Introduction
Breast cancer is a common type of non-skin malignant
tumor affecting women (1). A
previous study revealed that ~1.67 million breast cancer cases are
diagnosed each year worldwide (2).
Breast cancers with or without estrogen receptor (ER), progesterone
receptor (PR) and human epidermal growth factor receptor 2 (HER2)
can be divided into hormone-dependent and -independent breast
cancers (3,4). It is considered that ~15–20% of breast
cancer cells lack ER, PR and EGFR2, and thus are classed as
triple-negative breast cancers (TNBCs) (5,6). As
TNBCs lack hormone receptors, hormone or targeted therapies are not
effective in the treatment of TNBCs in clinical practice (7,8). Thus,
the development of novel treatment strategies for TNBCs is of
utmost importance.
Methotrexate (MTX) is known as a folate antagonist
used widely in the treatment of rheumatoid arthritis and cancer
(9,10). Low-dose MTX has been shown to exert
anti-inflammatory effects when used in the treatment of rheumatoid
arthritis; however, high-dose MTX has been shown to have
anti-proliferative cytotoxic activities with a number of
side-effects, such as renal damage (9,11,12).
Previous studies have suggested that MTX can induce cell
cytotoxicity which is related to increases in reactive oxygen
species (ROS) production (13,14).
Furthermore, a number of studies have demonstrated that MTX can
inhibit cell proliferation in various types of cancers including
lung cancer, lymphoma, leukemia and hepatoma (15–19).
However, to date, to the best of our knowledge, no previous study
has demonstrated that MTX alone can exert sufficient anticancer
effects on breast cancer. In order to enhance the anticancer
effects of MTX on breast cancer, combination treatment with MTX
with other agents has been considered (4,20,21).
Vitamin E is known as a fat-soluble vitamin with
anti-oxidative function (22).
Vitamin E has 8 variants, including 4 tocopherols (α-, β-, γ- and
δ-tocopherols) and 4 tocotrienols (α-, β-, γ- and δ-tocotrienols);
it has also been demonstrated to lower cancer risk (23–25).
α-tocopherol is a major variant found in vitamin E and has
anti-oxidative functions (26,27).
The majority of studies have suggested that γ- and δ-tocopherol and
γ- and δ-tocotrienol can inhibit breast cancer growth, while
α-tocopherol does not exert obvious anticancer activity in breast
cancer (25,28). Studies have demonstrated that γ- and
δ-tocopherol only inhibit ER-positive (ER+) breast
cancer, while γ- and δ-tocotrienol can inhibit both ER+
breast cancer and TNBC (23,28).
In addition, a previous study also revealed that high-dose (100 µM)
α-tocopherol inhibited cell proliferation in ER+ breast
cancer, including MCF-7 and T47D cells in a dose-dependent manner
(29). Previous studies have also
demonstrated that only α-tocopherol succinate (α-TOS) has potent
antioxidant activities, while γ- and δ-tocotrienol do not (22–27).
Clinical MTX treatment can induce oxidative stress resulting in
side-effects (9,11,12);
therefore, perhaps MTX-induced oxidative stress can be decreased by
the use of α-TOS. Therefore, whether α-tocopherol has potent
anticancer activities in breast cancer warrants further
investigation.
α-TOS is an analogue of α-tocopherol with anticancer
activity (30–32). Previous studies have revealed that
α-TOS has anticancer activities in various hormone-dependent breast
cancers, such as MCF-7, MDA-MB-435, 4T1 and MDA-MB-453 cells
(33–36). Another previous study demonstrated
that α-TOS induced the apoptosis of TNBC cells, such as MDA-MB-231
and SKBR-3 cells (37). However,
that study only demonstrated that apoptotic characteristics and Fas
signals were induced in α-TOS-treated cells. The other mechanisms
associated with α-TOS treatment in TNBC warrant further
investigation.
Based on the above-mentioned studies, MTX, vitamin E
and its analogue have been previously administered in the treatment
of breast cancers. The present study aimed to determine whether
combination treatment with MTX and vitamin E or its analogue may
have more potential for use in the treatment of TNBCs. The
anticancer effects on TNBC were determined following treatment with
MTX, α-tocopherol, α-TOS, MTX/α-tocopherol and MTX/α-TOS.
Materials and methods
Materials, reagents and
antibodies
Fetal bovine serum, Dulbecco's modified Eagle's
medium (DMEM), non-essential amino acids, L-glutamine and
penicillin/streptomycin were obtained from Gibco/Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). The MTT assay kit was obtained
from Bio Basic Canada Inc. (Markham, OT, Canada). Luminol,
lucigenin, α-tocopherol, α-TOS and Hoechst 33342 were purchased
from Sigma-Aldrich/Merck KGaA (Darmstadt, Germany). Anti-tubulin
(1:1,000; cat. no. BS1699) primary rabbit polyclonal antibody was
acquired from Bioworld Technology, Inc. (Louis Park, MN, USA).
Anti-cleaved poly(adenosine diphosphate-ribose) polymerase (PARP;
1:2000; cat. no. 9544) and anti-caspase-3 (1:1000; cat. no. 9665)
primary rabbit polyclonal antibodies and horseradish peroxidase
(HRP)-conjugated goat anti-rabbit IgG secondary antibody (1:2,000;
cat. no. 7074) were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA).
Cells and cell culture
The TNBC cell lines, MDA-MB-231 and MDA-MB-468, were
purchased from the Bioresource Collection and Research Center (Shin
Chu, Taiwan). The cells were cultured with DMEM supplemented with
10% fetal bovine serum, 0.1 mM non-essential amino acids, 2 mM
L-glutamine and 100 IU/ml penicillin/streptomycin and maintained at
37°C with a humidified atmosphere containing 5% CO2.
Cell survival rate assay
The cell survival rate was determined using an MTT
assay. The cells were cultured in 96-well dish (3×103
cells/well). Every 24 h, the control and experimental groups (MTX,
vitamin E, MTX/vitamin E treatments) were treated with the MTT kit.
The cells were incubated with MTT solution for 3 h at 37°C and the
formazan product was produced. The formazan product was dissolved
and the absorbance was determined at 570 nm (A570) using a
Multiskan™ FC Microplate Photometer (Molecular Devices LLC,
Sunnyvale, CA, USA). The survival rate (%) was calculated as [(A570
experimental group)/(A570 control group)] × 100%.
