Introduction
Hepatic steatosis is an early and common sign of
alcohol consumption and possible progression to alcoholic liver
disease (ALD), which is a major liver disease and an important
health issue in America and worldwide (1). ALD includes a series of phenotypes
ranging from simple steatosis to steatohepatitis, progressive
fibrosis, cirrhosis and hepatocellular carcinoma (2). The worldwide growing prevalence of ALD
suggests that more affordable treatments are needed.
Epidemiological studies have revealed an association
between an increased consumption of dietary flavonoids and a
reduced risk of dyslipidemia disease (3). The flavonoid naringenin
[5,7-dihydroxy-2-(4-hydroxyphenyl)chroman-4-one] has been
investigated as a possible candidate agent to protect against
inflammatory injuries (4).
Naringenin was found to improve glucose and insulin tolerance,
reduce hepatic lipid accumulation, and attenuate dyslipidemia and
atherosclerosis in mouse models (5,6).
Naringenin induced hepatic fatty acid oxidation, which reduces the
availability of lipids, particularly triglycerides, for the
assembly and secretion of apolipoprotein B-containing lipoproteins,
leading to reduced hepatic lipid accumulation and improved
dyslipidemia (6). Naringenin works
in a manner similar to that of metformin, a medicine used for type
2 diabetes, by reducing hepatic glucose production in hepatocytes
(7). The effects of naringenin on
dysregulated metabolism are related to reductions in adipose mass
and ectopic lipid deposition in the liver (5). Naringenin modulates the levels of
necrotic inflammation, reduces lipid and protein oxidation,
recruits the antioxidative defense system and markedly promotes
extracellular matrix degradation (8). Additionally, naringenin was found to
prevent diet-induced weight gain and adiposity by increasing
whole-body energy expenditure (5).
Other polyphenolic compounds prevented diet-induced adipose tissue
accumulation through a variety of mechanisms, including inhibition
of preadipocyte differentiation, lipolysis stimulation and
increased mitochondrial function (9). However, the role of naringenin in
alcohol-induced hepatic steatosis and its molecular mechanisms
remain to be fully elucidated.
Taking into account the above-mentioned findings,
the present study was undertaken to investigate the effects of
naringenin on alcohol-induced hepatic steatosis injury of zebrafish
larvae in vivo. To delineate the mechanisms by which
naringenin exerts its effects on alcohol metabolism,
anti-dyslipidemia and hepatocyte damage in ALD, we evaluated
several critical genes related to alcohol and lipid metabolism.
Morphological examinations of the whole zebrafish body and liver
were also performed to further substantiate the beneficial effects
of naringenin on pathological alterations induced by alcohol
exposure. For the first time, we evaluated the mechanisms by which
naringenin regulates alcohol and lipid homeostasis in zebrafish
larvae, and we conclude that naringenin can protect against
alcohol-induced metabolic dysregulation. Collectively, these
findings demonstrated that naringenin had marked lipid-lowering
potential, could normalize alcohol and lipid metabolism and could
prevent hepatic steatosis. The ability of naringenin to modulate
metabolic pathways linked to ALD suggests that citrus flavonoids
represent valuable tools in the search for regulators of alcohol
metabolism, lipid homeostasis and liver damage.
Materials and methods
Animal husbandry and treatments
Adult wild-type zebrafish (WT, AB strain) and a
liver-specific eGFP expression transgenic zebrafish line,
Tg(lfabp10α-eGFP), were maintained on a 14:10 h light:dark
cycle at 28°C. Embryos were collected following natural spawning
and raised at 28°C. All zebrafish protocols were approved by the
Institutional Animal Care and Use Committee of Southern Medical
University.
Larvae were exposed to 350 mmol/l ethanol (2% EtOH)
in fish water starting at 96–98 h post fertilization (hpf) for up
to 32 h as previously described (10). Zebrafish larvae in the model group
were randomly divided into 5 groups and placed in 6-well plates. We
dissolved monomers of naringenin in dimethyl sulfoxide (DMSO) and
diluted the stock solution with fish water to obtain different
concentrations. All groups were given 8 ml of water, and we added
appropriate naringenin concentrations to the drug-treated groups
and 0.1% DMSO (by volume concentration) to the DMSO group. The
6-well plates were placed in an incubator for 48 h; we then
observed and recorded the general situation of each group.
Oil Red O staining
Oil Red O staining was carried out as previously
described (11). Zebrafish larvae
were collected in 1.5 ml EP tubes and washed twice with
phosphate-buffered saline (PBS) after the animal experiments. We
then fixed whole larvae in 4% paraformaldehyde (PFA) overnight at
4°C. After the larvae were washed twice with PBS, they were
sequentially infiltrated with 20, 40, 80 and 100% propylene glycol
at room temperature for 15 min each and stained with 0.5% Oil Red O
in 100% propylene glycol at 65°C in the dark for 1 h. We washed the
stained larvae sequentially with 100, 80, 40 and 20% propylene
glycol for ~20 min each to fade the background color. Finally, the
stained larvae were washed with PBS and stored in 70% glycerol at
4°C. The larvae were observed and photographed on a bright-field
dissecting microscope (Olympus SZX10; Olympus, Tokyo, Japan).
H&E staining of paraffin
sections
Larvae were fixed in 4% PFA at 4°C overnight and
embedded in paraffin according to standard procedures (12). Then, 4 µm sections were stained with
hematoxylin and eosin (H&E), and the pathological changes in
the liver were observed and photographed using a light microscope
(Nikon Eclipse Ni-U; Nikon, Tokyo, Japan).
Nile Red staining
Whole zebrafish Nile Red staining was performed as
previously described (13). In
total, 0.5 mg of Nile Red was dissolved in 1 ml of acetone, and
then diluted with 75% glycerinum and 25% water. We washed the fixed
larvae 3 times with PBS. The larvae were then permeabilized with
0.1% Triton in citric acid solution at 65°C for 2 h, washed with
PBS, and stained with 0.5 µg/ml Nile Red at room temperature in the
dark for 50 min. After the larvae were washed with PBS, they were
stained with DAPI at room temperature in the dark for 10 min and
then washed 3 times with PBS. Finally, we photographed the stained
larvae using a confocal microscope (Nikon C2 Plus).
TUNEL staining
TUNEL assay was performed on paraffin-embedded liver
sections using an In Situ Cell Death Detection kit, POD
(Roche Diagnostics, Basel, Switzerland). Permeabilized larvae were
washed with PBS and stained with 1:50 TUNEL at 4°C in the dark
overnight. The next day, the larvae were stained with DAPI and
photographed as mentioned above.
