Introduction
The incidence of hepatocellular carcinoma (HCC) is
becoming the second leading cause of cancer-related death
worldwide, which accounting for approximately 800,000 deaths every
year (1). Hepatic resection and
liver transplantation have progressed in surgical procedures for
HCC. The improved outcomes are limited because of the frequent
recurrence, even after liver transplantation (2–5). Thus,
it is urgent to develop novel approaches for hepatocarcinoma
prevention and treatment. At present, chemotherapy is also a focus
for tumor treatment (6). Sorafenib,
the molecular targeting agent, was reported to improve survival
rates and outcomes in patients with non-resectable or early stage
HCC (7,8). However, sorafenib is the only approved
molecular targeted treatment for advanced HCC. Other targeted
agents are under investigation. Trials comparing new agents in
combination with sorafenib are ongoing. Combinations of systemic
targeted therapies with local treatments are being evaluated for
further improvement in HCC patient outcomes (9–11).
In recent years, increased data concerning the
traditional Chinese medicine with a remarkable activity on the
influence with tumor cell death pathway could guide tumor treatment
decisions and clinical management (12). Cantharidin, also together with its
acid form cantharidinate, was first extracted from Chinese blister
beetle, have been used in traditional Chinese medicine for many
years (13,14). Sodium cantharidinate has powerful
antitumor activity proved in clinical practices in recent years
(15). The compound directly
inhibits multiple malignant tumors, including myeloma, oral buccal
carcinoma, leukemia, gastric cancer, Colo205 CRC, and has low
toxic/adverse effects so far (16).
In recent years, researchers have confirmed through in vitro
experiments that sodium cantharidinate and its derivatives directly
kill liver cancer cell lines (17).
Autophagy is the natural, destructive cellular
mechanism that degrades damaged proteins and cytoplasm components
in lysosomes and thus maintains cellular homeostasis and supplies
substrates for energy generation. It is a critical pathway for
homeostasis, development and other pathophysiological processes
(18). Autophagy plays an important
role in the healthy and diseased liver (19, 20). Autophagy is
considered as an important cellular metabolic process (21). Its function on cell fate is
double-edged, which can promote cell survival, therefore may also
promote cell death via different mechanisms (22). Autophagy plays different roles
depending on the drug, cell type or time of drug action, and the
mechanism is not fully understood (23,24).
Therefore, the study of the dual role of autophagy may provide new
clues for tumor treatment. In the present study, we investigated
whether sodium cantharidinate induces the HepG2 cell line apoptosis
and whether it depends on the autophagy pathway.
Materials and methods
Reagents
Sodium cantharidinate, Hoechst 33258, MTT and RNase
were purchased from Sigma (St. Louis, MO, USA). Z-DEVD-FMK
(HY-12466, Caspase-3 inhibitor) was purchased from MedChem Express
(Monmouth Junction, NJ, USA). Propidium iodide and Annexin V-FITC
Apoptosis Detection kit was purchased from BD Biosciences (San
Jose, CA, USA). LC3 siRNA reagent kit was purchased from Cell
Signaling Technology (Danvers, MA, USA). Dulbecco's modified
Eagle's medium (DMEM), trypsin, fetal bovine serum (FBS), PBS,
penicillin and streptomycin were obtained from Gibco BRL Life
Technologies (Grand Island, NY, USA).
Preparation of sodium
cantharidinate
Sodium cantharidinate was dissolved in PBS (pH 7.2)
to prepare a stock solution at a concentration of 1.0 mM which was
stored at −20°C. DMEM complete medium was added to dilute the
sodium cantharidinate to the appropriate concentrations prior to
use.
HepG2 cell culture and treatment
HepG2 cells were routinely cultured in DMEM complete
medium which contains 50 U/ml antibiotics and 10% fetal bovine
serum (FBS) under the conditions of 5% CO2 at 37°C in
cell incubator (HERAcell 150i; Thermo Fisher Scientific, Waltham,
MA, USA). Following trypsinization to passage the cells in 75 T
flask 3–5 days, the cells were counted and reseed in 96-well plate
in DMEM complete medium without or with sodium cantharidinate for
MTT array or apoptosis detection.
The effect of sodium cantharidinate on
HepG2 proliferation
The inhibitory effect of sodium cantharidinate on
the proliferation of HepG2 cells were detected via MTT assays. All
experiment steps were performed following the instructions of the
kit. Briefly, the cells were seeded on 96-well plates at a density
of 5×104/ml at a volume of 200 µl per well. All groups
without or with sodium cantharidinate (0, 2.0, 5.0, 12.5 µM) were
incubated 6–24 h. MTT (1.0 mg/ml) was added to each well, and the
cells were incubated for 4 h. The MTT solution was then aspirated,
and 100 µl DMSO was added. The 96-well plates were read using a
microplate spectrophotometer (Synergy H1, BioTek, Winooski, VT,
USA) at 540 nm. The experiments were repeated in triplicate. The
inhibition percentage was calculated as (1 - the value in
experimental group / the value in the control group) ×100%.
