Introduction
BC is the second leading cause of cancer-related
deaths in women after lung cancer and it accounts for nearly 25% of
all cancers in females (1,2). Approximately 1.4 million women
worldwide are diagnosed with BC each year, and half a million BC
patients succumb to the disease (3). BC, which is comprised of several
different phenotypes, is a highly heterogeneous disease (4). According to studies (1,2), based
on the analysis of the expression of estrogen receptor (ER),
progesterone receptor (PR), human epidermal growth factor receptor
2 (HER2), and Ki-67, BC is classified into five subtypes, including
luminal A, luminal B, normal breast-like, HER2 and basal-like. One
of these subtypes, triple negative breast cancer (TNBC), is defined
by the lack of ER, PR and HER2 expression in tumor tissues, and it
accounts for 15–23.8% of BC patients (5). Compared with the other subtypes, TNBC
is frequently found in premenopausal women, particularly in young
women (<50 years), who may have a higher grade and a higher rate
of a cellular tumor antigen p53 mutation (6). TNBC is more prevalent in
African-American women and more aggressive than other molecular
subtypes of breast tumors (7).
Although the risk and roles of ER-positive cancer are well defined,
those for TNBC are not as well defined. Therefore, it is critical
to understand gene expression and genetic variability in the
etiology and pathogenesis of these types of BC.
Human trophoblast cell surface protein 2 (Trop2/
TACSTD2/M1S1/GA733-1), is a 36-kDa transmembrane protein that is
expressed primarily in the membrane surface of epithelial cells
(8). Trop2 has been revealed to
affect tumor proliferation, metastasis and invasion as an oncogene,
and it is overexpressed in various epithelial tumors, which may be
associated with aggressive tumor effects (9–12). In
our previous study, we found that Trop2 has an important role in
regulating stem cells and inducing EMT of gastric cancer cells.
However, little is known about the regulatory role of Trop2 in
TNBC.
E-cadherin is a type of adhesion molecule that
mainly exists in human and animal epithelium, and its main function
is to maintain normal epithelial cell morphology and structural
integrity (13–15). E-cadherin can regulate the close
adhesion of epithelial cells and its downregulation is a key factor
in epithelial neoplasm development (16). The aberrant structure of E-cadherin
was found in tumor cells, and the adhesion ability of cells was
decreased, which could easily lead to infiltration and distal
metastasis of cells to peripheral tissues (17). E-cadherin has been considered to be
an important component of intercellular adhesion and it is also a
key factor in initiating EMT transformation (18,19).
Decreased E-cadherin expression in cancers, which could induce the
EMT phenomenon has been a popular research area in recent years
(20). Many researchers have found
that inhibiting E-cadherin expression could be related to the
differentiation, invasion and metastasis of tumors (21,22).
In the present study, we examined the relationship
between Trop2 and E-cadherin in BC tissues obtained from Chinese
patients and compared the expression of two genes in the BC tissues
with matched adjacent non-tumor tissues.
Materials and methods
Tissue sample and clinical
information
We studied a cohort of 312 patients who were
diagnosed with primary BC from January 2009 to December 2012. A
total of 354 formalin-fixed, paraffin-embedded (FFPE) BC tissue
samples were investigated, including BC tissues (n=295) and matched
adjacent tissues (n=59). All of the tissue blocks were obtained
from the Department of Pathology, Nanjing First Hospital. Clinical
information about the tissues was collected, including age,
location, tumor size, tumor-node-metastasis (TNM) stage, ER/PR/HER2
status, Ki-67 expression, histological grade, lymph node status,
metastasis and overall survival (OS). In addition, 20 pairs of
freshly frozen BC tissues were obtained from the Department of
Pathology, Nanjing First Hospital. Written informed consent was
obtained before the patients underwent surgery. All the results
obtained from the study will have no effect on the patient's health
and future treatment. The study protocol was approved by the Human
Research Ethic Committee of Nanjing First Hospital.
qRT-PCR
We detected Trop2 and E-cadherin mRNA expression
levels in 20 pairs of human BC tissues, and then we compared them
with matched adjacent tissues. Cell RNA was extracted with TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and then reverse-transcribed into cDNA using a PrimeScript™ RT
reagent kit (Takara Bio, Inc., Otsu, Japan) according to the
manufacturer's protocol. Human GAPDH served as the internal
control. The primers used in the study were asfollows: GAPDH
forward, 5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse,
5′-GAAGATGGTGATGGGATTTC-3′; Trop2 forward,
5′-TGTCCTGATGTGATATGTCTGAG-3′ and reverse,
5′-GGGTGAGAGTGGGTTGGG-3′; E-cadherin forward,
5′-GACGCGGACGATGATGTGAAC-3′ and reverse,
5′-TTGTACGTGGTGGGATTGAAG-3′ (GenScript Co., Ltd., Nanjing, China).
qRT-PCR was performed on an ABI Prism 7500 HT Sequence Detection
System (Applied Biosystems; Thermo Fisher Scientific, Inc.) in
96-well plates. Relative expression levels were calculated as
ratios normalized against those of GAPDH. Results were normalized
to the respective internal controls. The Ct-value for each sample
was calculated using the ΔΔCt method, and the results were
expressed as 2−ΔΔCt.
