Introduction
Radiation-induced lung injury (RILI) is the most
common and the major obstacle prohibiting the high-dose radiation
necessary to eradicate cancer of the thoracic region. Researchers
have shown that 13–37% of patients receiving radical radiotherapy
for lung cancer will experience lung injury (1). Studies have shown that the main
pathophysiological features of RILI are an early phase of acute
pneumonitis and a later phase of fibrosis. There are many
hypotheses regarding RILI pathogenesis, and the perpetual cascade
of cytokine theory is widely accepted (2,3).
According to the theory, the response of normal lung tissue to
radiation injury is a dynamic and continuous process that involves
a variety of cytokines and multiple cell responses.
The proinflammatory cytokines tumour necrosis factor
(TNF)-α, interleukin (IL)-1 and IL-6 are likely key mediators in
the pathogenesis of radiation pneumonitis. Researchers have shown
that TNF-α, IL-6 and IL-1β are all increased in RILI (4). TNF-α is known to induce an acute phase
of the inflammatory response and play a role in tissue remodelling
(5). TNF-α can stimulate
neutrophilic granulocytes to express other cytokines, including
IL-1 and IL-6 (6). In addition,
TNF-α expression leads to the induction of TGF-β, which is a major
pro-fibrotic factor (7). IL-1 and
IL-6 are important immune regulatory factors that are involved in
the inflammatory response and fibrosis process. Studies have shown
that IL-l and IL-6 are circulating cytokine markers for radiation
pneumonitis (8).
Macrophages are thought to be the main source of
proinflammatory cytokines in the early stages of RILI. Hosoi and
colleagues showed that low doses of ionizing radiation in
macrophages could induce proinflammatory cytokines such as IL-1β
and IL-6 (9). Moreover, a study
showed that ionizing radiation led to macrophage-rich pneumonitis,
and Johnston and colleagues showed that the cell types
predominately recruited to the lung for 24 weeks post-irradiation
were macrophages and lymphocytes (10). These results indicate that
macrophages play an important role in RILI.
Nuclear factor κB (NF-κB) is a major switch that
regulates the body's inflammatory response. In recent studies,
ionizing radiation-activated NF-κB was shown to predominantly
upregulate genes involved in intercellular communication processes,
especially genes encoding cytokines and chemokines, such as IL-1β,
IL-6 and TNF-α (11,12). In addition, after high-dose
irradiation, the death and apoptosis of cells such as macrophages
can lead to the secretion of high-mobility group protein-1 (HMGB1),
which activates NF-κB by binding to Toll-like receptor-4 (TLR4),
resulting in the release of proinflammatory cytokines (13). Thus, the HMGB1/TLR4/NF-κB pathway
plays an important role in RILI.
The extracellular matrix (ECM) provides physical
support to tissues, and ECM remodelling is necessary in RILI.
Studies have shown that MMPs and tissue inhibitors of
metalloproteinases (TIMPs) are both involved in remodelling the
ECM. Yang et al showed that MMP-2 and MMP-9 are
overexpressed during the inflammatory response to RILI and degrade
collagen IV in the basement membrane (14).
In addition to inflammatory factors, oxidative
stress and hypoxia also play an important role in RILI. Studies
have shown that reactive oxygen species (ROS) can induce DNA damage
and initiate a series of repair reactions; these excessive repair
responses become the basis of lung injury. Several studies have
documented that antioxidants can reduce radiation-induced pulmonary
fibrosis. An important molecular source of ROS is the enzyme family
of NAD(P)H oxidases (Nox), and the Nox isoforms Nox1, Nox2 and Nox4
have been shown to be involved in pulmonary fibrosis in previous
studies (15–17).
Studies of RILI have shown that HIF-1α is activated
as early as 4 weeks post-irradiation and is accompanied by enhanced
oxidative stress, tissue hypoxia, and NF-κB activity (18). This finding indicates that the
NF-κB, ROS and HIF-1α pathways cooperate with each other during
irradiation-induced lung injury.
Several researchers have investigated the ways to
decrease the extent of RILI, but additional research efforts are
still needed (19). Recent studies
have revealed an immunomodulatory mechanism to limit inflammatory
reactions referred to as the cholinergic anti-inflammatory pathway.
This so-called cholinergic anti-inflammatory pathway is mediated by
acetylcholine, the principal neurotransmitter of the vagus nerve,
through a specific interaction with α7 cholinergic receptors
(α7-nAChR) on many cells, such as macrophages, neutrophils,
endothelial cells, epithelial cells and lymphocytes. Both
electrical stimulation of this pathway (via the vagus nerve) and
pharmacological stimulation of peripheral α7-nAChR have been shown
to have anti-inflammatory effects on several diseases, such as
rheumatoid arthritis (20),
mechanical ventilation-induced lung injury (21) and septic acute kidney injury
(22).
Although many studies have shown the protective
effect of α7-nAChR stimulation in inflammatory diseases, the
beneficial role of α7-nAChR in RILI is poorly understood. GTS-21,
an agonist of α7-nAChR, was reported to reduce acute lung injury
and mechanical ventilation-induced lung injury (21,23).
Thus, the aim of this study was to explore whether the α7-nAchR
agonist GTS-21 is a radioprotective agent for RILI. In this study,
we evaluated the histopathological differences between the GTS-21
and control groups in mouse lungs with or without radiation
treatment. Then, we investigated the effect of GTS-21 on TNF-α,
IL-1β and IL-6 expression in RILI. Finally, we investigated the
influence of GTS-21 on the HMGB1/TLR4/NF-κB pathway and ROS
production to demonstrate the mechanisms of reducing RILI.
Materials and methods
Animals
Eight-week-old female C57BL/6J mice (n=120) with an
approximate body weight of 20 g were purchased from the Animal
Center of Wuhan University (Wuhan, China). The mice, housed 5/cage,
were maintained in an environment with 60±5% humidity at 20±1°C
under a 12-h light/dark cycle. All experimental animals were housed
under specific-pathogen-free conditions for 1 week prior to the
initiation of the experiments. The studies were approved by the
Institutional Animal Care and Use Committee (IACUC), Wuhan
University, Hubei, China (AUP no. 2013065). All animal
experimentation was conducted conforming to the ‘Guiding Principles
for Research involving Animals and Human Beings’ (24).
The mice (n=5 for each time-point per group) were
divided into two experimental groups as follows: i) control group,
healthy mice that received phosphate-buffered saline (PBS); ii)
GTS-21 group, healthy mice that received GTS-21; iii) RT group,
healthy mice that received radiation and PBS; and iv) GTS-21+RT
group, healthy mice that received both radiation and GTS-21.
Cell culture
Murine RAW264.7 macrophages were obtained from the
Cell Resource Center of the Shanghai Institutes for Biological
Sciences of the Chinese Academy of Sciences. Cells were cultured in
DMEM (GE Healthcare Life Sciences/HyClone Laboratories, Logan, UT,
USA) containing 10% fetal bovine serum (FBS; GE Healthcare Life
Sciences/HyClone Laboratories) and 1% penicillin/streptomycin
solution at 37°C in a 5% CO2 atmosphere.
Radiation treatment
For thoracic radiation, mice were anaesthetized by
intraperitoneal application of 0.2 ml/20 g chloral hydrate 4%
(Melonepharma, Dalian, China) and immobilized by cloth surgical
tape. Then, the mice were irradiated with a single dose of 12 Gy
from a linear accelerator (Siemens Primus-Hi, Munich, Germany).
This dose was considered a regular dose to elicit a response from
lung disease (26). The beam was
6-MV photons at a dose rate of 1.886 Gy/min, the source-surface
distance (SSD) was 100 cm, and the radiation field (2.5×15 cm) was
set to cover the whole lung. After radiation, the mice were
maintained in a specific-pathogen-free environment and provided a
standard diet and water.
α7-nAchR agonist GTS-21
GTS-21 was purchased from Abcam (Cambridge, UK) as a
powder, diluted with sterilized water to a final concentration of
500 µg/ml, and then stored at 4°C. Mice received an i.p. injection
of 4 mg/kg GTS-21 (160 µl) or 160 µl of PBS daily for three days
with or without radiation. The dose was based on early studies that
demonstrated that the anti-inflammatory effects of GTS-21 in mice
were dose dependent and that 4 mg/kg GTS-21 significantly
attenuated cytokine production (26,27).
Furthermore, 4 mg/kg GTS-21 is a non-toxic dose according to the
manufacturer's instructions. GTS-21 was also diluted to different
concentrations (0, 10, 100, 1,000 and 10,000 nM) for RAW264.7 cell
treatment.
Tissue isolation
Mice in the GTS-21 and control groups were
sacrificed by cervical dislocation on days 1, 3 and 7 after GTS-21
treatment. Mice in the RT control and GTS-21+RT groups were
sacrificed by cervical dislocation before irradiation (day 0) and
at 1, 3, 7, 14 and 21 days and at 3 and 6 months post-irradiation.
The left lung lobes were excised for histopathology analyses and
measurement of hydroxyproline content. The right lung lobes were
snap-frozen in liquid nitrogen and later used for RNA and protein
isolation. Serum samples were analyzed using a Cytometric Bead
Array (CBA) kit (Becton Dickinson and Company, Franklin Lakes, NJ,
USA).