FCM for cell apoptosis
Annexin V-FITC and PI double staining flow cytometry
analyses were employed. The HepG2 cells were plated in 96-well
plates containing 200 µl medium at a density of 5×104
cells/well. The induction of apoptosis in the HepG2 cells were
examined without or with sodium cantharidinate (5.0 µM). After
culture, the cells were collected in 1.5 ml centrifuge tubes,
washed three times with cold PBS and binding buffer, and then
stained with Annexin V-FITC and PI (Annexin V-FITC Apoptosis
Detection kit; BD Biosciences) for apoptosis detection. Briefly,
HepG2 cells in centrifuge tubes were re-suspended in binding
buffer. Then, 5 µl of FITC-Annexin V was added to the tubes, which
were incubated for 10 min followed by the addition of 5 µl PI. The
samples were then incubated with PI for another 15 min and
immediately analyzed using a flow cytometer (FACScan; BD
Biosciences) with the Flowjo FACS analysis software. The cells in
the different portions represented the different cell states as
follows: the late-apoptotic cells were present in the upper right
portion, the viable cells were present in the lower left portion,
and the early apoptotic cells were present in the lower right
portion.
Western blotting
HepG2 cell lysates were separated by SDS-PAGE under
non-reducing conditions on a 10% polyacrylamide gel. The proteins
were then transferred onto PVDF membranes (GE Healthcare Life
Sciences, Piscataway, NJ, USA) by electroblotting. The membranes
were blocked with blocking buffer overnight at 4°C and then
incubated with the caspase-3, cleaved caspase-3, LC3-I and LC3-II
antibodies for 1.5 h at room temperature. The membranes were then
washed by washing buffer six times and incubated with
HRP-conjugated secondary antibodies for 1 h. After washing, protein
bands were visualized using an enhanced chemiluminescent system
(Thermo Fisher Scientific). The primary antibodies used (caspase-3,
cleaved caspase-3, LC3-I, LC3-II and β-actin) were all obtained
from Cell Signaling Technology.
Indirect immunofluorescence staining
and confocal laser microscopy
Hoechst 33258 (Sigma) were used to assessed
apoptotic nuclear changes. After treatment with 5.0 µM sodium
cantharidinate for 0, 6, 12, and 24 h, cells were fixed with 4%
paraformaldehyde, stained with Hoechst 33258 (2 µg/ml) for 30 min,
washed in PBS, and examined using Olympus FV1000 confocal laser
microscopy to reveal cell chromatin condensation. Briefly, HepG2
cells were cultured on coverslips overnight, then treated with 5.0
µM sodium cantharidinate for 6 h and rinsed with PBS at least three
times. Cells were fixed for 20 min with 4% paraformaldehyde after
incubation, then permeabilized with 0.1% Triton X-100 for 5 min,
finally blocked with bovine serum albumin. Then incubated with
primary antibodies against LC3 (1:50 dilution) overnight at 4°C,
then in FITC/Rhodamine Red-conjugated secondary antibodies (1:400
dilution) (all antibodies, Santa Cruz Biotechnology, CA, USA) for
0.5 h, and stained with Hoechst 33258 (2 µg/ml) for 2 min, washed
with PBS three times, and examined by confocal fluorescence
microscopy.
Statistical analysis
All data were analyzed and assessed for significance
using the Pearson omnibus normality test. All data are presented as
the mean ± the standard error of the mean. Mean values were
compared using paired t-tests (two groups) followed by the
Bonferroni correction for multiple comparison tests. p-values
<0.05 were considered to indicate a statistically significant
result. All statistical tests were performed with prism software
(GraphPad, San Diego, CA, USA).
