Introduction
Acute lymphoblastic leukemia (ALL) is a rapidly
progressing disease characterized by the progressive accumulation
of immature clonal cells in the bone marrow (BM). The molecular
pathogenesis of ALL involves the aberrant expression of
protooncogenes in several signaling pathways, chromosomal
translocations of transcription factors and hyperdiploidy (1). Currently, ~80% of all newly diagnosed
pediatric patients with ALL can become disease-free following
adequate treatment; however, a small number of children still
experience ALL relapse (2).
Treatment of relapsed ALL is largely ineffective, as the response
rate to chemotherapeutic drugs is only 10–20%, which is often
attributed to the effect of ATP-binding cassette (ABC) transporter
family members, multidrug resistance 1 (MDR1) and MDR-associated
protein (MRP) (3,4). The mechanisms of drug resistance are
associated with the overexpression of drug-efflux pumps, including
MDR1-encoded and membrane-located P-glycoprotein (P-gp) and MRP.
The overexpression of drug-efflux pumps promotes the cellular
escape of anticancer drugs, especially natural drugs and
anthracyclines, including vinca alkaloids, vinblastine, vincristine
(5) and doxorubicin. Therefore, it
is urgent to develop novel therapeutic strategies to increase
sensitivity of ALL to chemotherapeutic drugs.
The canonical Wnt/β-catenin signaling pathway is an
evolutionarily conserved cascade that controls a variety of
cellular activities, including cell proliferation, migration,
apoptosis and gene expression during embryonic development.
Previous studies have investigated the abnormal expression of
Wnt/β-catenin signaling pathway in solid cancer (6) and hematologic malignancies (7), including acute myeloid leukemia (AML)
and ALL. It has been indicated that MDR1 is activated by the
Wnt/β-catenin signaling pathway, potentially leading to
chemoresistance (8).
Because resistance to chemotherapy is a major
obstacle in successful treatment of relapsed ALL, it is
hypothesized that modulation of the Wnt/β-catenin signaling may
affect the expression of MDR1, and improve the sensitivity to
chemotherapeutic drugs. In the current study, a novel variant of
BALL-1, the B cell lineage of an ALL cell line, was selected to
mimic relapsed ALL. The new BALL-1 variant was resistant to
vincristine (VCR), an essential component in childhood ALL
therapies. In addition, multidrug resistance and increased levels
of several critical proteins in the Wnt/β-catenin signaling pathway
were identified in passaged BALL-1/VCR cells, consistent with those
of relapsed ALL. Subsequently, Dickkopf-related protein 1 (DKK1)
was used to inhibit the Wnt/β-catenin signaling pathway, and to
abolish the resistance in BALL-1/VCR and relapsed ALL cells.
Finally, the potential mechanism of drug resistance involving MDR1
and MRP was explored in the present study.
Materials and methods
Patient samples
Bone marrow samples from patients at first diagnosis
of ALL and relapsed ALL were collected at and provided by Shandong
University Qilu Hospital (Jinan, China). The primary cells were
separated from bone marrow by Ficoll-Hypaque centrifugation and
maintained in a fresh culture medium (RPMI-1640; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 20% fetal bovine
serum (FBS; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin. Informed consent was obtained from all
patients or their guardians. The protocol followed the Declaration
of Helsinki and was approved by Ethic Committee in Qilu Hospital of
Shandong University (no. KYLL-2017-253).
Materials
RPMI-1640 and FBS were obtained from Gibco (Thermo
Fisher Scientific, Inc.). Vincristine, vindesine (VDS),
doxorubicin, etoposide (VP16), mitoxantrone, cisplatin,
camptothecin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), and
prednisolone (Shandong Xinhua Pharmaceutical Co., Ltd., Zibo,
China) were serially diluted in RPMI and added to the culture media
at the indicated concentrations.
Cell lines
Wild-type BALL-1 (BALL-1/WT) and VCR-resistant
BALL-1 (BALL-1/VCR) human ALL cells were cultured at 37°C in 5%
CO2 in RPMI-1640 containing 10% FBS. The wild-type
BALL-1 cell line without mycoplasma contamination were donated by
Professor Dao-xin Ma (Key Laboratory of Shandong Province, Shandong
University Qilu Hospital, Jinan, China). The VCR-resistant variants
of BALL-1/WT cells were isolated by stepwise selection using
increasing concentrations of VCR, which started from 2X IC50 (970
M). When cells became confluent in the VCR containing medium, the
drug concentration was increased to 3X (1,455 M), 5X (2,425 M), 10X
(4,850 M), 20X (9,700 M), 30X (14,550 M), 50X (24,250 M) and 100X
IC50 (48,450 M), which was the maximal concentration. Following the
selection of BALL-1/VCR cells, they were sub-cultured in a medium
containing 6062.5 M VCR and were stably resistant to VCR for
several months.
DKK1-conditioned medium (DKK1-CM)
293T cells (donated by Tnstitute of Immunology,
Shandong University, Jinan, China) were cultured in Dulbecco's
modified Eagle's medium-conditioned medium (Biochrom, Ltd.,
Cambridge, UK) containing 10% FBS (HyClone; GE Healthcare Life
Sciences, Logan, UT, USA) and 1×106 cells were
transfected with 3 µg pcDNA3.1-DKK1 [designed and synthesized by
Shanghai GenePharma Co., Ltd. (Shanghai, China)] using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). The supernatant was collected as the
DKK1-conditioned media 48 h later and stored at −70°C for
subsequent experiments.
To inhibit the canonical Wnt signaling pathway,
BALL-1/VCR cells were seeded onto a 24-well plate at a density of
2×104 cells/well and treated with 1 ml DKK1-CM for 48
h.
Cytotoxicity assays
The effect of anticancer agents on cell viability
was assessed using MTT assay as described previously (9). In brief, cells (4–5×103 per
well) were seeded in 96-well plates and incubated for 24 h at 37°C.
Subsequently, the cells were exposed to varying concentrations of
anticancer drugs for a specific time before treated by 20 µl/well
MTT (5 mg/ml) for 4 h. Subsequently, dimethyl sulfoxide (DMSO) was
added to treat the cells for 10 min and the optical density in each
well was measured using a microplate reader (Bio-Rad 450; Bio-Rad
Laboratories, Inc., Rantoul, IL, USA) at 570 nm. The cell viability
ratio (%) was calculated based on the formula below: (A570
sample-A570 blank)/(A570 control-A570 blank) ×100. The IC50 value
of the cells was deemed as the drug dose that caused 50% of
absorbance reduction compared with DMSO-treated control cells. Each
experiment was performed in triplicate independently.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
In brief, total RNA was isolated from cells using
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) and RT was
performed using Quant reverse transcriptase (Beyotime Institute of
Biotechnology, Haimen, China) with incubation at 42°C for 30 min
and 85°C for 5 min. The sequences of the primers are listed in
Table I. qPCR was performed using
RealMasterMix (SYBR Green; Beyotime Institute of Biotechnology) for
30 cycles of amplification (95°C for 10 min followed by 30 cycles
of 95°C for 15 sec and 62°C for 1 min). The gene expression was
quantified using the comparative 2−ΔΔCq (10) method and then normalized to the
expression of GAPDH (11).
 | Table I.Primer information. |
Table I.