Tissue microarray (TMA) construction
and IHC
The TMAs were constructed at the Department of
Pathology, Nanjing First Hospital, (Nanjing, China) using the
Quick-Ray tissue system (Unitma, Co., Ltd., Seoul, Korea) manual.
Core tissue biopsies (2 mm in diameter) were obtained from 60
individuals. FFPE blocks were made and then arranged in new
recipient paraffin blocks. A total of 5 breast TMAs were created.
Tissue sections were deparaffinized and rehydrated through graded
alcohols. Endogenous peroxidase activity was blocked by incubation
in 3% H2O2. Tissues were then placed in 0.01
M citrate buffer (pH 6.0), and heated in a microwave for antigen
retrieval. Trop2 was detected using a monoclonal antibody rabbit
anti-human Trop2 (dilution 1:100) (cat. no. ab227689; Abcam,
Cambridge, UK), and a monoclonal antibody mouse anti-human
E-cadherin (dilution 1:100; cat. no. sc-8426; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). Reactions were detected with
an EnVision™ peroxidase kit (Dako; Agilent Technologies, Inc.,
Santa Clara, CA, USA). Tissues were then incubated in
3,3′-diaminobenzidine (Dako; Agilent Technologies, Inc.),
counterstained with hematoxylin, dehydrated through graded
alcohols, cleared in xylene, and coverslipped with permanent
mounting media. Staining was quantified in all of the tissues
without knowledge of clinical characteristics. Trop2 expression was
scored using the semi-quantitative H-score method, which takes into
account both the staining intensity and the percentage of cells at
that intensity (8). The following
staining intensity scores were used: 0 indicated no staining; 1+
indicated weak staining; 2+ indicated moderate staining; and 3+
indicated intense staining. The total number of cells at each
intensity level was multiplied by the corresponding intensity score
to yield an intensity percentage score. Final staining scores were
then calculated by summing the four intensity percentage scores;
the minimum possible final staining score was 0 (no staining) and
the maximum possible score was 300 (100% of cells with 3+ staining
intensity).
Cell lines and cell culture
The human BC cell lines MCF-7 and MDA-MB-231 and the
human breast epithelial cell line MCF-10A were preserved in our
laboratory. Cell lines were maintained at 37°C in RPMI-1640 medium
with 10% fetal bovine serum and 1% penicillin-streptomycin (all
from Gibco; Thermo Fisher Scientific, Inc). A humidified atmosphere
containing 5% CO2 was used to incubate the cells.
Protein extraction and western blot
analysis
Intracellular proteins were extracted using RIPA
Lysis and Extraction Buffer (Thermo Fisher Scientific, Inc.) based
on the manufacturer's protocol. Protein concentrations were
determined using a BCA Protein Assay kit (Beyotime Institute of
Biotechnology, Haimen, China). The same amounts of protein were
resolved by 10% SDS-PAGE and transferred to a polyvinylidene
difluoride (PVDF) membrane. The membrane was blocked for 1 h and
then incubated with primary antibodies (anti-Trop2 antibody or
anti-E-cadherin antibody) overnight at 4°C. After washing three
times with PBS-Tween-20 (PBST), the membrane was incubated with
secondary antibodies, including HRP-goat anti-rabbit secondary
antibody (cat. no. A12004-1; Amy Jet Scientific, Wuhan, China),
HRP-goat anti-mouse secondary antibody (A12003-1; Epigentek,
Farmingdale, NY, USA) at room temperature for 1 h. The membrane was
subsequently washed with PBST and incubated with SuperSignal West
Pico PLUS Chemiluminescent Substrate (Thermo Fisher Scientific,
Inc.) for 5 min. The specific bands were exposed to the ChemiDoc
XRS+ System (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
expression of GAPDH was used as the internal control.
Statistical analysis
The SPSS 19.0 statistical software package (SPSS,
Inc., Chicago, IL, USA) was utilized to analyze all of the
statistics. Unpaired Student's t-test was performed to compare two
groups. One-way ANOVA followed by Tukey's multiple comparison test
was used when three or more groups were compared. χ2
tests were performed to evaluate whether the expression levels of
Trop2 and E-cadherin were associated with clinicopathological
parameters. Kaplan-Meier analysis was used to estimate cumulative
patient survival. Survival curves were compared by log-rank test.
P<0.05 was considered to indicate a statistically significant
result.