Results
Sodium cantharidinate induces
apoptosis in HepG2 cells by caspase-3 activity
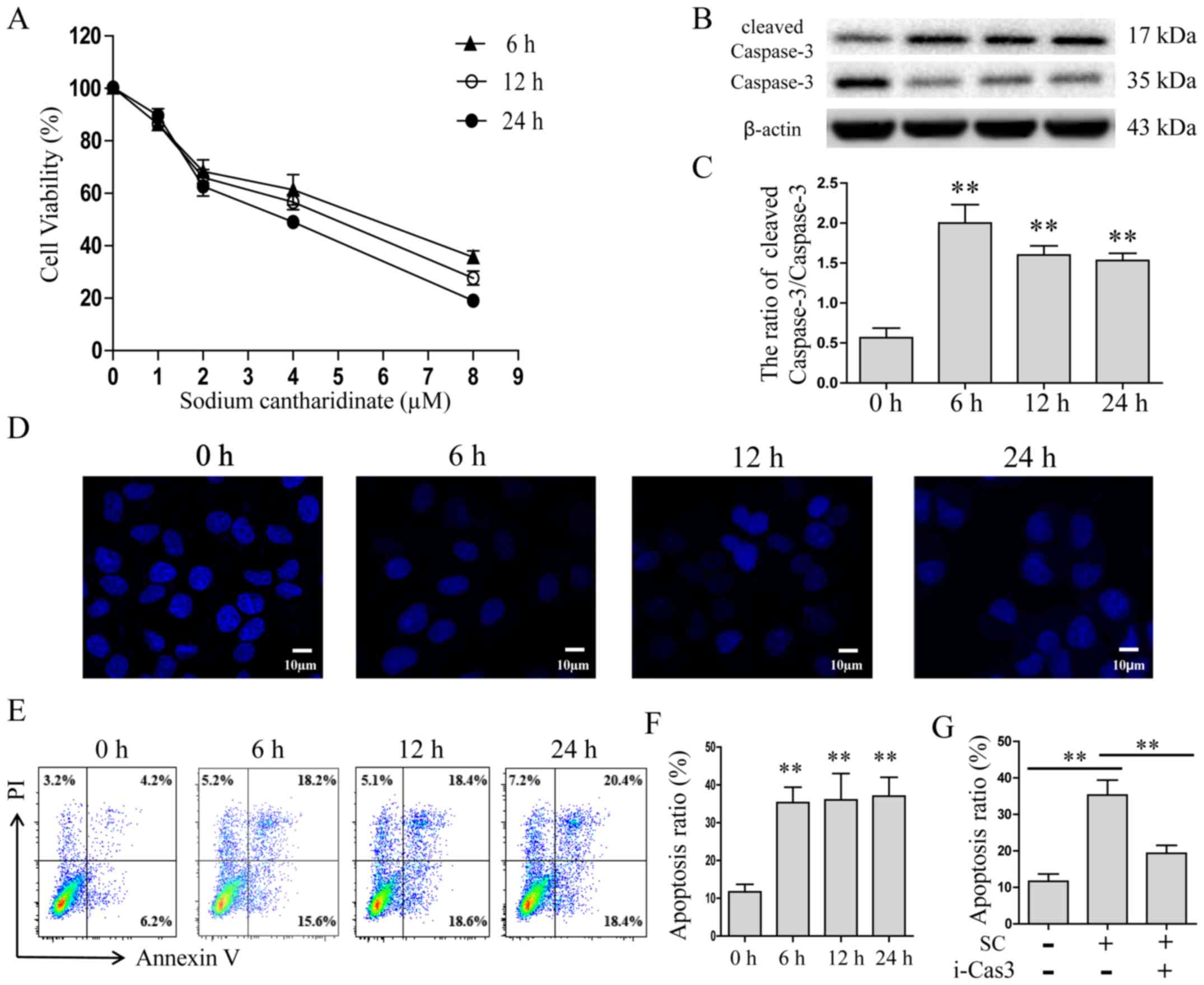
HepG2 cells were treated with different doses of
sodium cantharidinate for different time intervals, and MTT results
showed that sodium cantharidinate effectively inhibited the
proliferation of HepG2 cells in a dose- and time-dependent manner
(Fig. 1A). We then selected 5.0 µM
sodium cantharidinate for treatment of HepG2 cells at different
time intervals, and the apoptotic effector caspase-3 was detected
by western blotting. The results showed that the expression of
cleaved caspase-3 was increased at all three time points (4-fold
change, p<0.01, Fig. 1B and C).
The cell nucleus stained with Hoechst 33258 was observed using
confocal laser scanning microscopy. The results suggested that
sodium cantharidinate induced apoptosis in HepG2 cells, which keeps
consistency to the MTT array (Fig.
1D). Annexin V-FITC and PI double staining assay was also
performed to confirm the cytotoxicity of sodium cantharidinate on
HepG2 cells (Fig. 1E and F). The
results showed that comparing with the control group, the numbers
of early and late apoptotic cells increased significantly in the
treated group. The proportion of early and late apoptotic cells in
the sodium cantharidinate treatment group reached 37.2%, which was
greater than the proportion observed in the control group (10.1%,
p<0.01). To determine the effect of caspase-3 on the sodium
cantharidinate inducement of HepG2 cell apoptosis, a caspase-3
inhibitor, Z-DEVD-FMK (100 µM) was added to the well, or not. Cells
were treated by sodium cantharidinate for 6 h, then apoptosis was
determined by FACS. The proportion of apoptotic cells decreased
from 35.2% to 17.8% when the Z-DEVD-FMK was added (Fig. 1G, p<0.01). This finding indicated
that sodium cantharidinate significantly induced HepG2 cell
apoptosis by caspase-3 activity.
