Introduction
Colorectal cancer (CRC) is one of the most common
malignant neoplasms worldwide and the third leading cause of
cancer-related deaths in patients worldwide. CRC accounts for ~10%
of all cancer incidence and mortality (1,2). CRC
has the third highest incidence (after breast and lung cancer) and
the fourth highest mortality rate (after lung, liver and stomach
cancer) (3). In Saudi Arabia, CRC
is the second most prevalent malignancy among all ages (10.3%) and
is the commonest malignancy in males (11.8%) (4). CRC incidence is increasing in Saudi
Arabia and there is an urgent need to define novel
predictive/prognostic biomarkers.
CRC develops through multiple steps, with the
sequential acquisition of genetic alterations in key tumor
suppressors and oncogenes (5).
Recently De Rosa et al stated that there is an urgent need
to define novel predictive/prognostic biomarkers that may allow
surgeons and clinical oncologists to choose the appropriate therapy
for various patients (6,7).
The cathepsin family of lysosomal proteases has been
increasingly recognized for their altered expression in cancer
patients and their role in facilitating tumor progression.
Cathepsins are a group of multifunctional cysteine and aspartyl
proteases that regulate tumor growth, invasion, migration,
metastases and angiogenesis (8).
Cathepsin B (CTSB; is the HUGO-approved official gene symbol)
primarily functions as an endopeptidase within endolysosomal
compartments in normal cells.
However, during malignant transformation, the
regulation of CTSB can be altered at multiple levels resulting in
overproduction of CTSB which is of significant importance in
various pathologies and oncogenic processes (9). Regulation of CTSB can be altered at
multiple levels during malignant transformation in tumor cells, or
also in adjacent inflammatory cells, resulting in enhanced local
release. It has been suggested that CTSB is a promising target for
therapy, chemoprevention and molecular detection of neoplasia
(10). Activity of CTSB is known to
be important for tumorigenesis, angiogenesis, invasion and
metastases (11,12).
Increased levels of CTSB have been observed in
samples of primary and metastatic tumors in different types of
cancer (13–17). In clinical studies elevated levels
of CTSB expression have been reported in prostate cancer (18) gliomas (19) melanomas (20), breast cancer (21,22)
and lung squamous cell carcinoma (23).
Extracellular CTSB is involved in cell invasion
whereas intracellular CTSB may also mediate malignant properties of
CRC cells (24). It was recently
reported that 82% of CRC patients had tumors that expressed
enhanced CTSB (25). Other studies
suggested that CTSB expression or activity may peak during early
stage cancer and subsequently decline with advanced disease
(17,25). In either case, these reports point
to a possible role of CTSB in either or both early and late
alterations that lead to tumor formation in the colon.
In light of the above studies, and considering
important ethnic background differences, we used two strategies in
the present study. The first was to investigate CTSB levels in
Saudi Arabia patient sera at different tumor stages and the second
was to investigate mRNA gene and protein expression in paired
cancerous and normal adjacent tissue samples. The present study
demonstrated that the serum level of CTSB was significantly higher
in late stage CRC compared to early stage as well as higher in
tumor samples vs. healthy controls. The results also confirmed that
there was a significant increase in the CTSB expression at the mRNA
and protein levels in tumor tissue when compared to normal adjacent
mucosa in late stage CRC. Our conclusion is that CTSB is a
promising biomarker for predicting CRC progression and detecting
the late stages of the disease in Middle East populations.
Materials and methods
Clinical samples
Ninety samples of colorectal tumors and adjacent
normal tissue (at least 10 cm from each tumor) were obtained from
patients undergoing surgical resection between 2013 and 2015 at
King Khalid University Hospital, King Saud University Riyadh (KSA).
The patient clinicopathological characteristics are shown in
Table I. Tissues were collected
after obtaining the written informed consent of the patients,
according to the protocol approved by the Ethics Committee at King
Saud University. Paired tissue samples were frozen in liquid
nitrogen within 15 min from resection, and stored at −80°C until
used. Demographic characteristics, including age and gender, tumor
site and histological type of CRC were recorded. The histological
stage of the tumor was determined in accordance with the Union
International Cancer Control (UICC)-TNM staging system. Tumor
localization, UICC stage and tumor differentiation (grading) were
in accordance with the WHO classification (25), and documented in a database
prospectus. Furthermore, and in order to minimize possible
influences of radiotherapy and chemotherapy on CTSB status and
prognosis, all patients who had undergone neoadjuvant or adjuvant
therapy were excluded from this cohort.
 | Table I.Clinicopathological characteristics
of the CRC patients. |
Table I.
Clinicopathological characteristics
of the CRC patients.
|
Characteristics | No. of
patients |
|---|
| Mean age (58
years) |
|
<58 | 48 |
|
≥58 | 52 |
| Gender |
|
Male | 54 |
|
Female | 46 |
| Primary tumor |
|
Colon | 77 |
|
Rectum | 13 |
| Tumor staging
(UICC, 2010) |
| I | 5 |
| II | 39 |
|
III | 28 |
| IV | 28 |
Enzyme-linked immunosorbent assay
(ELISA)
A 5-ml blood sample from each participant patient
and healthy control subject was allowed to clot for 1 h at room
temperature. Each clotted sample was centrifuged at 5,000 rpm for
10 min. All sera were aliquoted and frozen at −80°C until used.
