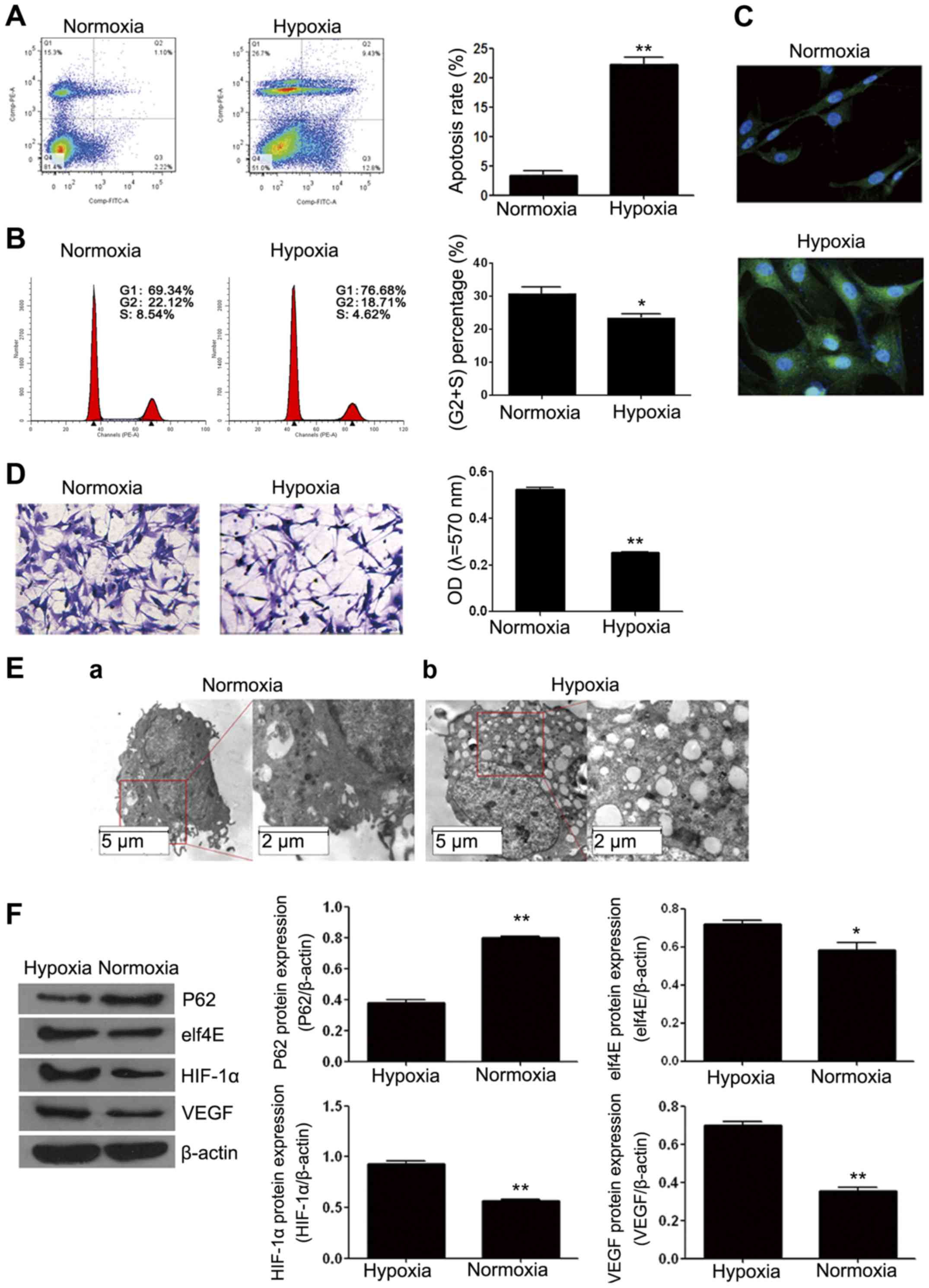
exerted hypoxia-induced miR-210/EFNA3 expression. We tested cell
cycle, apoptosis and autophagy of the cells after 24 h hypoxic
culture. Apoptosis increased greatly from ~3% under normoxic
conditions to ~20% (Fig. 3A). The
cell cycle arrested in G1 phase under hypoxic conditions (Fig. 3B).
