Introduction
Nasopharyngeal carcinoma (NPC) is the most prevalent
head and neck cancer in south China and east Asia, with the highest
annual incidence of more than 20 per 100,000 reported among the
Cantonese population (1,2). Unfortunately, most patients are
diagnosed at the advanced stage (III–IVb) during their first visit.
This is because the primary tumor is commonly located in a silent
area, and NPC exhibits high rates of local invasion and distant
metastasis (3,4). Despite using chemotherapy combined
with intensity-modulated radiotherapy, the outcome for advanced
stage patients remains unsatisfactory (5,6). Local
recurrence and distant metastasis are two major causes of treatment
failure. Therefore, novel biomarkers for early diagnosis and new
therapeutic targets are in high demand to aid in the treatment of
NPC patients (7,8).
NPC is etiologically associated with genetic and
environmental factors, but also with complex pathological processes
including squamous metaplasia of nasal mucosa and basal cell
dysplasia. It is commonly considered that nasopharyngeal
carcinogenesis is a multi-stage and multi-channel process of which
the detailed underlying molecular mechanisms remain to be
elucidated. SETDB1, a histone H3 lysine 9 (H3K9) methyltransferase,
was first identified as a SET (Su(var), Enhancer of zeste,
Trithorax) domain-containing protein following an in silico
search (9,10). SETDB1 helps regulate the
transcriptional repression of euchromatin and plays a central role
in the early development of embryonic stem cells by repressing a
subset of genes encoding for developmental regulators (11). Moreover, SETDB1 is a
recognized oncogene in many types of cancers including melanoma
(12), lung cancer (13–15),
glioma (16), hepatocellular
carcinoma (17–20), breast (21,22),
urothelial carcinoma (23) and
prostate cancer (24). These
observations support the hypothesis that aberrant histone
methylation leads to the activation or repression of critical genes
during tumorigenesis. However, little is known concerning the role
of SETDB1 in nasopharyngeal carcinogenesis. In the present study,
we first determined the expression of SETDB1 in NPC and further
explored its potential role in NPC progression.
Materials and methods
Tissue specimens
A tissue microarray block containing 152 NPC samples
and their clinic-pathological information was purchased from
Shanghai Outdo Biotech Co., Ltd. (Shanghai, China).
Paraffin-embedded nasopharynx mucosal tissues, between January 2006
and December 2008, were collected for control study from the
Department of Pathology at the Affiliated Hospital of Guangdong
Medical University. None of the patients received radiotherapy or
chemotherapy prior to biopsy. Informed consent was obtained in all
cases, and protocols were approved by the Institutional Ethics
Committee of the Affiliated Hospital of Guanddong Medical
University.
Immunohistochemical staining
Using the EnVision Detection System,
immunohistochemical staining assays were performed on tissue slides
according to the manufacturer's protocol (Dako). In brief, slides
were incubated with a SETDB1 primary antibody (1:100 dilution; cat.
no. HPA018142; Sigma-Aldrich, St. Louis, MO, USA) overnight at 4°C
and washed with phosphate-buffered saline (PBS). Next, the slides
were treated with horseradish peroxidase-conjugated secondary
antibodies (ChemMate™ EnVision+ HRP; cat. no. GK500705; Gene Tech
Co., Shanghai, China) before staining with diaminobenzidine and
counterstaining with hematoxylin. Negative controls were routinely
performed. All of the staining was evaluated and scored by two
independent pathologists. The score was a sum of the percentage of
positive-stained cells (0, 0%; 1, 1–10%; 2, 11–50%; 3, 56–75%; and
4, 76–100%) and the mean intensity of the staining (0, no staining;
1, weak staining; 2, moderate staining; and 3, strong staining). A
score of ≤4 was considered as weak or negative SETDB1 protein
expression, whereas a higher score was considered as
overexpression.
Cell lines
The human NPC cell lines HONE1, SUNE1, CNE1, CNE2,
5–8F and 6–10B were obtained from the Cancer Institute, Southern
Medical University (Guangzhou, China). These cell lines were
maintained in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% heat-inactivated
fetal bovine serum (FBS) and 100 U ml−1 of penicillin
and streptomycin (Gibco; Thermo Fisher Scientific, Inc.) at 37°C in
a humidified atmosphere containing 5% CO2. NP69
(immortalized normal human nasopharyngeal epithelial cell line) was
purchased from the Department of Physiology, Jinan University
(Guangzhou, China), and grown in defined keratinocyte-serum-free
medium supplemented with epidermal growth factor (EGF; Invitrogen;
Thermo Fisher Scientific, Inc.).
RT-qPCR
Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). Reverse transcription
was performed with 1 µg gDNA-Eraser-treated total RNA template,
using Oligo dT and reverse transcriptase (Takara Bio, Inc., Otsu,
Japan), according to the supplier's instructions. Quantitative PCR
was performed with SYBR Premix Ex Taq (Takara Bio) using an Applied
Biosystems 7500 Fast Real-Time PCR System (Life Technologies;
Thermo Fisher Scientific, Inc.). PCR primers included: SETDB1 sense
(5′-AAGACGTACTCAGGCCATGTCC-3′) and SETDB1 antisense
(5′-GACCCAAATGTCGCAGTCAG-3′); β-actin sense
(5′-TAAGAAGCTGCTGTGCTACG-3′) and β-actin antisense
(5′-GACTCGTCATACTCCTGCTT-3′). SETDB1 was amplified by 35 cycles at
94°C for 15 sec, 58°C for 30 sec, and 68°C for 2 min in order,
which was followed by a 10-min extension at 68°C. The ΔΔ method was
used to determine the relative levels of mRNA expression between
the experimental samples and controls as previously described
(25). The fold change in gene
expression was calculated as 2−ΔΔCq. PCR products were
electrophoresed on 1.2% agarose gel containing ethidium bromide and
visualized by UV-induced fluorescence.
Western blotting
Cell pellets were lysed on ice in RIPA buffer (1X
PBS, 1% NP-40, 0.1% SDS, 5 mM EDTA, 0.5% sodium deoxycholate and 1
mM sodium orthovanadate) with protease inhibitors. Protein extracts
were quantified using a bicinchoninic acid (BCA) protein assay
(Nanjing KeyGen Biotech, Co., Ltd., Nanjing, China), separated by
6% SDS-PAGE and analyzed by immunoblotting using primary antibodies
specific for human SETDB1 (1:800 dilution; cat. no. ab107225;
Abcam, Cambridge, UK), human β-actin (1:1,000 dilution; cat. no.
4970; Cell Signaling Technology, Inc., Danvers, MA, USA) and
affinity-purified goat anti-rabbit/mouse secondary antibody
(1:2,000 dilution; cat. nos. 7074 and 7076; Cell Signaling
Technology, Inc.).
Stable transfection regulates SETDB1
expression
The SETDB1 full coding sequence was cloned
into the pGLV5 lentiviral expression vector. Verified SETDB1
targeting short hairpin RNA (shRNA) sequences
(GCATGCGAATTCTGGGCAAGA) were cloned into the pGLV3 vectors.