Primer information.
| Gene | Forward sequence
(5′-3′) | Reverse sequence
(5′-3′) |
|---|
| Wnt3a |
CTTTGCAGTGACACGCTCAT |
GTGCTTCTCCACCACCATCT |
| Wnt5b |
CCAACTCCTGGTGGTCATTAGC |
TGGGCACCGATGATAAACATC |
| Wnt10a |
CTGGGTGCTCCTGTTCTTCCTA |
GAGGCGGAGGTCCAGAATG |
| Wnt14 |
GGGCAGACGGTCAAGCAA |
CCAGCCTTGATCACCTTCACA |
| Wnt16 |
GCCAATTTGCCGCTGAAC |
CGGCAGCAGGTACGGTTT |
| Fzd3 |
TGGCTATGGTGGATGATCAAAG |
TGGAGGCTGCCGTGGTA |
| Fzd6 |
ACAAGCTGAAGGTCATTTCCAAA |
GCTACTGCAGAAGTGCCATGAT |
| LRP5 |
CGTGATTGCCGACGATCTC |
TCCGGCCGCTAGTCTTGTC |
| LRP6 |
TTATGTGCCACACCCAAGTTCT |
CTGAGGGAGCTGATCATTGATTTA |
| GAPDH |
ATCACCATCTTCCAGGAGCG |
CCTGCTTCACCACCTTCTTG |
Flow cytometry
Apoptosis was evaluated by flow cytometry using
Annexin V/propidium iodide (PI) double staining (Invitrogen; Thermo
Fisher Scientific, Inc.). The analysis was performed using a Guava
EasyCyte 8HT flow cytometer (EMD Millipore, Billerica, MA, USA) for
a total of 50,000 counts. The results were analyzed using guavaSoft
3.1.1 (Merck KGaA).
Western blot analysis
The cells were lysed on ice in
radioimmunoprecipitation assay (RIPA) lysis buffer inhibitor
cocktail (Roche Applied Science, Mannheim, Germany) for 30 min.
After adding isopropanol on ice and melting at 37°C for three
times, lysate was boiled at 100°C for 5 min and centrifuged at
16,750 × g for 10 min, and supernatant containing nuclear protein
was collected. The protein concentrations were detected using a
bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology). For total proteins, the cells were lysed on ice in
RIPA lysis buffer inhibitor cocktail (Roche Applied Science) for 30
min. After 13,400 × g centrifugation for 10 min, the supernatant
containing total protein was collected. Equal amounts of protein or
nuclear protein (60 mg) from each sample were separated by SDS-PAGE
on 12% gels and transferred onto polyvinylidene difluoride
membranes (EMD Millipore), which were immunoblotted overnight at
4°C with primary antibodies against β-catenin (cat. no. ab16051;
Abcam, Cambridge, UK; 1:1,000), lymphoid enhancer binding factor 1
(LEF1; cat. no. MA5-14966; Thermo Fisher Scientific, Inc.; 1:1,000)
and GAPDH (cat. no. ab9485; Abcam; 1:1,000). Following washing
three times (5 min each time), the membranes were incubated with
horseradish peroxidase-conjugated secondary antibody (cat. no.
TA130005; OriGene Technologies, Inc., Beijing, China; 1:4,000) at
room temperature for 1 h, subsequently washed and visualized using
enhanced chemiluminescence (EMD Millipore).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Flow cytometry results were analyzed using guavaSoft 3.1.1 (Merck
KGaA). All statistical analyses were performed by two-way analysis
of variance followed by Bonferroni's multiple comparison test. All
statistical analyses were conducted using the GraphPad Prism 5
(GraphPad Software, Inc., La Jolla, CA, USA).
Results
BALL-1/VCR cells displayed multidrug
resistance
The vincristine-resistant BALL-1 ALL cell line
(BALL-1/VCR) was established by stepwise selection in increasing
concentrations of vincristine. As presented in Table II, the resistance of BALL-1/VCR
cells to vincristine and VDS was 25- and 22-fold of that in
BALL-1/WT cells, respectively. In addition, the resistance of the
BALL-1/VCR cell line to doxorubicin and VP16 was 9- and 5-fold of
BALL-1/WT cells, respectively. However, these cells exhibited
little cross-resistance (<4-fold resistance) to other drugs
including mitoxantrone, camptothecin and cisplatin.
 | Table II.Sensitivity of BALL-1/WT and
BALL-1/VCR cells to various anticancer agents. |
Table II.
Sensitivity of BALL-1/WT and
BALL-1/VCR cells to various anticancer agents.