Measurements of intracellular
H2O2
The production of cellular
H2O2 was determined using the
lucigenin-amplified chemiluminescence method (38,39).
The control and experimental samples (200 µl) were treated with 0.2
mmol/ml of luminol solution (100 µl) to determine the
H2O2 levels. All samples were analyzed and
observed for 5 min using a chemiluminescence analyzing system
(CLA-FSI; Tohoko Electronic Industrial Co., Ltd., Sendai,
Japan).
SDS electrophoresis and western blot
analysis
The control and experimental cells were treated with
lysis buffer (radio-immunoprecipitation assay buffer; cat. no.
20–188; EMD Millipore, Billerica, MA, USA). Following
centrifugation (16,000 × g; 4°C) for 30 min, proteins were obtained
from the supernatant layer. The protein concentration was
determined using a protein assay kit (cat. no. 23200; Thermo
Fischer Scientific, Inc.). A total of 40 µg protein was loaded and
separated on 13.3% SDS-PAGE under 80 volts. Separated proteins were
transferred onto PVDF membranes (EMD Millipore). The membranes were
firstly blocked with 5% non-fat milk at room temperature for 2 h.
After washing with phosphate-buffered saline (PBS) for 15 min (3
times), the membranes were treated with primary antibodies for 4 h
at room temperature, and the membranes were then washed with PBS
for 15 min (3 times). The membranes were subsequently incubated
with anti-rabbit HRP-conjugated secondary antibodies (1:2,000; cat.
no. 7074; Cell Signaling Technology, Inc.) at room temperature for
1 h. Finally, immunolabeled proteins were added and incubated with
Western Lightning® Chemiluminescence Plus reagent
(PerkinElmer, Inc., Waltham, MA, USA). The protein band was
observed and analyzed with a Luminescence Image Analysis system
(LAS-4000; FUJIFILM Electronic Materials Taiwan Co., Ltd., Taiwan)
and ImageJ 1.51j8 by Wayne Rasband (National Institutes of Health,
Bethesda, MD, USA).
Statistical analysis
All data were obtained and calculated from 3 or 4
independent experiments. Values are expressed as the means ± SD and
analyzed using one-way ANOVA (SPSS for Windows, version 10; SPSS,
Inc., Chicago, IL, USA) followed by Tukey's test for the
comparisons of group means. All statistical analyses were performed
using SAS for Windows, version 9.4. The level of significance was
set at a P-value <0.05.
Results
Combined treatment with MTX and
α-tocopherol suppresses the proliferation of TNBC cells
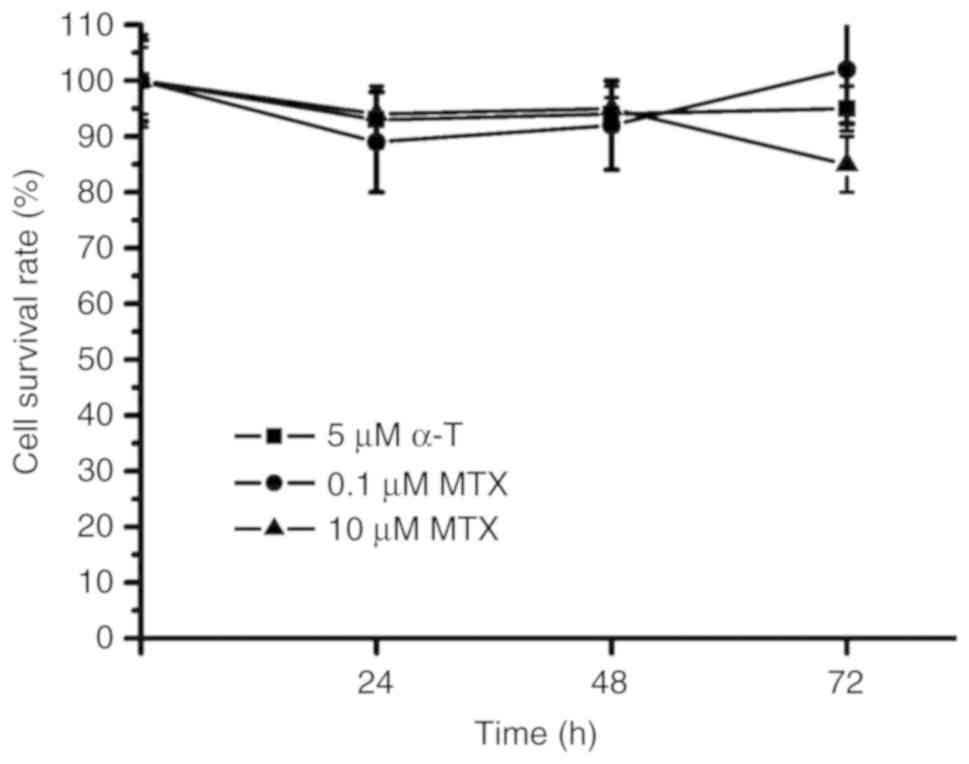
MTX and α-tocopherol were used to treat the TNBC
MDA-MB-231 cells. The survival rates were ~90% following 72 h of
treatment with 0.1 µM MTX, 10 µM MTX and 5 µM α-tocopherol in the
MDA-MB231 cells (Fig. 1). These
results indicated that MTX alone and α-tocopherol alone did not
effectively suppress the proliferation of the MDA-MB-231 cells.