Results
Trop2 and E-cadherin mRNA expression
in BC tissues compared with matched adjacent tissues
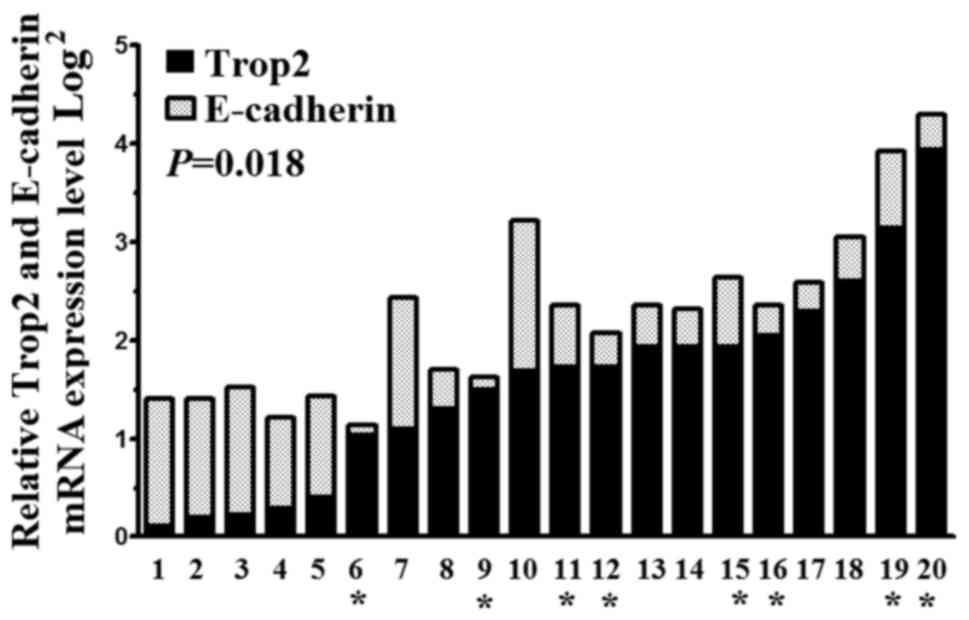
To study the levels of Trop2 and E-cadherin mRNA, we
used qRT-PCR in 20 pairs of BC tissues and matched adjacent
tissues. Trop2 and E-cadherin mRNA expression levels in BC tissues
were 1.55±0.78 and 0.70±0.38 fold higher than those in the matched
adjacent tissues, respectively (P=0.018). Notably, we also found
Trop2 and E-cadherin mRNA expression levels in the TNBC tissues
(T6, 9, 11, 12, 15, 16, 19 and 20) were 2.76±0.77 (P=0.013) and
0.54±0.21 (P=0.010) fold higher than those in other types of BC
(T1, 2, 3, 4, 5, 7, 8, 10, 13, 14, 17 and 18, respectively)
(Fig. 1).
Trop2 and E-cadherin protein
expression in BC tissues compared with matched adjacent
tissues
We used an IHC assay to detect Trop2 and E-cadherin
protein expression levels in BC tissues, and we found that Trop2
and E-cadherin were localized at the membrane and cytoplasm of the
BC cells (Fig. 2). Trop2 was
overexpressed in the BC tissues, while E-cadherin had a low
expression in BC tissues. We defined high and low Trop2/E-cadherin
expression levels based on OS in BC patients through the x-tile
software program for TMA data analysis. For Trop2, the cut-off of
130 was selected, and a score of 0–130 was considered to be low or
no expression, while 131–300 was considered to be a high
expression. For E-cadherin, a score of 0–120 was considered to be
low or no expression, while 121–300 was considered to be a high
expression.
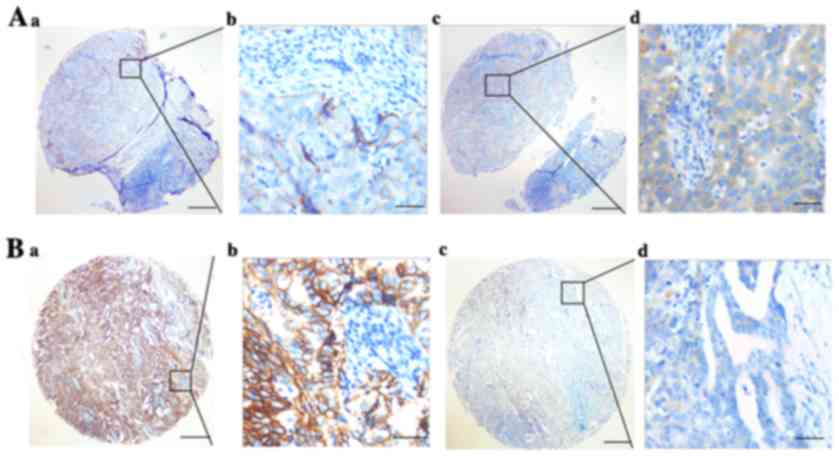 | Figure 2.Representative pattern of Trop2 and
E-cadherin protein expression in BC tissues on TMA sections. (A-a
and b) Low expression of Trop2 in matched adjacent tissues (IHC
score, 80); (A-c and d) high expression of E-cadherin in matched
adjacent tissues (IHC score, 180); (B-a and b) high expression of
Trop2 in BC tissues (IHC score, 300); and (B-c and d) low
expression of E-cadherin in BC tissues (IHC score, 50). Row a and c
are Trop2/E-cadherin staining with ×4 (bar, 500 µm), and row b and
d are Trop2/E-cadherin staining with ×40 (bar, 50 µm). BC, breast
cancer; IHC, immunohistochemistry; TMA, tissue microarray. |
High Trop2 and high E-cadherin
(T+E+), high Trop2 and low E-cadherin
(T+E−), low Trop2 and high E-cadherin
(T−E+), and low Trop2 and low E-cadherin
(T−E−) expression was detected in the BC
tissues, compared with those in the matched adjacent tissues. The
levels of T+E+, T+E−,
T−E+ and T−E−
expression in the TNBC tissues were 7 (7.3%), 68 (70.8%), 16
(16.7%), and 5 (5.2%), while in the BC tissues, they were 22
(11.1%), 102 (51.3%), 61 (30.7%), and 14 (7.0%), and compared with
those in matched adjacent tissues, they were 8 (13.6%), 13 (22.0%),
28 (47.5%), and 10 (16.9%), respectively (χ2=37.105,
P<0.001) (Table I).
 | Table I.Trop2 and E-cadherin expression in
breast cancer tissues. |
Table I.