Reverse transcription and quantitative
PCR (qPCR)
Total RNA was extracted from 20 larvae that had been
smashed with 1 ml syringe pumps, and the total RNA concentrations
were measured using a Thermo NanoDrop spectrophotometer. RNA was
reverse-transcribed into cDNA using a Takara reverse transcription
kit (Takara, Tokyo, Japan). Quantitative PCR was performed using
0.1 µmol/l of gene-specific primers, SYBR-Green SuperMix (Roche)
and a LightCycler® 96 (Roche). The qPCR conditions were
as follows: one cycle of 95°C for 10 min; followed by 45 cycles of
95°C for 10 sec, 60°C for 10 sec and 72°C for 10 sec; and a final
cycle of 95°C for 10 sec, 65°C for 60 sec and 97°C for 1 sec.
Rpp0 (ribosomal protein P0) was used as the reference; the
primer sequences are listed in Table
I. The mRNA level was calculated by the cycle threshold (Ct)
method (2−Ct (target)/2−Ct (rpp0)).
 | Table I.Primers used to quantify mRNA
levels. |
Table I.
Primers used to quantify mRNA
levels.
| Gene | FP sequence
(5′-3′) | RP sequence
(5′-3′) |
|---|
| rpp0 |
ctgaacatctcgcccttctc |
tagccgatctgcagacacac |
| cyp2y3 |
tattcccatgctgcactctg |
aggagcgtttacctgcagaa |
| cyp3a65 |
aaaccctgatgagcatggac |
caagtctttggggatgagga |
| chop |
aggaaagtgcaggagctgac |
ctccacaagaagaatttcctcc |
|
gadd45αa |
tggctttgtttgtgggactt |
tggaaaacagtccactgaga |
| hmgcra |
ctgaggctctggtggacgtg |
gatagcagctacgatgttggcg |
| hmgcrb |
cctgttagccgtcagtgga |
tctttgaccactcgtgccg |
| fasn |
gagaaagcttgccaaacagg |
gagggtcttgcaggagacag |
| fabp10α |
ttacgctcaggagaactacga |
ggatgtgggagaatcggtcag |
| edem1 |
gacagcagaaaccctcaagc |
catggccctcatcttgactt |
| echs1 |
agatgcagaatcgaaccttccaa |
gagatagcaaactcacatcctccg |
| fads2 |
tcatcgtcgctgttattctgg |
tgaagatgttgggtttagcgtg |
Statistical analysis
Statistical analysis was carried out using SPSS 16.0
statistical software (SPSS, Inc., Chicago, IL, USA). The results
are expressed as the mean ± standard error of the mean (SEM).
Statistical analysis was performed using an unpaired t-test or
one-way analysis of variance (ANOVA), followed by Tukey's multiple
comparison test with dependent experimental designs. One-way ANOVA
and t-tests were used for analysis. Differences were considered
statistically significance at P<0.05.
Results
An alcoholic fatty liver zebrafish
larva model was established
We chose to expose larvae to ethanol during a window
after the liver is formed (96 hpf) and before all the yolk is
utilized (5.5–6 dpf) to avoid the metabolic impact of fasting
(14). We defined acute exposure as
32 h, which is distinct from the chronic exposure that occurs in
alcoholics.
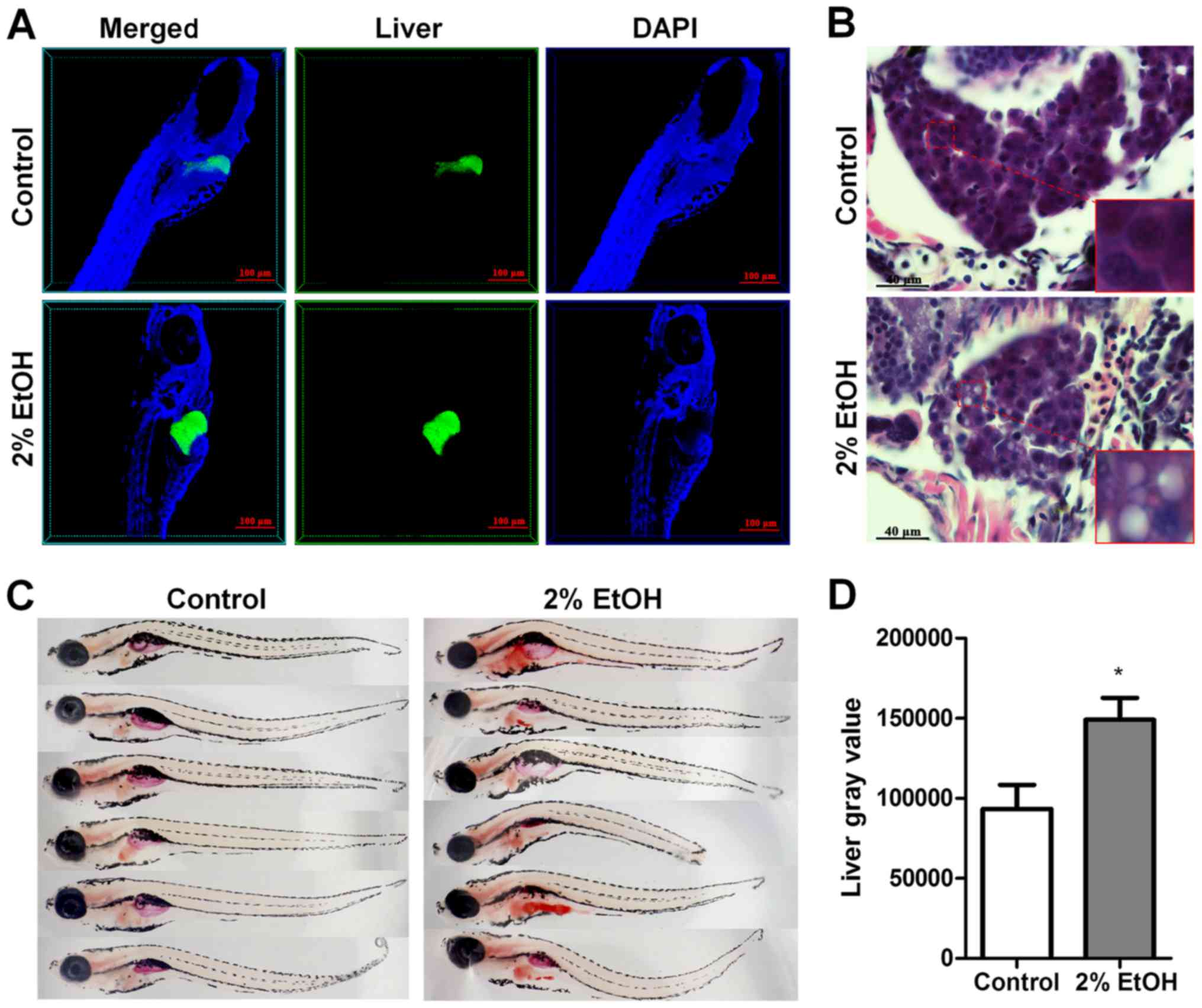
Previous studies indicate that ethanol causes
distinct morphological phenotypes, hepatomegaly and behavioral
abnormalities in nearly all larvae after 32 h of exposure to 350
mmol/l ethanol (12,15). In order to confirm the hepatic
morphological phenotypes, we tracked individual 4 dpf
Tg(lfabp10α-eGFP) larvae exposed to 350 mmol/l ethanol over
32 h and verified these findings in clear phase. Hepatomegaly and
lordosis were observed in nearly all larvae after 32 h of ethanol
exposure (Fig. 1A and C).