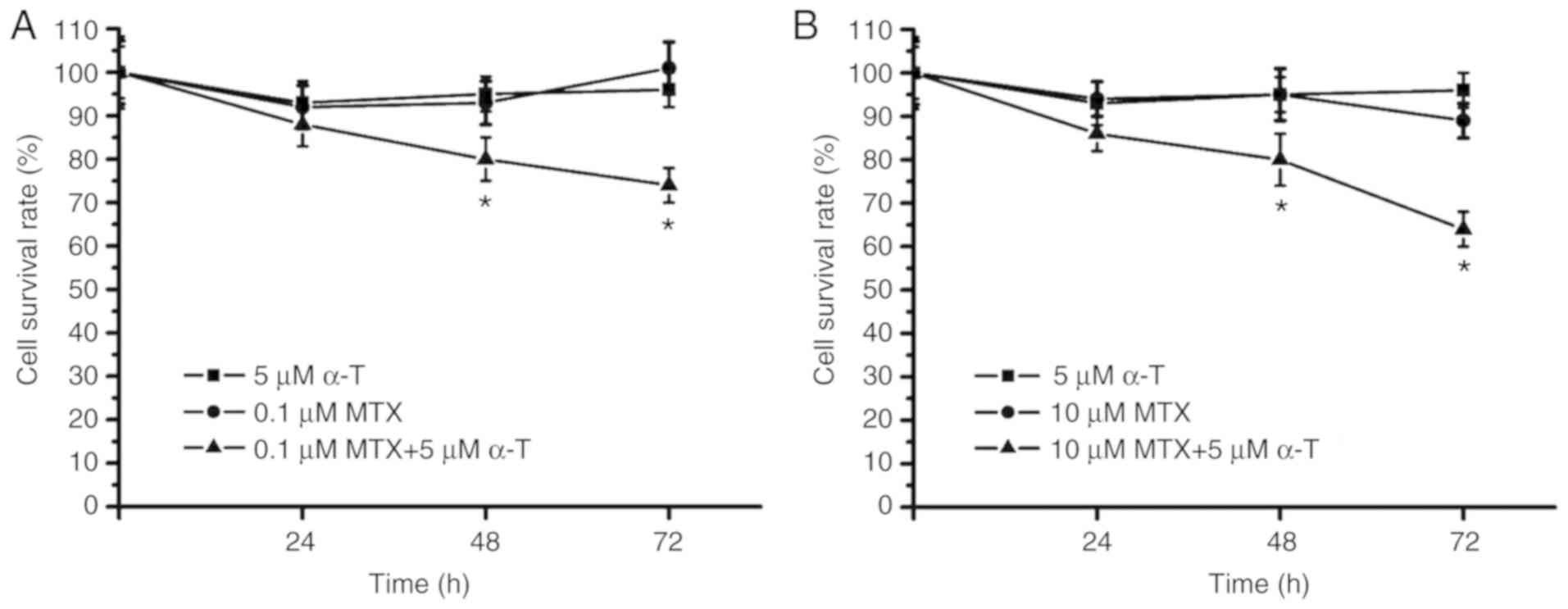
Combined treatment with MTX and α-tocopherol was then applied in
the MDA-MB-231 cells. The survival rate was ~75% following
treatment of the MDA-MB231 cells with 0.1 µM MTX plus 5 µM
α-tocopherol (Fig. 2A). In
addition, the survival rate was below 70% following treatment of
the MDA-MB231 cells with 10 µM MTX plus 5 µM α-tocopherol (Fig. 2B). As shown in Fig. 2, when compared with the group
treated with MTX alone, the group treated with MTX plus
α-tocopherol (MTX/α-tocopherol) exhibited significantly lower
survival rates at 48 and 72 h. These data suggested that
MTX/α-tocopherol treatment suppressed the proliferation of the
MDA-MB-231 cells. However, the survival rate was above 60% in the
MTX/α-tocopherol-treated group following 72 h of treatment. Thus,
it was considered that MTX/α-tocopherol may only attenuate cell
proliferation and thus MTX/α-tocopherol treatment may be not be an
effective strategy for clinical treatment.
α-tocopherol decreases the
H2O2 levels in MTX-treated cells
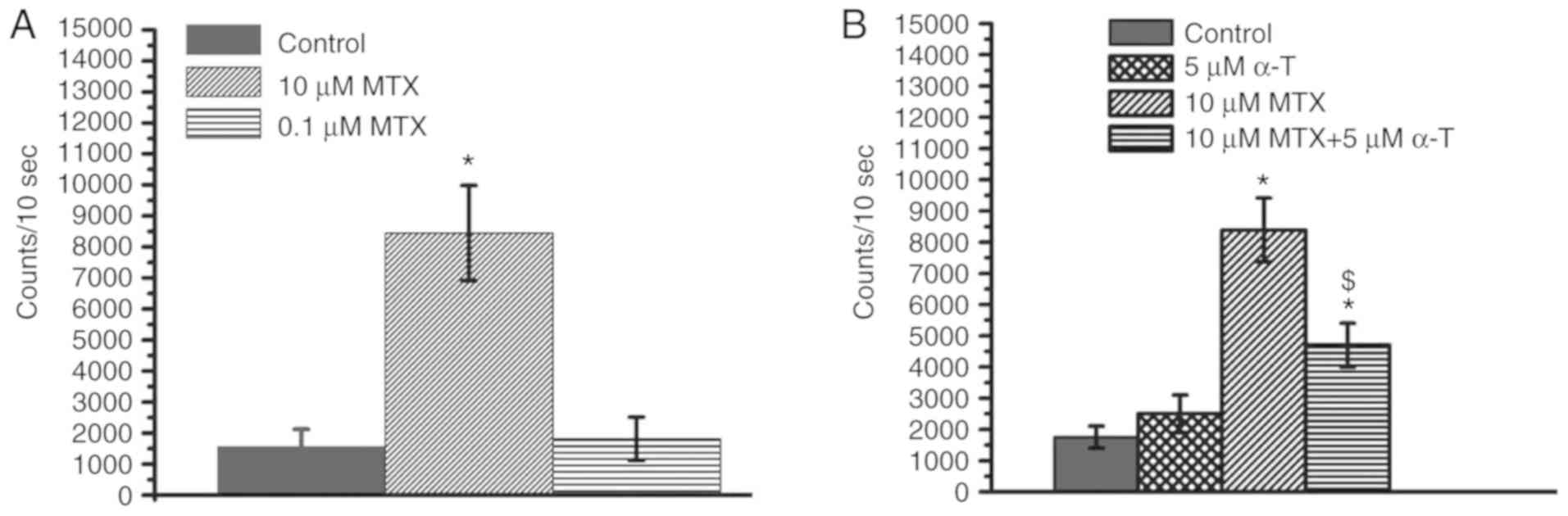
Previous studies have demonstrated that MTX can
induce an increase in ROS in cells, particularly
H2O2 levels, causing cell cytotoxicity
(14,19). In the present study, we wished to
determine whether MTX also induces an increase in
H2O2 levels in MDA-MB-231 cells. Compared to
the control group, treatment with 10 µM MTX significantly increased
the H2O2 levels in the MDA-MB-231 cells,
while treatment with 0.1 µM MTX did not markedly increase the
H2O2 levels (Fig.