Trop2 and E-cadherin expression in
breast cancer tissues.
|
Characteristics | n | Trop2
E-cadherin | + +(%) | + − (%) | − + (%) | − −(%) | Pearson
x2 37.105 | P-value
<0.001a |
|---|
| TNBC tissue | 96 |
| 7 (7.3) | 68 (70.8) | 16 (16.7) | 5 (5.2) |
|
|
| BC tissue | 199 |
| 22 (11.1) | 102 (51.3) | 61 (30.7) | 14 (7.0) |
|
|
| Matched adjacent
tissue | 59 |
| 8 (13.6) | 13 (22.0) | 28 (47.5) | 10 (16.9) |
|
|
Association of Trop2 and E-cadherin
expression with clinicopathological characteristics in BCs
Next, we investigated the relationship between
Trop2/E-cadherin protein expression and clinicopathological
variables in tissues of BC patients. The results indicated that the
T+E− expression in BC was associated with
lymph node status (χ2=36.688, P<0.001), metastasis
(χ2=42.958, P<0.001), TNM stage (χ2=15.91,
P<0.014), and TNBC (χ2=10.429, P<0.015). However,
we did not detect a significant association between Trop2 and
E-cadherin expression levels and tumor location, age and tumor size
(Table II).
 | Table II.Association of the expression level
of Trop2 and E-cadherin with clinicopathological characteristics in
BC patients. |
Table II.
Association of the expression level
of Trop2 and E-cadherin with clinicopathological characteristics in
BC patients.
| Characteristic | n | Trop2
E-cadherin | + + | + − | − + | − − | Pearson
x2 | P-value |
|---|
| Total | 295 |
|
|
|
|
| 38.089 |
<0.001a |
| Tumor location |
|
|
|
|
|
| 0.493 | 0.920 |
|
Left | 180 |
| 16 (8.9) | 105 (58.3) | 47 (26.1) | 12 (6.7) |
|
|
|
Right | 115 |
| 13 (11.3) | 65 (56.5) | 30 (26.1) | 7 (6.1) |
|
|
| Age (years) |
|
|
|
|
|
| 0.819 | 0.845 |
|
Premenopausal | 88 |
| 7 (8.0) | 51 (58.0) | 25 (28.4) | 5 (5.7) |
|
|
|
Postmenopausal | 207 |
| 22 (10.6) | 119 (57.5) | 52 (25.1) | 14 (6.8) |
|
|
| Tumor size
(cm) |
|
|
|
|
|
| 4.417 | 0.620 |
| ≤2 | 57 |
| 5 (8.8) | 29 (50.9) | 19 (33.3) | 4 (7.0) |
|
|
| >2
and ≤5 | 227 |
| 23 (10.1) | 136 (59.9) | 54 (23.8) | 14 (6.2) |
|
|
|
>5 | 10 |
| 0 (0.0) | 5 (50) | 4 (40.0) | 1 (10.0) |
|
|
| ER |
|
Positive | 126 |
| 16 (12.7) | 67 (53.2) | 36 (28.6) | 7 (5.6) | 3.378 | 0.337 |
|
Negative | 169 |
| 12 (7.7) | 103 (60.9) | 41(24.3) | 12 (7.1) |
|
|
| PR |
|
|
|
|
|
| 6.823 | 0.078 |
|
Positive | 122 |
| 17 (13.9) | 65 (53.3) | 29 (23.8) | 11 (9.0) |
|
|
|
negative | 173 |
| 12 (6.9) | 105 (60.7) | 48 (27.7) | 8 (4.6) |
|
|
| HER2 | 8.485 | 0.037a |