|
| IC50 (µM) |
|
|---|
|
|
|
|
|---|
| Drug | BALL-1/WT | BALL-1/VCR | Fold change |
|---|
| Vincristine | 514±30.8 | 12,858±1144 | 25 |
| Vindesine | 446±28.9 | 9,799±140 | 22 |
| Doxorubicin | 5.57±0.57 | 53.62±6.38 | 9.6 |
| VP16 | 0.92±0.10 | 4.60±0.32 | 5 |
| Mitoxantrone | 1.10±0.07 | 3.55±0.25 | 3.2 |
| Cisplatin | 5.03±0.47 | 12.03±0.07 | 2.4 |
| Camptothecin | 12.43±1.75 | 15.59±2.01 | 1.3 |
Activated Wnt/β-catenin signaling
pathway in BALL-1/VCR cells
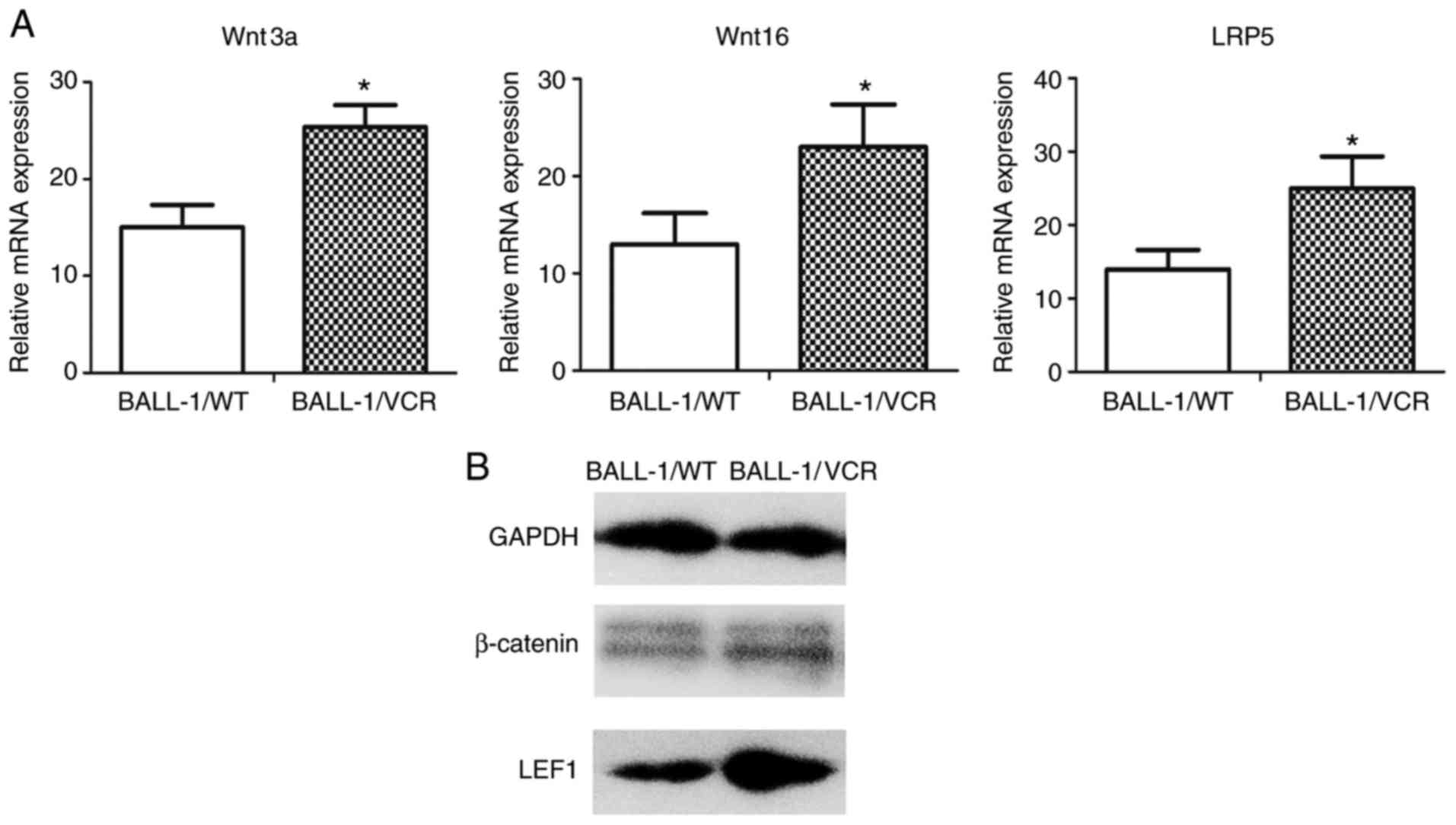
Expression levels of several Wnt family members and
their downstream signaling components were measured in BALL-1/WT
and BALL-1/VCR cells using RT-qPCR. The transcripts of Wnt family
members were expressed in both cell lines. However, in BALL-1/VCR
cells, levels of Wnt3a, Wnt5b, Wnt10a, Wnt14, Wnt16, Frizzled
(Fzd)3, Fzd6, LDL receptor related protein (LRP)5 and LRP6 were
significantly higher (Table III).
In addition, the expression of Wnt3a, Wnt16 and LRP5 were the
highest (Fig. 1A).
 | Table III.mRNA expression profile of Wnt family
members in BALL-1/WT and BALL-1/VCR cells. |
Table III.
mRNA expression profile of Wnt family
members in BALL-1/WT and BALL-1/VCR cells.
| Gene | BALL-1/WT | BALL-1/VCR | P-value |
|---|
| Wnt3a | 15.03±2.28 | 25.36±2.28 | <0.01 |
| Wnt5b | 11.09±2.60 | 17.93±2.81 | <0.01 |
| Wnt10a | 9.86±1.66 | 16.08±2.31 | <0.01 |
| Wnt14 | 9.67±3.02 | 15.91±3.36 | <0.01 |
| Wnt16 | 12.99±3.21 | 23.02±4.37 | <0.01 |
| Fzd3 | 9.59±2.43 | 13.80±3.51 | <0.01 |
| Fzd6 | 12.09±2.28 | 17.63±3.62 | <0.01 |
| LRP5 | 14.00±2.60 | 25.03±4.32 | <0.01 |
| LRP6 | 9.47±3.46 | 17.84±3.60 | <0.01 |
The canonical Wnt signaling pathway is activated by
the accumulation and nuclear translocation of β-catenin, which
binds to the transcription factors in the LEF/T-cell factor (TCF)
family. The expression of nuclear β-catenin and LEF1 was increased
significantly in BALL-1/VCR cells compared with that in BALL-1/WT
cells (Fig. 1B).
Increased chemo-sensitivity of
BALL-1/VCR cells treated with DKK1-CM
Subsequently, the Wnt/β-catenin signaling pathway in
BALL-1/VCR cells was inhibited by DKK1-CM. To assess the drug
sensitivity of BALL-1/VCR cells, the cells were treated with
anticancer drugs prior to and following the DKK1-CM treatment. As
presented in Fig. 2A, the cells
became sensitive to anticancer drugs, including VCR, VDS,
doxorubicin and etoposide, following DKK1-CM treatment, as was
revealed by their respective IC50 concentrations.