3A). These results indicated that MTX increases the
H2O2 levels in a dose-dependent manner.
α-tocopherol is known to have anti-oxidative activities (26,27).
In the present study, whether α-tocopherol can inhibit the
MTX-induced increase in H2O2 levels was
investigated. The results revealed that α-tocopherol decreased the
H2O2 levels in the MTX-treated cells
(Fig. 3B). Notably, as shown in
Figs. 2 and 3, the H2O2 levels
were not associated with cell proliferation in the MTX-treated and
MTX/α-tocopherol-treated MDA-MB-231 cells.
α-TOS induces cell cytotoxicity in
TNBC in a time- and concentration-dependent manner
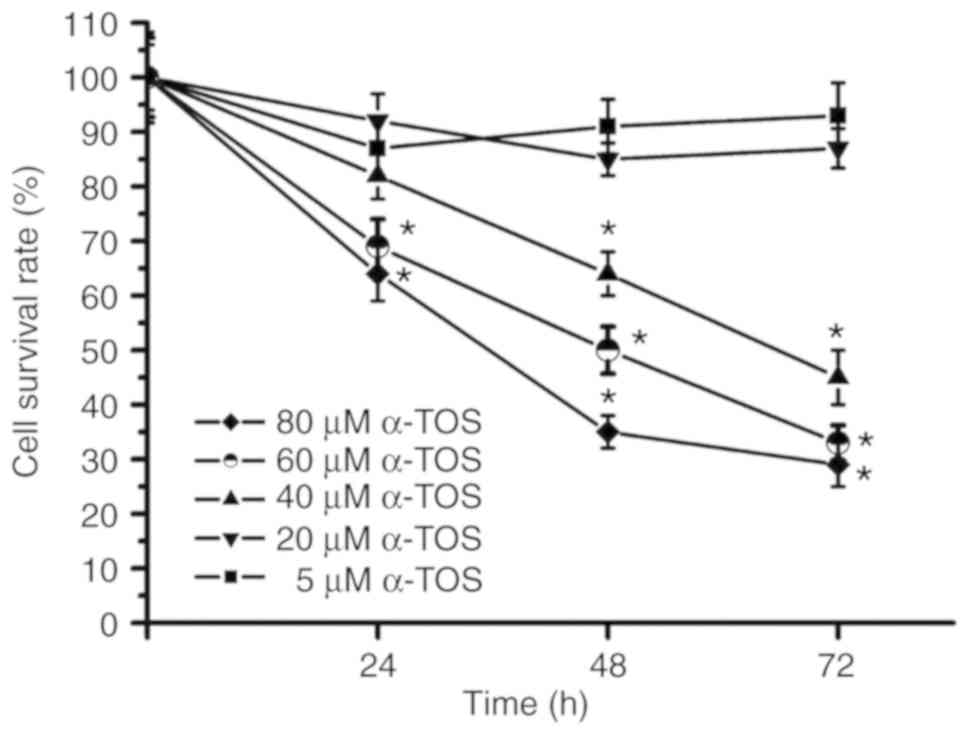
A previous study indicated that α-TOS induces the
apoptosis of and Fas activation in breast cancer cells (37). In the present study, the
concentration of α-TOS and the incubation time were further
investigated in the α-TOS-treated MDA-MB-231 cells. As shown in
Fig. 4, the cell survival rate was
~90% following treatment of the MDA-MB-231 cells with 5 and 20 µM
α-TOS for 72 h; however, the cell survival rate at 72 h was ~50%
following treatment with 40 µM α-TOS and below 50% following
treatment of the MDA-MB-231 cells with 60 and 80 µM α-TOS. These
results indicated that α-TOS induced cytotoxicity in a
concentration-dependent manner. Furthermore, following treatment
with 40, 60 and 80 µM α-TOS, the survival rates of the MDA-MB-231
cells gradually decreased during the 72-h treatment period. Thus,
α-TOS induced cytotoxicity in a time-dependent manner.
High- and low-dose MTX induces
distinct cytotoxicity in α-TOS-treated TNBC cells
Our data demonstrated that MTX/α-tocopherol
treatment suppressed TNBC cell proliferation, although
MTX/α-tocopherol induced-anticancer activity was ineffective
(Fig. 2). The present study further
investigated the anticancer effects on MDA-MB-231 cells following
treatment with MTX plus α-TOS, or α-tocopherol derivatives. The
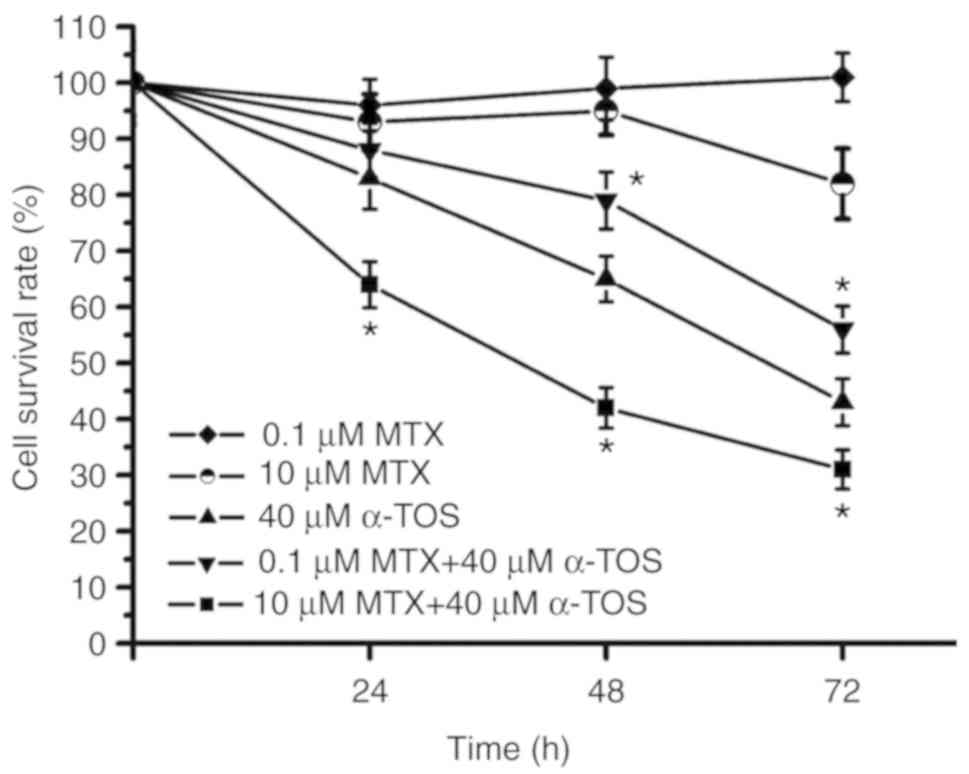
72-h cell survival rate was ~50% following treatment of the
MDA-MB-231 cell with 40 µM α-TOS alone (Fig. 5). Notably, the 72-h MDA-MB-231 cell
survival rate was ~35% in the group treated with 40 µM α-TOS plus
10 µM MTX; however, it was ~60% in the 40 µM α-TOS plus 0.1 µM MTX
group (Fig. 5). During the 72-h
treatment period, the survival rate curve of the group treated with
40 µM α-TOS plus 10 µM MTX was below that of the group treated with
40 µM α-TOS only. However, the survival rate curve of the group
treated with 40 µM α-TOS plus 0.1 µM MTX was above that of the
group treated with 40 µM α-TOS alone. Therefore, these results
indicated that high-dose MTX enhanced anticancer activity in
α-TOS-treated MDA-MB-231 cells, while low-dose MTX reduced
anticancer activity in α-TOS-treated MDA-MB-231 cells.