CTSB concentrations in the sera were determined by a human
cathepsin B kit using human CTSB ELISA kit (LS-F1026; Life Span
Biosciences, Inc., Seattle, WA, USA) according to the
manufacturer's instructions. The sera were analyzed in duplicate at
a dilution of 1:50.
Sample preparation
Total cellular RNA and protein were extracted
according to the manufacturer's instructions using the PARIS™ kit
(Ambion, Foster City, CA, USA) from frozen paired tumor and
adjacent normal tissue as recommended by the manufacturer.
Preparation of total RNA
Pure, concentrated RNA was eluted in buffer provided
as a kit ingredient, and stored at −80°C for further analysis. The
purity of the total RNA was assessed by measuring the A260/280
ratio (1.8–2.0), using an ultraviolet spectrophotometer. To remove
the contamination of genomic DNA, RNA samples were treated with
RNAse-free DNAase (Ambion). For cDNA synthesis, the High Capacity
cDNA kit was used to perform reverse transcriptase reaction (cat.
no. 4368814; Applied Biosystems, Foster City, CA, USA).
Real-time polymerase chain
reaction
PCR samples were prepared using Fast
SYBR®-Green Master Mix (cat. no. 4385612; Applied
Biosystems), and the following CTSB primers (Integrated DNA
Technologies, Leuven, Belgium) were used to detect the expression
of CTSB by RT-PCR: forward, 5′-CCAGGGAGCAAGACAGAGAC-3′ and reverse,
5′-GAGACTGGCGTTCTCCAAAG-3′; and GAPDH forward,
5′-TGCACCACCAACTGCTTAGC-3′ and reverse, 5′-GGCATGGACTGTGGTCATGA-3′.
The experiments were run on a ViiA™ 7 Real-Time PCR system. Results
of CTSB mRNA expression were measured relative to GAPDH gene
expression using the 2−ΔΔCt method.
Construction of tissue microarrays
(TMAs)
TMAs were constructed from paraffin-embedded tumor
blocks using a manual tissue arrayer (Array Mold kit D; IHC World,
Woodstock, MD, USA) as previously described (26). The detection of CTSB expression was
performed using streptavidin-biotinylated horseradish peroxidase
(S-ABC) kit (NovoLink Max Polymer Detection System; Novocastra,
Milton Keynes, UK) according to the manufacturer's instructions. As
a negative control, the same procedure was conducted with the
omission of the primary antibody. The expression of CTSB in tumor
and normal samples was analyzed using the eSlide capture device
(ScanScope CS; Aperio Technologies Inc., Vista, CA, USA).
Immunohistochemistry
Immunohistochemistry staining was carried out on
5-µm sections of TMA blocks. The detection of CTSB expression was
performed using streptavidin-biotinylated horseradish peroxidase
(S-ABC) kit (Novo Link Max Polymer Detection System; Novocastra).
Endogenous peroxidase activity was quenched with 3% hydrogen
peroxide in distilled H2O for 5 min, and then, the
slides were washed in Tris-buffered saline (TBS) for 10 min.
Non-specific binding of antibodies was blocked by incubation with
protein block (Novocastra) for 5 min. Subsequently, the slides were
incubated with human CTSB monoclonal mouse antibody (Abazyme,
Cambridge, MA, USA) primary antibody (1:100) for 1 h at room
temperature. Slides were washed in 3X TBS for 3 min, and then
incubated with biotinylated anti-mouse IgG (Novocastra) for 30 min.
Peroxidase was detected using diaminobenzedine (DAB) substrate
(Novocastra). Finally, slides were counterstained with Mayer's
hematoxylin (Novocastra). As a negative control, the same procedure
was conducted with the omission of the primary antibody. The
expression of CTSB in tumor and normal samples was analyzed using
the eSlide capture device (ScanScope CS).
Image analysis
High-resolution, whole-slide digital scans of all
TMA glass slides were created using a ScanScope slide scanner
(Aperio Technologies, Inc.) as previously described (26).
Western blotting
Four pairs of CRC tumors and adjacent normal tissue
protein samples were isolated from early and late stage CRC using
PARIS™ kit. Total protein concentration was determined using
Bradford protein reagent (Bio-Rad, Hercules CA, USA). Soluble
proteins (20 µg) were loaded on 10% precast TGX gels and were
analyzed by immunoblotting with anti-CTSB (dilution 1:100; EMD
Millipore, Billerica MA, USA). Reactivity was detected with
horseradish peroxidase-conjugated secondary antibodies and
chemiluminescence (Bio-Rad). Membranes were developed using C-Digit
Blot Scanner (LI-COR, Hamburg, Germany).
Statistical analysis
Statistical analysis was performed using Graph Pad
Prism software (version 5.0). The means between the two groups were
compared using Mann-Whitney test and paired t-test in order to
compare serum CTSB and mRNA levels in early and late tumor stage. A
P-value of ≤0.05 was considered significant.
Ethics statement
The present study was approved by the Ethics
Committee of King Khalid University Hospital, King Saud University.
Patient consent was obtained for the present study.