|
Positive | 108 |
| 8 (7.4) | 74 (68.5) | 20 (18.5) | 6 (5.6) |
|
|
|
Negative | 187 |
| 21 (11.2) | 96 (51.3) | 57 (30.5) | 13 (7.0) |
|
|
| Ki-67 |
|
|
|
|
|
| 5.864 | 0.118 |
|
Positive | 275 |
| 25 (9.1) | 162 (58.9) | 5 (25.0) | 16 (5.8) |
|
|
|
Negative | 20 |
| 4 (13.8) | 8 (40.0) | 72 (26.2) | 3 (15.0) |
|
|
| Lymph node
status |
| N0 | 187 |
| 20 (10.7) | 129 (69.0) | 27 (14.4) | 8 (7.4) | 38.688 |
<0.001a |
|
N1+2+3 | 108 |
| 9 (8.3) | 41 (38.0) | 50 (46.3) | 11 (5.9) |
|
|
| Metastasis |
| M0 | 182 |
| 18 (9.9) | 132 (72.5) | 23 (12.6) | 9 (4.9) | 42.958 |
<0.001a |
| M1 | 113 |
| 11 (9.7) | 38 (33.6) | 54 (47.8) | 10 (8.8) |
|
|
| TNM stage |
| 1 | 124 |
| 16 (12.9) | 60 (48.4) | 37 (29.8) | 11 (8.9) | 15.91 | 0.014b |
| 2 | 97 |
| 8 (8.2) | 68 (70.1) | 15 (15.5) | 6 (6.2) |
|
|
| 3 | 74 |
| 5 (6.8) | 42 (57.6) | 25 (33.8) | 2 (2.7) |
|
|
| TNBC |
|
|
|
|
|
| 10.429 | 0.015b |
|
Positive | 199 |
| 22 (11.1) | 102 (51.3) | 61 (30.7) | 14 (7.0) |
|
|
|
Negative | 96 |
| 7 (7.3) | 68 (70.8) | 16 (16.7) | 5 (5.2) |
|
|
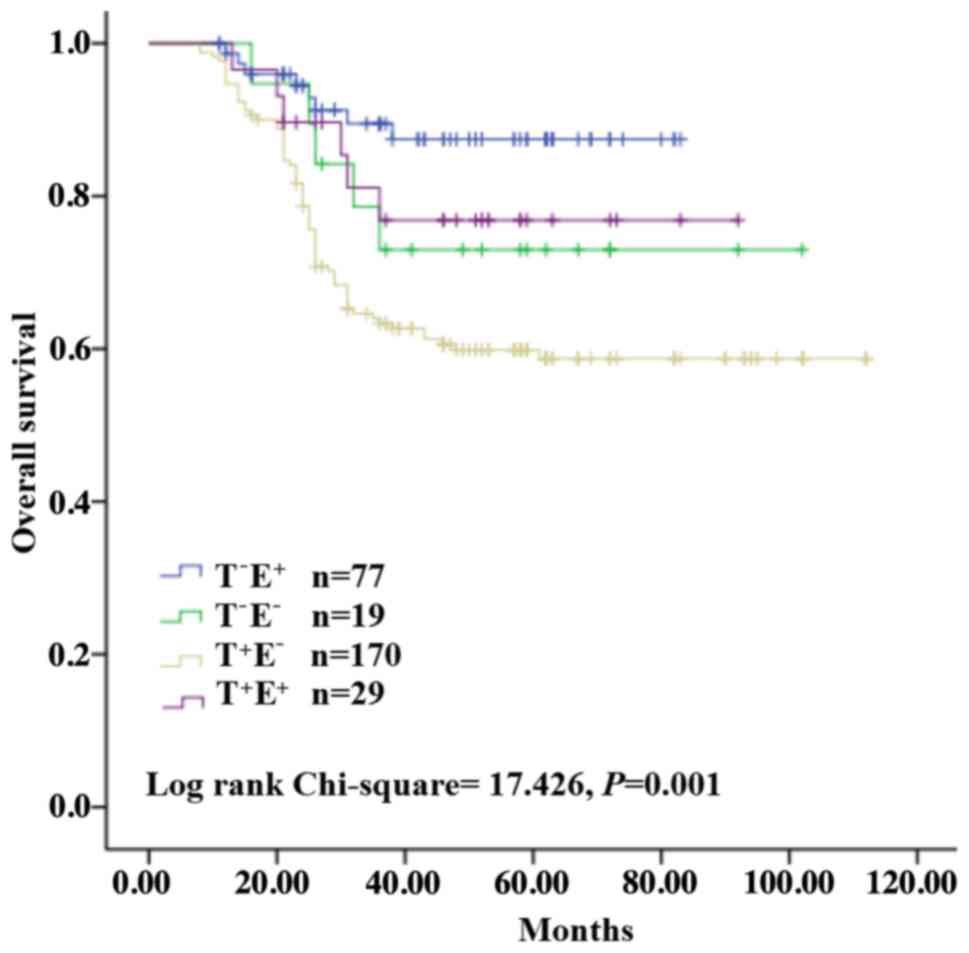
Prognostic value of Trop2 and
E-cadherin protein expression in BC
We also used univariate and multivariate analyses to
examine the possible prognostic factors for BC. The results
revealed that T+E− expression was
significantly associated with a shorter survival in univariate
analysis, along with lymph node status, metastasis, TNM stage and
TNBC. Multivariate analysis further indicated that
T+E− expression was associated with poor OS,
as did lymph node status, metastasis and TNBC (Table III). Kaplan-Meier survival curves
revealed that T+E− expression was associated
with poor OS (Fig. 3). These
results may help in our analysis of the 5-year survival rate of BC
patients.
 | Table III.Univariate and multivariate analysis
of prognostic markers for overall survival in breast cancer. |
Table III.
Univariate and multivariate analysis
of prognostic markers for overall survival in breast cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
|
| HR | P<|Z| | 95% CI | HR | P<|Z| | 95% CI |
|---|
| Trop2 and
E-cadherin |
|
T+E+ vs.
T+E+ vs. T−E− vs.
T−E+ | 1.656 | 0.001a | 1.241–2.209 | 1.743 | 0.037 | 0.518–2.066 |
| Location |
| Left
vs. right | 1.107 | 0.699 | 0.663–1.848 |
|
|
|
| Age (years) |
|
Premenopausal vs.
postmenopausal | 1.027 | 0.924 | 0.597–1.767 |
|
|
|
| Tumor size
(cm) |
| ≤2 vs.