Sodium cantharidinate induces HepG2
cell autophagy through LC3
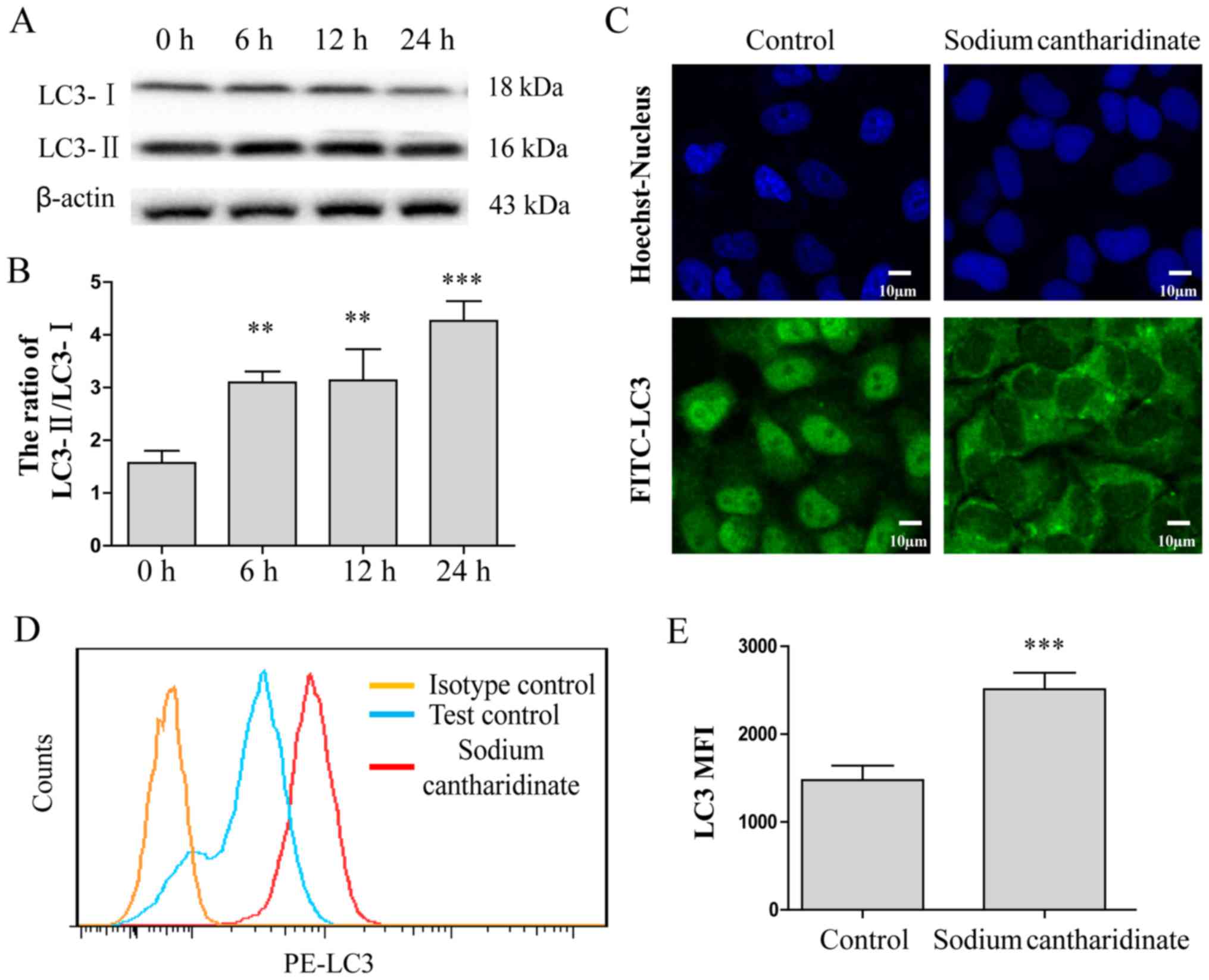
Studies suggest that autophagy may be involved in
the antitumor effect of drugs (25). Therefore, we analyzed the protein
expression of the autophagic maker protein LC3 in response to 5.0
µM sodium cantharidinate by western blotting. The results showed
that the protein expression ratio of LC3-II and LC3-I was
significantly increased by sodium cantharidinate treatment for 6–24
h (Fig. 2A and B). Furthermore,
indirect immunofluorescence showed that LC3 had translocated to the
cytoplasm, forming punctate aggregates, and the fluorescence
intensity of LC3 was also enhanced (Fig. 2C), suggesting that sodium
cantharidinate induced autophagy in HepG2 cells. The expression of
LC3 expressed in HepG2 cells was analyzed by flow cytometry. The
results are shown in Fig. 2D and E.
The LC3 expression level was much higher in HepG2 cells treated
with sodium cantharidinate than controls (MFI: 2508±165 vs.
1458±89, p<0.001). These results showed that sodium
cantharidinate induced HepG2 cell autophagy through LC3
pathway.
