Introduction
Despite the availability of advanced treatments for
cancers, they still present an important issue for human health.
Given the increase in cancer rates worldwide, the continued
discovery or improvement of more effective anticancer drugs is
required. One of the biggest problems of the current anticancer
treatment is the resistance of cancer cells to many anticancer
drugs. Many malignant tumors exhibit high resistance towards one
type of cell death, mainly apoptosis. For many years, the
characterization of apoptosis has been well studied in cancer
research. Therefore, most of the currently available anticancer
drugs have been designed to kill cancer cells by triggering
apoptosis. However, many cancer cells have become resistant to
these apoptosis-targeting anticancer drugs, which enables them to
overcome cell death and eventually aggravate the tumor. Thus, it is
essential to understand this mechanism in order to explore more
effective anticancer drugs. One of the strategies for overcoming
this problem may be to target other types of cell deaths along with
apoptosis. For a long time, eukaryotic cell death was divided into
two types: programmed cell death (PCD) of apoptosis and accidental
cell death of necrosis (1–5). However, emerging evidence has
suggested that necrosis is also a type of PCD. Therefore,
necroptosis, a new type of PCD, was proposed as apoptotic necrosis
by Teng et al (6). Many
recent studies have suggested that apoptosis and necroptosis are
very closely interconnected but undergo different pathways through
multiple signaling pathways depending on the stimuli and
environments (6–13). Therefore, we first tried to find
anticancer drugs that can induce necroptosis as well as
apoptosis.
In the effort of finding safe and effective
anticancer drugs from natural products, we initially focused on the
Schisandra chinensis fruit. Schisandra chinensis is
considered a traditional herbal medicine for the treatment and
alleviation of a variety of diseases (14–16).
Its chemical constituents include lignans, schisandrin,
deoxyschisandrin, pregomisin and gomisins, which have gained great
attention for their many cellular functions (14,17–21).
They regulate multiple signaling pathways involved in various
biological processes, such as vascular contractility, fibrosis,
inflammation, oxidative stress, adipogenesis, obesity and
anticancer effects (14,17–23).
Gomisin J, one of the extracts of Schisandra chinensis, has
been reported to have several activities, including anti-hepatic
effects against cardiovascular symptoms, preventive effect on
angiotensin II-induced hypertension, vascular relaxation,
anti-inflammatory effects and antiretroviral activity (23–27).
In the present study, we reported that gomisin J
from Schisandra chinensis possesses anticancer activity
against several different types of cancer cell lines by suppressing
cell growth and proliferation or by accelerating necroptosis as
well as apoptosis, in which it was much more effective,
particularly in apoptosis-resistant cancer cells.
Materials and methods
Preparation of gomisin J from
Schisandra chinensis fruit
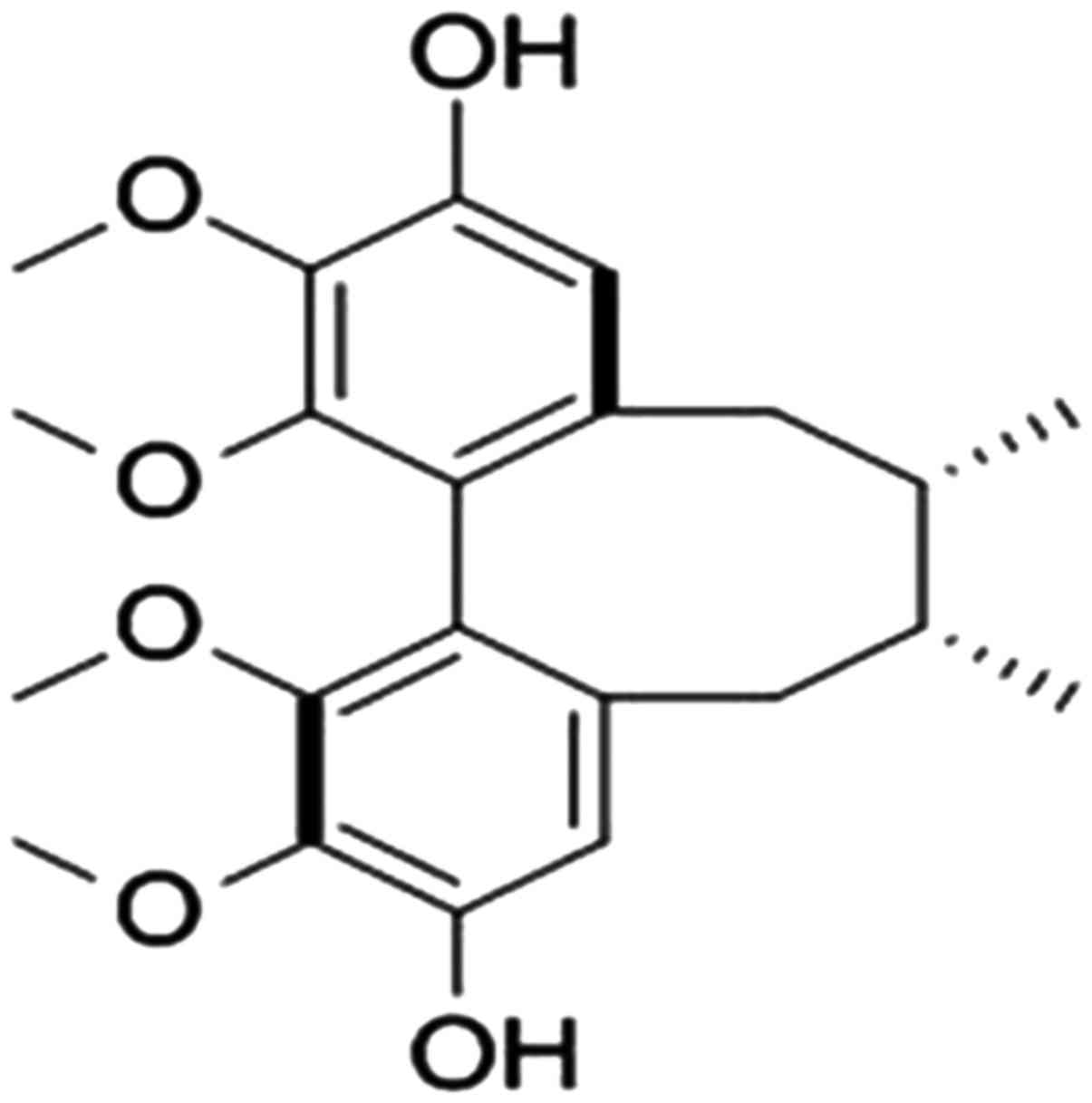
Gomisin J (PubChem CID: 3001686) (Fig. 1) was isolated from the fruits of
Schisandra chinensis following the previously published
method (28). It was dissolved in
dimethyl sulfoxide (DMSO) at a concentration of 10 mg dry weight/ml
and stored at −20°C.