Non-target control shRNA (TTCTCCGAACGTGTCACGTTTC) was included as a
negative control. The regenerated plasmid and its negative control
were respectively transfected into 293FT cells using Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturer's instructions. The viral supernatants were harvested,
filtered, and the virus titer was determined. Lentiviruses that
contained the SETDB1 shRNA and the negative control vector
were stably infected into the NPC cell line 5-8F, while those
containing the exogenous SETDB1 coding sequence were
infected into the CNE1 cell line. Flow cytometry assays were used
to sort stable integrants. Both RT-PCR and western blot analysis
confirmed SETDB1 expression.
Cell viability assay
The target cells were seeded in 96-well plates at a
density of 100 cells/well in 0.1 ml media. Following an overnight
incubation, 10 ml of Cell Counting Kit 8 (CCK-8; Dojundo
Laboratories, Kumamoto, Japan) was mixed with 100 ml of RPMI-1640
(Gibco; Thermo Fisher Scientific, Inc.) and added to each well. The
mixture was incubated at 37°C for 1.5 h before measuring the
absorbance at 450 nm over the next 96 h. All experiments were
performed in triplicate.
Cell cycle analysis
The cells cycle was analyzed as previously described
(26).
Transwell assays
Cell migration and invasion ability was assessed in
24-well Transwell inserts (8.0-µm pore size; Corning Inc., Corning,
NY, USA). For the migration assay, 200 µl of serum-free medium
containing 1×105 target cells was added to the top
chambers, while the lower chambers were filled with 500 µl complete
RPMI-1640 medium containing 10% FBS. Minor changes were implemented
for the cell invasion assay; the Transwell inserts were pre-coated
with diluted Matrigel (Sigma-Aldrich; Merck KGaA) and
3×105 cells were added to the top chambers. After 18–24
h of incubation, the cells that had migrated or invaded to the
undersurface of the membrane were fixed in 100% methanol and
stained with 0.1% crystal violet. Cells in five random fields were
visualized and counted under a microscope (Olympus CX41; Olympus
Corp., Tokyo, Japan).
Statistical analysis
SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA)
was used for the statistical analysis. Survival curves were plotted
following the Kaplan-Meier method and compared using the log-rank
test. The significance of various variables for survival was
analyzed by the Cox proportional hazards model (Enter model). The
significance of SETDB1 expression and the clinic pathological
characteristics was evaluated by the χ2 test.
Differences between groups were analyzed by one-way analysis of
variance with Tukey's post hoc test. Multiple-factors repetitive
measurement was performed by variance analysis. P<0.05 was
considered to indicate a statistically significant result.
Results
SETDB1 is frequently amplified in
human NPC
To explore the correlation of SETDB1 expression with
human NPC, we detected SETDB1 protein expression in 152
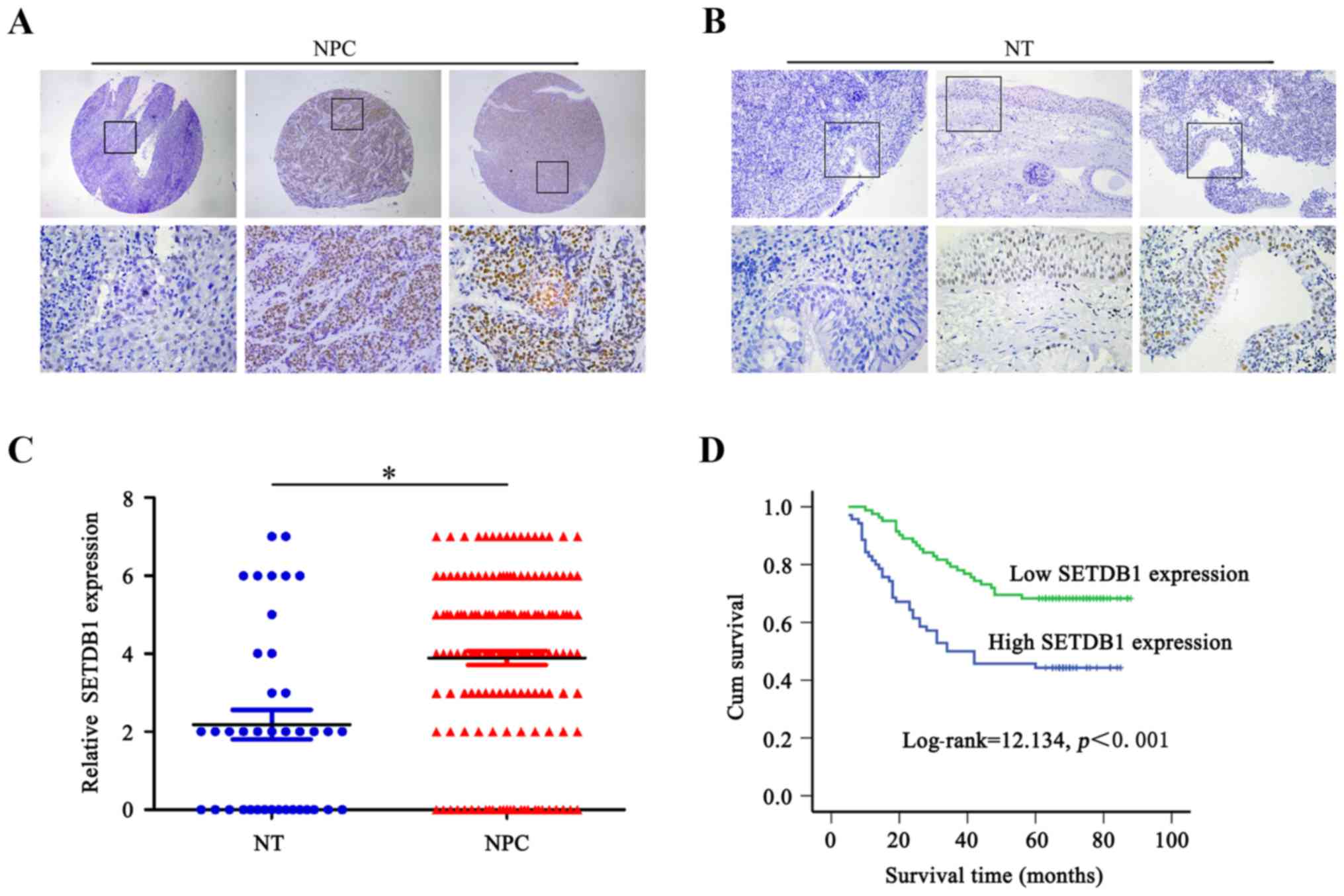
paraffin-embedded samples. As shown in Fig. 1A, SETDB1 showed positive nuclear
staining in NPC. One hundred and twenty-six out of 152 (82.9%)
patients exhibited SETDB1 amplification. Overexpression of SETDB1
protein was detected in 70 of the 126 (55.6%) NPC tissues, as
compared to only 8 of 39 (20.51%) nasopharynx mucosal tissues
(Fig. 1B and C). The overexpression
of SETDB1 protein was significantly different between NPC and
non-tumor samples (χ2=5.177, P=0.023).