After the larvae were exposed to 350 mmol/l ethanol
for 32 h, serious lipid accumulation was observed in the liver as
detected by H&E staining of the paraffin-embedded sections
(Fig. 1B) and Oil Red O staining of
whole larvae (Fig. 1C). The
quantification of positive Oil Red O staining in the liver was
performed using ImageJ software (Fig.
1D), which further confirmed that 32 h of exposure to 350
mmol/l ethanol successfully induced hepatic steatosis in zebrafish
larvae.
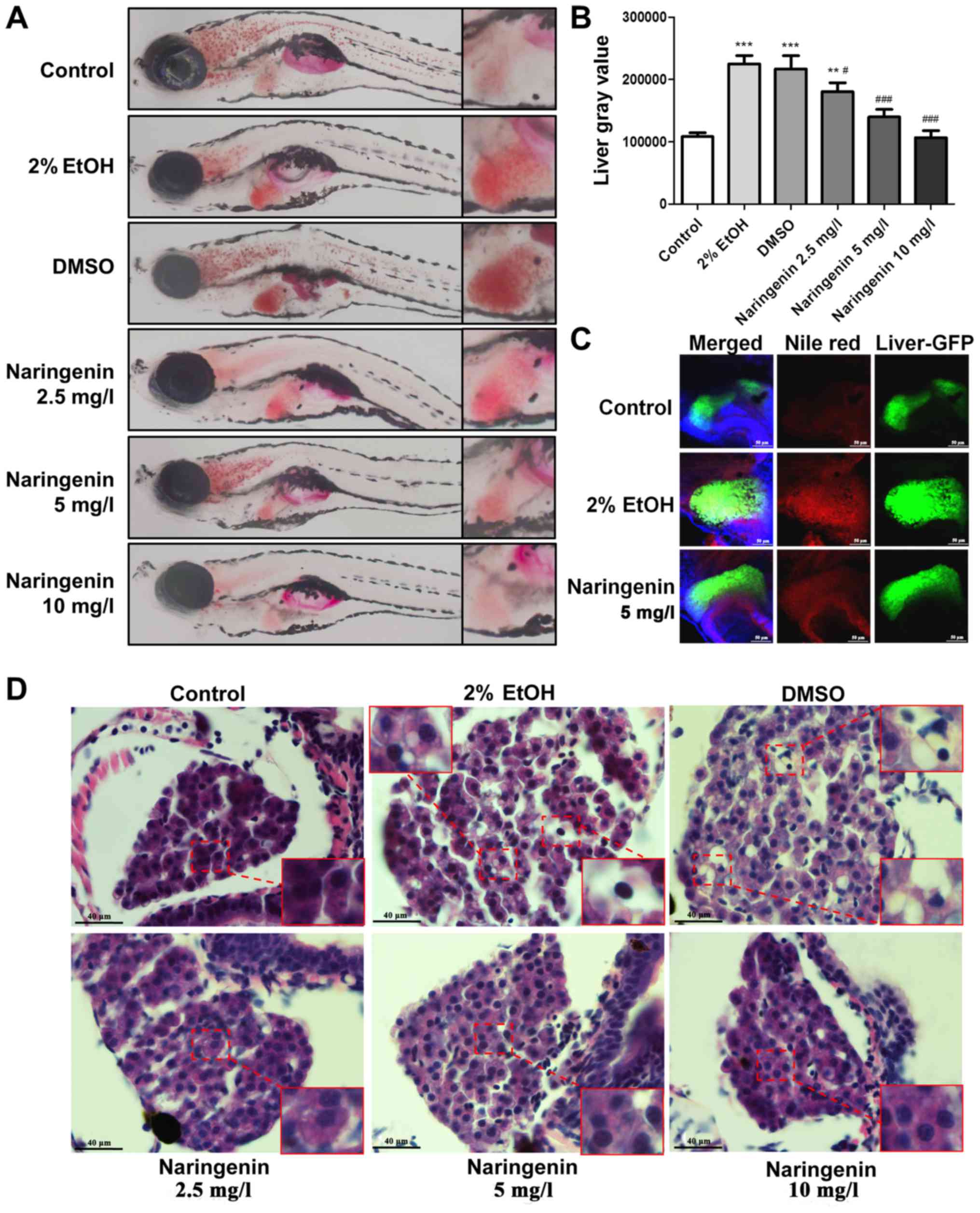
Naringenin reduces alcohol-induced
liver steatosis in zebrafish larvae
Although massive lipid deposition was visible in the
livers of larvae after ethanol exposure as detected by whole body
Oil Red O staining, notably, the obvious hepatic steatosis induced
by ethanol exposure was significant dose-dependently reduced by
naringenin (Fig. 2A). Based on the
Oil Red O staining results, hepatic steatosis was quantified in
terms of gray value using ImageJ software. The gray value analysis
further indicated that naringenin significantly downregulated
hepatic steatosis with a dose-dependent change and that 5 mg/l and
10 mg/l dosages almost reversed the alcoholic lipid accumulation in
larvae (Fig. 2B). In contrast, the
selective fluorescent staining (Nile Red) for intracellular lipid
droplets was used to detect the hepatic response to ethanol
exposure with or without naringenin treatment in
Tg(lfabp10α-eGFP) larvae. Consistent with the Oil Red O
staining results, naringenin treatment (5 mg/l, 48 h) significantly
reduced the alcohol-induced lipid droplet accumulation in the
livers of exposed larvae (Fig. 2C).
Furthermore, the hepatic pathologic change was confirmed by H&E
staining of paraffin-embedded larvae sections (Fig. 2D).