Inhibition of autophagy reduces sodium
cantharidinate-induced cell apoptosis
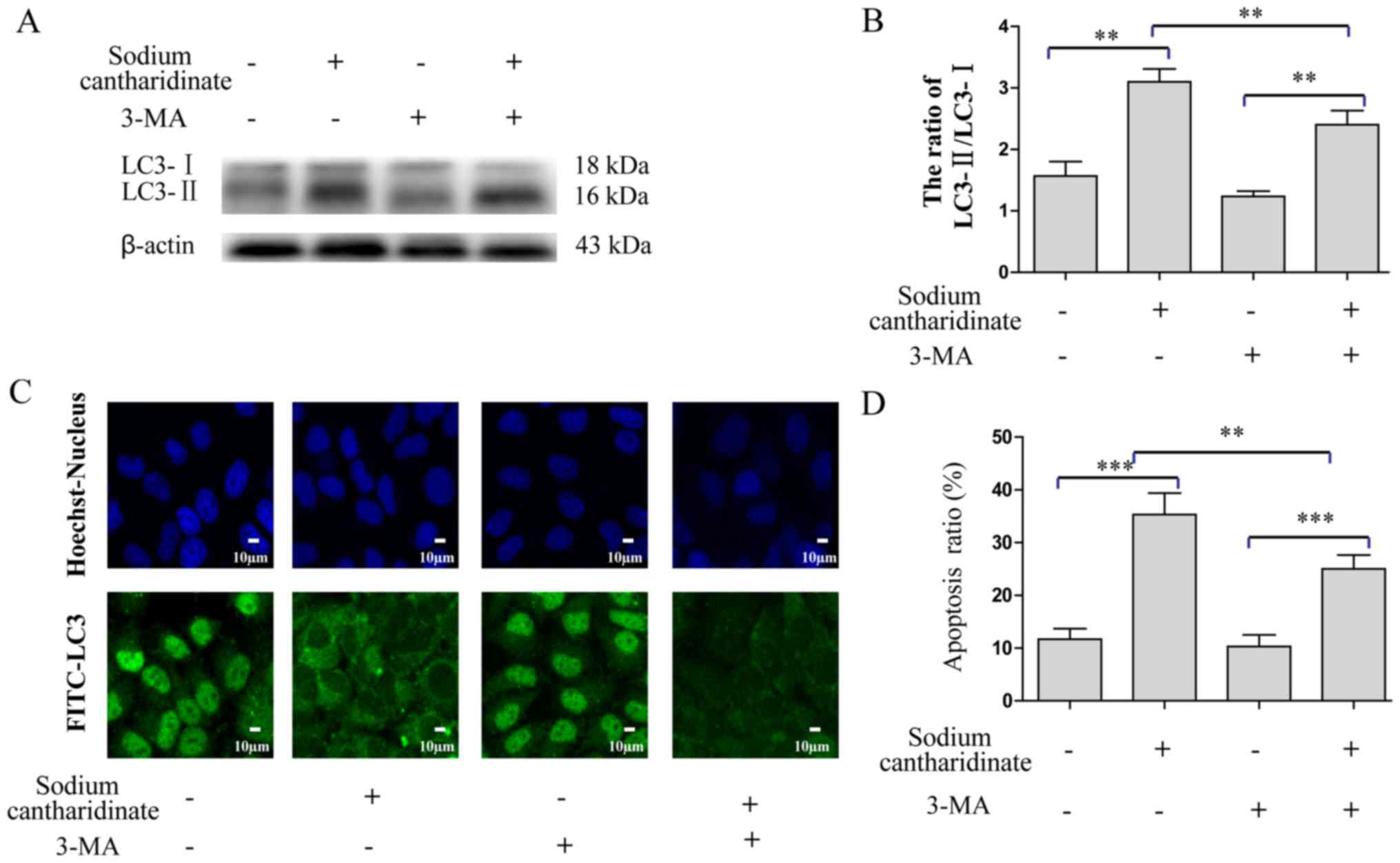
Previous research used 3-MA to inhibit autophagy and
prove that autophagy is involved in the growth inhibition of
hepatoma cells (26). Therefore, we
combined 5 mM 3-MA and 5 µM sodium cantharidinate treatment in
HepG2 cells for 6 h, and detected the protein expression of LC3-II
and LC3-I by western blotting. Sodium cantharidinate combined with
3-MA resulted in a reduction of protein expression ratio of LC3-II
and LC3-I compared with sodium cantharidinate alone (3-fold change,
p<0.01, Fig. 3A and B). Indirect
immunofluorescence demonstrated that LC3 was distributed in both
the cytoplasm and the nucleus, and the fluorescence intensity was
significantly reduced (Fig. 3C),
showing that 3-MA inhibited sodium cantharidinate-induced autophagy
effectively. Furthermore, Annexin V-FITC and PI double staining
assay was also performed to confirm the LC3 inhibitor influences
the cytotoxicity of sodium cantharidinate on HepG2 cells (Fig. 3D). The results showed that compared
with the control group, the numbers of early and late apoptotic
cells decreased significantly when 3-MA was combined with sodium
cantharidinate. The proportion of early and late apoptotic cells in
the sodium cantharidinate treatment group reached 37.2%, but it
decreased to 22.2% when 3-MA was added (p<0.01).