Histopathological analysis
Histological analysis of mouse tissues was performed
systematically at every time point after irradiation until 6
months, as previously described. In brief, parts of the left lung
were fixed with 4% formalin for 24 h and paraffin embedded.
Sections of 4 µm thickness were cut and stained with haematoxylin
and eosin (H&E) to determine the inflammation level and stained
with Masson's trichrome to detect collagen deposition. The fibrosis
score was quantified in a blinded manner by 2 independent
investigators using light microscopy, according to the criteria of
Hübner et al (28). The
hydroxyproline content was detected by an alkaline hydrolysis assay
kit (Jiancheng Biological Institution, Nanjing, China) that can
indirectly examine collagen content in the lungs. According to the
manufacturer's instructions, the calculation method for
hydroxyproline content was as follows: Content of hydroxyproline
(µg/mg, wet weight) = [absorbance (sample) - absorbance
(blank)]/[absorbance (standard) - absorbance (blank)] × 5 µg/ml ×
10 ml/weight (tissue).
Real-time PCR
Total RNA was extracted from lung tissue or cells
with Invitrogen™ TRIzol reagent (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) according to the manufacturer's protocol. cDNA
was synthesized from 5 µg of total RNA following the manufacturer's
instructions (RevertAid RT kit; Thermo Fisher Scientific, Inc.).
Quantification was performed with SYBR Premix Ex Taq™ (Takara Bio
Inc., Shiga, Japan) in a 20 ml reaction volume. The following
primers were used to amplify TLR-4, HMGB1 and MyD88: TLR-4 (forward
primer, 5′-AGTTTAGAGAATCTGGTGGCTGTG-3′ and reverse primer,
5′-TTCCCTGAAAGGCTTGGTCT-3′); HMGB1 (forward primer,
5′-ACAGCCATTGCAGTACATTGAG-3′ and reverse primer,
5′-TGCCCATGTTTAGTTGATTTCCC-3′); and MyD88 (forward primer,
5′-CCAGCGAGCTAATTGAGAAAAG-3′ and reverse primer,
5′-ATAGTGATGAACCGCAGGATAC-3′). Data analyses were performed using
the 2−ΔΔCq method (29).
Western blot analysis
After protein isolation and homogenization of the
frozen mouse lung, the protein content was determined, and equal
amounts of protein (50 µg/condition) were separated by SDS-PAGE and
transferred to a PVDF membrane (Millipore, Billerica, MA, USA) for
western blotting. The non-specific sites were blocked in 5% non-fat
dry milk at room temperature for 1 h. In this study, antibodies
directed against MyD88 (1:500; cat. no. 66660-1-l g; Proteintech
Group, Chicago, IL, USA), MMP2 (1:500; cat. no. 66366-1-l g;
Proteintech Group), MMP-9 (1:500; cat. no. 10375-2-AP; Proteintech
Group), HIF-1α (1:1,000; cat. no. ab187524; Abcam), TLR-4 (1:1,000;
cat. no. ab13556; Abcam), HMGB1 (1:1,000; cat. no. ab79823; Abcam)
and TIMP1 (1:1,000; cat. no. ab86482; Abcam) were used. The
secondary antibodies were goat anti-mouse horseradish peroxidase
antibody (cat. no. 1706516; Bio-Rad Laboratories, Inc., Hercules,
CA, USA) or goat anti-rabbit horseradish peroxidase antibody (cat.
no. 1662408; Bio-Rad Laboratories, Inc.) used at 1:20,000 or
1:10,000, respectively.
Immunohistochemistry
Slides were deparaffinized, rehydrated though a
graded series of ethanol and treated with 3%
H2O2 in H2O to quench endogenous
peroxidase activity. The specimens were incubated overnight at 4°C
with 100-fold diluted rabbit polyclonal antibody against NF-κB p65
(1:100; cat. no. 10745-1-AP; Proteintech Group) and goat
anti-rabbit secondary antibody (1:50; cat. no. a0208; Beyotime
Institute of Biotechnology, Haimen, China); diaminobenzidine (DAB)
was then used as the chromogen.
Dihydroethidium (DHE) ROS detection
assay
Unfixed frozen lung tissues were cut into 8-µm
sections and placed on glass slides. Slides were incubated with 1
µM dihydroethidium (DHE) in a light-protected, humidified chamber
at 37°C for 30 min, and then coverslipped. Tissue sections were
then visualized with an Olympus BX53 fluorescence microscope
(Olympus Corp., Tokyo, Japan), and fluorescence was detected with a
590 nm long-pass filter. Images were collected and stored
digitally.
RAW246.7 cells were seeded at 5×105
cells/well in 6-well plates. After cultivation overnight, cells
were treated with or without GTS-21 (100 nM) after 6 Gy
irradiation. After 24 h of cultivation, cells were incubated with 1
µM dihydroethidium (DHE) and placed in a chamber at 37°C for 30
min.
Serum cytokine measurements
Murine serum was collected from euthanized mice at
every time point post-irradiation. TNF-α, IL-1β and IL-6 in serum
from mice were determined using a CBA kit (BD Biosciences, San
Diego, CA, USA) according to the manufacturer's instructions.
MTT assays
RAW264.7 cells in the exponential growth phase were
seeded at a density of 5,000 cells/well in 96-well plates and
irradiated with 6 Gy. Then, the cells were treated with 200 µl of
0, 10, 100, 1,000 and 10,000 nM GTS-21 and cultured for 24, 48, 72,
96 and 120 h at 37°C in a humidified 5% CO2 incubator.
MTT assays were performed according to the manufacturer's
instructions (Beyotime Institute of Technology, Nanjing, China).
The absorbance values were determined at 570 nm on a Moduluse II
Microplate Multimode Reader (Turner BioSystems Inc., Sunnyvale, CA,
USA). All MTT assays were performed three times in
quintuplicate.
Statistical analyses
The data are presented as the mean ± SEM. The data
for the various time points were analyzed by one-way ANOVA and
unpaired two-tailed t-tests. Multiple comparisons between the
groups were performed using Tukey's post hoc test. P<0.05 were
considered statistically significant.
Results
GTS-21 reduces lung inflammatory
infiltrate and fibrosis in radiation-treated mice
Based on the results of H&E staining, GTS-21
treatment had little effect on lung tissue in the absence of
irradiation (Fig. 1A). Meanwhile,
radiation treatment of mice without GTS-21 treatment increased the
inflammatory response in the lungs over time, with the most notable
response at 21 days after radiation treatment. In contrast, the
GTS-21+RT group showed significantly lower inflammation on day 21
(Fig. 1B). Histological evaluation
revealed that the fibrosis level was significantly decreased in the
GTS-21+RT group compared with the RT control after 6 months but was
not significantly different between the GTS-21 and RT control
groups (Fig. 1C and D). To more
accurately evaluate the fibrosis level among the four groups, we
quantified lung fibrosis levels using a modified Ashcroft scale
based on hydroxyproline content. The fibrosis score was
significantly decreased in the GTS-21 treatment group compared with
the RT control group at 6 months after irradiation (3±0.63 and
4.8±0.44, respectively, P=0.001), while the RT control and GTS-21
groups showed no difference in the effect at 3 or 6 months
(Fig. 1E). We then used
hydroxyproline to test the fibrosis level of the lung in the four
groups at 6 months after irradiation. The results showed that the
hydroxyproline levels in the control and GTS-21 groups were not
different after 6 months. However, we observed significantly lower
expression of hydroxyproline in the GTS-21+RT group than in the RT
control group at 6 months (0.72±0.06 and 0.97±0.17 µg/mg,
respectively, P=0.03; Fig. 1F).