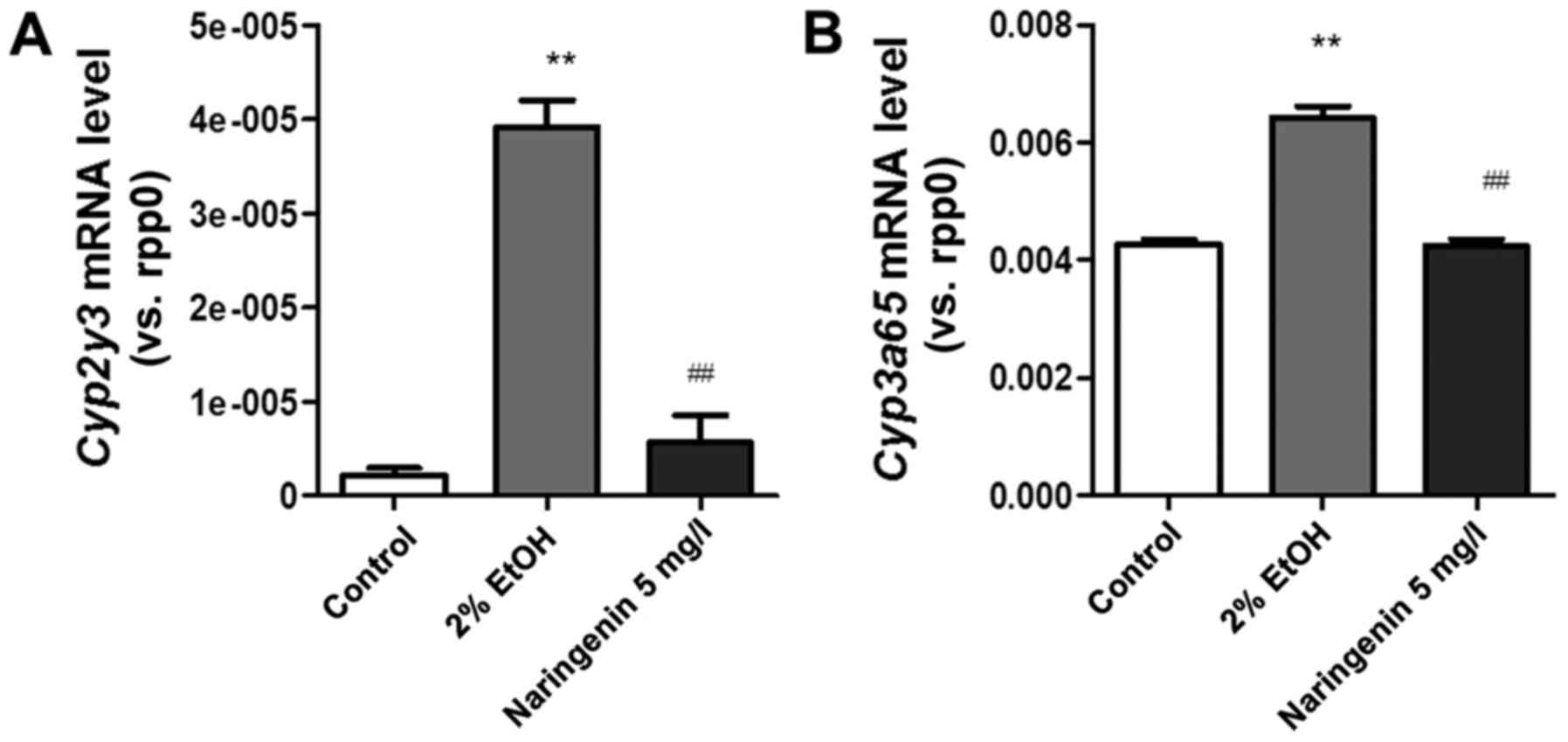
Naringenin treatment improves alcohol
metabolism in zebrafish larvae
Cytochrome P450 family 2 subfamily E member 1
(cyp2e1), the major enzyme that generates oxidative stress
by mediating ethanol metabolism, is proposed to play a major role
in ALD pathology in mammals. Cytochrome P450, family 2, subfamily
Y, polypeptide 3 (cyp2y3) and cyp2e1 are homologous
genes that are critical for ethanol metabolism in the zebrafish
liver (14). Upregulation of
cyp2y3 accelerates the speed of alcohol metabolism and
accumulation of acetaldehyde, which increase the quantity of liver
damage (14). Our data indicated
that the mRNA level of cyp2y3 was significantly elevated in
zebrafish larvae after alcohol exposure compared to the control
group, while naringenin treatment reversed this effect on the
cyp2y3 mRNA level (Fig. 3A).
Similar to cytochrome P450, family 3, subfamily A, polypeptide 65
(cyp3a65) (Fig. 3B), the
ortholog of cytochrome P450, family 3, subfamily A (cyp3a)
is also mainly expressed in the liver and plays a key role in the
metabolism of endogenous substrates and xenobiotics (16). The beneficial effects of naringenin
treatment may be due to the improvement of alcohol metabolism and
reduction of toxic substances.
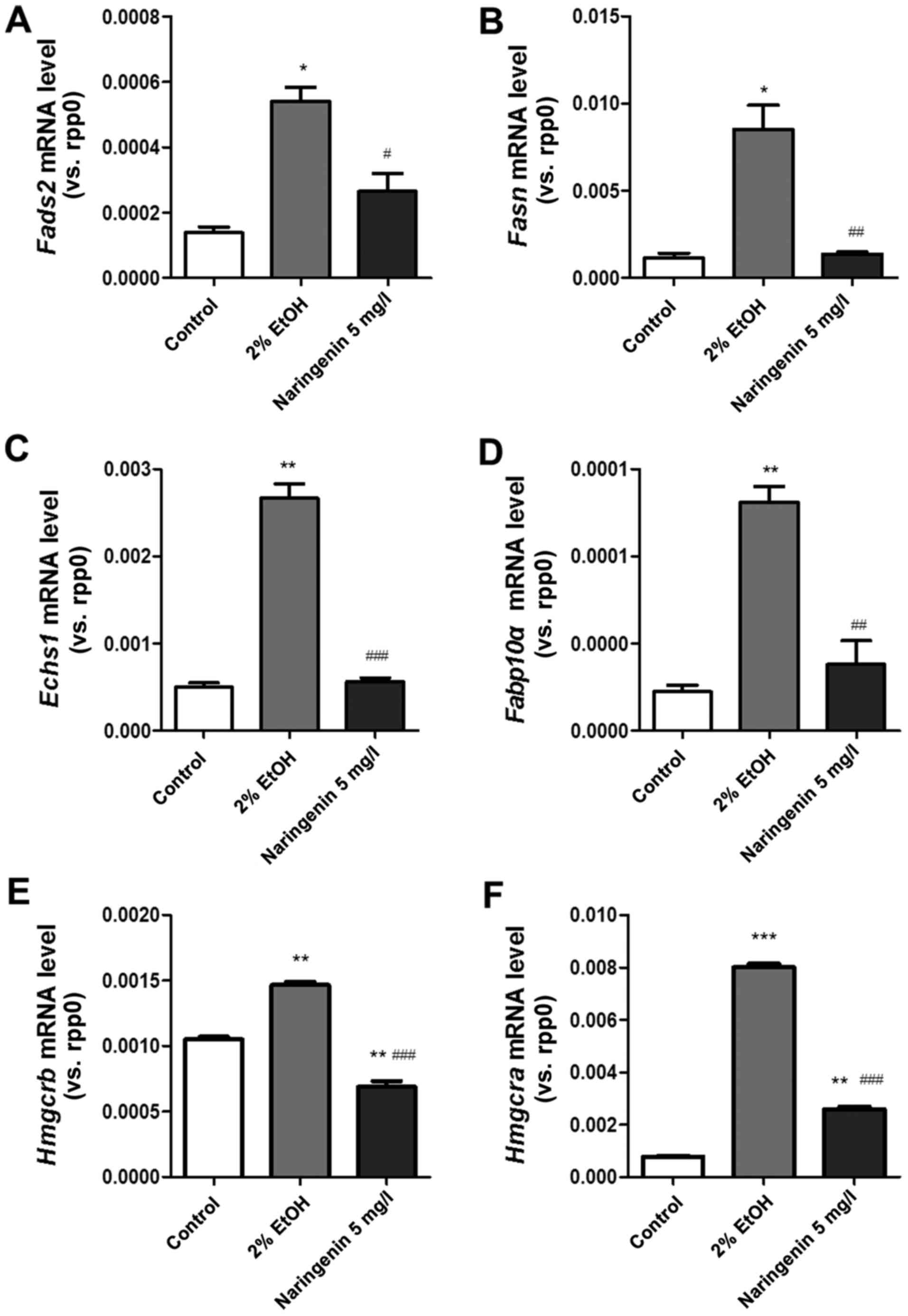
Naringenin treatment improves lipid
metabolism and protects zebrafish larvae against alcoholic
injury
We next evaluated whether naringenin could attenuate
lipid metabolism, improve lipid homeostasis and prevent
alcohol-induced hepatic steatosis. The key genes (fads2,
fasn, echs1, fabp10α, hmgcra and
hmgcrb) involved in lipid metabolism were chosen; these
genes are related to cholesterol biosynthesis, fatty acid synthase
and desaturase, and mitochondrial enzyme (17–21).