Results
Serum cathepsin B levels in CRC
patients
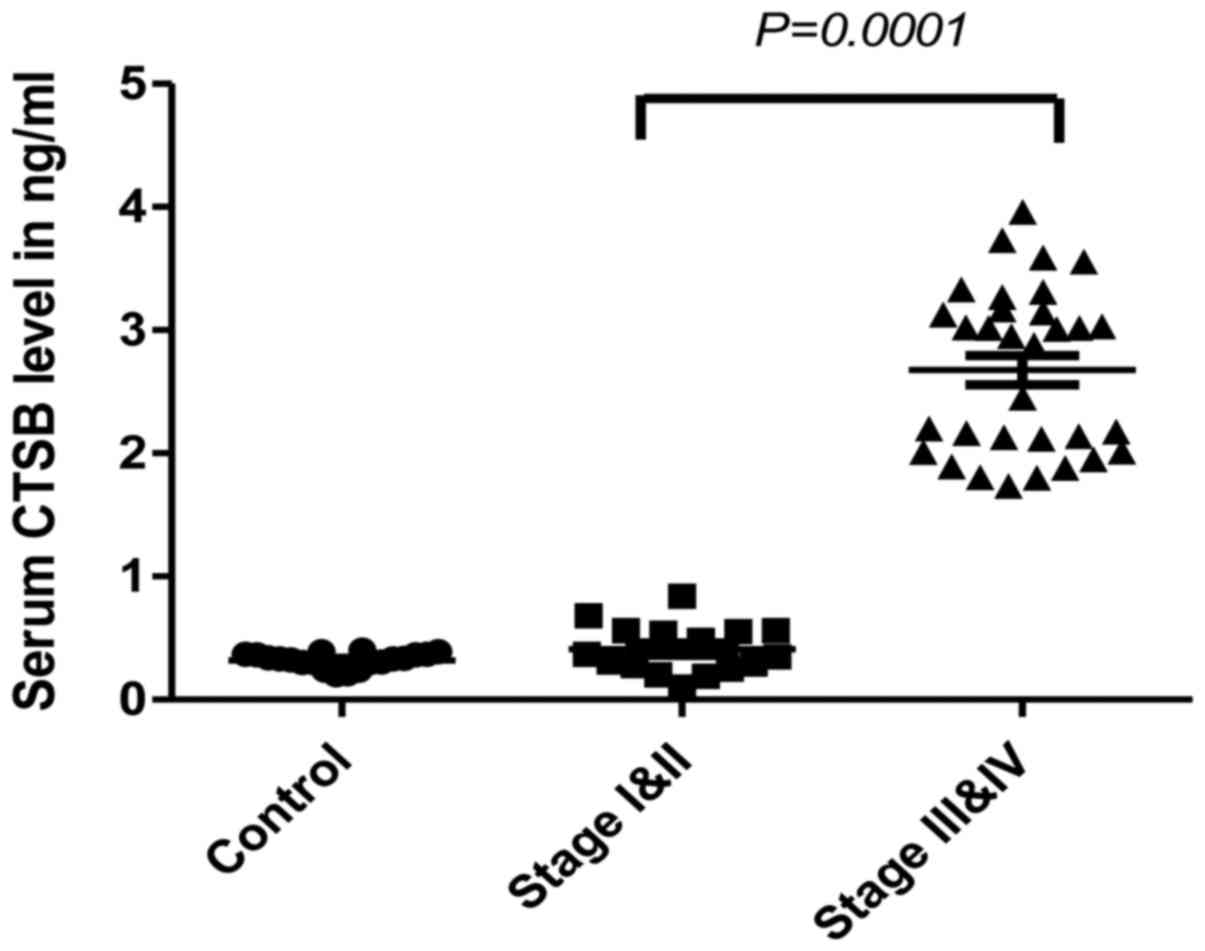
To evaluate the CTSB level in sera, two groups of
patients were analyzed: early and late stage CRC patients. The
level of CTSB was significantly increased in the sera of patients
with late stage CRC when compared to the healthy controls. The
medians were 2.9 and 0.33 ng/ml, respectively (P<0.0001)
(Fig. 1). There was no significant
difference between early stage cancer patients and healthy control
subjects.
