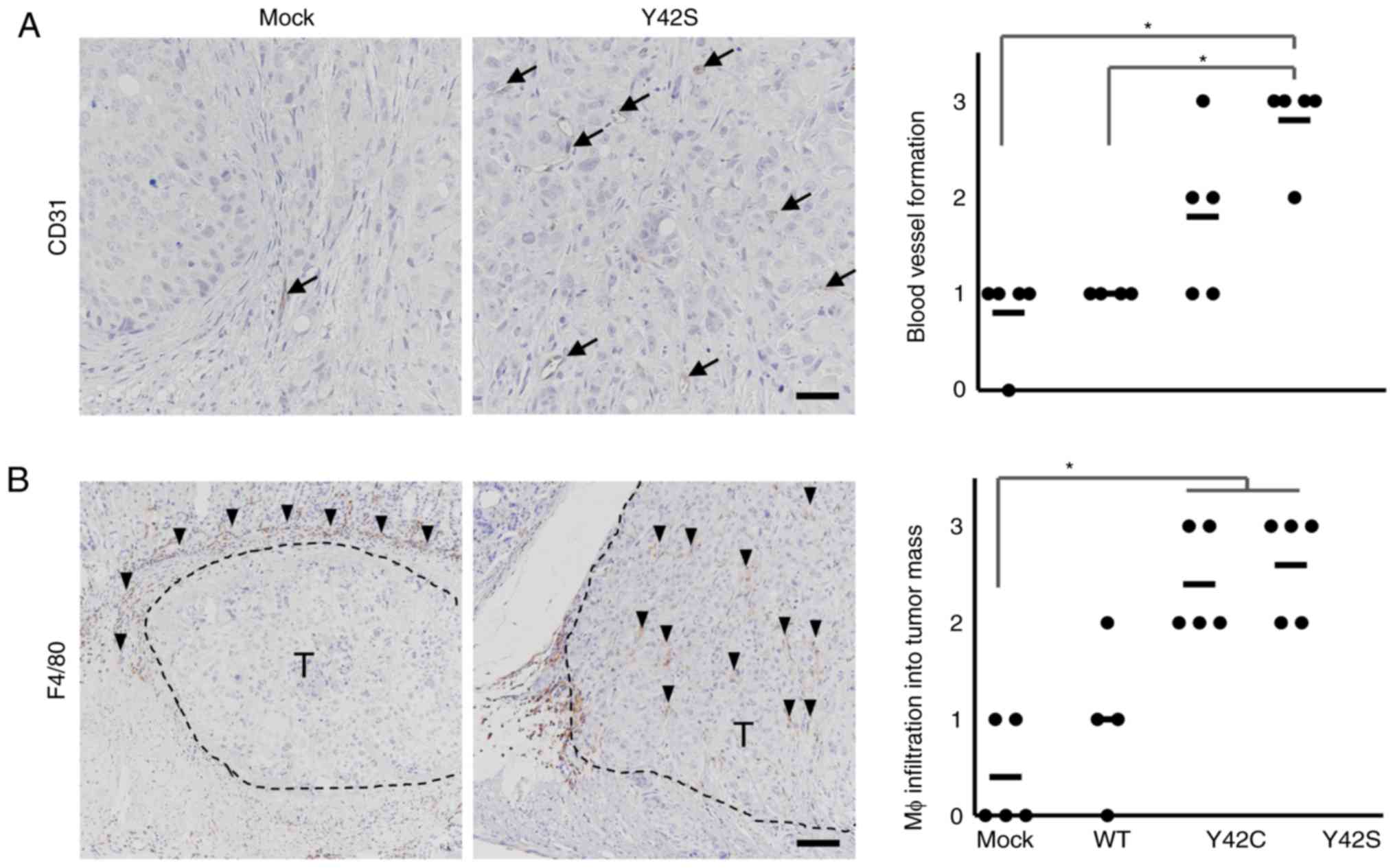
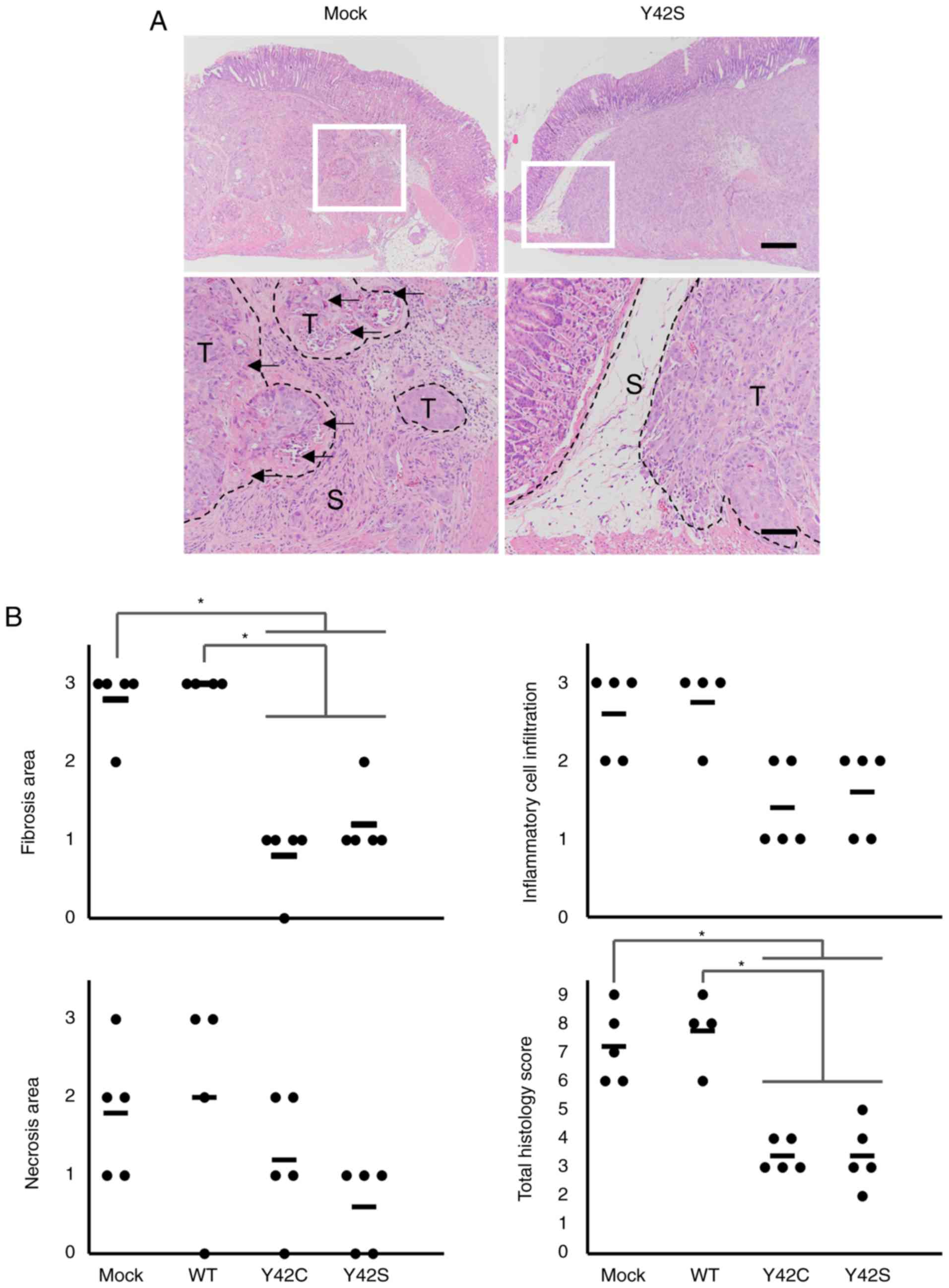
|
1
|
Ma J, Shen H, Kapesa L and Zeng S: Lauren
classification and individualized chemotherapy in gastric cancer.
Oncol Lett. 11:2959–2964. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen YC, Fang WL, Wang RF, Liu CA, Yang
MH, Lo SS, Wu CW, Li AF, Shyr YM and Huang KH: Clinicopathological
variation of lauren classification in gastric cancer. Pathol Oncol
Res. 22:197–202. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chiaravalli AM, Klersy C, Vanoli A,
Ferretti A, Capella C and Solcia E: Histotype-based prognostic
classification of gastric cancer. World J Gastroenterol.