Considering hypoxia could induce cell autophagy, we
measured the cell autophagy under hypoxic culture by IF and western
blotting. According to the results shown in Fig. 3C, during hypoxic culture, cellular
morphology changed greatly, cells became larger and round in shape,
which was associated with the cellular edema caused by too much
water in cells due to hypoxia-induced sodium potassium pump
function decrease. Besides, the IF staining results showed that the
expression level of LC3BII increased, and the fluorescent signal
around the cell nuclei significantly increased (Fig. 3C). According to Transwell
experiment, although the quantity of the cells entering into
Matrigel under the normoxic conditions was not significantly
different with that in hypoxia, the cell morphology changed
significantly, and under the hypoxic conditions, intercellular
interval was wider, cells became narrower and fusiform shape
(Fig. 3D). This may contribute to
hypoxia-induced epithelial to mesenchymal transition phenomenon.
According to the cell ultrastructure image by a transmission
electron microscope (Fig. 3E), we
found that during hypoxia, double membrane vacuole structures
occurred in cytoplasm, and apoptotic bodies in cytoplasm
significantly increased. Similarly, the results of western blotting
on the autophagy related protein P62 and elf4E also showed that,
after hypoxia P62 protein expression significantly decreased, while
the expression level of the autophagy effector elf4E increased
(Fig. 3F). Therefore, we concluded
that hypoxia enhanced apoptosis and autophagy. In addition,
according to Fig. 3F, HIF-1α and
VEGF expression increased after 24 h hypoxia; and considering
miR-210 expression increased and EFNA3 expression decreased during
hypoxia together (Fig. 2A), we
speculated that during hypoxia, the miR-210 enhancement of
schwannoma cells was induced by HIF-1α, and miR-210 regulated
vascularization by targeting EFNA3.
Mechanism of normoxia/hypoxia-induced