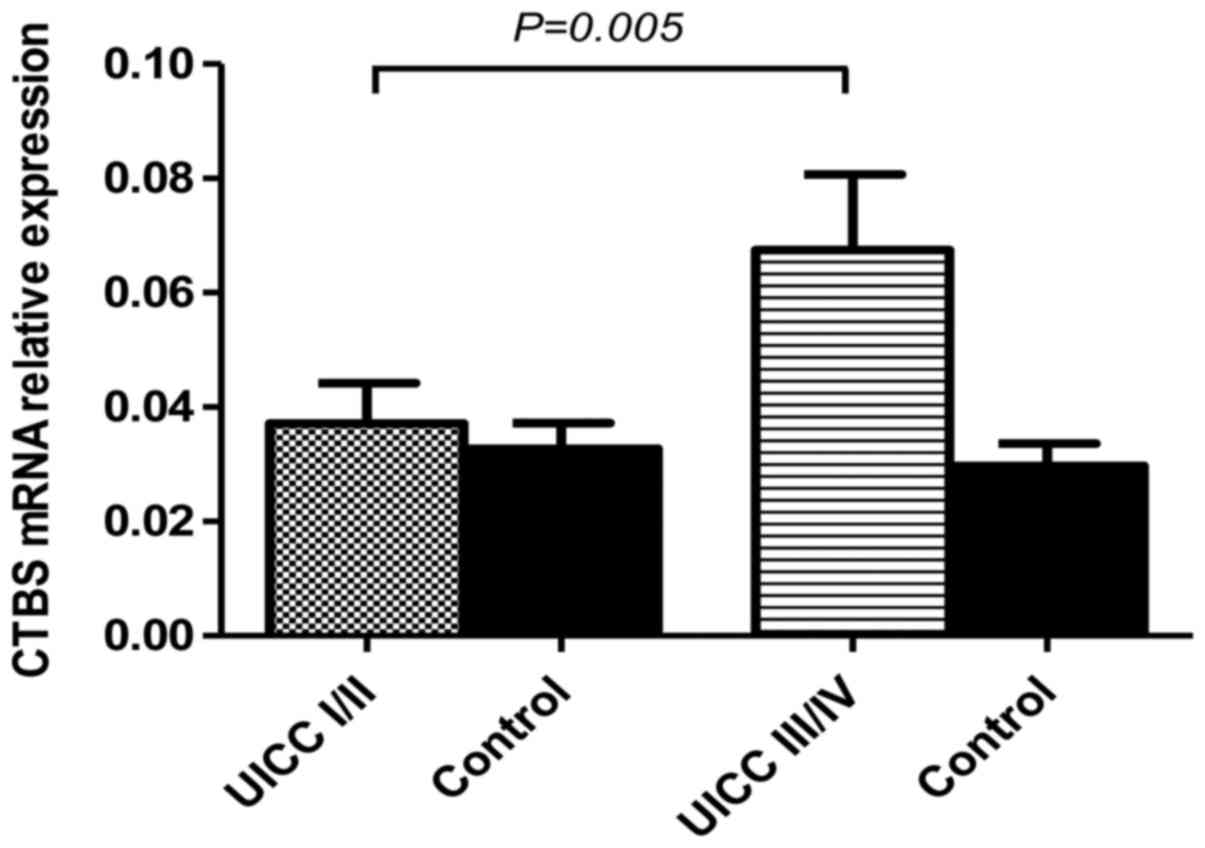
Cathepsin B mRNA is increased in late
stage CRC patients
CTSB was analyzed at the mRNA level in a series of
human paired specimens at early (stage I and II) and late stage
(III and VI) CRC. The relative levels of CTSB mRNA in tumor CRC
samples were significantly higher than levels in the adjacent
normal colorectal tissue (P<0.05). CTSB gene expression was also
elevated in late stage tumor samples, including patients with lymph
node metastases, when compared to early stage (P=0.0006). The mRNA
level in early stage CRC was significantly lower (0.04±0.01) than
the mRNA level in late stage tissues (0.07±0.02) (Fig. 2). These findings suggest that CTSB
mRNA was significantly higher in late stage CRC similar to the
differences noted in the serum CTSB levels.
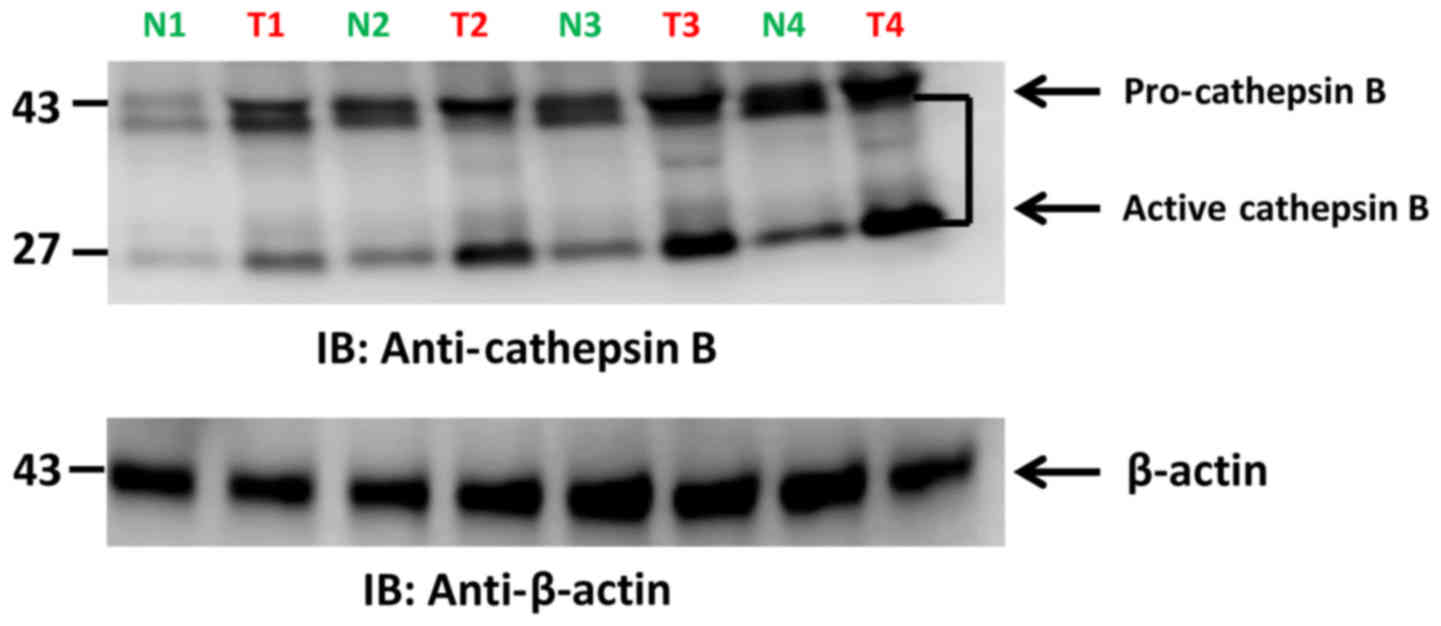
Cathepsin B protein expression in
tumor tissues
CTSB protein levels were analyzed in early vs. late
stage CRC tumor samples, and vs. adjacent normal tissue.