Cell lines and cell culture
Each cell line was cultured in the appropriate
media. Nine breast cancer cell lines (MCF7, MDA-MB-231, BT20,
BT549, T47D, SKBR3, MDA-MB-453, HS578T and MDA-MB-468), two colon
cancer cell lines (HCT116 and HT-29), and two cervical cancer cell
lines (HeLa and SiHa) were cultured in Dulbecco's modified Eagle's
medium (DMEM; Welgene, Inc., Daejeon, Korea) supplemented with 10%
fetal bovine serum (FBS; Gibco-BRL; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and 1 % antibiotic-antimycotic solution (cat. no.
15240-062; Gibco-BRL; Thermo Fisher Scientific, Inc.). Normal human
MCF10A mammary epithelial cell line was grown in DMEM/F12 medium
(cat. no. 11330-032; Invitrogen; Thermo Fisher Scientific, Inc.)
supplemented with 20 µg/ml epidermal growth factor (EGF) (cat. no.
E9644), 100 µg/ml cholera toxin (cat. no. C-8052), 10 µg/ml insulin
(cat. no. I-9278) and 0.5 mg/ml hydrocortisone (cat. no. H-0888;
all from Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), 5% horse
serum (cat. no. 16050-122; Invitrogen; Thermo Fisher Scientific
Korea Ltd., Seoul, Korea) and 1% antibiotic-antimycotic solution.
All cells were cultured at 37°C in a humidified atmosphere composed
of 95% air and 5% CO2. The SiHa cell line was obtained
from the Korean Cell Line Bank (KCLB; Seoul, Korea; cat. no. 30035)
and the other cell lines were purchased from the American Type
Culture Collection (ATCC; Mannassas, VA, USA).
Analysis of necroptosis and apoptosis
by western blot analysis
Each PCD was mainly analyzed through western
blotting with corresponding marker or regulatory proteins. In
brief, MCF7 and MDA-MB-231 cells (2×106/well) were
seeded in a 100-mm diameter culture dish containing DMEM and
treated with 30 µg/ml gomisin J for 72 h. Cells were centrifuged
(micro centrifuge; Smart R17 refrigerated; Hanil Science Co., Ltd.,
Daejeon Korea) at 3,000 × g at 4°C for 3 min, washed in ice-cold
phosphate-buffered saline (PBS) buffer, then lysed in radio
immunoprecipitation assay (RIPA) lysis buffer. The amount of
protein was quantified using a protein assay kit (Bio-Rad
Laboratories Korea, Seoul, Korea) and 10 or 30 µg of proteins were
loaded in each lane. Subsequently, they were separated on a 10 or
12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and then transferred onto a an Immobilon-P®
PVDF transfer membrane (cat. no. IPVH00010; EMD Millipore,
Billerica, MA, USA). Membranes were then blocked using 5% skimmed
milk in PBS for 30–60 min at 20–25°C and incubated overnight at 4°C
with the primary antibodies. After washing with Tris-buffered
saline containing 1% Tween-20 (product code T1027; CAS#9005-64-5;
Biosesang, Seongnam, Korea), membranes were incubated with the
corresponding secondary antibodies. Immunodetection was performed
using the PowerOpti-ECL Western Blotting Detection reagent (Bio-Rad
Laboratories). The antibodies used in this study were as follows: A
mouse monoclonal antibody against BAX (dilution 1:5,000; cat. no.
sc-20067) was purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). A rabbit polyclonal antibody against PARP
(dilution 1:3,000; cat. no. 9542S) was obtained from Cell Signaling
Technology (Danvers, MA, USA). A rabbit polyclonal antibody against
cyclophilin A (CypA) (dilution 1:3,000; cat. no. BML-SA296) was
supplied by Enzo Life Sciences, Inc. (Farmingdale, NY, USA). A goat
polyclonal antibody against γ-tubulin (dilution 1:5,000 dilution;
cat. no. sc-7396) was purchased from Santa Cruz Biotechnology, Inc.
(CA, USA). BAX and PARP were used to detect for apoptosis and
cyclophilin A (CypA) was used for necroptosis. Additionally,
γ-tubulin was used as a loading control. Necroptosis was checked by
assessing the levels of extracellular CypA biomarker protein that
were released from necroptotic cells to an extracellular location
(29). The result of the western
blot analysis was quantified using ImageJ software program (version
1.51u) (Image Processing and Analysis in Java; http://imagej.nih.gov/ij/) supplied by the National
Institutes of Health (Bethesda, MD, USA) and relative intensity vs.
β-actin is represented in the bar graphs.
Cell viability analysis
Cell viability was analyzed using a Cell Viability,
Proliferation and Cytotoxicity assay kit (EZ-CYTOX; cat. no.