Overexpression of SETDB1 was
indicative of poor survival for NPC patients
To investigate the prognostic value of SETDB1 for
NPC, we compared the overall survival of patients with high and low
SETDB1 protein expression. Kaplan-Meier analysis indicated that the
median survival of high SETDB1-expressing patients was 49.1 and
69.6 months for low SETDB1-expressing patients. Overexpression of
SETDB1 was significantly correlated with a poorer 5-year survival
rate of NPC patients (P=0.001, hazard ratio=0.442). Kaplan-Meier
survival curves were also significantly different for the low and
high SETDB1 expression patients (P<0.001, log-rank test)
(Fig. 1D).
Correlation between SETDB1 expression
and clinicopathological parameters
To determine whether SETDB1 expression could act as
an independent prognostic factor for NPC, univariate and
multivariate analyses were applied. Results indicated that SETDB1
protein expression was significantly related to overall survival
and was an independent prognostic factor in NPC patients (Table II). The relationship between the
SETDB1 expression and the clinicopathological characteristics was
also examined. As summarized in Table
I, no significant correlations were found between SETDB1
expression and any of the assessed clincopathological variables,
with the exception of N classification (P=0.035,
χ2=8.623).
 | Table II.Summary of overall survival analysis
by univariate and multivariate COX regression analyses. |
Table II.
Summary of overall survival analysis
by univariate and multivariate COX regression analyses.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Parameters | P-value | HR | 95% CI | P-value | HR | 95% CI |
|---|
| Age (years) |
|
|
|
|
|
|
| ≥50 vs.
<50 | 0.083 | 1.539 | 0.945–2.507 |
|
|
|
| Sex |
|
|
|
|
|
|
| Male
vs. female | 0.006 | 0.376 | 0.186–0.760 | 0.070 | 1.946 | 0.947–4.001 |
| T
classification |
|
|
|
|
|
|
| T0-2
vs. T3-4 | 0.006 | 0.489 | 0.294–0.814 | 0.221 | 0.640 | 0.314–1.308 |
| N
classification |
|
|
|
|
|
|
| N0 vs.
N1-3 | 0.037 | 0.532 | 0.294–0.961 | 0.175 | 1.531 | 0.828–2.833 |
| M
classification |
|
|
|
|
|
|
| N0 vs.
M1 | 0.000 | 0.116 | 0.045–0.302 | 0.001 | 5.606 | 2.086–15.064 |
| Clinical stage |
|
|
|
|
|
|
| I–II
vs. III–IV | 0.000 | 0.301 | 0.166–0.544 | 0.002 | 3.900 | 1.676–9.077 |
| SETDB1
expression |
|
|
|
|
|
|
| High
vs. low | 0.001 | 0.442 | 0.269–0.727 | 0.001 | 0.438 | 0.264–0.728 |
 | Table I.Correlation between the
clinicopathological variables and the expression of SETDB1
protein. |
Table I.
Correlation between the
clinicopathological variables and the expression of SETDB1
protein.
|
|
| SETHB1
expression |
|
|
|---|
|
|
|
|
|
|
|---|
|
Characteristics | n | Low | High | χ2 | P-value |
|---|
| All cases | 152 | 82 | 70 |
|
|
| Sex |
|
|
| 0.046 | 0.831 |
|
Male | 112 | 61 | 51 |
|
|
|
Female | 40 | 21 | 19 |
|
|
| Age (years) |
|
|
| 0.062 | 0.803 |
|
<50 | 82 | 45 | 37 |
|
|
|
≥50 | 70 | 37 | 33 |
|
|
| T
classification |
|
|
| 5.490 | 0.139 |
|
T0-1 | 12 | 9 | 3 |
|
|
| T2 | 63 | 32 | 31 |
|
|
| T3 | 36 | 23 | 13 |
|
|
| T4 | 41 | 18 | 23 |
|
|
| N
classification |
|
|
| 8.623 | 0.035 |
| N0 | 47 | 27 | 20 |
|
|
| N1 | 77 | 41 | 35 |
|
|
| N2 | 22 | 13 | 9 |
|
|
| N3 | 6 | 0 | 7 |
|
|
| Distant
metastasis |
|
|
| 2.398 | 0.121 |
|
Yes | 5 | 1 | 4 |
|
|
| No | 147 | 81 | 66 |
|
|
| Clinical stage |
|
|
| 0.033 | 0.855 |
|
I–II | 62 | 34 | 28 |
|
|
|
III–IV | 90 | 48 | 42 |
|
|
Expression of SETDB1 in NPC cell
lines
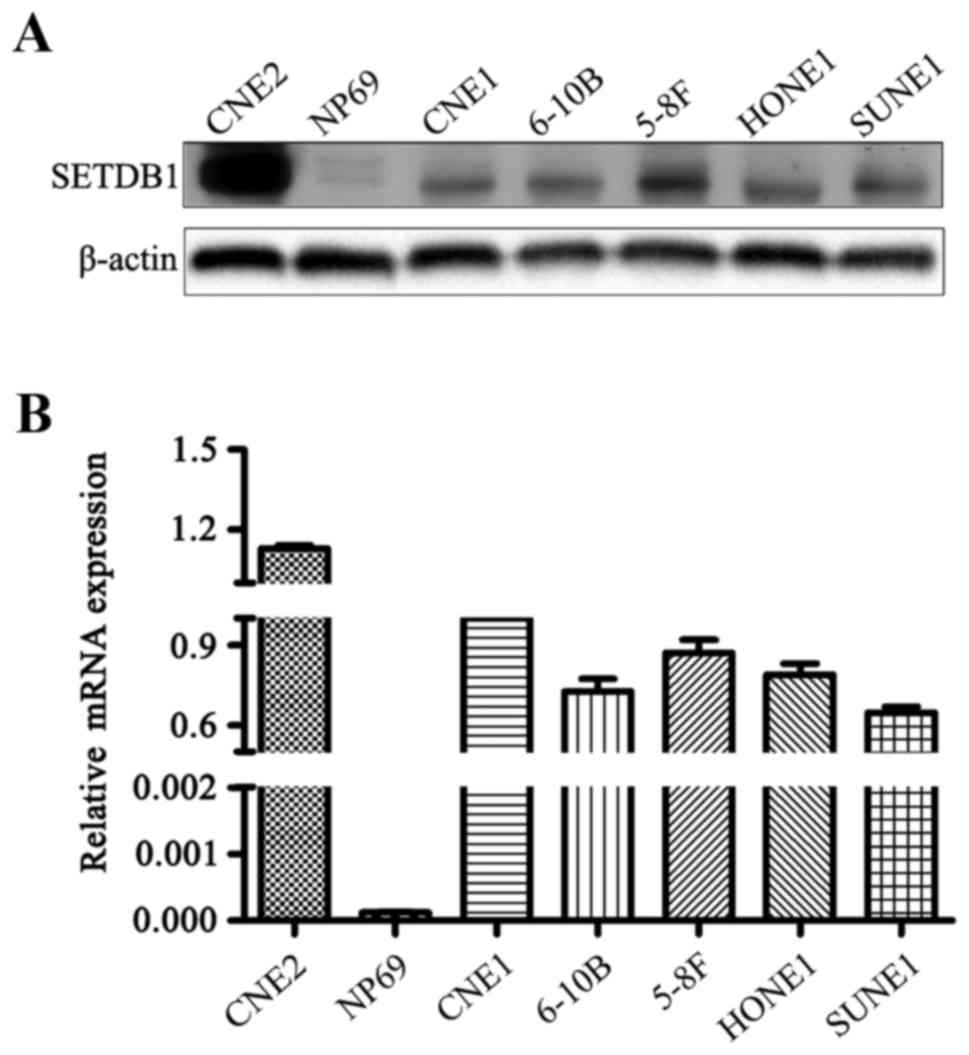
To detect the SETDB1 expression patterns in NPC cell
lines, RT-PCR and western blot analyses were performed. All six
human NPC cell lines exhibited higher SETDB1 mRNA expression levels
compared to the immortalized normal human nasopharyngeal epithelial
cell line NP69 (Fig. 2). Western
blot analysis confirmed that SETDB1 protein could be detected in
all NPC cell lines but not in NP69 cells. Taken together the data
suggest a potential role for SETDB1 in NPC tumorigenesis.