miR-210 regulation
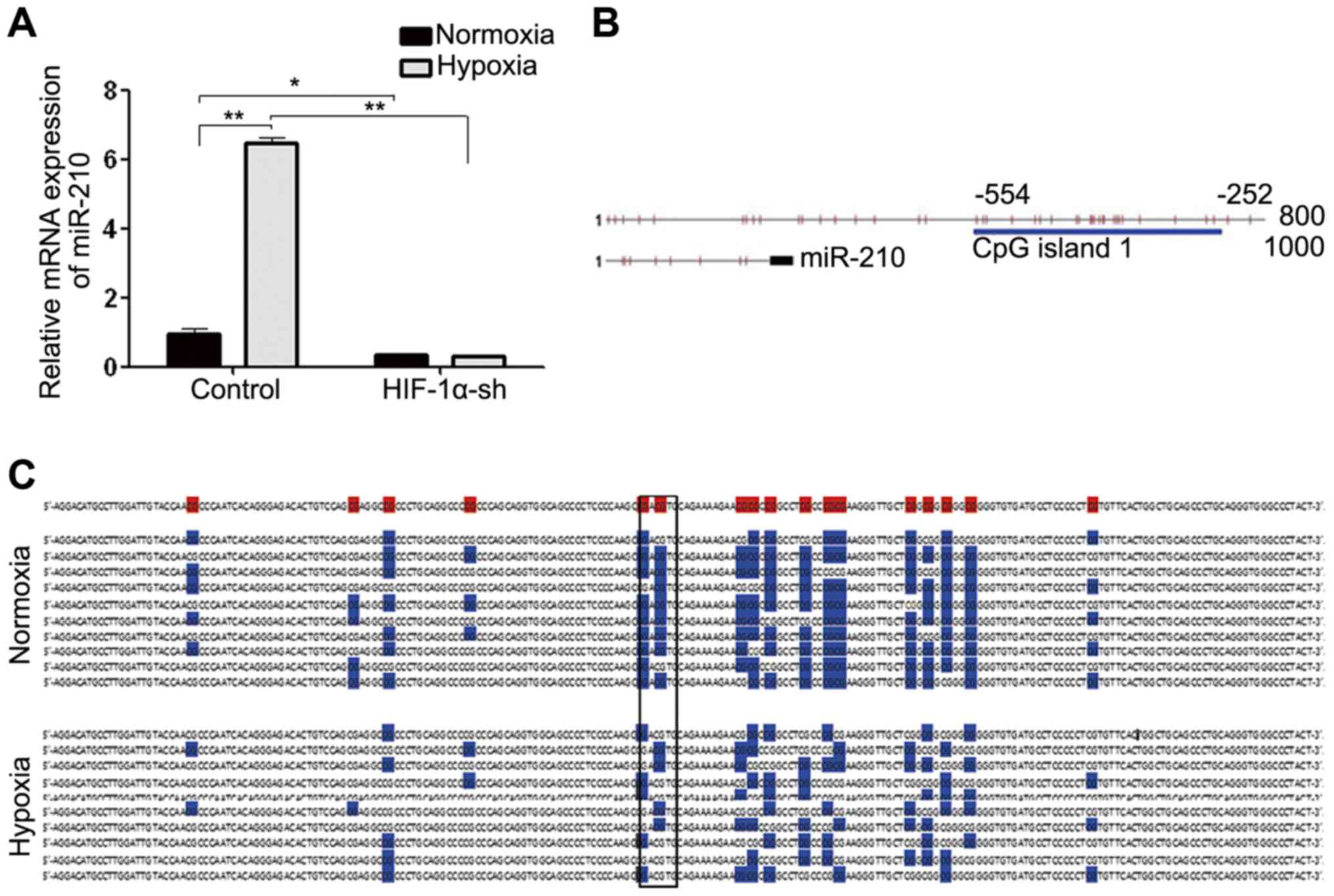
To investigate whether miR-210 upregulation during
hypoxia is induced by HIF-1α, we carried out HIF-1α silencing by
HIF-1α-shRNA, and then placed the HIF-1α loss-of-funciton cells
under normoxic/hypoxic conditions, respectively for 24 h. Hypoxia
can induce miR-210 upregulation, but after HIF-1α interference,
miR-210 expression level decreased significantly (Fig. 4A). This indicated that miR-210
expression did relate to HIF-1α expression, and HIF-1α may
positively regulate miR-210 expression.
We further studied the mechanism of how hypoxia
induced miR-210 upregulation. We predicted that there were abundant
CpG islands in the promoter region of rat miR-210 (Fig. 4B) by the online software Lilab
(http://www.urogene.org/cgi-bin/methprimer/methprimer.cgi),
CpG Island Searcher (http://cpgislands.usc.edu/), and EMBOSS explorer
(http://emboss.bioinformatics.nl/cgi-bin/emboss/cpgplot).
Moreover, the early methylation-specific PCR (MSP) preliminary
experiment results showed that under hypoxic conditions, the
miR-210 promoter region methylation level in cells decreased
significantly compared with that under normoxic conditions (data
not shown). We speculated that during hypoxia, demethylation
occurred in the miR-210 promoter region, which enabled more
hypoxia-induced HIF-1α to combine with HRE in the miR-210 promoter
region and activated miR-210 translation. So, we first tested the
methylation islands of the miR-210 promoter region by Bisulfite
sequencing PCR (BSP) under hypoxia, and then assessed whether HRE
combined with HIF-1α was included in these demethylated CpG
islands. The results showed that there were 17 CpG locuses in the
upstream −252 to −554 bp region of the miR-210 coding region, and
under the normoxic conditions, the methylation level of this CpG
islands segment was ~72.35%, while after 24 h hypoxic culture, the
methylation level dcreased significantly to ~44.70% (Fig. 4C).