Silence of LC3 inhibits autophagy to
reduce sodium cantharidinate-induced cell apoptosis
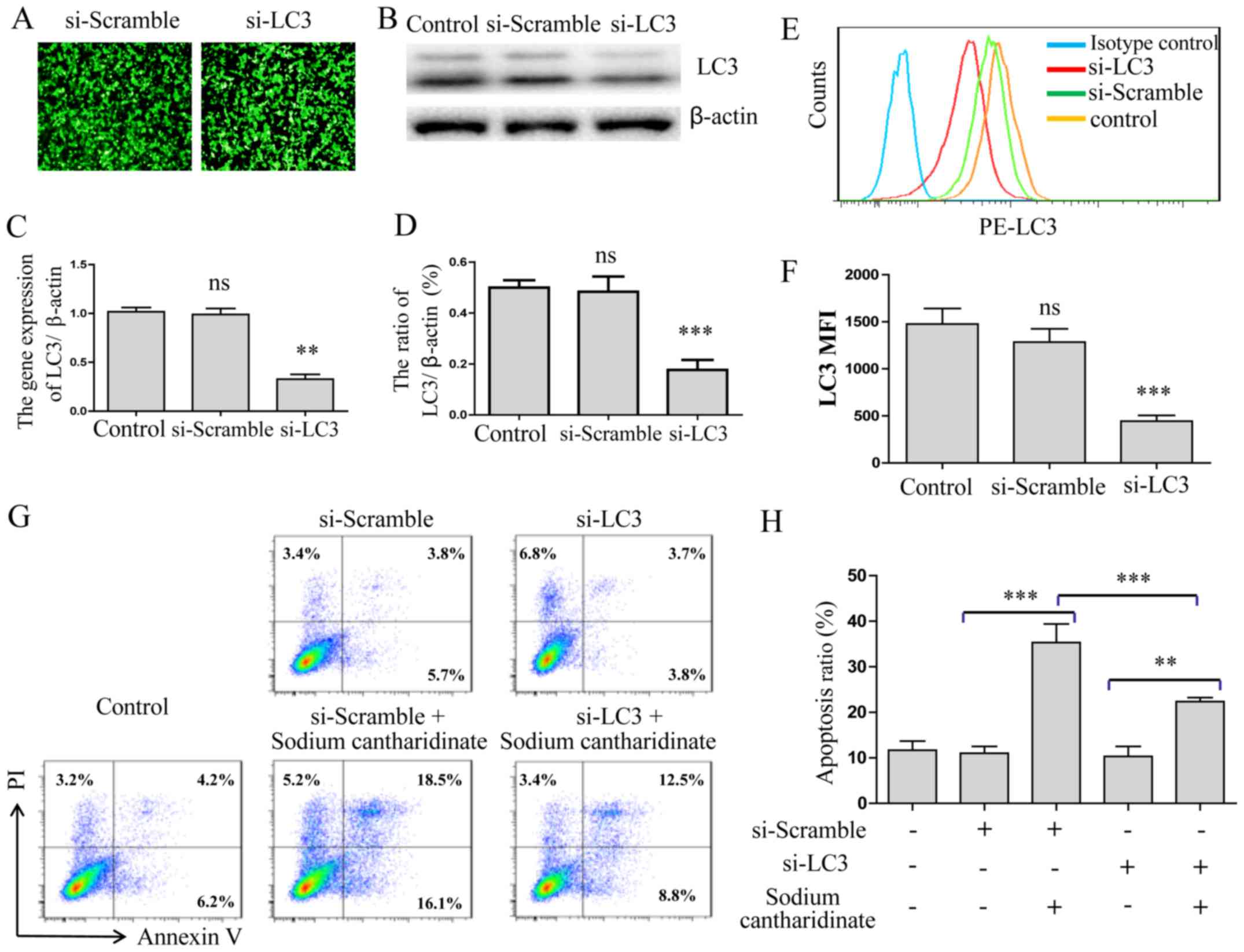
We applied RNAi technology to inhibit LC3 expression
on HepG2 cells. After the LC3 siRNA treatment of HepG2 cells for 24
h, we observed the expression rate of green fluorescent protein
(GFP) to be >85% by fluorescence microscopy (Fig. 4A). LC3 protein expression was
significantly decreased as detected by western blotting
(p<0.001, Fig. 4B and C) and
qRT-PCR (p<0.01, Fig. 4D). FACS
analysis showed that the MFI level of LC3 was also significantly
decreased (p<0.001, Fig. 4E and
F). Then, Annexin V-FITC and PI double staining assay was also
performed to confirm that the LC3 gene was silenced after sodium
cantharidinate treatment. The results showed that compared with the
control group, the numbers of early and late apoptotic cells
decreased significantly when sodium cantharidinate treatment of the
LC3 silenced HepG2 cells wre compared to the control cells
(p<0.001, Fig. 4G and H). These
results showed that LC3 autophagy pathway played an important role
in the sodium cantharidinate induced HepG2 cell apoptosis.