Alteration of SETDB1 expression
affects the proliferation ability of NPC cells in vitro
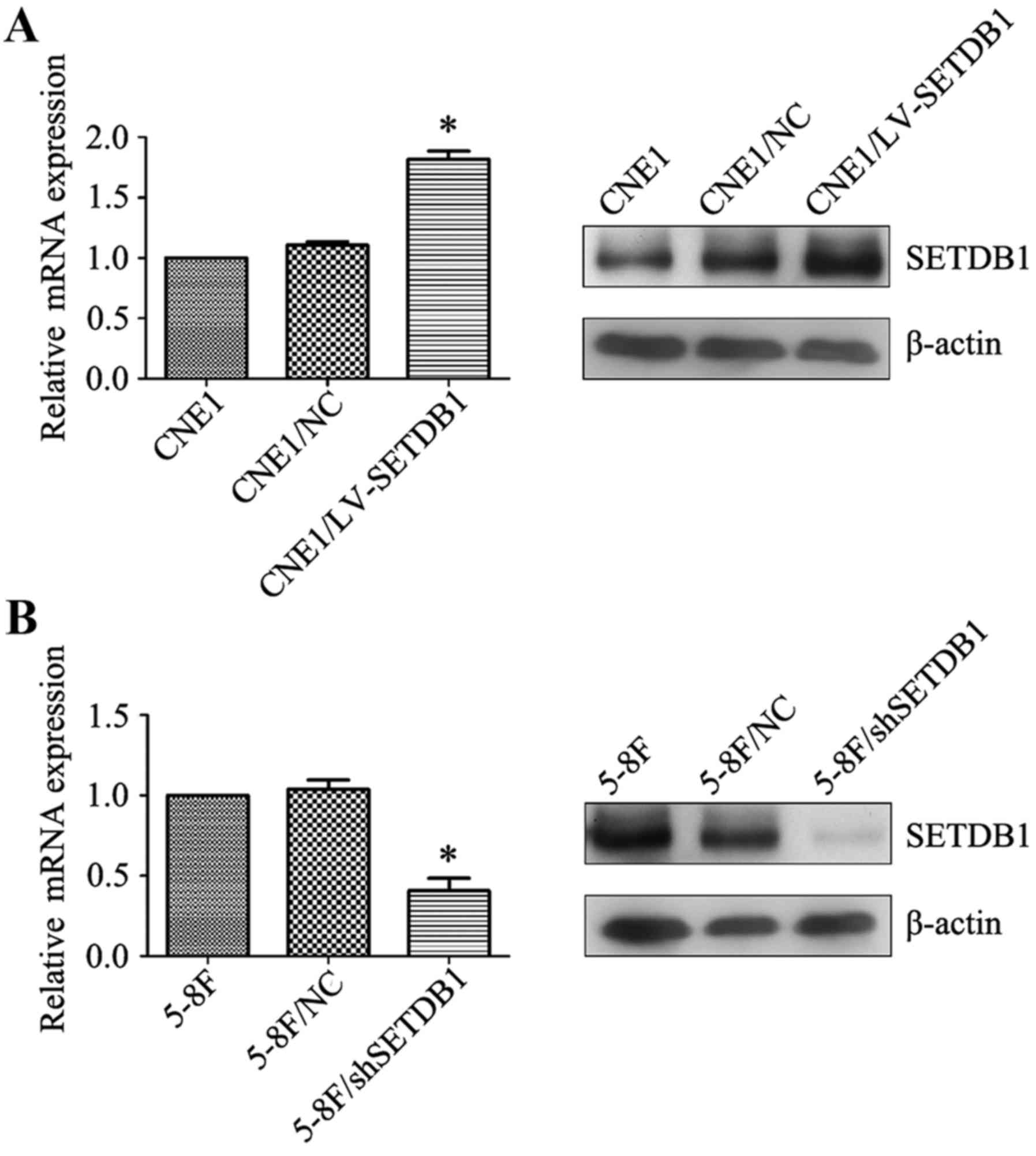
To explore the possible impact of altered
SETDB1 expression on the biological behavior of NPC, two
cell lines with either SETDB1 knockdown (5–8F/sh-SETDB1) or
overexpression (CNE1/LV-SETDB1) were constructed. Alteration of
SETDB1 mRNA and protein expression of these two stable cell lines
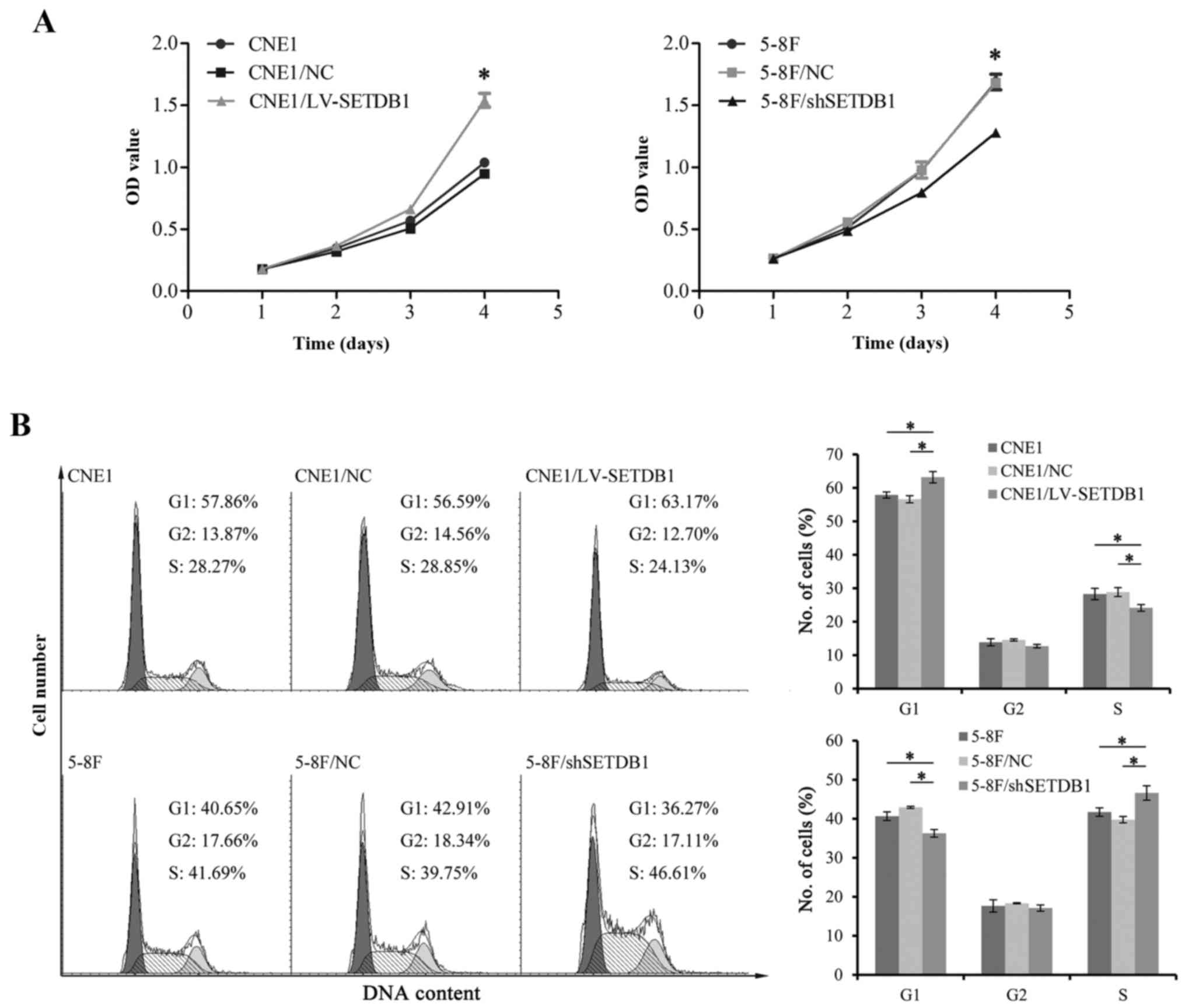
was confirmed by RT-PCR and western blot analysis (Fig. 3). Cell proliferation was analyzed
using CCK-8. Results showed that parental wild-type (WT) CNE1 cells
had a similar growth rate to negative controls over a 4 day
observation period. On day 4, the growth rate of CNE1/LV-SETDB1 had