Quantitative PCR results (Fig.
4A-F) indicated that alcoholic treatment induced significant
upregulation of zebrafish larva mRNA (fads2, fasn,
echs1, fabp10α, hmgcra and hmgcrb),
which was reversed by naringenin treatment.
Naringenin attenuates endoplasmic
reticulum stress and DNA damage in zebrafish larvae with alcoholic
injury
Endoplasmic reticulum stress and DNA damage play
critical roles in the pathogenesis of alcoholic liver injury
(14,22). We examined the anti-apoptotic
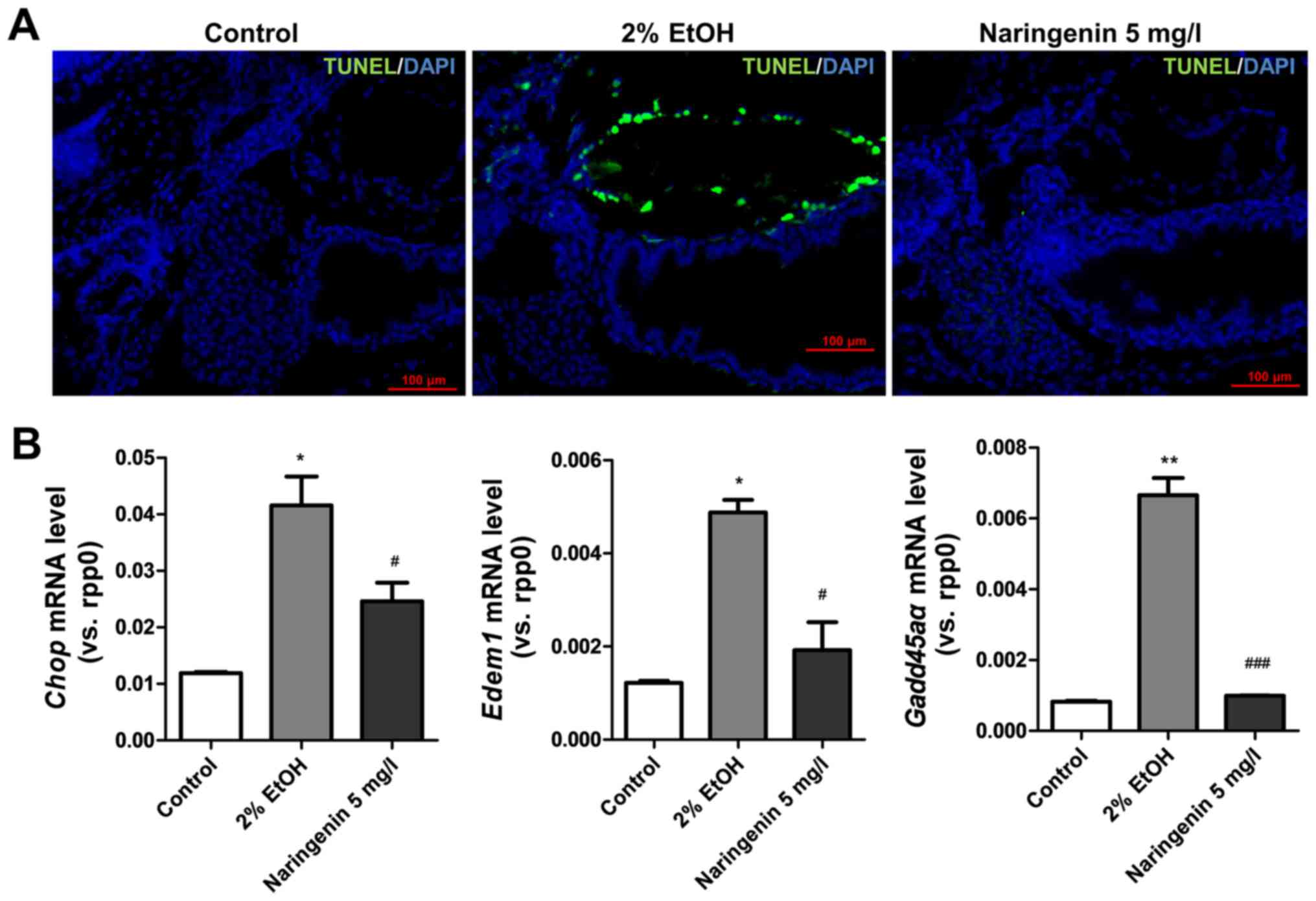
effects of naringenin on alcohol-induced liver steatosis by TUNEL
staining and compared the results with mRNA levels of DNA-damage
inducible transcript 3 (chop) (26) and growth arrest and DNA
damage-inducible, α, a (gadd45αa) (24), which are critical biomarkers of
endoplasmic reticulum stress and DNA damage. TUNEL staining
indicated that naringenin significantly reduced hepatocyte
apoptosis in zebrafish larvae with alcoholic injury (Fig. 5A). The detection of chop and
gadd45αa mRNA levels also indicated that naringenin reversed
the significantly increased mRNA levels of chop and
gadd45αa after alcohol treatment (Fig. 5B). Thus, naringenin inhibited
endoplasmic reticulum stress and DNA damage and reduced
ethanol-induced cell apoptosis in zebrafish larvae.