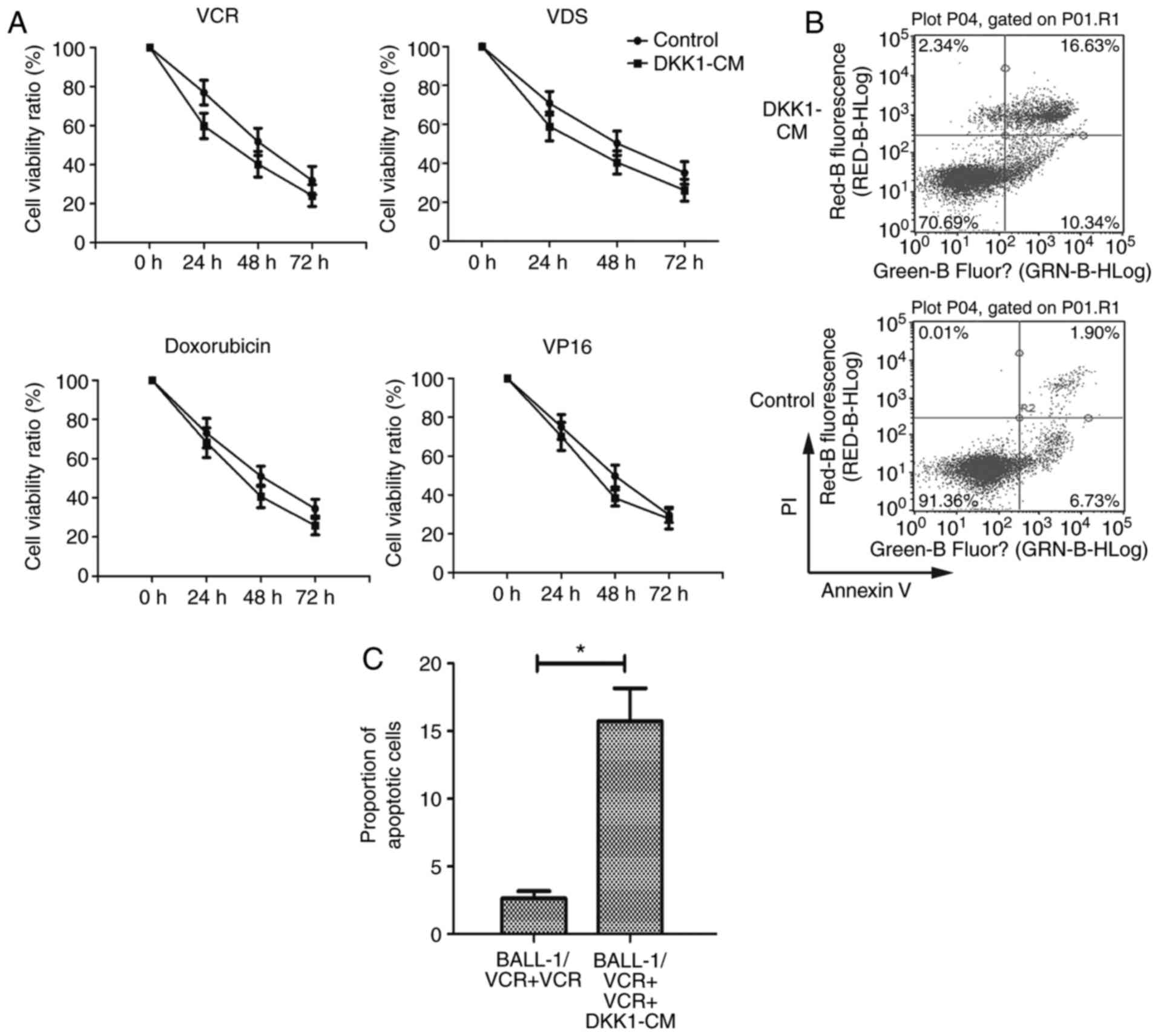 | Figure 2.Increased chemosensitivity in
BALL-1/VCR cells by Wnt inhibitor DKK1. (A) BALL-1/VCR cells were
treated with DKK1-CM for 48 h before adding VCR (12,125 M), VDS
(9,390 M), Doxorubicin (53 M) and VP16 (5 M). Cell viability was
assessed at 0, 24, 48 and 72 h by MTT assay. The data are expressed
as the mean ± standard deviation (% cell viability) of triplicate
experiments. (B) BALL-1/VCR cells were exposed to DKK1-CM for 48 h.
(B) Representative plots of 12 h cultures in the presence of VCR
(12,125 M). (C) The apoptosis of cells was quantified using Annexin
V and PI labeling and flow cytometry analysis. *P<0.01. VCR,
vincristine; VDS, vindesine; DKK1, Dickkopf-related protein 1;
VP16, etoposide; CM, conditioned media; PI, propidium iodide. |
To quantify the status of apoptosis in these cells,
they were analyzed by flow cytometry. The results showed that the
proportion of apoptotic cells was increased significantly following
the DKK1-CM treatment (Fig. 2B and
C).
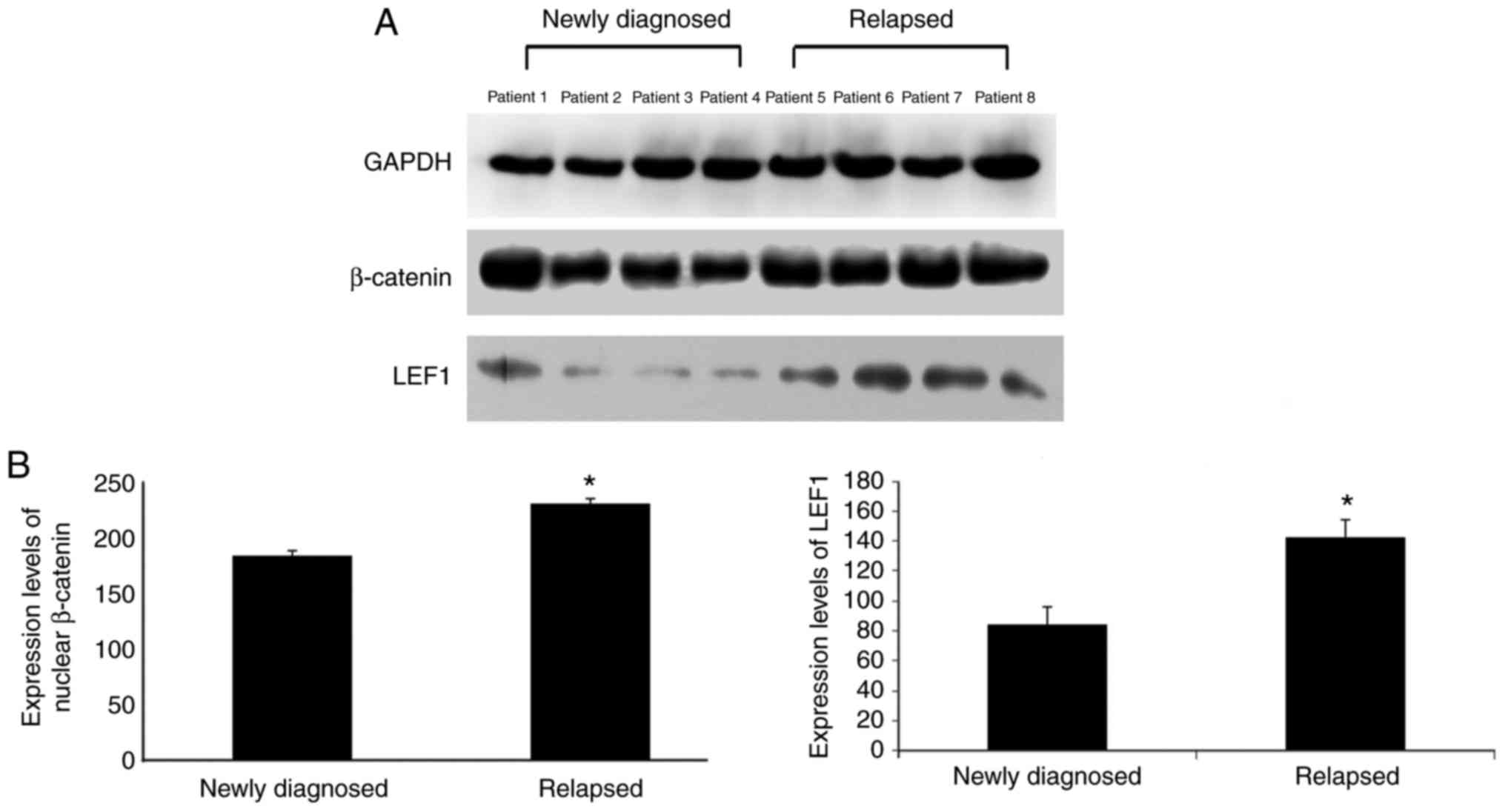
Wnt/β-catenin signaling of relapsed
ALL cells is over-activated with improved chemo-sensitivity
A total of four matched pairs of primary bone marrow
samples were collected from patients at the initial diagnosis of
ALL and relapsed ALL to evaluate activation of the Wnt/β-catenin
pathway. Expressions of nuclear β-catenin and LEF1 were observed in
samples from newly diagnosed patients with ALL and relapsed
patients with ALL. In addition, three of four samples from newly
diagnosed patients exhibited a significant decrease in expressions
of nuclear β-catenin and LEF1, whereas all relapsed samples
exhibited increased expressions of nuclear β-catenin and LEF1
(Fig. 3).