become significantly more rapid than that of the WT and negative
control cell lines (Fig. 4A).
Consistent with these results, flow cytometric analysis showed an
increased number of cells in the G1 phase and a decreased cell
percentage in the S phase of CNE1 cells with increased
SETDB1 expression (Fig. 4B).
As expected, contrasting results were observed with the 5-8F cell
line with knocked down SETDB1 expression. Together, the data
imply that SETDB1 may play a crucial role in the regulation of NPC
cell proliferation.
Alteration of SETDB1 expression
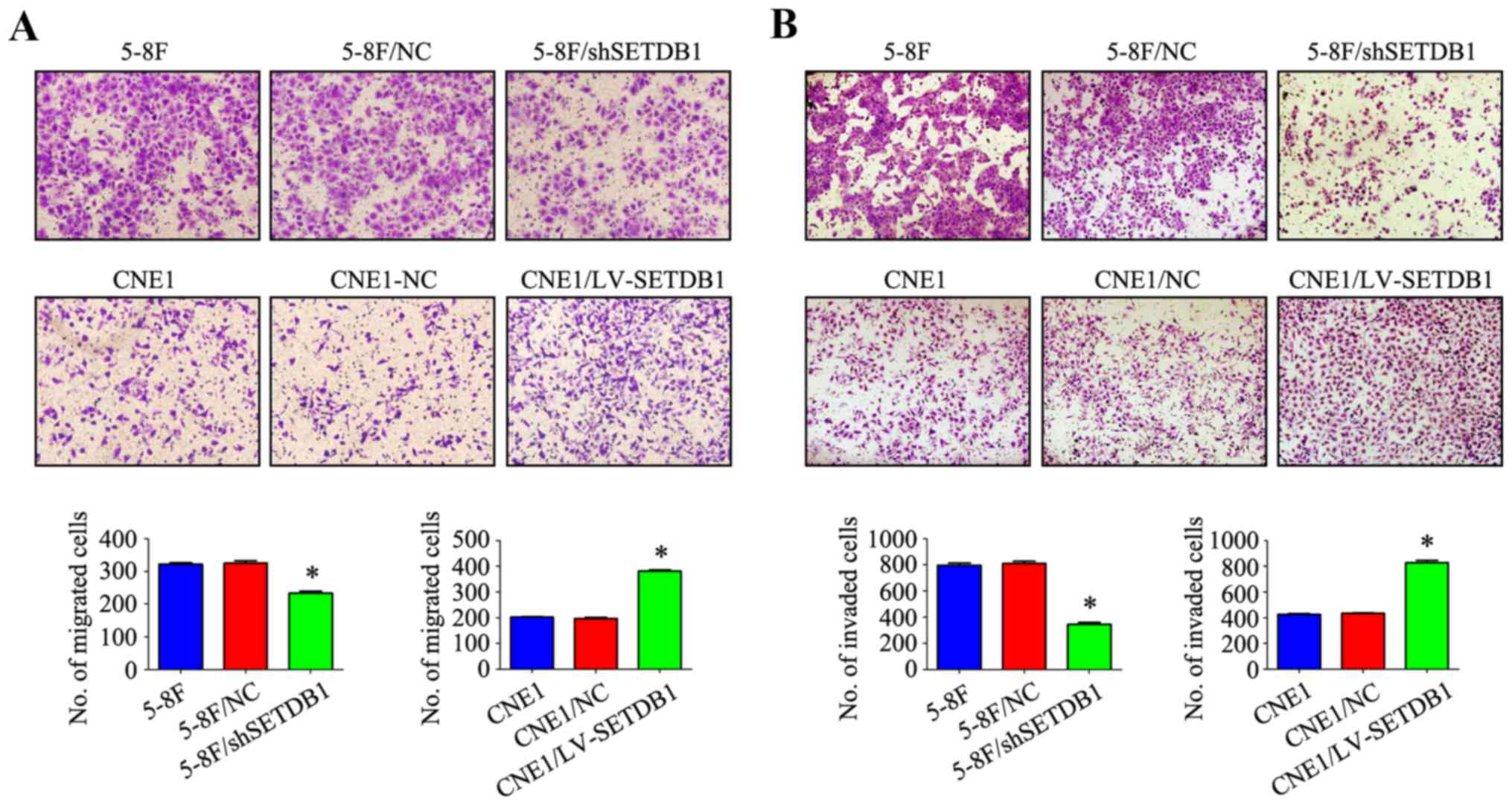
affects migration and invasion ability of NPC cells in vitro
We next focused on detecting whether SETDB1 affects
cell migration and invasion ability of NPC cells in vitro.