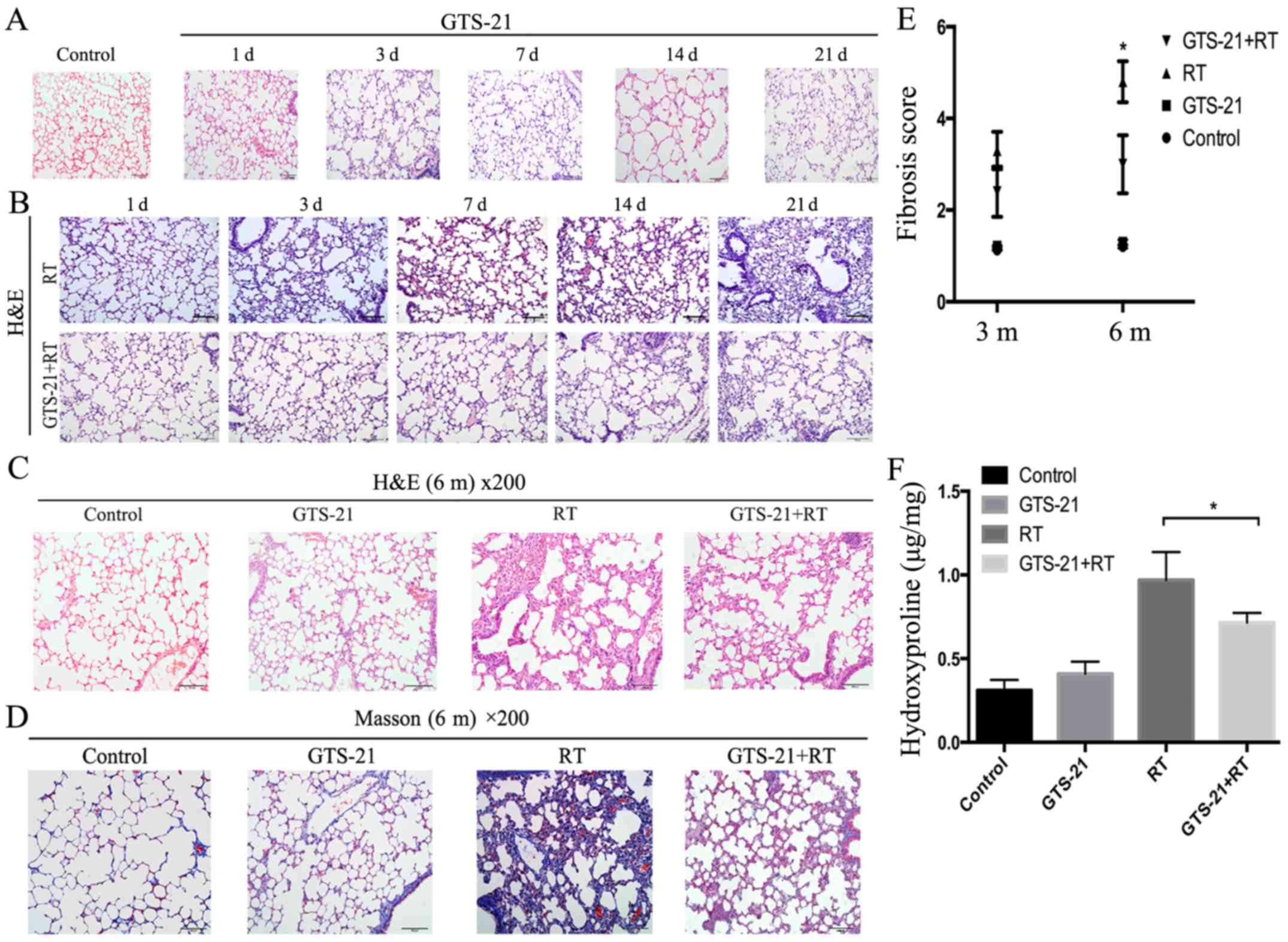 | Figure 1.GTS-21 reduces radiation-induced
histological signs of pulmonary injury. (A) Lungs were removed on
days (d) 1, 3, 7, 14 and 21 after GTS-21 or PBS treatment and
stained with haematoxylin and eosin (H&E). (B) Lungs were
removed at the indicated times after RT or GTS-21+RT and stained
with H&E to assess the extent of inflammation and fibrosis. (C
and D) H&E-stained and Masson's trichrome-stained sections
viewed at ×200 magnification at 6 months (m) after the following
treatments: PBS administration, GTS-21 administration, irradiation
or GTS-21 administration and irradiation treatment combined with
GTS-21 administration. (E) The fibrosis scores were calculated for
the four groups (control, GTS-21, RT and GTS-21+RT) at different
time-points. (F) The hydroxyproline content was detected in the
lungs removed from mice after PBS, GTS-21, radiation or GTS-21 and
radiation combination treatment for 6 months. Error bars represent
the SEM. *P<0.05. Groups: Control, healthy mice that received
phosphate-buffered saline (PBS); GTS-21, healthy mice that received
GTS-21; RT, healthy mice that received radiation and PBS; and
GTS-21+RT, healthy mice that received both radiation and
GTS-21. |
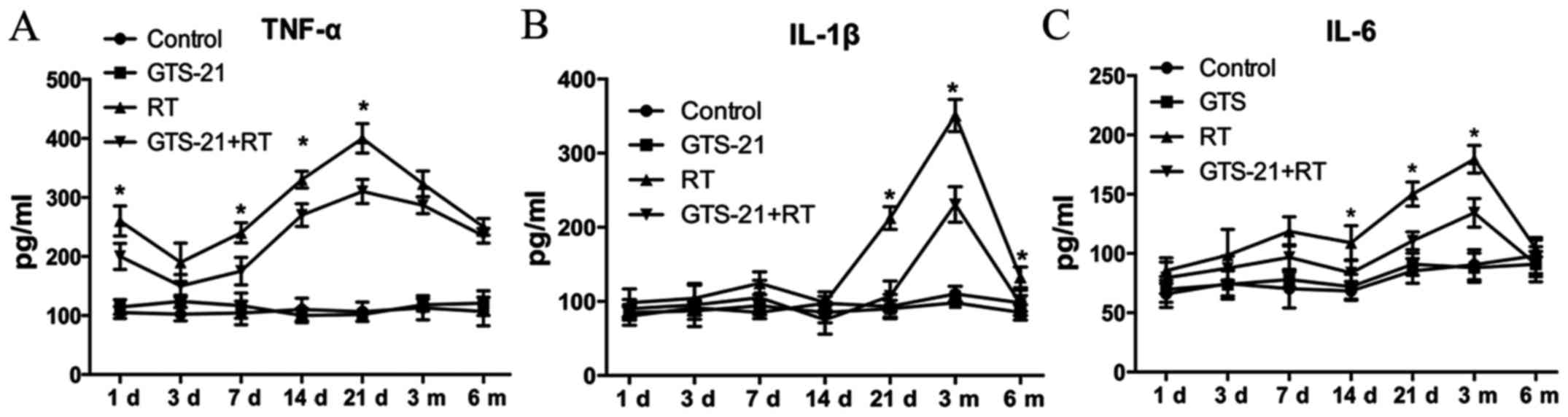
GTS-21 reduces the levels of TNF-α,
IL-1β and IL-6 in radiation-treated mice
To assess the inflammatory cytokine response to
GTS-21 treatment with or without radiation treatment, we measured
three specific proinflammatory cytokines (TNF-α, IL-1β and IL-6) in
the mouse sera. The results showed that TNF-α expression was
significantly decreased in the GTS-21+RT group compared to the RT
control group, especially at 21 days post-radiation (Fig. 2A). IL-1β expression was also lower
in the GTS-21+RT group than in the RT control group at 21 days
after irradiation, and it remained significantly lower at 3 and 6
months post-radiation. However, we found no significant difference
in IL-1β expression between the GTS-21+RT group and RT control
group during the early stages of the inflammatory response
(Fig. 2B). Fig. 2C shows that the level of IL-6 in the
GTS-21+RT group was significantly decreased at 14 and 21 days and 3
months after irradiation, while there was no significant difference
at other time points. Furthermore, we found that the levels of
TNF-α, IL-1β and IL-6 were not different between the control and
GTS-21 groups, indicating that GTS-21 had little effect on
proinflammatory cytokines without irradiation.