α-TOS- and MTX/α-TOS-induced
cytotoxicity is associated with caspase-3 activation and PARP
cleavage
Previous studies have demonstrated that both α-TOS
and MTX can induce cell cytotoxicity via caspase-3 activation
(19,40,41).
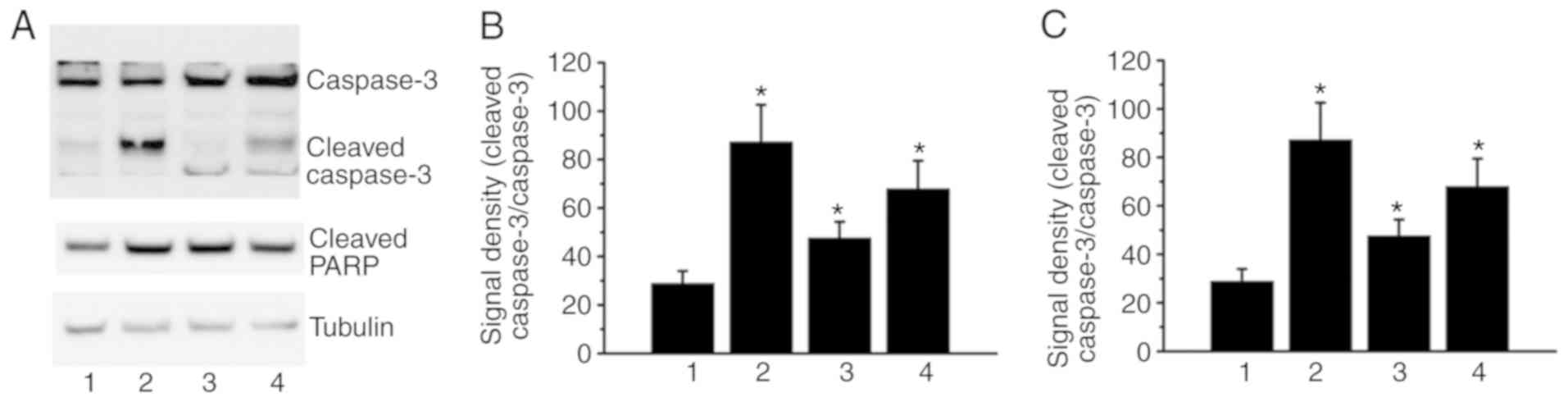
In the present study, as shown in Fig.
5, treatment with 40 µM α-TOS, 40 µM α-TOS/0.1 µM MTX and 40 µM
α-TOS/10 µM MTX induced cell cytotoxicity. Thus, caspase-3
activation and PARP, downstream of caspase-3, were investigated in
the present study. As determined by western blot analysis, the
ratio of cleaved caspase-3/pro-caspase-3 was increased in the
α-TOS- and α-TOS/MTX-treated cells (Fig. 6). In addition, PARP cleavage was
also observed in the α-TOS- and α-TOS/MTX-treated cells (Fig. 6). Therefore, these results indicate
that α-TOS and α-TOS/MTX may induce cell cytotoxicity via caspase-3
activation in MDA-MB-231 cells.
Discussion
Previous studies have indicated that vitamin E has
anti-oxidative functions in cancer chemotherapy, reducing chemical
agent-induced side-effects (42,43).
Furthermore, vitamin E can prolong the survival of patients with
gastric cancer (44) and can
attenuate prostate cancer metastasis (45). α-tocopherol is the most common form
found abundantly in vitamin E and displays anti-oxidative activity
(42,46). α-tocopherol is also used to decrease
chemical agent-induced side-effects in cancer therapy (47). The low dose of MTX (0.1 µM) used in
the present study, is the concentration that is used in clinical
practice for the treatment of rheumatoid arthritis. The high dose
of MTX (10 µM) used in the present study is the dose used in
clinical practice for cancer therapy. Currently, a dose of ≥10 µM
MTX is used in clinical practice for cancer therapy. On the other
hand, vitamin E is an antioxidant nutrient, and the dose range of
vitamin E used in clinical practice varies widely. Generally, the
dose of 5 to 80 µM vitamin E is used for combination treatment in
clinical practice for various diseases. Therefore, the dose range
of 5 to 80 µM vitamin E was used in the present study. A previous
study revealed that α-tocopherol enhances the anti-proliferative
effects of gefitinib in cells (48). However, α-tocopherol may attenuate
the anticancer activity of some chemical agents, such as tamoxifen
and crizotinib (49,50). The present study demonstrated that
treatment with α-tocopherol plus MTX decreased the proliferation of
MDA-MB-231 cells (Fig. 2).
Therefore, it was suggested that α-tocopherol may have different
anticancer effects when used in combination with different chemical
agents. However, the mechanisms through which α-tocopherol promotes
MTX- and gefitinib-induced cell proliferation and inhibits
tamoxifen- and crizotinib-induced anticancer activities remain
unclear.