EZ-3000; Seoul, Korea) according to the manufacturer's instructions
in triplicate. Briefly, cells (2×104/well) were seeded
in 96-well cell culture plates containing DMEM medium and exposed
to 1, 5, 10 or 30 µg/ml gomisin J. Cell viability was evaluated by
measuring absorbance at 450 nm in a Gemini XPA microplate reader
(Molecular Devices, San Jose, CA, USA). Cell viability was also
checked by Trypan blue staining under same conditions, in which the
number of live or dead cells were counted by using an EVE automatic
cell counter (NanoEnTek Inc., Pleasanton, CA, USA).
Statistical analysis
Statistical analysis was performed by using
Student's t-test to compare two different groups and one-way ANOVA
for multiple groups, followed by Bonferroni's multiple comparisons
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Cytotoxic effect of gomisin J on the
cell proliferation and viability of MCF7 and MDA-MB-231 breast
cancer cell lines
First, we tested the anticancerous effect of gomisin
J on the proliferation and viability of two human breast cancer
cell lines, MCF7 and MDA-MB-231. In particular, MCF7 was considered
to be a good model system for testing our hypothesis since it has
been reported to be highly resistant to many pro-apoptotic
anticancer drugs, most likely owing to the absence of the key
proteins necessary for apoptotic cell death (e.g., caspase-3). In
brief, MCF7 and MDA-MB-231 cell lines were plated onto 24-mm
culture dishes and allowed to form a confluent monolayer for 24 h.
These cells were screened with various concentrations of gomisin J
(0, 25, 50 and 100 µg/ml) for 5 days, and their cell density as
well as morphological changes were observed under an optical
microscope with IS capture software (KI-400F; Korea LabTech Corp.,
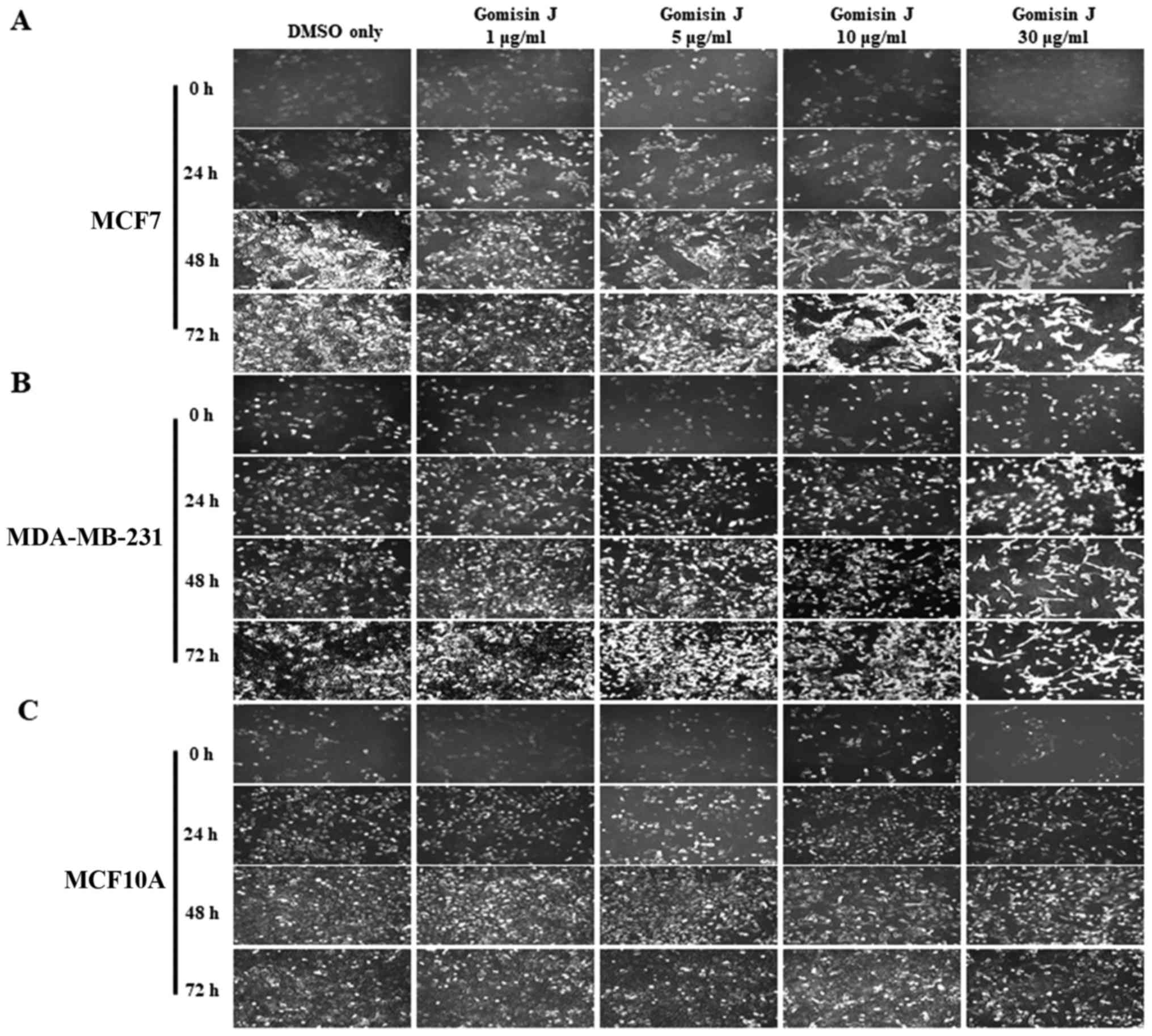
Seongnam, Korea). Notably, gomisin J exhibited a strong cytotoxic
effect on MCF7 and MDA-MB-231 cells at higher concentrations of 25
µg/ml (data not shown). Accordingly, the cytotoxicity of gomisin J
was quantified in detail by evaluating its effects on cell growth
rate (Fig. 2) and morphological
changes (Fig. 3) at four different
concentrations (0, 1, 5, 10 and 30 µg/ml) for 0, 24, 48 and 72 h.