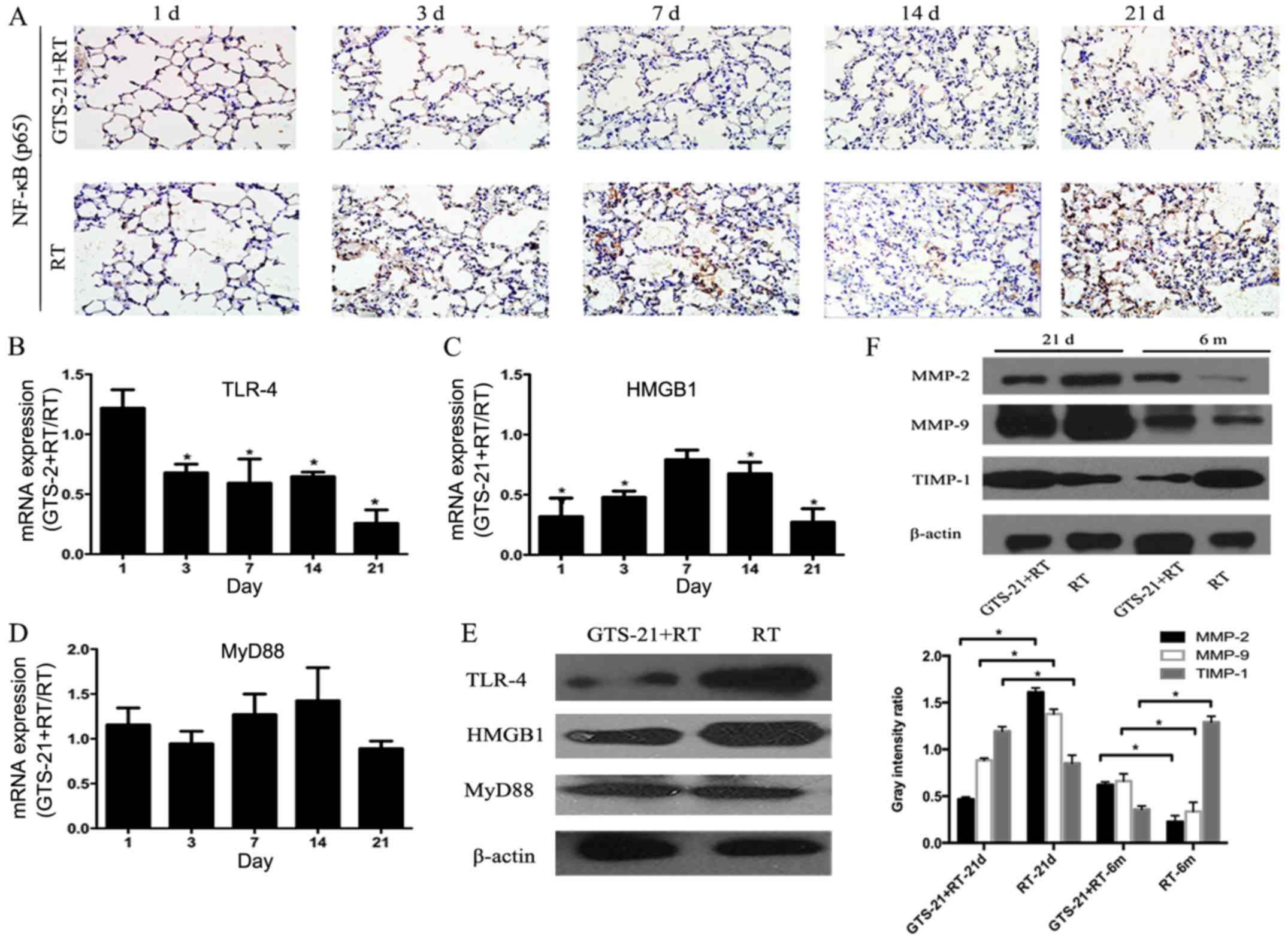
GTS-21 inhibits the HMGB1/TLR-4/NF-κB
pathway and regulates MMP/TIMP balance after irradiation
Since the NF-κB heterodimer p50/p65 is most often
activated in the radiation response and only p65 contains the
transactivation domain in its C-terminal region, the activity of
NF-κB can be evaluated by detecting the expression of p65. In this
study, we performed immunohistochemistry to detect NF-κB p65
expression in the GTS-21+RT and RT groups at the inflammatory stage
after irradiation (Fig. 3A). The
results showed that NF-κB p65 was significantly inhibited at 21
days in the GTS-21+RT group compared with the RT control. Then, we
compared the mRNA expression levels of TLR-4, HMGB1 and MyD88 in
the RT and GTS-21+RT groups at the inflammatory response stage. As
shown in Fig. 3B, the mRNA level of
TLR-4 in the GTS-21 treatment group trended towards an increase at
1 day after irradiation compared with the control, but was rapidly
decreased in the following days after irradiation. The most
significant fold change was revealed at 21 days post-radiation
(GTS-21+RT compared to RT control, 0.25±0.11, P≤0.05). HMGB1 mRNA
expression was immediately decreased after irradiation in the
GTS-21 treatment group compared with the RT control group
(GTS-21+RT compared to RT control, 0.31±0.15, P≤0.05) and continued
to decrease at the following time points, especially at 21 days
post-radiation (GTS-21+RT compared to RT control, 0.27±0.10,
P≤0.05). However, we did not find any differences in HMGB1
expression between the two groups at 7 days post-radiation
(Fig. 3C). Then, we examined the
mRNA level of MyD88 in the GTS-21+RT and RT control groups, but no
difference was found between them (Fig.
3D). Because the inflammatory response was significantly
different between the two groups on day 21 post-radiation, we chose
the time point of day 21 post-radiation to confirm the effect of
GTS-21 on the protein levels of TLR-4, HMGB1 and MyD88. The results
showed that TLR-4 and HMGB1 protein expression was significantly
reduced in mice treated with GTS-21. However, we did not observe
any alteration in the protein level of MyD88 (Fig. 3E). Furthermore, to elucidate the
effect of GTS-21 on extracellular matrix metalloproteinases that
are commonly involved in RILI, we examined their protein levels on
day 21 and at 6 months after irradiation. The results showed that
MMP-2 and MMP-9 protein expression was significantly reduced in
GTS-21-treated mice on day 21 and increased at 6 months after
irradiation compared with RT control mice. TIMP-1 was increased on
day 21 and decreased at 6 months after irradiation in the GTS-21
treatment group compared to the RT control group (Fig. 3F).
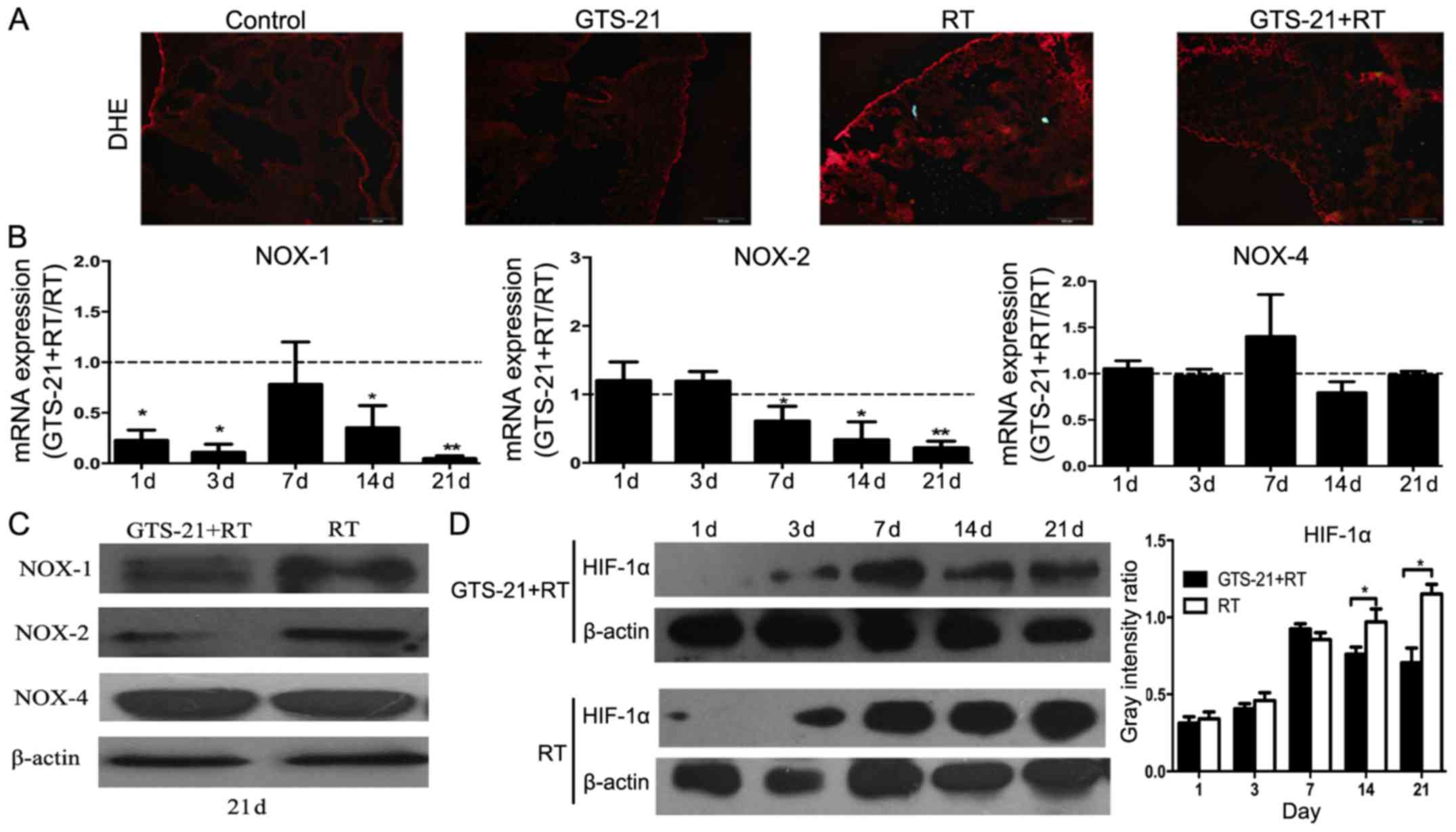
GTS-21 alleviates tissue hypoxia and
reduces ROS levels by inhibiting NOX-1 and NOX-2 in radiation
pneumonitis
To elucidate the effect of GTS-21 on ROS production
in radiation pneumonitis, we used DHE to analyze ROS in mouse lungs
on day 21 after irradiation. The results revealed that the ROS
level in lung tissues from the GTS-21+RT treatment group was
significantly reduced at 21 days after irradiation compared to that
from the RT control group. However, we did not observe any
difference in the GTS-21 and control groups without irradiation
(Fig. 4A). To further clarify the
relevant mechanisms, real-time PCR was used to detect the mRNA
levels of NOX1/2/4 in the GTS-21+RT and RT groups. The results
showed that the mRNA level of NOX-1 was reduced significantly in
the GTS-21 treatment group, except on day 7 after irradiation
(P<0.05); NOX-2 mRNA expression was also inhibited in the
GTS-21+RT group, especially at 21 days post-irradiation. There was
no difference in NOX-4 expression between the GTS-21+RT and RT
groups (Fig. 4B). Fig. 4C shows the corresponding NOX protein
levels on day 21 after irradiation in GTS-21-treated mice and
control mice, as evaluated by western blot analysis. Finally, we
found that the protein level of HIF-1α was significantly reduced at
14 and 21 days after irradiation in the GTS-21+RT group compared to
the RT control (Fig. 4D).