A recent study demonstrated that treatment with
vitamin C/MTX increased H2O2 levels,
resulting in cell cytotoxicity (4).
A number of studies have also revealed that high
H2O2 levels are associated with cell
cytotoxicity (51–53). However, the present study
demonstrated that α-tocopherol attenuated the MTX-induced
H2O2 levels (Fig.
3B), while treatment with α-tocopherol plus MTX exerted
anti-proliferative effects on MDA-MB-231 cells (Fig. 2). Therefore, it was suggested that
the anti-proliferative effects of treatment with α-tocopherol/MTX
may not be related to the H2O2 levels. The
mechanisms underlying the anti-proliferative activity of
α-tocopherol/MTX require further investigation in the future.
Previous studies have demonstrated that
cyclopentenone prostaglandins/α-TOS treatment and MTX/α-TOS
treatment have synergistic anticancer effects on oral squamous
carcinoma cells (54) and
osteosarcoma cells (55). Similar
to these studies, the data from the present study also demonstrated
that high-dose MTX/α-TOS had a synergistic anticancer effect on
MDA-MB-231 cells. The survival rate was below 50% following
treatment with high-dose MTX/α-TOS for 48 h (Fig. 5). Compared with the survival rate of
the high-dose MTX/α-TOS treatment group, the survival rate was
still above 60% following treatment with high-dose MTX/α-tocopherol
for 72 h (Fig. 2B). Therefore, it
was suggested that high-dose MTX/α-TOS may be a potential treatment
for TNBC, while high-dose MTX/α-tocopherol treatment may not be an
effective strategy for TNBC treatment.
The present study, as well as previous studies have
demonstrated that α-tocopherol/MTX and α-tocopherol/gefitinib
treatment exert synergistic anti-proliferative effects (48). However, previous studies have also
revealed that α-tocopherol/tamoxifen and α-tocopherol/crizotinib
treatment exert antagonistic anti-proliferative effects (49,50).
These studies indicate that α-tocopherol in combination with
different chemical agents has distinct anti-proliferative effects.
However, in the present study, high-dose MTX enhanced α-TOS-induced
cell cytotoxicity, while low-dose MTX antagonized α-TOS-induced
cell cytotoxicity in MDA-MB-231cells (Fig. 5). This result was similar to that of
a previous study (34). This
previous study demonstrated that high-dose sodium selenite promoted
α-TOS-induced cell cytotoxicity, while low-dose sodium selenite
attenuated α-TOS-induced cell cytotoxicity in MCF-7 cells. Based on
these studies, it was suggested that both synergistic and
antagonistic effects can arise in the same drug components in a
dose-dependent manner. Although the detailed signaling mechanisms
require further investigation, the results of the present study
suggested that caspase signaling (Fig.
6) had a similar mechanism when comparing the low- and
high-dose effects of MTX with α-TOS treatment. However, oxidative
stress (such as H2O2 levels; Fig. 3) may have different signaling
pathways when comparing the low- and high-dose effect of MTX with
α-TOS treatment. In addition, as shown in Fig. 5, high-dose MTX slightly reduced the
cell survival rate, while low-dose MTX slightly increased the cell
survival rate. These results may be related to the synergistic or
antagonistic anticancer effects caused by α-TOS/MTX treatment. Both
MDA-MB-231 and MDA-MB-468 were examined in the present study.
Similar results were obtained with the MDA-MB-468 cells (data not
shown).
In conclusion, the present study demonstrated that
combined treatment with MTX and α-tocopherol or α-TOS attenuated
the cell survival rate of TNBC cells. However, MTX/α-TOS exerted a
more potent anti-proliferative effect than MTX/α-tocopherol. In
addition, high-dose MTX enhanced the α-TOS-induced cytotoxic
effects, while low-dose MTX antagonized the α-TOS-induced cytotoxic
effects.
Acknowledgements
The authors would like to thank the Ministry of
Science and Technology, the Ministry of Health and Welfare, the
Taipei Tzu Chi Hospital and Drug Development Center, China Medical
University (Taiwan, ROC) for making the present study a
possibility.
Funding
The present study was supported by grants from the
Ministry of Science and Technology, Taiwan (grant nos. MOST106-
2320-B-039-051-MY3 and MOST107-2320-B-039-004), the Ministry of
Health and Welfare, Taiwan (grant no. MOHW107- TDU-B-212-112-015),
the Taipei Tzu Chi Hospital, Taiwan (grant nos. TCRD-TPE-106-35,
TCRD-TPE-106-36, TCRD- TPE-105-20 and TCRD-TPE-105-02) and the
study was also financially supported by the ‘Drug Development
Center, China Medical University’ from The Featured Areas Research
Center Program within the framework of the Higher Education Sprout
Project by the Ministry of Education (MOE) in Taiwan, ROC.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article or are available from
the corresponding author on reasonable request.
Authors' contributions
CWW and YLY performed the experiments, analyzed the
data and wrote the manuscript. YHC and YTH performed the
experiments and analyzed the data. GTY designed the experiments and
analyzed the data. All authors approved the final version of the
manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests
References
|
1
|
Fan J, Wu Y, Yuan M, Page D, Liu J, Ong
IM, Peissig P and Burnside E: Structure-leveraged methods in breast
cancer risk prediction. J Mac Learn Res. 17:852017.
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Robinson SP and Jordan VC: Antiestrogenic
action of toremifene on hormone-dependent, -independent, and
heterogeneous breast tumor growth in the athymic mouse. Cancer Res.
49:1758–1762. 1989.PubMed/NCBI
|
|
4
|
Wu CW, Liu HC, Yu YL, Hung YT, Wei CW and
Yiang GT: Combined treatment with vitamin C and methotrexate
inhibits triple-negative breast cancer cell growth by increasing
H2O2 accumulation and activating caspase-3
and p38 pathways. Oncol Rep. 37:2177–2184. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu YL, Chou RH, Liang JH, Chang WJ, Su KJ,
Tseng YJ, Huang WC, Wang SC and Hung MC: Targeting the EGFR/PCNA
signaling suppresses tumor growth of triple-negative breast cancer
cells with cell-penetrating PCNA peptides. PLoS One. 8:e613622013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Metzger-Filho O, Tutt A, de Azambuja E,
Saini KS, Viale G, Loi S, Bradbury I, Bliss JM, Azim HA Jr, Ellis
P, et al: Dissecting the heterogeneity of triple-negative breast
cancer. J Clin Oncol. 30:1879–1887. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Williams CB, Soloff AC, Ethier SP and Yeh
ES: Perspectives on epidermal growth factor receptor regulation in
triple-negative breast cancer: Ligand-mediated mechanisms of
receptor regulation and potential for clinical targeting. Adv
Cancer Res. 127:253–281. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Malaviya AN: Low-dose methotrexate
(LD-MTX) in rheumatology practice-a most widely misunderstood drug.