Discussion
Hepatic steatosis is the first response to alcohol
abuse (2) and may progress to more
severe liver diseases. Chronic steatosis is a prerequisite for
developing steatohepatitis and cirrhosis and makes hepatocytes
susceptible to damage (25).
Therefore, inhibiting hepatic lipid accumulation during alcohol
exposure may stop further liver damage. Naringenin acts in a manner
similar to metformin to reduce hepatic glucose production in
hepatocytes (7). In addition,
naringenin was found to improve various aspects of lipid
homeostasis and to mitigate adipose tissue inflammation in
vivo (6,7,26–28).
However, the effects of naringenin on alcoholic and metabolic
abnormalities have not been studied. The present study is the first
to use alcohol-exposed zebrafish to examine the effects of
naringenin on ethanol metabolism and major aspects of ALD
pathology, including steatosis, ER stress and apoptosis, in
zebrafish larvae. In the present study, we confirmed and expanded
on previous findings (12,15) that acute exposure of zebrafish
larvae to 350 mmol/l ethanol was suitable for establishing an
alcoholic fatty liver zebrafish model. Furthermore, we demonstrated
that naringenin treatment could prevent acute alcohol-induced
hepatic steatosis, dyslipidemia, and cell death. Naringenin
markedly attenuated alcohol and lipid metabolism and hepatic lipid
accumulation, collectively resulting in the attenuation of
steatosis.
The alcoholic fatty liver zebrafish larvae model is
easily and quickly generated. Obtaining liver tissue and blood from
zebrafish larvae is difficult and prevents the detection of mRNA
and protein expressed in the liver and the measurement of
biochemical parameters related to liver function; however,
zebrafish larvae have other advantages, including their short
breeding cycle and transparent soma. We can obtain vast larvae in a
short time, and it is easy to observe whole body staining of
larvae.
In the present study, we first detected the
anti-steatosis effect of naringenin against ethanol exposure in
zebrafish larvae. Both Oil Red O staining and H&E pathological
staining results showed that naringenin reduced alcoholic liver
steatosis in zebrafish larvae and that the therapeutic effects had
a dose-dependent tendency with the minimum and optimum naringenin
concentration (5 mg/l). After we confirmed the anti-steatosis
effect of naringenin, we examined the potential role of naringenin
in alcohol-induced cell death and injury. TUNEL staining showed
that apoptosis may contribute to alcoholic liver injury.
chop and gadd45αa are growth arrest and DNA damage
genes, respectively. chop regulates the transcription of
lipid metabolism genes (29), and
upregulation of the chop protein causes lipid metabolism disorders
in the liver (23). chop is
also known as a specific transcription factor of endoplasmic
reticulum stress. In contrast, ER degradation-enhancing
α-mannosidase-like protein 1 (edem1), a key gene involved in
the unfolded protein response, was increased significantly in
patients with steatosis (30).
Increased mRNA expressions of chop, gadd45αa and
edem1 after ethanol exposure indicated that endoplasmic
reticulum stress and DNA damage were more serious. In contrast,
less apoptosis and downregulation of chop, gadd45αa
and edem1 mRNA levels were induced by naringenin treatment.
Therefore, we confirmed that naringenin could suppress
alcohol-induced steatosis and damage in zebrafish larvae.
HMG-CoA reductases, including HMG coenzyme A
reductase a (hmgcra) and HMG coenzyme A reductase b
(hmgcrb), are key enzymes in lipid metabolism and mainly
regulate cholesterol biosynthesis genes (17,31).
Fatty acid synthase (fasn) regulates the synthesis and
desaturation of fatty acids (19).
Fatty acid desaturase 2 (fads2) is a dyslipidemia-related
gene that mainly metabolizes unsaturated fatty acids, is involved
in glucose metabolism and influences the concentrations of total
cholesterol, low-density lipoprotein cholesterol, high-density
lipoprotein cholesterol and triglycerides (18). Fatty acid-binding protein 10a
(fabp10α) is an intracellular fatty acid-binding protein
that is involved in intracellular lipid metabolism and fatty acid
transport (21). Enoyl-CoA
hydratase, short chain 1 (echs1), a gene that encodes a
mitochondrial enzyme, is related to the degradation of amino acids
and essential fatty acids, and echs1 mutations cause severe
metabolic disorders (20). The
fads2, fasn, echs1, fabp10α,
hmgcra and hmgcrb genes, which regulate lipid
metabolism, were significantly upregulated by ethanol exposure,
thus indicating that alcohol exposure caused disordered lipid
metabolism in larvae. Naringenin treatment significantly improved
lipid metabolism by reducing fads2, fasn,
echs1, fabp10α, hmgcra and hmgcrb mRNA
levels.
In contrast, cyp2y3 and cyp3a65, the
orthologs of cytochrome P450 CYP2 (cyp2) and cyp3a,
are required for ethanol metabolism mainly in zebrafish liver. In
zebrafish, the closest cyp2e1 homolog is cyp2y3,
which is 43% identical to the human protein (14). Blocking cyp2 homologs
significantly reduces alcohol metabolism and oxidative stress. In
addition, cyp3a65 plays a critical role in the metabolism of
endogenous substrates and xenobiotics (16). The roles of cyp2y3 and
cyp2e1 in biological disturbances are emphasized in the
initiation and progression of fatty liver disease caused by alcohol
consumption (14). Notably, our
data indicated that naringenin interruption could significantly
downregulate the elevated mRNA levels of cyp2y3 and
cyp3a65 in zebrafish larvae, which were induced by ethanol
exposure. The beneficial effects of naringenin treatment may be due
to the improvement of alcohol metabolism and reduction of toxic
substances. Combined with the above observations, we realized that
alcoholic injury in zebrafish larvae mainly resulted from
disordered lipid and alcohol metabolism and that naringenin could
improve the inordinate metabolism to inhibit alcoholic steatosis
and injury.