The effect of SETDB1 overexpression or knockdown on cell
migration capacity was tested with a Transwell assay. As shown in
Fig. 5A, cell migration increased
by nearly 100% in the SETDB1 overexpression group compared
with the WT and negative control cells. Accordingly, cell migration
was significantly decreased in the SETDB1-knockdown cells. Using a
Matrigel invasion assay, we observed that the number of cells able
to penetrate the Matrigel was 2-fold higher in CNE1/LV-SETDB1 cells
than that noted in the controls. In contrast, the invasion ability
of 5-8F/sh-SETDB1 cells exhibiting stable SETDB1 knockdown
was <0.5-fold vs. the controls (Fig.
5B). The data showed that SETDB1 overexpression or
knockdown could respectively promote or attenuate the migration and
invasion of NPC cells in vitro. These findings indicated
that the migration and invasion ability of NPC cells could be
correlated with the SETDB1 expression.
Discussion
NPC is a malignant tumor derived from nasopharyngeal
epithelial cells. It is widely accepted that NPC is a complicated
disease associated with multiple factors including genetics
(27,28), Epstein-Barr virus infection
(29–31) and the environment (32). Thus far, researchers have mainly
focused on improving our understanding of NPC pathogenesis
(33–36). However, we still need further
insight into the underlying molecular genetics of NPC, which would
enable future tailored patient prognostication and treatment
strategies. SETDB1, a histone lysine methyltransferase, plays an
important role in methylation and gene silencing (37,38).
Recent studies indicate that SETDB1 is markedly increased in
various types of human cancer, including melanoma, lung cancer,
hepatocellular carcinoma, breast, urothelial carcinoma and prostate
cancer, contributing to enhanced tumor growth and metastasis.
Hence, SETDB1 is a promising therapeutic target for cancer
(39–41). This study furthered the
understanding of SETDB1 and explored its potential role in
tumorigenesis and NPC.
To the best of our knowledge, the study presented
herein is the first to examine SETDB1 expression by
immunohistochemical staining of well-annotated NPC samples. We
observed that SETDB1 is frequently amplified in human NPC tissues,
with 126 out of 152 NPC patients exhibiting SETDB1 amplification.
The rate of overexpression of SETDB1 protein in tumor samples was
higher than that noted in nasopharynx mucosal tissues (55.6 vs.
20.51%, P<0.05). Kaplan-Meier survival analysis revealed a
significant correlation between SETDB1 expression and patient
survival where the higher the expression of SETDB1, the shorter the
survival time was for NPC patients. This result is in agreement
with previous observations of high SETDB1 expression levels in
various cancer tissues, which demonstrated that SETDB1
overexpression can be linked to a poor prognosis of NPC patients.
Furthermore, the association between SETDB1 expression and NPC
clinicopathologic variables was also examined. Overexpression of
SETDB1 was found to be significantly associated with N
classification. Consistent with its expression pattern in tissues,
SETDB1 expression was higher in 6 different NPC cell lines compared
with the normal human nasopharyngeal epithelial cell line NP69.
These results confirmed the clinical significance of SETDB1 as a
biomarker for NPC prognosis and the close relationship between
SETDB1 expression and tumorigenesis.
The role of SETDB1 in promoting tumor progression
has been investigated in a previous study. A study by Spyropoulou
et al (16) demonstrated
that SETDB1 knockdown by siRNA considerably reduced
proliferation, migration and clonogenic ability, and induced
apoptosis in glioma cell lines. Olcina et al (42) furthermore reported that increased
H3K9 methylation by SETDB1 is observed along with APAK (ATM and
p53-associated KZNF protein) repression in hypoxic conditions,
which was speculated to improve prognosis in colorectal cancer
cases. According to Zhang et al (22) miR-7 inhibited cancer metastasis and
inversed epithelial-mesenchymal transition of human breast cancer
stem cell via directly diminishing oncogenic SETDB1
expression. Additionally, Wong et al (43) revealed that SETDB1 expression was
markedly increased in liver cancer patients, due to activating
factors at the chromosomal, transcriptional and
post-transcriptional levels. Our preliminary work identified a
protein-protein interaction between SETDB1 and T cell lymphoma
invasion and metastasis 1 (Tiam1) in hepatocellular carcinoma (HCC)
according to yeast two-hybrid, which was confirmed by GST pull down
and crosslink immunoprecipitation assays. We also revealed that the
expression of SETDB1 was frequently upregulated in HCC tissues and
that this was positively correlated with Tiam1, resulting in HCC
progression (data not shown). Riviere and colleagues (44) showed that HBx relieves
chromatin-mediated transcriptional repression of hepatitis B viral
cccDNA involving SETDB1 histone methyltransferase. Thus, SETDB1 was
considered to be a new promising target in HCC therapy. Moreover,
SETDB1 is predicted to be involved in dedifferentiation such as the
development of induced pluripotent stem cells or in tumorigenesis
which involves conversion of somatic cells to cells with a more
plastic phenotype (11,45).
There is increasing evidence that supports the role
of SETDB1 in inducing invasion and metastasis in a wide range of
cancers. There are, however, no reports on SETDB1 and NPC, hence we
used the retroviral gene transfer method to alter the expression of
endogenous SETDB1 in NPC cells. The 5-8F, a high metastatic
potential NPC cell line, was genetically recombined to knock down
endogenous SETDB1, while a CNE1 cell line was used to
overexpress SETDB1. By knocking down SETDB1
expression we observed suppression of cell proliferation, migration
and invasion in vitro, and vice versa for SETDB1
overexpression. Our data were therefore in agreement with previous
findings for SETDB1 in other cancers and confirmed its important
role in promoting tumorigenesis.
In summary, the study presented herein identified an
association between SETDB1 expression and NPC. In order to
improve our understanding of the mechanism of carcinogenesis and
tumor progression in patients with NPC, future research will
require a larger set of samples and samples of different
pathological types as well as molecular, cellular and animal model
studies. As a potential biomarker for NPC it is imperative to
further analyze the predictive power of SETDB1 expression in future
studies.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Science Fund of China (grant no. 81502532), the Nature Science Fund
of Guangdong Province of China (grant no. 2014A030310101), the
Innovation and Strengthening School Project of Higher Education in
Guangdong Province (grant no. 2014KQNCX095) and the Guangdong
medical university research foundation (grant no. Z2014004).