Curr Rheumatol Rev. 12:168–176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Robien K, Boynton A and Ulrich CM:
Pharmacogenetics of folate-related drug targets in cancer
treatment. Pharmacogenomics. 6:673–689. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mori S, Hidaka M, Kawakita T, Hidaka T,
Tsuda H, Yoshitama T, Migita K and Ueki Y: Factors associated with
myelosuppression related to low-dose methotrexate therapy for
inflammatory rheumatic diseases. PLoS One. 11:e01547442016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maejima H, Watarai A, Nakano T, Katayama
C, Nishiyama H and Katsuoka K: Adverse effects of methotrexate in
three psoriatic arthritis patients. Rheumatol Int. 34:571–574.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kolli VK, Abraham P, Isaac B and
Selvakumar D: Neutrophil infiltration and oxidative stress may play
a critical role in methotrexate-induced renal damage. Chemotherapy.
55:83–90. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cağlar Y, Özgür H, Matur I, Yenilmez ED,
Tuli A, GÖnlüşen G and Polat S: Ultrastructural evaluation of the
effect of N-acetylcysteine on methotrexate nephrotoxicity in rats.
Histol Histopathol. 28:865–874. 2013.PubMed/NCBI
|
|
15
|
Yang W, Zou Y, Meng F, Zhang J, Cheng R,
Deng C and Zhong Z: Efficient and targeted suppression of human
lung tumor xenografts in mice with methotrexate sodium encapsulated
in all-function-in-one chimeric polymersomes. Adv Mater.
28:8234–8239. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Larsen EC, Devidas M, Chen S, Salzer WL,
Raetz EA, Loh ML, Mattano LA Jr, Cole C, Eicher A, Haugan M, et al:
Dexamethasone and high-dose methotrexate improve outcome for
children and young adults with high-risk B-acute lymphoblastic
leukemia: A report from children's oncology group study AALL0232. J
Clin Oncol. 34:2380–2388. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kansara R, Shenkier TN, Connors JM, Sehn
LH, Savage KJ, Gerrie AS and Villa D: Rituximab with high-dose
methotrexate in primary central nervous system lymphoma. Am J
Hematol. 90:1149–1154. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cipolleschi MG, Marzi I, Rovida E,
Olivotto M and Dello Sbarba P: Low-dose methotrexate enhances
cycling of highly anaplastic cancer cells. Cell Cycle. 16:280–285.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yiang GT, Chou PL, Hung YT, Chen JN, Chang
WJ, Yu YL and Wei CW: Vitamin C enhances anticancer activity in
methotrexate-treated Hep3B hepatocellular carcinoma cells. Oncol
Rep. 32:1057–1063. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barros S, Mencia N, Rodriguez L, Oleaga C,
Santos C, Noé V and Ciudad CJ: The redox state of cytochrome c
modulates resistance to methotrexate in human MCF7 breast cancer
cells. PLoS One. 8:e632762013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tanabe M: Combination chemotherapy of
mitomycin C and methotrexate was effective on metastatic breast
cancer resistant to Eribulin, Vinorelbine, and bevacizumab after
anthracycline, Taxane, and Capecitabine. Case Rep Oncol. 9:422–426.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Velthuis-te Wierik EJ, van den Berg H,
Weststrate JA, van het Hof KH and de Graaf C: Consumption of
reduced-fat products: Effects on parameters of anti-oxidative
capacity. Eur J Clin Nutr. 50:214–219. 1996.PubMed/NCBI
|
|
23
|
Smolarek AK, So JY, Burgess B, Kong AN,
Reuhl K, Lin Y, Shih WJ, Li G, Lee MJ, Chen YK, et al: Dietary
administration of δ- and γ-tocopherol inhibits tumorigenesis in the
animal model of estrogen receptor-positive, but not HER-2 breast
cancer. Cancer Prev Res. 5:1310–1320. 2012. View Article : Google Scholar
|
|
24
|
Constantinou C, Papas A and Constantinou
AI: Vitamin E and cancer: An insight into the anticancer activities
of vitamin E isomers and analogs. Int J Cancer. 123:739–752. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Smolarek AK and Suh N: Chemopreventive
activity of vitamin E in breast cancer: A focus on γ- and
δ-tocopherol. Nutrients. 3:962–986. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zapata GL, Guajardo MH and Terrasa AM: The
in vitro protective effect of alpha-tocopherol on oxidative injury
in the dog retina. Vet J. 177:266–272. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ekstrand-Hammarstrom B, Osterlund C,
Lilliehöök B and Bucht A: Vitamin E down-modulates
mitogen-activated protein kinases, nuclear factor-kappaB and
inflammatory responses in lung epithelial cells. Clin Exp Immunol.
147:359–369. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Comitato R, Nesaretnam K, Leoni G, Ambra
R, Canali R, Bolli A, Marino M and Virgili F: A novel mechanism of
natural vitamin E tocotrienol activity: Involvement of ERbeta
signal transduction. Am J Physiol Endocrinol Metab. 297:E427–E437.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chamras H, Barsky SH, Ardashian A,
Navasartian D, Heber D and Glaspy JA: Novel interactions of vitamin
E and estrogen in breast cancer. Nutr Cancer. 52:43–48. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gao Y, Qi X, Zheng Y, Ji H, Wu L, Zheng N
and Tang J: Nanoemulsion enhances α-tocopherol succinate
bioavailability in rats. Int J Pharm. 515:506–514. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kulikov AV, Vdovin AS, Zhivotovsky B and
Gogvadze V: Targeting mitochondria by α-tocopheryl succinate
overcomes hypoxia-mediated tumor cell resistance to treatment. Cell
Mol Life Sci. 71:2325–2333. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Angulo-Molina A, Reyes-Leyva J, López-Malo
A and Hernández J: The role of alpha tocopheryl succinate (α-TOS)
as a potential anticancer agent. Nutr Cancer. 66:167–176. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu W, Liao QY, Hantash FM, Sanders BG and
Kline K: Activation of extracellular signal-regulated kinase and
c-Jun-NH2-terminal kinase but not p38 mitogen-activated
protein kinases is required for RRR-α-tocopheryl succinate-induced
apoptosis of human breast cancer cells. Cancer Res. 61:6569–6576.