GTS-21 suppresses cell proliferation
and inhibits the HMGB1/TLR-4/NF-κB pathway and ROS production in
RAW264.7 cells after irradiation
Macrophages are the most active cells in lung
tissue, and they are considered to be the main source of
inflammatory cytokines in lung tissue after irradiation. Here, we
used murine RAW264.7 macrophages to confirm the effect of GTS-21 on
the inflammatory response in vitro. First, we used MTT
assays to detect whether GTS-21 can influence RAW264.7 cell
proliferation, and the result showed that RAW264.7 cell
proliferation was significantly inhibited with both low-dose (10
and 100 nM) and high-dose (1,000 and 10,000 nM) GTS-21 (Fig. 5A). Then, cells were treated with
three doses of GTS-21 immediately after irradiation, and the mRNA
levels of TLR-4, HMGB1, and MyD88 were examined by real-time PCR.
The results showed that each dose could significantly reduce the
mRNA levels of HMGB1 and TLR-4 but not MyD88, which was consistent
with the experimental data obtained in vivo. Since 100 nM
GTS-21 was sufficient to achieve the desired effect, we next used
100 nM GTS-21 to confirm its effect on the protein levels of
NF-κB(p65), TLR-4, HMGB1 and MyD88. The results showed that the
protein levels of NF-κB(p65), HMGB1 and TLR-4 in RAW264.7 cells
were also significantly reduced after GTS-21 treatment. Similarly,
there was no significant change in the expression of MyD88 between
the GTS-21+RT and RT groups (Fig.
5B). Finally, we confirmed the effect of GTS-21 on ROS
production in RAW264.7 cells after irradiation, and DHE staining
revealed that the ROS level was significantly decreased in the
GTS-21 group after 6 Gy irradiation when compared to the RT only
group (Fig. 5C).
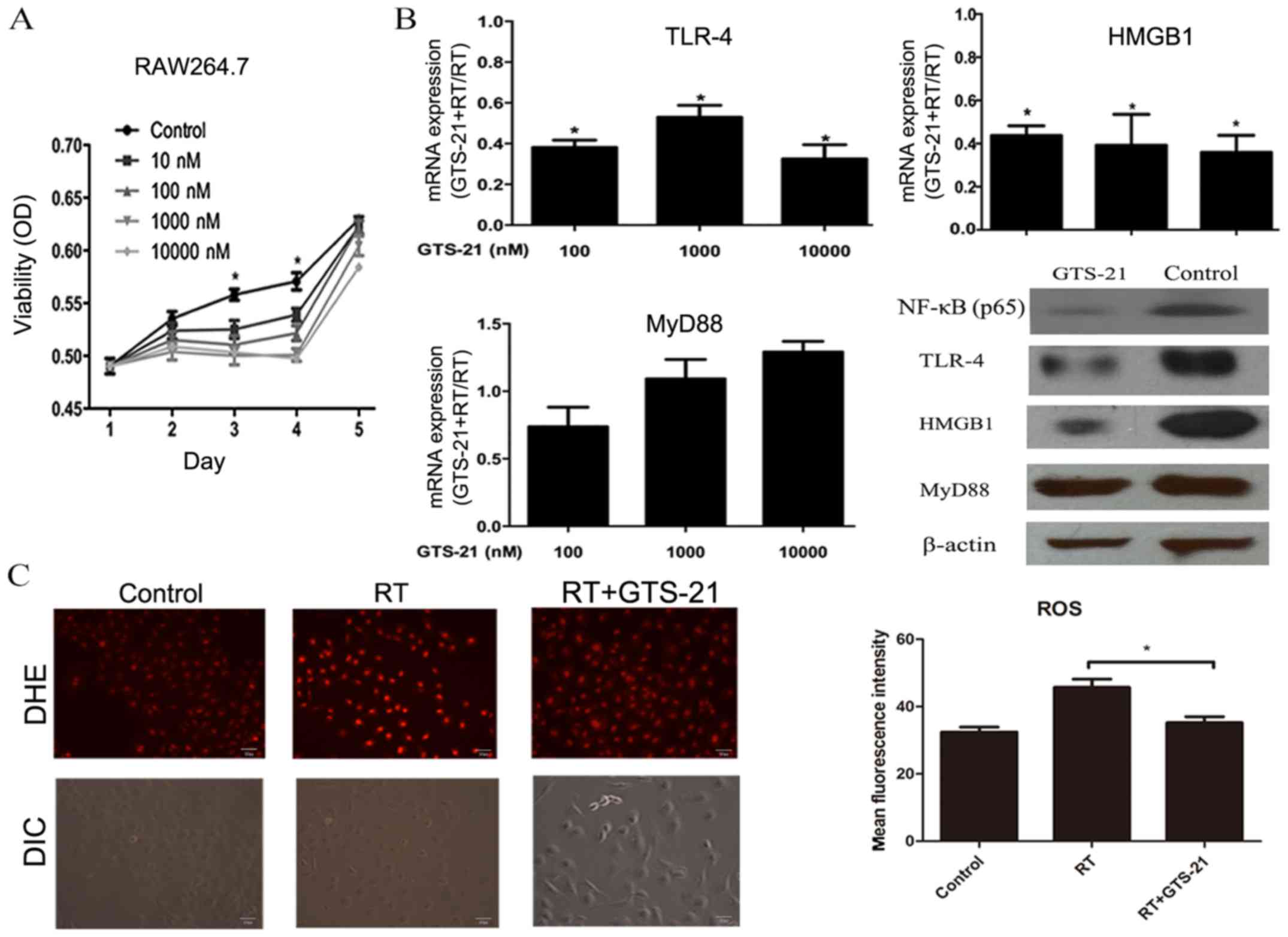 | Figure 5.Effect of GTS-21 on RAW264.7
macrophages after irradiation. (A) RAW264.7 cells were irradiated
at 6 Gy and treated with different concentrations of GTS-21 (0, 10,
100, 1,000 and 10,000 nM) after irradiation. After 1, 2, 3, 4 and 5
days, the cells were subjected to MTT assays. (B) RAW264.7 cells
were irradiated at 6 Gy and treated with or without GTS-21 (100,
1,000 and 10,000 nM). After 48 h, the mRNA levels of HMGB1, TLR4
and MyD88 were analyzed by real-time PCR. The protein levels of
NF-κB (p65), TLR4, HMGB1 and MyD88 were detected in cells that were
treated with or without GTS-21 (100 nM) at 48 h after irradiation.
(C) RAW264.7 cells were irradiated at 6 Gy and treated with or
without GTS-21 (100 nM). Unirradiated cells were used as negative
controls. After 24 h, ROS production in each group was observed by
DHE. Statistical data of fluorescence measurements are presented as
histograms (right). Magnification, ×200. Error bars represent the
SEM. *P<0.05. Groups: Control, healthy mice that received
phosphate-buffered saline (PBS); GTS-21, healthy mice that received
GTS-21; RT, healthy mice that received radiation and PBS; and
GTS-21+RT, healthy mice that received both radiation and
GTS-21. |
Discussion
Radiation-induced pneumonia and fibrosis often occur
as a complication of thoracic irradiation, which leads to reduced
radiation treatment doses and, subsequently, decreased efficiency
against cancer. However, there is a lack of effective therapies to
prevent this severe side-effect, which requires further study.
Our results demonstrated that stimulation of the
vagus nerve through the α7-nAChR agonist GTS-21 markedly reduced
the extent and severity of radiation-induced pneumonia and fibrosis
in mice. Moreover, the levels of proinflammatory cytokines such as
TNF-α, IL-1β and IL-6 were all reduced in mice treated with GTS-21
after irradiation. Finally, we demonstrated that the protective
effect of GTS-21 on RILI occurred partly through inhibition of the
HMGB1/TLR4/NF-κB pathway and ROS production. The results were
verified in vitro.
GTS-21 is a selective agonist of α7-nAChR with
anti-inflammatory and cognition-enhancing activities. In two phase
II clinical trials, volunteers were observed to have good tolerance
to GTS-21 (30,31). In the present study, we used the
recommended dose (4 mg/kg) of GTS-21 in mice after irradiation,
which resulted in decreased inflammatory infiltrate and
subsequently alleviated late fibrosis according to a
histopathological analysis. Similar results were shown by Leib and
colleagues, who showed that activation of the cholinergic pathway
through α7-nAChR could reduce inflammation and fibrosis in the
myocardium (32).
TNF-α, IL-1β and IL-6 have been shown to play a role
in the pathogenesis of RILI. Moreover, the results from previous
studies have shown that inhibiting TNF-α and IL-6 could reduce
radiation-induced lung fibrosis (33,34).
In addition, research from Zhao and colleagues showed that
standardized Myrtol could attenuate RILI by inhibiting TNF-α, IL-1β
and IL-6 expression (35). Thus,
inhibition of these proinflammatory cytokines may protect against
the development of RILI in mice. In the present study, we showed
that the agonist α7-nAChR led to reduced secretion of TNF-α
immediately after irradiation, and the most obvious difference was
observed at 21 days post-radiation. Furthermore, despite the low
expression of IL-6 and IL-1β observed in the GTS-21+RT group, the
differences in expression between the GTS-21+RT and RT groups were
observed only after 14 days. Rübe et al showed that IL-6 and
IL-1 expression was maintained at a low level during the early
inflammatory response and increased at 4 weeks after irradiation
(36). Therefore, we concluded that
the expression of IL-6 and IL-1β may be low at early time points
after irradiation, making it difficult to detect differences
between groups.