18:896–904. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kakiuchi M, Nishizawa T, Ueda H, Gotoh K,
Tanaka A, Hayashi A, Yamamoto S, Tatsuno K, Katoh H, Watanabe Y, et
al: Recurrent gain-of-function mutations of RHOA in diffuse-type
gastric carcinoma. Nat Genet. 46:583–587. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang K, Yuen ST, Xu J, Lee SP, Yan HH, Shi
ST, Siu HC, Deng S, Chu KM, Law S, et al: Whole-genome sequencing
and comprehensive molecular profiling identify new driver mutations
in gastric cancer. Nat Genet. 46:573–582. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cancer Genome Atlas Research Network, .
Comprehensive molecular characterization of gastric adenocarcinoma.
Nature. 513:202–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ushiku T, Ishikawa S, Kakiuchi M, Tanaka
A, Katoh H, Aburatani H, Lauwers GY and Fukayama M: RHOA mutation
in diffuse-type gastric cancer: A comparative clinicopathology
analysis of 87 cases. Gastric Cancer. 19:403–411. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stankiewicz TR and Linseman DA: Rho family
GTPases: Key players in neuronal development, neuronal survival,
and neurodegeneration. Front Cell Neurosci. 8:3142014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jaffe AB and Hall A: Rho GTPases:
Biochemistry and biology. Annu Rev Cell Dev Biol. 21:247–269. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nishizawa T, Nakano K, Harada A, Kakiuchi
M, Funahashi SI, Suzuki M, Ishikawa S and Aburatani H: DGC-specific
RHOA mutations maintained cancer cell survival and promoted cell
migration via ROCK inactivation. Oncotarget. 9:23198–23207. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Céspedes MV, Casanova I, Parreño M and
Mangues R: Mouse models in oncogenesis and cancer therapy. Clin
Transl Oncol. 8:318–329. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bibby MC: Orthotopic models of cancer for
preclinical drug evaluation: Advantages and disadvantages. Eur J
Cancer. 40:852–857. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Killion JJ, Radinsky R and Fidler IJ:
Orthotopic models are necessary to predict therapy of
transplantable tumors in mice. Cancer Metastasis Rev. 17:279–284.
1999. View Article : Google Scholar
|
|
14
|
Nakano K, Nishizawa T, Komura D, Fujii E,
Monnai M, Kato A, Funahashi SI, Ishikawa S and Suzuki M: Difference
in morphology and interactome profiles between orthotopic and
subcutaneous gastric cancer xenograft models. J Toxicol Pathol.
31:293–300. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Junttila MR and de Sauvage FJ: Influence
of tumour micro- environment heterogeneity on therapeutic response.
Nature. 501:346–354. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Makalowski W, Zhang J and Boguski MS:
Comparative analysis of 1196 orthologous mouse and human
full-length mRNA and protein sequences. Genome Res. 6:846–857.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bainer R, Frankenberger C, Rabe D, An G,
Gilad Y and Rosner MR: Gene expression in local stroma reflects
breast tumor states and predicts patient outcome. Sci Rep.