2001.PubMed/NCBI
|
|
34
|
Badr DM, Hafez HF, Agha AM and Shouman SA:
The combination of α-tocopheryl succinate and sodium selenite on
breast cancer: A merit or a demerit? Oxid Med Cell Longev 2016.
47416942016.
|
|
35
|
Wang XF, Xie Y, Wang HG, Zhang Y, Duan XC
and Lu ZJ: α-Tocopheryl succinate induces apoptosis in
erbB2-expressing breast cancer cell via NF-κB pathway. Acta
Pharmacol Sin. 31:1604–1610. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang D, Chuang HC, Weng SC, Huang PH,
Hsieh HY, Kulp SK and Chen CS: alpha-Tocopheryl succinate as a
scaffold to develop potent inhibitors of breast cancer cell
adhesion. J Med Chem. 52:5642–5648. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Turley JM, Fu T, Ruscetti FW, Mikovits JA,
Bertolette DC III and Birchenall-Roberts MC: Vitamin E succinate
induces Fas-mediated apoptosis in estrogen receptor-negative human
breast cancer cells. Cancer Res. 57:881–890. 1997.PubMed/NCBI
|
|
38
|
Lin BR, Yu CJ, Chen WC, Lee HS, Chang HM,
Lee YC, Chien CT and Chen CF: Green tea extract supplement reduces
D-galactosamine-induced acute liver injury by inhibition of
apoptotic and proinflammatory signaling. J Biomed Sci. 16:352009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yiang GT, Yu YL, Lin KT, Chen JN, Chang WJ
and Wei CW: Acetaminophen induces JNK/p38 signaling and activates
the caspase-9-3-dependent cell death pathway in human mesenchymal
stem cells. Int J Mol Med. 36:485–492. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Abe N, Shimizu T, Miyoshi N, Murata Y and
Nakamura Y: α-Tocopherol sensitizes human leukemia HL-60 cells to
apoptosis induced by benzyl isothiocyanate. Biosci Biotechnol
Biochem. 76:381–383. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Abo-Haded HM, Elkablawy MA, Al-Johani Z,
Al-Ahmadi O and El-Agamy DS: Hepatoprotective effect of sitagliptin
against methotrexate induced liver toxicity. PLoS One.
12:e01742952017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Peh HY, Tan WS, Liao W and Wong WS:
Vitamin E therapy beyond cancer: Tocopherol versus tocotrienol.
Pharmacol Ther. 162:152–169. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yüncü M, Bükücü N, Bayat N, Sencar L and
Tarakcioglu M: The effect of vitamin E and L-carnitine against
methotrexate-induced injury in rat testis. Turk J Med Sci.
45:517–525. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pyrhönen S, Kuitunen T, Nyandoto P and
Kouri M: Randomised comparison of fluorouracil, epidoxorubicin and
methotrexate (FEMTX) plus supportive care with supportive care
alone in patients with non-resectable gastric cancer. Br J Cancer.
71:587–591. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Drago JR, Nesbitt JA, Badalament RA and
Smith J: Chemotherapy and vitamin E in treatment of Nb rat prostate
tumors. In Vivo. 2:399–401. 1988.PubMed/NCBI
|
|
46
|
Fu JY, Htar TT, De Silva L, Tan DM and
Chuah LH: Chromatographic separation of vitamin E enantiomers.
Molecules. 22(pii): E2332017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dias MF, Sousa E, Cabrita S, Patricio J
and Oliveira CF: Chemoprevention of DMBA-induced mammary tumors in
rats by a combined regimen of alpha-tocopherol, selenium, and
ascorbic acid. Breast J. 6:14–19. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yiang GT, Chen JN, Lin PS, Liu HC, Chen SY
and Wei CW: Combined treatment with vitamin E and gefitinib has
synergistic effects to inhibit TGF-β1-induced renal fibroblast
proliferation. Mol Med Rep. 13:5372–5378. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Uchihara Y, Ueda F, Tago K, Nakazawa Y,
Ohe T, Mashino T, Yokota S, Kasahara T, Tamura H and Funakoshi-Tago
M: Alpha-tocopherol attenuates the anti-tumor activity of
crizotinib against cells transformed by NPM-ALK. PLoS One.
12:e01830032017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Peralta EA, Viegas ML, Louis S, Engle DL
and Dunnington GL: Effect of vitamin E on tamoxifen-treated breast
cancer cells. Surgery. 140:607–615. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chang H, Li C, Huo K, Wang Q, Lu L, Zhang
Q, Wang Y and Wang W: Luteolin prevents H2O2-induced apoptosis in
H9C2 cells through modulating Akt-P53/Mdm2 signaling pathway.
Biomed Res Int 2016. 51258362016.
|
|
52
|
Miyazato H, Taira J and Ueda K: Hydrogen
peroxide derived from marine peroxy sesquiterpenoids induces
apoptosis in HCT116 human colon cancer cells. Bioorg Med Chem Lett.
26:4641–4644. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang C, Wang G, Liu H and Hou YL:
Protective effect of bioactive compounds from Lonicera japonica
Thunb. Against H2O2-induced cytotoxicity using neonatal rat
cardiomyocytes. Iran J Basic Med Sci. 19:97–105. 2016.PubMed/NCBI
|
|
54
|
ElAttar TM and Virji AS: Inhibition of
growth in oral squamous carcinoma cells by cyclopentenone
prostaglandins: comparison with chemotherapeutic agents.
Prostaglandins Leukot Essent Fatty Acids. 56:461–465. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Alleva R, Benassi MS, Pazzaglia L,
Tomasetti M, Gellert N, Borghi B, Neuzil J and Picci P:
Alpha-tocopheryl succinate alters cell cycle distribution
sensitising human osteosarcoma cells to methotrexate-induced
apoptosis. Cancer Lett. 232:226–235. 2006. View Article : Google Scholar : PubMed/NCBI
|