NF-κB is a master switch in inflammation and the
central element of the acute innate immune response. Studies have
shown that NF-κB is immediately activated after exposure to
radiation and subsequently upregulates inflammatory cytokines,
chemokines and apoptosis-related factors, which promote
inflammation in tissue (11). Haase
and colleagues showed that NF-κB activation was sustained
throughout the progression of RILI in rats (37). Here, we showed that GTS-21 treatment
reduced the protein level of NF-κB(p65) during the early stage of
inflammation and clearly inhibited its expression on day 21 after
irradiation, which was the most severe period of the inflammatory
response, in the RT control group. The results indicate that GTS-21
can reduce radiation pneumonitis by inhibiting the activation of
NF-κB. Li and colleagues demonstrated that α7-nAChR agonists could
protect the liver from ischaemia-reperfusion injury by inhibiting
the expression of NF-κB(p65), which is consistent with the results
of our study (39). Café-Mendes and
colleagues also showed that α7-nAChR agonists decreased LPS-induced
activation of NF-κB in the neuroinflammatory system of the
hippocampus (40).
Toll-like receptor (TLR)-4 is an innate immune
receptor expressed by macrophages, dendritic cells, lymphocytes and
other immune cells (41). TLR-4 can
recognize endogenous ligands released by damaged or stressed
tissues, including HMGB1 (41).
This triggers downstream signaling cascades in a bifurcated fashion
via MyD88 and TRIF. A recent study showed that knockout of TLR-4 in
C57BL/6 mice significantly reduced radiation-induced pulmonary
fibrosis (42). In the present
study, we found that GTS-21 reduced TLR-4 transcript and protein
levels in mouse lungs compared with those in control lungs. This
result was consistent with that from a study by Kox et al
which showed that GTS-21 downregulated the monocyte cell-surface
expression of TLR4 during inflammation (43). However, we found that the expression
of MyD88 did not differ between the GTS-21+RT and RT groups after
irradiation, which indicated that GTS-21 had no effect on the MyD88
signaling pathway.
HMGB1 is present in all eukaryotic cells as a
conserved nuclear protein, and it can be secreted by the cell in
response to lipopolysaccharide, IL-1 or TNF-α stimulation.
Moreover, HMGB1 can bind to TLR-4 and activate NF-κB inflammatory
signaling pathways, which promote inflammatory cytokine release
(44–46). Studies have shown that radiation can
directly stimulate cells to secrete HMGB1 and increase the
expression of TLR-4 through a p53-dependent pathway (47,48).
Furthermore, as mentioned above, the HMGB1/TLR-4/NF-κB pathway can
be activated directly by radiation, which promotes an inflammatory
reaction. However, in this study, we found that the α7-nAChR
agonist GTS-21 reduced the transcript level of HMGB1 at the early
stage of inflammation and notably inhibited its expression on day
21 post-radiation. The protein level of HMGB1 on day 21 was also
decreased in the GTS-21 group. Sitapara and colleagues demonstrated
that GTS-21 effectively protected against mechanical
ventilation-induced lung injury by inhibiting HMGB1 release from
macrophages, which is consistent with the results of this study
(23).
The proteolytic activity of the MMPs is related to
extracellular matrix degradation, and it is precisely regulated by
TIMPs in tissue. Disruption of this balance is usually observed in
RILI. Li and colleagues showed that MMP inhibitors prevented
irradiation-induced lung injury in mice (49). In this study, we investigated
whether GTS-21 stimulation had an effect on extracellular MMPs and
TIMPs. Here, we showed that GTS-21 treatment reduced MMP-2/9
protein levels and increased TIMP-1 levels at the pulmonary
inflammation stage after irradiation. We further observed that
MMP-2/9 expression levels were higher while TIMP-1 expression
levels were lower in the GTS-21 treatment group than in the RT
control group during the pulmonary fibrosis stage. These results
indicated that GTS-21 may regulate the MMP/TIMP balance in
RILI.
ROS can immediately activate NF-κB and act as the
initiating factor of early inflammation after irradiation. Thus, to
investigate the effect of GTS-21 on ROS production in mouse lung
tissue after irradiation, we used DHE to detect ROS levels in the
lung tissue of GTS-21-treated and control mice at 21 days after
irradiation. We found that GTS-21 reduced ROS production on day 21
after irradiation compared to that in the controls. Studies by
Hiramoto et al (50) and
Navarro et al (51) showed
that α7-nAchR agonists could reduce the level of ROS, which is
similar to our findings.
NADPH oxidase is one of the major sources of ROS
generation. It is a multi-protein complex enzyme that is
functionally expressed in many cells. Recent studies have indicated
that NADPH oxidase-derived ROS play a pathophysiological role in
radiation-induced normal tissue injury (52). NOX-1/2/4 are the most widely studied
subunits in the present study. To further clarify the possible
mechanism by which GTS-21 inhibits ROS, we detected the expression
of NOX-1/2/4 in the lung tissues of the GTS-21+RT and RT groups
after irradiation. The results showed that GTS-21 could reduce the
expression of NOX-1/2 but had no effect on the expression of
NOX-4.
HIF-1α is closely related to radiation pneumonitis
and pulmonary fibrosis in mice. Researchers have speculated that
HIF-1α inhibitors may have protective effects on RILI. The present
study examined the expression of HIF-1α in lung tissue after 1, 3,
7, 14 and 21 days in the GTS-21-treated and control groups after
irradiation. The results showed that the expression of HIF-1α in
the GTS-21+RT group was lower than that in the control group 14
days post-radiation. The results indicated that GTS-21 could reduce
the expression of HIF-1α in lung tissue after irradiation. Since
GTS-21 and HIF-1α have not been demonstrated by any study to
directly interact, the reduction of HIF-1α may depend on the
improvement of the inflammatory environment, reduction of ROS
production and decrease in the degree of pulmonary fibrosis by
GTS-21.
Based on the results above, we found that GTS-21
inhibited the HMGB1/TLR-4/NF-κB pathway and reduced ROS production
in the RILI mouse model. The mechanism of GTS-21 in RILI is shown
in Fig. 6. As macrophages are
considered to be very important target cells in RILI and highly
express α7-nAchR, we further confirmed the effect of GTS-21 on
macrophages after irradiation. Our results showed that GTS-21
inhibited macrophage proliferation after irradiation. Jenkins and
colleagues found that local macrophage proliferation rather than
recruitment from the blood was a feature of Th2 inflammation
(53). Moreover, a previous study
from our group showed that RILI was also the result of a Th2-like
immune response (54). Thus, we
hypothesized that GTS-21 may alleviate RILI by inhibiting
macrophage proliferation. We found that GTS-21 could inhibit NF-κB
activation and suppress the expression of HMGB1 and TLR-4
transcripts and protein after irradiation, although it had no
effect on MyD88 expression. These results were consistent with the
in vivo results, which indicated that GTS-21 could reduce
RILI by inhibiting the HMGB1/TLR-4/NF-κB inflammatory pathway in
macrophages. Finally, we examined ROS production in macrophages
after irradiation with or without GTS-21 treatment, and the results
showed that GTS-21 also reduced ROS production in macrophages after
irradiation.