6:392402016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Komura D, Isagawa T, Kishi K, Suzuki R,
Sato R, Tanaka M, Katoh H, Yamamoto S, Tatsuno K, Fukayama M, et
al: CASTIN: A system for comprehensive analysis of cancer-stromal
interactome. BMC Genomics. 17:8992016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Motoyama T, Hojo H and Watanabe H:
Comparison of seven cell lines derived from human gastric
carcinomas. Acta Pathol Jpn. 36:65–83. 1986.PubMed/NCBI
|
|
20
|
Busuttil RA, Liu DS, Di Costanzo N,
Schröder J, Mitchell C and Boussioutas A: An orthotopic mouse model
of gastric cancer invasion and metastasis. Sci Rep. 8:8252018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yanagihara K, Takigahira M, Tanaka H,
Komatsu T, Fukumoto H, Koizumi F, Nishio K, Ochiya T, Ino Y and
Hirohashi S: Development and biological analysis of peritoneal
metastasis mouse models for human scirrhous stomach cancer. Cancer
Sci. 96:323–332. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gu Z, Eils R and Schlesner M: Complex
heatmaps reveal patterns and correlations in multidimensional
genomic data. Bioinformatics. 32:2847–2849. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Langmead B, Trapnell C, Pop M and Salzberg
SL: Ultrafast and memory-efficient alignment of short DNA sequences
to the human genome. Genome Biol. 10:R252009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Love MI, Huber W and Anders S: Moderated
estimation of fold change and dispersion for RNA-seq data with
DESeq2. Genome Biol. 15:5502014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Subramanian A, Tamayo P, Mootha VK,
Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub
TR, Lander ES and Mesirov JP: Gene set enrichment analysis: A
knowledge-based approach for interpreting genome-wide expression
profiles. Proc Natl Acad Sci USA. 102:15545–15550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mootha VK, Lindgren CM, Eriksson KF,
Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E,
Ridderstråle M, Laurila E, et al: PGC-1alpha-responsive genes
involved in oxidative phosphorylation are coordinately
downregulated in human diabetes. Nat Genet. 34:267–273. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chapman S, McDermott DH, Shen K, Jang MK
and McBride AA: The effect of Rho kinase inhibition on long-term
keratinocyte proliferation is rapid and conditional. Stem Cell Res
Ther. 5:602014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Suzuki M, Katsuyama K, Adachi K, Ogawa Y,
Yorozu K, Fujii E, Misawa Y and Sugimoto T: Combination of fixation
using PLP fixative and embedding in paraffin by the AMeX method is
useful for histochemical studies in assessment of immunotoxicity. J
Toxicol Sci. 27:165–172. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sato Y, Mukai K, Watanabe S, Goto M and
Shimosato Y: The AMeX method. A simplified technique of tissue
processing and paraffin embedding with improved preservation of
antigens for immunostaining. Am J Pathol. 125:431–435.
1986.PubMed/NCBI
|
|
30
|
Corliss BA, Azimi MS, Munson JM, Peirce SM
and Murfee WL: Macrophages: An inflammatory link between
angiogenesis and lymphangiogenesis. Microcirculation. 23:95–121.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Squadrito ML and De Palma M: Macrophage
regulation of tumor angiogenesis: Implications for cancer therapy.
Mol Aspects Med. 32:123–145. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Madeddu C, Gramignano G, Kotsonis P, Coghe
F, Atzeni V, Scartozzi M and Macciò A: Microenvironmental M1
tumor-associated macrophage polarization influences cancer-related
anemia in advanced ovarian cancer: Key role of interleukin-6.
Haematologica. 103:e388–e391. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mantovani A and Allavena P: The
interaction of anticancer therapies with tumor-associated
macrophages. J Exp Med. 212:435–445. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Quatromoni JG and Eruslanov E:
Tumor-associated macrophages: Function, phenotype, and link to
prognosis in human lung cancer. Am J Transl Res. 4:376–389.
2012.PubMed/NCBI
|
|
35
|
Yin S, Huang J, Li Z, Zhang J, Luo J, Lu C
and Xu H and Xu H: The prognostic and clinicopathological
significance of tumor-associated macrophages in patients with
gastric cancer: A meta-analysis. PLoS One. 12:e01700422017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ohgushi M, Matsumura M, Eiraku M, Murakami
K, Aramaki T, Nishiyama A, Muguruma K, Nakano T, Suga H, Ueno M, et
al: Molecular pathway and cell state responsible for
dissociation-induced apoptosis in human pluripotent stem cells.
Cell Stem Cell. 7:225–239. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chung W, Eum HH, Lee HO, Lee KM, Lee HB,
Kim KT, Ryu HS, Kim S, Lee JE, Park YH, et al: Single-cell RNA-seq
enables comprehensive tumour and immune cell profiling in primary
breast cancer. Nat Commun. 8:150812017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Azizi E, Carr AJ, Plitas G, Cornish AE,
Konopacki C, Prabhakaran S, Nainys J, Wu K, Kiseliovas V, Setty M,
et al: Single-cell map of diverse immune phenotypes in the breast
tumor microenvironment. Cell. 174:1293–1308.e36. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lambrechts D, Wauters E, Boeckx B, Aibar
S, Nittner D, Burton O, Bassez A, Decaluwé H, Pircher A, Van den
Eynde K, et al: Phenotype molding of stromal cells in the lung
tumor microenvironment. Nat Med. 24:1277–1289. 2018. View Article : Google Scholar : PubMed/NCBI
|