miR-210 plays important roles in
RT4-D6P2T schwannoma cell line under normoxic/hypoxic
conditions
In order to clarify the function of miR-210 on
schwannoma cells during hypoxia, we built miR-210 knockdown cell
model and then placed the model cells into normoxic/hypoxic
conditions for 24 h, respectively. As shown in Fig. 5A, compared with the negative control
group (anti-scramble), the proportion of the cells in the S+G2
phase significantly reduced in anti-miR-210 group, but the quantity
difference of the S+G2 phase between the normoxia and the hypoxia
group does not significant. The cell apoptosis rates increased
significantly in anti-miR-210 group in both the normoxia and the
hypoxia group (Fig. 5B). LC3B II
fluorescence intensities of anti-miR-210 group are lower than those
of the anti-scramble group, and higher (under hypoxic conditions)
than those of the cells cultured under normoxic conditions
(Fig. 5C).
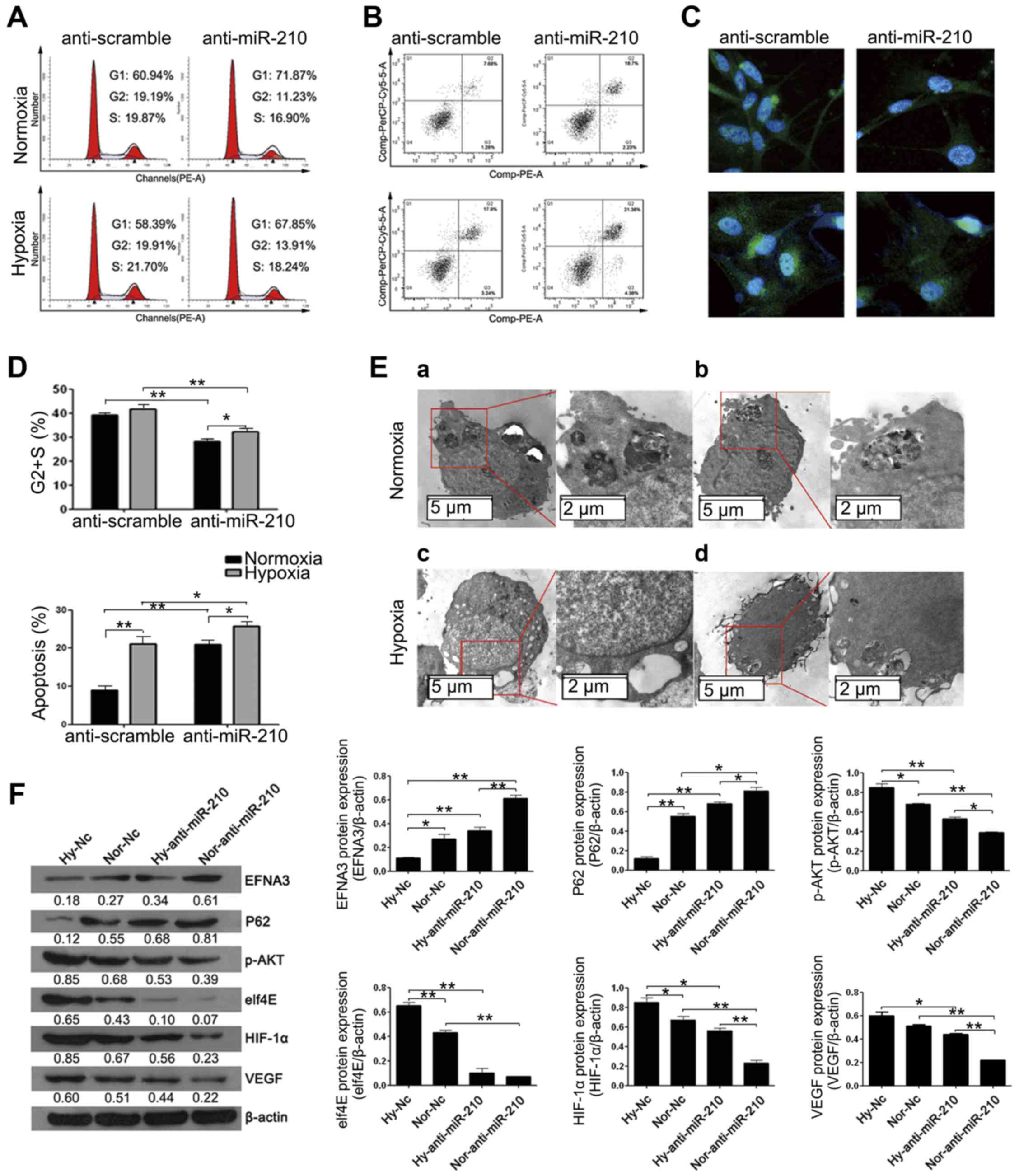 | Figure 5.miR-210 plays an important role in
cell function under normoxic/hypoxic conditions. (A) The flow
cytometry result on single stained cell cycle, statistical result
of (S+G2) % is shown in the figure (top of D). (B) Flow cytometry
results on double-stained cell apoptosis, statistical result of
(R3+R5) % is shown in the figure (bottom of D). (C) LC3BII/I
protein fluorescence intensity under ×400 microscope. (E) Cell
ultrastructure under transmission electron microscope, of which (a)
the negative control group cells under normoxic conditions, (b) the
groups without miR-210 function under normoxic conditions, (c) the
negative control group cells after 24 h hypoxic culture, (d) the
groups without miR-210 function after 24 h hypoxic culture. (F)
Western blot results on intracellular EFNA3, P62, elf4E, p-AKT,
HIF-1α, VEGF protein expression change grey-scale map and
statistical chart of each group. *P<0.05. **P<0.01. |
According to the transmission electron microscope
scanning results (Fig. 5E), under
normoxic conditions, cellular matrix density was uniform and with
few autolysosomes; after hypoxic culture, double membrane vacuole
structures occurred in cellular matrix, mitochondria breakage was
more serious, some damaged mitochondria were covered by
dual-membranes, and autophagy-induced cavitation in cells of the
miR-210 group were much less than those of the anti-scramble group.
This indicated that miR-210 could mediate cell autophagy. Western