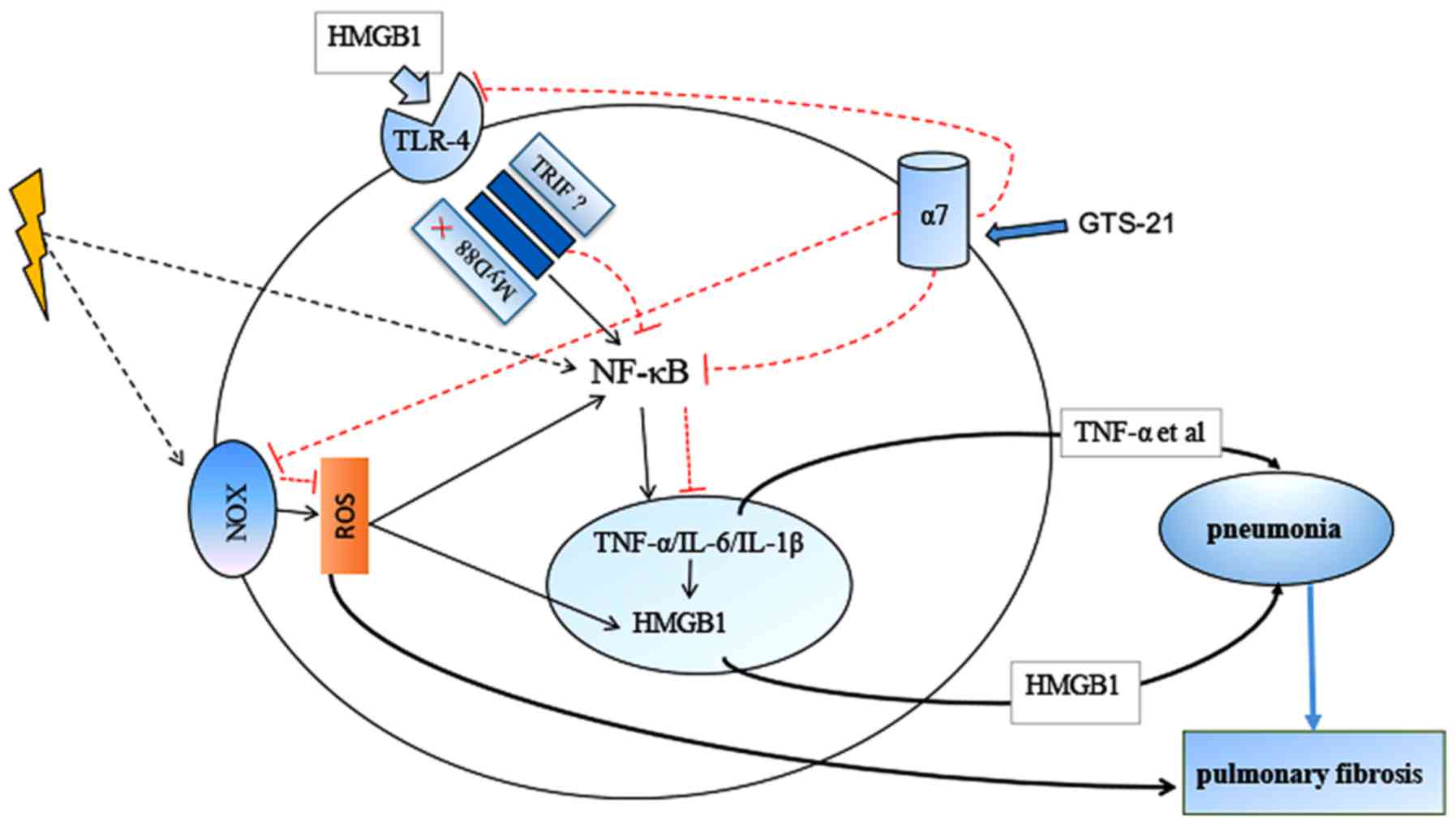 | Figure 6.Mechanism of GTS-21 protective role
in radiation-induced lung injury. After high-dose irradiation, the
death and apoptosis of cells such as macrophages can lead to the
secretion of high-mobility group protein-1 (HMGB1), which activates
TLR-4, subsequently activating NF-KB through MyD88 or
MyD88-independent pathway and resulting in the release of
proinflammatory cytokines such as TNF-α, IL-1β and IL-6. These
proinflammatory cytokines can also upregulate HMGB1, forming a
positive feedback loop that continues to promote inflammatory
signalling. GTS-21, an agonist of α7-nAChR, can inhibit TLR-4
through MyD88-independent pathway and inhibit HMGB1expresstions
which following inhibits NF-KB activation. Second, ROS can activate
NF-κB immediately and act as the initiating factor of early
inflammation after irradiation. GTS-21 reduced the expression of
NOX-1/2 and, thus, reduced radiation-induced ROS generation. |
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81272996) and the
Natural Science Foundation of Hubei Province (grant no.
2013CFA006).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
ZM and XT carried out most of the practical work and
drafted the manuscript. JC and YW contributed to the analysis and
interpretation of the data. YY and XL performed the animal
experiment. CY and SZ performed part of the molecular biology and
cell culture. CX participated in the study design and modified the
manuscript. All authors read and approved the manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The studies were approved by the Institutional
Animal Care and Use Committee (IACUC), Wuhan University, Hubei,
China (AUP no. 2013065).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Part of this work was presented as a poster at the
IASLC 17th World Conference on Lung Cancer (55).
References
|
1
|
Das SK, Zhou S, Zhang J, Yin FF, Dewhirst
MW and Marks LB: Predicting lung radiotherapy-induced pneumonitis
using a model combining parametric Lyman probit with nonparametric
decision trees. Int J Radiat Oncol Biol Phys. 68:1212–1221. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pahl HL: Activators and target genes of
Rel/NF-kappaB transcription factors. Oncogene. 18:6853–6866. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iademarco MF, McQuillan JJ, Rosen GD and
Dean DC: Characterization of the promoter for vascular cell
adhesion molecule-1 (VCAM-1). J Biol Chem. 267:16323–16329.
1992.PubMed/NCBI
|
|
4
|
Liu GD, Xia L, Zhu JW, Ou S, Li MX, He Y,
Luo W, Li J, Zhou Q, Yang XQ, et al: Genistein alleviates
radiation-induced pneumonitis by depressing Ape1/Ref-1 expression
to down-regulate inflammatory cytokines. Cell Biochem Biophys.
69:725–733. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wajant H, Pfizenmaier K and Scheurich P:
Tumor necrosis factor signaling. Cell Death Differ. 10:45–65. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Javaid K, Rahman A, Anwar KN, Frey RS,
Minshall RD and Malik AB: Tumor necrosis factor-alpha induces
early-onset endothelial adhesivity by protein kinase
Czeta-dependent activation of intercellular adhesion molecule-1.
Circ Res. 92:1089–1097. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sullivan DE, Ferris M, Pociask D and Brody
AR: Tumor necrosis factor-alpha induces transforming growth
factor-beta1 expression in lung fibroblasts through the
extracellular signal-regulated kinase pathway. Am J Respir Cell Mol
Biol. 32:342–349. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen Y, Williams J, Ding I, Hernady E, Liu
W, Smudzin T, Finkelstein JN, Rubin P and Okunieff P: Radiation
pneumonitis and early circulatory cytokine markers. Semin Radiat
Oncol. 12 1 Suppl 1:S26–S33. 2002. View Article : Google Scholar
|
|
9
|
Hosoi Y, Miyachi H, Matsumoto Y, Enomoto
A, Nakagawa K, Suzuki N and Ono T: Induction of interleukin-1beta
and interleukin-6 mRNA by low doses of ionizing radiation in
macrophages. Int J Cancer. 96:270–276. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Johnston CJ, Williams JP, Elder A, Hernady
E and Finkelstein JN: Inflammatory cell recruitment following
thoracic irradiation. Exp Lung Res. 30:369–382. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hellweg CE: The Nuclear Factor kappaB
pathway: A link to the immune system in the radiation response.
Cancer Lett. 368:275–289. 2005. View Article : Google Scholar
|
|
12
|
Chishti AA, Baumstark-Khan C, Koch K,
Kolanus W, Feles S, Konda B, Azhar A, Spitta LF, Henschenmacher B,
Diegeler S, et al: Linear energy transfer modulates
radiation-induced NF-kappa B activation and expression of its
downstream target genes. Radiat Res. 189:354–370. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pradere JP, Dapito DH and Schwabe RF: The
Yin and Yang of Toll-like receptors in cancer. Oncogene.
33:3485–3495. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang K, Palm J, Konig J, Seeland U,
Rosenkranz S, Feiden W, Rübe C and Rübe CE:
Matrix-Metallo-Proteinases and their tissue inhibitors in
radiation-induced lung injury. Int J Radiat Biol. 83:665–676. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Masamune A, Watanabe T, Kikuta K, Satoh K
and Shimosegawa T: NADPH oxidase plays a crucial role in the
activation of pancreatic stellate cells. Am J Physiol Gastrointest
Liver Physiol. 294:G99–G108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stas S, Whaley-Connell A, Habibi J, Appesh
L, Hayden MR, Karuparthi PR, Qazi M, Morris EM, Cooper SA, Link CD,
et al: Mineralocorticoid receptor blockade attenuates chronic
overexpression of the renin-angiotensin-aldosterone system
stimulation of reduced nicotinamide adenine dinucleotide phosphate
oxidase and cardiac remodeling. Endocrinology. 148:3773–3780. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hecker L, Vittal R, Jones T, Jagirdar R,
Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ and Thannickal
VJ: NADPH oxidase-4 mediates myofibroblast activation and
fibrogenic responses to lung injury. Nat Med. 15:1077–1081. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rabbani ZN, Mi J, Zhang Y, Delong M,
Jackson IL, Fleckenstein K, Salahuddin FK, Zhang X, Clary B,
Anscher MS and Vujaskovic Z: Hypoxia inducible factor 1alpha
signaling in fractionated radiation-induced lung injury: Role of
oxidative stress and tissue hypoxia. Radiat Res. 173:165–174. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mehta V: Radiation pneumonitis and
pulmonary fibrosis in non-small-cell lung cancer: Pulmonary
function, prediction, and prevention. Int J Radiat Oncol Biol Phys.
63:5–24. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koopman FA, Schuurman PR, Vervoordeldonk
MJ and Tak PP: Vagus nerve stimulation: A new bioelectronics
approach to treat rheumatoid arthritis? Best Pract Res Clin
Rheumatol. 28:625–635. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kox M, Pompe JC, Peters E, Vaneker M, van
der Laak JW, van der Hoeven JG, Scheffer GJ, Hoedemaekers CW and
Pickkers P: α7 nicotinic acetylcholine receptor agonist GTS-21
attenuates ventilator-induced tumour necrosis factor-alpha
production and lung injury. Br J Anaesth. 107:559–566. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chatterjee PK, Yeboah MM, Dowling O, Xue
X, Powell SR, Al-Abed Y and Metz CN: Nicotinic acetylcholine
receptor agonists attenuate septic acute kidney injury in mice by
suppressing inflammation and proteasome activity. PLoS One.