Intracellular CTSB protein expression was increased in late stage
when compared to adjacent normal mucosa. No differences were noted
in early cancer stages vs. adjacent normal tissue. The
intracellular/active form of CTSB was highly expressed in late
stage CRC samples (Fig. 3). These
findings suggest that CTSB is significantly overexpressed in the
late stage of CRC. Therefore active CTSB expression during late
disease stage (stage III and IV) may have value as a biomarker for
late stage CRC including patients with lymph node metastases.
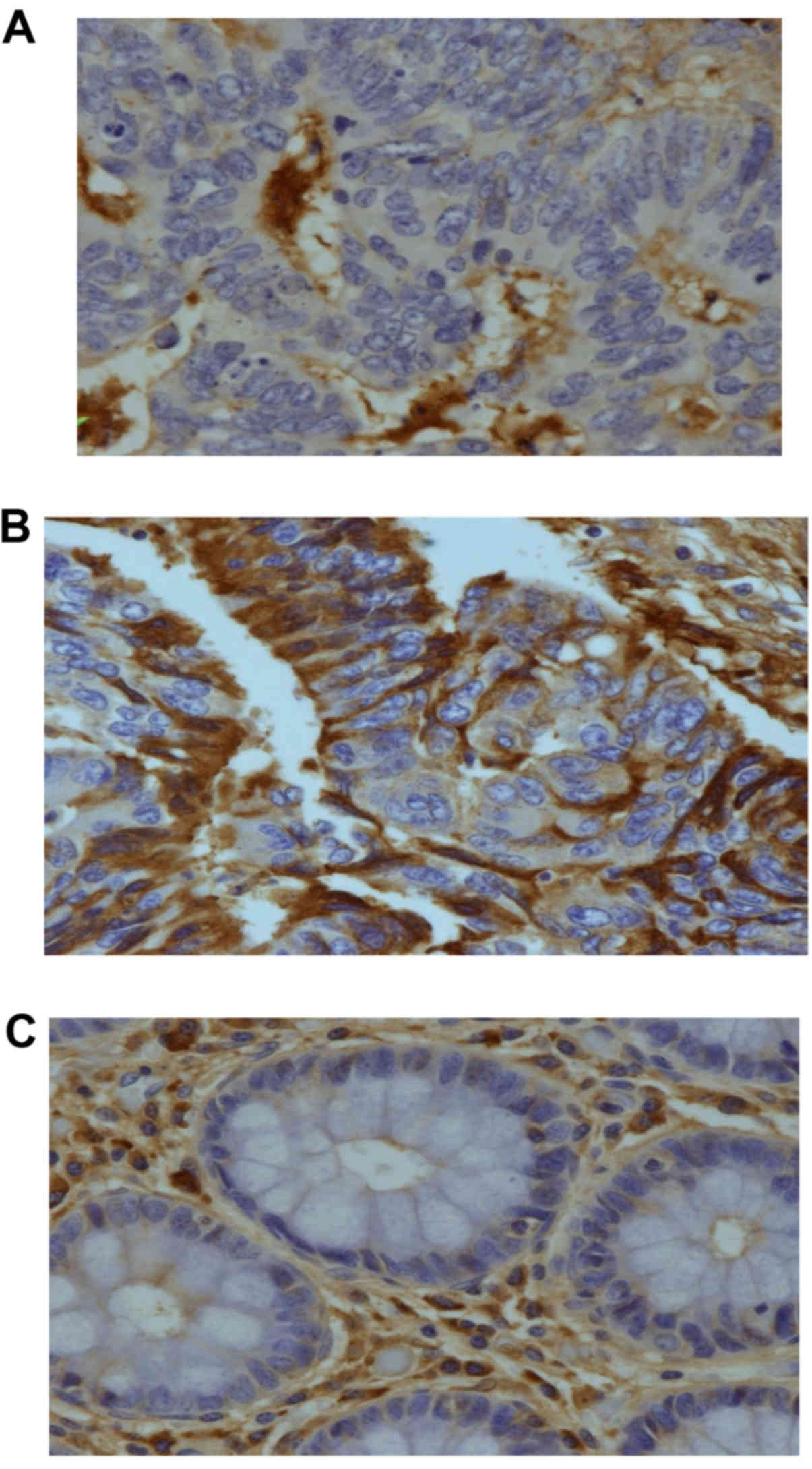
Immunostaining analysis of cathepsin
B
We performed immunohistochemistry of CTSB on tissue
microarray preparations of our paired tumor and adjacent normal
specimens. Positive cells displayed red-brown staining in the
cytoplasm. Late stage tumor tissues contained many CTSB-positive
tumor cells (Fig. 4C). In contrast
only a few were noted in early CRC stage samples (Fig. 4B). Normal colorectal tissues
contained very few positive epithelial cells or macrophages
(Fig. 4C). We performed a
semi-quantitative analysis on patient samples, and 80% of late
stage CRC samples showed strong positive staining while only 5%
samples exhibited strong staining in early stages (P<0.02). The
few early stage samples with significant staining may be in
transition from early to late stage. These results demonstrate that
CTSB expression is significantly increased at both the
transcription and translation levels likely giving rise to
increased serum CTSB levels observed in late stage CRC.