blot results on the autophagy related factors (P62 and elf4E) were
consistent with the aforementioned results (Fig. 5F). The expression level of HIF-1α
and VEGF protein also decreased when the miR-210 was knocked down,
while EFNA3 expression increased (Fig.
5F). This indicated that miR-210/EFNA3 was related with the
HIF-1α/VEGF-mediated neovascularization paths.
Discussion
According to this study, we found that under
hypoxia, HIF-1α, miR-210 and VEGF expression levels in RT4-D6P2T
cells were significantly higher, EFNA3 expression was significantly
lower, apoptosis higher, autophagy occurred, and cell cycle was
arrested. In addition, intracellular miR-210 expression
upregulation under hypoxia was found to be associated with its
transcription start region CpG island demethylation for the first
time.
It seems hypoxia can induce tumor cell apoptosis and
necrosis, and thus can inhibit excessive proliferation, but finally
it may do more harm than good. Because after apoptosis, necrotic
cells release inflammation signals to the tissue microenvironment
around them and recruit inflammatory cells of the immune system
(29,30), which can promote vasculogenesis,
cancer cell proliferation and infiltration, can also directly
activate the regulatory factors for adjacent viable cell
proliferation, such as IL-1α and finally promote tumor growth
(30).
Autophagy is an important cell physiological
reaction similar to apoptosis. Normal cells are at a low level, but
autophagy can be strongly activated under some cellular stress
states, of which the most significant stress state is nutrition
deficiency (31). The nutrition
deficiency can induce cellular autophagy level increase, and thus
is able to resist rather than enhance environmental stress
destruction process of tumor cells (32). In addition, in an adverse
environment, cells can shrink to a reversible dormant state via
autophagy contraction, which may enable some advanced tumors to
survive and finally relapse (33).
Our invasion experiment results showed that hypoxia
did not increase the invasion of cells, but caused fibrinoid
changes of cells, enabled them to grow filopodium, whose main
functions consisted of movement, adhesion, support, nutrition
absorption and phagocytosis, and thus increased invasion
capabilities of tumor cells. Thus, we can see that although hypoxia
has an unfavorable influence on the growth of the tumor, it is
still an important factor that can induce tumor progress
acceleration.