7:e353612012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sitapara RA, Antoine DJ, Sharma L, Patel
VS, Ashby CR Jr, Gorasiya S, Yang H, Zur M and Mantell LL: The α7
nicotinic acetylcholine receptor agonist GTS-21 improves bacterial
clearance in mice by restoring hyperoxia-compromised macrophage
function. Mol Med. 20:238–247. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
World Medical Association General
Assembly: Guiding principles for research involving animals and
human beings. Am J Physiol Cell Physiol. 280:3p after R1913.
2002.
|
|
25
|
Chen J, Tian X, Mei Z, Wang Y, Yao Y,
Zhang S, Li X, Wang H, Zhang J and Xie C: The effect of the TLR9
ligand CpG-oligodeoxynucleotide on the protective immune response
to radiation-induced lung fibrosis in mice. Mol Immunol. 80:33–40.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pavlov VA, Ochani M, Yang LH,
Gallowitsch-Puerta M, Ochani K, Lin X, Levi J, Parrish WR,
Rosas-Ballina M, Czura CJ, et al: Selective alpha7-nicotinic
acetylcholine receptor agonist GTS-21 improves survival in murine
endotoxemia and severe sepsis. Crit Care Med. 35:1139–44. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Giebelen IA, van Westerloo DJ, LaRosa GJ,
de Vos AF and van der Poll T: Local stimulation of alpha7
cholinergic receptors inhibits LPS-induced TNF-alpha release in the
mouse lung. Shock. 28:700–703. 2007.PubMed/NCBI
|
|
28
|
Hübner RH, Gitter W, Mokhtari NE El,
Mathiak M, Both M, Bolte H, Freitag-Wolf S and Bewig B:
Standardized quantification of pulmonary fibrosis in histological
samples. Biotechniques. 44:507–511. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Freedman R, Olincy A, Buchanan RW, Harris
JG, Gold JM, Johnson L, Allensworth D, Guzman-Bonilla A, Clement B,
Ball MP, et al: Initial phase 2 trial of a nicotinic agonist in
schizophrenia. Am J Psychiatry. 165:1040–1047. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kitagawa H, Takenouchi T, Azuma R, Wesnes
KA, Kramer WG, Clody DE and Burnett AL: Safety, pharmacokinetics,
and effects on cognitive function of multiple doses of GTS-21 in
healthy, male volunteers. Neuropsychopharmacology. 28:542–551.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Leib C, Goser S, Luthje D, Ottl R, Tretter
T, Lasitschka F, Zittrich S, Pfitzer G, Katus HA and Kaya Z: Role
of the cholinergic antiinflammatory pathway in murine autoimmune
myocarditis. Circ Res. 109:130–140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Saito-Fujita T, Iwakawa M, Nakamura E,
Nakawatari M, Fujita H, Moritake T and Imai T: Attenuated lung
fibrosis in interleukin 6 knock-out mice after C-ion irradiation to
lung. J Radiat Res. 52:270–277. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Przybyszewska M, Miloszewska J, Rzonca S,
Trembacz H, Pysniak K, Kotlarz A, Swoboda P, Zalewska M and Małecki
M: Soluble TNF-α receptor I encoded on plasmid vector and its
application in experimental gene therapy of radiation-induced lung
fibrosis. Arch Immunol Ther Exp. 59:315–326. 2011. View Article : Google Scholar
|
|
35
|
Zhao DY, Qu HJ, Guo JM, Zhao HN, Yang YY,
Zhang P, Cao K, Lei X, Cui JG, Liu C, et al: Protective effects of
myrtol standardized against radiation-induced lung injury. Cell
Physiol Biochem. 38:619–634. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rübe CE, Wilfert F, Palm J, König J,
Burdak-Rothkamm S, Liu L, Schuck A, Willich N and Rübe C:
Irradiation induces a biphasic expression of pro-inflammatory
cytokines in the lung. Strahlenther Onkol. 180:442–448. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Haase MG, Klawitter A, Geyer P, Alheit H,
Baumann M, Kriegel TM, Kasper M and Baretton GB: Sustained
elevation of NF-kappaB DNA binding activity in radiation-induced
lung damage in rats. Int J Radiat Biol. 79:863–877. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li F, Chen Z, Pan Q, Fu S, Lin F, Ren H,
Han H, Billiar TR, Sun F and Li Q: The protective effect of
PNU-282987, a selective alpha7 nicotinic acetylcholine receptor
agonist, on the hepatic ischemia-reperfusion injury is associated
with the inhibition of high-mobility group box 1 protein expression
and nuclear factor kappaB activation in mice. Shock. 39:197–203.
2013.PubMed/NCBI
|
|
39
|
Café-Mendes CC, Garay-Malpartida HM, Malta
MB, de Sá Lima L, Scavone C, Ferreira ZS, Markus RP and Marcourakis
T: Chronic nicotine treatment decreases LPS signaling through NF-κB
and TLR-4 modulation in the hippocampus. Neurosci Lett.
636:218–224. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Anders HJ, Banas B and Schlondorff D:
Signaling danger: Toll-like receptors and their potential roles in
kidney disease. J Am Soc Nephrol. 15:854–867. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yu M, Wang H, Ding A, Golenbock DT, Latz
E, Czura CJ, Fenton MJ, Tracey KJ and Yang H: HMGB1 signals through
toll-like receptor (TLR) 4 and TLR2. Shock. 26:174–179. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rhieu BH, Epperly MW, Cao S, Goff J,
Shields D, Franicola D, Wang H and Greenberger JS: Improved
longevity of hematopoiesis in long-term bone marrow cultures and
reduced irradiation-induced pulmonary fibrosis in Toll-like
receptor-4 deletion recombinant-negative mice. In Vivo. 28:441–448.
2014.PubMed/NCBI
|
|
43
|
Kox M, van Velzen JF, Pompe JC,
Hoedemaekers CW, van der Hoeven JG and Pickkers P: GTS-21 inhibits
pro-inflammatory cytokine release independent of the Toll-like
receptor stimulated via a transcriptional mechanism involving JAK2
activation. Biochem Pharmacol. 78:863–872. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Guijarro-Munoz I, Compte M,
Alvarez-Cienfuegos A, Alvarez-Vallina L and Sanz L:
Lipopolysaccharide activates Toll-like receptor 4 (TLR4)-mediated
NF-kappaB signaling pathway and proinflammatory response in human
pericytes. J Biol Chem. 289:2457–2468. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
He ZW, Qin YH, Wang ZW, Chen Y, Shen Q and
Dai SM: HMGB1 acts in synergy with lipopolysaccharide in activating
rheumatoid synovial fibroblasts via p38 MAPK and NF-kappaB
signaling pathways. Mediators Inflamm. 2013:5967162013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pusterla T, Nemeth J, Stein I, Wiechert L,
Knigin D, Marhenke S, Longerich T, Kumar V, Arnold B, Vogel A, et
al: Receptor for advanced glycation endproducts (RAGE) is a key
regulator of oval cell activation and inflammation-associated liver
carcinogenesis in mice. Hepatology. 58:363–373. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Candeias SM and Testard I: The many
interactions between the innate immune system and the response to
radiation. Cancer Lett. 368:173–178. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Menendez D, Shatz M, Azzam K, Garantziotis
S, Fessler MB and Resnick MA: The Toll-like receptor gene family is
integrated into human DNA damage and p53 networks. PLoS Genet.
7:e10013602011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li X, Ma D, Zha X, Quan D, Pan D, Sun M,
Hu B and Zhao B: Ilomastat, a synthetic inhibitor of MMPs, prevents
lung injury induced by γ-ray irradiation in mice. Oncotarget.
8:60789–60808. 2017.PubMed/NCBI
|
|
50
|
Hiramoto T, Chida Y, Sonoda J, Yoshihara
K, Sudo N and Kubo C: The hepatic vagus nerve attenuates
Fas-induced apoptosis in the mouse liver via alpha7 nicotinic
acetylcholine receptor. Gastroenterology. 134:2122–2131. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Navarro E, Buendia I, Parada E, León R,
Jansen-Duerr P, Pircher H, Egea J and Lopez MG: Alpha7 nicotinic
receptor activation protects against oxidative stress via
heme-oxygenase I induction. Biochem Pharmacol. 97:473–481. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yahyapour R, Motevaseli E, Rezaeyan A,
Abdollahi H, Farhood B, Cheki M, Rezapoor S, Shabeeb D, Musa AE,
Najafi M and Villa V: Reduction-oxidation (redox) system in
radiation-induced normal tissue injury: Molecular mechanisms and
implications in radiation therapeutics. Clin Transl Oncol.
20:975–988. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jenkins SJ, Ruckerl D, Cook PC, Jones LH,
Finkelman FD, van Rooijen N, MacDonald AS and Allen JE: Local
macrophage proliferation, rather than recruitment from the blood,
is a signature of TH2 inflammation. Science. 3(32): 1284–1288.
2011. View Article : Google Scholar
|
|
54
|
Han G, Zhang H, Xie CH and Zhou YF:
Th2-like immune response in radiation-induced lung fibrosis. Oncol
Rep. 26:383–388. 2011.PubMed/NCBI
|
|
55
|
Mei ZJ, Chen J, Xie CH, et al: α7-nAchR
Agonist GTS-21 Reduces Radiation-Induced Lung Injury by Inhibiting
HMGB1/TLR-4/NF-κB PathwayTopic: Biology, IASLC 17th World
Conference. Vienna: 2016
|