Availability of data and materials
The dataset generated or analyzed during this study
are available from the corresponding author on reasonable
request.
Authors' contributions
JH and WYH conceived and designed the study,
analyzed and interpreted the data and drafted the manuscript. ML
and JPZ jointly performed the experiments, DXJ, DHY and YZ
contributed to the clinical sample collection. YHX and ZHC
contributed to pathological analysis. LJP and ZHY reviewed the
manuscript and supervised the study. All authors read and approved
the manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Ethical approval for this study was obtained from
the Ethics Committee of Taizhou Hospital of Zhejiang Province and
the Affiliated Hospital of Guangdong Medical University. This study
does not contain any studies with animals performed by any of the
authors. Written informed consent was obtained from each individual
participant.
Patient consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Venugopal R, Bavle RM, Konda P,
Muniswamappa S and Makarla S: Familial cancers of head and neck
region. J Clin Diagn Res. 11:ZE01–ZE06. 2017.PubMed/NCBI
|
|
3
|
Cao SM, Simons MJ and Qian CN: The
prevalence and prevention of nasopharyngeal carcinoma in China.
Chin J Cancer. 30:114–119. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee AW, Ma BB, Ng WT and Chan AT:
Management of nasopharyngeal carcinoma: Current practice and future
perspective. J Clin Oncol. 33:3356–3364. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang MX, Li J, Shen GP, Zou X, Xu JJ,
Jiang R, You R, Hua YJ, Sun Y, Ma J, et al: Intensity-modulated
radiotherapy prolongs the survival of patients with nasopharyngeal
carcinoma compared with conventional two-dimensional radiotherapy:
A 10-year experience with a large cohort and long follow-up. Eur J
Cancer. 51:2587–2595. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ruuskanen M, Grenman R, Leivo I, Vahlberg
T, Mäkitie A, Saarilahti K, Wigren T, Korpela M, Voutilainen L,
Koivunen P, et al: Outcome of nasopharyngeal carcinoma in Finland:
A nationwide study. Acta Oncol. 57:251–256. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen X, Zhu X, Liang Z, Li L, Qu S, Chen K
and Pan X: Long-term outcomes of neoadjuvant chemotherapy followed
by concurrent chemoradiotherapy (CCRT) vs CCRT alone for
nasopharyngeal carcinoma in the era of intensity-modulated
radiation therapy using propensity score matching method.
OncoTargets Ther. 10:2909–2921. 2017. View Article : Google Scholar
|
|
8
|
Ribassin-Majed L, Marguet S, Lee AWM, Ng
WT, Ma J, Chan ATC, Huang PY, Zhu G, Chua DTT, Chen Y, et al: What
is the best treatment of locally advanced nasopharyngeal carcinoma?
An individual patient data network meta-analysis. J Clin Oncol.
35:498–505. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harte PJ, Wu W, Carrasquillo MM and Matera
AG: Assignment of a novel bifurcated SET domain gene, SETDB1, to
human chromosome band 1q21 by in situ hybridization and radiation
hybrids. Cytogenet Cell Genet. 84:83–86. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park I, Hwang YJ, Kim T, Viswanath ANI,
Londhe AM, Jung SY, Sim KM, Min SJ, Lee JE, Seong J, et al: In
silico probing and biological evaluation of SETDB1/ESET-targeted
novel compounds that reduce tri-methylated histone H3K9 (H3K9me3)
level. J Comput Aided Mol Des. 31:877–889. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kang YK: SETDB1 in early embryos and
embryonic stem cells. Curr Issues Mol Biol. 17:1–10.
2015.PubMed/NCBI
|
|
12
|
Ceol CJ, Houvras Y, Jane-Valbuena J,
Bilodeau S, Orlando DA, Battisti V, Fritsch L, Lin WM, Hollmann TJ,
Ferré F, et al: The histone methyltransferase SETDB1 is recurrently
amplified in melanoma and accelerates its onset. Nature.
471:513–517. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu PC, Lu JW, Yang JY, Lin IH, Ou DL, Lin
YH, Chou KH, Huang WF, Wang WP, Huang YL, et al: H3K9 histone
methyltransferase, KMT1E/SETDB1, cooperates with the SMAD2/3
pathway to suppress lung cancer metastasis. Cancer Res.
74:7333–7343. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lafuente-Sanchis A, Zúñiga Á, Galbis JM,
Cremades A, Estors M, Martínez-Hernández NJ and Carretero J:
Prognostic value of ERCC1, RRM1, BRCA1 and SETDB1 in early stage of
non-small cell lung cancer. Clin Transl Oncol. 18:798–804. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun QY, Ding LW, Xiao JF, Chien W, Lim SL,
Hattori N, Goodglick L, Chia D, Mah V, Alavi M, et al: SETDB1
accelerates tumourigenesis by regulating the WNT signalling
pathway. J Pathol. 235:559–570. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Spyropoulou A, Gargalionis A, Dalagiorgou
G, Adamopoulos C, Papavassiliou KA, Lea RW, Piperi C and
Papavassiliou AG: Role of histone lysine methyltransferases SUV39H1
and SETDB1 in gliomagenesis: Modulation of cell proliferation,
migration, and colony formation. Neuromolecular Med. 16:70–82.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chiba T, Saito T, Yuki K, Zen Y, Koide S,
Kanogawa N, Motoyama T, Ogasawara S, Suzuki E, Ooka Y, et al:
Histone lysine methyltransferase SUV39H1 is a potent target for
epigenetic therapy of hepatocellular carcinoma. Int J Cancer.
136:289–298. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rivière L, Gérossier L, Hantz O and
Neuveut C: Hepatitis B virus and chromatin remodeling: HBx
counteracts SETDB1/HP1/H3K9me3 transcriptional silencing. Med Sci.
32:455–458. 2016.(In French).
|
|
19
|
Cicchini C, Battistelli C and Tripodi M:
SETDB1 is a new promising target in HCC therapy. Chin Clin Oncol.