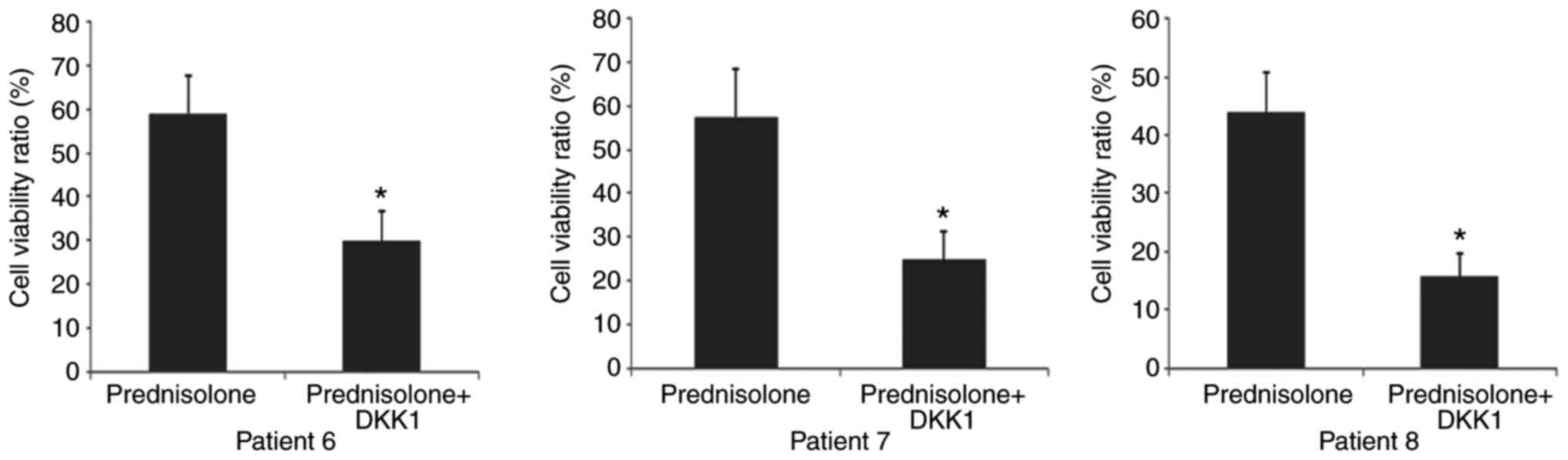
To investigate whether Wnt inhibition sensitizes
relapsed leukemic cells to anticancer drugs, changes in
chemo-sensitivity to prednisolone in leukemic blasts from three
relapsed samples were examined following DKK1-CM treatment.
Prednisolone was chosen because a previous study demonstrated that
relapsed ALL blasts exhibited strong resistance to glucocorticoids
(12). As expected, all relapsed
samples exhibited increased chemo-sensitivity in response to Wnt
inhibition (Fig. 4).
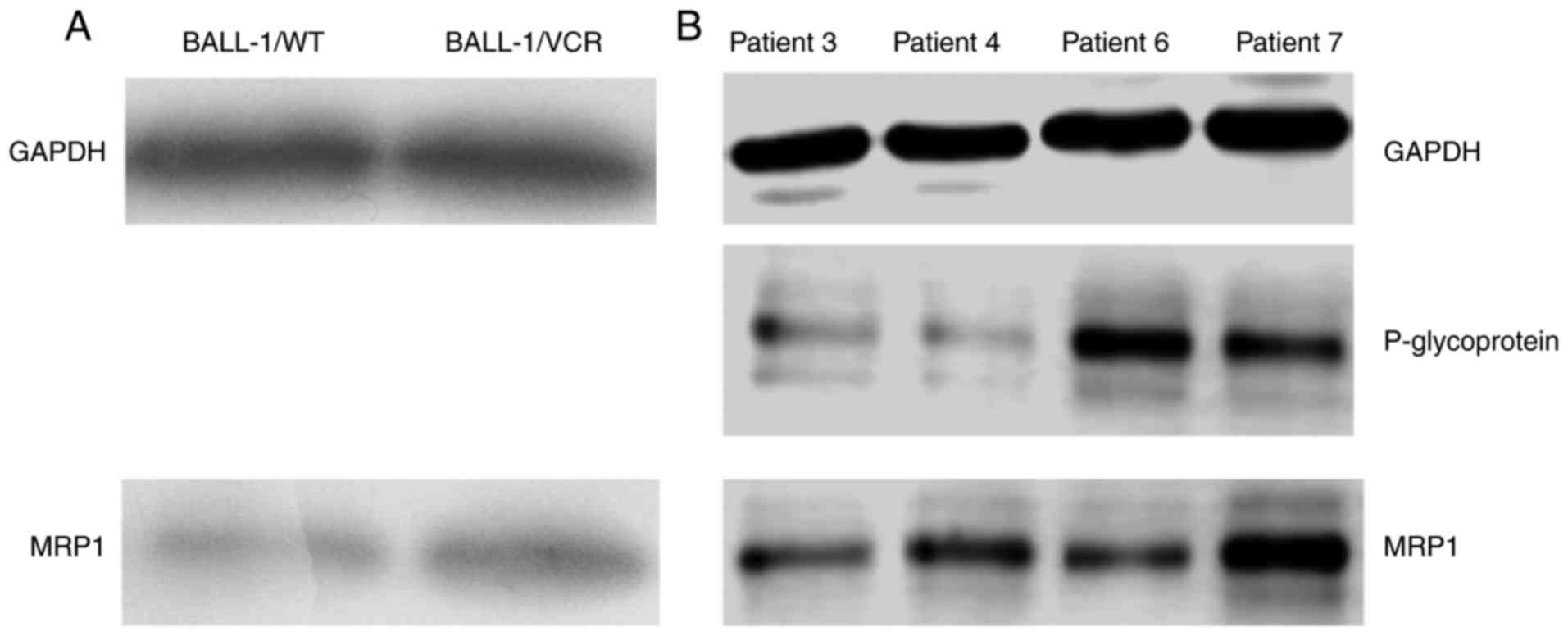
MDR1/P-gp protein and MRP
expressions
Overexpression of P-gp (MDR-1), which acts as a drug
efflux pump to decrease the intracellular accumulation of
anticancer drugs, is one of the major mechanisms underlying drug
resistance. Drug resistance may be also attributed to the
overexpression of proteins in the MRP family, which is a member of
the ABC transporter superfamily. In this study, the expression of
MDR1/P-gp and MRP1 in the cells was determined using western blot
analysis. The expression of P-gp was undetectable in both BALL-1/WT
and BALL-1/VCR cells, whereas the expression of MRP1 was increased
in BALL-1/VCR cells compared with WT (Fig. 5A). Notably, in the blasts from two
relapsed ALL samples, the expression of P-gp and MRP1 was
significantly increased compared with the blasts from newly
diagnosed ALL samples (Fig.