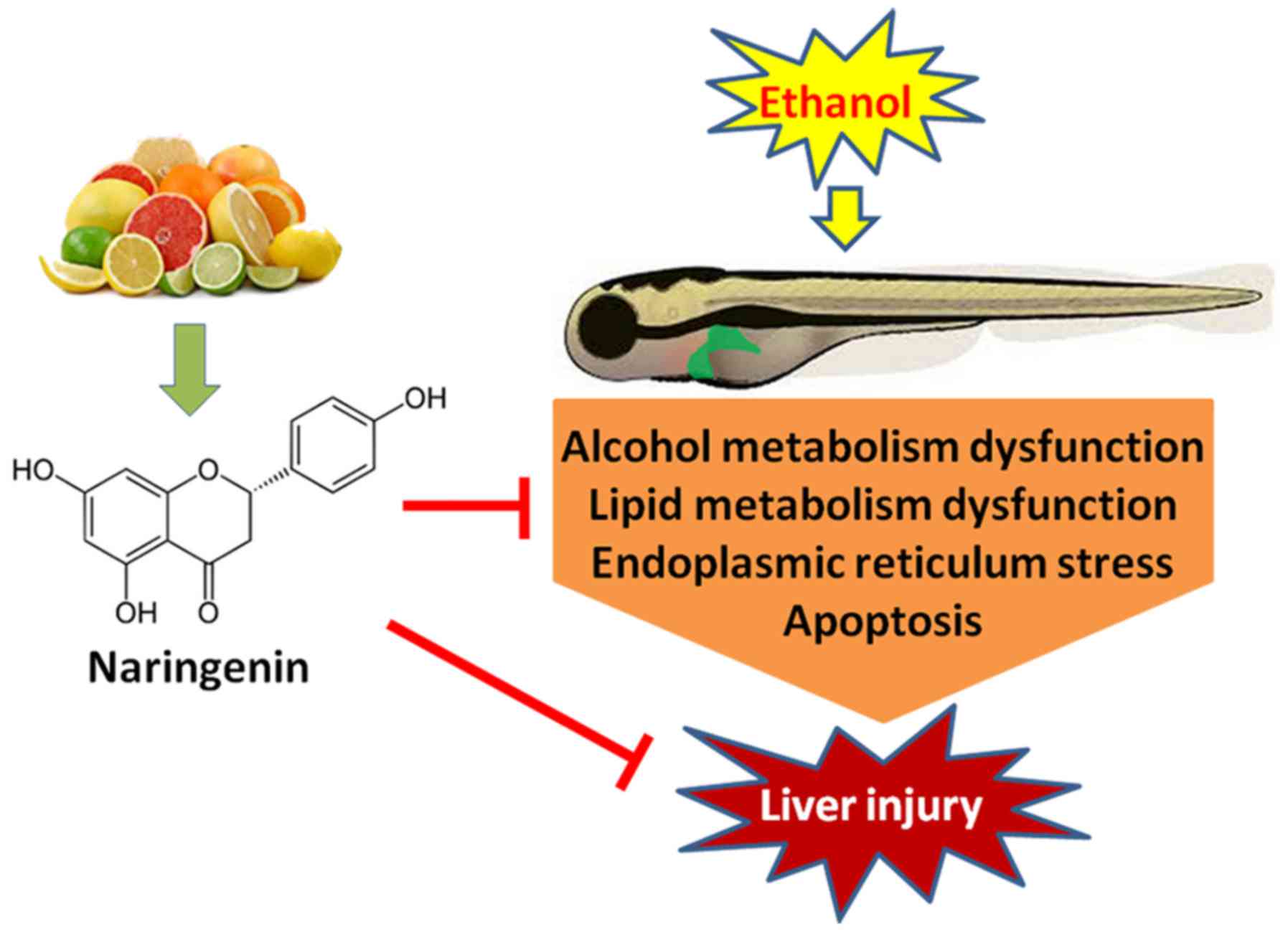
Finally, we summarize the critical roles of
naringenin in inhibiting alcohol-induced liver steatosis as showed
in a graphical illustration (Fig.
6). The present study demonstrated that naringenin inhibits
alcohol-induced liver steatosis and injury in zebrafish larvae by
reducing apoptosis and DNA damage and harmonizing alcohol and lipid
metabolism. Further experimentation is needed to illuminate the
pathway by which apoptosis is reduced and alcohol and lipid
metabolism is balanced. Naringenin readily accumulates in plasma
after ingesting orange juice, grapefruit juice and tomato paste or
sauce (5), suggesting the
bioavailability of naringenin in individuals who consume naringenin
food sources regularly. Further pre-clinical and human intervention
studies may help to determine whether naringenin can protect
against alcohol liver diseases and lipid metabolic syndrome in
humans.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (nos. 81603501 and 81302948),
the China Postdoctoral Science Foundation (2016M592508), the
Science and Technology Planning Project of Guangdong Province
(2014A020221097), the Administration of Traditional Chinese
Medicine of Guangdong Province (20162087), the Science and
Technology Planning Project of Guangzhou City (201508020014), and
the Scientific Research Initiative Program of Southern Medical
University (LX2015N003).
Glossary
Abbreviations
Abbreviations:
|
ALD
|
alcoholic liver disease
|
|
PBS
|
phosphate-buffered saline
|
|
PFA
|
paraformaldehyde
|
|
H&E
|
hematoxylin and eosin
|
|
qPCR
|
real-time quantitative PCR
|
|
hpf
|
h post-fertilization
|
|
dpf
|
days post-fertilization
|
|
rpp0
|
ribosomal protein P0
|
|
cyp2e1
|
cytochrome P450 family 2 subfamily E
member 1
|
|
cyp2y3
|
cytochrome P450, family 2, subfamily
Y, polypeptide 3
|
|
cyp3a65
|
cytochrome P450, family 3, subfamily
A, polypeptide 65
|
|
cyp3a
|
cytochrome P450, family 3, subfamily
A
|
|
chop
|
DNA-damage-inducible transcript 3
|
|
gadd45αa
|
growth arrest and DNA
damage-inducible, α, a
|
|
hmgcra
|
HMG coenzyme A reductase a
|
|
hmgcrb
|
HMG coenzyme A reductase b
|
|
fasn
|
fatty acid synthase
|
|
fads2
|
fatty acid desaturase 2
|
|
fabp10α
|
fatty acid binding protein 10a
|
|
edem1
|
ER degradation-enhancing
α-mannosidase-like protein 1
|
|
echs1
|
enoyl-CoA hydratase, short chain 1
|
References
|
1
|
Ni HM, Bhakta A, Wang S, Li Z, Manley S,
Huang H, Copple B and Ding WX: Role of hypoxia inducing factor-1β
in alcohol-induced autophagy, steatosis and liver injury in mice.
PLoS One. 9:e1158492014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Orman ES, Odena G and Bataller R:
Alcoholic liver disease: Pathogenesis, management, and novel
targets for therapy. J Gastroenterol Hepatol. 28 Suppl 1:S77–S84.
2013. View Article : Google Scholar
|
|
3
|
Akhlaghi M: Non-alcoholic fatty liver
disease: Beneficial effects of flavonoids. Phytother Res.
30:1559–1571. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moon PD, Choi IH and Kim HM: Naringenin
suppresses the production of thymic stromal lymphopoietin through
the blockade of RIP2 and caspase-1 signal cascade in mast cells.
Eur J Pharmacol. 671:128–132. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ke JY, Kliewer KL, Hamad EM, Cole RM,
Powell KA, Andridge RR, Straka SR, Yee LD and Belury MA: The
flavonoid, naringenin, decreases adipose tissue mass and attenuates
ovariectomy-associated metabolic disturbances in mice. Nutr Metab.