5:732016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fei Q, Shang K, Zhang J, Chuai S, Kong D,
Zhou T, Fu S, Liang Y, Li C, Chen Z, et al: Histone
methyltransferase SETDB1 regulates liver cancer cell growth through
methylation of p53. Nat Commun. 6:86512015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Regina C, Compagnone M, Peschiaroli A,
Lena A, Annicchiarico-Petruzzelli M, Piro MC, Melino G and Candi E:
Setdb1, a novel interactor of DeltaNp63, is involved in breast
tumorigenesis. Oncotarget. 7:28836–28848. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang H, Cai K, Wang J, Wang X, Cheng K,
Shi F, Jiang L, Zhang Y and Dou J: MiR-7, inhibited indirectly by
LincRNA HOTAIR, directly inhibits SETDB1 and reverses the EMT of
breast cancer stem cells by downregulating the STAT3 pathway. Stem
Cells. 32:2858–2868. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lindgren D, Sjödahl G, Lauss M, Staaf J,
Chebil G, Lövgren K, Gudjonsson S, Liedberg F, Patschan O, Månsson
W, et al: Integrated genomic and gene expression profiling
identifies two major genomic circuits in urothelial carcinoma. PloS
One. 7:e388632012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun Y, Wei M, Ren SC, Chen R, Xu WD, Wang
FB, Lu J, Shen J, Yu YW, Hou JG, et al: Histone methyltransferase
SETDB1 is required for prostate cancer cell proliferation,
migration and invasion. Asian J Androl. 16:319–324. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Adam L, Vadlamudi RK, McCrea P and Kumar
R: Tiam1 overexpression potentiates heregulin-induced lymphoid
enhancer factor-1/beta -catenin nuclear signaling in breast cancer
cells by modulating the intercellular stability. J Biol Chem.
276:28443–28450. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang J, Ye X, Guan J, Chen B, Li Q, Zheng
X, Liu L, Wang S, Ding Y, Ding Y and Chen L: Tiam1 is associated
with hepatocellular carcinoma metastasis. Int J Cancer. 132:90–100.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ooft M, van Ipenburg J, van Loo R, de Jong
R, Moelans C, Braunius W, de Bree R, van Diest P, Koljenović S,
Baatenburg de Jong R, et al: Molecular profile of nasopharyngeal
carcinoma: Analysing tumour suppressor gene promoter
hypermethylation by multiplex ligation-dependent probe
amplification. J Clin Pathol. 71:351–359. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Roy Chattopadhyay N, Das P, Chatterjee K
and Choudhuri T: Higher incidence of nasopharyngeal carcinoma in
some regions in the world confers for interplay between genetic
factors and external stimuli. Drug Discov Ther. 11:170–180. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakanishi Y, Wakisaka N, Kondo S, Endo K,
Sugimoto H, Hatano M, Ueno T, Ishikawa K and Yoshizaki T:
Progression of understanding for the role of Epstein-Barr virus and
management of nasopharyngeal carcinoma. Cancer Metastasis Rev.
36:435–447. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ooft ML, van Ipenburg JA, Braunius WW,
Zuur CI, Koljenović S and Willems SM: Prognostic role of tumor
infiltrating lymphocytes in EBV positive and EBV negative
nasopharyngeal carcinoma. Oral Oncol. 71:16–25. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim KY, Le QT, Yom SS, Ng RHW, Chan KCA,
Bratman SV, Welch JJ, Divi RL, Petryshyn RA and Conley BA: Clinical
utility of epstein-barr virus DNA testing in the treatment of
nasopharyngeal carcinoma patients. Int J Radiat Oncol Biol Phys.
98:996–1001. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dai J, Shen W, Wen W, Chang J, Wang T,
Chen H, Jin G, Ma H, Wu C, Li L, et al: Estimation of heritability
for nine common cancers using data from genome-wide association
studies in Chinese population. Int J Cancer. 140:329–336. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
He R, Hu Z, Wang Q, Luo W, Li J, Duan L,
Zhu YS and Luo DX: The role of long non-coding RNAs in
nasopharyngeal carcinoma: As systemic review. Oncotarget.
8:16075–16083. 2017.PubMed/NCBI
|
|
34
|
Fountzilas G, Psyrri A, Giannoulatou E,
Tikas I, Manousou K, Rontogianni D, Ciuleanu E, Ciuleanu T, Resiga
L, Zaramboukas T, et al: Prevalent somatic BRCA1 mutations shape
clinically relevant genomic patterns of nasopharyngeal carcinoma in
Southeast Europe. Int J Cancer. 142:66–80. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang Y, Liu W, Zhao Z, Zhang Y, Xiao H and
Luo B: Filaggrin gene polymorphism associated with Epstein-Barr
virus-associated tumors in China. Virus Genes. 53:532–537. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhen Y, Fang W, Zhao M, Luo R, Liu Y, Fu
Q, Chen Y, Cheng C, Zhang Y and Liu Z:
miR-374a-CCND1-pPI3K/AKT-c-JUN feedback loop modulated by PDCD4
suppresses cell growth, metastasis, and sensitizes nasopharyngeal
carcinoma to cisplatin. Oncogene. 36:275–285. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gao H, Yu Z, Bi D, Jiang L, Cui Y, Sun J
and Ma R: Akt/PKB interacts with the histone H3 methyltransferase
SETDB1 and coordinates to silence gene expression. Mol Cell
Biochem. 305:35–44. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schultz DC, Ayyanathan K, Negorev D, Maul
GG and Rauscher FJ III: SETDB1: A novel KAP-1-associated histone
H3, lysine 9-specific methyltransferase that contributes to
HP1-mediated silencing of euchromatic genes by KRAB zinc-finger
proteins. Genes Dev. 16:919–932. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Karanth AV, Maniswami RR, Prashanth S,
Govindaraj H, Padmavathy R, Jegatheesan SK, Mullangi R and
Rajagopal S: Emerging role of SETDB1 as a therapeutic target.
Expert Opin Ther Targets. 21:319–331. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ho YJ, Lin YM, Huang YC, Chang J, Yeh KT,
Lin LI, Gong Z, Tzeng TY and Lu JW: Significance of histone
methyltransferase SETDB1 expression in colon adenocarcinoma. APMIS.
125:985–995. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Na HH, Noh HJ, Cheong HM, Kang Y and Kim
KC: SETDB1 mediated FosB expression increases the cell
proliferation rate during anticancer drug therapy. BMB Rep.
49:238–243. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Olcina MM, Leszczynska KB, Senra JM, Isa
NF, Harada H and Hammond EM: H3K9me3 facilitates hypoxia-induced
p53-dependent apoptosis through repression of APAK. Oncogene.
35:793–799. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wong CM, Wei L, Law CT, Ho DW, Tsang FH,
Au SL, Sze KM, Lee JM, Wong CC and Ng IO: Up-regulation of histone
methyltransferase SETDB1 by multiple mechanisms in hepatocellular
carcinoma promotes cancer metastasis. Hepatology. 63:474–487. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rivière L, Gerossier L, Ducroux A, Dion S,
Deng Q, Michel ML, Buendia MA, Hantz O and Neuveut C: HBx relieves
chromatin-mediated transcriptional repression of hepatitis B viral
cccDNA involving SETDB1 histone methyltransferase. J Hepatol.
63:1093–1102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Thompson PJ, Dulberg V, Moon KM, Foster
LJ, Chen C, Karimi MM and Lorincz MC: Correction: hnRNP K
coordinates transcriptional silencing by SETDB1 in embryonic stem
cells. PLoS Genet. 12:e10063902016. View Article : Google Scholar : PubMed/NCBI
|