5B).
Therefore, the development of drug resistance in
BALL-1/VCR cells may be primarily attributed to the overexpression
of MRP1 rather than MDR1/P-gp. In addition, the development of drug
resistance in relapsed ALL may be associated with the
overexpression of MRP1 and MDR1/P-gp.
Discussion
Despite generally favorable outcomes of childhood
ALL, relapse still occurs with a dismal prognosis, thus it is
important to develop novel therapeutic modalities. Drug resistance
and early disease recurrence lead to limited survival of patients
with ALL (13). Previous attempts
to overcome drug resistance by increasing the dose of
chemotherapeutic agents have resulted in severe side effects and
even death. Therefore, new therapeutic modalities were employed to
suppress relevant signaling pathways and overcome drug
resistance.
In activation of the Wnt pathway, Wnt proteins bind
to cell surface receptors and induce a complex signaling cascade to
regulate cell growth and differentiation during hematopoiesis
(14). Considering that
hematological malignancies arise from immature hematopoietic stem
cells, one or more Wnt genes are often overexpressed and
functionally important in hematological malignancies (7,15).
Increasing evidence has indicated that the Wnt/β-catenin pathway
has a role in leukemia (16). For
example, the Wnt/β-catenin pathway is required for the development
of leukemia stem cells in AML (17). In addition, inhibition of the
Wnt/β-catenin signaling pathway leads to collateral
chemo-sensitivity in multidrug-resistant ALL cells (18), whereas aberrations of the
Wnt/β-catenin pathway induce cell death in B-cell ALL cell lines
(19). Hu et al (20) reported that Galectin-3 mediates drug
resistance in acute leukemia cells via the Wnt/β-catenin signaling
pathway.
In the current study, a drug-resistant variant of
the human ALL cell line BALL-1 (BALL-1/VCR) that had relatively
specific resistance to both doxorubicin and etoposide was used.
Furthermore, the role of Wnt family members and their downstream
signaling components in BALL-1/VCR cells was evaluated. Nuclear
β-catenin and LEF1 (one of the downstream targets of the Wnt
pathway) were selected as markers of Wnt/β-catenin pathway
activation. Over-activation of the Wnt/β-catenin signaling pathway
was observed in BALL-1/VCR and was identified as a potential
mechanism underlying ALL recurrence, consistent with the results
obtained in a previous study (12).
The importance of the Wnt/β-catenin pathway in leukemogenesis has
been reported previously (12,21,22).
For example, Dandekar et al (12) revealed that over-activation of the
Wnt signaling pathway may contribute to the chemo-resistance in
relapsed childhood ALL. Furthermore, treatment by two
small-molecule inhibitors of the Wnt/β-catenin signaling pathway
induced apoptosis of CLL cells in vitro and in vivo
(22). In addition, the inhibition
of the Wnt/β-catenin signaling pathway, which sensitizes the
resistant cells to chemotherapy, appears to be an attractive
strategy to maximize the chemotherapeutic potency of ALL.
During Wnt activation, Wnt proteins bind to cell
surface receptors encoded by the Fzd family, which allows β-catenin
to accumulate and to enter the nucleus so that it can interact with
TCF1 and LEF1 to recruit other proteins, thus promoting the
activation of Wnt target genes, leading to cell proliferation and
survival (23). Once Wnt binds to
its cell-surface receptor, which consists of Fzd, and LRP5 and 6,
it becomes essential for stabilization of β-catenin. In the current
study, the mRNA expression of Wnt3, Wnt5b, Wnt10a, Wnt14, Wnt16,
Fzd3, Fzd6, LRP5 and LRP6 was significantly upregulated in
BALL-1/VCR cells compared with wild-type BALL-1, while three of the
nine Wnt genes, Wnt 3a, Wnt16 and LRP5, were significantly
overexpressed in BALL-1/VCR.
Activation of the Wnt signaling pathway has been
broadly implicated in tumor formation, in which the transcriptional
repression of TCF1 has an important role (24). It has previously been demonstrated
that TCF1-knockout mice are prone to develop intestinal tumors and
highly metastatic thymic lymphoma (25,26).
Wnt3a, which has been confirmed as an agonist of the Wnt/β-catenin
signaling pathway, promotes the proliferation of mouse pro-B cells
in bone marrow by initiating a series of signaling events,
eventually leading to the β-catenin-dependent activation of the
LEF1 transcription factor. The overexpression of LEF-1 is strongly
associated with tumorigenesis of B-cell chronic lymphocytic
leukemia (27) and predicts
unfavorable outcomes of patients with B-precursor ALL (28). The presence of a complete
Wnt/Fzd/LRP/LEF1 gene expression signature in the BALL-1/VCR cells
suggests the functional importance of the canonical Wnt signaling
pathway. Therefore, the overexpression of Wnt family members,
nuclear β-catenin and LEF1, indicates over-activation of the Wnt
signaling pathway in BALL-1/VCR cells and the blasts from relapsed
ALL.
In this study, DKK1, a Wnt antagonist (29), was used to treat BALL-1/VCR cells
and inhibit the effect of Wnt/β-catenin signaling in these cells,
thus leading to an increased level of chemo-resistance. Activation
of the Wnt/β-catenin pathway can be competitively blocked with a
secreted form of Wnt antagonist, DKK1 (30). There are two possible mechanisms by
which DKK1 inhibits the Wnt signaling pathway: One is that DKK1
prevents the formation of Fz-LRP6 complex, which is necessary for
activation of the Wnt/β-catenin pathway; the other is that DKK1
interacts with the LRP/Kremen co-receptor complex and induces the
internalization of Wnt proteins, thus attenuating intensity of the
Wnt signaling pathway.