Since miRNAs can maintain pluripotency of cancer
stem cells/progenitor cells, and may become a molecule therapeutic
target or biomarker of cancers, they have attracted more and more
attentions from scholars (34).
However, the mechanism of how tumor cells regulate miRNA expression
has not been clearly revealed. Bioinformatics analysis results
showed that there were CpG islands in the transcription start site
(TSS) regions of 129 of 721 human miR genes (accounting for 18%) in
miRBase (http://www.mirbase.org/) (34). Some related references (35–40)
also showed that CpG island methylation or high methylation level
in TSS regions of miRNAs was thought to be inhibited by
hysterogenic (epigenetic) transcription. However, the relationship
between miR methylation and miR expression has not been completely
revealed, and the previous evidence on such a relationship is not
sufficient.
We discovered that hypoxia could induce miR-210
promoter region demethylation. Huang et al (17) revealed the locus where miR-210
promoter region could combine with HIF-1α as 5′-GACGTG-3′, upstream
−429 to −423 bp of miR-210 coding region. Our BSP test results
showed that there were 2 methylation loci in this region which
showed high methylation levels under normoxic conditions, while low
methylation levels under hypoxic conditions. This probably enhanced
the combining capacity of HIF-1α with HRE in miR-210 TSS region,
and further enhanced miR-210 transcriptional activity.
Moreover, in the oxygen-independent regulating
system, miR-210 can induce HIF-1α expression level increase via
inhibiting proly1 hydroxylases which can degrade HIF-1α (9). The repression of succinate
dehydrogenase complex subunit D (a HIF-1α inhibitor) by miR-210 can
also contribute to the maintence of HIF-1α activity, then further
promote miR-210 transcription and enhance the feedback regulation
(41). Such hypoxia-induced
HIF-1α/miR-210 feedback regulation mechanism directly promotes
schwannoma cell survival, autophagy protection, and tumor cell
changes from epithelial type to mesenchymal type during hypoxia,
and consequently increases possibility of malignancy
transformation. Therefore, miR-210 has the potential to become an
index for testing internal hypoxia of schwannoma tissues, and can
also be taken as an index for judging whether the tumor is of
malignancy transformation trend.
Similarly, we silenced miR-210 of RT4-D6P2T cells to
prove our conclusion, and compared the miR-210 functional role
under normoxic/hypoxic conditions. As anticipated, the results
showed miR-210 participated in many biological functions, such as
regulating RT4-D6P2T cell anti-apoptosis, autophagy protection and
invasion ability, and promoted tumor cell cycle. Thus, developing a
specific miR-210 inhibitor will be significant for neurilemmoma
treatment.
miR-210 can be taken as a biomarker for diagnosing
neurilemmoma hypoxia. Hypoxia-induced HIF-1α/miR-210 expression
upregulation can negatively regulate EFNA3 protein expression
level, and enhance tumor neovascularization. Hypoxia-induced
schwannoma cell apoptosis and autophagy level increase as well as
invasion potential increase can be reversed by anti-miR-210
treatment, and miR-210 is a potential neurilemmoma therapeutic
target.
Acknowledgements
This study was supported by the New Xiangya Talent
Project of The Third Xiangya Hospital of Central South University
(grant no. JY201516).
References
|
1
|
Masliah-Planchon J, Pasmant E, Luscan A,
Laurendeau I, Ortonne N, Hivelin M, Varin J, Valeyrie-Allanore L,
Dumaine V, Lantieri L, et al: MicroRNAome profiling in benign and
malignant neurofibromatosis type 1-associated nerve sheath tumors:
Evidences of PTEN pathway alterations in early NF1 tumorigenesis.
BMC Genomics. 14:4732013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Harris AL: Hypoxia - a key regulatory
factor in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Camps C, Saini HK, Mole DR, Choudhry H,
Reczko M, Guerra-Assunção JA, Tian YM, Buffa FM, Harris AL,
Hatzigeorgiou AG, et al: Integrated analysis of microRNA and mRNA
expression and association with HIF binding reveals the complexity
of microRNA expression regulation under hypoxia. Mol Cancer.