Discussion
To date, tumor node metastases (TNM) staging is
considered to be the ‘gold standard’ for tumor classification. It
offers valuable prognostic information and a guide to necessary
therapeutic decisions. However, patients presenting at a late
cancer stage are also a heterogeneous group which makes the
relationship between the TNM stage and patient prognosis very
complex (17). Stage II patients
who do not receive postoperative adjuvant chemotherapy sometimes
show similar disease outcome as stage III patients. Taking these
considerations into account, other biomarkers may be useful as an
adjunct to the TNM classification for the prediction of responses
to therapy and for the planning of personalized and ‘tailor made’
care (6).
In the present study, we investigated the level of
CTSB in blood and tissues using different quantitative immunoassays
to evaluate the possible use of CTSB as a biomarker in a specific
ethnic group. The present study showed that higher serum levels of
CTSB were associated with late stage colorectal cancer and lymph
node metastases which is in agreement with the study of Kos et
al who reported a significant correlation between serum CTSB
and late stage colorectal cancer (CRC) (27).
In addition, at the mRNA level, significant
differences in CTSB values were noted in late stage cancer when
compared to early stage and healthy control. This is in contrast to
the results published by other groups (28,29) in
which high tumor tissue mRNA was found to be increased in early
stage CRC (28,29). To focus on actual protein
expression, we investigated CTSB by western blotting in order to
confirm that intracellular/active CTSB was highly expressed in late
stage disease when compared to early stage tumors and to adjacent
normal mucosa. Recently, Bian et al found increased CTSB
mRNA and protein levels in CRC tumors irrespective of tumor stage
(24). These data are reminiscent
of the immunohistochemistry data reported by Chan et al
which showed increased CTSB protein expression in the majority of
CRC analyzed patients (558 cohort) and that this was also
independent of the tumor stage (25).
To date, numerous published analyses conclude that
CTSB plays a key role in the invasiveness of various carcinomas
(9,10,15,31).
Numerous studies have also reported CTSB expression in CRC
(15–17,25,27,32–34).
Cathepsin B has been reported to be an accurate indicator and a
marker to predict aggressiveness of many tumors such as advanced
endometrial cancer, glioblastoma cell lines, breast and advanced
endometrial cancer, and pancreatic adenocarcinoma (37–40).
Various studies concluded that an increased CTSB
level is associated with earlier local invasion than with the later
colonization of the metastatic process (30,31).
However, other published studies have reported seemingly
contradictory results, that is increased CTSB was correlated with
colonizing potential and tumor cell metastases (35). Specifically, Campo et al
reported that increased expression of CTSB correlates with tumor
progression and reduced survival of colorectal patients (36). These discrepancies in the results of
different investigations could be due to variations in the genetic
background of the populations studied.
In summary, our data provide support for clinical
application of preoperative serum CTSB levels in the detection of
late stage CRC. Serum CTSB concentrations were significantly higher
in patients with advanced CRC and local metastases. Although future
comprehensive studies on larger cohort numbers are required for a
definitive conclusion, the preoperative serum CTSB level may be
useful as a diagnostic and/or predictive biomarker for lymph node
metastases in patients with CRC at least in the Saudi population.
It is reasonable to anticipate that CTSB detection in advanced
stages may have therapeutic benefits as well in CRC. High
expression of CTBS in the tumor microenvironment particularly in
advanced stages can offer significant therapeutic benefit when
using CTBS inhibitors in the treatment of CRC. CTSB inhibitors
could be used in combination with known drugs or with nanoparticles
as nanotechnology significantly improves drug bioavailability and
the therapeutic index in cancer therapy. Combined therapy may
improve the therapeutic efficacy of the drug and reduce off-target
effects in order to enhance patient outcome.
To the best of our knowledge, the results of our
pilot study for the first time demonstrate the clinical utility of
CTSB as a blood and tissue-based biomarker to assess the stages in
CRC patients from Saudi Arabia. Thus, investigating CTSB as a
biomarker in a multi-central larger cohert may be useful in
monitoring the course of disease severity and treatment and,
therefore contribute to better management of CRC.