The resistance of cancer cell lines is associated
with multiple mechanisms, each of which has its own distinct
features. The multidrug resistance of classical MDR cell lines is
associated with reduced drug accumulation and the overexpression of
MDR1/P-gp, a membrane protein that functions as a drug efflux pump.
Although the susceptibility to VCR in BALL-1/VCR cells was 20-fold
lower than that in wild-type BALL-1/WT cells, there was no
detectable expression of MDR1 mRNA or P-gp in either cell lines.
Thus, P-gp is apparently not involved in the development of drug
resistance in BALL-1/VCR cells. Several cell lines, including a
mitoxantrone-resistant MCF7 cell line (31) and a VP16-resistant MCF7 cell line
(32), have been demonstrated to
exhibit an apparent defect in drug accumulation when the expression
of MDR1/P-gp is absent.
Several studies have demonstrated the overexpression
of MRP protein, which shares the homology with several members of
the ABC superfamily and is therefore thought to be involved in
multidrug resistance, in resistant cell lines (4,31,33).
When the expression of MRP in BALL-1/VCR and BALL-1 cells was
compared, it was observed that MRP expression was increased in
resistant cells. Therefore, it is possible that MRP overexpression
is involved in the development of the multidrug resistance
phenotype in BALL-1/VCR cells.
Clinical studies have also reported that the
overexpression of MDR-1 and MRP may contribute to the development
of cross-resistance to multiple anticancer agents (3,33).
Similarly, the overexpression of MRP was observed in BALL-1/VCR
cells and blasts isolated from relapsed ALL samples, whereas the
overexpression of P-glycoprotein was not observed. Therefore, the
molecular mechanism underlying the drug-resistance of relapsed ALL
may attributed to the increased expression of ABC transporters,
including MDR-1 and MRP proteins. However, ALL relapse not only
depends on chemotherapy resistance, but may also originate from
either major or minor clones present at the time of diagnosis
(34). Nevertheless, the
overexpression of MDR-1 and MRP may be involved in leukemia
relapse.
In conclusion, the current study produced a novel
BALL-1 variant cell line that was specifically resistant to VCR.
The expression of components in the Wnt signaling pathway of
BALL-1/VCR cells and blasts isolated from relapsed ALL samples
suggested an important role of the Wnt signaling pathway in ALL
relapse. Selective suppression of the Wnt signaling pathway using
DKK1 sensitized BALL-1/VCR cells anticancer agents. In addition,
the chemo-sensitivity to prednisolone in blasts from relapsed ALL
was restored by Wnt inhibition. As the resistance in BALL-1/VCR
cells is potentially attributed to the overexpression of MRP, drug
resistance in relapsed ALL may be associated with the
overexpression of MRP1 and MDR1/P-gp. Therefore, disruption of the
Wnt signaling pathway may have be of use for ALL treatment, while
targeting the Wnt signaling pathway with a more specific
pharmacologic antagonist, including antibodies and cytotoxins, is
an attractive therapeutic strategy for relapsed ALL.
Acknowledgements
The authors are appreciative to Professor Dao-xin Ma
(Department of Hematology, Qilu Hospital, Shandong University,
Jinan, China) and Institute of Immunology, Shandong University
(Jinan, China) for the cell line donation.
Funding
This work was supported by Scientific Research Fund
Project of Shandong University Qilu Hospital (grant nos. 2016QLQN18
and 2017QLQN19), Natural science fund of Shandong Province (grant
nos. ZR2014HM060, ZR2014HM093 and ZR2014HP037, ZR2017BH111) and
Shandong Province Key R&D Fund (grant no. 2017GSF218015).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JF conceptualized and designed the study, and
drafted the initial manuscript. LS carried out the study, reviewed
and revised the manuscript. YZ collected the samples, analyzed the
data and revised the manuscript. AZ collected the samples, analyzed
the data and revised the manuscript. NS collected the samples,
analyzed the data and revised the manuscript. DL coordinated and
supervised the data collection, and critically reviewed the
manuscript. BH analyzed the data and revised the manuscript. XJ
designed the data collection instruments, coordinated and
supervised the data collection, and critically reviewed the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Informed consent was obtained from all patients or
their guardians. The protocol followed the Declaration of Helsinki
and was approved by Ethic Committee in Qilu Hospital of Shandong
University (no. KYLL-2017-253).
Patient consent for publication
Informed consent was obtained from all patients or
their guardians.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Leonardi DB, Abbate M, Riccheri MC, Nuñez
M, Alfonso G, Gueron G, De Siervi A, Vazquez E and Cotignola J:
Improving risk stratification of patients with childhood acute
lymphoblastic leukemia: Glutathione-S-Transferases polymorphisms
are associated with increased risk of relapse. Oncotarget.
8:110–117. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jaime-Perez JC, Pinzon-Uresti MA,
Jimènez-Castillo RA, Colunga-Pedraza JE, González-Llano Ó and
Gómez-Almaguer D: Relapse of childhood acute lymphoblastic leukemia
and outcomes at a reference center in Latin America: Organomegaly
at diagnosis is a significant clinical predictor. Hematology.
23:1–9. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mahjoubi F and Akbari S: Multidrug
resistance-associated protein 1 predicts relapse in Iranian
childhood acute lymphoblastic leukemia. Asian Pac J Cancer Prev.
13:2285–2289. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zareifar S, Monabati A, Saeed A, Fakhraee
F and Cohan N: The association of glutathione S-transferase gene
mutations (including GSTT1 and GSTM1) with the prognostic factors
and relapse in acute lymphoblastic leukemia. Pediatr Hematol Oncol.
30:568–573. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hunyadi A, Csábi J, Martins A, Molnar J,
Balázs A, Tóth G and Backstabbing P-gp: Side-chain cleaved
ecdysteroid 2,3-dioxolanes hyper-sensitize MDR cancer cells to
doxorubicin without efflux inhibition. Molecules. 22(pii): pp.
E1992017, View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cai J, Fang L, Huang Y, Li R, Xu X, Hu Z,
Zhang L, Yang Y, Zhu X, Zhang H, et al: Simultaneous overactivation
of Wnt/β-catenin and TGFβ signalling by miR-128-3p confers
chemoresistance-associated metastasis in NSCLC. Nat Commun.