In both MCF7 and MDA-MB-231 cell lines, the cell growth rate was
highly delayed in comparison with those of the DMSO-only treated
control cells (Fig. 2A-D). In
addition, the number of cells decreased at relatively high
concentrations (30 µg/ml) after treatment for 72 h, which implied
the induction of cell death (Fig.
2A-D). These data indicated that gomisin J greatly suppressed
the proliferation and viability of cancer cells in a time- and
concentration-dependent manner. Notably, our data revealed that
MCF10A cells appeared to be much less sensitive to gomisin J
treatment, to a lesser degree than that observed in the breast
cancer cells, which may suggest reduced adverse or no side effects
in normal cells (Fig. 2E and F). In
a further experiment, examination under an optical microscope with
IS capture software (KI-400F; Korea LabTech Corp., Seongnam, Korea)
also revealed similar results as the cell growth data (Fig. 3).
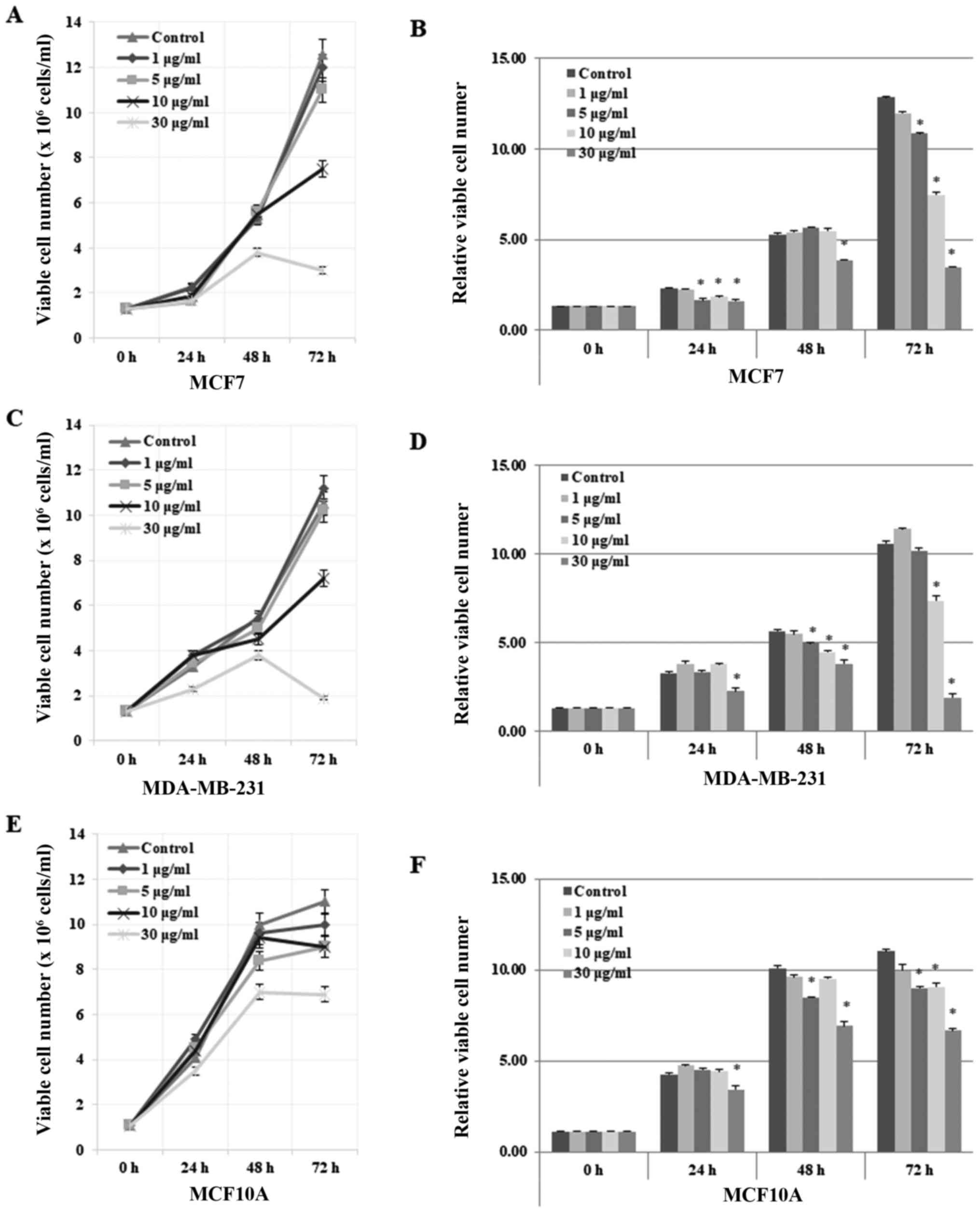 | Figure 2.Inhibitory effect of gomisin J on the
proliferation and viability of MCF7 and MDA-MB-231 cancer cell
lines but not on MCF10A normal cells. (A) MCF7, (C) MDA-MB-231 and
(E) MCF10A cells were grown in 100-mm cell culture plates and
treated with DMSO only (control), 1, 5, 10 or 30 µg/ml of gomisin J
for 0, 24, 48 or 72 h. Cell viability was assessed at the indicated
time-points by Trypan blue staining, in which the number of live or
dead cells were counted using an EVE automatic cell counter
(NanoEnTek Inc., Pleasanton, CA, USA). The data represent the means
± SDs from the three independent experiments. (B, D and F) Relative
viable cell number in MCF7, MDA-MB-231 and MCF10A cells,
respectively. Asterisks indicate a statistically significant
difference (*P<0.05). |
Collectively, our data indicated that gomisin J
functioned as an anticancer drug by inhibiting cell proliferation
and by inducing cell death in MCF7 and MDA-MB 231 cancer cells.