12:12015. View Article : Google Scholar
|
|
6
|
Mulvihill EE, Allister EM, Sutherland BG,
Telford DE, Sawyez CG, Edwards JY, Markle JM, Hegele RA and Huff
MW: Naringenin prevents dyslipidemia, apolipoprotein B
overproduction, and hyperinsulinemia in LDL receptor-null mice with
diet-induced insulin resistance. Diabetes. 58:2198–2210. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Purushotham A, Tian M and Belury MA: The
citrus fruit flavonoid naringenin suppresses hepatic glucose
production from Fao hepatoma cells. Mol Nutr Food Res. 53:300–307.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chtourou Y, Fetoui H, Jemai R, Ben Slima
A, Makni M and Gdoura R: Naringenin reduces cholesterol-induced
hepatic inflammation in rats by modulating matrix
metalloproteinases-2, 9 via inhibition of nuclear factor κB
pathway. Eur J Pharmacol. 746:96–105. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zar Kalai F, Han J, Ksouri R, El Omri A,
Abdelly C and Isoda H: Antiobesity effects of an edible halophyte
Nitraria retusa Forssk in 3T3-L1 preadipocyte differentiation and
in C57B6J/L mice fed a high fat diet-induced obesity. Evid Based
Complement Alternat Med. 2013:3686582013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Howarth DL, Yin C, Yeh K and Sadler KC:
Defining hepatic dysfunction parameters in two models of fatty
liver disease in zebrafish larvae. Zebrafish. 10:199–210. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dai W, Wang K, Zheng X, Chen X, Zhang W,
Zhang Y, Hou J and Liu L: High fat plus high cholesterol diet lead
to hepatic steatosis in zebrafish larvae: A novel model for
screening anti-hepatic steatosis drugs. Nutr Metab. 12:422015.
View Article : Google Scholar
|
|
12
|
Passeri MJ, Cinaroglu A, Gao C and Sadler
KC: Hepatic steatosis in response to acute alcohol exposure in
zebrafish requires sterol regulatory element binding protein
activation. Hepatology. 49:443–452. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Greenspan P, Mayer EP and Fowler SD: Nile
red: A selective fluorescent stain for intracellular lipid
droplets. J Cell Biol. 100:965–973. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsedensodnom O, Vacaru AM, Howarth DL, Yin
C and Sadler KC: Ethanol metabolism and oxidative stress are
required for unfolded protein response activation and steatosis in
zebrafish with alcoholic liver disease. 1213–1226. 2013.
|
|
15
|
Howarth DL, Passeri M and Sadler KC:
Drinks like a fish: Using zebrafish to understand alcoholic liver
disease. Alcohol Clin Exp Res. 35:826–829. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tseng H, Hseu T, Buhler D, Wang W and Hu
C: Constitutive and xenobiotics-induced expression of a novel CYP3A
gene from zebrafish larva. Toxicol Appl Pharmacol. 205:247–258.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Suganya S, Nandagopal B and Anbarasu A:
Natural inhibitors of HMG CoA reductase - an in silico approach
through molecular docking and simulation studies. J Cell Biochem.
99:1–6. 2016.
|
|
18
|
Tian Y, Zhang W, Zhao S, Sun Y, Bian Y,
Chen T, Du Y, Zhang J, Wang Z, Huang T, et al: FADS1-FADS2 gene
cluster confers risk to polycystic ovary syndrome. Sci Rep.
6:211952016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zappaterra M, Deserti M, Mazza R, Braglia
S, Zambonelli P and Davoli R: A gene and protein expression study
on four porcine genes related to intramuscular fat deposition. Meat
Sci. 121:27–32. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Olgiati S, Skorvanek M, Quadri M, Minneboo
M, Graafland J, Breedveld GJ, Bonte R, Ozgur Z, Van den Hout MC,
Schoonderwoerd K, et al: Paroxysmal exercise-induced dystonia
within the phenotypic spectrum of ECHS1 deficiency. Mov Disord.
31:1041–1048. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Storch J and Thumser AE: The fatty acid
transport function of fatty acid-binding proteins. Biochim Biophys
Acta. 1486:28–44. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ren Z, Wang X, Xu M, Yang F, Frank JA, Ke
ZJ and Luo J: Binge ethanol exposure causes endoplasmic reticulum
stress, oxidative stress and tissue injury in the pancreas.
Oncotarget. 7:54303–54316. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zheng X, Dai W, Chen X, Wang K, Zhang W,
Liu L and Hou J: Caffeine reduces hepatic lipid accumulation
through regulation of lipogenesis and ER stress in zebrafish
larvae. J Biomed Sci. 22:1052015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao XX, Zhang YB, Ni PL, Wu ZL, Yan YC
and Li YP: Protein arginine methyltransferase 6 (Prmt6) is
essential for early zebrafish development through the direct
suppression of gadd45αa stress sensor gene. J Biol Chem.
291:402–412. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Howarth DL, Lindtner C, Vacaru AM,
Sachidanandam R, Tsedensodnom O, Vasilkova T, Buettner C and Sadler
KC: Activating transcription factor 6 is necessary and sufficient
for alcoholic fatty liver disease in zebrafish. PLoS Genet.
10:e10043352014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cho KW, Kim YO, Andrade JE, Burgess JR and
Kim YC: Dietary naringenin increases hepatic peroxisome
proliferators-activated receptor α protein expression and decreases
plasma triglyceride and adiposity in rats. Eur J Nutr. 50:81–88.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Goldwasser J, Cohen PY, Yang E, Balaguer
P, Yarmush ML and Nahmias Y: Transcriptional regulation of human
and rat hepatic lipid metabolism by the grapefruit flavonoid
naringenin: Role of PPARalpha, PPARgamma and LXRalpha. PLoS One.
5:e123992010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Alam MA, Subhan N, Rahman MM, Uddin SJ,
Reza HM and Sarker SD: Effect of citrus flavonoids, naringin and
naringenin, on metabolic syndrome and their mechanisms of action.
Adv Nutr. 5:404–417. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rahman K, Liu Y, Kumar P, Smith T, Thorn
NE, Farris AB and Anania FA: C/EBP homologous protein modulates
liraglutide-mediated attenuation of non-alcoholic steatohepatitis.
Lab Invest. 96:895–908. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Congiu M, Ryan MC and Desmond PV: No
increase in the expression of key unfolded protein response genes
in HCV genotype 3 patients with severe steatosis. Virus Res.
160:420–423. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cocci P, Mosconi G and Palermo FA: Partial
cloning, tissue distribution and effects of epigallocatechin
gallate on hepatic 3-hydroxy-3-methylglutaryl-CoA reductase mRNA
transcripts in goldfish (Carassius auratus). Gene. 545:220–225.
2014. View Article : Google Scholar : PubMed/NCBI
|