13:282014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Semenza GL: Hypoxia-inducible factor 1:
Master regulator of O2 homeostasis. Curr Opin Genet Dev.
8:588–594. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ben-Yosef Y, Miller A, Shapiro S and Lahat
N: Hypoxia of endothelial cells leads to MMP-2-dependent survival
and death. Am J Physiol Cell Physiol. 289:C1321–C1331. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kitano H: Cancer as a robust system:
Implications for anticancer therapy. Nat Rev Cancer. 4:227–235.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Melillo G and Semenza GL: Meeting report:
Exploiting the tumor microenvironment for therapeutics. Cancer Res.
66:4558–4560. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nallamshetty S, Chan SY and Loscalzo J:
Hypoxia: A master regulator of microRNA biogenesis and activity.
Free Radic Biol Med. 64:20–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Valencia-Sanchez MA, Liu J, Hannon GJ and
Parker R: Control of translation and mRNA degradation by miRNAs and
siRNAs. Genes Dev. 20:515–524. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gee HE, Ivan C, Calin GA and Ivan M:
HypoxamiRs and cancer: From biology to targeted therapy. Antioxid
Redox Signal. 21:1220–1238. 2014.10.1089/ars.2013.5639. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen KC, Liao YC, Wang JY, Lin YC, Chen CH
and Juo SH: Oxidized low-density lipoprotein is a common risk
factor for cardiovascular diseases and gastroenterological cancers
via epigenomical regulation of microRNA-210. Oncotarget.
6:24105–24118. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang Y, Qu A, Liu J, Wang R, Liu Y, Li G,
Duan W, Fang Q, Jiang X, Wang L, et al: Serum miR-210 contributes
to tumor detection, stage prediction and dynamic surveillance in
patients with bladder cancer. PLoS One. 10:e01351682015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bertoli G, Cava C and Castiglioni I:
MicroRNAs: New biomarkers for diagnosis, prognosis, therapy
prediction and therapeutic tools for breast cancer. Theranostics.
5:1122–1143. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Humeau M, Vignolle-Vidoni A, Sicard F,
Martins F, Bournet B, Buscail L, Torrisani J and Cordelier P:
Salivary MicroRNA in pancreatic cancer patients. PLoS One.
10:e01309962015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dang K and Myers KA: The role of
hypoxia-induced miR-210 in cancer progression. Int J Mol Sci.
16:6353–6372. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang X, Ding L, Bennewith KL, Tong RT,
Welford SM, Ang KK, Story M, Le QT and Giaccia AJ:
Hypoxia-inducible mir-210 regulates normoxic gene expression
involved in tumor initiation. Mol Cell. 35:856–867. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang X, Le QT and Giaccia AJ: MiR-210 -
micromanager of the hypoxia pathway. Trends Mol Med. 16:230–237.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ying Q, Liang L, Guo W, Zha R, Tian Q,
Huang S, Yao J, Ding J, Bao M, Ge C, et al: Hypoxia-inducible
microRNA-210 augments the metastatic potential of tumor cells by
targeting vacuole membrane protein 1 in hepatocellular carcinoma.
Hepatology. 54:2064–2075. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hwang HW, Baxter LL, Loftus SK, Cronin JC,
Trivedi NS, Borate B and Pavan WJ: Distinct microRNA expression
signatures are associated with melanoma subtypes and are regulated
by HIF1A. Pigment Cell Melanoma Res. 27:777–787. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fasanaro P, D'Alessandra Y, Di Stefano V,
Melchionna R, Romani S, Pompilio G, Capogrossi MC and Martelli F:
MicroRNA-210 modulates endothelial cell response to hypoxia and
inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol
Chem. 283:15878–15883. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miao H and Wang B: EphA receptor signaling
- complexity and emerging themes. Semin Cell Dev Biol. 23:16–25.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Larson J, Schomberg S, Schroeder W and
Carpenter TC: Endothelial EphA receptor stimulation increases lung
vascular permeability. Am J Physiol Lung Cell Mol Physiol.