Induction of necroptosis and apoptosis
by gomisin J in MCF7 and MDA-MB-231 cells
Our main goal for cancer treatment is to understand
and overcome the resistance of malignant cancer cells to cell
death, mainly apoptosis. Our data revealed that gomisin J treatment
at concentrations above 30 µg/ml significantly induced cell death
in MCF7 and MDA-MB-231 cancer cells. In order to clarify whether
gomisin J can induce necroptosis as well as apoptosis, both cancer
cell lines were treated with either DMSO (as a control) or 30 µg/ml
of gomisin J for 72 h, and then the two types of PCD were assessed
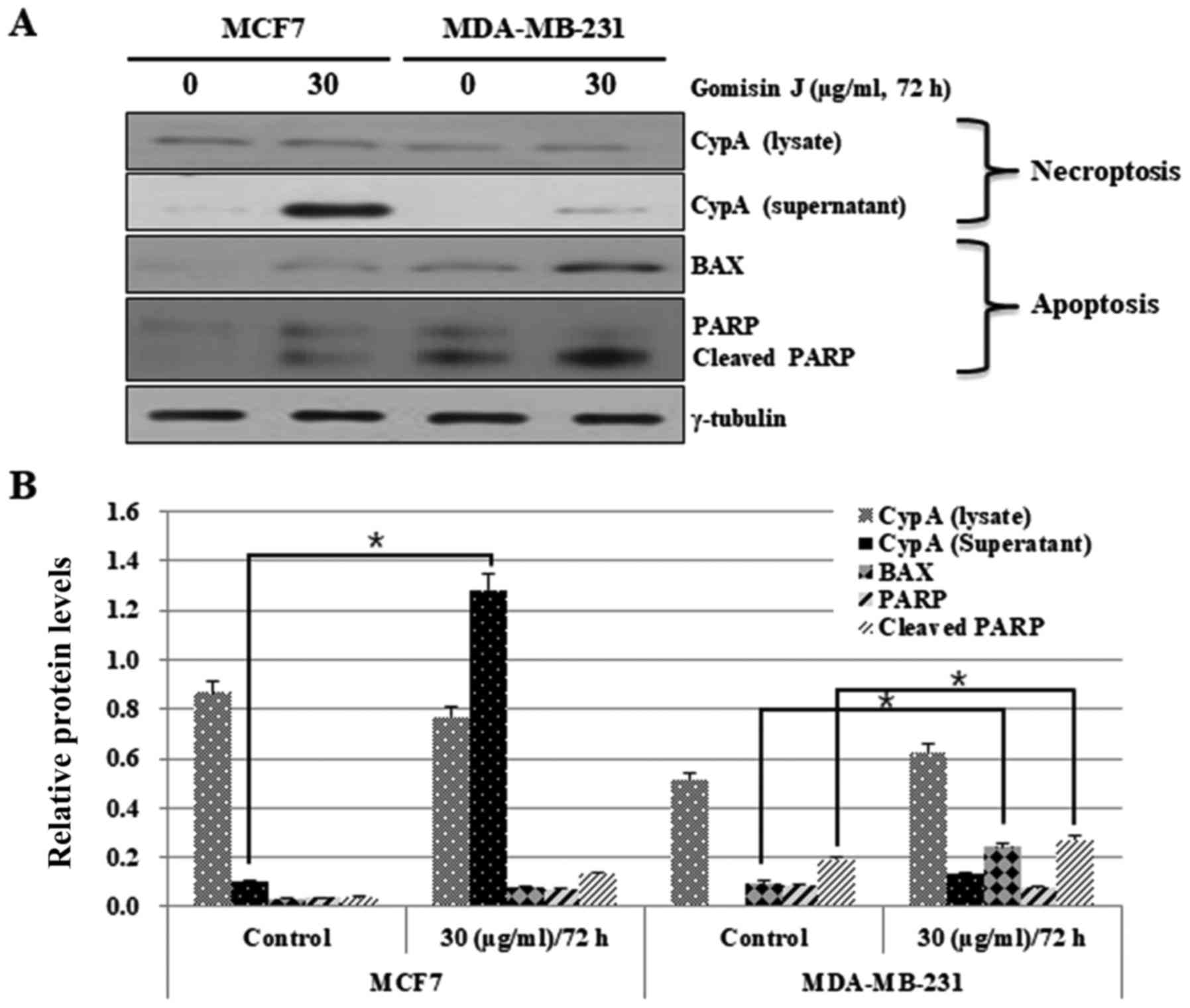
by employing western blot analysis. At first, we evaluated
necroptosis by assessing the levels of extracellular CypA biomarker
protein that were released from necroptotic cells. In this case,
the export of CypA into the extracellular space was greatly
increased in MCF7 cells but only slightly in MDA-MB-231 cells after
72 h, which indicated a much higher level of induction of
necroptosis in MCF7 cells compared with MDA-MB-231 cells (Fig. 4). Apoptosis was also investigated
through analysis of typical apoptotic markers, BAX and PARP. BAX
protein levels were significantly increased in MDA-MB-231 cells
(Fig. 4A and B). In contrast, they
were only slightly increased in MCF7 cells in comparison with that
in DMSO-treated control cells (Fig. 4A
and B). PARP was also cleaved at a much higher level in
MDA-MB-231 cells than in MCF-7 cells (Fig. 4A and B). These data again indicated
the high resistance of MCF7 cells to apoptosis.
Collectively, this result strongly indicated that
gomisin J can render apoptotic-resistant MCF7 cells more sensitive
to necroptosis than apoptosis.