8:158702017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ashihara E, Takada T and Maekawa T:
Targeting the canonical Wnt/β-catenin pathway in hematological
malignancies. Cancer Sci. 106:665–671. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu C, Cui C, Liu B, Zou S, Song H, Tian H,
Zhao J and Li Y: FERMT3 contributes to glioblastoma cell
proliferation and chemoresistance to temozolomide through integrin
mediated Wnt signaling. Neurosci Lett. 657:77–83. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma LS, Jiang CY, Cui M, Lu R, Liu SS,
Zheng BB, Li L and Li X: Fluopsin C induces oncosis of human breast
adenocarcinoma cells. Acta Pharmacol Sin. 34:1093–1100. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Si L, Tian H, Yue W, Li L, Li S, Gao C and
Qi L: Potential use of microRNA-200c as a prognostic marker in
non-small cell lung cancer. Oncol Lett. 14:4325–4330. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dandekar S, Romanos-Sirakis E, Pais F,
Bhatla T, Jones C, Bourgeois W, Hunger SP, Raetz EA, Hermiston ML,
Dasgupta R, et al: Wnt inhibition leads to improved
chemosensitivity in paediatric acute lymphoblastic leukaemia. Br J
Haematol. 167:87–99. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Scheijen B, Boer JM, Marke R, Tijchon E,
van Ingen Schenau D, Waanders E, van Emst L, van der Meer LT,
Pieters R, Escherich G, et al: Tumor suppressors BTG1 and IKZF1
cooperate during mouse leukemia development and increase relapse
risk in B-cell precursor acute lymphoblastic leukemia patients.
Haematologica. 102:541–551. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luis TC, Ichii M, Brugman MH, Kincade P
and Staal FJ: Wnt signaling strength regulates normal hematopoiesis
and its deregulation is involved in leukemia development. Leukemia.
26:414–421. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ge X and Wang X: Role of Wnt canonical
pathway in hematological malignancies. J Hematol Oncol. 3:332010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Undi RB, Gutti U, Sahu I, Sarvothaman S,
Pasupuleti SR, Kandi R and Gutti RK: Wnt signaling: Role in
regulation of haematopoiesis. Indian J Hematol Blood Transfus.
32:123–134. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang Y, Mallampati S, Sun B, Zhang J, Kim
SB, Lee JS, Gong Y, Cai Z and Sun X: Wnt pathway contributes to the
protection by bone marrow stromal cells of acute lymphoblastic
leukemia cells and is a potential therapeutic target. Cancer Lett.
333:9–17. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hamdoun S, Fleischer E, Klinger A and
Efferth T: Lawsone derivatives target the Wnt/β-catenin signaling
pathway in multidrug-resistant acute lymphoblastic leukemia cells.
Biochem Pharmacol. 146:63–73. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saba NS, Angelova M, Lobelle-Rich PA and
Levy LS: Disruption of pre-B-cell receptor signaling jams the
WNT/β-catenin pathway and induces cell death in B-cell acute
lymphoblastic leukemia cell lines. Leuk Res. Aug 10–2015.(Epub
ahead of print). doi: 10.1016/j.leukres.2015.08.002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu K, Gu Y, Lou L, Liu L, Hu Y, Wang B,
Luo Y, Shi J, Yu X and Huang H: Galectin-3 mediates bone marrow
microenvironment-induced drug resistance in acute leukemia cells
via Wnt/β-catenin signaling pathway. J Hematol Oncol. 8:12015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seke Etet PF, Vecchio L and Nwabo Kamdje
AH: Interactions between bone marrow stromal microenvironment and
B-chronic lymphocytic leukemia cells: Any role for Notch, Wnt and
Hh signaling pathways? Cell Signal. 24:1433–1443. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gandhirajan RK, Staib PA, Minke K, Gehrke
I, Plickert G, Schlösser A, Schmitt EK, Hallek M and Kreuzer KA:
Small molecule inhibitors of Wnt/beta-catenin/lef-1 signaling
induces apoptosis in chronic lymphocytic leukemia cells in vitro
and in vivo. Neoplasia. 12:326–335. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bejsovec A: Wnt pathway activation: New
relations and locations. Cell. 120:11–14. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Drager J, Simon-Keller K, Pukrop T, Klemm
F, Wilting J, Sticht C, Dittmann K, Schulz M, Leuschner I, Marx A
and Hahn H: LEF1 reduces tumor progression and induces
myodifferentiation in a subset of rhabdomyosarcoma. Oncotarget.
8:3259–3273. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu S, Zhou X, Steinke FC, Liu C, Chen SC,
Zagorodna O, Jing X, Yokota Y, Meyerholz DK, Mullighan CG, et al:
The TCF-1 and LEF-1 transcription factors have cooperative and
opposing roles in T cell development and malignancy. Immunity.
37:813–826. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tiemessen MM, Baert MR, Schonewille T,
Brugman MH, Famili F, Salvatori DC, Meijerink JP, Ozbek U, Clevers
H, van Dongen JJ and Staal FJ: The nuclear effector of
Wnt-signaling, Tcf1, functions as a T-cell-specific tumor
suppressor for development of lymphomas. PLoS Biol.
10:e10014302012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gutierrez A Jr, Tschumper RC, Wu X,
Shanafelt TD, Eckel-Passow J, Huddleston PM III, Slager SL, Kay NE
and Jelinek DF: LEF-1 is a prosurvival factor in chronic
lymphocytic leukemia and is expressed in the preleukemic state of
monoclonal B-cell lymphocytosis. Blood. 116:2975–2983. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kuhnl A, Gökbuget N, Kaiser M, Schlee C,
Stroux A, Burmeister T, Mochmann LH, Hoelzer D, Hofmann WK, Thiel E
and Baldus CD: Overexpression of LEF1 predicts unfavorable outcome
in adult patients with B-precursor acute lymphoblastic leukemia.
Blood. 118:6362–6367. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mao B, Wu W, Davidson G, Marhold J, Li M,
Mechler BM, Delius H, Hoppe D, Stannek P, Walter C, et al: Kremen
proteins are Dickkopf receptors that regulate Wnt/beta-catenin
signalling. Nature. 417:664–667. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yamamoto H, Sakane H, Yamamoto H, Michiue
T and Kikuchi A: Wnt3a and Dkk1 regulate distinct internalization
pathways of LRP6 to tune the activation of beta-catenin signaling.
Dev Cell. 15:37–48. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Morrow CS, Peklak-Scott C, Bishwokarma B,
Kute TE, Smitherman PK and Townsend AJ: Multidrug resistance
protein 1 (MRP1, ABCC1) mediates resistance to mitoxantrone via
glutathione-dependent drug efflux. Mol Pharmacol. 69:1499–1505.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sehested M, Friche E, Jensen PB and Demant
EJ: Relationship of VP-16 to the classical multidrug resistance
phenotype. Cancer Res. 52:2874–2879. 1992.PubMed/NCBI
|
|
33
|
Chen GK, Duran GE, Mangili A,
Beketic-Oreskovic L and Sikic BI: MDR 1 activation is the
predominant resistance mechanism selected by vinblastine in MES-SA
cells. Br J Cancer. 83:892–898. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sun C, Chang L and Zhu X: Pathogenesis of
ETV6/RUNX1-positive childhood acute lymphoblastic leukemia and
mechanisms underlying its relapse. Oncotarget. 8:35445–35459.
2017.PubMed/NCBI
|