Discussion
The incidence of hepatocellular carcinoma is
becoming the second leading cause of cancer-related death worldwide
accounting for approximately 800,000 deaths every year. Hepatic
resection and liver transplantation have progressed in surgical
procedures for HCC. However, the outcomes have improved only
slightly because of the frequent recurrence, even after liver
transplantation. The pathogenesis on HCC remains unclear, but the
genetic mutations of normal cells affected by environmental
deterioration or other risk factors become a generally accepted
carcinogenic factor (27).
Sodium cantharidinate kills liver cancer cell lines
directly, which provided the favorable theoretical basis for the
application of treatment of primary liver cancer (17). The present study demonstrated that
sodium cantharidinate was able to inhibit the proliferation of
HepG2 cells within the ranges of 2.0–12.5 µM and 6–24 h. Sodium
cantharidinate enhanced the apoptotic effector of caspase-3
activity and induced cell death. Nucleus stained with Hoechst 33258
and Annexin V-FITC and PI double staining is consistent with MTT
results. Caspase-3 activation could be initiated by many upstream
signal-regulated molecules (28–30).
Previous studies suggested that drugs could promote autophagy in
human cancer cell lines, prompting speculation that autophagy may
be involved in the antitumor effect (26). Some research also demonstrated that
oxidative stress can induce autophagy then inhibit the
proliferation of liver cancer cells (26). In this study, we found that LC3
punctate aggregates and nucleation appeared in HepG2 cells treated
with sodium cantharidinate, indicating that sodium cantharidinate
induced autophagy in HepG2 cells then caused cell death. The
results showed that the protein expression ratio of LC3-II and
LC3-I was significantly increased by sodium cantharidinate
treatment for 6–24 h on HepG2 cells.
To confirm how important autophagy pathway in the
sodium cantharidinate induced HepG2 cells apoptosis, the autophagy
inhibitor 3-MA was added to the cell culture system to observe
sodium cantharidinate-induced apoptosis of HepG2 cells. The results
showed that after HepG2 cells were treated with 3-MA, sodium
cantharidinate-induced apoptosis of HepG2 cells were reduced
greatly. We applied RNAi technology to inhibit LC3 expression.
After the LC3 siRNA treatment in HepG2 cells for 24 h, we observed
the expression of LC3 protein expression were significantly
decreased detected by western blotting. At the same time, the
numbers of early and late apoptotic cells decreased significantly.
Based on the results, we concluded that sodium cantharidinate
performed its antitumor effect by inducing autophagy on target
cells. In summary, this study found that sodium cantharidinate
acted to inhibit HepG2 cells by inducing autophagy. To our
knowledge, this is the first study revealing the exact mechanism of
sodium cantharidinate on inducing HepG2 cell apoptosis. Sodium
cantharidinate has potential for development as a new drug for
treatment of human HCC.
Acknowledgements
This study was supported in part by grants from the
Jilin Provincial Natural Science Foundation of China (no.
20140520014JH), the 4th Young Scientist Fund of Jilin University
(no. 2013068), the National Major Scientific, the Technological
Special Project for ‘Significant New Drugs Development’ (no.
2014ZX09303303), the Interdisciplinary Chemistry and Medicine
Foundation of Jilin University (JDYYJCHX004) and the National
Natural Science Foundation of China (no. 31470418, to Y.H.).
References
|
1
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shirabe K, Kanematsu T, Matsumata T,
Adachi E, Akazawa K and Sugimachi K: Factors linked to early
recurrence of small hepatocellular carcinoma after hepatectomy:
Univariate and multivariate analyses. Hepatology. 14:802–805. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamashita Y, Morita K, Iguchi T, Tsujita
E, Soejima Y, Taketomi A and Maehara Y: Surgical impacts of an en
bloc resection of the diaphragm for hepatocellular carcinoma with
gross diaphragmatic involvement. Surg Today. 41:101–106. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sakaguchi T, Suzuki S, Morita Y, Oishi K,
Suzuki A, Fukumoto K, Inaba K, Nakamura S and Konno H: Impact of
the preoperative des-gamma-carboxy prothrombin level on prognosis
after hepatectomy for hepatocellular carcinoma meeting the Milan
criteria. Surg Today. 40:638–645. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Taketomi A, Fukuhara T, Morita K,
Kayashima H, Ninomiya M, Yamashita Y, Ikegami T, Uchiyama H,
Yoshizumi T, Soejima Y, et al: Improved results of a surgical
resection for the recurrence of hepatocellular carcinoma after
living donor liver transplantation. Ann Surg Oncol. 17:2283–2289.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nault JC, De Reyniès A, Villanueva A,
Calderaro J, Rebouissou S, Couchy G, Decaens T, Franco D, Imbeaud
S, Rousseau F, et al: A hepatocellular carcinoma 5-gene score
associated with survival of patients after liver resection.