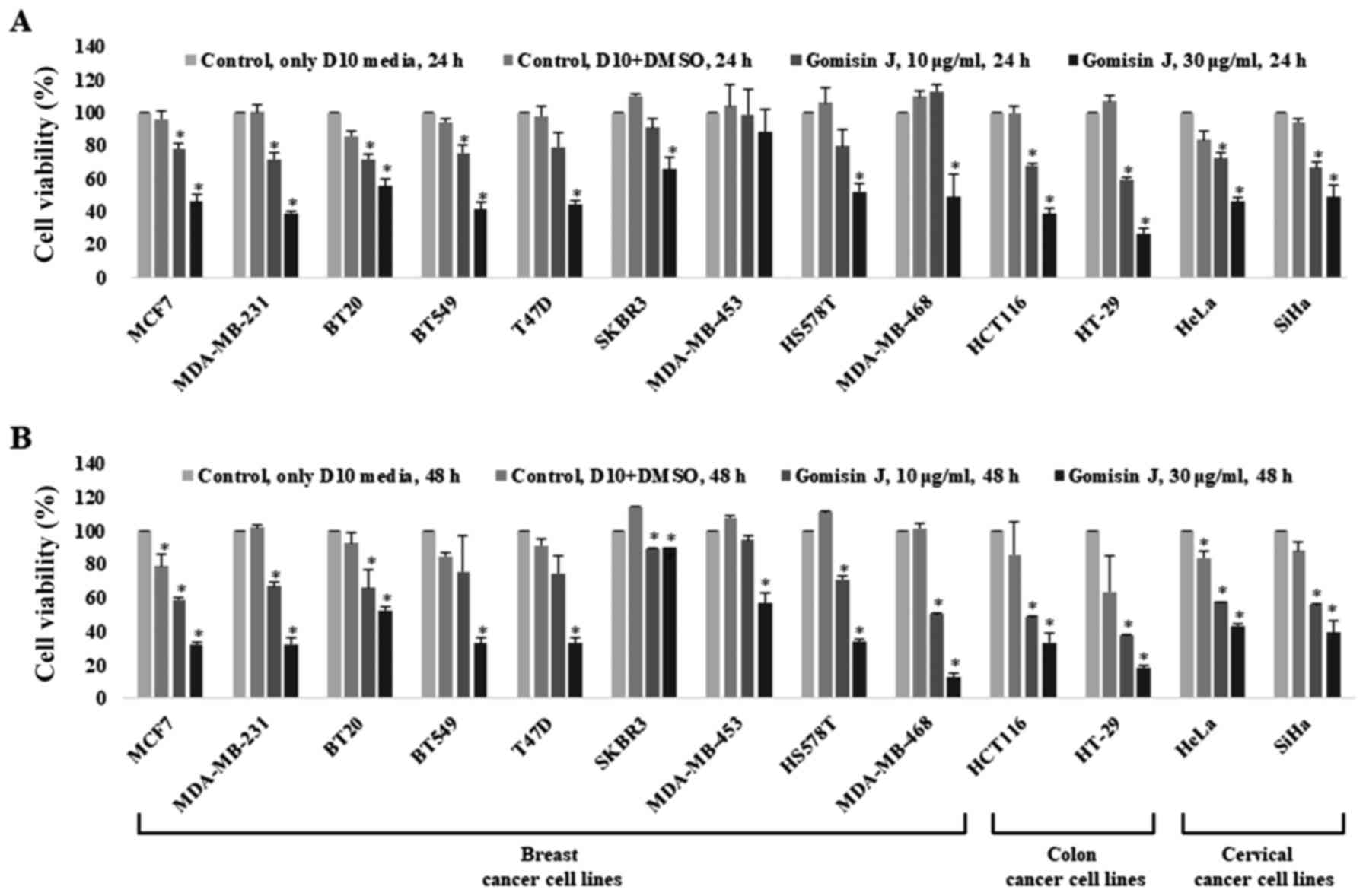
Cytotoxic effect of gomisin J on the proliferation
and viability of various cancer cell lines. The cytotoxicity of
gomisin J to MCF7 and MDA-MB-231 cells indicated the possibility of
similar effects on other cancer cell lines. Therefore, this
investigation was extended to different types of breast cancer
(BT20, BT549, T47D, SKBR3, MDA-MB-453, HS578T and MDA-MB-468),
colon cancer (HCT116 and HT-29) and cervical cancer (HeLa and SiHa)
cell lines. The cytotoxic effect of gomisin J on each cell line was
assessed as mentioned in Materials and methods after treatment of
the cells with either DMSO (control) or 10 or 30 µg/ml gomisin J
for 24 or 48 h. The cell viability markedly decreased in all tested
cancer cell lines treated with gomisin J in comparison with that in
untreated control cells (Fig. 5A and
B).
Collectively, our results indicated that gomisin J
has a strong inhibitory activity against the proliferation of
various cancer cells, indicating that the antitumor activity of
gomisin J may be used as an effective anticancer drug in various
types of cancer cells.
Discussion
Our ultimate goal is to identify new strategies that
can trigger more than one type of cell death in cancer cells. This
may lead to the discovery and development of much more effective
anticancer drugs. Since cancer cells promote tumorigenesis by
increasing resistance to apoptosis, we aimed to establish an
optimal strategy for the effective termination of
apoptosis-resistant cancer cells and to identify possible new
biomedical candidates. We initially identified anticancer drugs
that kill apoptosis-resistant cancer cells such as MCF7 by inducing
another type of PCD, necroptosis. The current results may present
an example of how to overcome the limitations of current anticancer
drugs.