>2 and ≤5 vs. >5 | 0.823 | 0.273 | 0.580–1.166 |
|
|
|
| ER |
|
Positive vs. negative | 0.993 | 0.977 | 0.619–1.594 |
|
|
|
| PR |
|
Positive vs. negative | 0.819 | 0.382 | 0.502–1.042 |
|
|
|
| HER2 |
|
Positive vs. negative | 2.192 | 0.092 | 0.919–1.309 |
|
|
|
| Ki-67 |
|
Positive vs. negative | 0.732 | 0.398 | 0.492–1.382 |
|
|
|
| Lymph node
status |
| N0 vs.
N1+2+3 | 11.11 |
<0.001a | 5.118–24.124 | 3.264 | 0.014a | 1.276–8.348 |
| Metastasis |
| M0 vs.
M1 | 10.29 |
<0.001a | 4.193–19.729 | 5.151 | 0.001a | 3.789–14.091 |
| TNM stage |
| 1 vs. 2
vs. 3 | 2.134 |
<0.001a | 1.723–2.634 | 1.592 | 0.062 | 0.983–1.869 |
| TNBC |
|
Positive vs. negative | 3.921 |
<0.001a | 2.732–5.823 | 3.029 |
<0.001a | 1.823–6.032 |
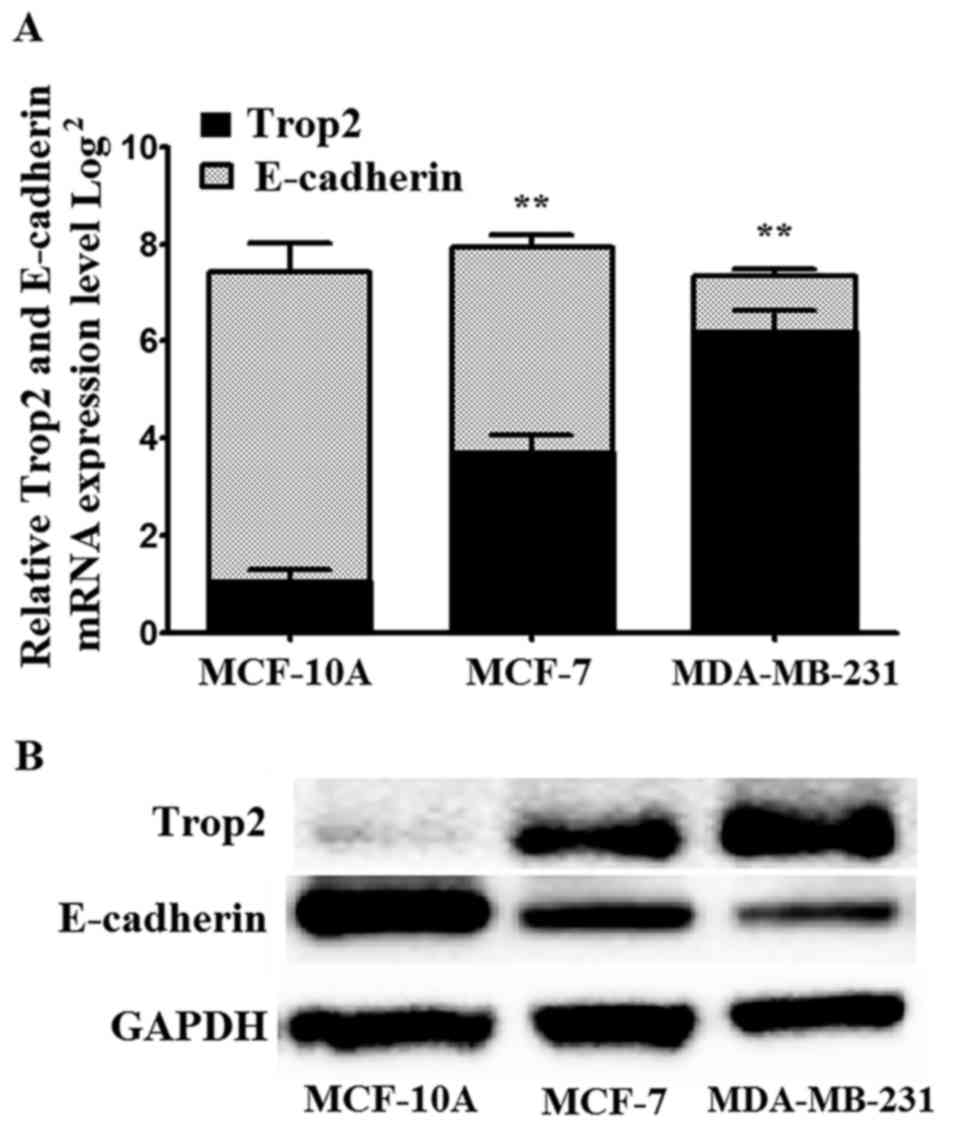
Trop2 and E-cadherin mRNA and protein
expression in BC cell lines
To better demonstrate the level of Trop2 and
E-cadherin expression in BC, we assessed the protein level of Trop2
and E-cadherin in two types of BC cell lines (MCF-7 and MDA-MB-231)
and the human breast epithelial cell line MCF-10A. Trop2 expression
was the lowest and E-cadherin was the highest in the MCF-10A cells
when compared with the BC cell lines. The MCF-7 and MDA-MB-231 cell
lines exhibited increased Trop2 and decreased E-cadherin expression
levels when compared with MCF-10A cells as determined by qRT-PCR
and western blot assay (Fig.