Gastroenterology. 145:176–187. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Balkwill F and Mantovani A: Inflammation
and cancer: back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
de Visser KE, Eichten A and Coussens LM:
Paradoxical roles of the immune system during cancer development.
Nat Rev Cancer. 6:24–37. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Posner MR: Paradigm shift in the treatment
of head and neck cancer: The role of neoadjuvant chemotherapy.
Oncologist. 10:(Suppl 3). 11–19. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bruix J, Gores GJ and Mazzaferro V:
Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut.
63:844–855. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kaseb AO, Abaza YM and Roses RE:
Multidisciplinary management of hepatocellular carcinoma. Recent
Results Cancer Res. 190:247–259. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gerber DE: Targeted therapies: A new
generation of cancer treatments. Am Fam Physician. 77:311–319.
2008.PubMed/NCBI
|
|
13
|
Honkanen RE: Cantharidin, another natural
toxin that inhibits the activity of serine/threonine protein
phosphatases types 1 and 2A. FEBS Lett. 330:283–286. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deng LP, Dong J, Cai H and Wang W:
Cantharidin as an antitumor agent: A retrospective review. Curr Med
Chem. 20:159–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsauer W, Lin JG, Lin PY, Hsu FL and
Chiang HC: The effects of cantharidin analogues on xanthine
oxidase. Anticancer Res. 17:2095–2098. 1997.PubMed/NCBI
|
|
16
|
Lin LH, Huang HS, Lin CC, Lee LW and Lin
PY: Effects of cantharidinimides on human carcinoma cells. Chem
Pharm Bull (Tokyo). 52:855–857. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yeh CB, Su CJ, Hwang JM and Chou MC:
Therapeutic effects of cantharidin analogues without bridging ether
oxygen on human hepatocellular carcinoma cells. Eur J Med Chem.
45:3981–3985. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ravikumar B, Sarkar S, Davies JE, Futter
M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M,
Korolchuk VI, Lichtenberg M, Luo S, et al: Regulation of mammalian
autophagy in physiology and pathophysiology. Physiol Rev.
90:1383–1435. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gual P, Gilgenkrantz H and Lotersztajn S:
Autophagy in chronic liver diseases: The two faces of Janus. Am J
Physiol Cell Physiol. 312:C263–C273. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim KY, Jang HJ, Yang YR, Park KI, Seo J,
Shin IW, Jeon TI, Ahn SC, Suh PG, Osborne TF, et al: Corrigendum:
SREBP-2/PNPLA8 axis improves non-alcoholic fatty liver disease
through activation of autophagy. Sci Rep. 6:377942016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ogier-Denis E and Codogno P: Autophagy: A
barrier or an adaptive response to cancer. Biochim Biophys Acta.
1603:113–128. 2003.PubMed/NCBI
|
|
22
|
Shintani T and Klionsky DJ: Autophagy in
health and disease: A double-edged sword. Science. 306:990–995.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mariño G and López-Otín C: Autophagy:
Molecular mechanisms, physiological functions and relevance in
human pathology. Cell Mol Life Sci. 61:1439–1454. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eskelinen EL: Maturation of autophagic
vacuoles in mammalian cells. Autophagy. 1:1–10. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guan J, Lo M, Dockery P, Mahon S, Karp CM,
Buckley AR, Lam S, Gout PW and Wang YZ: The xc-cystine/glutamate
antiporter as a potential therapeutic target for small-cell lung
cancer: Use of sulfasalazine. Cancer Chemother Pharmacol.
64:463–472. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guo W, Zhao Y, Zhang Z, Tan N, Zhao F, Ge
C, Liang L, Jia D, Chen T, Yao M, et al: Disruption of xCT inhibits
cell growth via the ROS/autophagy pathway in hepatocellular
carcinoma. Cancer Lett. 312:55–61. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bruix J, Reig M and Sherman M:
Evidence-based diagnosis, staging, and treatment of patients with
hepatocellular carcinoma. Gastroenterology. 150:835–853. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang M, Yan H, Li S and Yang J:
Rosmarinic acid protects rat hippocampal neurons from cerebral
ischemia/reperfusion injury via the Akt/JNK3/caspase-3 signaling
pathway. Brain Res. 1657:9–15. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Venkatesan RS and Sadiq AM: Effect of
morin-5′-sulfonic acid sodium salt on the expression of apoptosis
related proteins caspase 3, Bax and Bcl 2 due to the mercury
induced oxidative stress in albino rats. Biomed Pharmacother.
85:202–208. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mondal A and Bennett LL: Resveratrol
enhances the efficacy of sorafenib mediated apoptosis in human
breast cancer MCF7 cells through ROS, cell cycle inhibition,
caspase 3 and PARP cleavage. Biomed Pharmacother. 84:1906–1914.
2016. View Article : Google Scholar : PubMed/NCBI
|