Acknowledgements
The present study was funded by the King Saud
University, through the Vice Deanship of Research Chairs. We thank
Professor Ammar Al-Rikabi for his critical reading and appraisal of
this manuscript.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schoen RE, Pinsky PF, Weissfeld JL,
Yokochi LA, Church T, Laiyemo AO, Bresalier R, Andriole GL, Buys
SS, Crawford ED, et al: PLCO Project Team: Colorectal-cancer
incidence and mortality with screening flexible sigmoidoscopy. N
Engl J Med. 366:2345–2357. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mosli MH and Al-Ahwal MS: Colorectal
cancer in the Kingdom of Saudi Arabia: Need for screening. Asian
Pac J Cancer Prev. 13:3809–3813. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fearon ER and Vogelstein B: A genetic
model for colorectal tumorigenesis. Cell. 61:759–767. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
De Rosa M, Rega D, Costabile V, Duraturo
F, Niglio A, Izzo P, Pace U and Delrio P: The biological complexity
of colorectal cancer: Insights into biomarkers for early detection
and personalized care. Therap Adv Gastroenterol. 9:861–886. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Keppler D, Sameni M, Moin K, Mikkelsen T,
Diglio CA and Sloane BF: Tumor progression and angiogenesis:
Cathepsin B & Co. Biochem Cell Biol. 74:799–810. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mohamed MM and Sloane BF: Cysteine
cathepsins: Multifunctional enzymes in cancer. Nat Rev Cancer.
6:764–775. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gondi CS and Rao JS: Cathepsin B as a
cancer target. Expert Opin Ther Targets. 17:281–291. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marten K, Bremer C, Khazaie K, Sameni M,
Sloane B, Tung CH and Weissleder R: Detection of dysplastic
intestinal adenomas using enzyme-sensing molecular beacons in mice.
Gastroenterology. 122:406–414. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bengsch F, Buck A, Günther SC, Seiz JR,
Tacke M, Pfeifer D, von Elverfeldt D, Sevenich L, Hillebrand LE,
Kern U, et al: Cell type-dependent pathogenic functions of
overexpressed human cathepsin B in murine breast cancer
progression. Oncogene. 33:4474–4484. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lim IT, Meroueh SO, Lee M, Heeg MJ and
Mobashery S: Strategy in inhibition of cathepsin B, a target in
tumor invasion and metastasis. J Am Chem Soc. 126:10271–10277.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schmitt M, Jänicke F, Moniwa N,
Chucholowski N, Pache L and Graeff H: Tumor-associated
urokinase-type plasminogen activator: Biological and clinical
significance. Biol Chem Hoppe Seyler. 373:611–622. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Emmert-Buck MR, Roth MJ, Zhuang Z, Campo
E, Rozhin J, Sloane BF, Liotta LA and Stetler-Stevenson WG:
Increased gelatinase A (MMP-2) and cathepsin B activity in invasive
tumor regions of human colon cancer samples. Am J Pathol.
145:1285–1290. 1994.PubMed/NCBI
|
|
15
|
Khan A, Krishna M, Baker SP and Banner BF:
Cathepsin B and tumor-associated laminin expression in the
progression of colorectal adenoma to carcinoma. Mod Pathol.
11:704–708. 1998.PubMed/NCBI
|
|
16
|
Adenis A, Huet G, Zerimech F, Hecquet B,
Balduyck M, Peyrat JP and Cathepsin B: Cathepsin BL, and D
activities in colorectal carcinomas: Relationship with
clinico-pathological parameters. Cancer Lett. 96:267–275. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Troy AM, Sheahan K, Mulcahy HE, Duffy MJ,
Hyland JM and O'Donoghue DP: Expression of Cathepsin B and L
antigen and activity is associated with early colorectal cancer
progression. Eur J Cancer. 40:1610–1616. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sinha AA, Gleason DF, Deleon OF, Wilson MJ
and Sloane BF: Localization of a biotinylated cathepsin B
oligonucleotide probe in human prostate including invasive cells
and invasive edges by in situ hybridization. Anat Rec. 235:233–240.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rempel SA, Rosenblum ML, Mikkelsen T, Yan
PS, Ellis KD, Golembieski WA, Sameni M, Rozhin J, Ziegler G and
Sloane BF: Cathepsin B expression and localization in glioma
progression and invasion. Cancer Res. 54:6027–6031. 1994.PubMed/NCBI
|
|
20
|
Matarrese P, Ascione B, Ciarlo L, Vona R,
Leonetti C, Scarsella M, Mileo AM, Catricalà C, Paggi MG and
Malorni W: Cathepsin B inhibition interferes with metastatic
potential of human melanoma: An in vitro and in vivo study. Mol
Cancer. 9:2072010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sevenich L, Schurigt U, Sachse K, Gajda M,
Werner F, Müller S, Vasiljeva O, Schwinde A, Klemm N, Deussing J,
et al: Synergistic antitumor effects of combined cathepsin B and
cathepsin Z deficiencies on breast cancer progression and
metastasis in mice. Proc Natl Acad Sci USA. 107:pp. 2497–2502.