4).
Discussion
The invasion and metastasis of tumors refers to the
process of tumor cells being transferred to other tissues from the
original site (23–25). EMT is a developmental process in
which epithelial cells lose their phenotypes, such as E-cadherin
and N-cadherin, and mesenchymal cells gain their phenotypes, such
as vimentin and fibronectin. EMT is considered to be important in
the invasive and metastasis progression of cancer (26). EMT promotes migration and invasion,
accelerates stem cell properties, conduces immunosuppression, and
deters apoptosis and senescence (20). Both Trop2 and E-cadherin have a
great influence on EMT transformation.
In our previous study (unpublished data), we
confirmed that Trop2 upregulated the expression of the mesenchymal
phenotype and downregulated the epithelial phenotype, such as
E-cadherin and N-cadherin. We also demonstrated that Trop2 was
involved in β-catenin/TGF-β1-mediated EMT in gastric cancer. Lin
et al (27) have revealed
that Trop2 is highly expressed in BC patients and it is related to
the expression level of cyclin D1. However, the potential role of
Trop2 in the promotion of EMT in BC has not been fully studied.
In the present study, Trop2 mRNA was highly
expressed in BC tissues while E-cadherin mRNA was decreased in BC
tissues compared with those in matched para-carcinoma tissues,
results which were similar to a previous study (20). Notably, we also found that the Trop2
and E-cadherin mRNA expression levels in the TNBC tissues were
2.76±0.77 and 0.54±0.21 fold higher than those in other types of
BC. A similar result was found in protein expression. Our TMA
results also revealed that the
Trop2+/E-cadherin− expression was associated
with lymph node status, metastasis, TNM stage and
ER−/PR−/HER2−. Furthermore, a high
Trop2/low E-cadherin expression predicted poor OS in BC patients.
Lastly, we detected Trop2 and E-cadherin expression in BC cell
lines (MCF-7 and MDA-MB-231) and the normal breast epithelial cell
line MCF-10A, and we found that Trop2 had a higher expression while
E-cadherin had a lower level compared with that in the normal
breast epithelial cell line.
Different expression of Trop2/E-cadherin between
cancer tissues and adjacent tissues indicate that high Trop2/low
E-cadherin expression is connected with BC or even TNBC. Our
findings revealed that Trop2 promoted the migration and invasion of
BC cells by inducing the EMT phenomenon, resulting in lymph node
involvement and distant metastasis which always indicates poor
prognosis and short OS. The function of E-cadherin does the
opposite in BC.
Overall, high Trop2 and low E-cadherin expression
may predict poor prognosis and short survival in BC. Furthermore,
Trop2 and E-cadherin could be considered as therapeutic targets. In
the future, more studies should be performed to confirm the
mechanism of Trop2 and E-cadherin in vivo and in
vitro.
Acknowledgements
We thank all the patients enrolled in the study.
Funding
The present study was supported by grants from the
‘Six Talent Peaks Project’ in Jiangsu Province (no. WSN-068) and
the National Natural Science Foundation of China (no.
81601618).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
WBH and YCZ designed the study; WZ, XWK and XYZ
acquired the data and drafted the article; LZJ, JSW and XBY
analyzed and interpreted the data; ZDT, XLW, QL and BW revised the
article critically for important intellectual content. All authors
read and approved the manuscript and agree to be accountable for
all aspects of the research in ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
The study protocal was approved by the Human
Research Ethic Committee of Nanjing First Hospital. Written
informed consent was obtained before the patients underwent
surgery.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zeichner SB, Terawaki H and Gogineni K: A
review of systemic treatment in metastatic triple-negative breast
cancer. Breast Cancer (Auckl). 10:25–36. 2016.PubMed/NCBI
|
|
3
|
Abdel-Fatah TMA, Agarwal D, Liu DX,
Russell R, Rueda OM, Liu K, Xu B, Moseley PM, Green AR, Pockley AG,
et al: SPAG5 as a prognostic biomarker and chemotherapy
sensitivity predictor in breast cancer: A retrospective, integrated
genomic, transcriptomic, and protein analysis. Lancet Oncol.
17:1004–1018. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cetin I and Topcul M: Triple negative
breast cancer. Asian Pac J Cancer Prev. 15:2427–2431. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park YH, Jung HH, Ahn JS and Im YH: Statin
induces inhibition of triple negative breast cancer (TNBC) cells
via PI3K pathway. Biochem Biophys Res Commun. 439:275–279. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma CX, Cai S, Li S, Ryan CE, Guo ZF,
Schaiff WT, Lin L, Hoog J, Goiffon RJ, Prat A, et al: Targeting
Chk1 in p53-deficient triple-negative breast cancer is
therapeutically beneficial in human-in-mouse tumor models. J Clin
Invest. 122:1541–1552. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jitariu AA, Cimpean AM, Ribatti D and
Raica M: Triple negative breast cancer: The kiss of death.
Oncotarget. 8:46652–46662. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao W, Zhu H, Zhang S, Yong H, Wang W,
Zhou Y, Wang B, Wen J, Qiu Z, Ding G, et al: Trop2 is overexpressed
in gastric cancer and predicts poor prognosis. Oncotarget.