Our data revealed that gomisin J possesses
anticancer activity, as indicated by its ability to inhibit cancer
cell growth and to induce two types of PCD, apoptosis and
necroptosis. Notably, our data indicated that gomisin J may
predominantly induce non-apoptotic PCD, necroptosis rather than
apoptosis in cancer cell lines with defective apoptosis machinery,
such as MCF7. Our results also indicated that gomisin J may
sensitize those cancer cells to other types of cell deaths as well
as necroptosis and apoptosis. For example, our data revealed that
autophagy-mediated cell death was also induced in both MCF7 and
MDA-MB-231 cell lines at a high level (data not shown). Gomisin J
treatment started to only slightly induce the decrease of
p62/SQSTM1 expression level and the conversion from LC3-I to LC3-II
after 24 h, but significantly after longer exposure of 72 h (data
not shown). This result indicated that gomisin J treatment
stimulated autophagy at a relatively shorter exposure time,
probably for their survival. It is well known that many cancer
cells have acquired resistance to many anticancer drugs by
activating autophagy. However, prolonged treatment eventually
triggered autophagy-mediated cell death at a later time of 72 h, in
which cell death was induced. Considering the fact that gomisin J
induces autophagy-mediated cell death, which is promoted by the
inhibition of mTOR, we assumed that the PI3K/AKT/mTOR pathway could
be influenced by gomisin J (30–33),
which needs to be investigated in the future. In fact, Maharjan
et al proposed that gomisin G, a related compound of gomisin
J, blocked the AKT pathway that is upstream of mTOR (34). As gomisin J can enhance the efficacy
of anticancer drugs through the induction of two types of PCD in
cancer cells, our next project is to study this mechanism and to
identify the involved regulatory proteins or pathways. One of our
future studies will focus on the functional role of gomisin J in
mitochondria since mitochondria are known to be involved in many
types of cell death. In addition, it would be very useful to
perform a clonogenic survival assay to determine the long-term fate
of cells treated with gomisin J and to conduct animal experiments
such as xenografts in order to assess its effect in physiological
conditions. Collectively with the findings in other cancer cell
lines, the present data also indicated that gomisin J may be an
effective candidate for the inhibition of the proliferation or
induction of cell deaths in various cancer cell lines.
In summary, our results validate the potential use
of gomisin J from Schisandra chinensis fruits as an
anticancer agent for chemotherapy and the chemoprevention of many
different cancer cells including breast, colon and cervical cancer
cells. In particular, gomisin J appears to be more effective,
especially in cancer cells, with a high resistance to apoptosis
targeting anticancer drugs, since it can induce additional cell
death, necroptosis as well as apoptosis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Medical
Research Center Program (no. 2011-0030074) through the National
Research Foundation (NRF) grant funded by the Korean government
(MSIP).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SJ designed the general experimental plan, acquired
and analyzed the data, wrote and revised the manuscript. HIM and JY
purified the gomisin J and assisted in preparing the manuscript. SK
prepared the experimental materials and performed the western blot
analysis. NTNQ contributed in handling ImageJ program and performed
the statistical analysis. ZS, DDTL, HyegyeongL and HyojeongL
assisted in preparing the experimental materials and performed the
western blot analysis. MSL made substantial contribution to the
conception of the study and the experimental design, revised the
manuscript and gave the final approval for the publication of the
manuscript. All authors read and approved the manuscript and agree
to be accountable for all aspects of this research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chiorazzi N: Cell proliferation and death:
Forgotten features of chronic lymphocytic leukemia B cells. Best
Pract Res Clin Haematol. 20:399–413. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Danial NN and Korsmeyer SJ: Cell death:
Critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Günther C, Martini E, Wittkopf N, Amann K,
Weigmann B, Neumann H, Waldner MJ, Hedrick SM, Tenzer S, Neurath
MF, et al: Caspase-8 regulates TNF-α-induced epithelial necroptosis
and terminal ileitis. Nature. 477:335–339. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jäättelä M: Multiple cell death pathways
as regulators of tumour initiation and progression. Oncogene.
23:2746–2756. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nikoletopoulou V, Markaki M, Palikaras K
and Tavernarakis N: Crosstalk between apoptosis, necrosis and
autophagy. Biochim Biophys Acta. 1833:3448–3459. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Teng X, Degterev A, Jagtap P, Xing X, Choi
S, Denu R, Yuan J and Cuny GD: Structure-activity relationship
study of novel necroptosis inhibitors. Bioorg Med Chem Lett.
15:5039–5044. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Linkermann A: Beyond necroptosis -
regulated necrosis in the kidney. Cancer Res. 74((Suppl 19)):
SY29–04. 2014.
|
|
8
|
Linkermann A and Green DR: Necroptosis. N
Engl J Med. 370:455–465. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Su L, Quade B, Wang H, Sun L, Wang X and
Rizo J: A plug release mechanism for membrane permeation by MLKL.
Structure. 22:1489–1500. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vanden Berghe T, Linkermann A,
Jouan-Lanhouet S, Walczak H and Vandenabeele P: Regulated necrosis:
The expanding network of non-apoptotic cell death pathways. Nat Rev
Mol Cell Biol. 15:135–147. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vandenabeele P, Galluzzi L, Vanden Berghe
T and Kroemer G: Molecular mechanisms of necroptosis: An ordered
cellular explosion. Nat Rev Mol Cell Biol. 11:700–714. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang H, Sun L, Su L, Rizo J, Liu L, Wang
LF, Wang FS and Wang X: Mixed lineage kinase domain-like protein
MLKL causes necrotic membrane disruption upon phosphorylation by
RIP3. Mol Cell. 54:133–146. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou W and Yuan J: Necroptosis in health
and diseases. Semin Cell Dev Biol. 35:14–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Panossian A and Wikman G: Pharmacology of
Schisandra chinensis Bail. An overview of Russian research and uses
in medicine. J Ethnopharmacol. 118:183–212. 2008.