2010; View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sevenich L, Werner F, Gajda M, Schurigt U,
Sieber C, Müller S, Follo M, Peters C and Reinheckel T: Transgenic
expression of human cathepsin B promotes progression and metastasis
of polyoma-middle-T-induced breast cancer in mice. Oncogene.
30:54–64. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gong F, Peng X, Luo C, Shen G, Zhao C, Zou
L, Li L, Sang Y, Zhao Y and Zhao X: Cathepsin B as a potential
prognostic and therapeutic marker for human lung squamous cell
carcinoma. Mol Cancer. 12:1252013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bian B, Mongrain S, Cagnol S, Langlois MJ,
Boulanger J, Bernatchez G, Carrier JC, Boudreau F and Rivard N:
Cathepsin B promotes colorectal tumorigenesis, cell invasion, and
metastasis. Mol Carcinog. 55:671–687. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chan AT, Baba Y, Shima K, Nosho K, Chung
DC, Hung KE, Mahmood U, Madden K, Poss K, Ranieri A, et al:
Cathepsin B expression and survival in colon cancer: Implications
for molecular detection of neoplasia. Cancer Epidemiol Biomarkers
Prev. 19:2777–2785. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Al Obeed OA, Alkhayal KA, Al Sheikh A,
Zubaidi AM, Vaali-Mohammed MA, Boushey R, Mckerrow JH and Abdulla
MH: Increased expression of tumor necrosis factor-α is associated
with advanced colorectal cancer stages. World J Gastroenterol.
20:18390–18396. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leonard L, Gunderson, Jessup JM, Sargent
D, Greene F and Stewart Andrew: Revised TN categorization for colon
cancer based on national survival outcomes data. J Clin Oncol.
28:2010.PubMed/NCBI
|
|
28
|
Kos J, Nielsen HJ, Krasovec M, Christensen
IJ, Cimerman N, Stephens RW and Brünner N: Prognostic values of
cathepsin B and carcinoembryonic antigen in sera of patients with
colorectal cancer. Clin Cancer Res. 4:1511–1516. 1998.PubMed/NCBI
|
|
29
|
Sheahan K, Shuja S and Murnane MJ:
Cysteine protease activities and tumor development in human
colorectal carcinoma. Cancer Res. 49:3809–3814. 1989.PubMed/NCBI
|
|
30
|
Murnane MJ, Sheahan K, Ozdemirli M and
Shuja S: Stage specific increases in cathepsin B messenger RNA
content in human colorectal carcinoma. Cancer Res. 51:1 137–1142.
1991.
|
|
31
|
Tu C, Ortega-Cava CF, Chen G, Fernandes
ND, Cavallo-Medved D, Sloane BF, Band V and Band H: Lysosomal
cathepsin B participates in the podosome-mediated extracellular
matrix degradation and invasion via secreted lysosomes in v-Src
fibroblasts. Cancer Res. 68:9147–9156. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hirai K, Yokoyama M, Asano G and Tanaka S:
Expression of cathepsin B and cystatin C in human colorectal
cancer. Hum Pathol. 30:680–686. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Talieri M, Papadopoulou S, Scorilas A,
Xynopoulos D, Arnogianaki N, Plataniotis G, Yotis J and Agnanti N:
Cathepsin B and cathepsin D expression in the progression of
colorectal adenoma to carcinoma. Cancer Lett. 205:97–106. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shuja S, Sheahan K and Murnane MJ:
Cysteine endopeptidase activity levels in normal human tissues,
colorectal adenomas and carcinomas. Int J Cancer. 49:341–346. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Victor BC, Anbalagan A, Mohamed MM, Sloane
BF and Cavallo-Medved D: Inhibition of cathepsin B activity
attenuates extracellular matrix degradation and inflammatory breast
cancer invasion. Breast Cancer Res. 13:R1152011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Campo E, Muñoz J, Miquel R, Palacín A,
Cardesa A, Sloane BF and Emmert-Buck MR: Cathepsin B expression in
colorectal carcinomas correlates with tumor progression and
shortened patient survival. Am J Pathol. 145:301–309.
1994.PubMed/NCBI
|
|
37
|
Devetzi M, Scorilas A, Tsiambas E, Sameni
M, Fotiou S, Sloane BF and Talieri M: Cathepsin B protein levels in
endometrial cancer: Potential value as a tumour biomarker. Gynecol
Oncol. 112:531–536. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gopinathan A, Denicola GM, Frese KK, Cook
N, Karreth FA, Mayerle J, Lerch MM, Reinheckel T and Tuveson DA:
Cathepsin B promotes the progression of pancreatic ductal
adenocarcinoma in mice. Gut. 61:877–884. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Konduri S, Lakka SS, Tasiou A, Yanamandra
N, Gondi CS, Dinh DH, Olivero WC, Gujrati M and Rao JS: Elevated
levels of cathepsin B in human glioblastoma cell lines. Int J
Oncol. 19:519–524. 2001.PubMed/NCBI
|
|
40
|
Withana NP, Blum G, Sameni M, Slaney C,
Anbalagan A, Olive MB, Bidwell BN, Edgington L, Wang L, Moin K, et
al: Cathepsin B inhibition limits bone metastasis in breast cancer.
Cancer Res. 72:1199–1209. 2012. View Article : Google Scholar : PubMed/NCBI
|