7:6136–6145. 2016.PubMed/NCBI
|
|
9
|
McDougall AR, Tolcos M, Hooper SB, Cole TJ
and Wallace MJ: Trop2: From development to disease. Dev Dyn.
244:99–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Z, Jiang X and Zhang W: TROP2
overexpression promotes proliferation and invasion of lung
adenocarcinoma cells. Biochem Biophys Res Commun. 470:197–204.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cubas R, Zhang S, Li M, Chen C and Yao Q:
Trop2 expression contributes to tumor pathogenesis by activating
the ERK MAPK pathway. Mol Cancer. 9:2532010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guerra E, Trerotola M, Dell' Arciprete R,
Bonasera V, Palombo B, El-Sewedy T, Ciccimarra T, Crescenzi C,
Lorenzini F, Lorenzini F, et al: A bicistronic CYCLIN
D1-TROP2 mRNA chimera demonstrates a novel oncogenic mechanism
in human cancer. Cancer Res. 68:8113–8121. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu Y, Zhang L, Zhang L, Wang Y, Li H, Ren
XB, Wei F, Yu WW, Liu T, Wang X, et al: Long non-coding RNA HOTAIR
promotes tumor cell invasion and metastasis by recruiting EZH2 and
repressing E-cadherin in oral squamous cell carcinoma. Int J Oncol.
46:2586–2594. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bai ZT, Wu ZR, Xi LL, Li X, Chen P, Wang
FQ, Meng WB, Zhou WC, Wu XA, Yao XJ, et al: Inhibition of invasion
by N-trans-feruloyloctopamine via AKT, p38MAPK and EMT related
signals in hepatocellular carcinoma cells. Bioorg Med Chem Lett.
27:989–993. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang Y, Zhang J, Yan Y, Cai H, Li M, Sun
K, Wang J, Liu X, Wang J and Duan X: Low expression of Rap1GAP is
associated with epithelial-mesenchymal transition (EMT) and poor
prognosis in gastric cancer. Oncotarget. 8:8057–8068.
2017.PubMed/NCBI
|
|
16
|
Cong N, Du P, Zhang A, Shen F, Su J, Pu P,
Wang T, Zjang J, Kang C and Zhang Q: Downregulated microRNA-200a
promotes EMT and tumor growth through the wnt/β-catenin pathway by
targeting the E-cadherin repressors ZEB1/ZEB2 in gastric
adenocarcinoma. Oncol Rep. 29:1579–1587. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He R, Zhang FH and Shen N: LncRNA
FEZF1-AS1 enhances epithelial-mesenchymal transition (EMT) through
suppressing E-cadherin and regulating WNT pathway in non-small cell
lung cancer (NSCLC). Biomed Pharmacother. 95:331–338. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang J, Chen XY, Huang KJ, Wu WD, Jiang
T, Cao J, Zhou LS, Qiu ZJ and Huang C: Expression of FoxM1 and the
EMT-associated protein E-cadherin in gastric cancer and its
clinical significance. Oncol Lett. 12:2445–2450. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang ZS, Shen Y, Li X, Zhou CZ, Wen YG,
Jin YB and Li JK: Significance and prognostic value of Gli-1 and
Snail/E-cadherin expression in progressive gastric cancer. Tumour
Biol. 35:1357–1363. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chandra Mangalhara K, Manvati S, Saini SK,
Ponnusamy K, Agarwal G, Abraham SK and Bamezai RNK:
ERK2-ZEB1-miR-101-1 axis contributes to
epithelial-mesenchymal transition and cell migration in cancer.
Cancer Lett. 391:59–73. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ahmed NS, Ghatak S, El Masry MS, Gnyawali
SC, Roy S, Amer M, Everts H, Sen CK and Khanna S: Epidermal
E-Cadherin dependent β-catenin pathway is phytochemical inducible
and accelerates anagen hair cycling. Mol Ther. 25:2502–2512. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Garcia-Pedrero JM, Garcia-Cabo P, Ángeles
Villaronga M, Hermida-Prado F, Granda-Diaz R, Allonca E and Rodrigo
JP: Prognostic significance of E-cadherin and β-catenin expression
in HPV-negative oropharyngeal squamous cell carcinomas. Head Neck.
39:2293–2300. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Su SC, Hsieh MJ, Yang WE, Chung WH, Reiter
RJ and Yang SF: Cancer metastasis: Mechanisms of inhibition by
melatonin. J Pineal Res. 62:2017. View Article : Google Scholar
|
|
25
|
Pearlman RL, Montes de Oca MK, Pal HC and
Afaq F: Potential therapeutic targets of epithelial-mesenchymal
transition in melanoma. Cancer Lett. 391:125–140. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hao Y, Yang X, Zhang D, Luo J and Chen R:
Long noncoding RNA LINC01186, regulated by TGF-β/SMAD3, inhibits
migration and invasion through Epithelial-Mesenchymal-Transition in
lung cancer. Gene. 608:1–12. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin H, Zhang H, Wang J, Lu M, Zheng F,
Wang C, Tang X, Xu N, Chen R, Zhang D, et al: A novel human Fab
antibody for Trop2 inhibits breast cancer growth in vitro and in
vivo. Int J Cancer. 134:1239–1249. 2014. View Article : Google Scholar : PubMed/NCBI
|