295:L431–L439. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Genander M, Halford MM, Xu NJ, Eriksson M,
Yu Z, Qiu Z, Martling A, Greicius G, Thakar S, Catchpole T, et al:
Dissociation of EphB2 signaling pathways mediating progenitor cell
proliferation and tumor suppression. Cell. 139:679–692. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Uchiyama S, Saeki N and Ogawa K: Aberrant
EphB/ephrin-B expression in experimental gastric lesions and tumor
cells. World J Gastroenterol. 21:453–464. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Z, Liu Z, Liu B, Liu G and Wu S:
Dissecting the roles of Ephrin-A3 in malignant peripheral nerve
sheath tumor by TALENs. Oncol Rep. 34:391–398. 2015.PubMed/NCBI
|
|
27
|
Wang Z, Yin B, Wang B, Ma Z, Liu W and Lv
G: MicroRNA-210 promotes proliferation and invasion of peripheral
nerve sheath tumor cells targeting EFNA3. Oncol Res. 21:145–154.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mao L, Zhou Q, Zhou S, Wilbur RR and Li X:
Roles of apolipoprotein E (ApoE) and inducible nitric oxide
synthase (iNOS) in inflammation and apoptosis in preeclampsia
pathogenesis and progression. PLoS One. 8:e581682013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Galluzzi L and Kroemer G: Necroptosis: A
specialized pathway of programmed necrosis. Cell. 135:1161–1163.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Amaravadi RK and Thompson CB: The roles of
therapy-induced autophagy and necrosis in cancer treatment. Clin
Cancer Res. 13:7271–7279. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lu Z, Luo RZ, Lu Y, Zhang X, Yu Q, Khare
S, Kondo S, Kondo Y, Yu Y, Mills GB, et al: The tumor suppressor
gene ARHI regulates autophagy and tumor dormancy in human ovarian
cancer cells. J Clin Invest. 118:3917–3929. 2008.PubMed/NCBI
|
|
34
|
Du Y, Liu Z, Gu L, Zhou J, Zhu BD, Ji J
and Deng D: Characterization of human gastric carcinoma-related
methylation of 9 miR CpG islands and repression of their
expressions in vitro and in vivo. BMC Cancer. 12:2492012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Furuta M, Kozaki KI, Tanaka S, Arii S,
Imoto I and Inazawa J: miR-124 and miR-203 are epigenetically
silenced tumor-suppressive microRNAs in hepatocellular carcinoma.
Carcinogenesis. 31:766–776. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tsuruta T, Kozaki K, Uesugi A, Furuta M,
Hirasawa A, Imoto I, Susumu N, Aoki D and Inazawa J: miR-152 is a
tumor suppressor microRNA that is silenced by DNA hypermethylation
in endometrial cancer. Cancer Res. 71:6450–6462. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tsai KW, Wu CW, Hu LY, Li SC, Liao YL, Lai
CH, Kao HW, Fang WL, Huang KH, Chan WC, et al: Epigenetic
regulation of miR-34b and miR-129 expression in gastric cancer. Int
J Cancer. 129:2600–2610. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hildebrandt MA, Gu J, Lin J, Ye Y, Tan W,
Tamboli P, Wood CG and Wu X: Hsa-miR-9 methylation status is
associated with cancer development and metastatic recurrence in
patients with clear cell renal cell carcinoma. Oncogene.
29:5724–5728. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hashimoto Y, Akiyama Y, Otsubo T, Shimada
S and Yuasa Y: Involvement of epigenetically silenced microRNA-181c
in gastric carcinogenesis. Carcinogenesis. 31:777–784. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang C, Cai J, Wang Q, Tang H, Cao J, Wu L
and Wang Z: Epigenetic silencing of miR-130b in ovarian cancer
promotes the development of multidrug resistance by targeting
colony-stimulating factor 1. Gynecol Oncol. 124:325–334. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gorospe M, Tominaga K, Wu X, Fähling M and
Ivan M: Post-transcriptional control of the hypoxic response by
RNA-binding proteins and microRNAs. Front Mol Neurosci. 4:72011.
View Article : Google Scholar : PubMed/NCBI
|