|
|
15
|
Chun JN, Cho M, So I and Jeon JH: The
protective effects of Schisandra chinensis fruit extract and its
lignans against cardiovascular disease: A review of the molecular
mechanisms. Fitoterapia. 97:224–233. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Batchelor J and Miyabe K: Ainu economic
plants. Transactions of the Asiatic Society of Japan. R. Meiklejohn
Co. 51:198–240. 1893.
|
|
17
|
Park JY, Lee SJ, Yun MR, Seo KW, Bae SS,
Park JW, Lee YJ, Shin WJ, Choi YW and Kim CD: Gomisin A from
Schisandra chinensis induces endothelium-dependent and direct
relaxation in rat thoracic aorta. Planta Med. 73:1537–1542. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen P, Pang S, Yang N, Meng H, Liu J,
Zhou N, Zhang M, Xu Z, Gao W, Chen B, et al: Beneficial effects of
schisandrin B on the cardiac function in mice model of myocardial
infarction. PLoS One. 8:e794182013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Min HY, Park EJ, Hong JY, Kang YJ, Kim SJ,
Chung HJ, Woo ER, Hung TM, Youn UJ, Kim YS, et al:
Antiproliferative effects of dibenzocyclooctadiene lignans isolated
from Schisandra chinensis in human cancer cells. Bioorg Med Chem
Lett. 18:523–526. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Young Park J, Wook Yun J, Whan Choi Y, Ung
Bae J, Won Seo K, Jin Lee S, Youn Park S, Whan Hong K and Kim CD:
Antihypertensive effect of gomisin A from Schisandra chinensis on
angiotensin II-induced hypertension via preservation of nitric
oxide bioavailability. Hypertens Res. 35:928–934. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jang MK, Yun YR, Kim JH, Park MH and Jung
MH: Gomisin N inhibits adipogenesis and prevents high-fat
diet-induced obesity. Sci Rep. 7:403452017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yasukawa K, Ikeya Y, Mitsuhashi H, Iwasaki
M, Aburada M, Nakagawa S, Takeuchi M and Takido M: Gomisin A
inhibits tumor promotion by 12-O-tetradecanoylphorbol-13-acetate in
two-stage carcinogenesis in mouse skin. Oncology. 49:68–71. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oh SY, Kim YH, Bae DS, Um BH, Pan CH, Kim
CY, Lee HJ and Lee JK: Anti-inflammatory effects of gomisin N,
gomisin J, and schisandrin C isolated from the fruit of Schisandra
chinensis. Biosci Biotechnol Biochem. 74:285–291. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fujihashi T, Hara H, Sakata T, Mori K,
Higuchi H, Tanaka A, Kaji H and Kaji A: Anti-human immunodeficiency
virus (HIV) activities of halogenated gomisin J derivatives, new
nonnucleoside inhibitors of HIV type 1 reverse transcriptase.
Antimicrob Agents Chemother. 39:2000–2007. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim M, Lim SJ, Lee HJ, Kim SY and Nho CW:
Gomisin J inhibits oleic acid-induced hepatic lipogenesis by
activation of the AMPK-dependent pathway and inhibition of the
hepatokine fetuin-A in HepG2 cells. J Agric Food Chem.
63:9729–9739. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ye BH, Lee SJ, Choi YW, Park SY and Kim
CD: Preventive effect of gomisin J from Schisandra chinensis on
angiotensin II-induced hypertension via an increased nitric oxide
bioavailability. Hypertens Res. 38:169–177. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park JY, Choi YW, Yun JW, Bae JU, Seo KW,
Lee SJ, Park SY and Kim CD: Gomisin J from Schisandra chinensis
induces vascular relaxation via activation of endothelial nitric
oxide synthase. Vascul Pharmacol. 57:124–130. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Smejkal K, Slapetová T, Krmenčík P, Babula
P, Dall'Acqua S, Innocenti G, Vančo J, Casarin E, Carrara M,
Kalvarová K, et al: Evaluation of cytotoxic activity of Schisandra
chinensis lignans. Planta Med. 76:1672–1677. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Christofferson DE and Yuan J: Cyclophilin
A release as a biomarker of necrotic cell death. Cell Death Differ.
17:1942–1943. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nicoletti F, Fagone P, Meroni P, McCubrey
J and Bendtzen K: mTOR as a multifunctional therapeutic target in
HIV infection. Drug Discov Today. 16:715–721. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Feng H, Yang Z, Bai X, Yang M, Fang Y,
Zhang X, Guo Q and Ning H: Therapeutic potential of a dual mTORC1/2
inhibitor for the prevention of posterior capsule opacification: An
in vitro study. Int J Mol Med. 41:2099–2107. 2018.PubMed/NCBI
|
|
32
|
Donia M, Mangano K, Amoroso A, Mazzarino
MC, Imbesi R, Castrogiovanni P, Coco M, Meroni P and Nicoletti F:
Treatment with rapamycin ameliorates clinical and histological
signs of protracted relapsing experimental allergic
encephalomyelitis in Dark Agouti rats and induces expansion of
peripheral CD4+CD25+Foxp3+
regulatory T cells. J Autoimmun. 33:135–140. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Evangelisti C, Evangelisti C, Chiarini F,
Lonetti A, Buontempo F, Bressanin D, Cappellini A, Orsini E,
McCubrey JA and Martelli AM: Therapeutic potential of targeting
mTOR in T-cell acute lymphoblastic leukemia (Review). Int J Oncol.
45:909–918. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Maharjan S, Park BK, Lee SI, Lim Y, Lee K,
Kwon HJ and Gomisin G: Gomisin G inhibits the growth of
triple-negative breast cancer cells by suppressing AKT
phosphorylation and decreasing cyclin D1. Biomol Ther (Seoul).
26:322–327. 2018. View Article : Google Scholar : PubMed/NCBI
|